Abstract
Among the dementias, Alzheimer’s disease (AD) is the most commonly diagnosed, but there are still no effective drugs available for its treatment. It has been suggested that metallothionein-3 (MT-3) could be somehow involved in the etiology of AD, and in fact very promising results have been found in in vitro studies, but the role of MT-3 in vivo needs further analysis. In this study, we analyzed the role of MT-3 in a mouse model of AD, Tg2576 mice, which overexpress human Amyloid Precursor Protein (hAPP) with the Swedish mutation. MT-3 deficiency partially rescued the APP-induced mortality of females, and mildly affected APP-induced changes in behavior assessed in the hole-board and plus-maze tests in a gender-dependent manner. Amyloid plaque burden and/or hAPP expression were decreased in the cortex and hippocampus of MT-3-deficient females. Interestingly, exogenously administered Zn7MT-3 increased soluble Aβ40 and Aβ42 and amyloid plaques and gliosis, particularly in the cortex, and changed several behavioral traits (increased deambulation and exploration and decreased anxiety). These results highlight that the control of the endogenous production and/or action of MT-3 could represent a powerful therapeutic target in AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reflecting an ageing population, for most societies dementia is becoming a major health burden, representing high economic and quality-of-life costs. Alzheimer disease (AD) is far and away the most frequently diagnosed dementia and its pathognomonic signs include the presence of extracellular amyloid plaques (aggregated β-amyloid (Aβ) peptide) and intracellular neurofibrillary tangles (hyperphosphorylated tau protein deposits), which are commonly associated with marked oxidative stress and inflammation as well as synaptic loss and neuronal death of the affected brain regions [53]. The Aβ peptide, in its many possible forms (monomers, oligomers, fibrils, amyloid plaques, etc.), is considered the main causative agent of AD pathology, and results from the sequential proteolytic processing of the amyloid precursor protein (APP) by β- and γ-secretases through the “amyloidogenic pathway” [27].
It is widely accepted that Aβ, particularly in aggregated or fibrillar forms, triggers pro-inflammatory reactions of microglia and astrocytes, which usually surround the amyloid deposits [42]. Moreover, both Aβ accumulation and Aβ-triggered inflammatory response have been found to enhance the generation of free radicals and oxidative damage and hence the subsequent increase in the levels of lipidic, protein, and DNA oxidative stress markers and antioxidant enzymes present in AD brains and animal models of the disease [40, 92]. While an essential role for Aβ in the pathogenesis of AD is unquestionable, particularly given the genetic underpinnings of a subset of cases, experimental evidence suggests that other factors, such as bio-metals, trigger Aβ pathogenicity and downstream AD pathology [1, 12]. Zn, Cu, and Fe binding to Aβ results in its aggregation and precipitation and accordingly their levels have been found to be consistently increased within Aβ plaques [29, 91]. It seems plausible that a number of metal-binding proteins could have a role in AD pathophysiology, and indeed the metallothionein family may have a prominent role according to previous studies.
Metallothioneins (MTs) are low-molecular-weight (6–7 kDa), cysteine-rich proteins with high metal content (Zn(II), Cu(I)), which are subdivided into four subfamilies (MT-1–MT-4) in mammals; MT-1 and MT-2 are present in most tissues including the central nervous system (CNS) and are highly inducible by Zn, inflammation, and oxidative stress, whereas MT-3 and MT-4 are found primarily in the CNS and the stratified squamous epithelia, respectively [66, 100, 103, 104]. MT-3, originally named Growth Inhibitory Factor or GIF, was first identified in the brain by Uchida and coworkers and was proposed to be a major factor underlying Alzheimer disease; they found decreased levels of MT-3 in AD brains and demonstrated that MT-3 could inhibit neuronal growth and survival in vitro in the presence of AD brain extracts [98]. Whether MT-3 levels are decreased in AD is still debated [2, 9, 16, 21, 36, 43, 65, 96, 102, 105], whereas an inhibitory role of MT-3 on neuronal survival seems a consistent finding in the presence of AD brain extracts [41, 86, 89], and it may be the case with extracts from other species as well [11]. This has prompted speculation that MT-3 could interact with partner proteins to elicit its survival and/or other effects, and indeed a number of partners have been suggested [31, 32, 56, 59, 74]. In contrast, in the absence of brain extracts in culture, MT-3 is in fact a neuroprotective factor [25, 26, 36, 73, 76, 97]. On the other hand, MT-3 has prominent inhibitory effects on neuronal sprouting in vitro [26, 97] and in vivo [19].
MT-3 is mainly a neuronal protein but also an astrocytic protein in some conditions [68]. Its concentration is particularly high in the presynaptic vesicles of zinc-enriched neurons where it could have a role in synaptic Zn(II) regulation [22, 34, 68]. It is secretable [97], and in vitro studies on cultured cortical neurons demonstrate that it alleviates the neurotoxic effects of Aβ probably by a metal swap between Zn7MT-3 and soluble and aggregated Cu(II)-Aβ1–40 leading to suppression of reactive oxygen species (ROS) caused by the redox cycling of Cu(II)-Aβ [51, 73, 80]. Yet, MT-3 in fact can cause Aβ aggregation upon cysteine oxidation in Zn7MT-3 and subsequent Zn2+ release, moreover causing the formation of amyloid-type fibrils in contrast to amorphous Aβ40–42 aggregates formed by the addition of Zn2+ [30, 94]. Thus, MT-3 appears capable of modulating the metal-dependent aggregation pathway of Aβ, the metal-catalyzed radical production, and the final nature of the Aβ aggregates.
In summary, whether MT-3 will be a beneficial factor in AD neuropathology in vivo is unclear. To give insight in this matter, we have produced double transgenic mice presenting AD pathology (Tg2576 mouse AD model) in addition to MT-3 deficiency, and moreover we have studied the effect of chronic injection of Zn7MT-3 to Tg2576 mice. The results strongly suggest that attention must be paid to the role MT-3 in the human disease.
Materials and methods
Animals
The parental strains used in this study were mice deficient for MT-3 [34], kindly provided by Dr. Richard Palmiter, and the Tg2576 AD mouse model which expresses the human APP695 harboring the Swedish K670N/M671L mutations under the control of the hamster prion protein promoter [47] (Taconic Europe).
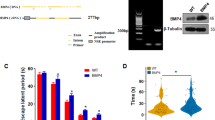
A double crossing strategy was designed to produce the desired transgenic mice in the same genetic background. In the first crossing, Tg2576 mice were crossed with MT3KO mice. From the resultant offspring (APP−/−/MT3+/− and APP+/−/MT3+/−), the APP+ were selected and backcrossed again with MT3KO mice to obtain the four genotypes that were used for the experiment: WT (APP−/−/MT3+/−), MT3KO (APP−/−/MT3−/−), APPWT (APP+/−/MT3+/−) and APPMT3KO (APP+/−/MT3−/−); the total number of mice per group engaged at weaning was 60, 81, 85, and 77, respectively (Fig. 1c). These initial large groups were needed because significant mortality is caused by the APP transgene. Depending on the behavioral, histological, or molecular analysis to be carried out and of the age of the animals, the number of mice per group differs. Behavioral analysis of young mice was carried out in a reduced single set of mice born only a few days apart to maximize homogeneity; those of old age were carried out in several separate sets of mice (since they were born over several months apart) keeping strictly the same environmental conditions, normally with all surviving mice, and then pooled. Genotype was determined by PCR (APP primers: 5′-gtg gat aac ccc tcc ccc agc cta gac ca-3′¸ 5′-ctg acc act cga cca ggt tct ggg t -3′ and 5′-tgg tgg tag ttg ggg tca gc -3′; MT-3 primers: 5′-cct agc acc cac cca aag agc tg-3′, 5′-ggt cct cac tgg cag cag ctg ca -3′ and 5′-ggc tct atg gct tat gag gcg ga -3′).
MT-3 deficiency reduces APP-induced lethality in females. Survival (a) and body weight (b) of 155 females (left) and 148 males (right) was followed-up from weaning to killing. Mice carrying hAPP transgene showed increased mortality rates and lower body weights than their controls as revealed by Kaplan–Meier and GEE tests, respectively. MT-3 deficiency clearly tended to diminish hAPP-associated mortality in females (p = 0.054), however, it had no effect on females’ body weight or in males survival and body weight. Chronic Zn7MT-3 injection did not significantly affect any of these parameters either (a, b). Filled diamond at least ≤0.005 vs. APP−. c χ2 analysis of the offspring genotyped at weaning revealed a normal ratio for the different genotypes
After weaning at 3 weeks of age, the animals were housed with free access to food and water in a 12-h dark-light cycle under constant temperature. All mouse care and experimental procedures were approved by the Ethics Committee in Human and Animal Experimentation from the Autonomous University of Barcelona. Body weight and mortality were monitored regularly from weaning until killing at approximately 14 months of age.
Complementing the experiment, a group of ten APPWT females was chronically administered with Zn7MT-3. Mice were injected subcutaneously once a week for 19 weeks (from 44 weeks of age until killing) with a dose of 54 μg/animal/week. Behavioral tests, killing, and the rest of the analysis were performed in parallel with the rest of the cohort. We did not include a group of mice injected with vehicle since the amount of mice engaged in the experiment did not recommend further splitting groups. It is unlikely though that a s.c. injection per week could have any significant effect on APP processing and plaques, considering that all the mice from weaning were subjected to substantial handling procedures (weighing, behavioral analysis, etc.) that were as stressful as the s.c. injection procedure might be.
Animals were killed by decapitation and the brain quickly removed on ice. The right hemisphere was dissected into cortex, hippocampus, cerebellum, and rest of the brain, frozen with liquid nitrogen, and stored at –80 °C. The left hemisphere was immersed in 4 % paraformaldehyde (PFA) and stored in 70 % ethanol at 4 °C.
Behavioral characterization
Mice were tested in the Hole Board (HB) and the Plus Maze (PM) paradigms at 4–5 and 14 months of age to assess activity, exploratory behavior, and anxiety as previously described [66]. Gender differences in emotional behavior have been widely described, and, particularly in the PM paradigm, it is generally accepted that females’ behavior is mainly driven by activity, whereas male behavior is more characterized by anxiety [38], thus making it imperative to separate the behavioral assessment of both genders.
Sensorimotor reflexes were evaluated at 11 and 14 months of age to assess the balance, reflexes, and strength of mice with a battery of tests. Briefly, to assess balance and general motor function, a horizontal flat rod (easy task) and a cylindrical rod (difficult task) (1 cm wide/diameter × 50 cm long and 40 cm from floor) were used. In both tests, mice were placed in the center of the rod for 20 s and the distance covered as well as the latency to fall were measured. Each animal was tested twice in each rod with 10-s inter-trial period. The forepaw grip capacity and strength were assessed in the coat hanger test that consists of a horizontal bar (1.8 mm diameter × 40 cm length) flanked by two diagonal bars (1.8 mm diameter × 22 cm length and inclined 35°) made of steel and placed at a height of 70 cm from the cushion-covered floor. For the first two trials, mice were placed in the center of the horizontal bar for 5 s and the ability of the animal to remain suspended by the forepaws was measured (prehensile ability test). For the last (3rd) trial, mice were placed in the center of the horizontal bar for 60 s, the distance covered and the latency to fall were measured (strength test).
Finally, spatial memory and learning were evaluated at 13 months of age in a subgroup of 25 females using the Morris Water Maze (MWM) as described in the companion paper (Manso et al., this volume).
Immunohistochemistry (IHC)
Fixed brains were paraffin-embedded and cut sagittally in 8-μm-thick sections for assessing the amyloid plaque load (primary antibody:4G8-Aβ17–24 1:5,000, Signet; secondary antibody:anti-mouse IgG biotin conjugate 1:400, Sigma) and astrogliosis (primary antibody:anti-GFAP 1:900, DakoCytomation; secondary antibody:biotinylated anti-rabbit IgG 1:300, Vector Laboratories) surrounding the plaques in cortex and hippocampus using standard procedures. For quantification, three non-consecutive sections per mouse were used. Photographs of cortex and hippocampus were taken with a Nikon Eclipse 400 microscope (4×). Images were transformed to a gray scale (8 bits) and analyzed using Scion Image software that computes, after the selection of a threshold, the area (number of positive pixels) and mean grey value (range from 1-white- to 256-black-) of the current selection. These measures were used to calculate the percentage of total area occupied by specific signal (area occupied by positive pixels/total area of the selection) × 100 and the volume in arbitrary units (AU) (area × mean grey value).
Protein extraction
Frozen cortex samples were weighed with a precision scale and fractionated using the high-fidelity extraction procedure described by Lesné et al. [64] that separates proteins in known cellular compartments (i.e., soluble/extracellular, cytoplasmic, membrane-associated, and insoluble-enriched protein fractions; the latter fraction was not used due to methodological problems) and allows quantifying and comparing four independent pools (fractions A–D) of transgene-derived Aβ species apart from the total cortex homogenates. Frozen hippocampus samples were weighed with a precision scale and homogenized by sonication in 50 mM Tris–HCl (pH 7.6), 0.01 % NP-40, 150 mM NaCl, 2 mM EDTA, 3 % SDS, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 % deoxycholate and protease inhibitor cocktail (Sigma-Aldrich).
All homogenates and fractions were stored at −80 °C. Protein concentration was estimated with the BCA protein assay according to the manufacturer’s instructions (Pierce).
Recombinant human MT-3 expression and purification
A pet-3d (Novagen) plasmid encoding for human MT-3 sequence was used for recombinant protein expression. Expression in Escherichia coli strain BL21(DE3)pLys and purification was performed as described previously [37]. The purity and correctness of the masses of the expressed proteins were confirmed by SDS-PAGE upon cysteine modification with monobromobimane [72] and ESI–MS analysis, respectively. The fully Zn(II)-loaded form Zn7MT-3 was prepared by metal reconstitution [99]. Zinc-to-protein ratios were determined by measuring protein concentration photometrically in 0.1 M HCl (ε220 = 53,000 M−1 cm−1) and metal content by flame atomic absorption spectroscopy (SpectrAA-110, Varian Inc.). Cysteine-to-protein ratios were determined via photometric sulfhydryl groups (CysSH) quantification upon their reaction with 2,2-dithiopyridine in 0.2 M sodium acetate/1 mM EDTA (pH 4) using ε343 of 7,600 M−1 cm−1 [79] and that of zinc by flame atomic absorption. In all cases, a zinc-to-protein ratio of 7.0 ± 0.3 and a CysSH-to-protein ratio of 20 ± 2 (MT-3 contains a total of 20 Cys residues) were obtained.
Western blotting
APP and APP fragments were assessed in cortex and hippocampus homogenates by WB using the 6E10 antibody (Aβ1–16 1:2,000, Signet) essentially as described elsewhere [64]. Briefly, samples were run on NuPage NOVEX Invitrogen bis–Tris 4–12 % 20-well gels at 130–160 V for 1 h. Membranes were blocked and finally developed with ECL reagent (Amersham). Images were captured and quantified using Bio-Rad Laboratories’ software QuantityOne ChemiDoc.
Only the specific bands that were present exclusively in the APP+ genotypes were quantified, i.e., APP/soluble APPα (≈90 kDa, 6E10 recognizes both of them and gel resolution is insufficient to obtain separated bands), a 14-kDa band that is considered by some authors as an oligomeric form of Aβ (trimer) [64], c-terminal fragment (CTF-)β (≈12 kDa, this band was also recognized using an anti-C-ter specific antibody; data not shown) and Aβ (≈4 kDa). To minimize loading differences, all the bands analyzed were corrected by β-actin.
Enzyme-linked immunosorbent assay (ELISA)
Determination of Aβ1–40 and Aβ1–42 content in cortex and hippocampus homogenates was done using a Sandwich ELISA commercial kit (Invitrogen) according to the manufacturer’s instructions. Absorbance at 450 nm was measured with Labsystems Multiskan Bichromatic microtiter plate reader.
Inductively coupled plasma-mass spectrometry (ICP-MS)
Determination of Cu, Mn, Fe, and Zn in the cortex of 14-month-old mice was done at the Servei d’Anàlisi Químic of the UAB. Briefly, samples were wet digested with HNO3 at 60 °C and further diluted into HNO3 1 %. ICP-MS was performed using an Agilent 7500ce spectrometer and samples were introduced using a glass nebulizer at a flow of 0.85 l/min.
Statistical analysis
Data was analyzed using the Statistical Package for Social Sciences (SPSS) version 17.0. Males and females were analyzed separately and only exceptionally combined gender analysis was done. Survival was analyzed using the Kaplan–Meier survival test, using genotype as a factor with four levels (WT, MT3KO, APPWT, APPMT3KO). The rest of the data was analyzed using the generalized linear model (GLZ) and generalized estimated equations (GEE) for repeated measures (i.e., body weight or acquisition in the MWM). In both cases, APP and MT-3 were used as factors with two levels each: APP+ and APP− for APP and MT3WT and MT3KO for MT-3. In addition, in GEE analysis, “time” was used as a within-subject factor. In parameters such as amyloidosis, which is not present in APP− genotypes, only APP+ genotypes were analyzed, using MT-3 as grouping factor. The Zn7MT-3 chronic administration experiment was analyzed using GLZ and Zn7MT-3 injection as main factor. Very occasionally, some animals were excluded from statistical analysis because its value was extreme according to SPSS criteria. Statistical significance was defined as p ≤ 0.05 and marginal significance as p ≤ 0.1.
Results
MT-3 deficiency rescues APP-induced mortality but not body weight loss in a gender-dependent manner
According to the literature, the expression of hAPP transgene increases mortality in both males and females, with the former being more conspicuously affected (≈60–70 % and ≈25 %, respectively) (Fig. 1a). Consistent with previous studies, APP+ genotypes had lower body weights than their APP− controls throughout the period studied (Fig. 1b). No significant effect due to MT-3 absence was observed neither in males’ survival (Fig. 1a) nor in both genders’ body weight (Fig. 1b); however, MT-3 deficiency clearly tended to prevent hAPP-induced mortality in females (p = 0.054) (Fig. 1a). Weekly injection of Zn7MT-3 protein to a subgroup of APPWT females from week 44 until killing did not significantly affect survival or body weight (Fig. 1a, b).
Analysis of the Mendelian distribution of the pups that were born was as expected, and there were no underrepresented genotypes (Fig. 1c), hence ruling out the occurrence of hAPP-induced intrauterine or perinatal (before weaning) mortality in this crossing.
Tg2576 behavioral phenotype is influenced by MT-3 deficiency and chronic Zn7MT-3 injection
Activity, exploratory behavior, and anxiety
As previously described, APP+ genotypes from both sexes and ages (4–5 and 14 months of age) clearly tended to be hypoactive compared to controls as suggested by the decreased number of rearings (Fig. 2c) and deambulations (Fig. 2d) in the HB and the lower number of entries in the closed arms of the PM (Fig. 3b). However, high variability precluded statistical significance in some of these parameters and only the number of rearings (Fig. 2c) and the number of entries in the closed arms (Fig. 3b) of young males and old females and the number of deambulations (Fig. 2d) of old females reached significance.
Hole-board test (HB) at 4–5 and 14 months of age. Exploratory behavior (a, b) and activity (c, d) were evaluated with the HB at 4–5 (young) and 14 (old) months of age. The number (a) and time (b) of head dipping (HD) were increased in both genders of APP+ mice, indicating an increased exploratory behavior of APP+ mice compared to controls. MT-3 absence in APP− decreased young males’ exploratory behavior and increased that of old females, while interestingly in young APP+ females MT-3 deficiency partly prevented from the hAPP-induced increases in exploratory activity (a, b). Chronic injection of Zn7MT-3 significantly increased exploratory activity (a, b). The number of rearings (c) and deambulations (d) tended to be decreased in APP+ genotypes from both sexes and at both ages, but MT-3 deficiency had no effect on mice activity. Chronic Zn7MT-3 injection significantly increased vertical and horizontal activity of APPWT females (c, d). Data represents mean ± SEM (n = 6–16 and 9–27 for young and old mice, respectively), and they were analyzed using GLZ with hAPP and MT-3 deficiency or Zn7MT-3 injection as factors.♦ p ≤ 0.05 vs. APP−;⋆ p ≤ 0.05 vs. MT3WT;♠ p ≤ 0.05 significant interaction between both factors;▼ p ≤ 0.01 vs. APPWT
Plus-Maze test (PM) at 4–5 and 14 months of age. The number of entries (a) and time spent (c) in the open arms (OA) of the PM, together with the percentage of entries [(nº entries OA/nº entries OA + nº entries closed arms, CA) × 100] (e) and the percentage of time spent in the OA [(time spent OA/time spent OA + time spent CA) × 100] (f) of the PM were increased in APP+ genotypes, specially at young ages evidencing its less anxious phenotype. MT-3 absence tended to increase anxiety of young females (c, f) and decreased it in young males (a). Interestingly, in old APP+ mice, MT-3 absence significantly prevented from the decreased anxiety levels observed in APPWT mice (a, c–f). Chronic Zn7MT-3 injection significantly decreased anxiety levels compared to APPWT females (a, c–f). The number of entries in the CA (b) was used as an activity measure; in line with HB results, APP+ mice tended to be hypoactive, and neither MT-3 absence nor Zn7MT-3 chronic injection affected this parameter. Data represents mean ± SEM (n = 6–16 and 9–27 for young and old mice, respectively), and they were analyzed using GLZ with hAPP and MT-3 deficiency or Zn7MT-3 injection as factors. ♦ p ≤ 0.05 vs. APP−; ⋆ p ≤ 0.05 vs. MT3WT; ♠ p ≤ 0.05 significant interaction between both factors; ▼ p ≤ 0.01 vs. APPWT
Regarding exploratory activity (number and time of head dipping in the HB), it was consistently increased in APP+ genotypes at both ages tested (Fig. 2a, b), although the effect, at least in young mice, was more obvious in APP+ females.
As expected, anxiety levels of APP+ genotypes were decreased compared to controls as evidenced by the increased number of entries (Fig. 3a), time spent (Fig. 3c), percentage of entries (Fig. 3e), and percentage of time spent (Fig. 3f) in the open arms of the maze. However, this effect was only clearly noticeable in young mice and tended to disappear in most of the parameters assessed in the old ones, perhaps due to the advanced age of testing.
MT-3 effects on behavior were complex and gender- and genotype-dependent. Thus, while MT-3 deficiency in APP− mice had no effect on activity (Figs. 2c, d, 3b), it tended to decrease and increase exploratory behavior of males and females, respectively (Fig. 2a, b). Gender also affected anxiety levels of young MT-3-deficient mice in a inverse manner, with females more anxious and the opposite for males. In old APP− mice, MT-3 deficiency showed similar effects for females but not for males (Fig. 3).
A different pattern emerged in APP+ mice, with some aspects of the behavioral phenotype significantly reversed by the absence of MT-3. For instance, MT-3 deficiency decreased the exploratory activity of young APP+ females (Fig. 2a, b), increased the activity in the HB (Fig. 2d), and diminished the anxiety levels of old males and females (Fig. 3a, c, e, f).
On the other hand, chronic injection of Zn7MT-3 into APP+ females significantly increased their activity in the HB (Fig. 2c, d) although not in the PM (Fig. 3b), increased their exploratory activity (Fig. 2a, b) and decreased their anxiety levels (Fig. 3a, c, d, e, f) compared to age-matched APPWT females.
Sensorimotor reflexes
Motor coordination and strength were evaluated in 11- and 14-month-old mice using a battery of three tests. At 11 months of age, the sensorimotor reflexes (balance, prehensile reflex, and strength) of mice from both genders were not affected either by hAPP transgene presence or by MT-3 absence (Fig. 4); however, MT-3 null males covered significantly less distance during the strength test compared to controls (not shown).
Sensorimotor reflexes at 11 and 14 months of age. The motor coordination (balance, prehensile reflex) and strength of mice from both sexes at 11 (11 mo) and 14 (14 mo) months of age was assessed using a battery of tests: the horizontal flat rod (a), the horizontal cylindrical rod (b), and the coat hanger test (c, d). In all tests, both the distance covered during the test and the latency to fall were measured but only the latter is shown. When the balance of the mice was tested in a flat rod (easy task) (a) all animals performed almost perfectly and no differences between genotypes could be observed at any age in any gender. When tested in the cylindrical rod (difficult task) (b), 11-mo. mice from both sexes showed no differences between genotypes. With further aging, mice balance tended to worsen and latencies to fall decreased. Noteworthy, the increased latency to fall observed in APPWT females compared to controls was prevented by MT-3 deficiency. On the other hand, Zn7MT-3 chronic injection significantly decreased the latency to fall. In the prehensile (c) and the strength test (d), no differences between genotypes could be observed in the 11-mo. mice, 14-mo. males, and Zn7MT-3-injected females; however, in 14-mo. females, MT-3 absence significantly increased the latency to fall. Data represents mean ± SEM (n = 5–15 and 10–38 for 11-mo. and 14-mo. mice, respectively), and they were analyzed using GLZ with hAPP and MT-3 deficiency or Zn7MT-3 injection as factors. ♦ p ≤ 0.05 vs. APP−; ⋆ p ≤ 0.05 vs. MT3WT; ♠ p ≤ 0.05 significant interaction between both factors; ▼ p ≤ 0.02 vs. APPWT
With further aging, the sensorimotor abilities of all genotypes tended to worsen, especially at the horizontal cylindrical rod (Fig. 4b) and the prehensile (Fig. 4c) and strength tests (Fig. 4d), although no changes could be observed in easier tasks such as the horizontal flat rod (Fig. 4a). At 14 months of age, MT-3-deficient females showed higher latencies to fall both in the prehensile test (Fig. 4c) and the strength test (Fig. 4d) than their age-matched controls. This trend was also apparent in the latency to fall from the cylindrical rod of APP− females (Fig. 4b), but noteworthy in APP+ females MT-3 deficiency decreased the latency to fall, and the same tendency was observed in males. No significant differences between males’ genotypes were observed in any of the other parameters measured (Fig. 4).
Chronic injection of Zn7MT-3 significantly increased the distance covered during the flat rod equilibrium test (data not shown) and decreased the latency to fall from the cylindrical rod (Fig. 4b) compared to controls, with no differences in the rest of variables measured (Fig. 4).
Spatial learning and memory
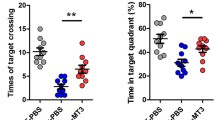
At 13 months of age, a cohort of 25 females was tested in the MWM. In the first phase of the test, the hidden platform test, all genotypes learned how to locate the submerged platform using external cues as evidenced by the decreased escape latencies through the training period (p < 0.001) (Fig. 5a), the increased percentage of time spent in the target quadrant (TQ) (Fig. 5c) and the increased number of target crossings (Fig. 5e). Nevertheless, no differences due to hAPP presence or MT-3 absence could be observed. When challenged to learn a new platform location in phase two, the four genotypes did not significantly differ either in the spatial memory and learning process (Fig. 5b) or in the retention (Fig. 5d). Moreover, the low percentage of time spent in the TQ (Fig. 5d) suggest that, probably due to the advanced age of testing, mice did not properly learn the task. In the third phase, the visible platform test (Fig. 5f), where mice have to switch to a stimulus–response strategy, all genotypes showed similar escape latencies the first testing day (day 15); interestingly, MT3KO females showed increased escape latencies compared to controls on the second day of testing (day 16), perhaps reflecting an inability of this group to change the search strategy.
Morris Water Maze at 13 months of age. Spatial memory and learning of 13-month-old females was assessed in the MWM. Despite that the mice learned through the training period of both the first and the second phase, no differences in the latency to reach the hidden platform (a, b), the percentage of time spent in the TQ (retention) (c, d), and the number of target crossings (e) were evident among genotypes. The visible platform test (f), which is better solved with a stimulus–response strategy rather than a spatial one, evidenced that MT-3 deficiency, at least on the second day of training, significantly increased escape latency. The dotted line in graphs c and d represents the percentage of time, that by chance and not because of a learning process, mice can spend in the TQ. Data represents mean ± SEM (n = 3–8), and they were analyzed using either GEE for repeated measures or GLZ for day by day analysis using APP and MT-3 as factors. ⋆ p < 0.01 vs. MT3WT
MT-3 deficiency decreases amyloid plaque load whereas chronic injection of Zn7MT-3 increases plaque load and the associated astrocytosis
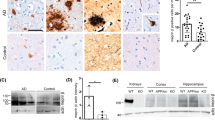
The amyloid plaque load of the 14-month-old mice was evaluated by IHC using 4G8 specific antibody (Fig. 6a, b). In general, MT-3 deficiency tended to decrease the amyloid plaque load in the hippocampus of both female and male mice, although statistical significance was only reached for the Aβ staining intensity of the hippocampus of females (Fig. 6a). In the cortex, no significant differences were observed.
Amyloid plaque burden and concomitant astrocytosis. Amyloid plaque content (a, b) and the concomitant astrocytosis (c, d) of 14-month-old mice were evaluated in hippocampus (a, c) and cortex (b, d) using two measures: the percentage of area occupied by specific immunostaining (left) and the intensity of staining (volume, right). In the hippocampus (a), MT-3 deficiency significantly decreased the amyloid staining intensity in females and the same trend could be observed in the percentage of area occupied by plaques and also in males’ measures. Zn7MT-3 chronic administration had no significant effect on hippocampus amyloidosis. In the cortex (b), MT-3 absence had no significant effect in any gender or measure but the trends were similar to the observed in the hippocampus. Zn7MT-3 chronic injection notably increased amyloid burden compared to controls. No differences or even decreased hippocampal astrocytosis (c) levels were found in APP+ females compared to controls. APP+ males, in contrast, presented increased astrocytosis levels although only the volume reached significance. MT-3 deficiency had no effect in any gender. Zn7MT-3 chronic injection significantly increased GFAP levels. In the cortex (d), both genders presented increased plaque-associated GFAP levels, however, no differences due to MT-3 absence were evident. Zn7MT-3 chronic administration significantly increased astrocytosis compared to controls. Results are mean ± SEM (n = 9–12), and they were analyzed using GLZ with hAPP and MT-3 deficiency, MT-3 deficiency or Zn7MT-3 injection as factors. ✦ p ≤ 0.05 vs. APPWT mice; ♦ p ≤ 0.05 vs. APP− mice ▼ p ≤ 0.02 vs. APPWT
The chronic injection of Zn7MT-3 to APPWT females notably increased the amyloid burden in the cortex compared to APPWT controls as evidenced by the increased percentage of area occupied by plaques and the increased volume (Fig. 6b). In contrast, hippocampal amyloid burden was not significantly affected by Zn7MT-3 injection, and, if anything, it tended to be decreased (p = 0.065 for the staining intensity) (Fig. 6a).
Amyloid plaques are commonly associated with gliosis, hence, IHC using anti-GFAP antibody was next assayed to evaluate astrocytosis (Fig. 6c, d). The most consistent results were obtained in the cortex, where GFAP staining was clearly increased in APP+ mice, and the chronic injection of Zn7MT-3 further increased it (Fig. 6d); MT-3 deficiency did not modify the phenotype of APP+ mice, but it tended to decrease GFAP staining of female APP− mice. The results for the hippocampus were less clear-cut since basal hippocampus GFAP immunostaining is already very high in control mice; yet, an increase of GFAP immunostaining was seen in APP+ male mice, and the injection of Zn7MT-3 increased GFAP staining (Fig. 6c).
MT-3 absence decreases hippocampal Aβ precursor protein (APP) in females only, while Zn7MT-3 injection increases APP proteolytic fragments
To characterize APP and its proteolytic fragments in the hippocampus, total homogenates from female mice were analyzed by WB (Fig. 7a, b) and ELISA (Fig. 7c). MT-3 deficiency significantly decreased the APP levels without affecting Aβ, CTF-β and the putative 14-kDa oligomer levels (Fig. 7a, b). The ELISA confirmed the WB results (Fig. 7c). Male hippocampi were also analyzed but MT-3 deficiency had no effect on the endpoints examined (data not shown).
Analysis of APP and its proteolytic fragments in the hippocampus of female mice. Total hippocampal homogenates were analyzed by WB and ELISA to further characterize APP and its proteolytic fragments. Representative WB band pattern (a) obtained with 6E10 antibody. b MT-3 absence decreased APP levels but not its proteolytic fragments while Zn7MT-3 injection increased all APP-derived fragments without affecting APP levels. ELISA analysis (c) evidenced that MT-3 injection significantly increased Aβ1–42 levels despite the same trend was observed for Aβ1–40. Data represents mean ± SEM (n = 10–22) and was analyzed using GLZ with MT-3 deficiency or Zn7MT-3 injection as factors. ✦ p ≤ 0.005 vs. APPWT mice ▼ p < 0.02 vs. APPWT
The analysis of Zn7MT-3-injected APP+ females showed that the levels of the different APP fragments were increased compared to non-injected females (Fig. 7a, b); further analysis by ELISA indicated that Aβ1–42 was probably the primary form increased by Zn7MT-3 chronic injection (Fig. 7c).
MT-3 absence decreases cortical Aβ precursor protein (APP) in females only, while Zn7MT-3 injection decreases APP and CTF-β
Consistent with hippocampal results, total cortex homogenates from APPMT3KO females showed decreased levels of APP, and in addition they also showed a trend for decreased CTF-β levels compared to APPWT females (Fig. 8a, c). No significant differences between genotypes could be observed when the different cortical fractions were analyzed by WB, although APP and CTF-β levels tended to be decreased in the extracellular-enriched fraction (p = 0.092 and p = 0.059, respectively) and CTF-β also in the membrane-associated enriched protein fraction (p = 0.060) (data not shown). Aβ1–40 and Aβ1–42 showed opposite tendencies in the membrane-associated enriched protein fraction as analyzed by ELISA (Fig. 8e), perhaps explaining the lack of differences observed in the analysis of Aβ by WB.
Analysis of APP and its proteolytic fragments in the cortex. Total cortical homogenates and different cortical fractions were analyzed by WB using 6E10 antibody. Representative WB pattern (a) of females’ total cortical homogenate and (b) of male’s membrane-associated enriched protein fraction. c MT-3 absence significantly decreased APP and CTF-β levels in females’ total cortical homogenates. d MT-3 absence significantly increased CTF-β levels in males’ membrane-associated enriched protein fraction. e Females’ membrane-associated enriched protein fraction analysis by ELISA revealed no differences between genotypes in any Aβ specie. Data represents mean ± SEM (n = 8–10), and was analyzed using GLZ with MT-3 deficiency or Zn7MT-3 injection as factors. ✦ p ≤ 0.02 vs. APPWT
WB analysis of total cortical homogenates from males showed no differences due to MT-3 deficiency in any of the quantified bands (data not shown); nevertheless, MT-3 absence increased CTF-β levels in the membrane-associated enriched protein fraction and tended to increase putative trimers and Aβ monomers (p = 0.095 and p = 0.054 respectively) (Fig. 8b, d). No differences could be observed in the other fractions analyzed (data not shown).
On the other hand, the injection of Zn7MT-3 to APPWT females significantly decreased APP levels compared to controls in total cortex (Fig. 9a, d) and in the extracellular (Fig. 9b, e) and the cytoplasmic-enriched protein fractions (Fig. 9c, f). Moreover, it also decreased CTF-β levels in total cortex (Fig. 9a, d) and the extracellular-enriched protein fraction (Fig. 9b, e). Further analysis of the membrane-associated enriched protein fraction by ELISA showed that chronic injection of Zn7MT-3 significantly increased Aβ1–40 levels and also the sum of both monomeric species (Aβ1–40 + Aβ1–42) (Fig. 9g).
Zn7MT-3-injected APPWT females present decreased cortical APP and CTF-β levels but increased Aβ1–40 levels. Representative WB band pattern of females’ total cortical homogenate (a), extracellular-enriched protein fraction (b), and cytoplasmic-enriched protein fraction (c), using 6E10 antibody. Zn7MT-3 injection significantly reduced both APP and CTF-β levels (d) in total cortex homogenates compared to controls. Zn7MT-3 injection significantly reduced APP and CTF-β levels (e) in the extracellular-enriched protein fraction. APP levels were also decreased in the cytoplasmic-enriched protein fraction (f) of Zn7MT-3-injected APPWT females compared to controls. ELISA analysis (g) of females’ membrane-associated enriched protein fraction showed that chronic Zn7MT-3 injection significantly increased Aβ1–40 levels and the sum of both species (Aβ1–40 + Aβ1–42) compared to controls. Data represents mean ± SEM (n = 9–10) and was analyzed using GLZ with MT-3 deficiency or Zn7MT-3 injection as factors. ▼ p ≤ 0.04 vs. APPWT
MT-3 deficiency tends to increase Cu levels in both genders
Metal content was analyzed in total cortical homogenates by ICP-MS in aged mice (14 months old) from both genders (Fig. 10). Neither hAPP presence nor MT-3 absence, nor Zn7MT-3 injection exerted any significant effect on females’ metal content, although MT-3 absence tended to increase Cu levels (p = 0.085) and Zn7MT-3 injection tended to increase Mn (p = 0.072), Fe (p = 0.093), and Zn (p = 0.067) levels compared to controls. Similarly, hAPP presence had no significant effect on males’ cortical metal content; MT-3 deficiency significantly increased Cu levels compared to controls although no effects could be observed in the other metals analyzed. Interestingly, MT-3 deficiency tended to decrease Zn levels in the presence of hAPP transgene but had no effect in its absence (p = 0.057).
Total Mn, Fe, Cu, and Zn content are not dramatically affected by hAPP presence, MT-3 absence and Zn7MT-3 injection. Total cortex homogenates from the 14-month-old mice from both sexes were analyzed by ICP-MS. No differences between genotypes were found in females, neither by hAPP presence, nor by MT-3 absence, nor by Zn7MT-3 injection. In males, MT-3 deficiency significantly increased Cu levels but had no effect in the rest of metals assessed. hAPP presence did not significantly affect metal content in this gender either. Results are μg of metal/g of tissue (mean ± SEM; n = 9–10), and they were analyzed using GLZ with hAPP and MT-3 deficiency as main factors. ⋆ p < 0.05 vs. MT3WT mice
Statistical GLZ combined analysis using APP, MT-3 and sex as factors revealed that females presented higher Mn and Zn levels than males (p < 0.02).
Discussion
The role of MT-3 in AD pathogenesis has long been controversial. Despite originally reported to be down-regulated in AD patients [95, 98, 105], no differences and even increased levels of MT-3 have also been found in AD patients as well as in mouse models of the disease [2, 15, 16, 36, 43]. In vitro data supports a neuroprotective role of MT-3 against Aβ-induced neurotoxicity [30, 51, 52, 73] with metal swap between Zn7MT-3 and Aβ-Cu(II) as the underlying mechanism [73]. Recent in vivo data also supports a protective role of MT-3 against Aβ toxicity in Drosophila [48], however the role of MT-3 in AD mice models is still unknown.
In the current study, we report for the first time in a transgenic (Tg) AD mouse model, the Tg2576, the involvement of MT-3 in AD progression. hAPP-induced lethality, a widely described phenomenon in hAPP Tg mice [8, 14, 33, 49, 82, 88], tended to be reduced in females when MT-3 was absent, suggesting a detrimental role of MT-3 isoform rather than a neuroprotective one at least in this gender. Since MT-3 effects have been reported to be dependent on the injury severity [17] and mortality was more severe in males, it is possible that in this context the MT-3 effect was blunted. However, body weight, which as expected was significantly reduced in APP+ genotypes from both genders (average 20–30 % reduction) [93], was not affected by MT-3. While the precise mechanisms are unclear, several reports support a role of reactive oxygen species in hAPP-induced mortality since SOD1 overexpression dramatically decreases mortality of APP mice [14, 49]. On the other hand, increased Cu bioavailability has also been shown to have a beneficial effect on life span in hAPP Tg mice [82]. Brain Cu levels in several of these models are found to be decreased [69, 70, 82] and this could be impairing SOD1 activity and other Cu (and Zn)-dependent enzymes thus promoting mortality [8]. Interestingly, but at odds with previous reports [34, 35], in our experiment, brain Cu levels of MT3KO genotypes from both genders tended to be increased compared to controls and could be modulating and normalizing SOD1 activity hence preventing premature death [8]. Moreover, considering the affinity and high metal-binding capacity and plasticity of the Zn-MT-3 isoform [78] it could be speculated that in MT-3 null mice bio-metals would be more bioavailable [83] and this could also be beneficial for preventing APP-induced lethality. Of notice SOD1 overexpression [10] but not increased Cu bioavailability [8, 88] protected against hAPP-related lower body weights suggesting different mechanisms underlying survival and body weight that could explain the lack of effect of MT-3 deficiency in the body weight observed in our experiment.
Increased Cu bioavailability in APP models has also been found to significantly reduce amyloid burden [8, 82], and in fact in our study cortical amyloid burden (measured by IHC) tended to be reduced in MT-3-deficient mice, and this reduction was also observed in the hippocampus although only females reached statistical significance. MT-3 concentration is particularly high in the presynaptic vesicles of zinc-enriched neurons of the cerebral cortex and hippocampus [34], with CA3 neurons of the hippocampus expressing the highest MT-3 levels [68]. The histochemically reactive Zn pool contained in these synaptic vesicles is the one thought to predominantly contribute to amyloid deposition [63] and even though under steady-state conditions MT-3 is not critical for the regulation of this pool [34] in stimulated conditions MT-3 has been proposed to have a role in synaptic Zn regulation and more concretely in its recycling [6]; moreover, the response of mice deficient for ZnT3 transporter, thus devoid of synaptic Zn, to kainic acid is comparable to that of ZnT3KO + MT3KO mice, suggesting that both ZnT3 and MT-3 function in the same pathway [22]. Since double Tg mice expressing hAPP and deficient for ZnT3 transporter present reduced levels of amyloid plaques [63] it is conceivable that MT-3 deficient mice in this context also present reduced amyloid burden. Analysis of amyloidosis by WB showed that in both regions assessed APP levels were decreased in MT-3 deficient females. Changes in APP levels modify the available substrate for α- and β-secretase proteolytic cleavage, however, CTF-β levels were only decreased in the cortex of MT3KO females while no differences in CTF-β or in the Aβ monomer were evident in hippocampus. No consistent effects due to MT-3 absence could be observed on amyloidosis in the male mice, perhaps reflecting a lower or even blunted effect of MT-3 in this gender as happened with survival.
Exogenous administration of MT-3 has been shown to exert a number of effects in the CNS in mouse models of brain injury where the blood–brain barrier (BBB) has been disrupted [17, 46, 81]. Noteworthy, the BBB of Tg2576 mice has been reported to be severely compromised in the vicinity of the amyloid plaques [28, 58], thus allowing the access of Zn7MT-3 to CNS. Chronic administration of Zn7MT-3 to Tg2576 females had no effect on survival or body weight (probably because these are early events and Zn7MT-3 administration began at 11 months of age), however, it markedly influenced amyloidosis and inflammation. In hippocampus, Zn7MT-3 injection increased oligomer (putative trimers) CTF-β and Aβ levels without altering APP synthesis, suggesting that Zn7MT-3 might be modifying secretase activity; as BACE can be modulated by copper/copper chaperones [4] the Zn7MT-3 effect on secretase activity might be related to the modulation of the metal binding properties of secretase. Accordingly, ELISA data showed increased levels Aβ1–42 in Zn7MT-3-injected females, nevertheless no significant differences in amyloid plaque content (measured by IHC) could be observed. Several authors suggest that, in vivo, Aβ1–42 trimers may be critical for the formation of higher-order non-fibrillar assemblies while dimers may follow a different pathway and may represent critical nucleation steps towards fibrillization and amyloid plaque formation [71] hence supporting the existence of separate and distinct aggregation pathways [77]. Interestingly, in vitro studies using cortical cultures exposed to Aβ [51] found that MT-3 protected from Aβ neurotoxicity and proposed that it may antagonize Aβ neurotoxic activity by abolishing the formation of fibrillar, SDS-insoluble aggregate forms and enhancing the formation of the amorphous, SDS-soluble Aβ aggregate forms [51]. In this scenario, MT-3 would increase the SDS-soluble Aβ pool by enhancing the formation of amorphous SDS-soluble Aβ aggregates but without necessarily modifying amyloid burden [73]. Moreover, the increased levels of Aβ1–42 (more prone to aggregate) or even MT-3 itself, might be favoring the oligomeric pathway over the fibrillization pathway, thus resulting in increased levels of Aβ1–42 trimers in the APPWT-injected females. Conversely, in cortex, chronic Zn7MT-3 injection markedly increased the cortex amyloid plaque content and decreased APP and CTF-β levels without affecting Aβ monomers or oligomers compared to non-injected APPWT females. Interestingly, when Aβ levels were measured by ELISA in the membrane-associated protein enriched fraction (that probably contains the less soluble pool of Aβ), Zn7MT-3 administration significantly increased Aβ1–40 and of Aβ1–40 + Aβ1–42 levels. Data obtained supports the notion that in the cortical region, the plaque formation pathway is favored over the oligomeric one since both the less soluble pool of Aβ and the amyloid burden are increased while the oligomeric forms are unaffected by Zn7MT-3 injection. Taken together, these data suggest that the effect of MT-3 is gender- and region-dependent. Moreover it suggests that MT-3 effect on amyloid deposition is likely to be independent from its effect on APP synthesis and processing.
The Tg2576 behavioral phenotype has been widely characterized, and there is divergent data reported for several behavioral traits. According to our previous reports, in general APP+ genotypes were hypoactive, had increased exploratory activity and were less anxious than their age-matched controls. However, due to the advanced age of testing some of these traits, i.e., anxiety, they had faded with aging. MT-3 deficiency did not dramatically affect any of these behavioral traits, but notably, Tg2576 behavioral phenotype was significantly influenced by MT-3 absence in some parameters. For instance, MT-3 absence significantly reduced the anxiety levels of old APP+ mice. On the other hand, chronic injection of Zn7MT-3 notably increased activity and exploratory activity and decreased anxiety levels compared to controls. Whether these effects are because MT-3 (absence/injection) is somehow exacerbating Tg2576 phenotype (less anxiety, increased exploratory activity) or because it is delaying age-related cognitive decline on APPWT mice we cannot know. The general motor condition of Tg2576 mice has also been described to be impaired even at early ages [5, 54, 55], however some authors report no differences in the Tg2576 and other AD models even at old ages [60, 61, 67]. In line with the latter reports, in our experiment general motor condition (balance, prehensile reflex and strength) was not affected by hAPP presence not even after widespread deposition of Aβ. MT-3 absence had no consistent effect in males at any age or in 11 months old females, however in 14 months old females it improved prehensile reflex and strength while in the APP+, as happened in other neurodegenerative scenarios, such as the G93A SOD1 model of Amyotrophic Lateral Sclerosis, it worsened the balance [85]. Paradoxically, chronic injection of Zn7MT-3 also impaired balance. Finally, impaired cognition has also been widely described in the Tg2576 and other AD mouse models [24, 84]; nevertheless when 13 months old females were tested in the MWM, no differences between genotypes emerged due to hAPP presence, in agreement with other reports [44]. Noteworthy, the low percentage of time that mice spent in the target quadrant, especially when the platform was re-located in a new position, suggests poor spatial retention perhaps related with the advanced age of the subjects tested. Similarly, MT-3 deficiency had no effects in spatial learning and memory [34, 57], however it impaired visible platform escape latency. Our results support that there is no obvious relationships between amyloid deposits and behavioral changes [45] and that age-related decline could be masking hAPP and/or MT-3 effects.
Aging, the main risk factor for AD, is invariably accompanied by depletion of sex steroids which have been shown to significantly contribute to AD risk in both women and men [101]. In general, epidemiological evidence supports an increased prevalence and severity of AD in women [3, 7, 90] Several AD mouse models, including the Tg2576 mice, also show prominent gender differences that can eventually be explained by sex hormones differences [13, 18, 20, 87]. Noteworthy, experiments crossing Tg2576 mice with mice lacking ZnT3 evidenced that with aging, females showed higher levels of synaptic zinc as well as increased levels of insoluble Aβ and amyloid plaques than males, and these differences between genders disappeared when ZnT3 transporter was absent, suggesting that synaptic Zn and its recycling, a process in which MT-3 has been suggested to be involved, have a crucial role in amyloid deposition [62]. Indeed several studies evidence that MT-3 is modulated by sexual steroids [23, 75] and suggest a relation between MT-3, Zn content and sex hormones [50]. Altogether our results showing clear gender-dependent changes are consistent with previous studies in both human and AD mouse models which demonstrate sexual dimorphism and a participation of sexual steroids in modulating several aspects of AD pathology.
Taken together we have demonstrated for the first time in a Tg AD mouse model that MT-3 is able to alter the Tg2576 phenotype in several aspects such as mortality and behavior in a gender-dependent manner. Moreover, we have also evidenced a complex effect (gender-, region- and severity-dependent) of MT-3 on the amyloid cascade that could be modulating the different aggregation pathways of Aβ. These results highlight that the control of the endogenous production and/or action of MT-3 could represent a powerful therapeutic target in AD.
References
Adlard PA, Bush AI (2006) Metals and Alzheimer’s disease. J Alzheimers Dis 10:145–163
Amoureux MC, Van Gool D, Herrero MT, Dom R, Colpaert FC, Pauwels PJ (1997) Regulation of metallothionein-III (GIF) mRNA in the brain of patients with Alzheimer disease is not impaired. Mol Chem Neuropathol 32:101–121
Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A (1999) Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurology 53:1992–1997
Angeletti B, Waldron KJ, Freeman KB, Bawagan H, Hussain I, Miller CC, Lau KF, Tennant ME, Dennison C, Robinson NJ, Dingwall C (2005) BACE1 cytoplasmic domain interacts with the copper chaperone for superoxide dismutase-1 and binds copper. J Biol Chem 280:17930–17937
Arendash GW, Gordon MN, Diamond DM, Austin LA, Hatcher JM, Jantzen P, DiCarlo G, Wilcock D, Morgan D (2001) Behavioral assessment of Alzheimer’s transgenic mice following long-term Abeta vaccination: task specificity and correlations between Abeta deposition and spatial memory. DNA Cell Biol 20:737–744
Aschner M (1997) Astrocyte metallothioneins (MTs) and their neuroprotective role. Ann N Y Acad Sci 825:334–347
Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA (2005) Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry 62:685–691
Bayer TA, Schäfer S, Simons A, Kemmling A, Kamer T, Tepest R, Eckert A, Schüssel K, Eikenberg O, Sturchler-Pierrat C, Abramowski D, Staufenbiel M, Multhaup G (2003) Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Abeta production in APP23 transgenic mice. Proc Natl Acad Sci USA 100:14187–14192
Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW (2004) Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci USA 101:2173–2178
Borg J, Chereul E (2008) Differential MRI patterns of brain atrophy in double or single transgenic mice for APP and/or SOD. J Neurosci Res 86:3275–3284
Bruinink A, Faller P, Sidler C, Bogumil R, Vašák M (1998) Growth inhibitory factor and zinc affect neural cell cultures in a tissue specific manner. Chem Biol Interact 115:167–174
Bush AI, Tanzi RE (2008) Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics 5:421–432
Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC (2001) Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am J Pathol 158:1173–1177
Carlson GA, Borchelt DR, Dake A, Turner S, Danielson V, Coffin JD, Eckman C, Meiners J, Nilsen SP, Younkin SG, Hsiao KK (1997) Genetic modification of the phenotypes produced by amyloid precursor protein overexpression in transgenic mice. Hum Mol Genet 6:1951–1959
Carrasco J, Adlard P, Cotman C, Quintana A, Penkowa M, Xu F, Van Nostrand WE, Hidalgo J (2006) Metallothionein-I and -III expression in animal models of Alzheimer disease. Neuroscience 143:911–922
Carrasco J, Giralt M, Molinero A, Penkowa M, Moos T, Hidalgo J (1999) Metallothionein (MT)-III: generation of polyclonal antibodies, comparison with MT-I + II in the freeze lesioned rat brain and in a bioassay with astrocytes, and analysis of Alzheimer’s disease brains. J Neurotrauma 16:1115–1129
Carrasco J, Penkowa M, Giralt M, Camats J, Molinero A, Campbell IL, Palmiter RD, Hidalgo J (2003) Role of metallothionein-III following central nervous system damage. Neurobiol Dis 13:22–36
Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ (2007) Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci 27:13357–13365
Ceballos D, Lago N, Verdu E, Penkowa M, Carrasco J, Navarro X, Palmiter RD, Hidalgo J (2003) Role of metallothioneins in peripheral nerve function and regeneration. Cell Mol Life Sci 60:1209–1216
Clinton LK, Billings LM, Green KN, Caccamo A, Ngo J, Oddo S, McGaugh JL, LaFerla FM (2007) Age-dependent sexual dimorphism in cognition and stress response in the 3xTg-AD mice. Neurobiol Dis 28:76–82
Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ (2002) Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J Neurosci Res 70:462–473
Cole TB, Robbins CA, Wenzel HJ, Schwartzkroin PA, Palmiter RD (2000) Seizures and neuronal damage in mice lacking vesicular zinc. Epilepsy Res 39:153–169
Cyr DG, Dufresne J, Pillet S, Alfieri TJ, Hermo L (2001) Expression and regulation of metallothioneins in the rat epididymis. J Androl 22:124–135
Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK (1999) Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci 2:271–276
Chung RS, Howells C, Eaton ED, Shabala L, Zovo K, Palumaa P, Sillard R, Woodhouse A, Bennett WR, Ray S, Vickers JC, West AK (2010) The native copper- and zinc-binding protein metallothionein blocks copper-mediated Abeta aggregation and toxicity in rat cortical neurons. PLoS ONE 5:e12030
Chung RS, Vickers JC, Chuah MI, Eckhardt BL, West AK (2002) Metallothionein-III inhibits initial neurite formation in developing neurons as well as postinjury, regenerative neurite sprouting. Exp Neurol 178:1–12
De Strooper B (2010) Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol Rev 90:465–494
Dickstein DL, Biron KE, Ujiie M, Pfeifer CG, Jeffries AR, Jefferies WA (2006) Abeta peptide immunization restores blood-brain barrier integrity in Alzheimer disease. FASEB J 20:426–433
Dong J, Atwood CS, Anderson VE, Siedlak SL, Smith MA, Perry G, Carey PR (2003) Metal binding and oxidation of amyloid-beta within isolated senile plaque cores: Raman microscopic evidence. Biochemistry 42:2768–2773
Durand J, Meloni G, Talmard C, Vašák M, Faller P (2010) Zinc release of Zn7-metallothionein-3 induces fibrillar type amyloid-β aggregates. Metallomics 2:741–744
El Ghazi I, Martin BL, Armitage IM (2006) Metallothionein-3 is a component of a multiprotein complex in the mouse brain. Exp Biol Med (Maywood) 231:1500–1506
El Ghazi I, Martin BL, Armitage IM (2010) New proteins found interacting with brain metallothionein-3 are linked to secretion. Int J Alzheimers Dis 2011:208634
El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD (2007) Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med 13:432–438
Erickson JC, Hollopeter G, Thomas SA, Froelick GJ, Palmiter RD (1997) Disruption of the metallothionein-III gene in mice: analysis of brain zinc, behavior, and neuron vulnerability to metals, aging, and seizures. J Neurosci 17:1271–1281
Erickson JC, Masters BA, Kelly EJ, Brinster RL, Palmiter RD (1995) Expression of human metallothionein-III in transgenic mice. Neurochem Int 27:35–41
Erickson JC, Sewell AK, Jensen LT, Winge DR, Palmiter RD (1994) Enhanced neurotrophic activity in Alzheimer’s disease cortex is not associated with down-regulation of metallothionein-III (GIF). Brain Res 649:297–304
Faller P, Hasler DW, Zerbe O, Klauser S, Winge DR, Vašák M (1999) Evidence for a dynamic structure of human neuronal growth inhibitory factor and for major rearrangements of its metal- thiolate clusters. Biochemistry 38:10158–10167
Fernandes C, González MI, Wilson CA, File SE (1999) Factor analysis shows that female rat behaviour is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol Biochem Behav 64:731–738
Gitter BD, Regoli D, Howbert JJ, Glasebrook AL, Waters DC (1994) Interleukin-6 secretion from human astrocytoma cells induced by substance P. J Neuroimmunol 51:101–108
Guglielmotto M, Giliberto L, Tamagno E, Tabaton M (2010) Oxidative stress mediates the pathogenic effect of different Alzheimer’s disease risk factors. Front Aging Neurosci 2:3
Hasler DW, Jensen LT, Zerbe O, Winge DR, Vašák M (2000) Effect of the two conserved prolines of human growth inhibitory factor (metallothionein-3) on its biological activity and structure fluctuation: comparison with a mutant protein. Biochemistry 39:14567–14575
Heneka MT, O’Banion MK, Terwel D, Kummer MP (2010) Neuroinflammatory processes in Alzheimer’s disease. J Neural Transm 117:919–947
Hidalgo J, Penkowa M, Espejo C, Martínez-Cáceres EM, Carrasco J, Quintana A, Molinero A, Florit S, Giralt M, Ortega-Aznar A (2006) Expression of metallothionein-I, -II, and -III in Alzheimer disease and animal models of neuroinflammation. Exp Biol Med (Maywood) 231:1450–1458
Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K (1998) Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med 4:97–100
Holcomb LA, Gordon MN, Jantzen P, Hsiao K, Duff K, Morgan D (1999) Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: lack of association with amyloid deposits. Behav Genet 29:177–185
Hozumi I, Uchida Y, Watabe K, Sakamoto T, Inuzuka T (2006) Growth inhibitory factor (GIF) can protect from brain damage due to stab wounds in rat brain. Neurosci Lett 395:220–223
Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274:99–102
Hua H, Münter L, Harmeier A, Georgiev O, Multhaup G, Schaffner W (2011) Toxicity of Alzheimer’s disease-associated Aβ peptide is ameliorated in a Drosophila model by tight control of zinc and copper availability. Biol Chem 392:919–926
Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA (1999) SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci 2:157–161
Iguchi K, Morihara N, Usui S, Hayama M, Sugimura Y, Hirano K (2011) Castration- and aging-induced changes in the expression of zinc transporter and metallothionein in rat prostate. J Androl 32:144–150
Irie Y, Keung WM (2001) Metallothionein-III antagonizes the neurotoxic and neurotrophic effects of amyloid beta peptides. Biochem Biophys Res Commun 282:416–420
Irie Y, Keung WM (2003) Anti-amyloid beta activity of metallothionein-III is different from its neuronal growth inhibitory activity: structure-activity studies. Brain Res 960:228–234
Ittner LM, Götz J (2011) Amyloid-β and tau—a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci 12:65–72
King DL, Arendash GW (2002) Behavioral characterization of the Tg2576 transgenic model of Alzheimer’s disease through 19 months. Physiol Behav 75:627–642
King DL, Arendash GW, Crawford F, Sterk T, Menendez J, Mullan MJ (1999) Progressive and gender-dependent cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer’s disease. Behav Brain Res 103:145–162
Knipp M, Meloni G, Roschitzki B, Vašák M (2005) Zn7metallothionein-3 and the synaptic vesicle cycle: interaction of metallothionein-3 with the small GTPase Rab3A. Biochemistry 44:3159–3165
Koumura A, Kakefuda K, Honda A, Ito Y, Tsuruma K, Shimazawa M, Uchida Y, Hozumi I, Satoh M, Inuzuka T, Hara H (2009) Metallothionein-3 deficient mice exhibit abnormalities of psychological behaviors. Neurosci Lett 467:11–14
Kumar-Singh S, Pirici D, McGowan E, Serneels S, Ceuterick C, Hardy J, Duff K, Dickson D, Van Broeckhoven C (2005) Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer’s disease are centered on vessel walls. Am J Pathol 167:527–543
Lahti DW, Hoekman JD, Tokheim AM, Martin BL, Armitage IM (2005) Identification of mouse brain proteins associated with isoform 3 of metallothionein. Protein Sci 14:1151–1157
Lalonde R, Dumont M, Staufenbiel M, Sturchler-Pierrat C, Strazielle C (2002) Spatial learning, exploration, anxiety, and motor coordination in female APP23 transgenic mice with the Swedish mutation. Brain Res 956:36–44
Lalonde R, Lewis TL, Strazielle C, Kim H, Fukuchi K (2003) Transgenic mice expressing the betaAPP695SWE mutation: effects on exploratory activity, anxiety, and motor coordination. Brain Res 977:38–45
Lee JY, Cole TB, Palmiter RD, Suh SW, Koh JY (2002) Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc Natl Acad Sci USA 99:7705–7710
Lee JY, Mook-Jung I, Koh JY (1999) Histochemically reactive zinc in plaques of the Swedish mutant beta-amyloid precursor protein transgenic mice. J Neurosci 19:RC10:1–5
Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH (2006) A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440:352–357
Liang WS, Dunckley T, Beach TG, Grover A, Mastroeni D, Ramsey K, Caselli RJ, Kukull WA, McKeel D, Morris JC, Hulette CM, Schmechel D, Reiman EM, Rogers J, Stephan DA (2008) Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: a reference data set. Physiol Genomics 33:240–256
Manso Y, Adlard PA, Carrasco J, Vašák M, Hidalgo J (2011) Metallothionein and brain inflammation. J Biol Inorg Chem 16:1103–1113
Massaad CA, Washington TM, Pautler RG, Klann E (2009) Overexpression of SOD-2 reduces hippocampal superoxide and prevents memory deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 106:13576–13581
Masters BA, Quaife CJ, Erickson JC, Kelly EJ, Froelick GJ, Zambrowicz BP, Brinster RL, Palmiter RD (1994) Metallothionein III is expressed in neurons that sequester zinc in synaptic vesicles. J Neurosci 14:5844–5857
Maynard CJ, Cappai R, Volitakis I, Cherny RA, Masters CL, Li QX, Bush AI (2006) Gender and genetic background effects on brain metal levels in APP transgenic and normal mice: implications for Alzheimer beta-amyloid pathology. J Inorg Biochem 100:952–962
Maynard CJ, Cappai R, Volitakis I, Cherny RA, White AR, Beyreuther K, Masters CL, Bush AI, Li QX (2002) Overexpression of Alzheimer’s disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J Biol Chem 277:44670–44676
Meilandt WJ, Cisse M, Ho K, Wu T, Esposito LA, Scearce-Levie K, Cheng IH, Yu GQ, Mucke L (2009) Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice. J Neurosci Res 29:1977–1986
Meloni G, Knipp M, Vašák M (2005) Detection of neuronal growth inhibitory factor (metallothionein-3) in polyacrylamide gels and by Western blot analysis. J Biochem Biophys Methods 64:76–81
Meloni G, Sonois V, Delaine T, Guilloreau L, Gillet A, Teissié J, Faller P, Vasák M (2008) Metal swap between Zn7-metallothionein-3 and amyloid-beta-Cu protects against amyloid-beta toxicity. Nat Chem Biol 4:366–372
Meloni G, Vašák M (2011) Redox activity of α-synuclein-Cu is silenced by Zn7-metallothionein-3. Free Radic Biol Med 50:1471–1479
Moffatt P, Séguin C (1998) Expression of the gene encoding metallothionein-3 in organs of the reproductive system. DNA Cell Biol 17:501–510
Montoliu C, Monfort P, Carrasco J, Palacios O, Capdevila M, Hidalgo J, Felipo V (2000) Metallothionein-III prevents glutamate and nitric oxide neurotoxicity in primary cultures of cerebellar neurons. J Neurochem 75:266–273
Necula M, Kayed R, Milton S, Glabe CG (2007) Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J Biol Chem 282:10311–10324
Palumaa P, Tammiste I, Kruusel K, Kangur L, Jornvall H, Sillard R (2005) Metal binding of metallothionein-3 versus metallothionein-2: lower affinity and higher plasticity. Biochim Biophys Acta 1747:205–211
Pedersen AO, Jacobsen J (1980) Reactivity of the thiol group in human and bovine albumin at pH 3–9, as measured by exchange with 2,2′-dithiodipyridine. Eur J Biochem 106:291–295
Pedersen JT, Hureau C, Hemmingsen L, Heegaard NH, Ostergaard J, Vašák M, Faller P (2012) Rapid exchange of metal between Zn(7)-metallothionein-3 and amyloid-β peptide promotes amyloid-related structural changes. Biochem 51:1697–1706
Penkowa M, Tió L, Giralt M, Quintana A, Molinero A, Atrian S, Vašák M, Hidalgo J (2006) Specificity and divergence in the neurobiological effects of different metallothioneins after brain injury. J Neurosci Res 83:974–984
Phinney AL, Drisaldi B, Schmidt SD, Lugowski S, Coronado V, Liang Y, Horne P, Yang J, Sekoulidis J, Coomaraswamy J, Chishti MA, Cox DW, Mathews PM, Nixon RA, Carlson GA, St George-Hyslop P, Westaway D (2003) In vivo reduction of amyloid-beta by a mutant copper transporter. Proc Natl Acad Sci USA 100:14193–14198
Pirev E, Ince Y, Sies H, Kröncke KD (2010) Heat shock but not cold shock leads to disturbed intracellular zinc homeostasis. J Cell Physiol 223:103–109
Pugh PL, Richardson JC, Bate ST, Upton N, Sunter D (2007) Non-cognitive behaviours in an APP/PS1 transgenic model of Alzheimer’s disease. Behav Brain Res 178:18–28
Puttaparthi K, Gitomer WL, Krishnan U, Son M, Rajendran B, Elliott JL (2002) Disease progression in a transgenic model of familial amyotrophic lateral sclerosis is dependent on both neuronal and non-neuronal zinc binding proteins. J Neurosci 22:8790–8796
Romero-Isart N, Jensen LT, Zerbe O, Winge DR, Vašák M (2002) Engineering of metallothionein-3 neuroinhibitory activity into the inactive isoform metallothionein-1. J Biol Chem 277:37023–37028
Rosario ER, Carroll J, Pike CJ (2010) Testosterone regulation of Alzheimer-like neuropathology in male 3xTg-AD mice involves both estrogen and androgen pathways. Brain Res 1359:281–290
Schäfer S, Pajonk FG, Multhaup G, Bayer TA (2007) Copper and clioquinol treatment in young APP transgenic and wild-type mice: effects on life expectancy, body weight, and metal-ion levels. J Mol Med (Berl) 85:405–413
Sewell AK, Jensen LT, Erickson JC, Palmiter RD, Winge DR (1995) Bioactivity of metallothionein-3 correlates with its novel beta domain sequence rather than metal binding properties. Biochemistry 34:4740–4747
Sinforiani E, Citterio A, Zucchella C, Bono G, Corbetta S, Merlo P, Mauri M (2010) Impact of gender differences on the outcome of Alzheimer’s disease. Dement Geriatr Cogn Disord 30:147–154
Suh SW, Jensen KB, Jensen MS, Silva DS, Kesslak PJ, Danscher G, Frederickson CJ (2000) Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains. Brain Res 852:274–278
Sultana R, Butterfield DA (2010) Role of oxidative stress in the progression of Alzheimer’s disease. J Alzheimers Dis 19:341–353
Toda T, Noda Y, Ito G, Maeda M, Shimizu T (2011) Presenilin-2 mutation causes early amyloid accumulation and memory impairment in a transgenic mouse model of Alzheimer’s disease. J Biomed Biotechnol 2011:617974
Tõugu V, Karafin A, Zovo K, Chung RS, Howells C, West AK, Palumaa P (2009) Zn(II)- and Cu(II)-induced non-fibrillar aggregates of amyloid-beta (1–42) peptide are transformed to amyloid fibrils, both spontaneously and under the influence of metal chelators. J Neurochem 110:1784–1795
Tsuji S, Kobayashi H, Uchida Y, Ihara Y, Miyatake T (1992) Molecular cloning of human growth inhibitory factor cDNA and its down-regulation in Alzheimer’s disease. EMBO J 11:4843–4850
Twine NA, Janitz K, Wilkins MR, Janitz M (2011) Whole transcriptome sequencing reveals gene expression and splicing differences in brain regions affected by Alzheimer’s disease. PLoS ONE 6:e16266
Uchida Y, Gomi F, Masumizu T, Miura Y (2002) Growth inhibitory factor prevents neurite extension and death of cortical neurons caused by high oxygen exposure through hydroxyl radical scavenging. J Biol Chem 277:32353–32359
Uchida Y, Takio K, Titani K, Ihara Y, Tomonaga M (1991) The growth inhibitory factor that is deficient in the Alzheimer’s disease brain is a 68 amino acid metallothionein-like protein. Neuron 7:337–347
Vašák M (1991) Metal removal and substitution in vertebrate and invertebrate metallothioneins. Methods Enzymol, 205:452–458
Vašák M, Meloni G (2011) Chemistry and biology of mammalian metallothioneins. J Biol Inorg Chem 16:1067–1078
Vest RS, Pike CJ (2012) Gender, sex steroid hormones, and Alzheimer’s disease. Horm Behav (in press). doi:10.1016/j.yhbeh.2012.04.006
Wang G, Zhang Y, Chen B, Cheng J (2003) Preliminary studies on Alzheimer’s disease using cDNA microarrays. Mech Ageing Dev 124:115–124
West AK, Hidalgo J, Eddins D, Levin ED, Aschner M (2008) Metallothionein in the central nervous system: Roles in protection, regeneration and cognition. Neurotoxicology 29:489–503
West AK, Leung JY, Chung RS (2011) Neuroprotection and regeneration by extracellular metallothionein via lipoprotein-receptor-related proteins. J Biol Inorg Chem, 16:1115–1122
Yu WH, Lukiw WJ, Bergeron C, Niznik HB, Fraser PE (2001) Metallothionein III is reduced in Alzheimer’s disease. Brain Res 894:37–45
Acknowledgments
The authors are grateful for grants from the Ministerio de Ciencia e Innovación y Cofinanciada por el Fondo Europeo de Desarrollo Regional (FEDER), SAF2002-01268, SAF2005-00671, SAF2008-00435, and SAF2011-23272 (J.H.). Y.M. acknowledges her Ph.D. fellowship (AP2005-0588). P.A. is supported by the National Health and Medical Research Council of Australia, The Australian Research Council, The Alzheimer’s Association (USA), and the Joan and Peter Clemenger Trust. A.B. is a paid consultant and shareholder of Prana Biotechnology Ltd, and a paid consultant of Adenoa Inc, and a shareholder of Brighton Biotech Inc. P.A is a paid consultant and shareholder of Prana Biotechnology Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manso, Y., Carrasco, J., Comes, G. et al. Characterization of the role of metallothionein-3 in an animal model of Alzheimer’s disease. Cell. Mol. Life Sci. 69, 3683–3700 (2012). https://doi.org/10.1007/s00018-012-1047-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1047-9