Abstract
Background
Nuciferine, a major bioactive component from the lotus leaf, has been reported to have notable anti-inflammatory activities such as renal inflammation and acute lung injury in previous studies. Mastitis is one of the most prevalent diseases in the dairy cattle, which causes large economic losses for the dairy industry. However, the effects of nuciferine on lipopolysaccharide (LPS)-induced mastitis have not been reported.
Methods and results
Here, we investigated the anti-inflammatory effects of nuciferine on LPS-induced mastitis in mice and illuminated its potential mechanism on the TLR4-mediated signaling pathway in mouse mammary epithelial cells (mMECs). Histopathological changes and myeloperoxidase (MPO) activity assay showed that nuciferine treatment significantly alleviated the LPS-induced injury of mammary gland flocculus, inflammatory cells infiltration. qPCR and ELISA assays indicated that nuciferine dose-dependently reduced the levels of TNF-α and IL-1β, which indicated that nuciferine might have therapeutic effects on mastitis. Furthermore, nuciferine treatment significantly decreased the expression of TLR4 in a dose-dependent manner. Besides, nuciferine was also found to suppress LPS-induced NF-κB activation.
Conclusion
These findings indicate that nuciferine potently ameliorates LPS-induced mastitis by inhibition of the TLR4-NF-κB signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mastitis is one of the most prevalent diseases in the dairy cattle, which causes large economic losses for the dairy industry [1, 2]. Mastitis is defined as inflammation of the mammary gland tissues and mainly characterized by edema and inflammatory cells infiltration [3]. A wide range of microorganisms can cause mastitis, including Gram-negative pathogen such as Escherichia coli (E. coli) [4].
Escherichia coli is a common pathogen which usually colonizes dairy cows, and represents a high risk of mastitis. Lipopolysaccharide (LPS), released from the surface of the cell membrane of E. coli, has been considered to be an important virulence factor for mastitis [5]. It is well known that the innate immune system recognizes the presence of pathogens ligands through a membrane receptors family known as Toll-like receptors (TLRs) [6]. TLR4 plays a key role in host defense against microbial infection, and recognizes exogenous ligands such as LPS [7]. Activation of TLR4 by LPS induces the activation of NF-κB pathway and eventually results in the production of pro-inflammatory cytokines, such as TNF-α and IL-1β [8]. These pro-inflammatory cytokines could lead to various inflammatory diseases, such as mastitis [9].
Over the last several decades, antibiotic has been used as a conventional drug against mastitis [10]. However, an increasing number of bacteria becomes resistant to antibiotic these years [11]. Furthermore, antibiotic residues are also harmful to people’s health. Thus, there is an urgent requirement to develop more effective and safe agents to treat mastitis. Many bioactive natural products from plants has attracted widespread attention for their therapeutic effects to inflammatory diseases because of their unique pharmacological profile and low toxicity [12, 13]. Nuciferine, an aromatic ring-containing alkaloid extracted from the lotus leaf, has been reported to possess extensive pharmacological activities, including stimulating insulin secretion, preventing hepatic steatosis and improving arrhythmia [14,15,16]. In addition, recent research has also suggested that nuciferine alleviated kidney inflammation through restraining the inflammation-related signaling pathways [17]. However, the effects of nuciferine on LPS-induced mastitis, as well as its possible molecular mechanisms on TLR4-mediated signaling pathway, are still unknown. Therefore, we investigated the protective effects of nuciferine on a LPS-induced mouse mastitis model, and elucidated the potential mechanism in mouse mammary epithelial cells (mMECs).
Materials and methods
Reagents
Nuciferine was provided by Shanghai Yuanye BioTechnology Co., Ltd. (purity ≥ 98%; Shanghai, China) (Fig. 1, the chemical structure of nuciferine that is an aromatic ring with an alkaloid). LPS (Escherichia coli 055:B5) was provided by Sigma (St. Louis, MO, USA). Mouse TNF-α and IL-1β ELISA kits were purchased from BioLegend (California, USA). Primary antibodies for TLR4, IκBα, NF-κB p65 and β-actin were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA). All other chemicals were of reagent grade.
Animal treatment and experimental groups
Sixty adult female BALB/c mice (6–8 weeks, 35–40 g weight) were purchased from the Experimental Animal Center of Wuhan University (Wuhan, China). The mice were housed in individual cages at 24 ± 1 °C with 65% humidity. All animals received food and sterile water ad libitum. All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals established by the US NIH and approved by the Ethical Committee on Animal Research at Huazhong Agricultural University.
The mice were randomly divided into five groups: control group, LPS group and nuciferine (10, 15, and 20 mg/kg) + LPS groups. Nuciferine was dissolved in dimethyl sulfoxide (DMSO) and diluted in Dulbecco’s modifed Eagle’s medium (DMEM) to give the final concentrations of 10, 15, and 20 mg/kg. The method for establishing the mastitis model was performed as previously described [3]. Briefly, lactating mice were anesthetized with sodium pentobarbital (intraperitoneal injection, 40 mg/kg) and then the near ends of the teats were cut. LPS (1 mg/mL) was infused into mammary gland (R4 and L4) through the duct of mammary gland using a 100-µL syringe. The control group received the equal amount of phosphate buffered saline (PBS). At 24 h after infused LPS, nuciferine groups received an intraperitoneal injection of different nuciferine concentrations (10, 15, and 20 mg/kg) three times (once every 6 h).
Then, all the mice were euthanized with CO2 inhalation after sodium pentobarbital anesthetized (40 mg/kg), and the mammary gland tissues were harvested and kept at − 80 °C.
Histopathological assay
Mammary tissues were excised and fixed with 4% paraformaldehyde, embedded in paraffin. After the sections (4 µm) were stained with hematoxylin and eosin (H&E), the pathological changes were observed under a light microscope (Olympus, Japan).
MPO activity assay
The infiltration of neutrophils in mammary tissues was assessed by MPO activity. Mammary gland tissues were homogenized with reaction buffer (w/v 1/9), and the MPO activity was determined with an myeloperoxidase (MPO) activity assay kit according to the manufacturer’s instructions (Nanjing Jiancheng Co., China).
Cell culture and treatment
Mouse primary mammary epithelial cells were performed as previously described [18, 19]. Mammary gland tissues from pregnant mice were harvested and minced into paste, and then digested by a collagenase I/II/trypsin mixture, collected the cells with centrifugation. The cells were cultured at 37 °C with 5% CO2.
The mMECs were pretreated with different concentrations of nuciferine (10, 15, 20 µg/mL) or dexamethasone (100 µg/mL) for 1 h, and then were stimulated with LPS (1 µg/mL) for 12 h. Cells that were not given any treatment were served as blank control. The concentrations of nuciferine used in the study were established based on previous studies [20].
Cell viability assay
The effects of nuciferine on mMECs viability were measured with 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay. The cells were treated with nuciferine (10, 15, 20 µg/mL) for 24 h. Subsequently, 20 µL of MTT was added in each well for 4 h at 37 °C. The supernatant was removed and dissolved with 100 µL DSMO in each well. The optical density (OD) value was read at 570 nm with a microplate reader (Thermo Scientific Multiskan MK3, USA). The value of cell viability was determined using the ratio of OD of treated to untreated cells as a control.
Cytokine assay
The effects of nuciferine on the levels of LPS-induced pro-inflammatory cytokines were determined in tissues and mMECs. The mammary gland tissues were homogenized with PBS, and then centrifuged and collected the supernatants. The protein concentration of these supernatants were normalized by BCA kit in each group. The supernatants of the mMECs with different treatment were also collected. And the levels of TNF-α and IL-1β were determined by ELISA kits following the manufacturer’s directions.
qPCR assay
Total RNA was extracted from tissues and mMECs using Trizol (Invitrogen, USA) according to the manufacturer’s instructions, and then cDNA was synthesized by reverse transcription. The expression levels of TNF-α and IL-1β were measured with qPCR. The PCR was performed using the SYBR green Plus reagent kit (Roche, Swissland) according to the manufacturer’s instructions. The expression levels were normalized by GAPDH using the 2− ΔΔCt comparative method. The primers used in qPCR are listed in Table 1.
Western blot analysis
The cells were dissociated by RIPA lysis buffer supplemented with protease inhibitor and centrifuged at 12,000g for 15 min at 4 °C. The protein concentrations were measured with a BCA protein assay kit (Thermo Scientific, MA, USA). Then, the proteins were separated by SDS–PAGE and transferred to a PVDF membrane. After blocking for 2 h with 5% skim milk, the membranes were incubated with the primary antibodies (1:1000 dilutions) at 4 °C overnight. Subsequently, the membranes were incubated with the secondary antibodies (1:5000 dilutions) for 1 h at room temperature. The protein levels were detected using the ECL Plus Western Blotting Detection System (ImageQuant LAS 4000mini, USA).
NF-κB DNA-binding activity assay
The DNA-binding activity of NF-κB p65 was measured using a TransAM NF-κB p65 kit (Active Motif, Carlsbad, CA) according to the manufacturer’s instructions. Briefly, the mMECs were pretreated with or without nuciferine for 1 h and then subjected to LPS for an additional 12 h. Subsequently, the nuclear protein was extracted and the protein concentration of each sample was measured with BCA assay. Finally, the nuclear NF-κB p65 DNA-binding activity was detected using a TransAM NF-κB p65 ELISA kit.
Statistical analysis
All values are expressed as the means ± S.E.M. The differences between groups were analyzed by one-way ANOVA. The results were considered statistically significant at P < 0.05.
Results
Effects of nuciferine on LPS-induced histopathological changes
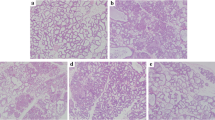
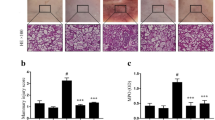
The effects of nuciferine on LPS-induced histopathological changes was detected by H&E staining. As shown in Fig. 2a–f, the control group displayed a normal structure of mammary glands with no histopathological changes. The LPS group showed severe pathologic changes, such as mammary gland flocculus injury, inflammatory cells infiltration, enhanced adipose tissue. In contrast, treatment with nuciferine significantly alleviated these histological changes induced by LPS. The histopathological changes in the mammary glands of mice were evaluated according to the score for injury degree such as the integrity of mammary tissues and the numbers of infiltrated inflammatory cells (Fig. 2g). The pathological grade was scored on a scale of 0–5 as previously described [21]. These findings were further confirmed by MPO assay (Fig. 2h).
Effects of nuciferine on LPS-induced mammary gland injury. Histopathological changes in mammary gland tissues (H&E). a Control group, b LPS group, c–e Nuciferine (10, 15, and 20 mg/kg) groups and f Dexamethasone group. g Histopathological grade score. h MPO activity assay. The red arrow was the tissue lesion area (the red arrow indicates the inflammatory cells infiltration in mammary gland tissues). Data represent the mean ± SEM (n = 5) of three replicates. #P < 0.05 vs. the control group. *P < 0.05 vs. the LPS group. (Color figure online)
Effects of nuciferine on cell viability
The potential cytotoxicity of nuciferine on mMECs was evaluated using the MTT assay. The results showed that cell viability was not affected by nuciferine administration (10, 15 and 20 µg/mL) (Fig. 3).
Effects of nuciferine on the levels of inflammatory cytokines
To investigate the effects of nuciferine on LPS-induced inflammatory cytokines production, the levels of TNF-α and IL-1β were determined by qPCR and ELISA assays. As shown in Figs. 4 and 5, the levels of TNF-α and IL-1β in the LPS group were dramatically increased relative to the control group (at least more than fourfold, p < 0.05). However, these increases were significantly suppressed by nuciferine or dexamethasone (such as the inhibition levels of TNF-α mRNA were 27.0%, 42.8%, 50.0% and 52.5%, respectively, p < 0.05). With increasing doses of nuciferine, the effects were more prominent.
Effects of nuciferine on the gene expression of pro-inflammatory cytokines in mammary tissues and cells. a TNF-α and IL-1β mRNA expression were measured by qPCR method in mammary tissues. b Expression of TNF-α and IL-1β were also detected by qPCR method in cells. GAPDH was used as the control. Data represent the mean ± SEM (n = 5) of three replicates. #P < 0.05 vs. the control group. *P < 0.05 vs. the LPS group
The effects of nuciferine on the protein levels of pro-inflammatory cytokines in mammary tissues and cells. a Protein levels of TNF-α and IL-1β were determined using a ELISA kit in mammary tissues. b Protein levels of TNF-α and IL-1β were measured by a ELISA kit in cells. Data represent the mean ± SEM (n = 5) of three replicates. #P < 0.05 vs. the control group. *P < 0.05 vs. the LPS group
Effects of nuciferine on TLR4 expression
TLR4 plays a critical role in the LPS-induced inflammatory response [22]. In this study, the expression of TLR4 was measured by Western blot. The results showed that the protein level of TLR4 was increased in the LPS group (the increase level of TLR4 was 124.7%, p < 0.05). Whereas nuciferine or dexamethasone significantly reduced the expression of TLR4 in a dose-dependent manner (the inhibition levels of TLR4 were 24.7%, 39.6%, 57.7% and 61.0%, respectively, p < 0.05, Fig. 6).
Effects of nuciferine on the NF-κB signaling pathway
In this study, the levels of NF-κB p65 and IκBα proteins were detected by Western blot method. As displayed in Fig. 7, LPS markedly induced phosphorylation of p65 and IκBα compared with the control group (the increase level of NF-κB p65 was 160.5%, p < 0.05). In contrast, the levels of phosphorylated p65 and IκBα were significantly decreased in the nuciferine groups (the inhibition levels of NF-κB p65 were 27.7%, 35.7%, 59.8% and 67.9%, respectively, p < 0.05).
Effects of nuciferine on the NF-κB p65 DNA-binding activity
To verify the effects of nuciferine on the NF-κB activation, we detected the DNA-binding activity of NF-κB p65 in nuclear extracts. As shown in Fig. 8, the DNA-binding activity of NF-κB p65 was remarkably increased after exposure to LPS (the increase level of NF-κB p65 was 400.0%, p < 0.05). However, pretreatment with nuciferine decreased LPS-induced increase in NF-κB p65 DNA-binding activity (the inhibition levels of NF-κB p65 were 21.6%, 40.1%, 55.7% and 61.6%, respectively, p < 0.05), suggesting nuciferine suppressed LPS-induced NF-κB activation.
Discussion
Mastitis is commonly recognized as an inflammatory response of the mammary gland caused by microbial infections, and has seriously hampered the development of dairy industry [2]. E. coli is one of the most common pathogenic microorganism and responsible for severe mastitis [23]. Although the efficacy of antibiotics in the treatment of mastitis is obvious, antibiotics residues in milk and dairy products are harmful to human health. Therefore, there is a medical need to develop a new therapeutic strategy against mastitis. Nuciferine is an aromatic ring-containing alkaloid extracted from the lotus leaf, and possesses various therapeutic effects such as hepatic steatosis [24]. And some recent studies have shown that nuciferine can alleviate renal injury by inhibiting renal injury inflammatory responses in rats [25]. A LPS-induced mouse mastitis model has been widely used as a practical approach to studying cow mastitis [19]. Therefore, we investigated the anti-inflammatory effect of nuciferine on LPS-induced mastitis and elucidated its possible mechanism in vitro.
In our study, it was found in the histopathological examination that LPS caused serious injury to the mammary gland tissues, such as mammary gland flocculus injury, inflammatory cells infiltration, enhanced adipose tissue, whereas administration of nuciferine improved the pathological changes. Furthermore, we also determined the effect of nuciferine on mMECs. The MTT assay showed that the doses of nuciferine used in the study were not cytotoxic, which was consistent with a previous study [20]. It has been well established that inflammatory cytokines play a pivotal role in the host defense against invading pathogenic microorganism [26]. Among these inflammatory cytokines, TNF-α and IL-1β are considered to be essential inflammatory mediators involved in the initiation and development of mastitis [27]. IL-1β is produced at the early phase of infection and recognized as a key inflammatory mediator [28]. TNF-α is an multi-functional pro-inflammatory cytokine secreted by activated macrophages, and can induce the production of other pro-inflammatory factors, such as IL-6 during the inflammatory response [29]. Proper levels of pro-inflammatory cytokine are important for immune responses against pathogens, but excessive cytokine production could cause severe cell damage [30]. Our results showed that nuciferine alleviated the inflammatory response LPS-stimulated mMECs by decreasing the production of these pro-inflammatory cytokines.
Toll-like receptor signaling is critical for immunity to a variety of intracellular pathogens [31]. TLR4 is an important immune receptor that is able to specifically recognize pathogen-associated molecules, such as LPS [32]. It had been reported that LPS can induce inflammatory responses through TLR4-NF-κB pathway [33]. To further explore the anti-inflammatory mechanism of action of nuciferine in LPS-stimulated mMECs, we then investigated the effects of nuciferine on TLR4 expression. The present results showed that the expression of TLR4 increased significantly in the LPS group but was decreased by nuciferine treatment. The NF-κB pathway plays a key role in regulating the expression of inflammatory cytokines [34,35,36]. NF-κB, a key nuclear transcription factor, has been reported to play an extremely important role in the regulation of pro-inflammatory cytokines production [37]. In the basal state, NF-κB p65 subunit is sequestered in the cytoplasm through association with an inhibitory protein IκBα [38]. Various inflammatory stimuli results in phosphorylation and degradation of IκBα, freeing NF-κB p65 subunit to translocate into the nucleus and promote the transcription of inflammatory genes [39, 40]. Our results showed that nuciferine inhibited the phosphorylation of p65 and IκBα in LPS-stimulated mMECs in a dose-dependent manner. Similar results were obtained from NF-κB p65 DNA-binding activity assay.
In summary, our results demonstrates that nuciferine has a anti-inflammatory effect in LPS-induced mastitis via suppressing the TLR4-NF-κB signaling pathway. Therefore, it is hoped that nuciferine may serve as a potential treatment for mastitis.
References
Taffurelli M, Pellegrini A, Santini D, Zanotti S, Di Simone D, Serra M. Recurrent periductal mastitis: surgical treatment. Surgery. 2016;160:1689–92.
LeBlanc SJ, Lissemore KD, Kelton DF, Duffield TF, Leslie KE. Major advances in disease prevention in dairy cattle. J Dairy Sci. 2006;89:1267–79.
Jiang KF, Zhao G, Deng GZ, Wu HC, Yin NN, Chen XY, et al. Polydatin ameliorates staphylococcus aureus-induced mastitis in mice via inhibiting tlr2-mediated activation of the p38 mapk/nf-κb pathway. Acta Pharmacol Sin. 2017;38(2):211–22.
Yang W, Zerbe H, Petzl W, Brunner RM, Gunther J, Draing C, et al. Bovine TLR2 and TLR4 properly transduce signals from Staphylococcus aureus and E. coli, but S. aureus fails to both activate NF-kappaB in mammary epithelial cells and to quickly induce TNFalpha and interleukin-8 (CXCL8) expression in the udder. Mol Immunol. 2008;45:1385–97.
Ibeagha-Awemu EM, Lee JW, Ibeagha AE, Bannerman DD, Paape MJ, Zhao X. Bacterial lipopolysaccharide induces increased expression of toll-like receptor (TLR) 4 and downstream TLR signaling molecules in bovine mammary epithelial cells. Vet Res. 2008;39:11.
Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511.
Roh E, Lee HS, Kwak JA, Jin TH, Nam SY, Jung SH, et al. MD-2 as the target of nonlipid chalcone in the inhibition of endotoxin LPS-induced TLR4 activity. J Infect Dis. 2011;203:1012–20.
Wu H, Zhao G, Jiang K, Chen X, Rui G, Qiu C, et al. Ifn-tau alleviates lipopolysaccharide-induced inflammation by suppressing nf-κB and mapks pathway activation in mice. Inflammation. 2016;39(3):1141–50.
Persson Waller K, Colditz IG, Lun S, Ostensson K. Cytokines in mammary lymph and milk during endotoxin-induced bovine mastitis. Res Vet Sci. 2003;74:31–6.
Kayitsinga J, Schewe RL, Contreras GA, Erskine RJ. Antimicrobial treatment of clinical mastitis in the eastern united states: the influence of dairy farmers' mastitis management and treatment behavior and attitudes. J Dairy Sci. 2017;100(2):1388–407.
Mehmeti I, Behluli B, Mestani M, Ademi A, Nes IF, Diep DB. Antimicrobial resistance levels amongst staphylococci isolated from clinical cases of bovine mastitis in Kosovo. J Infect Dev Ctries. 2016;10:1081–7.
Wu H, Zhao G, Jiang K, Chen X, Zhu Z, Qiu C, et al. Plantamajoside ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-kappaB and MAPK activation. Int Immunopharmacol. 2016;35:315–22.
Wu H, Zhao G, Jiang K, Li C, Qiu C, Deng G. Engeletin alleviates lipopolysaccharide-induced endometritis in mice by inhibiting TLR4-mediated NF-kappaB activation. J Agric Food Chem. 2016;64:6171–8.
Nguyen KH, Ta TN, Pham TH, Nguyen QT, Pham HD, Mishra S, et al. Nuciferine stimulates insulin secretion from beta cells-an in vitro comparison with glibenclamide. J Ethnopharmacol. 2012;142:488–95.
Guo F, Yang X, Li X, Feng R, Guan C, Wang Y, et al. Nuciferine prevents hepatic steatosis and injury induced by a high-fat diet in hamsters. PLoS One. 2013;8:e63770.
Ma W, Lu Y, Hu R, Chen J, Zhang Z, Pan Y. Application of ionic liquids based microwave-assisted extraction of three alkaloids N-nornuciferine, O-nornuciferine, and nuciferine from lotus leaf. Talanta. 2010;80:1292–7.
Wang MX, Liu YL, Yang Y, Zhang DM, Kong LD. Nuciferine restores potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Eur J Pharmacol. 2015;747:59–70.
Smalley MJ, Titley J, O’Hare MJ. Clonal characterization of mouse mammary luminal epithelial and myoepithelial cells separated by fluorescence-activated cell sorting. In Vitro Cell Dev Biol Anim. 1998;34:711–21.
Jiang K, Ma X, Guo S, Zhang T, Zhao G, Wu H, et al. Anti-inflammatory effects of rosmarinic acid in lipopolysaccharide-induced mastitis in mice. Inflammation. 2017;41(2):437–48.
Wu H, Yang Y, Guo S, Yang J, Jiang K, Zhao G, et al. Nuciferine ameliorates inflammatory responses by inhibiting the TLR4-mediated pathway in lipopolysaccharide-induced acute lung injury. Front Pharmacol. 2017;8:939. https://doi.org/10.3389/fphar.2017.00939.
Shao G, Tian Y, Wang H, Liu F, Xie G. Protective effects of melatonin on lipopolysaccharide-induced mastitis in mice. Int Immunopharmacol. 2015;29:263–8.
Zhang L, Sun D, Bao Y, Shi Y, Cui Y, Guo M. Nerolidol protects against lps-induced acute kidney injury via inhibiting tlr4/nf-κb signaling. Phytotherapy Res. 2017;31(3):459.
Yang Z, Yin R, Cong Y, Yang Z, Zhou E, Wei Z, et al. Oxymatrine lightened the inflammatory response of LPS-induced mastitis in mice through affecting NF-κB and MAPKs signaling pathways. Inflammation. 2014;37:2047–55.
Zhang DD, Zhang JG, Wu X, Liu Y, Gu SY, Zhu GH, et al. Nuciferine downregulates Per-Arnt-Sim kinase expression during its alleviation of lipogenesis and inflammation on oleic acid-induced hepatic steatosis in HepG2 cells. Fronti Pharmacol. 2015;6:238.
Wang MX, Zhao XJ, Chen TY, et al. Nuciferine Alleviates Renal Injury by Inhibiting Inflammatory Responses in Fructose-Fed Rats[J]. J Agric Food Chem. 2016;64(42):7899–910.
Hoeben D, Burvenich C, Trevisi E, Bertoni G, Hamann J, Bruckmaier RM, et al. Role of endotoxin and TNF-alpha in the pathogenesis of experimentally induced coliform mastitis in periparturient cows. J Dairy Res. 2000;67:503–14.
Zhu Y, Fossum C, Berg M, Magnusson U. Morphometric analysis of proinflammatory cytokines in mammary glands of sows suggests an association between clinical mastitis and local production of IL-1beta, IL-6 and TNF-alpha. Vet Res. 2007;38:871–82.
Gao XJ, Guo MY, Zhang ZC, Wang TC, Cao YG, Zhang NS. Bergenin plays an anti-inflammatory role via the modulation of MAPK and NF-kappaB signaling pathways in a mouse Model of LPS-induced mastitis. Inflammation. 2015;38:1142–50.
Gupta M, Babic A, Beck AH, Terry K. TNF-alpha expression, risk factors, and inflammatory exposures in ovarian cancer: evidence for an inflammatory pathway of ovarian carcinogenesis? Hum Pathol. 2016;54:82–91.
Lin YC, Schlievert PM, Anderson MJ, Fair CL, Schaefers MM, Muthyala R, et al. Glycerol monolaurate and dodecylglycerol effects on Staphylococcus aureus and toxic shock syndrome toxin-1 in vitro and in vivo. PLoS One. 2009;4:e7499.
Jiang K, Chen X, Zhao G, Wu H, Mi J, Qiu C, et al. IFN-tau plays an anti-inflammatory role in Staphylococcus aureus-induced endometritis in mice through the suppression of NF-kappaB pathway and MMP9 expression. J Interferon Cytokine Res. 2017;37:81–9.
Mateu A, Ramudo L, Manso MA, De Dios I. Cross-talk between TLR4 and PPARgamma pathways in the arachidonic acid-induced inflammatory response in pancreatic acini. Int J Biochem Cell Biol. 2015;69:132–41.
Zhang WJ, Frei B. Astragaloside IV inhibits NF-kappa B activation and inflammatory gene expression in LPS-treated mice. Mediators Inflamm. 2015;2015:274314.
Wu H, Zhao G, Jiang K, Chen X, Zhu Z, Qiu C, et al. Puerarin exerts an antiinflammatory effect by inhibiting NF-kB and MAPK activation in Staphylococcus aureus-induced mastitis. Phytother Res. 2016;30:1658–64.
Jiang K, Guo S, Zhang T, Yang Y, Zhao G, Shaukat A, et al. Downregulation of TLR4 by miR-181a provides negative feedback regulation to lipopolysaccharide-induced inflammation. Front Pharmacol. 2018;9:142. https://doi.org/10.3389/fphar.2018.00142.
Zhang F, Lu S, Jin S, Chen K, Li J, Huang B, et al. Lidanpaidu prescription alleviates lipopolysaccharide-induced acute kidney injury by suppressing the NF-κB signaling pathway. Biomed Pharmacother. 2018;99:245–52.
Morris KR, Lutz RD, Choi HS, Kamitani T, Chmura K, Chan ED. Role of the NF-kappaB signaling pathway and kappaB cis-regulatory elements on the IRF-1 and iNOS promoter regions in mycobacterial lipoarabinomannan induction of nitric oxide. Infect Immun. 2003;71:1442–52.
Jiang K, Zhang T, Yin N, Ma X, Zhao G, Wu H, et al. Geraniol alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis. Oncotarget. 2017;8:71038.
Li Q, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2:725–34.
Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, et al. 15-Deoxy-∆12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signaling pathway. Proc Natl Acad Sci USA. 2000;97:4844.
Acknowledgements
Fujian Provincial Key Laboratory for the Prevention and Control of Animal Infectious Diseases and Biotechnology (No. ZDSYS2017005).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible Editor: Andrew Roberts.
Rights and permissions
About this article
Cite this article
Chen, X., Zheng, X., Zhang, M. et al. Nuciferine alleviates LPS-induced mastitis in mice via suppressing the TLR4-NF-κB signaling pathway. Inflamm. Res. 67, 903–911 (2018). https://doi.org/10.1007/s00011-018-1183-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-018-1183-2