Abstract
Effects caused by the invasive American bullfrog (Lithobates catesbeianus) are poorly understood at the community level. This study assessed the effects of a small feral bullfrog population (at lag invasion phase) on the different components of native aquatic communities (phytoplankton, zooplankton, invertebrates, fish and amphibians) of Aceguá, Uruguay. Our interest focused on exploring the early local effects of this invasion. We explored whether there existed any difference in taxa richness, abundances and size structure in association to bullfrog invasion, using four seasonal sampling of all the lentic system around the foci (two invaded and five non-invaded ponds). We analysed the occurrence of differences between the invaded and the non-invaded communities: for the taxa richness we used rarefaction, for the abundances and body sizes we used mean tests, and for the tadpole developmental stages we used G-test. We only found statistically significant bullfrog effects, in fish and anuran larvae. The fish assemblage was favoured, reaching greater abundance and body size in the bullfrog invaded ponds. In these ponds, the nektonic tadpoles diminished their abundances, and the benthic tadpoles also reached greater body sizes, but decreasing their recruitment. Our results suggest that bullfrog invasion could have complex effects, acting asymmetrically, affecting different trophic paths, and depending on native species’ habits and attributes. Understanding these effects, in early invasion foci, has a great relevance to awareness of local environmental authorities and the implementation of management plans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasions are a major factor affecting global biodiversity, and are associated with the decline and the extinction of a large number of species (Clavero and Garcia-Berthou 2005; Bellard et al. 2016). Invasions have increased with rising commerce and this trend is expected to continue, especially in aquatic systems (Sala et al. 2000; Havel et al. 2015). Despite the importance of invasions, knowledge about their effects at different scales of ecological organization is scarce. Most of the existing empirical evidence comes from ecological studies of the effect of invasive species on a single native species and studies exploring interactions at the community level are scarce (White et al. 2006). This becomes even more complex if we consider that most of the available evidence comes from studies in the exponential growth phase, when invasive species increases their abundances, and so their effects are more noticeable. Unfortunately, the dynamics and interaction mechanisms during establishment (at early invasion phase, when population remains restricted in abundance and distribution), which could predict the invasion success, are poorly evaluated (Crooks 2005). Taken together, these poorly understood areas limit the development of management strategies (Simberloff 2004). This situation is even more noticeable in South America, where few species have been studied, mostly reporting their distribution or occurrence (Speziale et al. 2012).
An invasive species can interact differently with each component of the native community; these interactions could be positive, negative, or neutral (Rodriguez 2006). Larger scale studies on the effects of exotic species show the occurrence of drastic effects on communities and ecosystems, mediated by trophic cascades, alteration in nutrient cycling and habitat modification (Simberloff et al. 2013). Some studies have demonstrated that the introduction of large aquatic predators can produce radical changes in the invaded communities (Baxter et al. 2004; Bwanika et al. 2006). An interesting case is that of the American bullfrog Lithobates catesbeianus (Shaw, 1802) (= Rana catesbeiana), considered among the most harmful aquatic invasive species (Lowe et al. 2000). Given their ability to establish in large numbers and voracious appetite, bullfrogs can cause significant impacts on native biodiversity (Kraus 2009). Bullfrog can alter native communities via predation, competition or habitat modification (Kiesecker and Blaustein 1997a; Adams 2000; Kiesecker et al. 2001; Adams et al. 2003; Adams and Pearl 2007). At the same time, this species can be benefit from indirect trophic interactions with native and exotic fishes (Adams et al. 2003; Babbitt et al. 2003; Maezono and Miyashita 2003). Since most of the available evidence focuses on simple interactions (restricted to one or a few species), it is difficult to understand the effects of bullfrog invasion at the community level (Kraus 2009).
One could hypothesize that bullfrog invasion will have strong structural effects on native communities. Native prey would not be able to recognize this novel predator (Polo-Cavia et al. 2010), which due to its large body size and broad trophic niche, will be the top predator (Jancowski and Orchard 2013). Native species will be affected differentially, according to their habits and encounter probability (Pearl et al. 2004; reviewed by Adams and Pearl 2007; Kraus 2009). In addition, this strong new competitor at high densities, will affect those species that share resources (Kupferberg 1997; Boelter and Cechin 2007). In this context, amphibians will be one of the most affected groups (Blaustein and Kiesecker 2002; Boone et al. 2004; Li et al. 2011). As a consequence, we predict that these mechanisms will generate a simplified community structure at the local scale following bullfrog invasion. Bullfrog effects will be stronger in some native species abundances and body sizes, as a direct consequence of predation. Species escaping bullfrog predation will reach higher abundances and/or body sizes. Considering the strong effects reported for this invasive anuran, we should notice significant community changes during the lag phase.
Our objective was to explore the effects of a recently introduced bullfrog population across a wide range of taxa (from primary producers to vertebrates) in aquatic communities in Aceguá, Cerro Largo Department, northeastern Uruguay. This incipient invasion was restricted to a small number of lentic water bodies (lag phase according to Laufer et al. 2018). A methodological challenge of this study was the intrinsic problem of empirical evaluations of biological invasions at early stages: the low number of invaded communities, hampering comparisons and statistical analyses. Considering the importance of studying the early stages for invasion management (Simberloff 2014), we provided special emphasis in our methodology to counteract these empirical limitations. Therefore, we explored data recorded during four consecutive seasons with a large number of samples and including more than twice as many control (non-invaded) than invaded communities.
Materials and methods
Study site
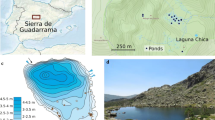
The locality of Aceguá is an upland area (approx. 220 m a.s.l.), located in the Cuenca Sedimentaria Gondwánica ecoregion. Two hydrographic basins exist in this site: the Laguna Merín lagoon and the Río Negro river. This region hosts important wildlife diversity, so its conservation is a priority for Uruguay (Laufer et al. 2009; Gobel et al. 2013; Brazeiro 2015). This region has a mean temperature of 23 °C in summer and 11 °C in winter; a mean relative humidity of 64% in summer and 86% in winter; and a mean monthly rain of 100 mm (distributed fairly evenly during the year, data available at meteorologia.com.uy). According to these characteristics, the region belongs to the Koppen “Caf” classification (Kottek et al. 2006).
At the studied location (31°53′36′′S, 54°09′26′′W) there are a series of artificial, permanent lentic water bodies built for agricultural uses including cattle farming. In 2007 a feral population of L. catesbeianus in establishment phase was detected there; in 2012 it was restricted to an approximate radius of 1.2 km around the site where the former bullfrog frog farm was located (Laufer and Gobel 2017; Laufer et al. 2018). We could detect that bullfrog invaded those water bodies that were nearer to the old farm (initial invasion focus, described in detail in Laufer et al. 2018). Considering the restricted bullfrog distribution, we could only find two permanent ponds (31°53′39.7″, 54°09′17.1″; 31°53′48.7″, 54°09′07.0″) which had a persistent bullfrog presence throughout the monitored period and were compared with other five nearby non-invaded systems, randomly selected and considered as control ponds (Online Resource 1). All the ponds were permanent, similar in bathymetry, and average distance between ponds was 1690 m. Those non-invaded systems were comparable to the invaded ones, and many of them were invaded in the years following this study, when bullfrog continued its expansion. In this sense, we did not observe selectivity within the studied ponds; bullfrog just invaded those systems located closer to the initial focus (Laufer et al. 2018). All studied systems had an average pH of 7.5, a conductivity of 73.5 µS/cm, a maximum depth of 2 m, 30% of their surface was covered by floating or emergent macrophytes and they were surrounded by natural grasslands where livestock grazes. All the information about areas, geographic coordinates, water quality, and macrophyte coverage of each studied system are provided in the Online Resource 1.
Native communities
Native aquatic vertebrate communities in the study area are relatively well known and comprised mainly of fish and amphibians (Laufer et al. 2009; Prigioni et al. 2011; Teixeira de Mello et al. 2011; Gobel et al. 2013). The most common fish were three Characidae, Hyphessobrycon anisitsi, Astyanax laticeps and Cheirodon interruptus. These small fish (less than 9 cm of total length) are usually found in large groups in local creeks, ponds and wetlands; they consume detritus, algae, small sized crustacean, and insect larva (Teixeira de Mello et al. 2011; Gobel et al. in press).
Natural lentic systems and water reservoirs are the common sites used for native anurans for foraging and reproduction. The most common tadpoles at the studied systems are Boana pulchella, Odontoprhynus americanus, Pseudis minuta, Scinax sp. (comprising S. granulatus and S. squalirostris) and Phyllomedusa iheringii. These tadpoles consume detritus and phytoplankton, with smaller amounts of crustaceans or insect larvae (Lajmanovich 1997; Echeverría et al. 2007). Boana pulchella (maximum total length 8 cm) and O. amaericanus (maximum total length 7 cm) are two common large benthic tadpoles that occur in a range of wetland habitats in the region. Both species have a relatively long larval phase, from March to November. Pseudis minuta is a large nektonic tadpole (maximum total length 9 cm) that inhabits permanent lentic water bodies. This species also shows a long larval period during the year, with most of the metamorphosis in spring and summer. Finally, Scinax sp. (maximum total length 4 cm) and P. iheringii (maximum total length 7 cm) have nektonic tadpoles, with shorter larval periods, from October to March (Moreira et al. 2007; Both et al. 2009).
Field sampling and laboratory analysis
We conducted four contiguous seasonal samplings over the course of 1 year, as follows: from May 24th to 27th, July 19th to 22nd, and October 18th to 22nd, in 2012, and February 4th to 7th, in 2013. We sampled phytoplankton, zooplankton, macroinvertebrates and aquatic vertebrates, in all seven ponds. We took three water samples from each pond at the same date, and then measured the chlorophyll a concentration for each, using a field fluorometer. The value of chlorophyll a concentration was obtained as the mean of the three samples. We evaluated the abundance and composition of zooplankton by five standardized filtrates of 2 l of water per pond, with a 68 µm pore size filter. These samples were subsequently analysed in the laboratory under a stereomicroscope, thus obtaining the number of individuals per litre (density) of each and every taxon found in the ponds. Finally, in order to study the larger body-sized organisms (macroinvertebrates, fish and tadpoles), we took standardized samples with a seine fishing net (5 × 1 m area, 0.5 cm mesh), hung vertically in the water with its bottom edge held down by weights and its top edge buoyed by floats. The use of this fishing gear allowed us to sample benthic and water column organisms. The members of our team entered the pond avoiding generating a disturbance in the area that would later be sampled. We performed two tows in each pond, one tow in the largest diameter and the other in the lowest diameter covering between 7 and 10 m, in each case. Tows were performed from the centre to the edge of the pond in order to cover the different strata of the system (benthos, water column and edge). Although the area did not strictly standardize samples, we increased the effort in largest ponds (Online Resource 1) and this was supported by the completeness in diversity evidenced by the rarefaction curves (see “Results”).
The collected vertebrates were sacrificed with an overdose of lidocaine hydrochloride and preserved with 4% formaldehyde. Macroinvertebrates were preserved in 70% alcohol. All the specimens were placed in the collections of the Museo Nacional de Historia Natural museum in Montevideo (MNHN). In the laboratory, we classified the specimens to the lowest possible taxonomic level—identifying family and species in the cases of macroinvertebrates and vertebrates, respectively (Ziegler and Maneyro 2008; Laufer et al. 2009; Mugnai et al. 2010; Teixeira de Mello et al. 2011). Then, we measured total length of macroinvertebrates, the length from mouth to the base of the caudal fin peduncle of fish, and the length from snout to the tail tip of tadpoles. We assigned developmental stage per Gosner (1960) for all tadpoles. For the most numerous samples, we randomly selected 80 organisms per taxon to measure and determine the developmental stage. We calculated an abundance index for each taxon in each pond, and in each sampling date, by dividing the number of collected individuals by the total distance towed by the net.
Data analysis
We compared taxa richness, abundance, and body size, between ponds with breeding bullfrogs (n = 2) and ponds without breeding bullfrogs (n = 5). We estimated and compared taxa richness through rarefaction procedure, employing the EcoSim software (Entsminger 2012). This approach accounts for patchiness in the data that result from natural levels of sample heterogeneity. As a result, the rarefaction curve is a plot of the number of species as a function of the number of samples. This analysis allows comparison of the richness of different samples, by analysing these curves and their 95% confidence intervals. Usually, this comparison is made for the cutoff point of the smallest sample (Chao and Jost 2012). For the analysis of rarefaction we considered the 37 taxa of macroinvertebrates, fish and amphibian species found in the whole area (the seven studied systems), however, 24 of them were present in very low frequency and therefore their data could not be used for the following analyses of body sizes and abundances. A complete list of these species, which includes the collection season and the invasion status of the system, can be seen in Online Resource 2. Then, we also analysed pond clustering using Bray–Curtis similarity index to quantify differences between samples, based on count data. This index ignores cases in which the species is absent in both communities, and is influenced by abundant species, rather than the rare species. The advantage of this method is that the scale is easy to understand: 0 means the samples are exactly the same, while 1 is the maximum difference that can be observed between two samples (Legendre and Legendre 1998).
We compared basal taxa density of the studied communities, by the repeated measures multivariate analysis of variance RM-MANOVA. Thus, we studied the possible occurrence of differences in the availability of basal resources in relation to bullfrog invasion. We analysed chlorophyll a concentration and density of cladocerans, copepods, nauplii, and rotifers, as response variables. Bullfrog adult and larval presence (invaded and non-invaded systems, Online Resource 1) were the independent variables. Also, we included total abundance index of native primary consumers (small omnivorous fish, amphibian larvae, and macroinvertebrate herbivores, following the classification criteria of Arim et al. 2010) as a covariable and sampling interval (included in the model in order to control annual variations) as a factor. We also included pond identity as a subject-factor in our analyses (Legendre and Legendre 1998).
We compared the abundance index of each taxon (for the most frequent macroinvertebrates, tadpoles, and fish, Online Resource 3) between invaded and non-invaded ponds with an analysis of variance (ANOVA). We considered season, and the presence-absence of fish and bullfrog as independent variables of the model. In this case, we added fish presence as a factor due to the strong available evidence that suggests that this variable largely determines the structure of amphibian larvae community (Hecnar and M’Closkey 1997; Porej and Hetherington 2005). We performed the ANOVA with a type III sum of squares analysis, which is appropriate for an unbalanced model (Sokal and Rohlf 2009).
We explored differences for each taxon—in each season—in body sizes (macroinvertebrates, fish, and amphibian larvae) and developmental stages (in tadpoles), between invaded and non-invaded ponds. Due to the absence of normality in these variables, we performed a Kruskal–Wallis test. Body size, represented by total length and development stage were considered as the response variable and bullfrog presence (Online Resource 1) was the explanatory variable.
We compared the frequency distribution of Gosner stage classes for the two most common tadpoles between invaded and non-invaded ponds. We used stage data from the sampling interval, in which the biggest range of body size was recorded, for both systems (October for B. pulchella and May for O. americanus). We evaluated the frequency distribution of Gosner’s stages in the invaded ponds through a G-test, considering the registered distribution in the non-invaded ponds as the null hypothesis. Finally, we analysed the densities of four size classes of the complete fish assemblage (obtained by quartiles 9.71–28.83 mm, 28.84–32.38 mm, 32.39–36.66 mm, and 36.67–85.86 mm) in the invaded and non-invaded ponds through a type III ANOVA with quadratic sum analysis. In all cases, we considered α = 0.05 as statistically significant (Sokal and Rohlf 2009) and we used R version 3.2.5 for analyses and graphs (R Core Team 2015).
Results
We did not observe significant differences in taxa richness (considering macroinvertebrates, native tadpoles and fish) between invaded communities and those non-invaded by bullfrog. The rarefaction analysis revealed an overlap in the confidence intervals of the different (invaded and non-invaded) communities (Fig. 1). This analysis also showed that our samples were representative of the studied communities, since the majority of them reached an asymptote. The cluster analysis by the Bray–Curtis similarity index also did not show a pattern related to bullfrog invasion, since invaded and non-invaded communities showed similarities (Online Resource 4).
The abundances of the non-vertebrate community components did not significantly differ between the invaded and non-invaded ponds. We observed no significant differences in the density of phytoplankton (assessed by the concentration of chlorophyll a) and zooplankton: cladocerans, copepods, nauplii, and rotifers (Wilks’ lambda = 0.75, F = 0.26, P = 0.9, df = 1; Fig. 2, Online Resource 5). For macroinvertebrates we observed a similar trend, where the abundances of the different families showed no statistically significant differences between invaded and non-invaded ponds (Fig. 3, Online Resource 6).
Mean density and standard deviation of the different zooplankton taxa measured in individuals per liter, and chlorophyll a concentration measured in relative fluorescence units (RFU), in invaded (black) and non-invaded (white) environments by Lithobates catesbeianus in Aceguá. Ro rotifers, Cl cladocerans, Co copepods, Na nauplii, Ch a chlorophyll a
Mean abundance index and standard deviation of macroinvertebrates, fish and amphibian larvae in invaded (black) and non-invaded (white) environments by Lithobates catesbeianus in Aceguá. Be Belostomatidae, Cor Corixidae, No Notonectidae, Coe Coenagrionidae, Li Libelulidae, A. l: Astyanax. laticeps, C. i: Cheirodon interruptus, H. a: Hyphessobrycon anisitsi, B.p: Boana pulchella, O. a: Odontophrynus americanus, S. sp: Scinax sp., P. m: Pseudis minuta. The symbol * indicates that the differences are statistically significant (ANOVA)
Abundance of several vertebrates’ species differed between invaded and non-invaded ponds. The Characid fish H. anisitsi was 20 times more abundant in the invaded than the non-invaded ponds (F = 8.58, P < 0.05, df = 1). Larvae of Scinax sp. (F = 6.30, P < 0.05, df = 1) and P. minuta (F = 4.94, P < 0.05, df = 1), were nine and ten times less abundant in the invaded systems. The other common tadpoles (B. pulchella and O. americanus) did not show significant differences in abundance index (Fig. 3, Online Resource 6).
Body sizes of the different community components showed significant differences between bullfrog invaded and non-invaded ponds (Fig. 4, Online Resource 7). Macroinvertebrates body size changes did not show a clear pattern related to bullfrog presence. In contrast, vertebrate species generally had larger sizes in the invaded ponds, with some exceptions. The fish H. anisitsi was 30%, and C. interruptus was 16% larger in the invaded ponds across all seasons sampled. Astyanax laticeps was 47% larger in the invaded ponds (with the exception of the autumn sample). A similar pattern was observed for two native amphibian species: the tadpole Boana pulchella was 32%, and that of O. americanus was 19% larger in the invaded ponds. In contrast, we did not detect significant differences in hylids larvae Scinax sp. and P. iheringii (species with a shorter larval period, detected only in summer). Pseudis minuta larvae were not detected in the invaded ponds (only two individuals in a single pond, in autumn; Online Resource 6).
Ratio of mean body size of each species in invaded and non-invaded ponds by bullfrog, in each sampled season. The circle indicates autumn, the triangle winter, the diamond spring and the square summer. The black-filled symbol shows that the body size differences are statistically significant and open symbol indicated not statistically differences. The species are the ones defined in Fig. 3, with the addition of Phyllomedusa iheringii
In several cases, tadpole Gosner stage significantly differed between invaded and non-invaded ponds (Fig. 5, Online Resource 8). Boana pulchella tadpoles were more advanced in Gosner stages in invaded ponds, in May (X2 = 45.12, P < 0.001, df = 1), in July (X2 = 14.84, P < 0.001, df = 1), and in October (X2 = 21.33, P < 0.001, df = 1). The same greater development was observed for O. americanus in May (X2 = 6.53, P = 0.01, df = 1). Contrary, Scinax sp. were less advanced in development stages in invaded ponds (X2 = 9.78, P = 0.002, df = 1). Phyllomedusa iheringii larvae did not show significant differences between invaded and non-invaded ponds (Fig. 5, Online Resource 8).
Ratio of mean Gosner’s developmental stages for different native tadpoles in invaded and non-invaded ponds by bullfrog, for each sampled season. The circle indicates autumn, the triangle winter, the diamond spring and the square summer. The black-filled symbol shows that the developmental stage differences are statistically significant and open symbol indicated not statistically differences
The larval developmental stages of B. pulchella (G = 33.39, P < 0.001, df = 4) and O. americanus (G = 46.28, P < 0.001, df = 4) differed between the invaded and non-invaded ponds. For both species, the invaded ponds had a lower frequency of early stages (less than 31 of Gosner) and a higher frequency of the advanced stages (Fig. 6). Finally, in reference to the fish assemblage, we observed that the invaded water bodies had a greater abundance of the two larger body size classes (mouth to peduncle length > 32 mm; F = 5.73, P = 0.04, df = 1; F = 11.15, P = 0.01, df = 1; Fig. 7, Online Resource 9).
Comparison of development stages frequencies of the two native tadpoles which showed the greatest change in body size associated with the presence of bullfrog. Here we compare the frequencies of the different classes of Gosner’s developmental stages, between ponds invaded (black) and non-invaded (white) by bullfrogs, in the season in which the greatest differences in average body size were observed: autumn for Odontophrynus americanus (a) and spring for Boana pulchella (b)
Discussion
Despite the early stage of the invasion in Aceguá, our results suggest that bullfrog are affecting some components of native communities. Bullfrog is a dangerous invading organism, being a large sized aquatic predator (Babbitt and Tanner 1998). This exotic anuran is two to three times larger (adults and larva respectively), than the average recorded for Aceguá native anurans. Its effects were evident in body sizes and abundances of aquatic vertebrates (fish and anuran larvae). Our analyses of community richness and diversity, density of plankton and abundances and sizes of macroinvertebrates did not show any effect attributable to bullfrog invasion. Our observations show the effects of an exotic predator during the invasion lag phase, in which it could be already structuring native communities by locally affecting some of its components. Although we had the limitation of the small number of communities sampled, we can assume that the differences observed would not respond to genetic differences, considering ponds proximity and the absence of environmental barriers, or to plastic responses to environment conditions, considering that all sampled ponds had the same water regime, and were similar in basal resources and macroinvertebrates predator’s abundances.
Considering the regional studies, it seems clear that bullfrog has a predatory role, mainly consuming invertebrates and amphibians (Silva et al. 2009; Leivas et al. 2012, 2013; Quiroga et al. 2015). Strong effects following bullfrog invasion are described in the literature, such as the declining of native species richness (e.g. Li et al. 2011; Batista et al. 2015, but see Both and Melo 2015), associated with various mechanisms such as predation, competition, and transmission of pathogens (Hirai 2004; Pearl et al. 2004; Wu et al. 2005; Garner et al. 2006, 2009). However, our results do not show a significant effect on the richness of the native communities. Probably, at this early stage of the invasion we should not expect to find an important effect on richness, but on other more sensitive community attributes.
Amphibians seem to be the most affected species elsewhere by bullfrog invasion (Kraus 2009). Due to the early invasion stage in Aceguá, we can assume that the native tadpoles cannot identify bullfrog as a predator and this makes them more vulnerable (Polo-Cavia et al. 2010). We observed two types of responses in native tadpoles: first a decrease in abundance of P. minuta and Scinax sp. (Fig. 3), and second, an increase in the average body size and developmental stage of B. pulchella and O. americanus (Figs. 4, 5). Both patterns could be related to predation and competition by bullfrogs, and the differences could be due to an asymmetry in the susceptibility of these native larvae.
The density of larvae of P. minuta and Scinax sp. could have been affected earlier because of their nektonic habits that make them more susceptible to bullfrog predation (Pearl et al. 2004). Alternatively, B. pulchella and O. americanus could have escaped predation due to their size and/or benthic habits, without affecting their abundances (Altig and Johnston 1989). Boana pulchella and O. americanus appeared with the highest averages of body size and Gosner’s stage in the invaded ponds (Figs. 4, 5), probably evidencing an increase in predation rate, rather than a response induced by environmental conditions (see Relyea 2007). We understand that this increase in body size (and development stage) would not be due to a decrease in competition pressure between native tadpoles, caused by the introduction of a new predator, considering the important biomass and consumption rate of bullfrog larvae in the invaded communities. This idea was reinforced in relation to the observed reduction of early larval stages frequencies (Fig. 6). Predation commonly affects smaller sizes individuals, affecting the recruitment and so increasing the mean larval size (Jara and Perotti 2010). Although adults of L. catesbeianus consume tadpoles of other species (Jancowski and Orchard 2013), the high density of bullfrog larvae present in the invaded sites (between 10 and 31 individuals per trawled meters) could also be playing an important role. Both stable isotope analysis and studies of stomach contents reveal that the trophic position of bullfrog larvae corresponds to a predator (Schiesari et al. 2009; Ruibal and Laufer 2012). In fact, Ruibal and Laufer (2012) suggest that these larvae might be excluding other species in the invaded systems by predation, especially by egg consumption. Moreover, Kiesecker and Blaustein (1997b) reported that bullfrog tadpoles actively prey on larvae of other anurans, especially on earlier stages, which are vulnerable because of their lower mobility and size. However, we cannot discard that the observed effects in the different native tadpoles respond to a mechanism of competitive exclusion, considering the large size, the high densities and consumption rate of bullfrog larvae (Online Resource 1). This phenomenon has been repeatedly invoked for different invaders and also for the bullfrog (Kiesecker and Blaustein 1997b; Kupferberg 1997; Kiesecker et al. 2001; Kraus 2009).
Although our interpretation is based on the important existing evidence of the direct effects of the bullfrog as predator, we cannot fail to consider that other mechanisms could explain the two types of responses observed in native tadpoles. One could be a change in foraging strategy in certain species or stages (Jara and Perotti 2010). In any case, the sampling method used should not be considered a cause, since it is an active method that covers a large area and diversity of strata within the pond. On the other hand, the recruitment of O. americanus and B. pulchella could be affected by a decrease in the reproduction of adults in the invaded ponds. However, our field observations do not show an inhibition of reproduction in the invaded ponds and most of the species present have synchronous reproduction pulses in the region (Both et al. 2008). In conclusion, the evidence seems to indicate a removal of individuals from the invaded aquatic systems, which could be associated with bullfrog predation.
A native amphibian that shares habits with the bullfrog is P. minuta. This anuran inhabits aquatic environments throughout its ontogeny, with adaptations in its adult stage to this medium, such as the development of interdigital membranes and dorsal eyes that allow it to observe over the surface (Santana et al. 2016). This overlap in habitat increase the chances of interactions. Considering that P. minuta is much smaller than bullfrog, it could be a very susceptible species. In fact, we could observe that this common species is practically absent in the water bodies invaded by bullfrog (Fig. 3). Both and Melo (2015) also reported the displacement of Pseudis by bullfrog invasion in Atlantic Forest water bodies in Brazil. Our observations suggest that this effect would be common to the different aquatic frogs of the region (Pseudis and Lysapsus), probably the most affected native amphibians.
The positive interaction that was observed in Aceguá was the response of abundance and body size of native fish assemblage in the invaded ponds. The magnitude of this pattern was such, that the density of larger sizes fish classes in the invaded ponds was two and a half times greater than in the non-invaded (Fig. 7). This could lead to an increased fitness in fish communities associated with the presence of bullfrogs. Adams et al. (2003) and Maezono and Miyashita (2003) reported a positive interaction between the bullfrog and invasive fish due to an indirect trophic interaction. Bullfrog tadpole, being tolerant to the presence of fish (Babbitt et al. 2003), takes advantage of this release of predators achieving a higher survival, and therefore greater abundances. While our observations also suggest a positive interaction, the underlying mechanism should not be the same. Meerhoff and collaborators (2007) stated that Uruguayan subtropical aquatic communities show different fish assemblages, characterized by a lower relative abundance of large-sized predators. Astyanax laticeps, H. anisitsi, and C. interruptus are small omnivores (consuming algae, zooplankton, and small invertebrates, Teixeira de Mello et al. 2011; Gobel et al. in press) that are not consumed by bullfrogs (unpublished diet data). The greater biomass of these fishes should be supported by a greater availability of trophic resources than in non-invaded ponds (Arim et al. 2010). Possibly, the presence of a large biomass of bullfrog tadpoles alters the energy flow pathways, through an indirect mechanism involving trophic resource availability (e.g. sediment removal; Smith et al. 2016). This increase in resources should not necessarily be visible in zooplankton abundance and could be masked by a high turnover due to fish consumption. Surely, cases of positive interactions to invasion are not as rare in nature as previously thought, but the lack of field studies at the community level may be limiting the evidence (Rodriguez 2006). This increase in fish density and abundance, could also affect habitat availability for other taxa, especially native amphibians (Semlitsch et al. 2015).
Our observations showed that not all the effects of a large exotic predator are negative (Townsend 2003; Rodriguez 2006; Duxbury et al. 2010; Li et al. 2011), and this should be evaluated at the community level (Simberloff et al. 2013). Bullfrog effects vary according to the considered taxa, but also according to the evaluated variables: size, growth rate, development, population structure, and abundance. Therefore, our data indicate that invasive predators could generate positive and negative effects, or also be neutral at their lag phase (Online Resources 7 and 8). Even an effect that could be identified as positive to body size for a native species may have been due to negative interactions addressing certain ontogenetic stages (Rodewald 2012).
Although a number of biotic and abiotic factors could have affected our observations, for instance, the low number of evaluated systems (that hinders the statistical analyses) is the main weakness of our study. This methodological defect is an intrinsic feature in the surveys of biological invasions at early stages. Invasive species effects may be masked by their low abundances at these stages, and then should be explored with sensible community attributes (Wiser et al. 1998; Parker et al. 1999). That’s probably why there is a strong bias towards the study of invasions in advanced stages, where the effects are notorious and clearly visible (Crooks 2005; Pyšek et al. 2008). Despite the methodological limitations, we emphasise the need for research at early invasion stages, for the understanding of involved mechanisms, and as a rapid conscience generator for decision makers (Puth and Post 2005; Pyšek et al. 2008; Simberloff et al. 2013). National environmental authorities should consider our observations, early showing the potential risk of bullfrog invasion in Uruguay and the region.
References
Adams MJ (2000) Pond permanence and the effects of exotic vertebrates on anurans. Ecol Appl 10:559–568. https://doi.org/10.1890/1051-0761(2000)010[0559:PPATEO]2.0.CO;2
Adams M, Pearl C (2007) Problems and opportunities managing invasive bullfrogs: is there any hope? In: Gherardi F (ed) Biological invaders in inland waters: profiles, distribution, and threats. Springer, Netherlands, pp 679–693
Adams MJ, Pearl CA, Bruce Bury R (2003) Indirect facilitation of an anuran invasion by non-native fishes. Ecol Lett 6:343–351. https://doi.org/10.1046/j.1461-0248.2003.00435.x
Altig R, Johnston GF (1989) Guilds of anuran larvae: relationships among developmental modes, morphologies, and habitats. Herpetol Monogr 3:81–109. https://doi.org/10.2307/1466987
Arim M, Abades SR, Laufer G et al (2010) Food web structure and body size: trophic position and resource acquisition. Oikos 119:147–153. https://doi.org/10.1111/j.1600-0706.2009.17768.x
Babbitt KJ, Tanner GW (1998) Effects of cover and predator size on survival and development of Rana utricularia tadpoles. Oecologia 114:258–262. https://doi.org/10.1007/s004420050444
Babbitt KJ, Baber MJ, Tarr TL (2003) Patterns of larval amphibian distribution along a wetland hydroperiod gradient. Can J Zool 81:1539–1552. https://doi.org/10.1139/z03-131
Batista M, Silva M, Barreto C (2015) Effects of introduction and decline of a bullfrog population (Lithobates catesbeianus) in a community of amphibians in the Cerrado from Central Brazil. Herpetol Notes 8:263–265
Baxter CV, Fausch KD, Murakami M, Chapman PL (2004) Fish invasion restructures stream and forest food webs by interrupting reciprocal prey subsidies. Ecology 85:2656–2663. https://doi.org/10.1890/04-138
Bellard C, Genovesi P, Jeschke JM (2016) Global patterns in threats to vertebrates by biological invasions. Proc R Soc B 283:20152454. https://doi.org/10.1098/rspb.2015.2454
Blaustein AR, Kiesecker JM (2002) Complexity in conservation: lessons from the global decline of amphibian populations. Ecol Lett 5:597–608. https://doi.org/10.1046/j.1461-0248.2002.00352.x
Boelter RA, Cechin SZ (2007) Impact of the bullfrog diet (Lithobates catesbeianus-Anura, Ranidae) on native fauna: case study from the region of Agudo-RS-Brazil. Nat Conserv 5:115–123
Boone MD, Little EE, Semlitsch RD (2004) Overwintered bullfrog tadpoles negatively affect salamanders and anurans in native amphibian communities. Copeia. https://doi.org/10.1643/CE-03-229R1
Both C, Melo AS (2015) Diversity of anuran communities facing bullfrog invasion in Atlantic forest ponds. Biol Invasions 17:1137–1147. https://doi.org/10.1007/s10530-014-0783-1
Both C, Kaefer ÍL, Santos TG, Cechin ST (2008) An austral anuran assemblage in the Neotropics: seasonal occurrence correlated with photoperiod. J Nat Hist 42:205–222. https://doi.org/10.1080/00222930701847923
Both C, Solé M, dos Santos TG, Cechin SZ (2009) The role of spatial and temporal descriptors for neotropical tadpole communities in southern Brazil. Hydrobiologia 624:125–138. https://doi.org/10.1007/s10750-008-9685-5
Brazeiro A (2015) Eco-regiones de Uruguay: Biodiversidad, presiones y conservación. Aportes a la estrategia nacional de biodiversidad. Facultad de Ciencias, CIEDUR, VS-Uruguay, SZU, Montevideo
Bwanika GN, Chapman LJ, Kizito Y, Balirwa J (2006) Cascading effects of introduced Nile perch (Lates niloticus) on the foraging ecology of Nile tilapia (Oreochromis niloticus). Ecol Freshw Fish 15:470–481. https://doi.org/10.1111/j.1600-0633.2006.00185.x
Chao A, Jost L (2012) Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93:2533–2547. https://doi.org/10.1890/11-1952.1
Clavero M, Garcia-Berthou E (2005) Invasive species are a leading cause of animal extinctions. Trends Ecol Evol 20:110–110. https://doi.org/10.1016/j.tree.2005.01.003
Crooks JA (2005) Lag times and exotic species: the ecology and management of biological invasions in slow-motion. Ecoscience 12:316–329. https://doi.org/10.2980/i1195-6860-12-3-316.1
Duxbury C, Holland J, Pluchino M (2010) Experimental evaluation of the impacts of the invasive catfish Hoplostemum littorale (Hancock, 1828) on aquatic macroinvertebrates. Aquat Invasions 5:97–102. https://doi.org/10.3391/ai.2010.5.1.11
Echeverría DD, Volpedo AV, Mascitti VI (2007) Diet of tadpoles from a pond in Iguazu National Park, Argentina. Gayana 71:8–15
Entsminger GL (2012) EcoSim professional: null modeling software for ecologists, v 1. Acquired Intelligence Inc., Kesey-Bear. and Pinyon Publishing, Montrose
Garner TW, Perkins MW, Govindarajulu P et al (2006) The emerging amphibian pathogen Batrachochytrium dendrobatidis globally infects introduced populations of the North American bullfrog, Rana catesbeiana. Biol Lett 2:455–459. https://doi.org/10.1098/rsbl.2006.0494
Garner TWJ, Walker S, Bosch J et al (2009) Life history tradeoffs influence mortality associated with the amphibian pathogen Batrachochytrium dendrobatidis. Oikos 118:783–791. https://doi.org/10.1111/j.1600-0706.2008.17202.x
Gobel N, Cortizas S, Mautone JM et al (2013) Predation of Pseudis Minuta Günther 1858, by Lethocerus annulipes (Heteroptera: Belostomatidae). Cuad Herpetol 27:63–63
Gobel N, Laufer G, Serra WS (in press) Density, body size and diet overlap of four fish species in artificial reservoirs in the Pampas, Uruguay. North West J Zool e171401
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Havel JE, Kovalenko KE, Thomaz SM, Amalfitano S, Kats LB (2015) Aquatic invasive species: challenges for the future. Hydrobiologia 750:147–170. https://doi.org/10.1007/s10750-014-2166-0
Hecnar SJ, M’Closkey RT (1997) The effects of predatory fish on amphibian species richness and distribution. Biol Conserv 79:123–131. https://doi.org/10.1016/S0006-3207(96)00113-9
Hirai T (2004) Diet composition of introduced bullfrog, Rana catesbeiana in the Mizorogaike pond of Kyoto, Japan. Ecol Res 19:375–380. https://doi.org/10.1111/j.1440-1703.2004.00647.x
Jancowski K, Orchard S (2013) Stomach contents from invasive American bullfrogs Rana catesbeiana (= Lithobates catesbeianus) on southern Vancouver island. B C Can NeoBiota 16:17–37. https://doi.org/10.3897/neobiota.16.3806
Jara F, Perotti M (2010) Risk of predation and behavioural response in three anuran species: influence of tadpole size and predator type. Hydrobiologia 644:313–324. https://doi.org/10.1007/s10750-010-0196-9
Kiesecker JM, Blaustein AR (1997a) Effects of introduced bullfrogs and smallmouth bass on microhabitat use, growth, and survival of sative red-legged frogs (Rana aurora). Conserv Biol 12:776–787. https://doi.org/10.1111/j.1523-1739.1998.97125.x
Kiesecker JM, Blaustein AR (1997b) Population differences in responses of red-legged frogs (Rana aurora) to introduced bullfrogs. Ecology 78:1752–1760. https://doi.org/10.1890/0012-9658(1997)078[1752:PDIROR]2.0.CO;2
Kiesecker JM, Blaustein AR, Miller CL (2001) Potential mechanisms underlying the displacement of native red-legged frogs by introduced bullfrogs. Ecology 82:1964–1970. https://doi.org/10.1890/0012-9658(2001)082[1964:PMUTDO]2.0.CO;2
Kottek M, Grieser J, Beck C, Rudolf B, Rubel F (2006) World map of the Köppen-Geiger climate classification updated. Meteorol Z 15:259–263. https://doi.org/10.1127/0941-2948/2006/0130
Kraus F (2009) Alien reptiles and amphibians A scientific compendium and analysis. Springer, Berlin
Kupferberg SJ (1997) Bullfrog (Rana catesbeiana) Invasion of a California river: the role of larval competition. Ecology 78:1736–1751. https://doi.org/10.1890/0012-9658(1997)078[1736:BRCIOA]2.0.CO;2
Lajmanovich RC (1997) Alimentación de larvas de anuros en ambientes temporales del sistema del río Paraná, Argentina. Doñana Acta Vertebr 24:191–202
Laufer G, Gobel N (2017) Habitat degradation and biological invasions as a cause of amphibian richness loss: a case report in Aceguá, Cerro Largo, Uruguay. Phyllomedusa 16:289–293. https://doi.org/10.11606/issn.2316-9079.v16i2p289-293
Laufer G, Canavero A, Núñez D, Maneyro R (2008) Bullfrog (Lithobates catesbeianus) invasion in Uruguay. Biol Invasions 10:1183–1189. https://doi.org/10.1007/s10530-007-9178-x
Laufer G, Piñeiro-Guerra JM, Pereira-Garbero R et al (2009) Distribution extension of Scinax aromothyella (Anura, Hylidae). Biota Neotrop 9:275–278. https://doi.org/10.1590/S1676-06032009000200028
Laufer G, Gobel N, Borteiro C, Soutullo A, Martinez-Debat C, de Sá RO (2018) Current status of American bullfrog, Lithobates catesbeianus, invasion in Uruguay and exploration of chytrid infection. Biol Invasions 20:285–291. https://doi.org/10.1007/s10530-017-1540-z
Legendre P, Legendre LF (1998) Numerical ecology, 2nd edn. Elsevier, Amsterdam
Leivas PT, Leivas FWT, Moura MO (2012) Diet and trophic niche of Lithobates catesbeianus (Amphibia: Anura). Zool Curitiba 29:405–412. https://doi.org/10.1590/S1984-46702012000500003
Leivas PT, Savaris M, Lampert S, Lucas EM (2013) Predation of Odontophrynus americanus (Anura: Odontophrynidae) by the invasive species Lithobates catesbeianus (Anura: Ranidae) in an Araucaria forest remnant in Southern Brazil. Herpetol Notes 6:603–606
Li Y, Ke Z, Wang Y, Blackburn T (2011) Frog community responses to recent American bullfrog invasions. Curr Zool 57:83–92. https://doi.org/10.1093/czoolo/57.1.83
Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the world’s worst invasive alien species: a selection from the global invasive species database. Invasive Species Specialist Group, Auckland
Maezono Y, Miyashita T (2003) Community-level impacts induced by introduced largemouth bass and bluegill in farm ponds in Japan. Biol Conserv 109:111–121. https://doi.org/10.1016/S0006-3207(02)00144-1
Meerhoff M, Clemente JM, Teixeira de Mello F, Iglesias C, Pedersen AR, Jeppesen E (2007) Can warm climate-related structure of littoral predator assemblies weaken the clear water in shallow lake? Glob Change Biol 13:1888–1897. https://doi.org/10.1111/j.1365-2486.2007.01408.x
Moreira LFB, Machado IF, Lace ARGM, Maltchik L (2007) Calling period and reproductive modes in an anuran community of a temporary pond in southern Brazil. S Am J Herpet 2:129–135. https://doi.org/10.2994/1808-9798(2007)2[129:CPARMI]2.0.CO;2
Mugnai R, Nessimian JL, Baptista DF (2010) Manual de identificação de macroinvertebrados aquáticos do estado do Rio de Janeiro: para atividades técnicas, de ensino e treinamento em programas de avaliação da qualidade ecológica dos ecossistemas lóticos. Technical Books Editora, São Paulo
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: Toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19. https://doi.org/10.1023/A:1010034312781
Pearl CA, Adams MJ, Bury RB, McCreary B (2004) Asymmetrical effects of introduced bullfrogs (Rana catesbeiana) on native ranid frogs in Oregon. Copeia 2004:11–20. https://doi.org/10.1643/CE-03-010R2
Polo-Cavia N, Gonzalo A, López P, Martín J (2010) Predator recognition of native but not invasive turtle predators by naïve anuran tadpoles. Anim Behav 80:461–466. https://doi.org/10.1016/j.anbehav.2010.06.004
Porej D, Hetherington TE (2005) Designing wetlands for amphibians: the importance of predatory fish and shallow littoral zones in structuring of amphibian communities. Wetl Ecol Manag 13:445–455. https://doi.org/10.1007/s11273-004-0522-y
Prigioni C, Borteiro C, Kolenc F (2011) Amphibia and reptilia, Quebrada de los Cuervos, departamento de Treinta y Tres, Uruguay. Check List 7:763–767. https://doi.org/10.15560/11021
Puth LM, Post DM (2005) Studying invasion: have we missed the boat? Ecol Lett 8:715–721. https://doi.org/10.1111/j.1461-0248.2005.00774.x
Pyšek P, Richardson DM, Pergl J, Jarošík V, Sixtová Z, Weber E (2008) Geographical and taxonomic biases in invasion ecology. Trends Ecol Evol 23:237–244. https://doi.org/10.1016/j.tree.2008.02.002
Quiroga LB, Moreno MD, Cataldo AA et al (2015) Diet composition of an invasive population of Lithobates catesbeianus (American bullfrog) from Argentina. J Nat Hist 49:1703–1716. https://doi.org/10.1080/00222933.2015.1005711
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for statistical computing, Vienna. http://www.R-project.org. Accessed 20 Feb 2018
Relyea RA (2007) Getting out alive: how predators affect the decision to metamorphose. Oecologia 152:389–400. https://doi.org/10.1007/s00442-007-0675-5
Rodewald AD (2012) Spreading messages about invasives. Divers Distrib 18:97–99. https://doi.org/10.1111/j.1472-4642.2011.00817.x
Rodriguez LF (2006) Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur. Biol Invasions 8:927–939. https://doi.org/10.1007/s10530-005-5103-3
Ruibal M, Laufer G (2012) Bullfrog Lithobates catesbeianus (Amphibia: Ranidae) tadpole diet: description and analysis for three invasive populations in Uruguay. Amphib Reptil 33:355–363. https://doi.org/10.1163/15685381-00002838
Sala OE, Chapin FS, Iii et al (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774. https://doi.org/10.1126/science.287.5459.1770
Santana DJ, de Medeiros Magalhães F, de Avelar V, Mângia S, Amado TF, Garda AA (2016) Calls and tadpoles of the species of Pseudis (Anura, Hylidae, Pseudae). Herpetol J 26:139–151
Schiesari L, Werner EE, Kling GW (2009) Carnivory and resource-based niche differentiation in anuran larvae: implications for food web and experimental ecology. Freshw Biol 54:572–586. https://doi.org/10.1111/j.1365-2427.2008.02134.x
Semlitsch RD, Peterman WE, Anderson TL, Drake DL, Ousterhout BH (2015) Intermediate pond sizes contain the highest density, richness, and diversity of pond-breeding amphibians. Plos One 10:e0123055. https://doi.org/10.1371/journal.pone.0123055
Silva ETD, Reis EPD, Feio RN, Filho OPR (2009) Diet of the invasive frog Lithobates catesbeianus (Shaw, 1802) (Anura: Ranidae) in Viçosa, Minas Gerais state, Brazil. South Am J Herpetol 4:286–294. https://doi.org/10.2994/057.004.0312
Simberloff D (2004) Community ecology: is it time to move on? (An American Society of Naturalists Presidential Address). Am Nat 163:787–799. https://doi.org/10.1086/420777
Simberloff D (2014) Biological invasions: what’s worth fighting and what can be won? Ecol Eng 65:112–121. https://doi.org/10.1016/j.ecoleng.2013.08.004
Simberloff D, Martin JL, Genovesi P et al (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66. https://doi.org/10.1016/j.tree.2012.07.013
Smith GR, Burgett AA, Temple KG, Sparks KA (2016) Differential effects of bluegill sunfish (Lepomis macrochirus) on two fish-tolerant species of tadpoles (Anaxyrus americanus and Lithobates catesbeianus). Hydrobiologia 773:77–86. https://doi.org/10.1007/s10750-016-2680-3
Sokal RR, Rohlf FJ (2009) Introduction to biostatistics, 2nd edn. Dover Publications, New York
Speziale KL, Lambertucci SA, Carrete M, Tella JL (2012) Dealing with non-native species: what makes the difference in South America? Biol Invasions 14:1609–1621. https://doi.org/10.1007/s10530-011-0162-0
Teixeira de Mello F, González-Bergonzoni I, Loureiro M (2011) Peces de agua dulce de Uruguay. PPR-MGAP, Montevideo
Townsend CR (2003) Individual, population, community, and ecosystem consequences of a fish invader in New Zealand streams. Conserv Biol 17:38–47. https://doi.org/10.1046/j.1523-1739.2003.02017.x
White EM, Wilson JC, Clarke AR (2006) Biotic indirect effects: a neglected concept in invasion biology. Divers Distrib 12:443–455. https://doi.org/10.1111/j.1366-9516.2006.00265.x
Wiser SK, Allen RB, Clinton PW, Platt KH (1998) Community structure and forest invasion by an exotic herb over 23 years. Ecology 79:2071–2081. https://doi.org/10.1890/0012-9658(1998)079[2071:CSAFIB]2.0.CO;2
Wu Z, Li Y, Wang Y, Adams MJ (2005) Diet of introduced bullfrogs (Rana catesbeiana): predation on and diet overlap with native frogs on Daishan island, China. J Herpetol 39:668–674. https://doi.org/10.1670/78-05N.1
Ziegler L, Maneyro R (2008) Clave para la identificación de los anfibios de Uruguay (Chordata: Amphibia). Universidad de la República, Montevideo
Acknowledgements
GL and NG thank the Agencia Nacional de Investigación e Innovación (ANII) (POS_NAC_2015_1_109517) and the Programa de Desarrollo de las Ciencias Básicas (PEDECIBA), Uruguay, for their postgraduate grants. GL is a member of the Sistema Nacional de Investigadores (SNI), Uruguay. NG and SC thank the ANII for the Beca de Iniciación. We all thank the support of the Rufford Foundation and the local habitants of the studied sites for their contributions during fieldwork. All the used animal welfare protocols were authorized by the National Commission for Animal Experimentation, Museo Nacional de Historia Natural (Code 013/11).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gobel, N., Laufer, G. & Cortizas, S. Changes in aquatic communities recently invaded by a top predator: evidence of American bullfrogs in Aceguá, Uruguay. Aquat Sci 81, 8 (2019). https://doi.org/10.1007/s00027-018-0604-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-018-0604-1