Abstract
Ecological interactions play a fundamental role in determining the genetic structure of plant species in time and space. The demography of the Andean Puya hamata has been linked to fire regimes and hummingbird behaviour, which might modify the plant’s population genetic structure. Naturally, poor dispersal results in patches of genetically related plants, a pattern intensified further by burning which promotes seedling germination around parent plants. Later, when these plants flower, large patches are attractive to territorial hummingbirds which prevent visits by traplining hummingbird species, carrying pollen from likely unrelated plants. To explore this hypothesis, a genetic study of P. hamata using microsatellite markers was conducted with (i) isolated and grouped adults in two size categories of patches, and (ii) seeds collected from the same patches and isolated individuals. Isolated individual plants presented a higher observed heterozygosity with close to zero inbreeding. Adult plants from large patches showed a lower observed heterozygosity and higher inbreeding than plants from other spatial contexts. Seed genetic structure displayed a gradient of diversity: lower at patch centres but higher at patch edges, in small patches, and for isolated infructescences. The spatial context of these plants, especially the contrast between large patch centres and other situations, determines the genetic diversity of their seeds via hummingbird foraging behaviour. Territorial hummingbirds restrict gene flow in and out of large patches, but traplining hummingbirds maintain genetic diversity among isolated plants, small patches, and plants at the edges of large patches. Our study illustrates the need to consider interactions between land use, plants, and their pollinators when considering genetic diversity at the landscape scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The high-elevation tropical Andean páramo ecosystem is characterized by rich plant diversity and endemism (Madriñán et al. 2013; Sklenar et al. 2011). Additionally, páramos are hotspots for ecosystems services, including providing water reservoirs, carbon sequestration, irrigation, and rural livelihood improvement (Madriñán et al. 2013). However, páramo ecosystems face challenges like climate change (Aide et al. 2019; Carilla et al. 2018) and anthropogenic perturbations (Keating 2007; Vásquez et al. 2015) that threaten their ecological integrity. These challenges produce regional landscape transformations and ecosystem modifications (Aide et al. 2019), which strongly affect plant richness as well as biotic and abiotic interactions that shape the genetic structure of ecological communities (Gilman et al. 2010; Young and Leon 2007).
Pollination and seed dispersal are keystone interactions that shape plant species’ genetic structure (Midgley et al. 1991; Valenta et al. 2017). Within the context of climate change, these species interactions could alter, modifying communities and creating new scenarios for species diversification (Buermann et al. 2011; Gilman et al. 2010). These new scenarios could lead to novel ecological connections that may reduce plants’ genetic diversity and reproductive output through genetic drift and inbreeding (Chase et al. 1996; Fadrique et al. 2018). Thus, understanding the biotic and abiotic interactions that shape current plant populations’ genetic diversity in the páramo is important to be able to handle the unpredictable landscape transformations caused by climate change.
Puya hamata L.B. Sm. (Bromeliaceae) is a good biological model for exploring the impact of species interaction and environmental constraints on genetic diversity. P. hamata is a conspicuous giant basal rosette that forms dense local populations in humid páramos in Ecuador and Colombia (Schmidt Jabaily and Sytsma 2013) and has multiple ecological interactions with vertebrates (Garcia-Meneses and Ramsay 2014). Its seed dispersal is mainly by gravity and wind, and it has restricted seed mobility, which explains its aggregated distribution (Benzing 2000; Miller 1988). Additionally, P. hamata seeds have a higher germination rate, seedling recruitment, and survival in fire-prone páramos (Laegaard 1992). The plant is adapted to fire, with thermal insulation of its meristem, and seed germination occurs only at temperatures above 14 °C, which are often found in more open vegetation, often in recently burned tussock grassland (Garcia-Meneses and Ramsay 2014; Ramsay and Oxley 1996). These traits result in the formation of high-density patches of young plants derived from one or several parents in recently burned páramos (Garcia-Meneses and Ramsay 2014). These patches vary from isolated individuals to large patches with > 100 individuals, and this mosaic of patches covers dozens of square kilometers on the páramo. Thus, the páramo fires promote seed germination, but have only limited effects on the growth rate and survival of adult plants.
Moreover, P. hamata is a hummingbird-pollinated plant offering a concentrated nectar resource that attracts hummingbirds with a high metabolic rate (Altshuler and Dudley 2006; Woods and Ramsay 2001). Competition for nectar among pollinators often results in different foraging strategies (territorial or traplining; Rappole and Schuchmann 2003) that promote or inhibit the transfer of genetic material among the plants. This behaviour has been previously linked to the spatial distribution of nectar for P. hamata pollinators (Garcia-Meneses and Ramsay 2012; Woods and Ramsay 2001) and other high-elevation Andean species like Oreocallis grandiflora (Hazlehurst and Karubian 2016). Resource availability defines foraging behaviour that eventually structures plant population genetic diversity.
In summary, the fire response of P. hamata would be expected to drive spatial and genetic structure in adult plants, while hummingbird foraging behaviour, in response to the spatial pattern of flowering plants, would be expected to affect the genetic structure of seeds. However, P. hamata’s fine-scale genetic structure has not yet been explored; population genetic studies have focused mainly on P. raimondii (Hornung-Leoni et al. 2013; Sgorbati et al. 2004).
This study explores P. hamata populations’ genetic diversity and structure in the northern Andes of Ecuador. We will test the following ecological hypotheses related to P. hamata’s dispersal and pollination using molecular markers, following ideas proposed by Garcia-Meneses and Ramsay (2012): (i) we expect individuals in patches to have low genetic diversity and high rates of inbreeding, due to the plant’s limited seed dispersal and germination response to fire, while isolated individuals’ genetic composition would be more diverse, because they come from many provenances; (ii) we expect that seeds from plants in the centre of large patches will exhibit lower genetic diversity and higher inbreeding (better defended by territorial hummingbirds) than those from patch edges, smaller patches, or isolated individuals (influenced more by traplining hummingbirds).
Materials and methods
Model system and study site
The genus Puya (Bromeliaceae) comprises conspicuous rosettes widely distributed on the páramos from Costa Rica to northern Argentina and Chile (Sklenar et al. 2011). P. hamata is a rosette that forms locally clumped populations in humid páramos between 3300 and 3700 m asl in Colombia, Ecuador and Peru (Miller and Silander 1991; Schmidt Jabaily and Sytsma 2013). P. hamata has a rosette reaching > 2 m in diameter and a 4-m tall inflorescence with around 1000 flowers (Garcia-Meneses and Ramsay 2012), attracting hummingbirds (Miller 1986).
The study area comprises the humid páramos on the southern slopes of Volcán Chiles on the Ecuador-Colombian border (Carchi Province, UTM coordinates: 18 N 173248 89318), between 3400 and 4200 m asl, with an annual precipitation of 1000–1500 mm and constant environmental humidity (Hofstede et al. 1998). This area is owned by Comuna La Esperanza farmers in Tufiño who, in the past, have used burning as an agricultural tool associated with livestock grazing, leading to a landscape mosaic of areas with different times since fire and patches of P. hamata of different ages (Garcia-Meneses and Ramsay 2014).
Sampling method
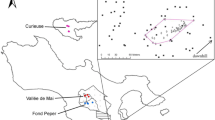
The experimental design comprised two surveys across a variety of spatial contexts for P. hamata: (i) leaf sampling to explore the genetic structure of adult individuals; and (ii) seed sampling to assess their genetic structure. Leaves were sampled in August 2013 from 20 isolated individuals (I), 35 individuals from three small patches of > 5 and < 20 individuals (S1 n = 12; S2 n = 10; S3 n = 13); and 39 individuals from three large patches, each > 100 individuals (L1 n = 13, L2 n = 13, L3 n = 13). In total, 94 individuals were sampled (Fig. 1).
Study site. The location of the 74 P. hamata adults (red color): 15 isolated plants (circles: I66–I80), small patches (squares: S1, S2, S3), and large patches (triangles: L1, L2, L3). The location of the 20 P. hamata infructescence (blue color): isolated plants (circles: I3, I4, I5, I7, I8, I10), small patches (squares: S2, S3, S4, S7), and large patches (triangle: LC1–LC5 + LE1–LE5)
Seed sampling occurred in February 2014 and comprised eight infructescences from isolated individuals (I), six infructescences from individuals in small patches (S), and 14 infructescences from individuals from a single large patch of which seven were from individuals on the patch edge (LE) and seven from individuals in the patch centre (LC). From each infructescence, we randomly selected 10 seeds from different sections of the infructescence. A total of 28 infructescences were collected (280 seeds).
DNA isolation, primer screening, and genotyping
DNA extraction from the leaf samples followed Doyle (1991)’s method. DNA extraction from seeds was done using the Wizard genomic DNA Purification Kit according to the manufacturer’s instructions. A set of five microsatellite markers (simple sequence repeats or SSRs) of genera Aechmea (Bromeliaceae) and 15 SSRs of Ananas (Bromeliaceae) were chosen to be transferred to P. hamata. Polymerase chain reaction (PCR) amplifications followed Goetze et al. (2013) and Wöhrmann and Weising (2011). PCR products were separated by electrophoresis in 6% denatured polyacrylamide gels and silver nitrate staining. Samples without an amplification of at least four loci were not considered for the study.
Statistical analysis
Genetic data derived from adult individuals and infructescences were evaluated at two different levels: (i) genetic structure and (ii) patch arrangement. Genetic structure was evaluated with GenAlEx 6.5 (Peakall and Smouse 2006) using 10,000 permutations. The inbreeding coefficient (Fis), the number of private alleles (PA, as a measure of flow gene), average expected heterozygosity (He, genetic diversity), and observed heterozygosity (Ho) were calculated for adults and seeds. Allelic richness (AR) within each patch and isolated individuals was computed by the rarefaction method implemented in HP-RARE (Kalinowski, 2005). Deviations from Hardy–Weinberg equilibrium (HWE) and linkage disequilibrium for adults and seeds were computed using GENEPOP v4.2 with default parameters (Raymond and Rousset 1995). Statistical differences among the genetic indexes were computed with a Mann–Whitney U test using XLSTAT for Excel (Addinsoft Inc, New York, USA).
A genetic distance-based analysis (patch arrangement) was conducted to visualize the genetic composition among adults and seeds. A principal coordinate analysis (PCoA) was performed using Codom-Genotypic distance as implemented by GenAlEx 6.5. Seeds PCoA was conducted using a pooled data set of seeds for each infructescence. A second PCoA was conducted among P. hamata adults. A Bayesian clustering analysis was performed to determine genetic similarity among adults and seeds using Structure v2.3 (Pritchard et al. 2000). An admixture mode and simulations were performed with a burn-in length of 1,000,000 repetitions followed by 2,000,000 Markov-Chain Monte Carlo replicates. The number of distinct genetic clusters (K) present in the data set was from 1 to 10 using 5 iterations per K. We used the ΔK method (Evanno et al. 2005) as implemented in STRUCTURE HARVESTER v.0.6.94 (Earl and von Holdt 2012) to detect the number of K that best fits the data.
Three distance matrices were created: a spatial matrix using distance (m) between pairs of plants, calculated with Esri ArcMapTM 10.2.0.3348 (Environmental Systems Research Institute, Redlands, CA, USA); a genetic matrix using Codom-Genotypic distance between plants; and an altitude matrix of altitudinal distance (m) between plants. A Mantel test analysis was conducted to examine relationships between the matrices independently for adults and seeds using GenAlEx 6.5 with 10,000 permutations.
Results
Transfer of molecular markers and DNA isolation
Six out of 20 SSRs’ loci were successfully transferred to P. hamata: five loci of Ananas comosus and one SSR of Aechmea caudata. Among the loci transferred, Acom 64.22 was monomorphic, while the others were polymorphic ranging from 3 to 13 alleles (Table 1). We used the five polymorphic loci for the genetic analysis. Of the 94 adult individuals, only 74 gave a positive signal for DNA isolation (Table 2). P. hamata seeds are less than 2 mm in size and were, therefore, troublesome for the DNA isolation and PCR amplification. Of the 280 seeds sampled, only 187 seeds (grouped in 20 infructescences) gave a positive signal for DNA isolation (Table 3). Linkage disequilibrium was not significant (P > 0.05) for any pair of loci when tested within clusters of adults and seeds (Online Resource 1), supporting the assumption of loci independence. Significant deviations from HWE were computed within patches for adults and seeds (P < 0.05; Tables 2, 3).
Diversity and genetic structure
The allelic richness showed no evident pattern among patches. Meanwhile, the observed heterozygosity (Ho) was higher for the small patch S3 and for isolated individuals, and expected heterozygosity (He) was higher for isolated individuals. Private alleles were only present for isolated plants and ones from large patches. Plants in large patches tended to be more homozygous than the HWE expectation (range 0.17–0.32; Table 2).
PCoA analysis placed the 74 adult plants into three weakly defined clusters (A, B, C; Fig. 2), which correlated somewhat with the large patches in this study: A (n = 32) included 7/10 L1 individuals plus others; B (n = 16) included all L2 individuals plus four individuals from other patches; and C (n = 21) included 7/12 L3 individuals plus others. Five individuals from small patches and isolated individuals were placed outside these analysis clusters (Fig. 2a). By contrast, Bayesian analysis recognized only two groups (ΔK = 2, Fig. 2b): K1 (n = 23) corresponded to PCoA cluster B and K2 (n = 51) included the rest of the individuals (Online Resource 2). K1 mostly comprised individuals from altitudes ≥ 3980 masl, while K2 those from < 3980 masl (Fig. 2c).
a Principal coordinates analysis (PCoA) of 74 P. hamata adult individuals. The two PCoA axes explain 63.18% of the variance. Isolated plants (orange circles), small patches (squares: light orange S1, orange S2, dark orange S3), and large patches (triangles: light orange L1, orange L2, dark orange L3) are shown. Only the 23 individuals clustered by K1 are named in the PCoA. b Schematic representation of Bayesian analysis by STRUCTURE with the respective Delta K/K plot. c Geographical location of the P. hamata adult individuals based on the Bayesian analysis
The allelic richness in seeds decreased from those of small patches to those from plants in the centre of large patches (Table 3), with significant differences for S-LE (Mann–Whitney U test: U = 15; P = 0.057) and S-LC (U = 20; P = 0.02). Only isolated plants had private alleles. Statistically significant differences were detected for genetic diversity among S–LC (U = 20; P = 0.02) and LE–LC (U = 19.5; P = 0.01); and for observed heterozygosity between S–LC (U = 19; P = 0.03) and LC–LE (U = 20; P = 0.02).
Four PCoA clusters were derived from the 20 infructescences (W, X, Y, Z; Fig. 3). W comprised mostly isolated infructescences, X all small patches, Y mostly edges of large patches, and Z all centres of large patches (Fig. 3a). Bayesian analysis with 187 seeds from the 20 infructescences detected three groups (ΔK = 3, Fig. 3b; Online Resource 3). This grouping pattern strongly matched those from PCoA, where K1 corresponded to W, K2 to Z + Y, and K3 to X. Unlike PCoA, Bayesian analysis did not detect genetic differentiation between infructescences from the large patches’ centres and edges.
a Principal coordinates analysis (PCoA) of 20 P. hamata seeds/infructescences. The two PCoA axes explain 62.03% of the variance. Isolated plants (blue circles), small patches (blue squares), centres of large patches (blue triangles), and edges of large patches (blue diamonds) are shown. b Schematic representation of Bayesian analysis by STRUCTURE: K1 (n = 50), K2 (n = 88) and K3 (n = 49), with the respective Delta K/K plot. c Geographical location of the seeds/infructescences based on the Bayesian analysis
Nearby patches tended to be genetically more similar than expected by chance, and genetic differences increased linearly with spatial distances and altitude (Table 4).
Discussion
Effect of fire on the genetic structure of P. hamata individuals
Isolated adults and small patches had higher values for both expected and observed heterozygosity than larger patches. In addition, larger patches presented high values of inbreeding. Limited seed dispersal in Puya explains the higher inbreeding found in large patches, where most plants would be expected to derive from the same parent after fire had opened up the vegetation canopy (Garcia-Meneses and Ramsay 2014). By contrast, the higher genetic diversity among isolated plants and small patches can be explained by their probable origin as rare, unconnected seed dispersal events from distant parents. Thus, the spatial context of adult Puya plants is linked to their heterozygosity, allelic richness and inbreeding levels.
The genetic diversity of P. hamata adults in this study was relatively high and corresponded to a wider genetic pool when compared with some other Puya species. For instance, Sgorbati et al. (2004), applying AFLP and cpSSRs markers, found a low and extremely uniform genetic diversity among P. raimondii populations distributed in Peru’s central and southern Andes, which suggests a single progenitor causes genetic uniformity among and within P. raimondii populations. Conversely, Hornung-Leoni et al. (2013), working with several P. raimondii populations from Peru’s central Andes and using AFLP markers, reported a high level of genetic variation among populations. Geographical bias in sampling and limitations in technical tools could explain this significant difference (Hornung-Leoni et al. 2013). In particular, the nature of the genetic marker types needs to be interpreted carefully, because the unknown marker properties like mutation rate would affect the levels of variation. Our study revealed higher levels of genetic diversity at a finer spatial scale than the aforementioned studies. It would be possible to observe both high and low genetic diversity within the same Puya population, depending on whether samples were taken from isolated individuals or ones from the centres of large patches. The spatial context of the sampled plants should be accounted for in future studies, and samples of isolated individuals would provide better estimates of genetic diversity for an area than samples from within large patches of P. hamata.
The moderate levels of genetic diversity exhibited by P. hamata plants (mean He= 0.46 ± 0.07 SD) are comparable with other Andean plant studies at similar spatial scale and with similar molecular markers. Almeida et al. (2013) reported higher genetic diversity values (range He = 0.70–0.76) for perennial evergreen Lasiocephalus ovatus populations, but Vásquez et al. (2016) found several populations of the long-lived semelparous giant rosette Lupinus alopecuroides had lower diversity (range He = 0–0.51).
Effect of pollinator behaviour on the genetic structure of seeds of P. hamata
A fine-scale genetic structure was detected among P. hamata seeds. This pattern can be explained by (i) the isolated or aggregated spatial context of infructescences, and (ii) the location of infructescences within larger patches (edge or centre). There was a diversity gradient from the centres of large patches (less diversity) to the edges of large patches, small patches, and isolated plants (more diversity), consistent with the hypothesis of Garcia-Meneses and Ramsay (2012). Traplining hummingbirds, moving from plant to plant at a landscape scale, forage more frequently on P. hamata inflorescences in isolated, small-patch or patch-edge contexts, while the centres of larger patches are well defended by territorial hummingbirds against traplining competitors (Woods and Ramsay 2001). This favours outcrossing in isolated, small-patch or patch-edge contexts and boosts genetic diversity. The territorial defence of patch centres reduces the probability of incorporating new genetic material from other populations (Groom 1998; Woods and Ramsay 2001). Territory size for the Aglaeactis cupripennis hummingbird varied from 0.03 to 0.54 ha in Ecuadorian and Peruvian montane forest (Céspedes et al. 2019), but the concentrated nectar sources in large P. hamata patches are likely to result in smaller end of this range in the páramo. Nevertheless, territorial individuals are often unable to defend all plants in their territory simultaneously, allowing trapliners to enter territories for short feeding bouts, especially at the margins. The emergence of traplining or territoriality as foraging strategies in hummingbirds has been linked to ecological and evolutionary strategies to reduce competition between these pollinators (Rappole and Schuchmann 2003) and is likely to be robust in the face of changes in hummingbird composition from place to place. In places where A. cupripennis is absent, other hummingbird species have been observed to establish territories around P. hamata patches (Miller 1988), maintaining the barrier for outcrossing in patch centres.
Altitude partially explained the genetic relationships of adults and seeds, with some differentiation between individuals at higher versus lower altitudes. This was expected, since altitude controls the altitudinal movement of genotypes (Almeida et al. 2013) as well as hummingbird movements (Buermann et al. 2011; Ohsawa and Ide 2008; Rappole and Schuchmann 2003). However, seeds from small patches of P. hamata were defined as a single, discrete genetic cluster, despite the altitudinal range they occupied (3647–4042 m). Although spatially and altitudinally distant, genetic flux existed among these small patches, which resulted in similar genetic composition. It seems likely that traplining hummingbirds transfer genes among these patches over a wide altitudinal range. Traplining hummingbirds not only visit widely separated individuals (Gill 1988), but also fly over greater altitudinal ranges compared to territorial hummingbirds (Barbará et al. 2007; López-Segoviano et al. 2018). In our study, the territorial species, A. cupripennis, is more often found in cloud forest at lower altitudes, but forages in the lower reaches of páramo grasslands, especially during Puya flowering events.
There is some evidence in our study for different pollinator behaviour for small patches compared with isolated plants. Seeds were genetically distinct for these categories (Fig. 3). We do not have an evidenced explanation for this pattern, but we speculate that different traplining species might partition their foraging, such that some species might specialise on small patches, while others focus on isolated plants. This would require confirmation with behavioural studies.
The incorporation of isolated plants and small patches into traplining by pollinators could move alleles among larger patches of Puya, with the isolated plants and small patches acting as stepping stones, in the manner suggested by Howe and Miriti (2004). In this regard, the ecological and genetic contribution of isolated individuals located between forest remnants has already been reported in the context of tropical forest ecology (Chase et al. 1996; Fuchs and Hamrick 2010; Guevara et al. 1992).
Conclusion
The spatial context of P. hamata plants at a landscape scale is driven by burning, common throughout the páramo grasslands of the Andes and Central America (Horn and Kappelle 2009). P. hamata patchiness in association with burning has been noted several times (Garcia-Meneses and Ramsay 2014; Laegaard 1992; Miller and Silander 1991), and in its absence, Puya species would be rarer and the population would consist mostly of isolated individuals or small patches. Only in the presence of burning would large patches of Puya be expected. Thus, the genetic patterns reported here are the outcome of the interaction of burning influences on patchiness of adult plants (driven by germination requirements and poor seed dispersal) and pollinator behaviour in response to patchiness of nectar resources (specifically territoriality).
Andean mountains are particularly at risk from climate change (Urrutia and Vuille 2009), with more rapid change expected at higher elevations (Pepin et al. 2015). The impact of this warming, alongside land-use changes, makes it difficult to predict species’ responses (Buermann et al. 2011; Frei et al. 2010) and the influence of burning on future páramo landscapes. Temperature increases at the páramo-forest ecotone will favour more agricultural activity and, consequently, increase anthropogenic fires (Anderson et al. 2011). In addition, potentially drier conditions in the páramo zone could favour the rapid spread of fires forming larger patches (Ruiz Carrascal et al. 2011), changing the balance of large patches versus small patches and isolated plants. The dominance of larger patches in a population could mean a loss of genetic diversity in P. hamata. It is clear from our work that isolated and small-patch Puya plants are a significant genetic resource that should be taken into account when developing management strategies for these plants and their pollinators.
In conclusion, poorly dispersed P. hamata seeds with particular germination requirements lead to the formation of large patches of closely related plants in burned landscapes. Territorial hummingbirds restrict gene flow in and out of these large patches, but traplining hummingbirds maintain genetic diversity among small patches and isolated plants. These traplining foragers also introduce genetic diversity to plants at the edges of large patches. All of this is consistent with the findings of Garcia-Meneses and Ramsay (2012) on reproductive output and their predictions on gene flow. Our study illustrates the need to consider interactions between land use, plants, and their pollinators to maintain genetic diversity at the landscape scale.
Data availability
The datasets generated during the current study are available at the DRYAD repository: https://doi.org/10.5061/dryad.g79cnp5kq.
References
Aide TM, Grau HR, Graesser J et al (2019) Woody vegetation dynamics in the tropical and subtropical Andes from 2001 to 2014: satellite image interpretation and expert validation. Glob Change Biol 25:2112–2126. https://doi.org/10.1111/gcb.14618
Almeida JP, Montúfar R, Anthelme F (2013) Patterns and origin of intraspecific functional variability in a tropical alpine species along an altitudinal gradient. Plant Ecol Divers 6:423–433. https://doi.org/10.1080/17550874.2012.702137
Altshuler DL, Dudley R (2006) The physiology and biomechanics of avian flight at high altitude. Integr Comp Biol 46:62–71. https://doi.org/10.1093/icb/icj008
Anderson EP, Marengo J, Villalba R et al (2011) Consequences of climate change for ecosystems and ecosystem services in the Tropical Andes. In: Herzog SK, Martínez R, Jørgensen PM, Tiessen H (eds) Climate change and biodiversity in the Tropical Andes. Inter-American Institute for Global Change Research, São José dos Campos, pp 1–18
Barbará T, Martinelli G, Fay MF et al (2007) Population differentiation and species cohesion in two closely related plants adapted to neotropical high-altitude ‘inselbergs’, Alcantarea imperialis and Alcantarea geniculata (Bromeliaceae). Mol Ecol 16:1981–1992. https://doi.org/10.1111/j.1365-294X.2007.03272.x
Benzing DH (2000) Bromeliaceae: profile of an adaptive radiation. Cambridge University Press, Cambridge
Buermann W, Chaves JA, Dudley R et al (2011) Projected changes in elevational distribution and flight performance of montane Neotropical hummingbirds in response to climate change. Glob Change Biol 17:1671–1680. https://doi.org/10.1111/j.1365-2486.2010.02330.x
Carilla J, Halloy S, Cuello S et al (2018) Vegetation trends over eleven years on mountain summits in NW Argentina. Ecol Evol 8:11554–11567. https://doi.org/10.1002/ece3.4602
Céspedes LN, Pavan LI, Hazlehurst JA, Jankowski JE (2019) The behavior and diet of the Shining Sunbeam (Aglaeactis cupripennis): a territorial high-elevation hummingbird. Wilson J Ornithol 131:24–34. https://doi.org/10.1676/18-79
Chase MR, Moller C, Kesseli R, Bawa KS (1996) Distant gene flow in tropical trees. Nature 383:398–399. https://doi.org/10.1038/383398a0
Doyle J (1991) DNA protocols for plants. In: Hewitt GM, Johnston AWB, Young JPW (eds) Molecular techniques in taxonomy. Springer, Berlin, pp 283–293. https://doi.org/10.1007/978-3-642-83962-7_18
Earl DA, von Holdt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361. https://doi.org/10.1007/s12686-011-9548-7
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620. https://doi.org/10.1111/j.1365-294X.2005.02553.x
Fadrique B, Báez S, Duque Á et al (2018) Widespread but heterogeneous responses of Andean forests to climate change. Nature 564:207–212. https://doi.org/10.1038/s41586-018-0715-9
Frei E, Bodin J, Walther G-R (2010) Plant species’ range shifts in mountainous areas—all uphill from here? Bot Helv 120:117–128. https://springerlink.bibliotecabuap.elogim.com/article/10.1007%2Fs00035-010-0076-y
Fuchs EJ, Hamrick JL (2010) Genetic diversity in the endangered tropical tree, Guaiacum sanctum (Zygophyllaceae). J Hered 101:284–291. https://doi.org/10.1093/jhered/esp127
Garcia-Meneses PM, Ramsay PM (2012) Pollinator response to within-patch spatial context determines reproductive output of a giant rosette plant. Basic Appl Ecol 13:516–523. https://doi.org/10.1016/j.baae.2012.08.011
Garcia-Meneses PM, Ramsay PM (2014) Puya hamata demography as an indicator of recent fire history in the páramo of El Ángel and Volcán Chiles, Ecuador-Colombia. Caldasia 36:53–69. https://doi.org/10.15446/caldasia.v36n1.43891
Gill FB (1988) Trapline foraging by hermit hummingbirds: competition for an undefended, renewable resource. Ecology 69:1933–1942. https://doi.org/10.2307/1941170
Gilman SE, Urban MC, Tewksbury J et al (2010) A framework for community interactions under climate change. Trends Ecol Evol 25:325–331. https://doi.org/10.1016/j.tree.2010.03.002
Goetze M, Louzada RB, Wanderley MdGL et al (2013) Development of microsatellite markers for genetic diversity analysis of Aechmea caudata (Bromeliaceae) and cross-species amplification in other bromeliads. Biochem Syst Ecol 48:194–198. https://doi.org/10.1016/j.bse.2012.12.022
Groom MJ (1998) Allee effects limit population viability of an annual plant. Am Nat 151:487–496. https://doi.org/10.1086/286135
Guevara S, Meave J, Moreno-Casasola P, Laborde J (1992) Floristic composition and structure of vegetation under isolated trees in neotropical pastures. J Veg Sci 3:655–664. https://doi.org/10.2307/3235833
Hazlehurst JA, Karubian JO (2016) Nectar robbing impacts pollinator behavior but not plant reproduction. Oikos 125:1668–1676. https://doi.org/10.1111/oik.03195
Hofstede R, Lipss J, Jongsma W (1998) Geografía, ecología y forestación de la sierra alta del Ecuador: revisión de la literatura. Ediciones Abya-Yala, Quito
Horn SP, Kappelle M (2009) Fire in the Páramo ecosystems of Central and South America. In: Cochrane MA (ed) Tropical fire ecology: climate change, land use, and ecosystem dynamics. Springer Praxis Books, Heidelberg, pp 505–539. https://doi.org/10.1007/978-3-540-77381-8_18
Hornung-Leoni CT, Sosa V, Simpson J, Gil K (2013) Genetic variation in the emblematic Puya raimondii (Bromeliaceae) from Huascaran National Park, Peru. Crop Breed Appl Biotechnol 13:67–74. https://doi.org/10.1590/S1984-70332013000100008
Howe HF, Miriti MN (2004) When seed dispersal matters. Bioscience 54:651–660. https://doi.org/10.1641/0006-3568(2004)054%5b0651:WSDM%5d2.0.CO;2
Jabaily RS, Sytsma KJ (2013) Historical biogeography and life-history evolution of Andean Puya (Bromeliaceae). Bot J Linn Soc 171:201–224. https://doi.org/10.1111/j.1095-8339.2012.01307.x
Kalinowski ST (2005) HP-RARE 1.0: a computer program for performing rarefaction on measures of allelic richness. Mol Ecol Notes 5:187–189. https://doi.org/10.1111/j.1471-8286.2004.00845.x
Keating PL (2007) Fire ecology and conservation in the high tropical Andes: observations from northern Ecuador. J Lat Am Geogr 6:43–62. https://muse.jhu.edu/article/212941
Laegaard S (1992) Influence of fire in the grass Páramo vegetation of Ecuador. In: Balslev H, Luteyn JL (eds) Páramo: an Andean ecosystem under human influence. Academic, London, pp 151–170
López-Segoviano G, Bribiesca R, Arizmendi M (2018) The role of size and dominance in the feeding behaviour of coexisting hummingbirds. Ibis 160:283–292. https://doi.org/10.1111/ibi.12543
Madriñán S, Cortés AJ, Richardson JE (2013) Páramo is the world’s fastest evolving and coolest biodiversity hotspot. Front Genet 4:192. https://doi.org/10.3389/fgene.2013.00192
Midgley JJ, Bond WJ, Jarzembowski EA, Grubb PJ (1991) How important is biotic pollination and dispersal to the success of the angiosperms? Philos Trans R Soc B 333:209–215. https://doi.org/10.1098/rstb.1991.0069
Miller GA (1986) Pubescence, floral temperature and fecundity in species of Puya (Bromeliaceae) in the Ecuadorian Andes. Oecologia 70:155–160. https://doi.org/10.1007/BF00377126
Miller GA (1988) The population biology and physiological ecology of species of Puya (Bromeliaceae) in the Ecuadorian Andes. PhD Thesis, University of Connecticut
Miller GA, Silander JA Jr (1991) Control of the distribution of giant rosette species of Puya (Bromeliaceae) in the Ecuadorian páramos. Biotropica 23:124–133. https://www.jstor.org/stable/i316804
Ohsawa T, Ide Y (2008) Global patterns of genetic variation in plant species along vertical and horizontal gradients on mountains. Glob Ecol Biogeogr 17:152–163. https://doi.org/10.1111/j.1466-8238.2007.00357.x
Peakall R, Smouse PE (2006) GenAlEx 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295. https://doi.org/10.1093/bioinformatics/bts460
Pepin N, Bradley RS, Diaz HF et al (2015) Elevation-dependent warming in mountain regions of the world. Nat Climate Change 5:424–430. https://doi.org/10.1038/nclimate2563
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Ramsay PM, Oxley ERB (1996) Fire temperatures and postfire plant community dynamics in Ecuadorian grass paramo. Vegetatio 124:129–144. https://doi.org/10.1007/BF00045489
Rappole JH, Schuchmann K-L (2003) Ecology and evolution of hummingbird population movements and migration. In: Berthold P, Gwinner E, Sonnenschein E (eds) Avian migration. Springer, Berlin, pp 39–51. https://doi.org/10.1007/978-3-662-05957-9_3
Raymond M, Rousset F (1995) GENEPOP (Version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249. https://doi.org/10.1093/oxfordjournals.jhered.a111573
Ruiz Carrascal D, Arroyave Maya MdP, Gutiérrez Lagoueyte ME, Zapata Jaramillo PA (2011) Increased climatic stress on High-Andean ecosystems in the Cordillera Central of Colombia. In: Herzog SK, Martínez R, Jørgensen PM, Tiessen H (eds) Climate change and biodiversity in the Tropical Andes. Inter-American Institute for Global Change Research, São José dos Campos, pp 182–191
Sgorbati S, Labra M, Grugni E (2004) A survey of genetic diversity and reproductive biology of Puya raimondii (Bromeliaceae), the endangered Queen of the Andes. Plant Biol 6:222–230. https://doi.org/10.1055/s-2004-817802
Sklenar P, Duskova E, Balslev H (2011) Tropical and temperate: evolutionary history of Páramo flora. Bot Rev 77:71–108. https://doi.org/10.1007/s12229-010-9061-9
Urrutia R, Vuille M (2009) Climate change projections for the tropical Andes using a regional climate model: temperature and precipitation simulations for the end of the 21st century. J Geophys Res Atmos. https://doi.org/10.1029/2008JD011021
Valenta K, Nevo O, Martel C, Chapman CA (2017) Plant attractants: integrating insights from pollination and seed dispersal ecology. Evol Ecol 31:249–267. https://doi.org/10.1007/s10682-016-9870-3
Vásquez DLA, Balslev H, Sklenář P (2015) Human impact on tropical-alpine plant diversity in the northern Andes. Biodivers Conserv 24:2673–2683. https://doi.org/10.1007/s10531-015-0954-0
Vásquez DLA, Balslev H, Hansen MM, Sklenář P, Romoleroux K (2016) Low genetic variation and high differentiation across sky island populations of Lupinus alopecuroides (Fabaceae) in the northern Andes. Alpine Bot 126:135–142. https://doi.org/10.1007/s00035-016-0165-7
Wöhrmann T, Weising K (2011) In silico mining for simple sequence repeat loci in a pineapple expressed sequence tag database and cross-species amplification of EST-SSR markers across Bromeliaceae. Theor Appl Genet 123:635–647. https://doi.org/10.1007/s00122-011-1613-9
Woods S, Ramsay PM (2001) Variability in nectar supply: implications for high-altitude hummingbirds. In: Ramsay PM (ed) The ecology of Volcán Chiles: high-altitude ecosystems on the Ecuador-Colombia border. Pebble & Shell, Plymouth, pp 209–217
Young KR, Leon B (2007) Tree-line changes along the Andes: implications of spatial patterns and dynamics. Philos Trans R Soc B 362:263–272. https://doi.org/10.1098/rstb.2006.1986
Acknowledgements
The Ministerio del Ambiente del Ecuador gave us permission to collect the samples (permits 015-1C-DFP and 001-IC-FLO-DPAC). Thanks to J. Karubian for his comments to this manuscript.
Funding
This work was supported by the Pontificia Universidad Católica del Ecuador [Dinámica genética de Puya J13165/I13165].
Author information
Authors and Affiliations
Contributions
PMR and RM had the original idea and designed the research; GR, RM, and PMR collected plant material, and conducted genetic and statistical analyses; RM, PMR, and GR wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
Data and findings presented in this manuscript have not been published and are not under consideration for publication elsewhere. All the authors have approved this submission and all persons entitled to authorship have been named. The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rivadeneira, G., Ramsay, P.M. & Montúfar, R. Fire regimes and pollinator behaviour explain the genetic structure of Puya hamata (Bromeliaceae) rosette plants. Alp Botany 130, 13–23 (2020). https://doi.org/10.1007/s00035-020-00234-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00035-020-00234-7