Abstract
In Lake Grande de Peñalara, an originally fishless small high mountain lake in the Central Iberian Peninsula, brook trout (Salvelinus fontinalis) was introduced in the 1970s, and then eradicated 30 years later using gillnets. In this study, we investigated the time-course and changes in macroinvertebrates and zooplankton communities, before and after the eradication, by studying their richness and several biological and ecological traits of macroinvertebrates. Macroinvertebrates richness increased from 13 taxa coexisting with fish, up to a maximum of 27 taxa after the eradication. Rare groups usually affected by fish predation, e.g. swimmers in surface and open waters, showed high dispersal and recolonization capabilities, while those with burrowing, interstitial or crawler habits maintained their presence, and even with the presence of fish given their advantage of hiding from being directly sighted by fish. Taxa with affinities for rare habitats within the lake (e.g. macrophyte beds) occasionally appeared 4–6 years after eradication. In contrast, zooplankton assemblage did not significantly change in richness in the 10 years after eradication. No new species of cladocerans or copepods appeared after fish removal, but 4 new rotifer taxa appeared and 5 disappeared. This was apparently more related to a change in water quality or trophic status as a consequence of the fish removal than to the direct effect of fish removal on rotifers. Zooplankters were significantly smaller, on average, before fish eradication rather than later, indicating that the community responded to the change in predation pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most high mountain lakes are naturally fishless because their remote locations in steep terrain make it difficult for fishes to go upstream to reach them. If fish naturally colonized some of these lakes in the ancient past, they needed to be adapted to very harsh conditions such as scarce food resources in oligotrophic or ultra-oligotrophic waters, and very cold waters with winter ice-cover during several months which, furthermore, could generate deep oxygen depletion. In naturally fishless high mountain lakes, the effects of the introduction of non-native fish have been illustrated for many different regions of the world. Decreases in larger-sized zooplankton and benthic invertebrates after fish introductions have been observed in North American lakes, specifically in California (Bradford et al. 1998; Knapp et al. 2001), Colorado (Lafrancois et al. 2003), Utah (Carlisle and Hawkins 1998) and Western Canada (Donald et al. 2001; Leavitt et al. 1994, Parker et al. 2001; Schindler et al. 2001), as well as in Europe lakes in Northern Italy (Magnea et al. 2013; Tiberti et al. 2014), France (Cavalli et al. 2001), Austria (Schabetsberger et al. 2009), Portugal (Boavida and Gliwicz 1996), and Poland (Gliwicz 1980; Sienkiewicz and Gąsiorowski 2016). Negative effects of fish introduction in lakes have also been recorded for amphibians (Braña et al. 1996), submerged macrophytes (Gacia et al. 2018) and natural fish assemblages (Miró and Ventura 2015) in Spain, and these introductions also resulted in increases in the abundance of pathogens and virulent bacteria in Northern Italian lakes (Pastorino et al. 2018). However, no significant differences were found for large zooplankton species densities or for the composition of the zooplankton assemblages in a few cases when comparing lakes with and without fish, such as in Switzerland (Winder et al. 2001) and Bolivia (Aguilera et al. 2006). A similar lack of effects was also reported for benthic invertebrate assemblages in lakes of New Zealand (Wissinger et al. 2006). Benthic macroinvertebrates and zooplankton are the most affected by these fish introductions when they become the main food resource for the new predators (De Mendoza et al. 2012; Gliwicz and Rowan 1984; McNaught et al. 1999; Schindler and Parker 2002; Sienkiewicz and Gąsiorowski 2016; Tiberti et al. 2014), although other indigenous fish can be affected or they can even become extinct (Nelson and Paetz 1992). The cascade effects on the whole lake ecosystem of fish introductions affected trophic status (Sienkiewicz and Gąsiorowski 2016) by enhancing phosphorus recycling, making it available for algal production (Leavitt et al. 1994). In small lakes, large zooplankton species such as Daphnia can frequently become extinct because of fish predation. As a result, smaller zooplankters such as rotifers (eg. Keratella, Polyarthra) can increase their densities (Schindler and Parker 2002).
Long-term (i.e. decades) effects of introduced fish in high mountain lakes, which are usually located in areas with strong barriers for dispersal of organisms (Donald et al. 2001), pose the question of: to what extent does the recovery towards the ecosystem’s original status become possible if the main pressure (introduced fish) is reduced or removed? Some aquatic invertebrates have adult phases whereby they are able to fly relatively long distances and colonise new aquatic water bodies, but alpine environments display landscape barriers which impede or difficult dispersal (Bitušík et al. 2017). Amphibians, if they have found refuge in small fishless ponds, can recolonise lakes once they are restored to a fishless condition if there are no physical barriers and long distances between lakes (Knapp et al. 2007; Tiberti et al. 2014). Some zooplankton species have resting stages which means that they can maintain their viability for long periods in the sediments (Brendonck and De Meester 2003; Schindler and Parker 2002) although, presumably, after some decades this ability would be minimised by burying due to sedimentation processes. Moreover, the probability of colonization of water bodies located in remote areas is lower.
After the total elimination of fish in some high mountain lakes, the invertebrate assemblages showed different degrees of recovery or changes depending on aspects such as the lake morphometry, the existence and quality of refuge habitats (Carlisle and Hawkins 1998), and the presence of resting eggs in sediments (McNaught et al. 1999; Parker et al. 2001; Schindler and Parker 2002). A decade or even longer periods were needed in some lakes to approach the original or natural communities’ state (Donald et al. 2001; Schindler and Parker 2002). Thus, the resilience of biological communities after the eradication of fish species that were introduced in the past in Alpine lacustrine ecosystems can be influenced by multiple factors (Donald et al. 2001; Knapp et al. 2001; Knapp and Matthews 1998; Knapp and Sarnelle 2008; McNaught et al. 1999; Parker et al. 2001; Sarnelle and Knapp 2004; Tiberti 2017; Tiberti et al. 2018).
The functional structure of macroinvertebrate assemblages, based on combinations of their species traits, can be used to detect differences among them and responses caused by fish predation, and therefore, they can be used to describe the stability of the community linked to the resistance (or resilience) to taxa disappearance through predation (Usseglio-Polatera et al. 2000; 2001). Species traits are attributes describing preferences and adaptations to environmental conditions related to biological, physiological and ecological aspects, and they can be characterized at the genus or even family taxonomic level in order to study and describe the diversity of macroinvertebrate communities (Tachet et al. 2000; Usseglio-Polatera et al. 2000). Functional diversity of biological communities, represented by functional traits of species, can help in the assessment of ecosystems disturbance, processes and biodiversity (Villéger et al. 2010). Therefore, the study of advantageous or disadvantageous biological and ecological traits of macroinvertebrates to avoid fish predation, such as body size, locomotion, dispersal mechanisms, or life cycle duration, can be a useful tool in the evaluation of restoration measures and the assessment of the recovery ability or resilience of aquatic systems, such as the transit towards an original fishless scenario.
Most of the Iberian high mountain lakes are naturally fishless, and only a few native fish species are present in some of them (Doadrio 2001). During the last century, in order to expand the possibilities of sport fishing in mountain areas, some non-native fish species were released for angling in many Iberian high mountain lakes. Most of them were salmonid species, which are well adapted to these cold environments (Gacia et al. 2018; García-Berthou et al. 2015; Miró and Ventura 2015; Toro et al. 2006; Ventura et al. 2017). Brook trout (Salvelinus fontinalis) is native to the NE United States where it lives in lakes, streams, estuaries, or even with anadromous habits (Scott and Scott 1988). This species has been introduced in many high mountain lakes because it is well adapted to oligotrophic waters, surviving under the ice over long periods, and requires cold waters (optimum temperature range of 10–14 °C) and oxygen concentrations greater than 5 mg/l (Ficke et al. 2009; Scott and Scott 1988). Adults feed mainly on insects or small fish, and only small emergent fry (< 3 cm) feed on zooplankton or macroscopic crustaceans (Karas 1997; Tiberti et al. 2016). In the Iberian Peninsula, this species was introduced for the first time in the late nineteenth century, but was spread mainly during the middle of the twentieth century in some high mountain lakes that are located in different mountain ranges (Doadrio 2001; Elvira and Almodóvar 2001; Granados et al. 2006; Toro 2007), including Lake Grande de Peñalara, which is located in the Iberian Central Range.
For several decades, there has been an increasing trend to develop programmes for the eradication of exotic fish, which have been introduced into aquatic ecosystems, with the aim of restoring the ecological balance of these ecosystems. Several and diverse eradication techniques have been used, obtaining varied results according to the type of ecosystem and the introduced species (Donaldson and Cooke 2016). In the Iberian Peninsula, the ecological impact of the allochthonous fish introduction in aquatic ecosystems has, to the best of our knowledge, not yet been well studied, although the still scarce specific studies in rivers and lakes warn about the possible magnitude of this problem (García-Berthou et al. 2015). To date, in Spain only one experience of eradication of an introduced fish (Cyprinus carpio) has been carried out with success, in two Mediterranean lowland lakes, obtaining promising results in the recovery of these ecosystems and their associated biological communities (Maceda-Veiga et al. 2017; Torres-Esquivias et al. 2009).
In 1992, a monitoring and restoration project involving the Lake Grande de Peñalara Alpine area (Central Iberian Range) was implemented by the regional Government (Toro and Granados 2002).This project included the removal of the introduced salmonid brook trout (Salvelinus fontinalis) in Lake Grande de Peñalara, with annual monitoring of the invertebrate community to control the recovery process prior to, during, and after fish removal.
Our main hypotheses are: 1. Brook trout, a non-native fish species which is introduced into a small Iberian high mountain lake, during the nearly 30 years of its presence, would have modified the structure and composition of the aquatic invertebrate community, with the latter being its main food resource; 2. After brook trout removal, a recovery of the aquatic invertebrate community towards a community structure closer to a natural fishless condition is expected; and 3. Changes in the aquatic invertebrate community would be mediated by the main biological and ecological traits that provide aquatic invertebrates better chances of survival and colonization.
Materials and methods
Study site
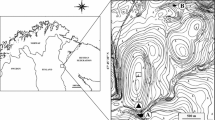
Lake Grande de Peñalara is a small and relatively shallow high mountain lake, formed by glacial overdeepening, which is located in the National Park of Sierra de Guadarrama, in the Iberian Central Range (Spain), at 2019 m a.s.l. (Table 1 and Fig. 1). The lake bottom is mainly silty, with relatively low organic matter content (13%), and blocks, stones, pebbles and sandy material along the shores. No submerged macrophytes are present. The lake is discontinuous cold polymictic, according to the classification of Lewis (1983), as it is ice covered during ca. 4 months. There is one small temporary inlet creek at its northwest shore, a permanent outlet at the southern shore (Fig. 1), and diffuse water inlets that are located along the northeast shore. More detailed information of this lake and its catchment can be found in Granados et al. (2006), Toro and Granados (2002), and Toro et al. (2006).
This lake was originally fishless (Toro 2007), and brook trout were introduced for angling in the 1970s. The lack of adequate and heterogeneous habitats for the refuge of invertebrates, such as submerged macrophytes communities, could have facilitated a strong predation effect on large size and swimming invertebrates after fish stocking, as described for other sites (Carlisle and Hawkins 1998; De Mendoza and Catalan 2010).
Re-colonization of the lake by drifting aquatic invertebrates is unlikely since there are no ponds or wetlands located upstream in the lake catchment, and only one temporary small inlet creek could act as a possible source of macroinvertebrates. The nearest ponds or small lakes are located farther than 200–300 m and in a hydrological sense they are disconnected from the lake (Fig. 1).
Fish eradication
The eradication of brook trout in Lake Grande de Peñalara was conducted using 5 multi-mesh nylon gillnets (Nordic Survey Net), consisting of 12 panels (1.5 m height × 2.5 m length) of different mesh sizes (5, 6.25, 8, 10, 12.5, 15.5, 19.5, 24, 29, 35, and 55 mm stretched net from knot to knot) in a standard randomised order (design according to Appelberg et al. 1995). The gillnets were set perpendicular to the lake shore during the whole year with removal of fish every few days (except during the winter period when the nets stayed under the ice cover for several months). Gillnets were removed, deep cleaned and placed again every 1 or 2 months. Nets were installed at the beginning of August 1999, ending by July 2004, two years after the last three fish were collected in the spring of 2002 (just after the ice-cover period), thus declaring the complete brook trout eradication according to criteria of Tiberti et al. (2018). Although gillnetting is a more expensive and time consuming method than other systems such as rotenone, it is very efficient and more ecologically friendly for such small mountain lakes (Knapp and Matthews 1998).
Sampling of aquatic invertebrates
Sampling of benthic macroinvertebrates was performed over a period of 22 years (with 1 to 8 campaigns per year) during the period of 1986–2018, and also during the ice-free period (Table 2). Samples were collected with D-nets (350 µm mesh size) by sweeping the surface of stirred substrate up to 1 m depth during 30 min, from all of the existing littoral habitats of the lake (silts, sands, gravels, rocks), in a proportion that is relative to its coverage. Zooplankton samples were collected from an inflatable boat or from the ice-cover over the deepest part of the lake (4.8 m deep) by taking 1–3 vertical hauls with a conical plankton net (55 µm mesh size and 26 cm diameter) from the lake bottom to the surface. Zooplankton sampling was performed at least annually from 1991 to 2013, with a variable number of campaigns every year (see Table 2), both during the ice covered periods and the ice-free periods. Even though it was not possible to carry out the same number of campaigns every year due to the lack of ongoing funded research projects during the 30 years of the study, the possible differences in the sampling frequency each year did not appear to significantly affect the number of taxa found. In fact, in both periods (before and after the eradication), years with fewer campaigns show a number of taxa similar to those of years with a higher number of campaigns (Table 2; Fig. 2).
Number of taxa per year (richness) of macroinvertebrates found in Lake Grande de Peñalara before, during and after the eradication of brook trout. Years without any taxa data were not sampled (see Table 2)
Benthic macroinvertebrates and zooplankton samples were preserved in 4% formalin from 1990 to 2004, subsequently macroinvertebrates were preserved in 70% ethanol and zooplankton samples were preserved in lugol (2005–2018). Taxa were identified to the lowest possible taxonomic level under stereo (10–40×) and inverted (10–100×) microscopes for macroinvertebrates and zooplankton, respectively, by using different taxonomic keys. The entire sample (sediment/water) was completely analysed for macroinvertebrates. For the counting of zooplankton, the sample was divided into aliquots and up to 250 individuals for each group were counted for both rotifers and crustaceans, respectively, although counts are not available for all the years. In addition, we measured the length of a minimum of ten individuals for each stage of each species in each sample. The measurement of the body length was used as a surrogate of individual size.
Biological indicators
Biological and ecological traits of macroinvertebrates have been used to assess the response and recovery of these communities after the eradication of brook trout in Lake Grande de Peñalara (Table 3). According to Tachet et al. (2000), species, genus and family level can be used to describe the functional diversity (e.g. traits) of macroinvertebrate communities (Usseglio-Polatera et al. 2000). Each trait has different modalities with a score assigned to each taxon according to its affinity for that category, ranging from 0 (“no affinity”) to a maximum value of 5 (“high affinity”), following a fuzzy coding approach (Chevenet et al. 1994; Tachet et al. 2000). The main advantage of the fuzzy coding procedure is that it allows the analysis of information from various sources and for intermediate values of each category, transforming continuous data into categories (Aşan and Senturk 2012). A taxon could get scores for some or all codes for a trait. A trait profile (e.g. ecological) can be compared to a frequency distribution of the taxon’s affinity for the different modalities of the particular trait. Selected traits according to the information given by Chevenet et al. (1994) and Tachet et al. (2000) were: (i) those showing a potential relationship with the resistance or capability to avoid predation by fish (size, preference of habitat and locomotion), (ii) those related to adaptation to the presence of predatory fish, and (iii) those linked to the ability to colonise new favourable habitats (life and reproduction cycles, aquatic stages and dispersal; Cavalli et al. 1997; Eggers 1982; Schilling et al. 2009; Knapp et al. 2001; Sánchez et al. 2007; Tiberti et al. 2014, 2018; Wattiez 1981).
Data analysis
Composition and richness of benthic invertebrates and zooplankton assemblages, before and after the eradication of brook trout, were compared. A hierarchical cluster analysis (HCA) of year assemblage data (input dataset: Table 4 and Fig. 6) was performed as the most suitable exploratory method, due to the low number of cases (22 years for macroinvertebrates and 16 for zooplankton) and taxa (45 for macroinvertebrates and 22 for zooplankton), many of them with only presence/absence data available (Gore 2000). HCA defines groups of years and taxa, and assigns group memberships based on the degree of similarity between observations, but it does not make any distinction between dependent and independent variables. Squared Euclidian Distance among taxa/years was adopted as a dissimilarity index, and Ward’s minimum variance (Ward 1963) as the most appropriate clustering method to maximise the differences between clusters (Gore 2000).
For macroinvertebrates, a fuzzy correspondence analysis (FCA) (Chevenet et al. 1994) was used to analyse the relative affinity of the sampled years (before, during and after eradication) for the different categories of traits that were selected as indicators of the resistance to fish predation by fish and the ability to colonise and establish in the different lake habitats, respectively. It is based on information about taxa that assumes several categories of the same trait with different degrees of affinity (Dolédec and Chevenet 1994). This kind of analysis has been successfully used in previous studies for the assessment of changes in macroinvertebrate assemblages, before and after experiencing different pressures (Brand and Miserendino 2014; Arce et al. 2014; Desrosiers et al. 2019). A matrix of years (equivalent to “samples”) and traits was constructed by multiplying two matrices: taxa-traits and taxa-years, by transforming the result to percentages (%) of each modality for each year. This matrix of years and traits represents the relevance of each trait and its modalities in the macroinvertebrate assemblage existing in that year, where the effect of the presence/absence of fish could play an important role. The R package Factoclass was used to combine the factorial method (Chessel et al. 2004) and cluster analyses to perform the multivariate exploration of the data matrix (Pardo and Del Campo 2007), as well as to obtain a partition of the dataset and the characterization of each of the classes.
Regarding the zooplankton community composition, we checked the possible effects of fish presence and temporal variation, both intraannual and interannual, through redundancy analysis (RDA). We used the abundance of each zooplankton taxon as response variables. The predictive variables were three explicative factors: the fish presence, the month and the year when the sample was obtained. The fish presence was considered as a category by defining three periods: before, during, and after eradication. The Hellinger transformation was used for the species abundance data. The explicative variables of the RDA were tested through a forward selection procedure with a double stopping criterion based on the alfa significance and the adjusted R2 calculated with all explaining variables (Blanchet et al 2008).
In order to seek differences among the pre-eradication period, the eradication period, and thereafter, as well as to reveal possible differences in the zooplankton lengths as a function of fish presence in the lake and of the temporal variation, we performed an analysis of variance (ANOVA) of the zooplankton lengths, both for the whole assemblage as well as for the different species, as response variables. We considered the temporal variation in the analysis by including again the sampling month and year as predictors. For the whole assemblage analysis we also used the taxon and the developmental stage as predictors. Moreover, we also performed ANOVAs to seek for differences in the ratio of crustacean to rotifer using the eradication period, the month and the year as predictors and the ratio as the response variable.
Statistical analyses were performed using IBM SPSS Statistics (IBM Corp. Released 2016) for cluster analyses. RStudio (RStudio Team 2015) was used for fuzzy correspondence analysis with the package Factoclass (Pardo and Del Campo 2007), whereas the vegan package was used for RDA (Oksanen et al. 2017). ANOVAs were performed with the basic functions of RStudio.
Results
Brook trout eradication
Once the eradication activities were started, for the first year (1999) a total of 239 individuals were captured; for the second year (2000) 318 were captured; for the third year (2001) the catches were between 20 and 40 individuals (this number is approximate, due to poor conservation of the fish after being trapped under the ice for several months during the winter period); and finally, the last 3 fish were collected in 2002, just after the ice-cover period. Gillnets remained installed until the summer of 2004 (July), without capturing any additional fish. After that date, no more fish were observed in the lake. Brook trout has also been removed from the lake outlet downstream (Bosch et al. 2019), hence natural recolonization is not possible.
Macroinvertebrate fauna changes
The composition and richness of the macroinvertebrate assemblages changed during and after the eradication of brook trout in Lake Grande de Peñalara. Mean taxa richness per year increased from 9 in the years prior to eradication, to 18 during the eradication process, and to 22 in the subsequent fishless years, reaching a maximum of 27 taxa in 2011 (Table 4 and Fig. 2). The absence (zero value) of a taxon in a given year should be considered as the taxon was not captured in the sampling campaigns, although its absence from the lake could not be demonstrated.
From the cluster analyses, Ward’s dendrogram grouping years (Fig. 3a) resulted in two major dissimilar clusters (Y1 and Y2) (rescaled distance of combined clusters = 25), with one of clusters (Y2) being divided into two relatively dissimilar groups (rescaled distance of combined clusters = 11). All three were clearly separated by the fish presence/absence in the lake as follows: Y1 = Years with presence of brook trout; Y21 = Years during eradication process and first year after eradication, and Y22 = Two or more years after eradication, without brook trout in the lake.
Dendrograms of classification analysis of years (a) and taxa (b) according to matrix of Table 4. Groups (Y prefix for years and T prefix for taxa) are defined according to criteria of fish presence/absence for years and frequency of appearance and fish presence/absence for taxa. See the text for explanations of the clusters codes
Ward’s dendrogram for taxa (Fig. 3b) resulted in two major dissimilar clusters (T1 and T2) (rescaled distance of combined clusters = 25), both of which were divided into two relative low dissimilar groups (rescaled distance of combined clusters = 5 and 3), related to frequency of appearance and to fish presence/absence. Group T1, characterized by high frequency taxa (continuous annual presence), is divided into two groups: T11 = High frequency taxa and coexistence with fishes and after eradication; T12 = High frequency taxa appearing during eradication and later. Instead, group T2, which always corresponds to the eradication process or later, is characterized by a low-medium frequency of appearance (discontinuous annual presence), and is divided into two groups: T21 = Low frequency taxa with appearance since the first year of sampling (2001) during the eradication process, and later; T22 = Low or medium frequency taxa with appearance mostly in 2002, during the eradication, and/or later. T22 can be divided into 5 groups with a low dissimilarity between them (rescaled distance of combined clusters = 2): T221, T222 and T223 include taxa with appearance mostly in 2002 and/or later (mostly 1–3 years). T224 includes taxa with appearances from the last two years of sampling during eradication process (2001–2002) to 4 years later (2006), and T225 includes taxa with appearances 6 years (2008) or later after the eradication process.
The assignments of selected trait categories for each taxon found in Lake Grande de Peñalara from 1986 to 2018 are shown in Table 5. Results of Fuzzy Correspondence Analysis (FCA) show that the information on the first 2 axes explains most of the variability (78.0%) in traits and therefore, it is sufficient to analyse the first factorial plane (Fig. 5). All trait categories (Fig. 4a) are ordered along the first axis according to their affinity with the distribution of years grouping along the same first axis (Fig. 4b). The years have been classified into three groups (Fig. 4b), indicating a gradient of presence/absence of fish in the lake as follows: I. Before fish eradication; II. Eradication period; III. After fish eradication. Figure 5 shows trait correlations with axis 1–2 in the FCA.
Distribution of a the different modalities of traits (see codes in Table 3) and b years on the F1-F2 factorial plane as result of the Fuzzy Correspondence Analysis (FCA). Years are classified into 3 groups: I. Before fish eradication; II. Years during eradication; III. After fish eradication
Distribution of modalities of the taxa traits on the first two axes of the fuzzy correspondence analysis (FCA) (data from matrix traits/years). Each modality in each trait plot is located at the weighted average of the year position (dot) representing that one. Correlation ratios with both axis and ordination plot scale are indicated. These ratios represent proportions of the total variance explained by each axis to quantify the distance among modalities of a variable. Table with values and cumulative proportion of inertia (100% = 1) for the Fuzzy Correspondence Analysis of relative affinity of sampled years for the different categories of selected traits is shown
Regarding locomotion and the relation with substrate, taxa with crawler (u4), burrower (u5), interstitial (u6) or temporally attached (u7) habits showed a better response to fish predation, and they are related to the group of years with fish presence (group I). On the contrary, the categories of fliers (u1), surface swimmers (u2) and swimmers in open water (u3), less advantageous to avoid predatory pressure, are significantly related to the years after eradication. This trait shows the highest correlation rate (0.094) with Axis 1 of the factorial plane (Fig. 5).
The microhabitat trait shows a similar, although less marked, response to the previous trait along the first axis (Fig. 4a). The microhabitat categories related to the taxa found in years with fish presence, boulders, cobbles and pebbles (t1), gravels (t2), sands (t3), silt (t4) and twigs or roots (t7), located in the gradient of Axis 1, also coincide with those most characteristic and predominant microhabitats in the lake. However, the categories of the microhabitat trait that are related to the taxa that appeared in the years after fish eradication correspond to less frequent or often lacking habitats in the lake: beds of macrophytes and filamentous algae (t5), microphytes (t6), organic detritus and litter (t8), and mud (t9).
With respect to dispersion mechanisms, this trait reaches the second highest correlation with the first axis of the factorial plane (correlation rate = 0.022) (Figs. 4, 5). Taxa with aquatic passive dispersion (f1) or aerial passive dispersion (f3) are correlated with the years with the presence of brook trout, and taxa with active aquatic dispersion (f2) and active aerial dispersion (f4) are correlated with the years after the eradication.
The different aquatic stages in which a taxon can be found in the aquatic environment are also reflected in a gradient along Axis 1. Thus, the aquatic stages of larvae (d2), and especially pupae (d3), are more typically the taxa which coexisted with fish. On the contrary, the forms of eggs (d1) and especially the aquatic adult stage (d4), are more representative of taxa which appeared in the lake after fish eradication.
Short life cycles (≤ 1 year) (b1) and low number (< 1) of reproductive cycles per year (c1) are traits related to taxa coexisting with brook trout, although these traits do not show a high correlation with Axis 1 (0.003 and 0.007, respectively) (Fig. 5).
Finally, taxon size shows a weaker ordination in the factorial plane regarding the transition from fish presence to a fishless condition (correlation with Axis 1 = 0.005) (Fig. 5), with largest sizes (a4, a5 and a6) closer to years with fish presence, and smaller sizes (a1, a2 and a3) slightly closer to fishless years. A gradient that is somewhat more related to Axis 2 (correlation rate = 0.007) was found for this trait (Fig. 5), which could represent a transition between the years of the eradication process (2001–2002) and the subsequent years with fish already eradicated. Thus, the attributes of large taxon sizes (a5 and a6) are located in the plane that is most related to the transition years of eradication, in contrast to the category of smaller size (a1) which is positioned in the opposite extreme of Axis 2.
Zooplankton
The annual richness of the zooplankton assemblages did not show any significant change when comparing the periods before (mean value = 9; n = 6) and after (mean value = 9.6; n = 10) the eradication of brook trout in Lake Grande de Peñalara (Fig. 6). However, and regarding clustering analyses, Ward’s dendrogram for years (Fig. 7a) resulted in two major dissimilar clusters (Y1 and Y2) (rescaled distance of combined clusters = 25), with one of the clusters (Y2) being divided into two relative dissimilar groups Y21 and Y22) (rescaled distance of combined clusters = 8), and all three significantly being related to the presence/absence of fishes in the lake: Y1 = Years with presence of brook trout; Y21 = A group of 4 years which include one year before eradication, two following years just after eradication, and one year 9 years after eradication; and Y22 = Three or more years after eradication (except 2011, included in Y21), without brook trout in the lake.
Time course of the zooplankton assemblages during the period 1991–2012 in Lake Grande de Peñalara. For each species, the years when its presence has been detected are shaded in green. Species abundances (in bars) (individuals/m3) are only available for 1997 and the period 2003–2012. The blue lines link the dates of greatest abundance each year with quantitative data
Dendrograms of classification analysis of years (a) and taxa (b) according to data from Fig. 6. Groups are defined according to criteria of fish presence/absence for years and frequency of appearance and fish presence/absence for taxa
Ward’s dendrogram for taxa (Fig. 7b) based on presence/absence data resulted in two major dissimilar clusters (T1and T2) (rescaled distance of combined clusters = 25), both divided into two relative low dissimilar groups (rescaled distance of combined clusters = 5 and 6), related to both the frequency of taxa appearance and presence/absence of fish respectively. Group T1 is divided into two groups, and it is characterized by low (T11) or medium (T12) taxa appearance (discontinuous annual presence): T11 = Low or rare taxa, including 6 taxa which appeared only when fish were present, 3 taxa which appeared only when fish were absent, and 1 taxa which appeared both in years with presence and absence of fish; T12 = Medium frequency taxa with appearance both in years with presence and absence of fish. Group T2 comprised species with almost continuous occurrence, and is divided into two subgroups: T21 includes species mainly appearing after the eradication of the fish; and T22 includes taxa with very frequent or continuous appearance both before and after the eradication process.
Figure 6 also shows both the presence/absence data, as well as the absolute abundance of each zooplankton species in years when the samples were analysed quantitatively. Because only one year is available before eradication with quantitative data (1997), to be compared with data from the 10 years after eradication (2003–2012), a detailed analysis of these absolute abundance data has not been performed. Nevertheless, notable differences are observed for some species between the maximum annual abundance values (nº indiv./m3) reached after the eradication with the values found in 1997, when the brook trout was present in the lake. Ceriodaphnia reticulata had a density of 67,000 ind./m3 at the end of the summer of 1997, and during the period of 2003–2012 its density was never higher than 14,645 ind./m3. Similarly, Eucyclops serrulatus and Asplanchna priodonta, with abundances (ind./m3) of 4100 and 22,600 in 1997, and maxima of 414 and 3440 within the 10 years following eradication, respectively, dropped their abundance almost by one order of magnitude. In contrast, species such as Tropocyclops prasinus or Keratella quadrata showed maximum values (ind./m3) of 6650 and 11,900, respectively, in 1997, but reaching annual maxima of 75,400 and 98,100, respectively, in the period of 2003–2012, increasing by nearly one order of magnitude.
The RDA showed both intraannual and interannual differences in the composition of the zooplankton assemblages as both, the month and the year, were selected using Blanchet et al (2008) criteria for the forward selection procedure, whereas the time series period (defined by fish presence), was not selected. The RDA had a good performance (r2 = 0.32) when solely including these two temporal variables. We found that the four species which dominated the zooplankton composition, Polyarthra vulgaris, K. quadrata, Tropocyclops vulgaris and C. reticulata, were associated with different months and years (Fig. 8). P. vulgaris was related to spring months and was also mostly linked to the period prior to the eradication. Then, the summer months were dominated by K. quadrata, the autumn samples showed the dominance of the crustaceans T. vulgaris and C. reticulata, and by the end of the autumn and the winter the dominance turned to K. quadrata. As for the year, before the eradication the community was dominated by K. quadrata, and during the eradication it moved towards a community with an increasing importance of the crustaceans. From 2003 to 2005, K. quadrata and C. reticulata were dominant, whereas from 2006 to 2009 P. vulgaris dominated, and then there was a crustacean dominance in 2011 and 2012.
Five new rotifer taxa (Trichotria tetractis, Colurella/Euchlanis, Filinia opoliensis, Kellicotia longispina and Lecane lunaris) were found exclusively after fish eradication, whereas five other taxa disappeared (Polyarthra remata, P. dolichoptera, Filinia longiseta, Tetramastix opoliensis and Brachionus calyciflorus). P. vulgaris showed a continuous presence in the lake after fish removal but it was only found in one of the years before eradication, whereas P. dolichoptera and P. remata were only present during fish stocking, and then they disappeared after fish eradication.
Globally, the zooplankton assemblage was significantly smaller (shorter length, p < 0.001) before fish eradication (mean length 157 µm) than during the eradication period (mean length 185 µm) and thereafter (mean length 208 µm). Additionally, there were significant differences among years. In years 2003, 2007 and 2009, the average lengths were significantly bigger, whereas in 2004 and 2005 they were significantly smaller. The crustacean/rotifer ratio was also significantly higher after the fish removal (6.8% more than the mean p < 0.001), which could also explain the bigger sizes after eradication since, as aforementioned, crustaceans are generally bigger than rotifers. Furthermore, we also found significant differences in species sizes for the different periods. For instance, we found significant differences (p < 0.001) in the length of females of the copepod E. serrulatus, the biggest zooplankton species in the lake, with a mean length of 836 µm before the eradication, and 932 µm afterwards. However, other species of smaller sizes showed no significant size changes.
Discussion
The analysis of trait profiles and presence/absence data of invertebrate taxa found in Lake Grande de Peñalara, before, during and after the eradication of introduced alien brook trout, helps to interpret the changes in the composition of their communities throughout the eradication process, as related to the fish predatory pressure. Feeding habits of the brook trout in lake ecosystems are mainly benthic-littoral (Power et al. 2002), where the habitat heterogeneity and inputs of allochthonous fauna, as an important food source (Rolla et al. 2018), are high. Lake Grande de Peñalara is a relatively small (6964 m2) and shallow (4.8 m maximum depth) lake, where the proportion of littoral habitat is very high compared to that of pelagic habitat. Therefore, the response of the assemblages of benthic or littoral invertebrates is expected to be very rapid in response to changes in the levels of pressures such as fish predation.
The macroinvertebrates richness (nº of taxa) (Fig. 2) already showed a clear annual increase during the brook trout eradication in 2001 and 2002. Most fish catches occurred in 1999 (239 ind.) and 2000 (318 ind.), and consequently predatory pressure radically decreased from 2001, when only a few dozen individuals were captured, and 2002, when the last 3 fish were caught. The macroinvertebrate assemblages of the lake during the period where brook trout inhabited the lake included only 13 taxa, 9 of them with a repeated appearance every sampled year. However, to avoid sampling biases, we have considered a taxon as absent when it did not appear over a period of at least 10 years (e.g. taxa that are not found in the presence of brook trout: Acilius, Berosus, Gerris), whereas we considered a taxon as having an uncertain absence when it has appeared intermittently in the 10 years period (e.g. for the post-eradication period, taxa that are found for only a few of those years), which could be due to two reasons: (a) the difficulty of capturing as being elusive or scarce (e.g. Gyrinus, Dytiscus); (b) the concerned taxon reaches the lake sporadically, but does not colonise that lake due to the difficulty in finding a suitable habitat or adequate conditions (e.g. Amphinemura, Aeshnidae).
Macroinvertebrates classification (Fig. 3b) shows a group, Group T11, that is very well defined by its biological and ecological traits which confers them a good resistance to the presence of predatory fish. These are burrowers and they have interstitial habits (Chironomidae, Oligochaeta, Pisidium, Sialis) or crawler (Allogamus, Athripsodes, Habrophlebia, Limnephilinae, Oulimnius) locomotion and substrate beneficial habits, thus helping them to hide from fish vision (Knapp et al. 2001; Tiberti et al. 2014). Chironomidae are usually reported as a frequent and high density group in sediments of alpine lakes where fish are present (Gilinsky 1984; Lafrancois et al. 2003). On the contrary, swimmers are potentially one of the more sensitive groups to fish predation. Fliers (u1), surface swimmers (u2) and open water swimmers (u3) are significantly related to the years after the eradication, given that these are unfavourable traits which would allow brook trout predation. Similarly, Tiberti et al. (2018) reported an increase in swimmers and clingers in alpine lakes of the Western Italian Alps after the eradication of brook trout. Moreover, in eastern North America, Schilling et al. (2009) found more abundant, active and free-swimming macroinvertebrate taxa in fishless lakes, whereas Evans (1989) recorded the disappearance of some Heteroptera (i.e. notonectids and corixids) and coleoptera larvae (Dytiscidae), just 3 months after the introduction of brook trout in a lake. On the other hand, Heteroptera (e.g. Artocorixa, Corixa, Gerris, Hesperocorixa, Notonecta, Pleidae, Sigara, Veliidae) have a high capacity of active dispersion, both aerial (f4) and aquatic (f2), which facilitates their rapid colonization once the eradication was effective in Lake Grande de Peñalara. Most of them were absent or rare during fish presence because of their swimmer habits (u2 and u3) in surface (e.g. Gerris, Gyrinus) and open waters (other Heteroptera), making them very visible to fish, but they appeared as the first group of lake colonisers at the beginning of the eradication process (Groups T12, T221 and T222 in Fig. 3b), but always being present thereafter. The absence of macrophytes in this lake, which would serve as a spatial refuge from fish predation, probably precluded the presence of these swimmer species as demonstrated by other authors (Gilinsky 1984; Leppä et al. 2003). For instance, in a study of 82 alpine lakes in the Pyrenees Mountain Range (NE Iberian Peninsula), the presence of salmonids and macrophytes were more decisive for the distribution of dytiscids (swimming Coleopterans) than temperature (De Mendoza et al. 2012). In that study, the dytiscid Agabus bipustulatus, a typical swimmer with a wide thermal distribution on the Iberian Peninsula, was mainly found in cold high mountain lakes that are fishless. In the Southern Lake Grande de Peñalara, which is located in a warmer climate than the Pyrenean lakes, A. bispustulatus (and also A. nebulosus) were only found when the eradication of brook trout had already started, but they appeared almost constantly thereafter, which shows its high sensitivity to fish predation. The capacity for aerial dispersion was also very important for some of the new colonisers, since there are no significant inlets to the lake allowing drifting processes. The aerial dispersal mode would also play an important role in the sensitivity of macroinvertebrate taxa to be captured by adult fish before the eradication. Accordingly, Sánchez et al. (2007) observed a large number of aerial preys, as opposed to the benthic preys, consumed by adult fish in five alpine lakes in the nearby Gredos Mountains (Iberian Central Range).
The macroinvertebrate taxa included in groups T224 and T225 (Fig. 3b) appeared in the lake 4 to 6 years after the complete eradication of the brook trout, respectively. All of them were relatively rare or with a low frequency of appearance in these years, and their trait affinities for microhabitats were related to macrophytes and filamentous algae (t5), microphytes (t6), organic detritus and litter (t8) and mud (t9), all of them being scarce or lacking in the lake. In contrast to other aquatic insects (e.g. Chironomidae, some mayflies, stoneflies or caddisflies) with a limited dispersal potential between alpine lakes (Bitušík et al. 2017), the dispersal mechanisms of these taxa (e.g. Acilius, Aeshnidae, Artocorixa, Berosus, Hemisphaera, Hydrochus, Hydrophilidae) are mainly aerial and active (f4), most of them with winged adult stages. Their sporadic appearances probably correspond to a continuous flux of individuals which reach the lake from short distances (nearby ponds in the area) but do not effectively colonise and reproduce there (Hawkins et al. 2000), due to the lack of suitable habitats. They continuously contribute to the maintenance of a higher richness after the eradication of brook trout. These taxa could, therefore, be considered as suitable indicators of environmental recovery of these types of aquatic ecosystems (Resh et al. 2005). Schilling et al. (2009) have pointed out that some of the T22 Group families (i.e. Dytiscidae, Gyrinidae, Aeshnidae, Corixidae or Notonectidae) which were recorded in Lake Grande de Peñalara after fish eradication, are robust indicators of fish absence in lakes. Therefore, they should not be ignored or excluded in community analyses as outliers, although some authors (Hawkins et al. 2000) consider them unimportant with respect to quantifying the resistance and resilience of the faunal community.
In contrast to the macroinvertebrates, the richness of the zooplankton taxa kept similar annual mean values (Fig. 6), before and after the eradication of the brook trout in Lake Grande de Peñalara. These results are in accordance with some studies in alpine lakes of the Swiss Alps (Winder et al. 2001) and the Italian Alps (Stoch et al. 2019), where fish presence was excluded as a main factor in explaining the occurrence or absence of large zooplankton species, and in Western Canadian mountain lakes, where Donald et al. (2001) compared fishless lakes with lakes where salmonids were introduced, and noting the low planktivorous efficiency of the brook trout. Even though an eradication experience in the Western Italian Alps (Tiberti et al. 2018) showed that the biomass of larger zooplankton taxa increased after fish eradication, our results reveal how, in Lake Grande de Peñalara, not so large zooplankton species such as Tropocyclops prasinus, Ceriodaphnia sp. or Alona sp. did not show any permanent increase in the trend of their abundance after fish eradication.
Several authors have reported increases in rotifer abundance after lake fish stocking due to fish predation on large crustaceans (McNaught et al. 1999). In our study we have insufficient data on rotifer abundance prior to fish eradication, but the appearance of 4 new taxa (Trichotria tetrctis, Colurella/Euchlanis, Filinia opoliensis and Kellicotia longispina) and the disappearance of 5 taxa (Polyarthra remata, P. dolichoptera, Filinia longiseta, Tetramastix opoliensis and Brachionus calyciflorus) after fish eradication have been recorded, showing a significant change in the composition of rotifer assemblages in the lake. Among the rotifer species that were found in the lake throughout the whole of the studied period, Polyarthra vulgaris, Asplanchna priodonta, K. longispina and Keratella quadrata are also very common species in other European high mountain lakes (Catalan et al. 2009; Miracle 1978; Tonolli and Tonolli 1951). K. quadrata is the most abundant and perennial (pre- and post-eradication) rotifer species in Lake Grande de Peñalara, and it has also been reported as being the most abundant rotifer in other high mountain lakes in the Iberian Central Range; i.e. in Serra da Estrela (Boavida and Gliwicz 1996) and in Sierra de Gredos (Toro and Granados 2001).
There is no clear relationship between the changes of every single rotifer species and fish effects. The new and continuous record of K. longispina after fish eradication has a good fit with the results that were obtained by Schindler and Parker (2002) in lakes from the Canadian Rockies, where this species was dominant before fish introductions in the 1960s, but contrasting results were obtained by McNaught et al. (1999) for a small alpine lake in the Canadian Rocky Mountains. This species was abundant during fish stocking, and disappeared after fish removal, being replaced by Polyarthra dolichoptera, which in turn was present in Lake Grande de Peñalara only during the fish stocking period. Similarly, the absence of P. dolichoptera after fish removal was observed by Stenson (1982, 1983) in a Swedish lake, where P. vulgaris remained after fish eradication. Changes in phytoplankton community and oxygen content and temperature were noted as possible reasons for these changes. P. dolichoptera, a cold-stenothermal species, would tolerate low oxygen levels in the water column (Stenson 1983) during a higher productivity period with fish presence in Lake Grande de Peñalara, and P. vulgaris could have increased its population with the improvement of water quality and oligotrophy trend of the lake, and the phytoplankton changes after fish eradication shown by Toro and Granados (2002) and Toro et al. (2006). Therefore, some changes in zooplankton composition and structure in Lake Grande de Peñalara after brook trout eradication could be more related to indirect effects of fish removal such as shifts in phytoplankton composition, productivity, and trophic status, rather than to the effects of direct predation. Since rotifers can be good indicators of the lake trophic status (Berzins and Pejler 1989), the disappearance of species indicating a higher trophic status, such as Brachionus sp. and F. longiseta (Ejsmont-Karabin 2012; Gannon and Stemberger 1978; Velasco et al. 2005) could be related to an improvement in the trophic status of the lake after fish eradication.
Although zooplankton did not show important changes in species richness, differences in the composition could be found, with a predominance of rotifers over crustaceans before and during the eradication, whereas the crustaceans increased their relative importance after fish eradication. Equally, Knapp et al. (2001) and Tiberti et al. (2018) found that the abundances of large crustaceans were lower in fish stocked lakes, whereas small crustaceans and rotifers were more abundant. In these lakes, predation by brook trout showed important effects on bigger zooplankton, especially in those exceeding 1 mm length. In Lake Grande de Peñalara, the big species did not exceed this length, and therefore the impact of brook trout could have been more limited. However, some changes could be seen after the eradication, as the mean of the lengths of the community individuals increased due to both the increase in size of the biggest species and to the increasing dominance of crustaceans over rotifers.
To our knowledge, the resilience of zooplankton species is not yet well established. Some authors have pointed to a high resilience due to their high dispersal rates (Louette and Meester 2005), even higher for cladocerans than for copepods (McNaught et al. 1999), as well as by their capacity to keep resting eggs in sediments (Parker et al. 1996). Instead, Donald et al. (2001) suggested an average of 19 years to recover the original zooplankton assemblages after fish removal, arguing difficulties for recovery because of the physical dispersal barriers in mountain ranges. Aspects such as predation by macroinvertebrates on zooplankton, the length of fish permanence (long residence time would decrease the size of the egg bank of sensitive taxa in the sediments), or low lake depths (a deeper lake would facilitate the coexistence of fishes and large zooplankton species providing refuge in deep habitats) have been suggested as relevant factors for the resilience of the zooplankton assemblages (Donald et al. 1994; Knapp et al. 2001; Knapp and Sarnelle 2008; McNaught et al. 1999). Unfortunately, to date, there are no similar reference sites with and without fishes in Lake Grande de Peñalara surroundings with available data to be compared and used for testing these hypotheses.
The lack of information on zooplankton composition prior to the fish stocking in Lake Grande de Peñalara makes it difficult to know whether or not a natural state is reached after fish removal, or if fish stocking did not affect zooplankton community. A study on feeding ecology of brook trout in five alpine lakes in the nearby Sierra de Gredos (Iberian Central Range, ca. 130 km from Lake Grande de Peñalara) showed a diet composition based mainly on macroinvertebrates, with no record of crustaceans on a total of 49 stomachs that were analysed (Sánchez et al. 2007). However, that particular study was carried out solely in the summer months, when the food resource is mainly composed of macroinvertebrates, while other authors (Cavalli et al. 2001) have reported a fish diet in alpine lakes based also on zooplankton (copepods) during the ice cover period. We do not know if any large zooplankton species (e.g. large crustaceans) with predatory capacity on smaller crustaceans or rotifers (McNaught et al. 1999) were present before fish stocking, which may not have returned to the lake after fish removal. If these large species were present, the current lack of these top predators in the food chain could hinder the attainment of an original equilibrium in the zooplankton composition. For instance, Winder et al. (2003) observed that the composition and relative dominance of some zooplankton species under fish predation in a high mountain Swiss lake were determined by the food web structure (including invertebrate predation or zooplankton competition) and the trophic status. According to previous experiences, it is not expected to reach a stability in the establishment of a community structure that matches the hypothetically existing stability prior to the fish introduction for a period of at least 10 (McNaught et al. 1999; Schindler and Parker 2002) to 19 years (Knapp et al. 2001). Although there is no clear evidence of significant changes in the zooplankton assemblages before and after the eradication of the brook trout in Lake Grande de Peñalara, the period of our study was already within this range, but changes should already have occurred or, at least, they should have shown evident trends, if any existed. Likewise, Symons and Shurin (2016) found similar zooplankton assemblages in lower elevation fish-containing and fishless lakes, while high-elevation lakes showed different communities with and without fishes. Higher summer temperatures in this Mediterranean high mountain lake type, in comparison with higher latitude lakes, could also be a factor influencing this apparent similitude before and after eradication in Lake Grande de Peñalara.
Because of aspects such as the small size, the simple habitats displayed in the lake, the low diversity of the zooplankton community, and the simplicity of the food web of this alpine ecosystem, higher sensitivity (or fragility) to the introduction of a top predator in the food web would be expected (Cavalli et al. 2001; Knapp et al. 2005). This, together with the low resilience shown by zooplankton, would mean that the impact of the introduction of fish on biological communities in these types of lakes, in addition to being significant, would last longer than desired. Additionally, it is interesting to consider the possibility of a process of adaptability of the ecosystem (i.e. considering the zooplankton community) instead of a resilience type of dynamics, if the disturbance caused by brook trout is over a long enough period that would be a driver to a different end than the pre-disturbance state (Müller et al. 2016). In such a case, no future short- or medium-term shifts towards a hypothetically previous state would be expected, and the ecosystem could go towards a different and, perhaps, less stable organization, or return to the baseline over the long term, which could take decades (Spears et al. 2017).
Finally, recent climate warming, with the already registered reduction in cold extremes and moderate precipitation decrease in the Iberian Peninsula in the last decades (Brunet et al. 2007; Vicente-Serrano et al. 2017) could also have affected the response of biological communities and lake food web structure towards an unexpected state under predation pressure (Symons and Shurin 2016) and perhaps also after fish eradication. Warming affects metabolism and trophic interactions in lakes, and it is suspected of reducing the resilience of food webs to introduced predators, which eliminate the large size zooplankton species, diminishing the capacity of species replacement to buffer the fish effect (Symons and Shurin 2016). Shallowness, together with the small size of the lake and also the small catchment sizes, added to the low topographic shading in Lake Grande de Peñalara, are factors that make this lake very sensitive to climate warming (Hamerlík et al. 2014; Novikmec et al. 2013).
In summary, after the eradication of a salmonid that was introduced into a small mountain lake in south-western Europe, communities of aquatic invertebrates showed different degrees of recovery. Very early changes have been observed since the beginning of eradication in macroinvertebrate assemblages (especially in swimmer species) towards richer communities, probably being similar to those that would be found before fish introduction, showing a high recovery capacity due to the dispersal abilities of the already extinct taxa due to fish predation. On the contrary, zooplankton have not undergone significant changes that could be directly related to the introduction and eradication of the brook trout, displaying either a low sensitivity to fish predation, or a very low recovery capacity, since a response and return to its theoretical original conditions would be expected in the studied period exceeding 10 years after eradication. However, rotifer composition has shown changes, but they are apparently more related to water quality (i.e. trophic status) or other environmental variables as a possible indirect consequence of fish eradication, and zooplankton assemblage was significantly smaller in length before the fish eradication than during the eradication period and after. Palaeolimnological studies of subfossil remains of zooplankton species (e.g. cladocerans) in this lake, or surveys of invertebrate communities in nearby fishless lakes, would be essential for addressing the assessment of the ecological resilience of these southern European high mountain lakes.
References
Aguilera X, Declerck S, De Meester L, Maldonado M, Ollevier F (2006) Tropical high Andes lakes: a limnological survey and an assessment of exotic rainbow trout (Oncorhynchus mykiss). Limnologica 36:258–268
Appelberg M, Berger HM, Hesthagen T, Kleiven E, Kurkilahti M, Raitaniemi J, Rask M (1995) Development and intercalibration of methods of Nordic freshwater fish monitoring. Water Air Soil Pollut 85:883–888
Arce E, Archaimbault V, Mondy CP, Usseglio-Polatera P (2014) Recovery dynamics in invertebrate communities following water-quality improvement: taxonomy- vs trait-based assessment. Freshw Sci 33:1060–1073
Aşan Z, Senturk S (2012) An application of fuzzy coding in multiple correspondence analysis for transforming data from continuous to categorical. J Mult Valued Logic Soft Comput 18:355–370
Berzins B, Pejler B (1989) Rotifer occurrence and trophic degree. Hydrobiologia 182:171–180
Bitušík P, Svitok M, Novikmec M, Trnková K, Hamerlík L (2017) Biological recovery of acidified alpine lakes may be delayed by the dispersal limitation of aquatic insect adults. Hydrobiologia 790:287–298
Blanchet FG, Legendre P, Borcard D (2008) Forward selection of explanatory variables. Ecology 89:2623–2632
Boavida MJ, Gliwicz M (1996) Limnological and biological characteristics of the alpine lakes of Portugal. Limnetica 12:39–45
Bosch J, Bielby J, Martin-Beyer B, Rincón P, Correa-Araneda F, Boyero L (2019) Eradication of introduced fish allows successful recovery of a stream-dwelling amphibian. PLoS ONE 14(4):e0216204
Bradford DF, Cooper SD, Jenkins TM Jr, Kratz K, Sarnelle O, Brown AD (1998) Influences of natural acidity and introduced fish on faunal assemblages in California alpine lakes. Can J Fish Aquat Sci 55:2478–2491
Braña F, Frechilla L, Oriazola G (1996) Effects of introduced fish on amphibians assemblages in mountian lakes of Northern Spain. Herpetol J 6:145–148
Brand C, Miserendino ML (2014) Biological traits and community patterns of Trichoptera at two Patagonian headwater streams affected by volcanic ash deposition. Zool Stud 53:1
Brendonck L, De Meester L (2003) Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491:65–84
Brunet M, Jones PD, Sigro J, Saladie O, Aguilar E, Moberg A, Della-Marta PM, Lister D, Walther A, Lopez D (2007) Temporal and spatial temperature variability and change over Spain during 1850–2005. J Geophys Res. https://doi.org/10.1029/2006JD008249
Carlisle DM, Hawkins CP (1998) Relationships between invertebrate assemblage structure, 2 Trout species, and habitat structure in Utah mountain lakes. J N Am Benthol Soc 17:286–300
Catalan J, Grazia Barbieri M, Bartumeus F, Bitusik P, Botev I, Brancelj A, Cogalniceanu D, Manca M, Marchetto A, Ognjanova-Rumenova N et al (2009) Ecological thresholds in European alpine lakes. Freshw Biol 54:2494–2517
Cavalli L, Chappaz R, Bouchard P, Brun G (1997) Food availability and growth of the brook trout, Salvelinus fontinalis (Mitchill), in a French Alpine lake. Fish Manag Ecol 4:167–177
Cavalli L, Miquelis A, Chappaz R (2001) Combined effects of environmental factors and predator-prey interactions on zooplancton assembalges in five high alpine lakes. Hydrobiologia 455:127–135
Chessel D, Dufour AB, Thioulouse J (2004) The ade4 Package-I: onetable methods. R News 4:5–10
Chevenet F, Dolédec S, Chessel D (1994) A fuzzy coding approach for the analysis of long-term ecological data. Freshw Biol 31:295–309
De Mendoza G, Catalan J (2010) Lake macroinvertebrates and the altitudinal environmental gradient in the Pyrenees. Hydrobiologia 648:51
De Mendoza G, Rico E, Catalan J (2012) Predation by introduced fish constrains the thermal distribution of aquatic Coleoptera in mountain lakes. Freshw Biol 57:803–814
Desrosiers M, Usseglio-Polatera P, Archaimbault V, Larras F, Méthot G, Pinel-Alloul B (2019) Assessing anthropogenic pressure in the St. Lawrence River using traits of benthic macroinvertebrates. Sci Total Environ 649:233–246
Doadrio I (2001) Atlas y Libro Rojo de los Peces Continentales de España. Dirección General de Conservación de la Naturaleza-Consejo Superior de Investigaciones Científicas. Madrid. https://www.miteco.gob.es/es/biodiversidad/temas/inventarios-nacionales/inventario-especies-terrestres/inventario-nacional-de-biodiversidad/ieet_peces_atlas.aspx
Dolédec S, Chevenet F (1994) Fuzzy correspondence analysis. Sci Direct 158:1–20
Donald DB, Stewart R, Mayhood DW (1994) Coexistence of fish and large Hesperodiaptomus species (Crustacea: Calanoida) in subalpine and alpine lakes. Can J Zool 72:259–261
Donald DB, Vinebrooke RD, Anderson RS, Syrgiannis J, Graham MD (2001) Recovery of zooplankton assemblages in mountain lakes from the effects of introduced sport fish. Can J Fish Aquat Sci 58:1822–1830
Donaldson LA, Cooke SJ (2016) The effectiveness of non-native fish eradication techniques in freshwater ecosystems: a systematic review protocol. Environ Evid 5:12
Eggers DM (1982) Planktivore preference by prey size. Ecology 63:381–390
Ejsmont-Karabin J (2012) The usefulness of zooplankton as lake ecosystem indicators: rotifers trophic state index. Pol J Ecol 60:339–350
Elvira B, Almodóvar A (2001) Freshwater fish introductions in Spain: facts and figures at the beginning of the 21st century. J Fish Biol 59:323–331
Evans RA (1989) Responses of limnetic insect populations of two acidic, fishless lakes to liming and brook trout (Salvelinus fontinalis) introductions. Can J Fish Aquat Sci 46:342–351
Ficke AD, Peterson DP, Janowsky B (2009) Brook trout (Salvelinus fontinalis): a Technical Conservation Assessment. USDA Forest Service, Rocky Mountain Region. https://www.fs.fed.us/r2/projects/scp/assessments/brooktrout.pdf. Accessed 26 May 2019
Gacia E, Buchaca T, Bernal-Mendoza N, Sabás I, Ballesteros E, Ventura M (2018) Non-native minnows threaten quillwort populations in high mountain shallow lakes. Front Plant Sci 9:329. https://doi.org/10.3389/fpls.2018.00329
Gannon JE, Stemberger RS (1978) Zooplankton (especially crustaceans and rotifers) as indicators of water quality. Trans Am Microsc Soc 97:16–35
García-Berthou E, Almeida D, Benejam L, Magellan K, Bae MJ, Casals F, Merciai R (2015) Impacto ecológico de los peces continentales introducidos en la Península Ibérica. Ecosistemas 24:84–89
Gilinsky E (1984) The role of fish predation and spatial heterogeneity in determining benthic community structure. Ecology 65:455–468
Gliwicz ZM (1980) Extinction of planktonic cladoceran species from alpine lakes stocked with fish planktivores. In: Horie S (ed) Paleolimnology of Lake Biwa and the Japanese Pleistocene, 8. Kyoto University, Kyoto, pp 3–22
Gliwicz ZM, Rowan M (1984) Survival of Cyclops abyssorum tatricus (Copepoda, Crustacea) in alpine lakes stocked with planktivorous fish. Limnol Oceanogr 29:1290–1299
Gore P (2000) Cluster Analysis. In: Tinsley H, Brown S (eds) Handbook of applied multivariate statistics and mathematical modeling. Academic Press, New York, pp 297–321
Granados I, Toro M, Rubio-Romero A (2006) Laguna Grande de Peñalara, 10 años de seguimiento limnológico. Comunidad de Madrid, Consejería de Medio Ambiente y Ordenación del Territorio, Dirección General del Medio Natural, Madrid
Hamerlík L, Svitok M, Novikmec M, Očadlík M, Bitušík P (2014) Local, among-site, and regional diversity patterns of benthic macroinvertebrates in high altitude waterbodies: do ponds differ from lakes? Hydrobiologia 723:41–52
Hawkins CP, Norris RH, Gerritsen J, Hughes RM, Jackson SK, Johnson RK, Stevenson RJ (2000) Evaluation of the use of landscape classifications for the prediction of freshwater biota: synthesis and recommendations. J N Am Benthol Soc 19:541–556
IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp
Karas N (1997) Brook trout. Lyons and Burford, New York
Knapp RA, Matthews KR (1998) Eradication of nonnative fish by gill netting from a small mountain lake in California. Restor Ecol 6:207–213
Knapp RA, Sarnelle O (2008) Recovery after local extinction: factors affecting re-establishment of alpine lake zooplankton. Ecol Appl 18:1850–1859
Knapp RA, Matthews KR, Sarnelle O (2001) Resistance and resilience of alpine lake fauna to fish introductions. Ecol Monogr 71:401–421
Knapp RA, Hawkins CP, Ladau J, McClory JG (2005) Fauna of Yosemite national park lakes has low resistance but high resilience to fish introductions. Ecol Appl 15:835–847
Knapp RA, Boiano DM, Vredenburg VT (2007) Removal of nonnative fish results in population expansion of a declining amphibian (mountain yellow-legged frog, Rana muscosa). Biol Cons 135:11–20
Lafrancois BM, Carlisle DM, Nydick KR, Johnson BM, Baron JS (2003) Environmental characteristics and benthic invertebrate assemblages in Colorado mountain lakes. Western North American Naturalist 63:137–154
Leavitt PR, Schindler DE, Paul AJ, Hardie AK, Schindler DW (1994) Fossil pigment records of Phytoplankton in Trout-stocked Alpine Lakes. Can J Fish Aquat Sci 51:2411–2423
Leppä M, Hämäläinen H, Karjalainen J (2003) The response of benthic macroinvertebrates to whole-lake biomanipulation. Hydrobiologia 498:97–105
Lewis WM Jr (1983) A revised classification of lakes based on mixing. Can J Fish Aquat Sci 40:1779–1787
Louette G, Meester LD (2005) High dispersal capacity of cladoceran zooplankton in newly founded communities. Ecology 86:353–359
Maceda-Veiga A, López R, Green AJ (2017) Dramatic impact of alien carp Cyprinus carpio on globally threatened diving ducks and other waterbirds in Mediterranean shallow lakes. Biol Cons 212:74–85
Magnea U, Sciascia R, Paparella F, Tiberti R, Provenzale A (2013) A model for high-altitude alpine lake ecosystems and the effect of introduced fish. Ecol Model 251:211–220
McNaught AS, Schindler DW, Parker BR, Paul AJ, Anderson RS, Donald DB, Agbeti M (1999) Restoration of the food web of an alpine lake following fish stocking. Limnol Oceanogr 44:127–136
Miracle R (1978) Composición específica de las comunidades zooplanctónicas de 153 lagos de los Pirineos y su interés biogeográfico. Oecologia aquatica 3:167–191
Miró A, Ventura M (2015) Evidence of exotic trout mediated minnow invasion in Pyrenean high mountain lakes. Biol Invasions 17:791–803
Müller F, Bergmann M, Dannowski R, Dippner JW, Gnauck A, Haase P, Jochimsen MC, Kasprzak P, Kröncke I, Kümmerlin R, Küster M, Lischeid G, Meesenburg H, Merz C, Millat G, Müller J, Padisák J, Schimming CG, Schubert H, Schult M, Selmeczy G, Shatwell T, Stoll S, Schwabe M, Soltwedel T, Straile D, Theuerkauf M (2016) Assessing resilience in long-term ecological data sets. Ecol Ind 65:10–43
Nelson JS, Paetz MJ (1992) The Fishes of Alberta, 2nd edn. The University of Alberta Press, Edmonton, Alberta
Novikmec M, Svitok M, Kočický Š, Bitušik P (2013) Surface water temperature and ice cover of Tatra mountains lakes depend on altitude, topographic shading, and bathymetry. Arct Antarct Alp Res 45:77–87
Oksanen JF, Blanchet, FG, Friendly, M, Kindt, R, Legendre P, McGlinn D, Minchin PR, O’Hara B, Simpson GL, Solymos P, Stevens H, Szoecs E, Wagner H (2017) 'vegan'. Community Ecology Package. R. Package Version 2.4–2
Pardo CE, Del Campo PC (2007) Combinación de métodos factoriales y de análisis de conglomerados en R: el paquete FactoClass. Revista Colombiana de Estadística 30:231–245
Parker BR, Wilhelm FM, Schindler DW (1996) Recovery of Hesperodiaptomus arcticus populations from diapausing eggs following elimination by stocked salmonids. Can J Zool 74:1292–1297
Parker BR, Schindler DW, Donald DB, Anderson RS (2001) The effects of stocking and removal of a nonnative salmonid on theplankton of an Alpine lake. Ecosystems 4:334–345
Pastorino P, Polazzo F, Bertoli M, Santi M, Righetti M, Pizzul E, Prearo M (2018) Consequences of fish introductions in fishless Alpine lakes: preliminary notes from a sanitary point of view. Turk J Fish Aquat Sci. https://doi.org/10.4194/1303-2712-v20_01_01
Power M, Power G, Caron F, Doucett RR, Guiguer KR (2002) Growth and dietary niche in Salvelinus fontinalis as revealed by stable isotope analysis. Environ Biol Fishes 64:75–85
Resh VH, Beche LA, McElravy EP (2005) How common are rare taxa in long-term benthic macroinvertebrate surveys? J N Am Benthol Soc 24:976–989
Rolla M, Biffoni G, Brighenti S, Iacobuzio R, Liautaud K, Pasquaretta C, Tiberti R (2018) Predation by introduced fish can magnify the terrestrial arthropod subsidies in mountain lakes. Can J Fish Aquat Sci 75:1453–1464
RStudio Team (2015) RStudio: Integrated Development for R. RStudio, Inc., Boston, MA URL https://www.rstudio.com/
Sánchez J, Cobo F, González MA (2007) Biología y la alimentación del salvelino, Salvelinus fontinalis (Mitchill, 1814), en cinco lagunas glaciares de la Sierra de Gredos (Ávila, España). Nova Acta Cientifica Compostelana (Bioloxía) 16:129–144
Sarnelle O, Knapp RA (2004) Zooplankton recovery after fish removal: limitations of the egg bank. Limnol Oceanogr 49:1382–1392
Schabetsberger R, Luger MS, Drozdowski G, Jagsch A (2009) Only the small survive: monitoring long-term changes in the zooplankton community of an Alpine lake after fish introduction. Biol Invasions 11:1335–1345
Schilling EG, Lofton CS, Huryn AD (2009) Macroinvertebrates as indicators of fish absence in naturally fishless lakes. Freshw Biol 54:181–202
Schindler DW, Parker BR (2002) Biological pollutants: alien fishes in mountain lakes. Water Air Soil Pollut Focus 2:379–397
Schindler DE, Knapp RA, Leavitt PR (2001) Alteration of nutrient cycles and algal production resulting from fish introductions into mountain lakes. Ecosystems 4:308–321
Scott WB, Scott MG (1988) Atlantic fishes of Canada. Can Bull Fish Aqua Sci 219:731
Sienkiewicz E, Gąsiorowski M (2016) The effect of fish stocking on mountain lake plankton communities identified using palaeobiological analyses of bottom sediment cores. J Paleolimnol 55:129–150
Spears BM, Futter MN, Jeppesen E, Huser BJ, Ives S, Davidson TA, Adrian R, Angeler DG, Burthe SJ, Carvalho L, Daunt F, Gsell AS, Hessen DO, Janssen ABG, Mackay EB, May L, Moorhouse H, Olsen S, Søndergaard M, Woods H, Thackeray SJ (2017) Ecological resilience in lakes and the conjunction fallacy. Nat Ecol Evol 1:1616–1624
Stenson JAE (1982) Fish impact on rotifer community structure. Hydrobiologia 87:57–64
Stenson JAE (1983) Changes in the relative abundance of Polyarthra vulgaris and P. dolichoptera, following the elimination of fish. Hydrobiologia 104:269–273
Stoch F, Vagaggini D, Margaritora FG (2019) Macroecological and spatial patterns in the distribution of cladocerans in Alpine lakes. Limnetica 38:119–136
Symons CC, Shurin JB (2016) Climate constrains lake community and ecosystem responses to introduced predators. Proc R Soc B Biol Sci 283:20160825
Tachet H, Richoux P, Bournaud M, Usseglio-Polatera P (2000) Invertébrés d'eau douce. Systématique, biologie, écologie. CNRS Editions, Paris
Tiberti R (2017) Can satellite ponds buffer the impact of introduced fish on newts in a mountain pond network? Aquat Conserv Mar Freshw Ecosyst 28:457–465
Tiberti R, von Hardenberg A, Bogliani G (2014) Ecological impact of introduced fish in high altitude lakes: a case of study from the European Alps. Hydrobiologia 724:1–19
Tiberti R, Brighenti S, Canedoli C, Iacobuzio R, Pasquini G, Rolla M (2016) The diet of introduced brook trout (Salvelinus fontinalis; Mitchill, 1814) in an alpine area and a literature review on its feeding ecology. J Limnol 75:488–507
Tiberti R, Bogliani G, Brighenti S, Iacobuzio R, Liautaud K, Rolla M, von Hardanberg A, Bassano B (2018) Recovery of high mountain Alpine lakes after the eradication of introduced brook trout Salvelinus fontinalis using non-chemical methods. Biol Invasions 21:875–894
Tonolli L, Tonolli V (1951) Ossevacioni sulla biologia ed ecologia di 170 popolamenti zooplanctonici di laghi italiani di alta quota. Memorie dell’Istituto Italiano di Idrobiología “Dottore Marco Marchi” 6:53–136
Toro M (2007) Las lagunas del Macizo de Peñalara (Sierra de Guadarrama): de los primeros naturalistas y científicos a los problemas de conservación a comienzos del siglo XXI. Boletín de la Real Sociedad Española de Historia Natural, Sección Biológica 102:127–148
Toro M, Granados I (2001) Las lagunas del Parque Regional de la Sierra de Gredos. Monografías de la Red de Espacios Naturales de Castilla y León, Serie Técnica: Junta de Castilla y León, Valladolid
Toro M, Granados I (2002) Restoration of a small high mountain lake after recent tourist impact: the importance of limnological monitoring and palaeolimnology. Water Air Soil Pollut Focus 2:295–310
Toro M, Granados I, Robles S, Montes C (2006) High mountain lakes of the Central Range (Iberian Peninsula): Regional limnology and environmental changes. Limnetica 25:217–252
Torres-Esquivias JA, Arenas González RM, Fernández Delgado C (2009) La malvasía cabeciblanca (Oxyura leucocephala) de nuevo en la Laguna de Zóñar. Oxyura Revista sobre las Zonas Húmedas 12:41–48
Usseglio-Polatera P, Bournaud M, Richoux P, Tachet H (2000) Biological and ecological traits of benthic freshwater macroinvertebrates: relationships and definition of groups with similar traits. Freshw Biol 43:175–205
Usseglio-Polatera P, Philippe R, Bournaud M, Tachet H (2001) A functional classification of benthic macroinvertebrates based on biological and ecological traits: application to river condition assessment and stream management. Archiv fuer Hydrobiol Suppl 139:53–83
Velasco JL, Álvarez M, García Sánchez-Colomer M (2005) Comunidades planctónicas de los lagos de montaña de Neila (Burgos, España). Ecología 19:75–94
Ventura M, Tiberti R, Buchaca T, Buñay D, Sabás I, Miró A (2017) Why should we preserve fishless high mountain lakes? In: Catalan J, Ninot JM, Añiz M (eds) High mountain conservation in a changing world. Springer-Verlag, Cham, pp 181–205
Vicente-Serrano SM, Rodríguez-Camino E, Domínguez-Castro F, El Kenawy A, Azorín-Molina C (2017) An updated review on recent trends in observational surface atmospheric variables and their extremes over Spain. Geogr Res Lett 43:209–232
Villéger S, Ramos J, Flores D, Mouillot D (2010) Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol Appl 20:1512–1522
Ward JH (1963) Hierarchical groupings to optimize an objective function. J Am Stat Assoc 58:236–244
Wattiez C (1981) Biomasse du zooplancton et productivity des cladoceres d'eaux de degre trophique different. Ann Limnol 17:219–236
Winder M, Monaghan MT, Spaak P (2001) Have human impacts changed alpine zooplankton diversity over the past 100 years? Artic Antarct Alpine Res 33:467–475
Winder M, Bürgi HR, Spaak P (2003) Mechanisms regulating zooplankton populations in a high-mountain lake. Freshw Biol 48:795–809
Wissinger SA, McIntosh AR, Greig HS (2006) Impacts of introduced brown and rainbow trout on benthic invertebrate communities in shallow New Zealand lakes. Freshw Biol 51:2009–2028
Acknowledgements
This work has been supported by the National Park of Sierra de Guadarrama (Regional Government of Madrid). Work by A.C. is currently funded by the project CLIMAWET (CGL2015-69557-R). The authors are very grateful for the help given in the taxonomic determinations by Andrés Mellado (macroinvertebrates) and Juan José Aldasoro, Manuel García Sánchez-Colomer and Santiago Robles (zooplankton), and for the assistance provided in the statistical analysis by Andrés Mellado, Jorge R. Sánchez, and Ignacio González.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toro, M., Granados, I., Rubio, Á. et al. Response of the aquatic invertebrate community to the eradication of an exotic invasive fish 30 years after its introduction into an Iberian alpine lake. Aquat Sci 82, 55 (2020). https://doi.org/10.1007/s00027-020-00728-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-020-00728-w