Abstract
Taste receptors were first identified on the tongue, where they initiate a signaling pathway that communicates information to the brain about the nutrient content or potential toxicity of ingested foods. However, recent research has shown that taste receptors are also expressed in a myriad of other tissues, from the airway and gastrointestinal epithelia to the pancreas and brain. The functions of many of these extraoral taste receptors remain unknown, but emerging evidence suggests that bitter and sweet taste receptors in the airway are important sentinels of innate immunity. This review discusses taste receptor signaling, focusing on the G-protein–coupled receptors that detect bitter, sweet, and savory tastes, followed by an overview of extraoral taste receptors and in-depth discussion of studies demonstrating the roles of taste receptors in airway innate immunity. Future research on extraoral taste receptors has significant potential for identification of novel immune mechanisms and insights into host-pathogen interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been previously proposed that the immune system is the mammalian “sixth sense” [1–3]. While our senses of sight, smell, hearing, touch, and taste allow us to consciously perceive our external environment, our immune system similarly detects the presence of potentially dangerous foreign chemicals and organisms, albeit internally and often subconsciously. Looking at the immune system from this viewpoint, it is perhaps not surprising but nonetheless very exciting that recent studies have demonstrated that several mechanisms of mammalian innate immunity utilize components of sensory signal transduction. Chemosensory G-protein-coupled receptors (GPCRs) that were originally identified as “taste” receptors have now been found in many tissues outside the tongue (Table 1), and bitter and sweet taste GPCRs have recently been found to be sentinels of defense against infection in the airway, where they function as a novel arm of innate immunity, as described in more detail below. Emerging evidence [4] also supports the hypothesis that taste receptors serve similar immune roles in at least some of the other tissues in which they are expressed. Because these chemosensory receptors are likely involved in many biological processes beyond taste, it is important that researchers in other fields understand the physiology and cell biology of chemosensory receptors. Thus, the focus of this review is on taste receptor signaling mechanisms, extra-oral taste receptors, and the recently identified roles of taste receptors in the regulation of airway epithelial innate immunity.
The biology of taste and taste receptors
Overview of taste signaling
Taste GPCRs were first identified in the type II taste cells of the tongue. These receptors signal information to the brain regarding the nutritive value and/or potential toxicity of ingested foods and beverages [5, 6]. Our sensory perception of foods and beverages is called flavor [7], which is a complex sensation made up of taste, smell, and texture, also called “mouth feel.” Flavor can also include pain, as in the case of capsaicin- or CO2-meditated activation of nociceptor neurons during ingestion of foods containing chili peppers or carbonated beverages [8, 9], respectively. However, there are only five well-defined types of tastes that are detected by the sensory cells of the taste buds of the tongue [10]. These are sweet, salty, sour, bitter, and savory (also known as umami, which is the taste of savory amino acids such as glutamate). Sweet, salty, and umami tastes reveal the presence of sugars, sodium chloride, and amino acids, respectively. These are generally perceived as beneficial nutrients, and they result in a pleasing taste. In contrast, sour and bitter tastes are often perceived as unpleasant; they signal the presence of potentially harmful chemicals. Sour taste can alert the body to the presence of spoiled foods by detecting lactic acid products from bacterial fermentation, while bitter can signal the presence of toxic plant alkaloids such as strychnine [11].
There is thought to be only one major type of receptor type for sweet (T1R2/3 [11–15]), salty (the epithelial sodium channel or ENaC [16–20]), savory (T1R1/3 [14, 21–24]), and sour (acid-sensing ion channels or ASICs [20, 24–26]). Bitter sensation is unique in that there are multiple G-protein coupled receptor (GPCR) isoforms tuned to a wide array of different bitter compounds. These are known as T2R receptors [6, 27–30] (Fig. 1a), and humans have at least 25 different functional T2R isoforms [15, 31]. T2R bitter taste receptors are found in taste bud cells known as type II cells, sometimes also called receptor cells [32, 33]. Type II cells also contain a second family of taste GPCRs, known as T1R receptors. The T1R family contains only 3 isoforms, T1R1, T1R2, and T1R3; (Fig. 1b–c), which oligomerize to make up the receptors for umami (T1R1 + T1R3) and sweet (T1R2 + T1R3). However, it is important to note that an alternative T1R3 homo-oligomeric form of a sweet taste receptor has also been recently proposed to exist in pancreatic beta cells [34–37] and adipocytes [38]. Additionally, some type II taste cells have been observed to express only T1R3 without T1R1 or T1R2 [31, 39], and T1R3 homo-oligomers have been proposed to affect calcium and magnesium taste [40]. Thus, the true range of oligomerization states of T1Rs and the resulting functional consequences are not yet fully clear. Biochemical studies of T1R and T2R oligomerization have relied on heterologous expression systems, including HEK293 cells, often utilizing tagged versions of the receptors [41, 42]. In vivo biochemistry has so far been hampered in particular by low levels of protein expression and poor antibody specificity [43]. It still remains to be determined if and/or how the in vivo oligomerization of T1Rs and T2Rs changes their pharmacological profiles or otherwise alters their physiological responses.
G-protein coupled receptors (GPCRs) involved in bitter, sweet, and umami taste. a Bitter taste receptors are generally believed to be primarily composed of homo- or hetero-oligomers of isoforms of the taste receptor 2 (T2R) family [6, 27–31]. Most T2R isoforms have been shown to co-immunoprecipitate with other T2R isoforms co-expressed in heterologous expression systems [41, 42]. However, while most bitter responsive type II taste cells express multiple T2Rs, the state of T2R oligomerization in vivo is almost completely unknown. Additionally, the EC50 values for receptors do not appear to be shifted by co-expression of different T2Rs in the same cells, as measured through calcium signaling in heterologous expression systems in vitro [41, 42]. However, potential effects of T2R oligomerization in type II taste cell signaling in vivo are unknown. It remains unclear whether each T2R oligomer signals independently or cooperatively. b, c Umami and sweet receptors are made up of oligomers of the taste receptor 1 (T1R) family. T1R1 and T1R3 oligomers form umami receptors [14, 21–23], while T1R2 and T1R3 oligomers form sweet receptors [11–15]. Both T1R and T2R family members are believed to have similar structures to other 7-transmembrane domain GPCRs, but T1Rs are believed to have more extensive extracellular N-termini than do T2Rs. The N-termini of T1Rs are thought to contain multiple ligand binding sites [12, 22, 31, 39, 47]
Within type II taste cells, T1R and T2R signaling is thought to occur through identical intracellular pathways (Fig. 2) [32, 44]. Stimulation of these receptors activates intracellular calcium signals that cause the type II cells to release ATP [45, 46] to activate purinergic receptors on presynaptic cells and afferent sensory fibers [11, 44, 47]. While these bitter, sweet, and umami GPCRs share common intracellular signaling pathways, the differential transmission of these three taste sensations occurs, at least in part, at a cellular level. The majority of type II taste cells respond to only one type of taste: bitter, sweet, or umami [33, 48–50], depending on their expression of T2Rs, T1R2 + T1R3, or T1R1 + T1R3, respectively. Each taste bud contains multiple type II cells that are differentially coded for bitter, sweet, or umami sensation. However, we still do not yet fully understand how these three tastes are discriminated, as some type II cells have been found to express multiple types of receptors and respond to multiple types of tastes [48–50].
Signal transduction pathway of bitter (T2R), sweet (T1R2/3), and umami (T1R1/3) GPCRs in type II taste cells of the tongue. As described in the text and reviewed in [11, 32], ligand binding to taste GPCRs results in Ca2+ signaling through two G-protein-coupled pathways. Gβγ activation of phospholipase C isoform β2 (PLCβ2) results in production of inositol 1,4,5-trisphosphate (IP3), which activates the IP3 receptor (IP3R), an intracellular ion channel that allows calcium (Ca2+) release from the intracellular endoplasmic reticulum (ER) calcium stores [254]. Simultaneously, Gα-gustducin activates phosophodiesterases (PDEs), which reduce the levels of cyclic-AMP (cAMP) and decrease protein kinase A (PKA) activity [28]. PKA can phosphorylate and inhibit the activity of the type III IP3R [255, 256], the major IP3R isoform found in type II taste cells [257–259], thus reduction of PKA activity can enhance IP3R3-mediated calcium signaling. Calcium activates the plasma membrane-localized cation channel TRPM5 [137, 138], causing depolarization of cellular membrane potential, activation of voltage-gated sodium (Na+) channels [260], and generation of an action potential that results in ATP release [11] through the CALHM1 ion channel [45, 46] and subsequent purinergic neurotransmission of taste sensations
It is important to note that the focus of this review is on T1R sweet and T2R bitter taste GPCRs, which have been implicated in innate immunity as described below. However, other GPCRs are expressed on taste cells of the tongue. Most notable are the free fatty acid receptor 1 (FFA1; also known as GPR40) and GPR120, which detects omega-3 fatty acids [51, 52]. These may even constitute a sixth taste sensation, the taste of fats, which have previously been thought to be mainly detected through texture. Fat preference may also involve the glycoprotein CD36 (also known as fatty acid translocase or FAT) [53–61]. However, the function of these proteins in human fat taste and fat preference is not yet fully understood and remains an active area of research. Any roles for these fatty acid receptors or other taste cell GPCRs in innate immunity have not been identified.
Extraoral taste receptors: identification and potential roles in immunity
Recent studies have determined that the expression of T2R bitter and T1R sweet taste GPCRs extends far beyond the tongue (reviewed in [11, 44, 62–65]). These receptors have been found in organs as diverse as the brain, pancreas, bladder, and testes, and they have been termed “extraoral” taste receptors. A representative list of known extraoral taste GPCR expression and some of the known roles of these receptors are shown in Table 1. Research has elucidated several of the downstream signaling pathways of extraoral T2Rs and T1Rs, but for the most part their physiological roles remain to be determined. We also lack information about the identities of physiological ligands for most of these receptors in other tissues. Oral T2Rs detect poisonous ingested chemicals like toxic plant products, and oral T1Rs detect sugar in nutrient-rich foods [11, 15, 27, 66, 67]; however, it is unclear what agonists might activate extraoral receptors in tissues that do not come into direct contact with ingested food. Nonetheless, because many compounds used as medications are known to have a bitter taste [67], one important implication of the discovery of extraoral T2R bitter receptors is that extraoral T2Rs may be a mechanism underlying some off-target drug effects [63], reinforcing the need to better understand the role these receptors play in human biology.
In the case of T2R bitter receptors, we and other researchers have hypothesized that at least some extraoral T2Rs may detect bitter components of products secreted by pathogenic bacteria or fungi. The initial evidence for this comes from studies of solitary chemosensory cells (SCCs) in the mouse nose, which express both T2R and T1R receptors [68–77]. The Finger lab at the University of Colorado showed in 2010 that these SCCs exhibit intracellular calcium signals in response to acyl-homoserine lactones (AHLs) [72], which are quorum-sensing molecules secreted by gram-negative bacteria such as the airway pathogen Pseudomonas aeruginosa [78, 79]. As many lactones are known to be bitter [80], this result suggested that AHLs activate one or more extraoral T2R receptors, which has now been experimentally confirmed [81, 82] and will be described in greater detail below. It is highly likely that there are other bitter or sweet products secreted by pathogenic microorganisms that are detected by extra-oral T2Rs or T1Rs, respectively.
A role for T2Rs in innate immunity is particularly intriguing, as T2Rs have a uniquely high density of naturally occurring genetic variants [83]. This variation contributes to the complex individual taste preferences for bitter foods such as green leafy vegetables [84] as well as beverages such as coffee [85], scotch [86], and beer [86]. We hypothesize that if T2Rs are important in innate or adaptive immunity, the genetic variation that causes differences in T2R receptor function governing taste preferences may cause variation in how cells from different individuals detect and respond to infection. In other words, susceptibility to protection against infections might result from genetic variations in T2Rs that cause reduced or enhanced receptor function, respectively. It has long been thought that genetic components underlie susceptibility to certain types of infections [87], particularly respiratory infections [88–90]. As we will discuss below, recent evidence has now validated this hypothesis by demonstrating that T2Rs do indeed recognize bacterial products and that genetic variation in at least one human T2R isoform can alter susceptibility to bacterial infection.
Role of bitter and sweet taste receptors in upper respiratory innate immunity
Overview airway epithelial innate immunity
The upper respiratory tract consists of the nose and sinuses, termed the sinonasal cavity. In addition to warming and humidifying inspired air, the sinonasal cavity is also the front line of defense of the respiratory tract [91–97]. Host-pathogen interactions occur with every breath containing aerosolized fungal spores, bacteria, and virus particles [94]. However, in most individuals, the upper and lower airways remain free of pathological bacterial infection. This is largely due to the multiple first-line innate immune mechanisms that work in concert to defend the sinonasal epithelium (Fig. 3). The primary physical defense is the process of mucociliary clearance [91, 92, 98–103]. The airway surface is lined by a mucus gel made up of cross-linked glycosylated mucin macromolecules produced by airway secretory cells; the carbohydrate sidechains of the mucins create “sticky” binding sites that trap airway pathogens and particulates in the mucus [91, 92, 98, 103, 104]. The spatially and temporally coordinated beating of ciliated epithelial cells then transports the debris- and pathogen-laden mucus from the upper and lower respiratory passages toward the throat, where the mucous/pathogens/debris mixture is cleared by swallowing or expectoration [91, 92, 98, 103]. Mucociliary clearance is complemented by the secretion of antimicrobial compounds, including proteins such as lysozyme, lactoferrin, cathelicidins, and defensins [105], as well as the generation of reactive oxygen and nitrogen species (ROS/RNS) that can have direct antibacterial, anti-fungal, and antiviral effects [106, 107]. Nitric oxide (NO) is thought to be a particularly important RNS defense mechanism in the sinuses. High levels of nitric oxide synthase (NOS), the enzyme that generates NO radicals from arginine, are expressed in the sinonasal epithelium [108, 109]. NO and its reactive derivatives have direct bactericidal effects [106, 107], and it is thought that NO produced by the sinuses diffuses through the mucous and is important for preventing infection. Altered NO levels have been linked to chronic rhinosinusitis and other airway diseases [110–114]. Finally, when the above innate defenses are not enough, epithelial cells can also secrete cytokines and chemokines that can recruit dedicated immune cells and activate inflammatory pathways [105].
Mechanisms of epithelial innate immunity in the airway. As described in the text and reviewed in [105, 261], inhaled viruses, bacteria, and fungi are trapped by sticky mucus created by mucin macromolecules secreted by secretory goblet cells. Trapped pathogens are removed from the airway by mucociliary transport, which is driven by ciliary beating and is dependent upon regulation of ion and fluid transport by epithelial cells that regulates the mucus viscosity. In addition to mucociliary transport, direct pathogen killing or inactivation can occur via the secretion of antimicrobial peptides as well as the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). During longer-term exposure to pathogens, epithelial cells can also secrete cytokines to recruit dedicated immune cells and activate inflammatory pathways
When these upper respiratory innate defenses fail, infection can result. Chronic rhinosinusitis (CRS) is a disease of chronic infection and inflammation of the nose and sinuses [91, 92], frequently requiring prolonged medical therapy and severely decreasing quality of life [91, 92]. In fact, CRS patients report worse quality-of-life scores for physical pain and social functioning than those suffering from chronic obstructive pulmonary disease (COPD), congestive heart failure, or angina [115, 116]. In addition to severely reducing quality of life, sinus and nasal infections can “seed” lower airway infections and/or exacerbate existing lower airway diseases [117], making these infections an important public health burden. Elucidating innate airway epithelial defense mechanisms and identifying novel therapeutic targets is particularly important in light of the rising prevalence of antibiotic resistant bacteria in patients with upper airway infections and CRS [97, 118–122]. Interestingly, as we will describe below, many innate defense pathways in the sinonasal cavity are regulated by taste GPCRs.
The T2R38 bitter taste receptor in human upper airway cilia detects bacterial quorum-sensing molecules and stimulates nitric oxide (NO) production
In addition to being the “engines” driving mucociliary clearance, airway ciliated cells have also long been known to function in immune detection through the expression of Toll-like receptors (TLRs [96, 97, 123, 124]). TLRs recognize conserved structures called pathogen-associated molecular patterns (PAMPs), including lipoteichoic acid (LTA; recognized by TLR2) from gram-positive bacteria, lipopolysaccharide (LPS; recognized by TLR4) from gram-negative bacteria, and bacterial flagellin (recognized by TLR5) [96, 97]. In 2009, the Welsh Lab at the University of Iowa published the observation that human bronchial epithelial cells also express T2R bitter taste receptors that activate calcium responses to increase ciliary beating [125], proposing that this process is a response that exists to clear noxious chemicals. Interestingly, these bronchial T2Rs were found to be localized within the motile cilia themselves [125], demonstrating that motile cilia are “sensory organelles.” This finding was highly novel, as traditionally there are two distinct classifications of animal cell cilia. Primary or sensory cilia are expressed on nearly every cell type in the body with one single primary cilium per cell, containing a 9 + 0 microtubule structure and functioning in diverse sensory roles [126–128]. Motile cilia, however, are expressed only in specialized epithelial cells and exhibit a 9 + 2 microtubule structure, occurring at 100–300 per cell [129]. Motile cilia had long been thought to be solely responsible for mechanical transport of fluid/mucus, as in the airway epithelial (Fig. 2) and during development of embryonic left–right asymmetry in vertebrates [130]. However, the identification of chemosensory taste receptors within motile cilia suggested that they, like primary cilia, also serve a sensory role.
We examined the expression of bitter taste receptors in motile cilia of the upper respiratory tract to determine if they, too, express T2R receptors and whether these receptors might detect bacterial products and play a role in innate immunity. We found that human sinonasal ciliated epithelial cells express the bitter taste receptor T2R38, which was indeed localized to the motile cilia [81]. T2R38 function was studied in human tissue explants as well as air–liquid interface (ALI) cultures of primary sinonasal cells. ALI cultures are a state-of-the-art respiratory cell culture model that mimics a polarized respiratory epithelium with well-differentiated ciliated cells [131–135]. When ciliated epithelial cells were stimulated with T2R38-specific agonists, such as phenylthiocarbamide (PTC), they exhibited low-level calcium responses that activated NOS to drive robust intracellular NO production [81]. This signaling pathway depended on two important components of the canonical taste signal transduction cascade, TRPM5 [11, 136–138] and phospholipase C isoform β2 (PLCβ2) [11] (Fig. 2), as shown by pharmacological inhibition [81].
High levels of NOS are expressed in the cilia and microvilli of the sinonasal epithelium [108, 109], and NO production is thought to be an important airway defense mechanism [110, 139, 140]. NO released by the airway epithelium is believed to be able to rapidly diffuse inside bacteria, where its reactive derivative S-nitrosothiols and peroxynitrites can damage DNA or membrane lipids and inactivate enzymes containing sulfhydryl groups, thiol groups, or metal cofactors [106, 107]. We found that the NO produced during T2R38 activation acted in two ways. First was as a second messenger to increase mucociliary clearance through guanylyl cyclase and PKG activation, which increases ciliary beating [129]. Secondly, the NO also diffused into the airway surface liquid (ASL) and had direct bacteridical effects against P. aeruginosa [81]. In this study, we also identified the two major P. aeruginosa AHLs, N-butyryl-L-homoserine lactone (C4HSL) and N-3-oxo-dodecanoyl-L-homoserine lactone (C12HSL) [79] as T2R38 agonists [81]. Using a Wt P. aeruginosa strain (PAO1) as well as a strain mutated for the enzymes that synthesize AHLs (strain PAO-JP2; ΔlasI, ΔrhlI; [141]), we demonstrated that T2R38 detects physiological concentrations of AHLs to activate calcium-dependent NO-production, suggesting that T2R38 functions in airway ciliated cells as a sentinel receptor to detect bacteria and regulate innate immune responses. Because many types of gram-negative bacteria secrete AHLs [142], this is likely a general innate immune mechanism against many pathogenic gram-negative bacteria. A diagram of T2R38 function in sinonasal ciliated epithelial cells is shown in Fig. 4.
T2R38 bitter taste receptor regulation of airway epithelial innate immunity. Reading from left to right, acyl-homoserine lactone (AHL) molecules are secreted by gram-negative bacteria to regulate quorum sensing. These AHL molecules activate T2R38 expressed in human sinonasal cilia [81] and yet-unidentified T2Rs in mouse nasal cilia [82], which results in activation of PLCβ2, which liberates IP3 and causes initiation of a calcium (Ca2+) signal that activates nitric oxide synthase (NOS)-dependent nitric oxide (NO) production. Because the NOS activation is rapid (within seconds) and Ca2+-dependent, it is likely that the NOS isoforms involved are of the endothelial NOS (eNOS) family [262], known to be expressed in the airway [263]. NO production has two distinct effects. The first is activation of cellular protein kinase G (PKG), which phosphorylates ciliary proteins [129, 264] to increase ciliary beating and mucociliary transport [81, 82]. NO additionally diffuses directly into the airway surface liquid, where it has direct bactericidal effects [81]
While there is no clear T2R38 homologue between humans and mice, mice do express T2R receptors capable of responding to PTC [71, 143–150]. We found that mouse nasal ciliated epithelial cells likewise respond to PTC or AHL stimulation with a calcium-dependent NO response that increases mucociliary clearance [82]. The use of TRPM5-/- and PLCβ2-/- knockouts mouse cultures supported the requirement for these two signal transduction components. Interestingly, the airway epithelial T2R38 response was completely intact in mice knocked out for the Gα protein Gα-gustducin [82], an essential component of taste signaling in the type II taste cells of the tongue [11, 14, 28, 151–154]. While taste-receptor signaling has been previously observed to be partially intact in the absence of Gα-gustducin [14, 21, 154–156], to our knowledge, this finding that a T2R-receptor-linked signaling pathway is completely independent of Gα-gustducin is unique [82]. Additional research is needed to more clearly determine the signal transduction mechanisms of T2R38 and any other T2Rs that are localized to airway cilia.
To determine if T2R38 may be a marker for bacterial infection, we examined the effects of common human polymorphisms in the TAS2R38 gene, which encodes T2R38, on airway innate immune responses. TAS2R38 is one of the most well-studied TAS2R genes [157–161]. TAS2R38 has 2 common polymorphisms in Caucasian populations, one encoding a functional receptor and one encoding a nonfunctional receptor. The differences in the resulting proteins are at amino acid positions 49, 262, and 296. The functional T2R38 receptor contains proline (P), alanine (A), and valine (V) residues while the nonfunctional T2R38 contains alanine (A), valine (V), and isoleucine (I) at these positions, respectively [157]. It has been suggested that the loss of the valine at the third position in the AVI variant prevents receptor activation [162–164]. Homozygous AVI/AVI individuals (~30 % frequency in Caucasian populations) are “non-tasters” for the T2R38-specific agonists PTC (also known as phenylthiourea or PTU) and 6-propyl-2-thiouracil (PROP) [157]. PAV/PAV individuals (~20 % frequency in Caucasian populations [157]) are termed “super tasters” for these agonists, while AVI/PAV heterozygotes have varying intermediate levels of taste [157, 165].
We found that the AHL-induced antibacterial responses of human sinonasal epithelial cells correlate with these genetic polymorphisms. Epithelial cells derived from PAV/PAV “supertaster” individuals exhibited markedly enhanced NO production, mucociliary clearance, and bacterial killing compared with AVI/PAV heterozygote or AVI/AVI “non-taster” cells [81]. Furthermore, preliminary clinical data suggested that PAV/PAV T2R38 “supertasters” are less susceptible to gram-negative sinonasal infection than PAV/AVI or AVI/AVI patients who have lower levels of T2R38 function [81]. Now, further clinical studies have demonstrated that T2R38 supertasters are less susceptible to CRS [166–168]. Prospective clinical studies of T2R38 genotype and CRS/infection susceptibility, including patient outcomes, are currently ongoing. However, these data have already established the T2R38 pathway as a potential therapeutic target to promote innate immune responses in patients with upper respiratory infections. However, there is a large subset of patients that would be sub-optimally responsive to treatment with T2R38 agonists (i.e., PAV/AVI and AVI/AVI individuals). It is thus still necessary to further define the T2R38-mediated signaling pathway in airway epithelial cells as well as identify other T2Rs that activate similar innate immune responses. Additionally, more research is needed to determine whether AVI/AVI individuals are more susceptible to infections in other tissues where taste receptors are expressed and may contribute to innate immunity, including the lungs or gut epithelium.
Solitary chemosensory cells (SCCs) use both T2R bitter and T1R sweet taste receptors to regulate upper respiratory innate immunity through antimicrobial peptide secretion
Beyond the T2R bitter taste receptors in ciliated epithelial cells, the upper airway also contains dedicated chemosensory cells, known as solitary chemosensory cells (SCCs), which express both bitter and sweet taste receptors [68, 69, 71–77, 169–171]. The term “solitary chemosensory cell” was first used to describe the chemosensory epithelial cells found in fish [172–174], which exhibit an elongated morphology with heavy neuronal innervation. Morphologically similar cells were later discovered in the upper respiratory tracts of alligators [175] and mammals, including mice, rats, and humans [68, 70–75, 170, 176]. These cells have been classified as SCCs based on their elongated morphology as well as their expression of chemosensory signal transduction components, including T2R bitter and T1R sweet “taste” receptors [68–76, 171, 176, 177], as will be described below.
Immunofluorescence, in situ hybridization, and imaging of TRPM5-GFP-labeled and gustducin-GFP-labeled mouse airways have demonstrated that mouse nasal SCCs express important components of the canonical taste signaling pathway that are known to be important for taste receptor signaling in the cells of the tongue, including Gα-gustducin, PLCβ2, and TRPM5 [68, 71–73, 173]. When mouse nasal SCCs are stimulated with bitter compounds such as denatonium benzoate or with bacterial AHL quorum sensing molecules, they exhibit intracellular calcium responses that cause ACh release to activate trigeminal afferent nerves that are peptidergic nociceptors [68, 71, 72], resulting in breath-holding [72] and inflammatory responses [77]. The breath-holding response, which presumably exists to limit further inhalation of toxic compounds, is similar to what is observed with nasal application of capsaicin, which directly activates the TRPV1 ion channel localized to airway trigeminal nociceptor neurons [72]. These trigeminal nociceptors can also release several types of neuropeptides into the local airway environment, including vasoactive intestinal peptide (VIP), substance P, and calcitonin gene related peptide (CGRP) [178–182]. It is thus very possible that SCC activation in vivo also results in local responses such as enhanced ciliary beating [98] or fluid secretion from submucosal exocrine glands [183–186]. However, any ability of SCC-activation to regulate these processes in vivo remains to be experimentally confirmed.
SCCs have only recently been identified in humans [74–76]. In addition to T2R38, which we showed is expressed in ciliated cells [81], other researchers using reverse-transcription (rt)-PCR initially demonstrated expression of T2R4, T2R14, and T2R46 in preparations from the inferior and middle turbinates, septum, and uncinate process of the human sinonasal cavity [75]. SCC-like cells, expressing the bitter receptor T2R4, the umami receptor component T1R1, and the sweet receptor component T1R2, were initially found in the human vomeronasal duct [74]. More recently, we used immunofluorescence microscopy to identify T2R47- and T1R2/3-expressing SCC-like cells in ALI cultures derived from cells isolated from surigical specimens obtained from a variety of sinoasal anatomical regions [76]. It remains to be determined whether there are differences in SCC numbers or distribution throughout the sinonasal cavity, but it appears that SCCs can be isolated and cultured from many different regions of the sinonasal cavity.
We studied SCC physiology in ALI cultures from humans and mice as well as human sinonasal explants [76]. ALI cultures have been previously been shown to contain ciliated, goblet, and basal cells [131–135], and we now know that human and mouse ALIs also contain SCCs, as described below. When human sinonasal ALIs or inferior turbinate tissue explants were stimulated with denatonium benzoate, a bitter agonist that was previously used to stimulate mouse nasal SCCs [68, 71–73], an intracellular calcium response was observed that originated from discrete cells. This calcium response initiated a calcium wave that spread to the surrounding cells through carbenoxolone-sensitive and 18α-glycerrhetinic acid-sensitive gap junctions [76]. Initiation of the calcium signal required components of the canonical taste signaling pathway including α-gustducin, PLCβ2, the inositol 1,4,5-trisphosphate (IP3) receptor, and TRPM5, as demonstrated by both pharmacology in human cultures as well as using cultures from knock-out mice [76]. Injection of the human denatonium-responsive cells with a fluorescent dye revealed a morphology identical to SCCs [76]. Subsequent immunofluorescence microscopy revealed the co-expression of T2R47 and T1R3 in ALI cultures in cells with a similar non-ciliated SCC-like morphology. The pharmacological profile of the bitter agonists that induced human SCC responses (denatonium benzoate, absinthin, parthenolide, and amarogentin; [187]) suggested a role for T2R isoforms T2R10, and T2R46, and T2R47 (also known as T2R30) [76]. Interestingly, human SCC responses were activated by neither T2R38-specific agonists nor P. aeruginosa AHLs, as previously shown for mice SCCs [68, 71–73, 77, 176]. This may reflect a species-specific difference, with T2R38-expressing ciliated epithelial cells mediating the primary response to AHLs in humans rather than SCCs. It is yet unknown which, if any, bacterial or fungal products activate T2Rs in human nasal SCCs, though the strong antimicrobial response evoked by their stimulation (discussed below) strongly suggests that they are activated in response to infection. Microbes secrete numerous products in addition to AHLs, including exotoxins, metabolic products, and other quorum-sensing molecules, such as autoinducer 2 (AI-2 [188]) and various autoinducer peptides [189, 190]. Further identification of T2R isoforms expressed in SCCs as well as screening of these T2Rs with bacterial and fungal compounds and/or conditioned-media will likely elucidate more bitter products secreted by airway pathogens.
Surprisingly, it was noted that denatonium-induced calcium responses in ALI cultures were blocked in a dose-dependent fashion by apical sugars such as glucose and sucrose [76]. This inhibition was mimicked by a non-metabolizable artificial sweetener, sucralose, a potent T1R2/3 agonist [14, 15, 22]. The glucose or sucralose inhibition was reversed by the T1R2/3 antagonists lactisole [12, 191, 192] and amiloride [193], but not by inhibitors of glucose transporters such as phloretin and phlorizin [76]. The data were supported by studies using T1R3 knock-out mice, which lack functional T1R2/3 receptors [194, 195]; ALI cultures derived from Wt mice exhibited sugar-mediated inhibition of T2R SCC calcium responses that were abolished in cultures derived from T1R3 knock-out mice [76]. Together, the immunocytochemical and physiological data confirm that sinonasal SCCs express both bitter and sweet taste receptors, which function in antagonistic physiological roles. The physiological and clinical significance of this finding will be discussed below.
Unlike T2R38, the SCC T2R stimulation did not activate nitric oxide production, nor did it activate cytokine secretion [76]. Instead, stimulation of SCC T2Rs activated robust secretion of antimicrobial peptides, including β-defensins 1 and 2. The defensin secretion required propagation of the calcium signal to the surrounding epithelial cells [76]. The secretion of these β-defensins appeared to occur directly from the surrounding ciliated and non-ciliated epithelial cells, based on immunofluorescence localization of β-defensins in ALI cultures and the loss of the immunofluorescence signal after stimulation with the bitter T2R agonist denatonium. The antimicrobial secretions were found to have activity against a spectrum of both gram-positive (Staphylococcus epidermis and methicillin-resistant Staphylococcus aureus) as well as gram-negative (Pseudomonas aeruginosa and Klebsiella pneumoniae) bacteria [76]. The antimicrobial peptide secretion observed during SCC T2R stimulation was immediate; the majority occurred within 5 min. In contrast, enhanced antimicrobial peptide secretion in response to stimulation of TLRs was observed to take up to 12 h [76]. It has long been known that TLRs in epithelial cells up-regulate mRNA for antimicrobial peptides such as defensins [96, 97]. Based on these observations, the TLR- and T2R-mediated defensin responses are distinguished by very different time scales, with T2Rs mediating a more rapid/immediate release of already-synthesized antimicrobial peptides. Thus, while TLRs are important for sustained responses through enhanced antimicrobial production, T2Rs are important for regulating rapid antimicrobial responses through more immediate antimicrobial release.
Calcium imaging and quantification of β-defensin release from human sinonasal explants suggest that ALI cultures accurately reflected the in vivo responses of the epithelial cells. Furthermore, the SCC/T2R-mediated epithelial antimicrobial peptide secretion is unique to the human upper airway; cultures derived from human bronchial tissue samples did not exhibit similar localized SCC-mediated calcium signals or antimicrobial peptide secretion [76]. Rather, the bronchial epithelial cell responses to bitter agonist were more global, as previously reported by others [125], likely reflecting the role of ciliated cells, rather than SCCs, as the primary sites of expression of T2Rs in the lower airway. Additionally, while many components of T2R-initiated calcium signaling appeared to be similar in human and mouse nasal ALI cultures, and stimulation of mouse nasal SCCs yielded a calcium wave, it did not result in release of antimicrobial peptides.
As described above, in the mouse, denatonium-responsive T2R signaling in vivo is linked to activation of trigeminal neurons and breath holding responses [68, 71–73, 170, 173] and activation of inflammation [77]. The data obtained so far suggest that human sinonasal SCCs and denatonium-responsive T2Rs are linked to more local responses regulating innate immunity. It cannot yet be ruled out that a similar trigeminally-mediated response exists in humans and mice, though the data so far suggest that the localized SCC immune response via release of antimicrobial peptides is not present in the mouse and thus illustrate a major difference between human and mouse SCCs. The role of SCCs in trigeminal activation in humans awaits further investigation. Experiments in ALI cultures, which lack neuronal innervation, cannot be used to determine whether SCCs activate trigeminal neurons in the human upper airway. Further in vivo experiments are required to determine if this is the case, but performing such experiments in human subjects may prove difficult. It is possible that clinically-relevant in vivo studies of SCCs and their regulation of local immune pathways will require the identification and use of an animal model that can better recapitulate the human sinonasal physiology, potentially the rabbit [196], sheep [197], or pig [198]. However, if human SCCs do activate trigeminal nerves, they may likewise trigger the release of neuropeptides such as substance P or VIP. Such neurotransmitters could trigger airway submucosal gland secretion [185, 199, 200], increase secretory or ciliary beating responses from airway epithelial cells themselves [98], and/or activate inflammation [77].
Perhaps the most surprising revelation about human SCC physiology was the inhibition of T2R bitter receptor responses during T1R2/3 sweet receptor stimulation. As mentioned above, T1R2/3 sweet receptor activation by physiologically-relevant concentrations of glucose or by artificial sweeteners inhibited both T2R-activated calcium signaling as well as antimicrobial peptide secretion in both ALIs as well as tissue explants [76]. Interestingly, the concentrations of glucose normally found in the ASL (~0.5 mM), which appear to be sufficient to activate T1R2/3 expressed in human nasal SCCs, are 10-100-fold lower than the concentrations required to activate T1R2/3 in in vitro heterologous expression systems [22] or to activate T1R2/3-dependent sweet taste [201]. However, like airway T1R sweet receptors, T1R sweet receptors expressed in pancreatic β-cells [202] and gut endocrine [203] also respond to lower sugar concentrations than oral T1R2/3. While oral T1R2/3 sweet receptors appear to be tuned to the higher concentrations of sugars found in foods, extra-oral T1R2/3s appear to be tuned to sugar concentrations that are physiologically relevant to the tissues in which they are found. Whether the differences in extra-oral and oral T1R sweet receptor sugar sensitivity are accounted for by altered post-translational modification, changes in stoichiometry, coupling to alternative signaling pathways, or expression of tissue-specific accessory subunits remains to be determined.
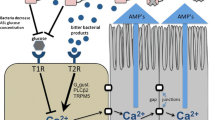
Nonetheless, T1R2/3 expression in the airway plays an important role in attenuation of antimicrobial responses. We hypothesize that, in vivo, sinonasal T1R2/3 sweet receptors are activated by glucose that is always present in the airway surface liquid, albeit at low levels in healthy individuals. Glucose is normally present in airway surface liquid because it tonically leaks through the epithelium via paracellular pathways. Glucose uptake via apical glucose transporters such as facilitative-diffusion GLUT transporters as well as sodium linked glucose transporters (SLGTs) keep healthy airway surface liquid glucose around 0.5 mM or less, or approximately ten-fold below fasting serum levels [204–206]. However, 0.5 mM glucose was sufficient to partially attenuate the SCC T2R antimicrobial response by approximately half [76]. We hypothesize that T1R2/3 may act as a “rheostat” to control the magnitude of the T2R response depending on the glucose concentration in the airway surface liquid. Depletion of ASL glucose concentration via bacterial glucose consumption may signal the onset of a bona fide infection and play a role in the activation of T2R-mediated AMP secretion. The T1R2/3 sweet receptors in SCCs may function to desensitize SCC T2Rs to bitter compounds secreted by some bacteria during low-level colonization, but this desensitization is relieved when bacterial numbers increase enough to cause depletion of ASL glucose. A model of this proposed mechanism is shown in Fig. 5.
Nasal solitary chemosensory cell (SCC)- and taste receptor-dependent regulation of airway innate immunity. Reading from left to right, bitter chemicals are secreted by microbes during infection. Some of these molecules, which are yet unidentified but are distinct from AHLs, activate the T2R bitter receptors expressed in solitary chemosensory cells (SCCs), which activates a Gα-gustducin (Gα-gust.)-dependent and PLCβ2-dependent calcium (Ca2+) response that propagates to surrounding epithelial cells via gap junctions [76]. In human, but not mouse, sinonasal epithelial cells, this calcium signal causes the surrounding cells to secrete antimicrobial peptides (AMPs), including β-defensins, which directly kill both gram-positive and gram-negative bacteria. Airway surface liquid (ASL) glucose (~0.5 mM in healthy individuals [76]) normally attenuates T2R-mediated signaling through activation of T1R2/3 sweet receptors, except during times of infection, when bacteria likely decrease ASL glucose concentration by consuming and metabolizing the glucose. Reduction of ASL glucose relieves the T1R2/3-mediated inhibition of T2R signaling and AMP secretion [76]. In mice, SCC activation by bitter compounds results in acetylcholine (ACh) release and activation of trigeminal neurons [77]; it remains to be determined if this mechanism also exists in the human nasal epithelium. For purposes of simplicity and clarity, T2R receptors present in nasal ciliated cells are not shown in this figure
While intriguing, this hypothesis requires further study in vivo. However, if validated, it may have very novel clinical implications. As stated above, normal ASL glucose concentration from healthy individuals is approximately 0.5 mM or less [76, 204–206]. However, we found that the mean glucose concentration in nasal secretions from patients with CRS was approximately three to four fold higher than healthy individuals (P < 0.01) [76]. As discussed above, glucose homeostasis in the ASL is the result of a balance of tonic glucose leakage through the airway epithelium as well as uptake into the airway cells via apical transporters [204–206]. Upsetting this balance can alter ASL glucose concentration, as observed in diabetic patients with elevated blood glucose levels (hyperglycemia) who have a resulting increased flux of glucose into the ASL and have elevated ASL glucose [206, 207]. CRS patients have elevated ASL glucose independent of blood glucose levels. It is likely that the higher CRS glucose concentrations derive from increased leak caused by breakdown of the epithelial barrier as a consequence of chronic infection and inflammation [204], which likely varies with individual patient disease [204]. It has been demonstrated in vitro that pro-inflammatory mediators increase paracellular glucose flux in human bronchial cells and disrupt tight junctions in human sinonasal cells [208, 209].
The higher ASL glucose concentrations in diabetic patients [204] may contribute to previous observations that diabetics are more prone to some airway infections than non-diabetics [204, 210]. Recently, in a retrospective study of CRS patients, diabetics were found to be more likely to have intraoperative microbiology cultures that included gram-negative bacteria such as P. aeruginosa [211]. Previously, it has been speculated that keeping the ASL glucose concentrations low is important for keeping the airways sterile because it limits the nutrients available for bacteria to consume [204–206]. However, high ASL glucose in CRS or diabetic patients may actually facilitate airway infections by an additional mechanism of repressing T2R-mediated responses in SCCs through over-activation of T1R2/3 sweet receptors. This could predispose patients with elevated glucose levels to infection by limiting the normal SCC responses to bitter molecules produced by bacteria. Topical application of T1R2/3 antagonists like lactisole may restore the ability of sinonasal epithelial cells to mount an appropriate antimicrobial response to bitter bacterial molecules secreted during infection, and may be a useful therapy for some patients while avoiding conventional antibiotics.
It also remains to be determined if there is any correlation between susceptibility to airway infections and polymorphisms in the TAS1R2 and TAS1R3 genes, which encode T1R2 and T1R3. TAS1R polymorphisms have been identified which alter the response of T1R2/3 receptors to sugars [158, 160, 212]. Increased sugar sensitivity of T1R2/3 in the airway might lead to increased repression of T2R signaling in SCCs, potentially limiting antimicrobial responses. The potential role of the T1R2/3 sweet receptor polymorphisms in airway disease is strongly supported by a recent study of Canadian CRS patients and healthy individuals showing allele frequency differences of >10 % for 16 different single nucleotide polymorphisms in TAS1R genes [168]. Further genetic studies of TAS1R2/3 in airway diseases are needed.
Taste receptors in other airway cell types
Airway taste receptor expression extends beyond ciliated cells and sinonasal SCCs. There are also other chemosensory cells identified in other regions of the mouse airway that await further investigation in human. In mice, tracheal chemosensory cells have been identified, which are called “brush cells” due to their apical tuft of microvilli [4, 177, 213–215]. Mouse tracheal brush cells express T2Rs that are activated by AHLs, including C12HSL, to stimulate ACh release that activates trigeminal-nerve-mediated breath-holding responses [4, 177, 213, 214]. A chemosensory role for human tracheal brush cells has not yet been identified, but it is possible that human tracheal brush cells are linked to local antimicrobial responses similarly to human nasal SCCs. This requires further experimentation using human tracheal ALI cultures and tissue explants. Bronchial smooth muscle cells also express T2Rs that mediate bronchodilation [216–219]. It is unknown whether smooth muscle T2Rs respond to endogenous yet-unidentified host signaling molecules or to bitter molecules from pathogens that penetrate the epithelium. Further research is needed to identify how T2Rs in both brush cells and smooth muscle cells contribute to host-pathogen interactions as well as airway innate immunity.
Conclusions and remaining questions
As described above, the emerging data suggest that T2R bitter and T1R sweet taste receptors constitute a novel sentinel detection system in the upper airway epithelium. Multiple receptors are expressed in different airway cell types, including T2R38 in ciliated cells and T2R47 and T1R2/3 in SCCs. These two different cell types regulate different antibacterial defense mechanisms [76, 81]. Of particular interest are the differences between nasal and bronchial taste receptor responses as well as the differences between human and mouse SCCs, as described above. This suggests that taste receptors have evolved to function in highly specialized roles in different tissues as well as in different species. The ability of human SCC T2R-activated antimicrobial secretions to kill a broad range of bacteria, including antibiotic-resistant S. aureus [76] suggests that this antimicrobial pathway may be a promising therapeutic target. It is also possible that the airway T2R pathways may be involved in antifungal [220] or antiviral [221] responses as well. Importantly, at least one of these taste receptors, T2R38, appears to be part of an interkingdom “eavesdropping” system by which mammalian host cells can intercept bacterial quorum sensing communications [81]. Research into determining whether other bacterial compounds are perceived as bitter is ongoing. A better understanding of the different T2R isoforms expressed in ciliated cells and SCCs will speed identification of potential compounds that stimulate one or both of these pathways.
Additionally, more research is needed to examine the role of bitter and sweet taste receptors in other epithelia beyond the airway (Table 1). It is very logical to hypothesize that at least some extra-oral T1Rs and T2Rs receptors play immune roles beyond the airway. Their roles in airway epithelial innate immunity may only represent the “tip of the iceberg” of the true scope of the importance of taste receptors to immunity. Supporting this, it was recently demonstrated that chemosensory brush cells of the rodent urethra, which express both T2R bitter and T1R1/3 umami receptors, respond to the bitter compound denatonium and the umami agonist monosodium glutamate, resulting in release ACh to activate the bladder detrusor muscle [4]. Moreover, these chemosensory cells also respond to a heat-inactivated uropathogenic E. coli strain, suggesting that these chemosensory cells function to detect infecting bacteria and trigger their expulsion.
It is becoming increasingly clearer that taste receptors have important roles beyond simply that of gustation. The chemosensory functions of T1Rs and T2Rs as sentinels of innate immunity may partly explain why these receptors are so widely expressed throughout the body. While variations in the genes encoding these bitter and sweet GPCRs have long been known to control human food and beverage preferences, they may be even more important to human biology than previously thought if these genes also impact susceptibility to infection. It is very probable that future studies of extra-oral taste receptors will reveal additional novel insights into immune pathways as well as interkingdom-signaling mechanisms that play key roles in host-pathogen interactions between mammalian cells and invading pathogens. Differences in chemical signals and activation of chemosensory receptors might also one day be found to play a role in how host cells differentiate between pathogenic and commensal or symbiotic bacteria.
Abbreviations
- ACh:
-
Acetylcholine
- AHL:
-
Acyl-homoserine lactone
- AMP:
-
Antimicrobial peptide
- ASL:
-
Airway surface liquid
- ATP:
-
Adenosine trisphophate
- C4HSL:
-
N-butyryl-L-homoserine lactone
- C12HSL:
-
N-3-oxo-dodecanoyl-L-homoserine lactone
- CALHM1:
-
Calcium homeostasis modulator isoform 1
- cAMP:
-
Cyclic adenosine monophosphate
- CGRP:
-
Calcitonin gene-related peptide
- COPD:
-
Chronic obstructive pulmonary disease
- CRS:
-
Chronic rhinosinusitis
- CSF:
-
Cerebrospinal fluid
- ENaC:
-
Epithelial sodium channel
- ER:
-
Endoplasmic reticulum
- GPCR:
-
G-protein–coupled receptor
- IP3 :
-
Inositol 1,4,5-trisphosphate
- IP3R3:
-
Inositol trisphosphate receptor isoform 3
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- PDE:
-
Phosphodiesterase
- PKA:
-
cAMP-dependent protein kinase A
- PKG:
-
cGMP-dependent protein kinase G
- PLCβ2:
-
Phospholipase C isoform β2
- PROP:
-
Propylthiouracil
- PTC:
-
Phenylthiocarbamide, also known as phenylthiourea
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- SCC:
-
Solitary chemosensory cell
- T1R:
-
Taste receptor family 1 protein isoform
- T2R:
-
Taste receptor family 2 protein isoform
- TAS1R :
-
Taste receptor family 1 gene
- TAS2R :
-
Taste receptor family 2 gene
- TLR:
-
Toll-like receptor
- TRPM5:
-
Transient receptor potential cation channel subfamily M isoform
References
Blalock JE (2005) The immune system as the sixth sense. J Intern Med 257(2):126–138. doi:10.1111/j.1365-2796.2004.01441.x
Blalock JE, Smith EM (2007) Conceptual development of the immune system as a sixth sense. Brain Behav Immun 21(1):23–33. doi:10.1016/j.bbi.2006.09.004
Bedford FL (2011) The missing sense modality: the immune system. Perception 40(10):1265–1267
Deckmann K, Filipski K, Krasteva-Christ G, Fronius M, Althaus M, Rafiq A, Papadakis T, Renno L, Jurastow I, Wessels L, Wolff M, Schutz B, Weihe E, Chubanov V, Gudermann T, Klein J, Bschleipfer T, Kummer W (2014) Bitter triggers acetylcholine release from polymodal urethral chemosensory cells and bladder reflexes. Proc Natl Acad Sci USA 111(22):8287–8292. doi:10.1073/pnas.1402436111
Margolskee RF (2005) Teaching resources. Sensory systems: taste perception. Sci STKE 290:tr20. doi:10.1126/stke.2902005tr20
Margolskee RF (1993) The molecular biology of taste transduction. BioEssays 15(10):645–650. doi:10.1002/bies.950151003
Beauchamp GK, Mennella JA (2011) Flavor perception in human infants: development and functional significance. Digestion 83(Suppl 1):1–6. doi:10.1159/000323397
Wise PM, Wolf M, Thom SR, Bryant B (2013) The influence of bubbles on the perception carbonation bite. PLoS One 8(8):e71488. doi:10.1371/journal.pone.0071488
Viana F (2011) Chemosensory properties of the trigeminal system. ACS Chem Neurosci 2(1):38–50. doi:10.1021/cn100102c
Breslin PA, Huang L (2006) Human taste: peripheral anatomy, taste transduction, and coding. Adv Otorhinolaryngol 63:152–190. doi:10.1159/000093760
Kinnamon SC (2012) Taste receptor signalling—from tongues to lungs. Acta Physiol (Oxf) 204(2):158–168. doi:10.1111/j.1748-1716.2011.02308.x
Cui M, Jiang P, Maillet E, Max M, Margolskee RF, Osman R (2006) The heterodimeric sweet taste receptor has multiple potential ligand binding sites. Curr Pharm Des 12(35):4591–4600
Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF (2001) Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 28(1):58–63. doi:10.1038/88270
Ozeck M, Brust P, Xu H, Servant G (2004) Receptors for bitter, sweet and umami taste couple to inhibitory G protein signaling pathways. Eur J Pharmacol 489(3):139–149. doi:10.1016/j.ejphar.2004.03.004
Margolskee RF (2002) Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem 277(1):1–4. doi:10.1074/jbc.R100054200
Lin W, Finger TE, Rossier BC, Kinnamon SC (1999) Epithelial Na + channel subunits in rat taste cells: localization and regulation by aldosterone. J Comp Neurol 405(3):406–420
Kretz O, Barbry P, Bock R, Lindemann B (1999) Differential expression of RNA and protein of the three pore-forming subunits of the amiloride-sensitive epithelial sodium channel in taste buds of the rat. J Histochem Cytochem 47(1):51–64
Eylam S, Spector AC (2003) Oral amiloride treatment decreases taste sensitivity to sodium salts in C57BL/6 J and DBA/2 J mice. Chem Senses 28(5):447–458
Liu L, Leonard AS, Motto DG, Feller MA, Price MP, Johnson WA, Welsh MJ (2003) Contribution of Drosophila DEG/ENaC genes to salt taste. Neuron 39(1):133–146
Ben-Shahar Y (2011) Sensory functions for degenerin/epithelial sodium channels (DEG/ENaC). Adv Genet 76:1–26. doi:10.1016/B978-0-12-386481-9.00001-8
He W, Yasumatsu K, Varadarajan V, Yamada A, Lem J, Ninomiya Y, Margolskee RF, Damak S (2004) Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J Neurosci 24(35):7674–7680. doi:10.1523/JNEUROSCI.2441-04.2004
Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E (2002) Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 99(7):4692–4696. doi:10.1073/pnas.072090199
Rong M, He W, Yasumatsu K, Kokrashvili Z, Perez CA, Mosinger B, Ninomiya Y, Margolskee RF, Damak S (2005) Signal transduction of umami taste: insights from knockout mice. Chem Senses 30(Suppl 1):i33–i34. doi:10.1093/chemse/bjh099
Beauchamp GK (2009) Sensory and receptor responses to umami: an overview of pioneering work. Am J Clin Nutr 90(3):723S–727S. doi:10.3945/ajcn.2009.27462E
Benarroch EE (2014) Acid-sensing cation channels: structure, function, and pathophysiologic implications. Neurology 82(7):628–635. doi:10.1212/WNL.0000000000000134
Holzer P (2009) Acid-sensitive ion channels and receptors. Handb Exp Pharmacol 194:283–332. doi:10.1007/978-3-540-79090-7_9
Shimada S, Ueda T, Ishida Y, Yamamoto T, Ugawa S (2006) Acid-sensing ion channels in taste buds. Arch Histol Cytol 69(4):227–231
Kinnamon SC, Margolskee RF (1996) Mechanisms of taste transduction. Curr Opin Neurobiol 6(4):506–513
Wong GT, Gannon KS, Margolskee RF (1996) Transduction of bitter and sweet taste by gustducin. Nature 381(6585):796–800. doi:10.1038/381796a0
Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS (2000) A novel family of mammalian taste receptors. Cell 100(6):693–702
Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ (2000) T2Rs function as bitter taste receptors. Cell 100(6):703–711
Scott K (2004) The sweet and the bitter of mammalian taste. Curr Opin Neurobiol 14(4):423–427. doi:10.1016/j.conb.2004.06.003
Iwata S, Yoshida R, Ninomiya Y (2014) Taste transductions in taste receptor cells: basic tastes and moreover. Curr Pharm Des 20(16):2684–2692
Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ (2003) Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112(3):293–301
Kojima I, Nakagawa Y, Ohtsu Y, Medina A, Nagasawa M (2014) Sweet taste-sensing receptors expressed in pancreatic beta-cells: sweet molecules act as biased agonists. Endocrinol Metab (Seoul) 29(1):12–19. doi:10.3803/EnM.2014.29.1.12
Nakagawa Y, Nagasawa M, Mogami H, Lohse M, Ninomiya Y, Kojima I (2013) Multimodal function of the sweet taste receptor expressed in pancreatic beta-cells: generation of diverse patterns of intracellular signals by sweet agonists. Endocr J 60(10):1191–1206
Medina A, Nakagawa Y, Ma J, Li L, Hamano K, Akimoto T, Ninomiya Y, Kojima I (2014) Expression of the glucose-sensing receptor T1R3 in pancreatic islet: changes in the expression levels in various nutritional and metabolic states. Endocr J 61(8):797–805
Nakagawa Y, Ohtsu Y, Nagasawa M, Shibata H, Kojima I (2014) Glucose promotes its own metabolism by acting on the cell-surface glucose-sensing receptor T1R3. Endocr J 61(2):119–131
Masubuchi Y, Nakagawa Y, Ma J, Sasaki T, Kitamura T, Yamamoto Y, Kurose H, Kojima I, Shibata H (2013) A novel regulatory function of sweet taste-sensing receptor in adipogenic differentiation of 3T3-L1 cells. PLoS One 8(1):e54500. doi:10.1371/journal.pone.0054500
Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS (2001) Mammalian sweet taste receptors. Cell 106(3):381–390
Tordoff MG, Shao H, Alarcon LK, Margolskee RF, Mosinger B, Bachmanov AA, Reed DR, McCaughey S (2008) Involvement of T1R3 in calcium-magnesium taste. Physiol Genomics 34(3):338–348. doi:10.1152/physiolgenomics.90200.2008
Kuhn C, Bufe B, Batram C, Meyerhof W (2010) Oligomerization of TAS2R bitter taste receptors. Chem Senses 35(5):395–406. doi:10.1093/chemse/bjq027
Kuhn C, Meyerhof W (2013) Oligomerization of sweet and bitter taste receptors. Methods Cell Biol 117:229–242. doi:10.1016/B978-0-12-408143-7.00013-X
Behrens M, Born S, Redel U, Voigt N, Schuh V, Raguse JD, Meyerhof W (2012) Immunohistochemical detection of TAS2R38 protein in human taste cells. PLoS One 7(7):e40304. doi:10.1371/journal.pone.0040304
Li F (2013) Taste perception: from the tongue to the testis. Mol Hum Reprod 19(6):349–360. doi:10.1093/molehr/gat009
Taruno A, Matsumoto I, Ma Z, Marambaud P, Foskett JK (2013) How do taste cells lacking synapses mediate neurotransmission? CALHM1, a voltage-gated ATP channel. BioEssays 35(12):1111–1118. doi:10.1002/bies.201300077
Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK (2013) CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495(7440):223–226. doi:10.1038/nature11906
Liman ER, Zhang YV, Montell C (2014) Peripheral coding of taste. Neuron 81(5):984–1000. doi:10.1016/j.neuron.2014.02.022
Roper SD (2013) Taste buds as peripheral chemosensory processors. Semin Cell Dev Biol 24(1):71–79. doi:10.1016/j.semcdb.2012.12.002
Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD (2007) Breadth of tuning and taste coding in mammalian taste buds. J Neurosci 27(40):10840–10848. doi:10.1523/JNEUROSCI.1863-07.2007
Yoshida R, Shigemura N, Sanematsu K, Yasumatsu K, Ishizuka S, Ninomiya Y (2006) Taste responsiveness of fungiform taste cells with action potentials. J Neurophysiol 96(6):3088–3095. doi:10.1152/jn.00409.2006
Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S (2010) Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci 30(25):8376–8382. doi:10.1523/JNEUROSCI.0496-10.2010
Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM (2010) GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142(5):687–698. doi:10.1016/j.cell.2010.07.041
Ozdener MH, Subramaniam S, Sundaresan S, Sery O, Hashimoto T, Asakawa Y, Besnard P, Abumrad NA, Khan NA (2014) CD36- and GPR120-mediated Ca(2)(+) signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology 146(4):995–1005. doi:10.1053/j.gastro.2014.01.006
Sclafani A, Zukerman S, Ackroff K (2013) GPR40 and GPR120 fatty acid sensors are critical for postoral but not oral mediation of fat preferences in the mouse. Am J Physiol Regul Integr Comp Physiol 305(12):R1490–R1497. doi:10.1152/ajpregu.00440.2013
Martin C, Passilly-Degrace P, Gaillard D, Merlin JF, Chevrot M, Besnard P (2011) The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One 6(8):e24014. doi:10.1371/journal.pone.0024014
Godinot N, Yasumatsu K, Barcos ME, Pineau N, Ledda M, Viton F, Ninomiya Y, le Coutre J, Damak S (2013) Activation of tongue-expressed GPR40 and GPR120 by non caloric agonists is not sufficient to drive preference in mice. Neuroscience 250:20–30. doi:10.1016/j.neuroscience.2013.06.043
Gilbertson TA, Khan NA (2014) Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog Lipid Res 53:82–92. doi:10.1016/j.plipres.2013.11.001
Dramane G, Akpona S, Simonin AM, Besnard P, Khan NA (2011) Cell signaling mechanisms of gustatory perception of lipids: can the taste cells be the target of anti-obesity agents? Curr Med Chem 18(22):3417–3422 BSP/CMC/E-Pub/2011/251 [pii]
Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P (2005) CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest 115(11):3177–3184. doi:10.1172/JCI25299
Khan NA, Besnard P (2009) Oro-sensory perception of dietary lipids: new insights into the fat taste transduction. Biochim Biophys Acta 1791(3):149–155. doi:10.1016/j.bbalip.2009.01.001
Abdoul-Azize S, Selvakumar S, Sadou H, Besnard P, Khan NA (2014) Ca2 + signaling in taste bud cells and spontaneous preference for fat: unresolved roles of CD36 and GPR120. Biochimie 96:8–13. doi:10.1016/j.biochi.2013.06.005
Laffitte A, Neiers F, Briand L (2014) Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr Opin Clin Nutr Metab Care. doi:10.1097/MCO.0000000000000058
Clark AA, Liggett SB, Munger SD (2012) Extraoral bitter taste receptors as mediators of off-target drug effects. FASEB J 26(12):4827–4831. doi:10.1096/fj.12-215087
Depoortere I (2014) Taste receptors of the gut: emerging roles in health and disease. Gut 63(1):179–190. doi:10.1136/gutjnl-2013-305112
Yamamoto K, Ishimaru Y (2013) Oral and extra-oral taste perception. Semin Cell Dev Biol 24(3):240–246. doi:10.1016/j.semcdb.2012.08.005
Gilbertson TA, Damak S, Margolskee RF (2000) The molecular physiology of taste transduction. Curr Opin Neurobiol 10(4):519–527
Mennella JA, Spector AC, Reed DR, Coldwell SE (2013) The bad taste of medicines: overview of basic research on bitter taste. Clin Ther 35(8):1225–1246. doi:10.1016/j.clinthera.2013.06.007
Finger TE, Bottger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL (2003) Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci USA 100(15):8981–8986. doi:10.1073/pnas.1531172100
Sbarbati A, Osculati F (2003) Solitary chemosensory cells in mammals? Cells Tissues Organs 175(1):51–55
Tizzano M, Merigo F, Sbarbati A (2006) Evidence of solitary chemosensory cells in a large mammal: the diffuse chemosensory system in Bos taurus airways. J Anat 209(3):333–337. doi:10.1111/j.1469-7580.2006.00617.x
Gulbransen BD, Clapp TR, Finger TE, Kinnamon SC (2008) Nasal solitary chemoreceptor cell responses to bitter and trigeminal stimulants in vitro. J Neurophysiol 99(6):2929–2937. doi:10.1152/jn.00066.2008
Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, Finger TE (2010) Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci USA 107(7):3210–3215. doi:10.1073/pnas.0911934107
Tizzano M, Cristofoletti M, Sbarbati A, Finger TE (2011) Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med 11:3. doi:10.1186/1471-2466-11-3
Braun T, Mack B, Kramer MF (2011) Solitary chemosensory cells in the respiratory and vomeronasal epithelium of the human nose: a pilot study. Rhinology 49(5):507–512. doi:10.4193/Rhin
Barham HP, Cooper SE, Anderson CB, Tizzano M, Kingdom TT, Finger TE, Kinnamon SC, Ramakrishnan VR (2013) Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int Forum Allergy Rhinol 3(6):450–457. doi:10.1002/alr.21149
Lee RJ, Kofonow JM, Rosen PL, Siebert AP, Chen B, Doghramji L, Xiong G, Adappa ND, Palmer JN, Kennedy DW, Kreindler JL, Margolskee RF, Cohen NA (2014) Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest 124(3):1393–1405. doi:10.1172/JCI72094
Saunders CJ, Christensen M, Finger TE, Tizzano M (2014) Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc Natl Acad Sci USA 111(16):6075–6080. doi:10.1073/pnas.1402251111
Pearson JP, Passador L, Iglewski BH, Greenberg EP (1995) A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA 92(5):1490–1494
Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ (2012) The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev 76(1):46–65. doi:10.1128/MMBR.05007-11
Chadwick M, Trewin H, Gawthrop F, Wagstaff C (2013) Sesquiterpenoids lactones: benefits to plants and people. Int J Mol Sci 14(6):12780–12805. doi:10.3390/ijms140612780
Lee RJ, Xiong G, Kofonow JM, Chen B, Lysenko A, Jiang P, Abraham V, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Beauchamp GK, Doulias P-T, Ischiropoulos H, Kreindler JL, Reed DR, Cohen NA (2012) T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest 122(11):4145–4159
Lee RJ, Chen B, Redding KM, Margolskee RF, Cohen NA (2014) Mouse nasal epithelial innate immune responses to Pseudomonas aeruginosa quorum-sensing molecules require taste signaling components. Innate Immun 20(6):606–617. doi:10.1177/1753425913503386
Kim U, Wooding S, Ricci D, Jorde LB, Drayna D (2005) Worldwide haplotype diversity and coding sequence variation at human bitter taste receptor loci. Hum Mutat 26(3):199–204. doi:10.1002/humu.20203
Li D, Zhang J (2014) Diet shapes the evolution of the vertebrate bitter taste receptor gene repertoire. Mol Biol Evol 31(2):303–309. doi:10.1093/molbev/mst219
Hayes JE, Wallace MR, Knopik VS, Herbstman DM, Bartoshuk LM, Duffy VB (2011) Allelic variation in TAS2R bitter receptor genes associates with variation in sensations from and ingestive behaviors toward common bitter beverages in adults. Chem Senses 36(3):311–319. doi:10.1093/chemse/bjq132
Lanier SA, Hayes JE, Duffy VB (2005) Sweet and bitter tastes of alcoholic beverages mediate alcohol intake in of-age undergraduates. Physiol Behav 83(5):821–831. doi:10.1016/j.physbeh.2004.10.004
Lionakis MS, Netea MG, Holland SM (2014) Mendelian genetics of human susceptibility to fungal infection. Cold Spring Harb Perspect Med 4(6):a019638. doi:10.1101/cshperspect.a019638
Greisner WA 3rd, Settipane GA (1996) Hereditary factor for nasal polyps. Allergy Asthma Proc 17(5):283–286
Cohen NA, Widelitz JS, Chiu AG, Palmer JN, Kennedy DW (2006) Familial aggregation of sinonasal polyps correlates with severity of disease. Otolaryngol Head Neck Surg 134(4):601–604. doi:10.1016/j.otohns.2005.11.042
Hamilos DL (2007) Chronic rhinosinusitis patterns of illness. Clin Allergy Immunol 20:1–13
Antunes MB, Gudis DA, Cohen NA (2009) Epithelium, cilia, and mucus: their importance in chronic rhinosinusitis. Immunol Allergy Clin North Am 29(4):631–643. doi:10.1016/j.iac.2009.07.004
Cohen NA (2006) Sinonasal mucociliary clearance in health and disease. Ann Otol Rhinol Laryngol Suppl 196:20–26
Genoway KA, Philpott CM, Javer AR (2011) Pathogen yield and antimicrobial resistance patterns of chronic rhinosinusitis patients presenting to a tertiary rhinology centre. J Otolaryngol Head Neck Surg 40(3):232–237
Hamilos DL (2013) Host-microbial interactions in patients with chronic rhinosinusitis. J Allergy Clin Immunol. doi:10.1016/j.jaci.2013.06.049
Kingdom TT, Swain RE Jr (2004) The microbiology and antimicrobial resistance patterns in chronic rhinosinusitis. Am J Otolaryngol 25(5):323–328 S0196070904000341 [pii]
Ooi EH, Wormald PJ, Tan LW (2008) Innate immunity in the paranasal sinuses: a review of nasal host defenses. Am J Rhinol 22(1):13–19. doi:10.2500/ajr.2008.22.3127
Ramanathan M Jr, Lane AP (2007) Innate immunity of the sinonasal cavity and its role in chronic rhinosinusitis. Otolaryngol Head Neck Surg 136(3):348–356. doi:10.1016/j.otohns.2006.11.011
Lee RJ, Chen B, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Cohen NA (2013) Vasoactive intestinal peptide regulates sinonasal mucociliary clearance and synergizes with histamine in stimulating sinonasal fluid secretion. FASEB J 27(12):5094–5103. doi:10.1096/fj.13-234476
Zhao KQ, Cowan AT, Lee RJ, Goldstein N, Droguett K, Chen B, Zheng C, Villalon M, Palmer JN, Kreindler JL, Cohen NA (2012) Molecular modulation of airway epithelial ciliary response to sneezing. FASEB J 26(8):3178–3187. doi:10.1096/fj.11-202184
Sleigh MA, Blake JR, Liron N (1988) The propulsion of mucus by cilia. Am Rev Respir Dis 137(3):726–741
Eliezer N, Sade J, Silberberg A, Nevo AC (1970) The role of mucus in transport by cilia. Am Rev Respir Dis 102(1):48–52
Gudis D, Zhao KQ, Cohen NA (2012) Acquired cilia dysfunction in chronic rhinosinusitis. Am J Rhinol Allergy 26(1):1–6. doi:10.2500/ajra.2012.26.3716
Gudis DA, Cohen NA (2010) Cilia dysfunction. Otolaryngol Clin North Am 43(3):461–472. doi:10.1016/j.otc.2010.02.007
Knowles MR, Boucher RC (2002) Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest 109(5):571–577
Parker D, Prince A (2011) Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol 45(2):189–201. doi:10.1165/rcmb.2011-0011RT
Fang FC (1997) Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Invest 99(12):2818–2825. doi:10.1172/JCI119473
Marcinkiewicz J (1997) Nitric oxide and antimicrobial activity of reactive oxygen intermediates. Immunopharmacology 37(1):35–41 S0162310996001683 [pii]
Deja M, Busch T, Bachmann S, Riskowski K, Campean V, Wiedmann B, Schwabe M, Hell B, Pfeilschifter J, Falke KJ, Lewandowski K (2003) Reduced nitric oxide in sinus epithelium of patients with radiologic maxillary sinusitis and sepsis. Am J Respir Crit Care Med 168(3):281–286. doi:10.1164/rccm.200207-640OC
Degano B, Valmary S, Serrano E, Brousset P, Arnal JF (2011) Expression of nitric oxide synthases in primary ciliary dyskinesia. Hum Pathol 42(12):1855–1861. doi:10.1016/j.humpath.2011.01.027
Haight JS, Djupesland PG, Qjan W, Chatkin JM, Furlott H, Irish J, Witterick I, McClean P, Fenton RS, Hoffstein V, Zamel N (1999) Does nasal nitric oxide come from the sinuses? J Otolaryngol 28(4):197–204
Naraghi M, Deroee AF, Ebrahimkhani M, Kiani S, Dehpour A (2007) Nitric oxide: a new concept in chronic sinusitis pathogenesis. Am J Otolaryngol 28(5):334–337. doi:10.1016/j.amjoto.2006.10.014
Phillips PS, Sacks R, Marcells GN, Cohen NA, Harvey RJ (2011) Nasal nitric oxide and sinonasal disease: a systematic review of published evidence. Otolaryngol Head Neck Surg 144(2):159–169
Ricciardolo FL (2003) Multiple roles of nitric oxide in the airways. Thorax 58(2):175–182
Zhang Y, Endam LM, Filali-Mouhim A, Bosse Y, Castano R, Desrosiers M (2011) Polymorphisms in the nitric oxide synthase 1 gene are associated with severe chronic rhinosinusitis. Am J Rhinol Allergy 25(2):e49–e54. doi:10.2500/ajra.2011.25.3588
Gliklich RE, Metson R (1995) The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg 113(1):104–109
Khalid AN, Quraishi SA, Kennedy DW (2004) Long-term quality of life measures after functional endoscopic sinus surgery. Am J Rhinol 18(3):131–136
Hens G, Hellings PW (2006) The nose: gatekeeper and trigger of bronchial disease. Rhinology 44(3):179–187
Bhattacharyya N, Kepnes LJ (2008) Assessment of trends in antimicrobial resistance in chronic rhinosinusitis. Ann Otol Rhinol Laryngol 117(6):448–452
Marcinkiewicz J, Strus M, Pasich E (2013) Antibiotic resistance: a “dark side” of biofilm-associated chronic infections. Pol Arch Med Wewn 123(6):309–313
Rujanavej V, Soudry E, Banaei N, Baron EJ, Hwang PH, Nayak JV (2013) Trends in incidence and susceptibility among methicillin-resistant Staphylococcus aureus isolated from intranasal cultures associated with rhinosinusitis. Am J Rhinol Allergy 27(2):134–137. doi:10.2500/ajra.2013.27.3858
Manes RP, Batra PS (2012) Bacteriology and antibiotic resistance in chronic rhinosinusitis. Facial Plast Surg Clin North Am 20(1):87–91. doi:10.1016/j.fsc.2011.10.010
Kennedy JL, Borish L (2013) Chronic rhinosinusitis and antibiotics: the good, the bad, and the ugly. Am J Rhinol Allergy 27(6):467–472. doi:10.2500/ajra.2013.27.3960
Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, Prince A (2004) Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol 30(6):777–783. doi:10.1165/rcmb.2003-0329OC
Greene CM, McElvaney NG (2005) Toll-like receptor expression and function in airway epithelial cells. Arch Immunol Ther Exp (Warsz) 53(5):418–427
Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ (2009) Motile cilia of human airway epithelia are chemosensory. Science 325(5944):1131–1134. doi:10.1126/science.1173869
Singla V, Reiter JF (2006) The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313(5787):629–633. doi:10.1126/science.1124534
Satir P, Christensen ST (2008) Structure and function of mammalian cilia. Histochem Cell Biol 129(6):687–693. doi:10.1007/s00418-008-0416-9
Takeda S, Narita K (2012) Structure and function of vertebrate cilia, towards a new taxonomy. Differentiation 83(2):S4–S11. doi:10.1016/j.diff.2011.11.002
Salathe M (2007) Regulation of mammalian ciliary beating. Annu Rev Physiol 69:401–422. doi:10.1146/annurev.physiol.69.040705.141253
Babu D, Roy S (2013) Left-right asymmetry: cilia stir up new surprises in the node. Open Biol 3(5):130052. doi:10.1098/rsob.130052
Lai Y, Chen B, Shi J, Palmer JN, Kennedy DW, Cohen NA (2011) Inflammation-mediated upregulation of centrosomal protein 110, a negative modulator of ciliogenesis, in patients with chronic rhinosinusitis. J Allergy Clin Immunol 128(6):e11207–e11215. doi:10.1016/j.jaci.2011.09.001
Ramanathan M Jr, Lane AP (2007) A comparison of experimental methods in molecular chronic rhinosinusitis research. Am J Rhinol 21(3):373–377
Antunes MB, Woodworth BA, Bhargave G, Xiong G, Aguilar JL, Ratner AJ, Kreindler JL, Rubenstein RC, Cohen NA (2007) Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques 43(2):195–196 198, 200 passim 000112531 [pii]
Dimova S, Brewster ME, Noppe M, Jorissen M, Augustijns P (2005) The use of human nasal in vitro cell systems during drug discovery and development. Toxicol In Vitro 19(1):107–122. doi:10.1016/j.tiv.2004.07.003
Woodworth BA, Antunes MB, Bhargave G, Palmer JN, Cohen NA (2007) Murine tracheal and nasal septal epithelium for air-liquid interface cultures: a comparative study. Am J Rhinol 21(5):533–537. doi:10.2500/ajr.2007.21.3068
Perez CA, Margolskee RF, Kinnamon SC, Ogura T (2003) Making sense with TRP channels: store-operated calcium entry and the ion channel Trpm5 in taste receptor cells. Cell Calcium 33(5–6):541–549
Zhang Z, Zhao Z, Margolskee R, Liman E (2007) The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci 27(21):5777–5786. doi:10.1523/JNEUROSCI.4973-06.2007
Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF (2002) A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5(11):1169–1176. doi:10.1038/nn952
Gustafsson LE, Leone AM, Persson MG, Wiklund NP, Moncada S (1991) Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun 181(2):852–857
Maniscalco M, Sofia M, Pelaia G (2007) Nitric oxide in upper airways inflammatory diseases. Inflamm Res 56(2):58–69. doi:10.1007/s00011-006-6111-1
Pearson JP, Pesci EC, Iglewski BH (1997) Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol 179(18):5756–5767
Li Z, Nair SK (2012) Quorum sensing: How bacteria can coordinate activity and synchronize their response to external signals? Protein Sci. doi:10.1002/pro.2132
Whitney G, Harder DB (1994) Genetics of bitter perception in mice. Physiol Behav 56(6):1141–1147
Wu SV, Chen MC, Rozengurt E (2005) Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol Genomics 22(2):139–149. doi:10.1152/physiolgenomics.00030.2005
Whitney G, Harder DB (1986) Phenylthiocarbamide (PTC) preference among laboratory mice: understanding of a previously “unreplicated” report. Behav Genet 16(6):605–610
St John SJ, Pour L, Boughter JD Jr (2005) Phenylthiocarbamide produces conditioned taste aversions in mice. Chem Senses 30(5):377–382 bji032 [pii] 0.1093/chemse/bji032
Nelson TM, Munger SD, Boughter JD Jr (2003) Taste sensitivities to PROP and PTC vary independently in mice. Chem Senses 28(8):695–704
Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E (2002) Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA 99(4):2392–2397. doi:10.1073/pnas.042617699
Chen MC, Wu SV, Reeve JR Jr, Rozengurt E (2006) Bitter stimuli induce Ca2 + signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2 + channels. Am J Physiol Cell Physiol 291(4):C726–C739. doi:10.1152/ajpcell.00003.2006
Jeon TI, Seo YK, Osborne TF (2011) Gut bitter taste receptor signalling induces ABCB1 through a mechanism involving CCK. Biochem J 438(1):33–37. doi:10.1042/BJ20110009
Ruiz-Avila L, Wong GT, Damak S, Margolskee RF (2001) Dominant loss of responsiveness to sweet and bitter compounds caused by a single mutation in alpha -gustducin. Proc Natl Acad Sci USA 98(15):8868–8873. doi:10.1073/pnas.151235798
Hoon MA, Northup JK, Margolskee RF, Ryba NJ (1995) Functional expression of the taste specific G-protein, alpha-gustducin. Biochem J 309(Pt 2):629–636
McLaughlin SK, McKinnon PJ, Margolskee RF (1992) Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature 357(6379):563–569. doi:10.1038/357563a0
Caicedo A, Pereira E, Margolskee RF, Roper SD (2003) Role of the G-protein subunit alpha-gustducin in taste cell responses to bitter stimuli. J Neurosci 23(30):9947–9952
He W, Danilova V, Zou S, Hellekant G, Max M, Margolskee RF, Damak S (2002) Partial rescue of taste responses of alpha-gustducin null mice by transgenic expression of alpha-transducin. Chem Senses 27(8):719–727
Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, Spector AC (2005) Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses 30(4):299–316. doi:10.1093/chemse/bji025
Bufe B, Breslin PA, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim UK, Drayna D, Meyerhof W (2005) The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol 15(4):322–327. doi:10.1016/j.cub.2005.01.047
Bachmanov AA, Bosak NP, Lin C, Matsumoto I, Ohmoto M, Reed DR, Nelson TM (2014) Genetics of taste receptors. Curr Pharm Des 20(16):2669–2683 CPD-E-PUB-54566 [pii]
Guo SW, Reed DR (2001) The genetics of phenylthiocarbamide perception. Ann Hum Biol 28(2):111–142
Mennella JA, Pepino MY, Reed DR (2005) Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics 115(2):e216–e222. doi:10.1542/peds.2004-1582
Reed DR, Knaapila A (2010) Genetics of taste and smell: poisons and pleasures. Prog Mol Biol Transl Sci 94:213–240. doi:10.1016/B978-0-12-375003-7.00008-X
Tan J, Abrol R, Trzaskowski B, Goddard WA 3rd (2012) 3D Structure Prediction of TAS2R38 Bitter Receptors Bound to Agonists Phenylthiocarbamide (PTC) and 6-n-Propylthiouracil (PROP). J Chem Inf Model. doi:10.1021/ci300133a
Biarnes X, Marchiori A, Giorgetti A, Lanzara C, Gasparini P, Carloni P, Born S, Brockhoff A, Behrens M, Meyerhof W (2010) Insights into the binding of Phenyltiocarbamide (PTC) agonist to its target human TAS2R38 bitter receptor. PLoS One 5(8):e12394. doi:10.1371/journal.pone.0012394
Floriano WB, Hall S, Vaidehi N, Kim U, Drayna D, Goddard WA 3rd (2006) Modeling the human PTC bitter-taste receptor interactions with bitter tastants. J Mol Model 12(6):931–941. doi:10.1007/s00894-006-0102-6
Lipchock SV, Mennella JA, Spielman AI, Reed DR (2013) Human bitter perception correlates with bitter receptor messenger RNA expression in taste cells. Am J Clin Nutr 98(4):1136–1143. doi:10.3945/ajcn.113.066688
Adappa ND, Howland TJ, Palmer JN, Kennedy DW, Doghramji L, Lysenko A, Reed DR, Lee RJ, Cohen NA (2013) Genetics of the taste receptor T2R38 correlates with chronic rhinosinusitis necessitating surgical intervention. Int Forum Allergy Rhinol 3(3):184–187
Adappa ND, Zhang Z, Palmer JN, Kennedy DW, Doghramji L, Lysenko A, Reed DR, Scott T, Zhao NW, Owens D, Lee RJ, Cohen NA (2013) The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int Forum Allergy Rhinol. doi:10.1002/alr.21253
Mfuna Endam L, Filali-Mouhim A, Boisvert P, Boulet LP, Bosse Y, Desrosiers M (2014) Genetic variations in taste receptors are associated with chronic rhinosinusitis: a replication study. Int Forum Allergy Rhinol 4(3):200–206. doi:10.1002/alr.21275
Sbarbati A, Merigo F, Osculati F (2010) Eukaryotic vs. prokaryotic chemosensory systems. Biomed Pharmacother 64(4):233–239. doi:10.1016/j.biopha.2009.06.015
Gulbransen B, Silver W, Finger TE (2008) Solitary chemoreceptor cell survival is independent of intact trigeminal innervation. J Comp Neurol 508(1):62–71. doi:10.1002/cne.21657
Osculati F, Bentivoglio M, Castellucci M, Cinti S, Zancanaro C, Sbarbati A (2007) The solitary chemosensory cells and the diffuse chemosensory system of the airway. Eur J Histochem 51(Suppl 1):65–72
Kotrschal K (2000) Taste(s) and olfaction(s) in fish: a review of specialized sub-systems and central integration. Pflugers Arch 439(3 Suppl):R178–R180
Tizzano M, Finger TE (2013) Chemosensors in the nose: guardians of the airways. Physiology (Bethesda) 28(1):51–60. doi:10.1152/physiol.00035.2012
Whitear M (1992) Solitary chemoreceptor cells. In: Hara TJ (ed) Chemoreception in Fishes. Chapman and Hall, London, pp 103–125
Hansen A (2007) Olfactory and solitary chemosensory cells: two different chemosensory systems in the nasal cavity of the American alligator, Alligator mississippiensis. BMC Neurosci 8:64. doi:10.1186/1471-2202-8-64
Lin W, Ogura T, Margolskee RF, Finger TE, Restrepo D (2008) TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J Neurophysiol 99(3):1451–1460. doi:10.1152/jn.01195.2007
Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Muhlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, Baal N, Weihe E, Schutz B, Kotlikoff M, Ibanez-Tallon I, Kummer W (2011) Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci USA 108(23):9478–9483. doi:10.1073/pnas.1019418108
Baraniuk JN, Kaliner MA (1990) Neuropeptides and nasal secretion. J Allergy Clin Immunol 86(4 Pt 2):620–627
Dinh QT, Groneberg DA, Mingomataj E, Peiser C, Heppt W, Dinh S, Arck PC, Klapp BF, Fischer A (2003) Expression of substance P and vanilloid receptor (VR1) in trigeminal sensory neurons projecting to the mouse nasal mucosa. Neuropeptides 37(4):245–250 S0143417903000659 [pii]
Fang SY, Shen CL (1997) Neuropeptidergic innervation of human nasal mucosa in various pathological conditions. Proc Natl Sci Counc Repub China B 21(1):8–12
Mendonca JC, Dolci JE (2005) Neuropeptide immunofluorescence in human nasal mucosa: assessment of the technique for vasoactive intestinal peptide (VIP). Braz J Otorhinolaryngol 71(2):123–131 S0034-72992005000200002 [pii]/S0034-72992005000200002
Mosimann BL, White MV, Hohman RJ, Goldrich MS, Kaulbach HC, Kaliner MA (1993) Substance P, calcitonin gene-related peptide, and vasoactive intestinal peptide increase in nasal secretions after allergen challenge in atopic patients. J Allergy Clin Immunol 92(1 Pt 1):95–104
Ianowski JP, Choi JY, Wine JJ, Hanrahan JW (2007) Mucus secretion by single tracheal submucosal glands from normal and CFTR knock-out mice. J Physiol 580:301–314
Ianowski JP, Choi JY, Wine JJ, Hanrahan JW (2008) Substance P stimulates CFTR-dependent fluid secretion by mouse tracheal submucosal glands. Pflugers Arch 457:529–537
Lee RJ, Foskett JK (2010) cAMP-activated Ca2 + signaling is required for CFTR-mediated serous cell fluid secretion in porcine and human airways. J Clin Invest 120(9):3137–3148
Lee RJ, Foskett JK (2012) Why mouse airway submucosal gland serous cells do not secrete fluid in response to cAMP stimulation. J Biol Chem 287(45):38316–38326. doi:10.1074/jbc.M112.412817
Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M (2010) The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses 35(2):157–170. doi:10.1093/chemse/bjp092
Pereira CS, Thompson JA, Xavier KB (2013) AI-2-mediated signalling in bacteria. FEMS Microbiol Rev 37(2):156–181. doi:10.1111/j.1574-6976.2012.00345.x
Frederix M, Downie AJ (2011) Quorum sensing: regulating the regulators. Adv Microb Physiol 58:23–80. doi:10.1016/B978-0-12-381043-4.00002-7
Abraham WR (2006) Controlling biofilms of gram-positive pathogenic bacteria. Curr Med Chem 13(13):1509–1524
Jiang P, Cui M, Zhao B, Liu Z, Snyder LA, Benard LM, Osman R, Margolskee RF, Max M (2005) Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J Biol Chem 280(15):15238–15246. doi:10.1074/jbc.M414287200
Jiang P, Cui M, Zhao B, Snyder LA, Benard LM, Osman R, Max M, Margolskee RF (2005) Identification of the cyclamate interaction site within the transmembrane domain of the human sweet taste receptor subunit T1R3. J Biol Chem 280(40):34296–34305. doi:10.1074/jbc.M505255200
Imada T, Misaka T, Fujiwara S, Okada S, Fukuda Y, Abe K (2010) Amiloride reduces the sweet taste intensity by inhibiting the human sweet taste receptor. Biochem Biophys Res Commun 397(2):220–225. doi:10.1016/j.bbrc.2010.05.088
Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF (2003) Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301(5634):850–853. doi:10.1126/science.1087155
Lemon CH, Margolskee RF (2009) Contribution of the T1r3 taste receptor to the response properties of central gustatory neurons. J Neurophysiol 101(5):2459–2471. doi:10.1152/jn.90892.2008
Chiu AG, Antunes MB, Feldman M, Cohen NA (2007) An animal model for the study of topical medications in sinusitis. Am J Rhinol 21(1):5–9
Ha KR, Psaltis AJ, Tan L, Wormald PJ (2007) A sheep model for the study of biofilms in rhinosinusitis. Am J Rhinol 21(3):339–345
Wang JC, Hathorn I, Habib AR, Chang E, Javer AR (2013) Evaluation of domestic and Yucatan swine nasal sinus anatomy as models for future sinonasal research of medications delivered by standard instruments used in functional endoscopic sinus surgery. Int Forum Allergy Rhinol 3(2):150–156. doi:10.1002/alr.21081
Trout L, Corboz MR, Ballard ST (2001) Mechanism of substance P-induced liquid secretion across bronchial epithelium. Am J Physiol Lung Cell Mol Physiol 281(3):L639–L645
Choi JY, Khansaheb M, Joo NS, Krouse ME, Robbins RC, Weill D, Wine JJ (2009) Substance P stimulates human airway submucosal gland secretion mainly via a CFTR-dependent process. J Clin Invest 119(5):1189–1200
Schiffman SS, Booth BJ, Sattely-Miller EA, Graham BG, Gibes KM (1999) Selective inhibition of sweetness by the sodium salt of ± 2-(4-methoxyphenoxy)propanoic acid. Chem Senses 24(4):439–447
Kyriazis GA, Soundarapandian MM, Tyrberg B (2012) Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci USA 109(8):E524–E532. doi:10.1073/pnas.1115183109
Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM (2007) Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 104(38):15069–15074. doi:10.1073/pnas.0706890104
Garnett JP, Baker EH, Baines DL (2012) Sweet talk: insights into the nature and importance of glucose transport in lung epithelium. Eur Respir J 40(5):1269–1276. doi:10.1183/09031936.00052612
Kalsi KK, Baker EH, Fraser O, Chung YL, Mace OJ, Tarelli E, Philips BJ, Baines DL (2009) Glucose homeostasis across human airway epithelial cell monolayers: role of diffusion, transport and metabolism. Pflugers Arch 457(5):1061–1070. doi:10.1007/s00424-008-0576-4
Pezzulo AA, Gutierrez J, Duschner KS, McConnell KS, Taft PJ, Ernst SE, Yahr TL, Rahmouni K, Klesney-Tait J, Stoltz DA, Zabner J (2011) Glucose depletion in the airway surface liquid is essential for sterility of the airways. PLoS One 6(1):e16166. doi:10.1371/journal.pone.0016166
Baker EH, Clark N, Brennan AL, Fisher DA, Gyi KM, Hodson ME, Philips BJ, Baines DL (1985) Wood DM (2007) Hyperglycemia and cystic fibrosis alter respiratory fluid glucose concentrations estimated by breath condensate analysis. J Appl Physiol 102(5):1969–1975. doi:10.1152/japplphysiol.01425.2006
Rogers GA, Den Beste K, Parkos CA, Nusrat A, Delgaudio JM, Wise SK (2011) Epithelial tight junction alterations in nasal polyposis. Int Forum Allergy Rhinol 1(1):50–54. doi:10.1002/alr.20014
Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, Kast JI, Akdis CA (2012) Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immunol 130(5):1087–1096, e1010. doi:10.1016/j.jaci.2012.05.052
Koziel H, Koziel MJ (1995) Pulmonary complications of diabetes mellitus pneumonia. Infect Dis Clin North Am 9(1):65–96
Zhang Z, Adappa ND, Lautenbach E, Chiu AG, Doghramji L, Howland TJ, Cohen NA, Palmer JN (2014) The effect of diabetes mellitus on chronic rhinosinusitis and sinus surgery outcome. Int Forum Allergy Rhinol. doi:10.1002/alr.21269
Fushan AA, Simons CT, Slack JP, Manichaikul A, Drayna D (2009) Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Curr Biol 19(15):1288–1293. doi:10.1016/j.cub.2009.06.015
Krasteva G, Canning BJ, Papadakis T, Kummer W (2012) Cholinergic brush cells in the trachea mediate respiratory responses to quorum sensing molecules. Life Sci 91(21–22):992–996. doi:10.1016/j.lfs.2012.06.014
Saunders CJ, Reynolds SD, Finger TE (2013) Chemosensory brush cells of the trachea. A stable population in a dynamic epithelium. Am J Respir Cell Mol Biol 49(2):190–196. doi:10.1165/rcmb.2012-0485OC
Sbarbati A, Osculati F (2005) A new fate for old cells: brush cells and related elements. J Anat 206(4):349–358. doi:10.1111/j.1469-7580.2005.00403.x
An SS, Wang WC, Koziol-White CJ, Ahn K, Lee DY, Kurten RC, Panettieri RA Jr, Liggett SB (2012) TAS2R activation promotes airway smooth muscle relaxation despite beta(2)-adrenergic receptor tachyphylaxis. Am J Physiol Lung Cell Mol Physiol 303(4):L304–L311. doi:10.1152/ajplung.00126.2012
Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB (2010) Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16(11):1299–1304. doi:10.1038/nm.2237
Robinett KS, Deshpande DA, Malone MM, Liggett SB (2011) Agonist-promoted homologous desensitization of human airway smooth muscle bitter taste receptors. Am J Respir Cell Mol Biol 45(5):1069–1074. doi:10.1165/rcmb.2011-0061OC
Robinett KS, Koziol-White CJ, Akoluk A, An SS, Panettieri RA Jr, Liggett SB (2014) Bitter taste receptor function in asthmatic and nonasthmatic human airway smooth muscle cells. Am J Respir Cell Mol Biol 50(4):678–683. doi:10.1165/rcmb.2013-0439RC
Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, Buchner J, Schaller M, Stange EF, Wehkamp J (2011) Reduction of disulphide bonds unmasks potent antimicrobial activity of human beta-defensin 1. Nature 469(7330):419–423. doi:10.1038/nature09674
Wilson SS, Wiens ME, Smith JG (2013) Antiviral mechanisms of human defensins. J Mol Biol 425(24):4965–4980. doi:10.1016/j.jmb.2013.09.038
Lee RJ, Cohen NA (2013) The emerging role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis. Am J Rhinol Allergy 27(4):283–286. doi:10.2500/ajra.2013.27.3911
Cohen SP, Buckley BK, Kosloff M, Garland AL, Bosch DE, Cheng G Jr, Radhakrishna H, Brown MD, Willard FS, Arshavsky VY, Tarran R, Siderovski DP, Kimple AJ (2012) Regulator of G-protein signaling-21 (RGS21) is an inhibitor of bitter gustatory signaling found in lingual and airway epithelia. J Biol Chem 287(50):41706–41719. doi:10.1074/jbc.M112.423806
Pulkkinen V, Manson ML, Safholm J, Adner M, Dahlen SE (2012) The bitter taste receptor (TAS2R) agonists denatonium and chloroquine display distinct patterns of relaxation of the guinea pig trachea. Am J Physiol Lung Cell Mol Physiol 303(11):L956–L966. doi:10.1152/ajplung.00205.2012
Grassin-Delyle S, Abrial C, Fayad-Kobeissi S, Brollo M, Faisy C, Alvarez JC, Naline E, Devillier P (2013) The expression and relaxant effect of bitter taste receptors in human bronchi. Respir Res 14:134. doi:10.1186/1465-9921-14-134
Krasteva G, Hartmann P, Papadakis T, Bodenbenner M, Wessels L, Weihe E, Schutz B, Langheinrich AC, Chubanov V, Gudermann T, Ibanez-Tallon I, Kummer W (2012) Cholinergic chemosensory cells in the auditory tube. Histochem Cell Biol 137(4):483–497. doi:10.1007/s00418-012-0911-x
Elliott RA, Kapoor S, Tincello DG (2011) Expression and distribution of the sweet taste receptor isoforms T1R2 and T1R3 in human and rat bladders. J Urol 186(6):2455–2462. doi:10.1016/j.juro.2011.07.083
Dehkordi O, Rose JE, Fatemi M, Allard JS, Balan KV, Young JK, Fatima S, Millis RM, Jayam-Trouth A (2012) Neuronal expression of bitter taste receptors and downstream signaling molecules in the rat brainstem. Brain Res 1475:1–10. doi:10.1016/j.brainres.2012.07.038
Singh N, Vrontakis M, Parkinson F, Chelikani P (2011) Functional bitter taste receptors are expressed in brain cells. Biochem Biophys Res Commun 406(1):146–151. doi:10.1016/j.bbrc.2011.02.016
Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE (2009) Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci 3:12. doi:10.3389/neuro.07.012.2009
Shin YJ, Park JH, Choi JS, Chun MH, Moon YW, Lee MY (2010) Enhanced expression of the sweet taste receptors and alpha-gustducin in reactive astrocytes of the rat hippocampus following ischemic injury. Neurochem Res 35(10):1628–1634. doi:10.1007/s11064-010-0223-2
Singh N, Chakraborty R, Bhullar RP, Chelikani P (2014) Differential expression of bitter taste receptors in non-cancerous breast epithelial and breast cancer cells. Biochem Biophys Res Commun 446(2):499–503. doi:10.1016/j.bbrc.2014.02.140
Foster SR, Porrello ER, Purdue B, Chan HW, Voigt A, Frenzel S, Hannan RD, Moritz KM, Simmons DG, Molenaar P, Roura E, Boehm U, Meyerhof W, Thomas WG (2013) Expression, regulation and putative nutrient-sensing function of taste GPCRs in the heart. PLoS One 8(5):e64579. doi:10.1371/journal.pone.0064579
Hao S, Dulake M, Espero E, Sternini C, Raybould HE, Rinaman L (2009) Central Fos expression and conditioned flavor avoidance in rats following intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol 296(3):R528–R536. doi:10.1152/ajpregu.90423.2008
Dotson CD, Zhang L, Xu H, Shin YK, Vigues S, Ott SH, Elson AE, Choi HJ, Shaw H, Egan JM, Mitchell BD, Li X, Steinle NI, Munger SD (2008) Bitter taste receptors influence glucose homeostasis. PLoS One 3(12):e3974. doi:10.1371/journal.pone.0003974
Bezencon C, le Coutre J, Damak S (2007) Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses 32(1):41–49. doi:10.1093/chemse/bjl034
Janssen S, Laermans J, Verhulst PJ, Thijs T, Tack J, Depoortere I (2011) Bitter taste receptors and alpha-gustducin regulate the secretion of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci USA 108(5):2094–2099. doi:10.1073/pnas.1011508108
Kokrashvili Z, Mosinger B, Margolskee RF (2009) Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr 90(3):822S–825S. doi:10.3945/ajcn.2009.27462T
Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP (2005) Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans 33(Pt 1):302–305. doi:10.1042/BST0330302
Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP (2007) T1R3 and gustducin in gut sense sugars to regulate expression of Na + -glucose cotransporter 1. Proc Natl Acad Sci USA 104(38):15075–15080. doi:10.1073/pnas.0706678104
Sclafani A (2007) Sweet taste signaling in the gut. Proc Natl Acad Sci USA 104(38):14887–14888. doi:10.1073/pnas.0707410104
Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Daly K, Ionescu C, Bravo D, Shirazi-Beechey SP (2010) Expression of Na +/glucose co-transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. Br J Nutr 104(5):637–646. doi:10.1017/S0007114510000917
Steinert RE, Gerspach AC, Gutmann H, Asarian L, Drewe J, Beglinger C (2011) The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). Clin Nutr 30(4):524–532. doi:10.1016/j.clnu.2011.01.007
Gerspach AC, Steinert RE, Schonenberger L, Graber-Maier A, Beglinger C (2011) The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiol Endocrinol Metab 301(2):E317–E325. doi:10.1152/ajpendo.00077.2011
Geraedts MC, Takahashi T, Vigues S, Markwardt ML, Nkobena A, Cockerham RE, Hajnal A, Dotson CD, Rizzo MA, Munger SD (2012) Transformation of postingestive glucose responses after deletion of sweet taste receptor subunits or gastric bypass surgery. Am J Physiol Endocrinol Metab 303(4):E464–E474. doi:10.1152/ajpendo.00163.2012
Shirazi-Beechey SP, Daly K, Al-Rammahi M, Moran AW, Bravo D (2014) Role of nutrient-sensing taste 1 receptor (T1R) family members in gastrointestinal chemosensing. Br J Nutr 1:8. doi:10.1017/S0007114513002286
Meyer-Gerspach AC, Wolnerhanssen B, Beglinger C (2014) Gut sweet taste receptors and their role in metabolism. Front Horm Res 42:123–133. doi:10.1159/000358321
Malaisse WJ, Vanonderbergen A, Louchami K, Jijakli H, Malaisse-Lagae F (1998) Effects of artificial sweeteners on insulin release and cationic fluxes in rat pancreatic islets. Cell Signal 10(10):727–733
Xu J, Cao J, Iguchi N, Riethmacher D, Huang L (2013) Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol Hum Reprod 19(1):17–28. doi:10.1093/molehr/gas040
Li F, Zhou M (2012) Depletion of bitter taste transduction leads to massive spermatid loss in transgenic mice. Mol Hum Reprod 18(6):289–297. doi:10.1093/molehr/gas005
Meyer D, Voigt A, Widmayer P, Borth H, Huebner S, Breit A, Marschall S, de Angelis MH, Boehm U, Meyerhof W, Gudermann T, Boekhoff I (2012) Expression of Tas1 taste receptors in mammalian spermatozoa: functional role of Tas1r1 in regulating basal Ca(2)(+) and cAMP concentrations in spermatozoa. PLoS One 7(2):e32354. doi:10.1371/journal.pone.0032354
Mosinger B, Redding KM, Parker MR, Yevshayeva V, Yee KK, Dyomina K, Li Y, Margolskee RF (2013) Genetic loss or pharmacological blockade of testes-expressed taste genes causes male sterility. Proc Natl Acad Sci USA 110(30):12319–12324. doi:10.1073/pnas.1302827110
Voigt A, Hubner S, Lossow K, Hermans-Borgmeyer I, Boehm U, Meyerhof W (2012) Genetic labeling of Tas1r1 and Tas2r131 taste receptor cells in mice. Chem Senses 37(9):897–911. doi:10.1093/chemse/bjs082
Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF (1999) Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci 2(12):1055–1062. doi:10.1038/15981
Giovannucci DR, Groblewski GE, Sneyd J, Yule DI (2000) Targeted phosphorylation of inositol 1,4,5-trisphosphate receptors selectively inhibits localized Ca2 + release and shapes oscillatory Ca2 + signals. J Biol Chem 275(43):33704–33711. doi:10.1074/jbc.M004278200
Yule DI, Straub SV, Bruce JI (2003) Modulation of Ca2 + oscillations by phosphorylation of Ins(1,4,5)P3 receptors. Biochem Soc Trans 31(Pt 5):954–957
Clapp TR, Stone LM, Margolskee RF, Kinnamon SC (2001) Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci 2:6
Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, Ninomiya Y, Mikoshiba K (2007) Abnormal taste perception in mice lacking the type 3 inositol 1,4,5-trisphosphate receptor. J Biol Chem 282(51):37225–37231. doi:10.1074/jbc.M705641200
Miyoshi MA, Abe K, Emori Y (2001) IP(3) receptor type 3 and PLCbeta2 are co-expressed with taste receptors T1R and T2R in rat taste bud cells. Chem Senses 26(3):259–265
Gao N, Lu M, Echeverri F, Laita B, Kalabat D, Williams ME, Hevezi P, Zlotnik A, Moyer BD (2009) Voltage-gated sodium channels in taste bud cells. BMC Neurosci 10:20. doi:10.1186/1471-2202-10-20
Waterer GW (2012) Airway defense mechanisms. Clin Chest Med 33(2):199–209. doi:10.1016/j.ccm.2012.03.003
Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(7):829–837. doi:10.1093/eurheartj/ehr304 837a-837d
Shaul PW (2002) Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol 64:749–774. doi:10.1146/annurev.physiol.64.081501.155952
Stout SL, Wyatt TA, Adams JJ, Sisson JH (2007) Nitric oxide-dependent cilia regulatory enzyme localization in bovine bronchial epithelial cells. J Histochem Cytochem 55(5):433–442. doi:10.1369/jhc.6A7089.2007
Acknowledgments
Some of the research described in this review was supported by a grant from the Flight Attendants Medical Research Institute (082478) and a philanthropic contribution from the RLG Foundation Inc., both to N.A.C.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, R.J., Cohen, N.A. Taste receptors in innate immunity. Cell. Mol. Life Sci. 72, 217–236 (2015). https://doi.org/10.1007/s00018-014-1736-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1736-7