Abstract
Thyroid hormones participate in the development and homeostasis of several organs and tissues. It is well documented that they act via nuclear receptors, the TRs, which are transcription factors whose function is modulated by the hormone T3. Importantly, T3-induced physiological response within a cell depends on the specific TR expression and on the T3 bioavailability. However, in addition to this T3-dependent control of TR functionality, increasing data show that the action of TRs is coordinated and integrated with other signaling pathways, specifically at the level of stem/progenitor cell populations. By focusing on the intestinal epithelium of both amphibians and mammals we summarize here new data in support of a role for thyroid hormones and the TR nuclear receptors in stem cell biology. This new concept may be extended to other organs and have biological relevance in therapeutic approaches aimed to target stem cells such as tissue engineering and cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
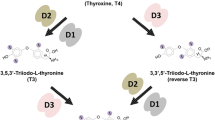
Thyroid hormones (THs) are key regulators of several aspects of development and homeostasis [1, 2]. The THs are synthesized by the thyroid gland and this process is regulated through the hypothalamus–pituitary–thyroid axis [3]. The levels of circulating THs are not, however, indicative of a specific cellular TH status. In fact, hormone transport and metabolism determine the intracellular levels of l-thyroxine, or T4, and 3,5,3′-l-triiodothyronine, or T3. Both of these hormones are actively transported across the cell membrane by specific transporter proteins, of which monocarboxylate transporter-8 and organic anion-transporting polypeptide-1c are the best-characterized [4–7]. Three iodothyronine deiodinase selenoenzymes (Dio1, Dio2, and Dio3) regulate TH’s activation and catabolism [8]. Dio1 and Dio2 catalyze the 5′-deiodination of T4 to its active metabolite T3. Conversely, Dio3 catalyzes the irreversible 5-deiodination of T4 and T3 to their inactive metabolites rT3 (3,5,5′-T3, or reverse T3) and 3,3′-T2 [8].
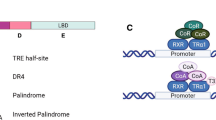
The thyroid hormone T3 is considered the active form of THs, because of its binding to the thyroid hormone nuclear receptors (TRs) that are transcription factors belonging to the nuclear receptor superfamily [9]. The main characteristic of the TRs is the presence of a DNA- and a hormone-binding domain, known as DBD and HBD, respectively (Fig. 1a). Two genes, TRα and TRβ, code for the TRs, each of them is responsible for the production of different isoforms (Fig. 1a) by alternative splicing or the use of different promoters [1]. TRα1, TRβ1, and TRβ2 are bona fide nuclear receptors (i.e., presence of DBD and HBD), while the TRα2 isoform retains the DBD but lacks the HBD, then behaving as a dominant negative vis-à-vis of the receptor [10]. The TRs bind specific DNA sequences named thyroid hormone response elements (TREs), which are generally located within the genomic non-coding regions of the target genes. The canonical TRE consensus is a tandem of AGGTCA sequences in direct repetition that are separated by four base pairs, named the Direct Repeat 4 (DR4) [1]. However, the TREs present in the promoters of the target genes often differ from the consensus sequence in terms of their arrangements as palindromes or inverted palindromes or in the number of nucleotides that separate the tandem sequences (Fig. 1b) [11]. The existence of this variety of TREs may help explain the different modalities of transcriptional modulation by TRs (tissue-specificity or activation vs. repression) [1].
Schematic representation of the various isoforms encoded by TRα or TRβ genes and of their DNA binding motifs. a The upper panel shows the different domains involved in TR function. These include the DNA-Binding Domain (DBD) and the Hormone-Binding Domain (HBD), which are specifically present in the TRα1, TRβ1 and TRβ2 proteins, which are bona fide T3 nuclear receptors. The TRα2 isoform lacks the HDB. Other functional regions of the TRs include cofactor-binding domains (located in A/B, D, and E) and dimerization domains (located in C and E). AF-1 and AF-2 domains are important for transcriptional activation. b Different arrangements of thyroid hormone responsive elements (TRE). The TREs are constituted by repetitions of two half-sites (upper panel) in different arrangements as indicated
Recent reviews, including ours, have summarized the characteristics and mode of action of the TRs [12–20]. For the specific aim of this review, we will focus on the current knowledge of the functions of THs and TRs on intestinal progenitor/stem cell biology. In fact, there is a compelling interest in the field of intestinal stem cell biology for several reasons. First, from a fundamental point of view, the mammalian intestinal epithelium is the fastest renewing tissue in homeostatic condition, and this process depends on stem cell activity [21]. Second, new data showed that cancer stem cells derive from physiological stem cells and described them as the cells at the origin of cancer development and maintenance [22]. These last findings are of importance for translational research aimed at developing new therapeutic approaches in patients. In both cases, understanding how the intestinal stem cells are able to receive, integrate, and respond to specific stimuli, such as THs, is of fundamental importance to better define their physiology and to understand the mechanisms of stem cell transformation leading to cancer.
Thyroid hormones and the TRs on intestinal physiology
Even if there is no clear-cut demonstration of TH/TR action on stem cell biology, there are increasing data in this direction, and several reports demonstrated that THs could influence somatic stem cell biology and affect progenitor cell fate [20]. The THs and their receptors TRs control the balance between cell proliferation and cell differentiation in several organs and tissues during development as well as in adulthood [23]. The paradigm is the amphibian metamorphosis that is triggered by an increase of circulating THs levels and of TRβ expression [24]. In mammals, among the organ targets, we recall the nervous system where THs and TRs play multiple actions [25]. Regarding their involvement in precursor cell biology, it has been shown that they are involved in neurogenesis during development [26], by controlling the correct number of progenitors in specific areas such as the fetal neocortex [27] or the telencephalon [28]. Moreover, a major role for the liganded TRα1 receptor has been unveiled in the adult neurogenic areas such as the subventricular zone [29, 30] or the hippocampus [31, 32]. Other well-characterized progenitor/stem cell targets include those of the skin [33], and intriguingly the embryonic stem cells that upon T3 treatment can massively differentiate toward a cardiomyocyte lineage [34].
Last but not least, a well-established target of the THs and TRs is the developing and the adult gut [24, 35]. We will summarize in this section the current knowledge concerning the intestinal epithelium organization and architecture as well as the action of THs and TRs on intestinal development and homeostasis with a particular emphasis on stem cell biology.
The intestinal mucosa structure and function
Investigations into amphibian metamorphosis during the early 20th century offered the first evidence of THs’ key role in the regulation of gastrointestinal development. In fact, THs trigger and control the whole metamorphosis process. Indeed, the gastrointestinal tract undergoes dramatic remodeling, which includes a phase of apoptosis followed by a burst in cell proliferation [35, 36]. Comparative studies, focused on the intestinal postnatal development in mammals, have also shed light on THs’ central role during the maturation at weaning time [35]. Notably, in mammals, THs and the TRα gene have an important function in both development and in the homeostatic control of this organ [37, 38].
In both mammals and amphibians, the intestine presents a tubular morphology developed along its proximo-distal axis, composed of three tissue layers. The outer layer is constituted by smooth muscles organized in circular-inner and longitudinal-outer layers. These muscles are mainly involved in the peristalsis, under the control of the parasympathetic nervous system. The middle layer, or submucosa, consists of fibrous connective tissue. Finally, the inner surface is constituted by the epithelium, organized as a sheet of polarized columnar cells [39]. The intestinal epithelium is in charge of processing and absorbing nutrients. In mammals, the absorptive surface of the small intestine (SI) is strongly enhanced by the presence of protrusions into the lumen and by invaginations into the submucosa, respectively the villi and crypts of Lieberkühn (Fig. 2a). At least seven different cell types have been identified in this tissue but only four are considered the main cytotypes: (1) the enterocytes, responsible for nutrient absorption that represent the vast majority of villous cells; (2) the goblet cells, which produce a protective mucus layer and are scattered throughout the epithelium; (3) the enteroendocrine cells, which secrete digestive hormones, and (4) the Paneth cells, present only in the SI, which reside at the bottom of the crypts and provide antimicrobial peptides [40].
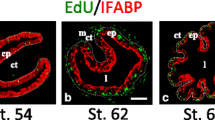
Organization of the adult mammalian small intestine and expression domain of the TR genes. a The scheme illustrates the intestinal epithelium organization into proliferative compartments (the crypts) and differentiated compartments (the villi). b In crypts, somatic stem cells are present, which self-renew and give rise to undifferentiated progenitors that proliferate, differentiate while migrating, and are eventually shed in the lumen after apoptosis. Yellow, Paneth cells; Red, stem cells; Green, secretory progenitors; Blue, absorptive progenitors. TRα (c) and TRβ (d) driven LacZ expression on intestinal sections from TRα+/0 or TRβ+/- mice. Pictures show β-galactosidase activity in the different cell types; c crypts, sm smooth muscle, v villi. The dotted bars indicate the limit between the crypts and the villi. Bar 15 μm
The main characteristic of the intestinal epithelium is its rapid and continuous renewal; its homeostasis involves several processes and the integration of multiple signaling pathways. This renewal is maintained by the presence of a proliferative compartment that is located in the interfold regions of the intestine, where the stem cells are located. In mammals, these regions are defined as crypts of Lieberkühn, and the stem cells reside near their bottom (Fig. 2b) [41]. These cells self-renew and give rise to proliferative progenitors that differentiate as they migrate along the vertical axis. Finally, the cells are exfoliated into the lumen after death by apoptosis [21, 41]. In amphibians, the adult epithelium renews along the trough-crest axis of the intestinal folds, with a mechanism that is similar to that of the mammalian crypt-villus axis [42]. Stem cells have also been described in the interfold regions of the adult amphibian epithelium; these cells give rise to proliferating progenitors that differentiate, migrate, and die [42].
The continuous cell renewal of the intestinal epithelium is regulated by fine cross-regulations between several pathways, including Wnt, Hedgehog, Notch, BMP, and THs [21, 42–44]. These pathways play a central role in intestinal development and homeostasis, and the molecular basis of their action has begun to be characterized in both mammals and amphibians [43–45]. However, our knowledge of the intra- and inter-regulations occurring between the different signaling pathways or their specific functions is still unclear and sometimes puzzling.
The intestinal epithelial stem cells
As said, the intestinal epithelium is a highly dynamic tissue with a rapid and perpetual renewal [43] that depends on the activity of somatic intestinal epithelial stem cells (ISCs) [46]. The ISCs are multipotent cells characterized by a very long self-renewal capability; their progeny fill in the so-called “transit-amplifying” zone (TA), composed of highly proliferating progenitors that differentiate while migrating. Proliferation ceases when cells reach the crypt–villus boundary, thus the villi contain only post-mitotic cells [43, 46].
Studies on the ISCs started during the 1970s, when Cheng and Leblond [47] stated that all epithelial cell lineages of the intestine are monoclonal populations are derived from a single stem cell; they defined the crypt base columnar (CBC) cells as ISCs based on morphological criteria and on their position at the bottom of the crypts, between the Paneth cells. Successively, [3H]-thymidine labeling retention experiments combined with bromodeoxyuridine pulse showed that ISCs are prevalently quiescent (steady state) and that they are located at the +4 position counting from the crypt base, just above the Paneth cells [48]. This model was confirmed by other approaches [49] and supports the concept of “dormant” somatic stem cells. It was only in 1999 that Bjerknes and Cheng clearly demonstrated that the ISCs are capable of generating all the intestinal cytotypes: by using chemical mutagenesis and following the inheritance pattern of specific mutations, they showed that the intestinal crypts contain a population of somatic multipotent stem cells that are located between the +1 and +4 positions from the bottom of the crypts [50]. The more recent identification of specific ISCs markers has led to important advances in the characterization of ISC biology and their role in intestinal homeostasis, repair, and cancer [46]. Currently, a dozen ISC markers have been proposed but few of them have been characterized and validated as bona fide markers. Among them, the most studied is the leucine-rich-repeat-containing G-protein-coupled receptor 5 (Lgr5), which is a Wnt target with an expression domain restricted to the crypts. Using reporter mouse lines, Barker and colleagues showed that (1) Lgr5-driven expression is initiated and confined to CBC cells, (2) CBCs are able to generate all epithelial lineages over a 60-day period, thus proving that they are multipotent ISCs; (3) contrariwise to the acquired notion on slow cycling somatic stem cells, they also provided evidence that CBCs cycle actively (every 24 h) and are responsible for epithelial homeostasis [51]. Finally, the isolation of Lgr5+ stem cells also demonstrated that they contain significant telomerase activity, that progressively decreases in the TA progenitors and then is absent in differentiated cells [52]. On the other side, a subpopulation of slowly cycling ISCs located around the +4 position of the crypts and specifically expressing the reverse transcriptase component of murine telomerase (mTert), can also give rise to Lgr5+-CBC stem cells [53]. Lineage-tracing approaches demonstrated that mTert+ cells generate all differentiated intestinal cell types at low frequency under basal conditions and at higher frequencies following injury [53]. Similar lineage-tracing strategies have been also used to follow the fate of the B lymphoma Mo-MLV insertion region 1 homolog (Bmi1)-positive cells. In fact, Bmi1-CreER mice crossed with a lacZ reporter mouse model showed a specific activity restricted to the +4 position above the Paneth cells [54]. Bmi1 belongs to the Polycomb group gene family, which was originally thought to regulate the self-renewal and proliferation of normal and leukemic stem cells [55]. Ablation of the Bmi1+ population induces disorganized intestinal mucosa and the loss of crypts, supporting the hypothesis of an impaired stem cell function [54]. Both Lgr5+ and Bmi1+ cells share the ability to proliferate, expand, self-renew, and give rise to all the differentiated intestinal epithelium cell lineages. However, the expression of Bmi1 is observed in a minority of the crypts in the proximal small intestine but results absent from the rest of the intestinal tract [54]. According to the possibility that mammals use more than one molecularly distinguishable adult ISC population, the homeodomain-only protein (Hopx) has been proposed as a new marker of +4 ISCs, which also shows a stem cell hierarchy and/or plasticity between CBCs and +4 stem cells [56]. In agreement with these different observations, a new mouse model based upon the expression of the RNA-binding protein Musashi 1, confirmed the existence of two populations of ISCs, which differ for their position, ISCs marker expression and cell cycle activity [57].
Altogether, these different findings demonstrate the existence of a complex stem-cell zone within the intestinal crypts, where the cells are characterized by the expression of different markers and display diverse cell cycle and symmetric/asymmetric cell division properties [58, 59]. These observations clearly reveal a highly complicated scenario, far from being clearly understood.
TH and TRs in development, homeostasis, and cancer
In-depth studies of intestinal remodeling during amphibians metamorphosis or mouse postnatal maturation at weaning have provided great insights into understanding the function of THs and TRs in the intestine [12]. A large body of literature exists regarding the cellular and molecular mechanisms at the basis of the gut remodeling regulated by THs in amphibians. In particular, the increase of THs levels induces a wave of massive apoptosis of the larval epithelium followed by a surge of cell proliferation within the surviving cells to generate an adult epithelium [60]. On the contrary, in mammals, no dramatic postnatal changes occur, and postnatal maturation consists of an increase in mucosal growth with a burst in cell proliferation [35]. Interestingly, THs level increase significantly in rodents during the second postnatal week, corresponding to the weaning period [61]. At that time, structural and functional intestinal remodeling takes place, and THs stimulate extensive mucosal growth and initiate the onset of adult-type digestive enzymes expression in the enterocytes [35].
TH signaling depends on the specific expression pattern/domain of the different players involved in THs signal reception and metabolism. In amphibians, the intestinal expression of the deiodinase selenoenzyme Dio2 increases and that of the deiodinase selenoenzyme Dio3 decreases at the time of the climax [62], when the gut remodeling activity is very high [60]. This modulation of both Dio2 and Dio3 expression can correlate with the increased level of local T3 synthesis and be responsible for the increased cell proliferation in the neo-forming adult epithelium [60]. In mammals, the three deiodinases appear poorly expressed during intestinal development until the adult stages, suggesting limited deiodinase activity in this organ. In particular in rat, only Dio1 is expressed, and its levels are very low compared with those observed in liver or the skin [63]. Our own studies in mouse adult intestine further support this observation [64]. Regarding the expression of the TRs, it has been reported that TRα is present at a low level in the pre-metamorphic intestine of tadpoles, whereas TRβ expression strongly increases after the surge of THs level [60]. Both TRs demonstrated to play a fundamental role during the process of gut remodeling [65]. The situation appears different in mammals, since a clear-cut function of TRβ in intestinal physiology has not been established [66], even if TRβ locus is able to drive LacZ expression specifically in villi cells of TRβ+/− mice [67] (Fig. 2d), suggesting that TRβ is expressed in differentiated epithelial cells. Furthermore, data from Hodin and colleagues showed a developmental regulation of TRα1 and TRβ1 expression in the postnatal intestine [68], and we also confirmed these results and showed the dynamic expression of TRα1 during postnatal development [66, 69]. Moreover, we showed that TRα1 expression domain is restricted to the intestinal crypts [37, 69] and to the smooth muscle layers [66], as also illustrated in Fig. 2c by the β-galactosidase staining of intestinal sections from TRα+/0 animals that recapitulate TRα gene expression domain [70].
Our extensive analysis using engineered mice established that THs mainly regulate the proliferation of crypt epithelial precursors during both maturation at weaning and homeostasis at adulthood [12]. Our data indicated that this function specifically depends on the TRα1 receptor [66], coherent with its restricted expression domain at the levels of the crypts [69], and implicate the activation of specific gene networks [37]. These findings are summarized in Fig. 3. Intriguingly, from a molecular point of view, the TH-dependent developmental programs of Xenopus and mouse show several similarities, as discussed in other reviews [44].
TH signaling and TRα1-dependent activation of the Wnt pathway in the intestinal epithelial precursors. THs (T3 and T4, green stars) enter the cells via specific transporters belonging to the monocarboxylate transporter (MCT) and organic anion-transporting polypeptide (OATP) protein families. Both T3 and T4 can be metabolized by the deiodinases. Dio1 and Dio2 catalyze the synthesis of T3 (red stars); Dio3 degrades both T4 and T3 into inactive forms. In the intestinal epithelium, only Dio1 mRNA has been detected. Our work showed that T3 binding to TRα1 receptor induces the transcription of the Ctnnb1 (encoding β-catenin) and of Sfrp2 (soluble-frizzled related protein 2) genes. The sFRP2-secreted protein functionally interacts with frizzled (Fzd), alone or in combination with Wnt, to stabilize β-catenin and to activate Wnt target genes. The canonical Wnt pathway acts via the formation of a complex between Wnt/Fzd/LRP, leading to the transduction of the extracellular signal. This in turn blocks the degradation complex and stabilizes β-catenin, which shuttles into the nucleus and activates the Wnt target genes
Until recently, only limited data described the role of THs in adult intestinal physiology, such as metabolic processes of absorption and secretion of nutrients [35, 71, 72]. The recent description of mutations in the TRα1 receptor in patients provides a novel perspective on the role of TRα1 in this organ. These mutations result in a non-functional receptor, which competes with the wild-type receptor [73, 74]. Patients present characteristics of hypothyroidism, including high levels of circulating TSH, delayed bone maturation, and impaired brain development. Together with these defects, they suffer from altered intestinal functionality characterized by reduced bowel movements. The enteric nervous system controls these movements through the smooth muscle tissues [75], suggesting that the lack of TRα1 function affects the physiology of one or both mesenchymal derivatives. One of the reports mentions that there are no overt abnormalities of the colon mucosa upon histological examination [73], suggesting that detailed studies will be necessary to better define the origin of the reduced bowel motility due to the TRα1 mutation. Notably, reduced ileal muscular activity has been described in TRα−/− mice (which lack the expression of TRα1 and TRα2 but retain the expression of the short TRΔα isoforms [76]), together with a substantial reduction of crypt cell proliferation; this phenotype is stronger than those described for other TRα gene knockout mice [70, 77, 78]. In the light of these observations, it is worth speculating that in the context of the TRα−/− background, the short isoforms could mimic the dominant negative action of the TRα1 mutations described in the patients. This hypothesis is consistent with the previously reported function of these short isoforms, as negative modulators of the TRs or of other nuclear receptors [79].
Several reports have indicated that mutant TRs or altered TH statuses are involved in various cancers [80–87]. Similar to their organ-/tissue-specific action [44], both tumor-inducer or tumor-suppressor roles have been described [88], making it quite difficult to draw a general picture. Regarding an action of altered TH levels and cancers of the gastrointestinal tract, only in the case of hepatocarcinomas (HCC) a clear correlation has been established between hypothyroidism and HCC [88], whereas contrasting results in the literature described both increased and decreased levels of hormones in the development of human breast and colon cancers [83, 85]. In HCC, it has also been shown that T3 controls Cathepsin H gene transcription [89]. The resulting up-regulation of Cathepsin H expression favors cancer cell migration and invasion, suggesting a link between THs and invasive cancer [89]. A similar regulation, however, has not been observed in normal intestinal crypts [37] or in intestinal tumors (our unpublished observations), indicating again the existence of a organ/tissue-specific regulation. The deiodinases appear important actors of THs activity [8] and a complex interplay between Dio2 (i.e., high cellular T3), Dio3 (i.e., low cellular T3) and sonic hedgehog [90] or Wnt [91] has been described in skin tumors or in colon cancer cell lines, respectively. In this last case, Dio3 is up-regulated by Wnt signal resulting in a positive effect on cell proliferation. These results on colon cell lines appear in contradiction with our data that described a positive correlation between TH levels and cell proliferation in mouse intestinal crypts both in vivo and in primary cultures [37, 69]. This contradiction, however, may be quickly solved because a different biological status (i.e., physiological vs. pathological condition) can explain the different cellular outcomes downstream of TH’s signal. Concerning the TRs, it has been shown that their mutation [84, 86, 87] or aberrant expression [81, 84, 86, 87, 92] is associated with gastrointestinal tumors. In particular, TRβ gene is frequently methylated and its expression strongly decreased in colon cancer [93], whereas it is still unclear whether in this same context TRα gene expression is altered. However, our own data on animal models strongly suggest that the pro-proliferative action of THs and TRα1 on crypt cells may play a major role in tumor development. Taking together the limited, and sometimes contradictory, information regarding the function of THs and the control of their activity by the deiodinases [83, 85] or the mutation/altered expression of the TRs [81, 84, 86, 87, 92] in human gastrointestinal physiopathology, we propose a model (Fig. 4) in which the signaling by THs and TRs can have different outcomes when dealing with normal epithelial progenitors or with tumoral cells, as also suggested by Brown et al. [88]. Moreover, we also speculate that the action of the mutated TRα1 receptors in patients might also result in a reduction of the intestinal epithelial progenitor/stem cell proliferation. The interference with the functionality of the wild-type TRα1 can be particularly deleterious in pathological conditions when unaffected proliferative capacities of the precursor cells are absolutely required, such as epithelial regeneration after gut resection [94] or inflammation [95].
Action of THs on intestinal epithelium in development and homeostasis versus cancer. The scheme summarizes the direct (arrow) and the indirect (connector) effects of THs via TRs on the Wnt effectors and targets, which in turn regulate cell proliferation. Intriguingly, increased levels of Dio3 have been shown in human colon cancer, possibly due to the increased Wnt activity in those lesions. It remains, however, to be established whether a relation exist between Dio3 and TRs in this specific context
THs and TRs in stem cell biology
Evidence supports the assumption that THs and TRα1 are involved in intestinal epithelial progenitor/stem cell physiology, given that they can influence their proliferative capacity [12]. Moreover, the regenerative properties of the epithelium after γ-ray induced DNA damage, are strongly affected by the lack of TRα1 expression [96], and the targeted overexpression of TRα1 in the intestinal epithelium (vil-TRα1 mice) induces crypt hyperplasia, hyperproliferation, and adenoma development [38]. In particular, the induced aberrant villi architecture can be due to increased crypt fission [97], which reflects enhanced stem cell activity [98]. In favor of this assumption, TRα1 overexpression in a tumor-prone model (vil-TRα1/Apc mice) accelerates the intestinal tumorigenic process [38].
A specific mechanism of TH-TR action on the intestinal stem cell biology, which includes self-renewal and multipotency, has not been described yet, whereas, as said, several findings in support of this action have been reported [12]. In particular, in Xenopus there is not a clearly defined stem cell population in the larval epithelium at the tadpole stage, but stem cells appear together with the generation of the adult epithelium; intriguingly, both processes are under the control of THs [99]. Moreover, several genes that are suitable or putative markers of ISCs in mammals are strongly and transiently up-regulated by the surge in THs levels during this phase of stem cell appearance [100]. Among them, Musashi1 is regulated by THs in metamorphic gut in tadpoles as well as in the developing mouse intestine [101]. This gene, however, is not directly regulated at the transcriptional level, indicating that complex cell interactions or other mechanisms involving up-stream regulator(s), such as the Wnt pathway, could control Musashi1 expression [101, 102].
Crosstalk between TH-TRα1 and the Wnt pathway
The TRα1 receptor interacts at multiple levels with the Wnt pathway in the intestinal epithelial precursors to control crypt proliferation in physio-pathological conditions [12].
The Wnt pathway is essential for proper intestinal development and homeostasis and its deregulation is strongly correlated to gut carcinogenesis [103]. The major actor of canonical Wnt signaling is the β-catenin. The binding of Wnt to the Fzd receptor leads to increased β-catenin stabilization and to its translocation to the nucleus, where it acts as a transcriptional co-factor by associating with members of the Tcf/Lef (T cell factor/lymphoid-enhancing factor) family of transcription factors [104, 105]. Other signaling pathways such as Notch [106] as well as extracellular secreted proteins, including Wnt inhibitory factor (Dikkopf, Cerberus, and secreted Frizzled-Related Protein), can modulate the Wnt signaling [107].
T3-liganded TRα1 activates the proliferation of the mouse intestinal epithelium precursors by modulating genes involved in cell cycle control and components of the Wnt pathway [37, 69]. Indeed, the TRα1 receptor is a direct transcriptional regulator of the Ctnnb1 gene, which encodes for the β-catenin. The increased expression of β-catenin, in turn, activates its targets such as cyclins D1 and D2 as well as c-Myc [69]. Moreover, the secreted frizzled-related protein sFRP2 was also characterized as a direct target of TRα1, acting as a positive regulator of the canonical Wnt pathway in intestinal progenitors in vitro, as summarized in Fig. 3 [37]. Given the role of the Wnt pathway in gut tumorigenesis [103], we tested the hypothesis that the alteration of TRα1 expression may have a tumor-inducer potentiality in mouse intestine [38]. Our results showed that TRα1 overexpression in vil-TRα1 mice induced crypt hyperplasia and hyper-proliferation, but was not able per se to promote cancer. Conversely, it can cooperate with an activated Wnt pathway (vil-TRα1/Apc mice) in the induction of aggressive gut tumors [38]. In an effort to define the mechanisms involved in this cooperation, we also reported that TRα1 and the β-catenin/Tcf4 complex can physically and functionally interact, resulting in the reciprocal modulation of their activity [64].
This complex scenario of TRα1 and Wnt cross-regulations in the context of the intestinal epithelium underlines a key role of TRα1 at the level of the proliferative compartment, including TA cells and ISCs, where Wnt is strongly active [103] and TRα1 is specifically expressed [66].
Conclusions
The importance of THs in development and homeostasis has been first suggested in thyroid-related human pathologies. In fact, the original name for hypothyroidism, myxedema, refers to the edema-like associated skin condition only subsequently connected to alterations in THs status [108, 109]. Insufficient TH levels during development were also shown to have clinical consequences such as neurological damage and cretinism [110], whereas detrimental effects of hyperthyroidism on the skeleton have been first described in 1891 [111]. Hypothyroidism has been commonly associated with intestinal constipation and recent papers described a TRα1 mutation in patients with severe peristalsis impairment [73, 74]. Noteworthy, to try to understand how THs alteration can influence a specific tissue/organ it is necessary to keep into account not only the expression pattern of the TR receptors but also the local availability of the hormone, which in turn depends on the abundance of the THs transporters and of specific deiodinases expression.
We summarized here evidence that TH signaling through the TRs stimulates the proliferation of the intestinal epithelial precursors in both amphibians and mammals. Moreover, this process is strongly correlated with a set of common regulated TH-target genes and signaling pathways [37]. However, discrepancies also exist when comparing these two models [12, 44], indicating that an in-depth comparative analysis is still lacking. Nevertheless, despite this evident lack of knowledge, we underlined here several findings in favor of the existence of a TH-dependent signaling on gut stem cell biology in both models. In fact, we described a certain number of similarities, strongly suggesting a convergent molecular mechanism at the level of these peculiar cells. We believe that increasing the body of knowledge in this specific area will help to define the molecular basis of developmental abnormalities and of diseases such as cancer.
References
Yen PM, Ando S, Feng X, Liu Y, Maruvada P, Xia X (2006) Thyroid hormone action at the cellular, genomic and target gene levels. Mol Cell Endocrinol 246(1–2):121–127
Tata JR (2006) Amphibian metamorphosis as a model for the developmental actions of thyroid hormone. Mol Cell Endocrinol 246(1–2):10–20
Fredric E, Wondisford M (2004) Lessons learned from TR-beta mutant mice. In: Beck-Peccoz P (ed) Syndromes of hormone resistance on the hypothalamic-pituitary-thyroid axis, Kluwer Academic Publishers, Uttar Pradesh, India, pp 109–118
Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S (2006) Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology 147(9):4036–4043
Refetoff S, Dumitrescu AM (2007) Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract Res Clin Endocrinol Metab 21(2):277–305
Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ (2004) Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364(9443):1435–1437
Visser TJ (2007) Thyroid hormone transporters. Horm Res 68(Suppl 5):28–30
Bianco AC, Kim BW (2006) Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest 116(10):2571–2579
Laudet V (1997) Evolution of the nuclear receptor superfamily: early diversification from an ancestral orphan receptor. J Mol Endocrinol 19(3):207–226
Koenig RJ, Lazar MA, Hodin RA, Brent GA, Larsen PR, Chin WW, Moore DD (1989) Inhibition of thyroid hormone action by a non-hormone binding c-erbA protein generated by alternative mRNA splicing. Nature 337(6208):659–661
Hartong R, Wang N, Kurokawa R, Lazar MA, Glass CK, Apriletti JW, Dillmann WH (1994) Delineation of three different thyroid hormone-response elements in promoter of rat sarcoplasmic reticulum Ca2 + ATPase gene. Demonstration that retinoid X receptor binds 5′ to thyroid hormone receptor in response element 1. J Biol Chem 269(17):13021–13029
Sirakov M, Plateroti M (2011) The thyroid hormones and their nuclear receptors in the gut: from developmental biology to cancer. Biochim Biophys Acta 1812(8):938–946
Sirakov M, Skah S, Nadjar J, Plateroti M (2013) Thyroid hormone’s action on progenitor/stem cell biology: new challenge for a classic hormone? Biochim Biophys Acta 1830(7):3917–3927
Davis PJ, Leonard JL, Davis FB (2008) Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol 29(2):211–218
Davis PJ, Davis FB, Mousa SA, Luidens MK, Lin HY (2011) Membrane receptor for thyroid hormone: physiologic and pharmacologic implications. Annu Rev Pharmacol Toxicol 51:99–115
Lazar MA (2003) Thyroid hormone action: a binding contract. J Clin Invest 112(4):497–499
Tata JR (2011) Looking for the mechanism of action of thyroid hormone. J Thyroid Res 2011:730630
Cheng SY, Leonard JL, Davis PJ (2010) Molecular aspects of thyroid hormone actions. Endocr Rev 31(2):139–170
Brent GA (2012) Mechanisms of thyroid hormone action. J Clin Invest 122(9):3035–3043
Pascual A, Aranda A (2013) Thyroid hormone receptors, cell growth and differentiation. Biochim Biophys Acta 1830(7):3908–3916
van der Flier LG, Clevers H (2009) Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71:241–260
Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H (2009) Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457(7229):608–611
Puzianowska-Kuznicka M, Pietrzak M, Turowska O, Nauman A (2006) Thyroid hormones and their receptors in the regulation of cell proliferation. Acta Biochim Pol 53(4):641–650
Su Y, Damjanovski S, Shi Y, Shi YB (1999) Molecular and cellular basis of tissue remodeling during amphibian metamorphosis. Histol Histopathol 14(1):175–183
Santisteban P, Bernal J (2005) Thyroid development and effect on the nervous system. Rev Endocr Metab Disord 6(3):217–228
Patel J, Landers K, Li H, Mortimer RH, Richard K (2011) Thyroid hormones and fetal neurological development. J Endocrinol 209(1):1–8
Mohan V, Sinha RA, Pathak A, Rastogi L, Kumar P, Pal A, Godbole MM (2012) Maternal thyroid hormone deficiency affects the fetal neocorticogenesis by reducing the proliferating pool, rate of neurogenesis and indirect neurogenesis. Exp Neurol 237(2):477–488
Chen C, Zhou Z, Zhong M, Zhang Y, Li M, Zhang L, Qu M, Yang J, Wang Y, Yu Z (2012) Thyroid hormone promotes neuronal differentiation of embryonic neural stem cells by inhibiting STAT3 signaling through TRalpha1. Stem Cells Dev 21(14):2667–2681
Lemkine GF, Raj A, Alfama G, Turque N, Hassani Z, Alegria-Prevot O, Samarut J, Levi G, Demeneix BA (2005) Adult neural stem cell cycling in vivo requires thyroid hormone and its alpha receptor. Faseb J 19(7):863–865
Lopez-Juarez A, Remaud S, Hassani Z, Jolivet P, Pierre SJ, Sontag T, Yoshikawa K, Price J, Morvan-Dubois G, Demeneix BA (2012) Thyroid hormone signaling acts as a neurogenic switch by repressing Sox2 in the adult neural stem cell niche. Cell Stem Cell 10(5):531–543
Kapoor R, Ghosh H, Nordstrom K, Vennstrom B, Vaidya VA (2011) Loss of thyroid hormone receptor beta is associated with increased progenitor proliferation and NeuroD-positive cell number in the adult hippocampus. Neurosci Lett 487(2):199–203
Kapoor R, Desouza LA, Nanavaty IN, Kernie SG, Vaidya VA (2012) Thyroid hormone accelerates the differentiation of adult hippocampal progenitors. J Neuroendocrinol 24(9):1259–1271
Tiede S, Bohm K, Meier N, Funk W, Paus R (2010) Endocrine controls of primary adult human stem cell biology: thyroid hormones stimulate keratin 15 expression, apoptosis, and differentiation in human hair follicle epithelial stem cells in situ and in vitro. Eur J Cell Biol 89(10):769–777
Lee YK, Ng KM, Chan YC, Lai WH, Au KW, Ho CY, Wong LY, Lau CP, Tse HF, Siu CW (2010) Triiodothyronine promotes cardiac differentiation and maturation of embryonic stem cells via the classical genomic pathway. Mol Endocrinol 24(9):1728–1736
Henning SJ, Rudbin DC, Shulman J (1994) Ontogeny of the intestinal mucosa. In: Johnson LR (ed) Physiology of the gastrointestinal tract, 3rd edn. Raven Press, New York, pp 571–601
Heimeier RA, Das B, Buchholz DR, Fiorentino M, Shi YB (2010) Studies on Xenopus laevis intestine reveal biological pathways underlying vertebrate gut adaptation from embryo to adult. Genome Biol 11(5):R55
Kress E, Rezza A, Nadjar J, Samarut J, Plateroti M (2009) The frizzled-related sFRP2 gene is a target of thyroid hormone receptor alpha1 and activates beta-catenin signaling in mouse intestine. J Biol Chem 284(2):1234–1241
Kress E, Skah S, Sirakov M, Nadjar J, Gadot N, Scoazec JY, Samarut J, Plateroti M (2010) Cooperation between the thyroid hormone receptor TRalpha1 and the WNT pathway in the induction of intestinal tumorigenesis. Gastroenterology 138(5):1863–1874
Randall D, Burggren W, French K (2002) Eckert Animal Physiology mechanisms and adaptation. Freeman and Company, New York
Gerbe F, Legraverend C, Jay P (2012) The intestinal epithelium tuft cells: specification and function. Cell Mol Life Sci 69(17):2907–2917
Stappenbeck TS, Wong MH, Saam JR, Mysorekar IU, Gordon JI (1998) Notes from some crypt watchers: regulation of renewal in the mouse intestinal epithelium. Curr Opin Cell Biol 10(6):702–709
Ishizuya-Oka A, Shi YB (2008) Thyroid hormone regulation of stem cell development during intestinal remodeling. Mol Cell Endocrinol 288(1–2):71–78
Sancho E, Batlle E, Clevers H (2004) Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol 20:695–723
Kress E, Samarut J, Plateroti M (2009) Thyroid hormones and the control of cell proliferation or cell differentiation: paradox or duality? Mol Cell Endocrinol 313(1–2):36–49
Buchholz DR, Heimeier RA, Das B, Washington T, Shi YB (2007) Pairing morphology with gene expression in thyroid hormone-induced intestinal remodeling and identification of a core set of TH-induced genes across tadpole tissues. Dev Biol 303(2):576–590
Barker N, Clevers H (2010) Lineage tracing in the intestinal epithelium. Curr Protoc Stem Cell Biol, Chp. 5:Unit5A 4
Cheng H, Leblond CP (1974) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat 141(4):461–479
Chwalinski S, Potten CS, Evans G (1988) Double labelling with bromodeoxyuridine and [3H]-thymidine of proliferative cells in small intestinal epithelium in steady state and after irradiation. Cell Tissue Kinet 21(5):317–329
Potten CS (1977) Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature 269(5628):518–521
Bjerknes M, Cheng H (1999) Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 116(1):7–14
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449(7165):1003–1007
Schepers AG, Vries R, van den Born M, van de Wetering M, Clevers H (2011) Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J 30(6):1104–1109
Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT (2011) Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA 108(1):179–184
Sangiorgi E, Capecchi MR (2008) Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40(7):915–920
Lessard J, Sauvageau G (2003) Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 423(6937):255–260
Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA (2011) Interconversion between intestinal stem cell populations in distinct niches. Science 334(6061):1420–1424
Cambuli FM, Rezza A, Nadjar J, Plateroti M (2013) Musashi1-Egfp Mice, a new tool for differential isolation of the intestinal stem cell populations. Stem Cells 31(10):5
Carlone DL, Breault DT (2012) Tales from the crypt: the expanding role of slow cycling intestinal stem cells. Cell Stem Cell 10(1):2–4
Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H (2010) Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143(1):134–144
Ishizuya-Oka A, Shi YB (2005) Molecular mechanisms for thyroid hormone-induced remodeling in the amphibian digestive tract: a model for studying organ regeneration. Dev Growth Differ 47(9):601–607
Hadj-Sahraoui N, Seugnet I, Ghorbel MT, Demeneix B (2000) Hypothyroidism prolongs mitotic activity in the post-natal mouse brain. Neurosci Lett 280(2):79–82
Cai L, Brown DD (2004) Expression of type II iodothyronine deiodinase marks the time that a tissue responds to thyroid hormone-induced metamorphosis in Xenopus laevis. Dev Biol 266(1):87–95
Bates JM, Germain DL, Galton VA (1999) Expression profiles of the three iodothyronine deiodinases, D1, D2, and D3, in the developing rat. Endocrinology 140(2):844–851
Sirakov M, Skah S, Lone IN, Nadjar J, Angelov D, Plateroti M (2012) Multi-level interactions between the nuclear receptor TRalpha1 and the WNT effectors beta-catenin/Tcf4 in the intestinal epithelium. PLoS One 7(4):e34162
Das B, Matsuda H, Fujimoto K, Sun G, Matsuura K, Shi YB (2010) Molecular and genetic studies suggest that thyroid hormone receptor is both necessary and sufficient to mediate the developmental effects of thyroid hormone. Gen Comp Endocrinol 168(2):174–180
Plateroti M, Chassande O, Fraichard A, Gauthier K, Freund JN, Samarut J, Kedinger M (1999) Involvement of T3Ralpha- and beta-receptor subtypes in mediation of T3 functions during postnatal murine intestinal development. Gastroenterology 116(6):1367–1378
Gauthier K, Chassande O, Plateroti M, Roux JP, Legrand C, Pain B, Rousset B, Weiss R, Trouillas J, Samarut J (1999) Different functions for the thyroid hormone receptors TRalpha and TRbeta in the control of thyroid hormone production and post-natal development. EMBO J 18(3):623–631
Hodin RA, Meng S, Chamberlain SM (1994) Thyroid hormone responsiveness is developmentally regulated in the rat small intestine: a possible role for the alpha-2 receptor variant. Endocrinology 135(2):564–568
Plateroti M, Kress E, Mori JI, Samarut J (2006) Thyroid hormone receptor alpha1 directly controls transcription of the beta-catenin gene in intestinal epithelial cells. Mol Cell Biol 26(8):3204–3214
Gauthier K, Plateroti M, Harvey CB, Williams GR, Weiss RE, Refetoff S, Willott JF, Sundin V, Roux JP, Malaval L, Hara M, Samarut J, Chassande O (2001) Genetic analysis reveals different functions for the products of the thyroid hormone receptor alpha locus. Mol Cell Biol 21(14):4748–4760
Jumarie C, Malo C (1994) Alkaline phosphatase and peptidase activities in Caco-2 cells: differential response to triiodothyronine. Vitro Cell Dev Biol Anim 30A(11):753–760
Matosin-Matekalo M, Mesonero JE, Laroche TJ, Lacasa M, Brot-Laroche E (1999) Glucose and thyroid hormone co-regulate the expression of the intestinal fructose transporter GLUT5. Biochem J 339(Pt 2):233–239
Bochukova E, Schoenmakers N, Agostini M, Schoenmakers E, Rajanayagam O, Keogh JM, Henning E, Reinemund J, Gevers E, Sarri M, Downes K, Offiah A, Albanese A, Halsall D, Schwabe JW, Bain M, Lindley K, Muntoni F, Khadem FV, Dattani M, Farooqi IS, Gurnell M, Chatterjee K (2012) A mutation in the thyroid hormone receptor alpha gene. N Engl J Med 366(3):243–249
van Mullem A, van Heerebeek R, Chrysis D, Visser E, Medici M, Andrikoula M, Tsatsoulis A, Peeters R, Visser TJ (2012) Clinical phenotype and mutant TRalpha1. N Engl J Med 366(15):1451–1453
Furness JB (2012) The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9(5):286–294
Fraichard A, Chassande O, Plateroti M, Roux JP, Trouillas J, Dehay C, Legrand C, Gauthier K, Kedinger M, Malaval L, Rousset B, Samarut J (1997) The T3R alpha gene encoding a thyroid hormone receptor is essential for post-natal development and thyroid hormone production. EMBO J 16(14):4412–4420
Plateroti M, Gauthier K, Domon-Dell C, Freund JN, Samarut J, Chassande O (2001) Functional interference between thyroid hormone receptor alpha (TRalpha) and natural truncated TRDeltaalpha isoforms in the control of intestine development. Mol Cell Biol 21(14):4761–4772
Wikstrom L, Johansson C, Salto C, Barlow C, Campos BA, Baas F, Forrest D, Thoren P, Vennstrom B (1998) Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor alpha 1. EMBO J 17(2):455–461
Chassande O, Fraichard A, Gauthier K, Flamant F, Legrand C, Savatier P, Laudet V, Samarut J (1997) Identification of transcripts initiated from an internal promoter in the c-erbA alpha locus that encode inhibitors of retinoic acid receptor-alpha and triiodothyronine receptor activities. Mol Endocrinol 11(9):1278–1290
Gonzalez-Sancho JM, Garcia V, Bonilla F, Munoz A (2003) Thyroid hormone receptors/THR genes in human cancer. Cancer Lett 192(2):121–132
Horkko TT, Tuppurainen K, George SM, Jernvall P, Karttunen TJ, Makinen MJ (2006) Thyroid hormone receptor beta1 in normal colon and colorectal cancer-association with differentiation, polypoid growth type and K-ras mutations. Int J Cancer 118(7):1653–1659
Cheng SY (2003) Thyroid hormone receptor mutations in cancer. Mol Cell Endocrinol 213(1):23–30
Rose DP, Davis TE (1981) Plasma thyronine levels in carcinoma of the breast and colon. Arch Intern Med 141(9):1161–1164
Wang CS, Lin KH, Hsu YC (2002) Alterations of thyroid hormone receptor alpha gene: frequency and association with Nm23 protein expression and metastasis in gastric cancer. Cancer Lett 175(2):121–127
Iishi H, Tatsuta M, Baba M, Okuda S, Taniguchi H (1992) Enhancement by thyroxine of experimental carcinogenesis induced in rat colon by azoxymethane. Int J Cancer 50(6):974–976
Lin KH, Zhu XG, Hsu HC, Chen SL, Shieh HY, Chen ST, McPhie P, Cheng SY (1997) Dominant negative activity of mutant thyroid hormone alpha1 receptors from patients with hepatocellular carcinoma. Endocrinology 138(12):5308–5315
Lin KH, Zhu XG, Shieh HY, Hsu HC, Chen ST, McPhie P, Cheng SY (1996) Identification of naturally occurring dominant negative mutants of thyroid hormone alpha 1 and beta 1 receptors in a human hepatocellular carcinoma cell line. Endocrinology 137(10):4073–4081
Brown AR, Simmen RC, Simmen FA (2013) The role of thyroid hormone signaling in the prevention of digestive system cancers. Int J Mol Sci 14(8):16240–16257
Wu SM, Huang YH, Yeh CT, Tsai MM, Liao CH, Cheng WL, Chen WJ, Lin KH (2011) Cathepsin H regulated by the thyroid hormone receptors associate with tumor invasion in human hepatoma cells. Oncogene 30(17):2057–2069
Dentice M, Luongo C, Huang S, Ambrosio R, Elefante A, Mirebeau-Prunier D, Zavacki AM, Fenzi G, Grachtchouk M, Hutchin M, Dlugosz AA, Bianco AC, Missero C, Larsen PR, Salvatore D (2007) Sonic hedgehog-induced type 3 deiodinase blocks thyroid hormone action enhancing proliferation of normal and malignant keratinocytes. Proc Natl Acad Sci USA 104(36):14466–14471
Dentice M, Luongo C, Ambrosio R, Sibilio A, Casillo A, Iaccarino A, Troncone G, Fenzi G, Larsen PR, Salvatore D (2010) beta-Catenin regulates deiodinase levels and thyroid hormone signaling in colon cancer cells. Gastroenterology 143(4):1037–1047
Modica S, Gofflot F, Murzilli S, D’Orazio A, Salvatore L, Pellegrini F, Nicolucci A, Tognoni G, Copetti M, Valanzano R, Veschi S, Mariani-Costantini R, Palasciano G, Schoonjans K, Auwerx J, Moschetta A (2009) The intestinal nuclear receptor signature with epithelial localization patterns and expression modulation in tumors. Gastroenterology 138(2):636–648
Markowitz S, Haut M, Stellato T, Gerbic C, Molkentin K (1989) Expression of the ErbA-beta class of thyroid hormone receptors is selectively lost in human colon carcinoma. J Clin Invest 84(5):1683–1687
Thompson JS, Saxena SK, Sharp JG (2000) Regulation of intestinal regeneration: new insights. Microsc Res Tech 51(2):129–137
Koch S, Nusrat A (2012) The life and death of epithelia during inflammation: lessons learned from the gut. Annu Rev Pathol 7:35–60
Kress E, Rezza A, Nadjar J, Samarut J, Plateroti M (2008) The thyroid hormone receptor-alpha (TRalpha) gene encoding TRalpha1 controls deoxyribonucleic acid damage-induced tissue repair. Mol Endocrinol 22(1):47–55
Park HS, Goodlad RA, Wright NA (1995) Crypt fission in the small intestine and colon. A mechanism for the emergence of G6PD locus-mutated crypts after treatment with mutagens. Am J Pathol 147(5):1416–1427
Snippert HJ, Schepers AG, van Es JH, Simons BD, Clevers H (2014) Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep 15(1):62–69
Ishizuya-Oka A, Hasebe T, Buchholz DR, Kajita M, Fu L, Shi YB (2009) Origin of the adult intestinal stem cells induced by thyroid hormone in Xenopus laevis. FASEB J 23(8):2568–2575
Shi YB, Hasebe T, Fu L, Fujimoto K, Ishizuya-Oka A (2011) The development of the adult intestinal stem cells: insights from studies on thyroid hormone-dependent amphibian metamorphosis. Cell Biosci 1(1):30
Rezza A, Skah S, Roche C, Nadjar J, Samarut J, Plateroti M (2010) The overexpression of the putative gut stem cell marker Musashi-1 induces tumorigenesis through Wnt and Notch activation. J Cell Sci 123(Pt 19):3256–3265
van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G, Pals S, Eilers M, Medema R, Clevers H (2002) The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111(2):241–250
Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127(3):469–480
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17(1):9–26
Liu T, Lee YN, Malbon CC, Wang HY (2002) Activation of the beta-catenin/Lef-Tcf pathway is obligate for formation of primitive endoderm by mouse F9 totipotent teratocarcinoma cells in response to retinoic acid. J Biol Chem 277(34):30887–30891
Fre S, Pallavi SK, Huyghe M, Lae M, Janssen KP, Robine S, Artavanis-Tsakonas S, Louvard D (2009) Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci USA 106(15):6309–6314
Kawano Y, Kypta R (2003) Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116(Pt 13):2627–2634
Ord W (1878) On myxoedema, a term proposed to be applied to an essential condition in the “cretinoid” affection occasionally observed in middle-aged women. Med Chir Trans (Lond) 61:57
Horsley V (1885) The brown lectures on pathology. Br Med J 1:111–115
Pitt-Rivers R (1963) Biochemistry and physiology of thyroid hormones. NY State J Med 63:43–49
Ross DS (1994) Hyperthyroidism, thyroid hormone therapy, and bone. Thyroid 4(3):319–326
Acknowledgments
The work in the Plateroti lab is supported by the Institut National pour le Cancer (Grant INCA-2009-175), the ANR Blanc ThRaSt (ANR-11-BSV2-019) and by the Département du Rhône de la Ligue contre le cancer (Grant No. 88283).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sirakov, M., Kress, E., Nadjar, J. et al. Thyroid hormones and their nuclear receptors: new players in intestinal epithelium stem cell biology?. Cell. Mol. Life Sci. 71, 2897–2907 (2014). https://doi.org/10.1007/s00018-014-1586-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1586-3