Abstract
Mastitis is a major disease in humans and other animals and is characterized by mammary gland inflammation. It is a major disease of the dairy industry. Bergenin is an active constituent of the plants of genus Bergenia. Research indicates that bergenin has multiple biological activities, including anti-inflammatory and immunomodulatory properties. The objective of this study was to evaluate the protective effects and mechanism of bergenin on the mammary glands during lipopolysaccharide (LPS)-induced mastitis. In this study, mice were treated with LPS to induce mammary gland mastitis as a model for the disease. Bergenin treatment was initiated after LPS stimulation for 24 h. The results indicated that bergenin attenuated inflammatory cell infiltration and decreased the concentration of NO, TNF-α, IL-1β, and IL-6, which were increased in LPS-induced mouse mastitis. Furthermore, bergenin downregulated the phosphorylation of nuclear factor-kappaB (NF-κB) and mitogen-activated protein kinases (MAPK) signaling pathway proteins in mammary glands with mastitis. In conclusion, bergenin reduced the expression of NO, TNF-α, IL-1β, and IL-6 proinflammatory cytokines by inhibiting the activation of the NF-κB and MAPKs signaling pathways, and it may represent a novel treatment strategy for mastitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Mastitis is a major disease restricting the dairy industry worldwide [1], and results in decreased milk production, increased health care costs, and many other problems [2]. Additional studies are necessary to develop new prophylactic and therapeutic approaches for bovine mastitis. Mastitis is the inflammation of the mammary gland, caused by infecting pathogenic microorganisms [3, 4]. Escherichia coli is the primary gram-negative bacterial pathogen that causes mastitis [5]. E. coli and its toxins may damage mammary epithelial cells and induce the epithelial cells to release inflammatory cytokines, causing an acute, severe inflammatory response [6].
Lipopolysaccharide (LPS), the main component of the outer membranes of E. coli, may trigger the inflammatory response [7, 8]. It has been shown that injection of LPS into mammary gland induces mastitis similar to E. coli infection [9]. The costs associated with experimental bovine mastitis are prohibitive; therefore, the use of smaller animals is required. Recently, LPS was diffusely used in studies of the mouse model of mastitis [10, 11]. Here, our study was performed with the mouse model of mastitis by injecting LPS into the mammary gland.
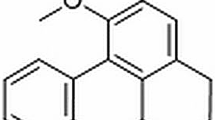
The antimicrobials used in the treatment of clinical mastitis may induce biofilm formation by E. coli [12], increasing the bacterial drug resistance [13]. An effective drug for mastitis is necessary for clinical therapy. Bergenin (Fig. 1), an active constituent of the plants of the genus Bergenia, is used as a folk medicine for the treatment of many diseases [14, 15]. Studies report that it has multiple biological activities, such as anti-oxidant [16], anti-inflammatory [17], anti-malarial [18], neuroprotective [19], and immunomodulatory [20] properties. The anti-inflammatory action of bergenin is associated with potent inhibition of the inflammatory mediators, NO and TNF-a, as demonstrated in macrophages stimulated with LPS [21]. The exact molecular mechanism of bergenin’s anti-inflammatory effects remains unclear, and there is little data about the anti-inflammatory effect of bergenin on mastitis. Therefore, the present study was performed to determine the effect of bergenin in the mouse model of LPS-induced mastitis and its mechanism of action.
MATERIALS AND METHODS
Animals
In the present study, 60 adult female BALB/c mice (6–8 weeks old, weighing 40–45 g) were used. All mice were provided by the center of Experimental Animals of Baiqiuen Medical College of Jilin University. All animal experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Jilin University.
Experimental Groups and Administration
Bergenin (Purity >99.9 %) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China) (Fig. 1). It was dissolved in physiological saline at concentrations of 100, 50, and 25 mg/kg. LPS (E. coli 055:B5) was diluted in sterile PBS and adjusted to a concentration of 0.2 mg/ml. The mice were divided into three groups as follows:
-
(1)
The LPS-stimulated group (LPS) was the mouse model of mastitis without drug treatment. A 100-μl syringe with a 30-gauge blunt needle was used to inoculate 50 μl LPS into the mammary gland through both the L4 (on the left) and R4 (on the right) of the abdominal mammary glands.
-
(2)
The bergenin administration group (BAG) was subjected to mastitis by LPS and intraperitoneally administered bergenin at 100, 50, and 25 mg/kg, injected four times at 6, 12, 18, and 24 h after LPS stimulation for 24 h.
-
(3)
The dexamethasone administration group (DEX) was subjected to mastitis by LPS and intraperitoneally administered dexamethasone.
-
(4)
The control group (CG) in which the mice received no treatment.
After the treatment, the mice were euthanized by CO2 inhalation, and the mammary gland was harvested and stored at −80 °C until analysis.
Histological Analysis
Immediately after euthanasia, the mammary tissues were harvested and fixed in 10 % formalin. After 1 week, the fixed tissues were embedded in paraffin, deparaffinized with xylene, and rehydrated with graded alcohol for staining. The tissue sections were stained with hematoxylin and eosin (H&E) and visualized with a microscope (Olympus, Japan).
Cytokine Enzyme-Linked Immunosorbent Assays
The mammary gland tissues were weighed and homogenized with phosphate-buffered saline (w/v 1/9) on ice and then centrifuged at 2000g for 40 min at 4 °C. Finally, the supernatants were collected to determine the levels of TNF-α, IL-1β, and IL-6 using the mouse enzyme-linked immunosorbent assay (ELISA) kits (BioLegend, Inc, San Diego, CA, USA) in accordance with the manufacturer’s instructions.
NO Assays (mol/kg)
The mammary gland tissues were weighed and homogenized with phosphate-buffered saline (w/v 1/9) on ice and then deproteinized with 10 % zinc sulfate. Nitrite and nitrate may produce NO. The total NO concentrations were determined using the acidic Griess reaction via the reduction of nitrate to nitrite by vanadium (III) chloride [22]. The nitric oxide concentrations were measured using a spectrophotometer in accordance with the manufacturer’s instructions of the kit (Nanjing Jiancheng BIO, Inc, NJ, China). The absorbance at 540 nm was measured using a microplate reader (Molecular Devices) after 10 min of incubation at room temperature.
Quantitative Real-Time Polymerase Chain Reaction
The total RNA from the mammary gland tissues (50 mg tissue; n = 3/diet group) was extracted using Trizol. The concentration and purity of the total RNA were determined spectrophotometrically at 260/280 nm. The cDNA was generated using the Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific, MA). The synthesized cDNA was diluted fivefold with sterile water and stored at −80 °C. The Primer Premier Software (PREMIER Biosoft International, USA) was used to design specific primers for IκB, nuclear factor-kappaB (NF-κB), p38, ERK, JNK, and β-actin based on known sequences (Table 1). The mRNA expression was measured via quantitative real-time polymerase chain reaction (qRT-PCR) using the SYBR Green Plus reagent kit (Roche, Swiss) and a 7500 Fast Real-Time PCR System (Applied Biosystems, USA). The amplification efficiency for each gene was determined using the DART-PCR program [23]. The mRNA relative abundance was calculated according to the method of Pfaffl [24], accounting for gene-specific efficiencies, and was normalized to the mean expression of β-actin.
The results (fold changes) were expressed as 2-ΔΔCt, in which ΔΔCt = (Ct IκB/NF-κB/p38/ERK/JNK -Ctβ-actin)t-(CtiNOS/IκB/NF-κB/p38/ERK/JNK-Ctβ-actin)c, where CtIκB/NF-κB/p38/ERK/JNK and Ctβ-actin) are the cycle thresholds for carp IκB/NF-κB/p38/ERK/JNK and β-actin genes in the different treated groups, respectively; t is the treatment group, and c is the control group.
Western Blot Analysis
The mammary tissue samples were homogenized, and the total protein was extracted with the T-PER Tissue Protein Extraction Reagent according to the manufacturer’s protocols. The protein concentrations were determined using the BCA protein assay. For Western blot analysis, each sample with an equal amount of protein (50 μg) was fractionated on 10 % SDS polyacrylamide gels, electrophoretically transferred onto polyvinylidene difluoride (PVDF) membrane, and blocked in 5 % skim milk in tris-Tween-buffered saline (TTBS) for 2 h at room temperature. The membranes were incubated with primary antibodies (1:1000 dilution) (Beverly, MA, USA) at 4 °C overnight. β-actin was used as a control. After washing the membrane with TTBS, incubation with a 1:5000 dilution (vol/vol) of secondary antibody (Buckinghamshire, UK) was performed for 1 h at room temperature. The specifically bound peroxidase was detected with the Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific, MA, USA). The images were captured using a MicroChemi 4.2 system (DNR Bio Imaging Systems, Jerusalem, Israel).
Data Analysis
The statistical analysis of the cytokine assays and mRNA level was performed using the SPSS statistical software for Windows (version 13; SPSS Inc., Chicago, IL, USA). The significance was determined via a one-way ANOVA using a significance level of P < 0.05. The data were assessed using the Tukey-Kramer method for multiple comparisons. All values were expressed as the means ± SD.
RESULTS
Pathological Histology Analysis
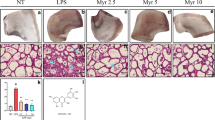
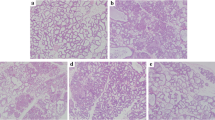
The mammary gland tissues from all groups were harvested at 24 h after LPS stimulation and bergenin treatment. The tissues sections were subjected to hematoxylin eosin (HE) staining. No pathological lesions were observed in the control group (Fig. 2a). There were mild pathological lesions in the DEX group (Fig. 2c). In the LPS-stimulated group without bergenin treatment (Fig. 2b), the inflammatory cells, including neutrophils and macrophages, were observed in the acinus. Some acini were injured. Many of the mammary epithelial cells were destroyed. The organizational structure of the mammary lobules was incomplete. However, bergenin inhibited the pathological damage caused by LPS. In the bergenin administration group, the inflammatory cell infiltration was decreased and the acini and lobules were not destroyed. The histopathological changes were ameliorated at drug concentrations of 25, 50, and 100 mg/kg (Fig. 2d, e, f).
Effect of Bergenin on Inflammatory Cytokines in LPS-Stimulated Mammary Gland
LPS-induced mastitis is related to many types of inflammatory cytokines. To determine the effect of bergenin on mammary glands with mastitis, the NO, TNF-α, IL-β, and IL-6 concentrations in the mammary gland were measured (Fig. 3). Compared to CG, stimulation with LPS led to a significant increase in NO, TNF-α, IL-1β, and IL-6 production. These increases were significantly inhibited by bergenin. These results indicate that bergenin significantly suppresses the production of NO, TNF-α, IL-1β, and IL-6 in a dose-dependent manner, with increasing concentrations of bergenin (25, 50, and 100 μg/ml). The production of these cytokines was slightly increased in the DEX group.
Inflammatory cytokines levels in mammary tissue. NO assays using the Griess reaction. TNF-α, IL-1β, and IL-6 ELISAs. The data represent 1 ml mammary homogenate supernatant and are presented as the means ± SEM (n = 10). CG control group, LPS LPS-stimulated group. The 25, 50, and 100 mg/kg were bergenin administration groups. #P <0.01 is significantly different from the CG. *P <0.05 is significantly different from the LPS group.
Regulation of the NF-κB Pathway by Bergenin in the Mammary Gland of LPS-Induced Mastitis Mice
NF-κB, bound to its inhibitor, IκB, is an important signaling molecule for development of inflammation. NF-κB pathway activation may enhance the transcription of proinflammatory genes. In the present study, the mRNA and protein expressions for IκB and NF-κB were measured using quantitative RT-PCR and Western blot analysis as shown in Fig. 4. Compared to the CG, there was a significant increase (P < 0.05) of the IκB and NF-κB mRNA and protein levels in the LPS group, bergenin groups, and the DEX group. Compared to the LPS group, the IκB and NF-κB mRNA levels were not significantly different from those of the (P > 0.1) bergenin groups. The LPS-stimulated group exhibited significantly higher levels of IκBα and P65 phosphorylation than the control group and treatment groups. However, bergenin significantly suppresses IκBα and P65 protein phosphorylation, with increasing concentrations of bergenin (25, 50, and 100 μg/ml).
Effect of bergenin on NF-κB activation. Western blot and qPCR were performed to detect the NF-κB and IκB protein and mRNA levels. Protein phosphorylation was also detected. β-actin was used as the control. CG is the control group, and the mice were untreated. LPS is the LPS-stimulated group. DEX is the dexamethasone administration group. The bergenin administration groups received 25, 50, and 100 mg/kg bergenin post-LPS stimulation. The values are the means ± SEM (n = 10). #P <0.01 is significantly different from the CG. *P <0.05 is significantly different from LPS.
Effect of Bergenin on the MAPK Pathway in Mammary Glands from LPS-Induced Mastitis Mice
The mitogen-activated protein kinase (MAPK) pathway may also mediate the development of an inflammatory response. The MAPKs are p38, JNK, and ERK. To determine whether MAPKs are affected by bergenin, the MAPKs were further examined. The mRNA, protein, and phosphorylation levels of all MAPKs were significantly increased in the LPS group (Fig. 5). There was no significant difference for the (P > 0.1) mRNA levels of JNK, ERK, and p38 between the LPS group and the bergenin administration groups. However, the results indicated that bergenin inhibited the LPS-induced phosphorylation of p38, ERK, and JNK. This effect is significant with increasing concentrations of bergenin (25, 50, and 100 μg/ml) (Fig. 5).
Effect of bergenin on MAPK activation. Western blot was performed to detect p38, ERK, and JNK protein levels and phosphorylation. The qPCR was performed to detect the mRNA of p38, ERK, and JNK. β-actin was used as the control. CG is the control group, and the mice were untreated. LPS is the LPS-stimulated group. DEX is the dexamethasone administration group. The bergenin administration groups received 25, 50, and 100 mg/kg bergenin post-LPS stimulation. The values are presented as the means ± SEM (n = 10). #P < 0.01 is significantly different from the CG. * P <0.05 is significantly different from LPS.
DISCUSSION
Mastitis, a serious disease for humans and animals [25], occurs as two types, clinical and subclinical mastitis [26]. Additional studies are necessary to develop new prophylactic and therapeutic approaches for mastitis and have been performed in the mouse model. The present study successfully used this model. Based on the histopathological observation, a severe inflammatory response was induced by LPS in the mammary gland tissues. The inflammatory cells, including neutrophils and macrophages, were observed in the acinus. Several acini were injured. Many of the mammary epithelial cells were destroyed. The organizational structure of the mammary lobules was incomplete. The results were the same as those of previous studies [6, 10, 11]. However, bergenin significantly inhibited the injury severity of the mammary gland. Although some inflammatory cells were among the acini in the bergenin administration group, the acinar structure was not destroyed. Previous studies reported that bergenin had a protective effect [18, 27]. For the first time, our results indicated that bergenin was protective to mammary gland tissues.
The expression of the proinflammatory cytokines, TNF-α, IL-1β, and IL-6, were increased by mastitis in the mammary gland [28]. A previous study showed that bergenin is a potent inhibitor of the inflammatory mediators, NO and TNF-α [21]. In the present study, NO, TNF-α, IL-1β, and IL-6 were analyzed. The result showed that bergenin significantly suppressed the production of NO, TNF-α, IL-1β, and IL-6, which were induced by LPS in a dose-dependent manner, and this was significant with increasing concentrations of bergenin (25, 50, and 100 μg/ml). These cytokines are involved in the host defense and pathophysiological progression of infectious and inflammatory diseases [29]. NO and TNF-α are early cytokines. Proinflammatory cytokines are indicators of inflammation [30, 31]. Previous studies reported that NO and TNF-α rapidly increase with LPS [32, 33], and our result was consistent with this. IL-1β and IL-6 play important roles in the regulation of the host immune responses [34]. Many studies reported that mice lacking IL-1β expression have increased susceptibility to infective inflammation [35]. This indicated that bergenin exerted its anti-inflammatory effect by reducing the expression of IL-1β in the mammary gland. The optimal levels of NO, TNF-α, IL-1β, and IL-6 are important to defense; however, excess concentrations mediate systemic inflammation, with destructive rather than protective effects on the host [36]. In the present study, we determined that bergenin was anti-inflammatory because it regulated NO, TNF-α, IL-1β, and IL-6 and prevented LPS-induced mastitis destruction of the mammary gland.
The expression of inflammatory cytokines is modulated by the NF-κB and MAPK pathways. To further assess the mechanism by which bergenin inhibits cytokine production, the effects of bergenin on the expression of IκB, NF-κB, and MAPKs (JNK, ERK, and p38) were examined. Upon phosphorylation, the NF-κB subunit, p65, dissociates from IκB and the inhibitory protein of NF-κB and translocates into the nucleus, where it initiates the transcription of inflammatory cytokines [37, 38]. ERK, JNK, and p38 are the primary MAPKs [39]. In the present study, the expression of NF-κB, IκB, and MAPK mRNAs was increased in the LPS group and the bergenin administration group. There were no significant differences between the groups (P > 0.1). The phosphorylation of the NF-κB and IκB proteins was attenuated in the bergenin administration group and was dose-dependent, demonstrating that bergenin decreased the dissociation of IκB and NF-κB and inhibited the subsequent translocation of NF-κB p65 into the nucleus. The expression of NO, TNF-α, IL-1β, and IL-6 was reduced. The phosphorylation of ERK, JNK, and p38 was significantly suppressed in the bergenin administration group. Many studies showed that the inhibition of phosphorylation of ERK, JNK, and p38 played an anti-inflammatory role in mastitis [6, 10, 28]. Both NF-κB and MAPK appear to be critical mediators of the inflammatory response. The present study demonstrated that bergenin inhibits the phosphorylation of NF-κB, IκB, and MAPKs and has beneficial effects in mastitis.
CONCLUSION
Bergenin may alleviate the inflammatory reaction of mastitis caused by LPS. Bergenin suppressed the production of proinflammatory mediators that were upregulated by LPS and regulated by the NF-κB and MAPK signaling pathways in the mammary gland with LPS-induced mastitis. The results of this study suggest that bergenin appears to be an effective drug for mastitis and may be used for clinical therapy.
References
Viguier, C., S. Arora, N. Gilmartin, K. Welbeck, and R. O’Kennedy. 2009. Mastitis detection: current trends and future perspectives. Trends in Biotechnology 27: 486–493.
Melchior, M.B., H. Vaarkamp, and J. Fink-Gremmels. 2006. Biofilms: a role in recurrent mastitis infections? Veterinary Journal 171: 398–407.
Carneiro, D.M.V.F., P.F. Domingues, and A.K. Vaz. 2009. Innate immunity of the bovine mammary gland: response to infection. Ciencia Rural 39: 1934–1943.
Bradley, A.J. 2002. Bovine mastitis: an evolving disease. Veterinary Journal 164: 116–128.
Silva, V.O., L.O. Soares, A. Silva Junior, H.C. Mantovani, Y.F. Chang, and M.A. Moreira. 2014. Biofilm formation on biotic and abiotic surfaces in the presence of antimicrobials by escherichia coli isolates from cases of bovine mastitis. Applied and Environmental Microbiology 80: 6136–6145.
Song X, Zhang W, Wang T, Jiang H, Zhang Z, Fu Y, et al. 2014. Geniposide plays an anti-inflammatory role via regulating TLR4 and downstream signaling pathways in lipopolysaccharide-induced mastitis in mice. Inflammation.
Atabai, K., and M.A. Matthay. 2002. The pulmonary physician incritical care. 5: acute lung injury and the acute respiratory distress syndrome: denitions and epidemiology. Thorax 57: 452–458.
Rubenfeld, G.D., E. Caldwell, E. Peabody, J. Weaver, D.P. Martin, M. Neff, et al. 2005. Incidence and outcomes of acute lung injury. The New England Journal of Medicine 353: 1685–1693.
Oliver, S., and L. Calvinho. 1995. Influence of inflammation on mammary gland metabolism and milk composition. Journal of Animal Science 73: 18–33.
Fu, Y.H., B. Liu, X.S. Feng, Z.C. Liu, D.J. Liang, F.Y. Li, et al. 2013. Lipopolysaccharide increases toll-like receptor 4 and downstream toll-like receptor signaling molecules expression in bovine endometrial epithelial cells. Veterinary Immunology and Immunopathology 151: 20–27.
Li, F., D. Liang, Z. Yang, T. Wang, W. Wang, X. Song, et al. 2013. Astragalin suppresses inflammatory responses via down-regulation of NF-kappaB signaling pathway in lipopolysaccharide-induced mastitis in a murine model. International Immunopharmacology 17: 478–482.
Costa, J.C., F. Espeschit Ide, F.A. Pieri, A. Benjamin Ldos, and M.A. Moreira. 2012. Increased production of biofilms by Escherichia coli in the presence of enrofloxacin. Veterinary Microbiology 160: 488–490.
Odenholt, I. 2001. Pharmacodynamic effects of subinhibitory antibiotic concentrations. International Journal of Antimicrobial Agents 17: 1–8.
Uniyal, S.K., K.N. Singh, P. Jamwal, and B. Lal. 2006. Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. Journal of Ethnobiology and Ethnomedicine 2: 14–21.
Ahmed, E., M. Arshad, M. Ahmad, M. Saeed, and M. Ishaque. 2004. Ethnopharmacological survey of some medicinally important plants of Galliyat areas of NWFP, Pakistan. Asian Journal of Plant Sciences 3: 410–415.
Heitor, A., L. Izandina, P. Gilmar, D. Piló-Veloso, and A. Antônio-Flávio. 2008. Antioxidant activity of (+)-bergenin-a phytoconstituent isolated from the bark of Sacoglottis uchi Huber (Humireaceae). Organic and Biomolecular Chemistry 6: 2713–2718.
Swarnalakshmi, T., M.G. Sethuraman, N. Sulochana, and R. Arivudainambi. 1984. Antimicrobial activity of bergenin from Endopleura uchi. Current Science 53: 917.
Uddin, G., A. Sadat, and B.S. Siddiqui. 2014. Comparative antioxidant and antiplasmodial activities of 11-O-galloylbergenin and bergenin isolated from Bergenia ligulata. Tropical Biomedicine 31: 143–148.
Takahashi, H., M. Kosaka, Y. Watanabe, K. Nakade, and Y. Fukuyama. 2003. Synthesis and neuroprotective activity of bergenin derivatives with antioxidant activity. Bioorganic & Medicinal Chemistry 11: 1781–1788.
Nazir, N., S. Koul, M.A. Qurishi, S.C. Taneja, S.F. Ahmad, S. Bani, et al. 2007. Immunomodulatory effect of bergenin and norbergenin against adjuvant-induced arthritis—a flow cytometric study. Journal of Ethnopharmacology 112: 401–405.
Shah, M.R., M. Arfan, H. Amin, Z. Hussain, M.I. Qadir, M.I. Choudhary, et al. 2012. Synthesis of new bergenin derivatives as potent inhibitors of inflammatory mediators NO and TNF-alpha. Bioorganic & Medicinal Chemistry Letters 22: 2744–2747.
Atakisi, O., H. Oral, E. Atakisi, O. Merhan, S. Metin Pancarci, A. Ozcan, et al. 2010. Subclinical mastitis causes alterations in nitric oxide, total oxidant and antioxidant capacity in cow milk. Research in Veterinary Science 89: 10–13.
Peirson S.N., Butler J.N., Foster R.G. 2003. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Research 31.
Pfaffl, M.W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research 29: e45.
Seegers, H., C. Fourichon, and F. Beaudeau. 2003. Production effects related to mastitis and mastitis economics in dairy cattle herds. Veterinary Research 34: 475–491.
Kim, K.W., J. Im, J.H. Jeon, H.G. Lee, C.H. Yun, and S.H. Han. 2011. Staphylococcus aureus induces IL-1 beta expression through the activation of MAP kinases and AP-1, CRE and NF-kappa B transcription factors in the bovine mammary gland epithelial cells. Comparative Immunology and Microbiology 34: 347–354.
Nazir, N., S. Koul, M.A. Qurishi, M.H. Najar, and M.I. Zargar. 2011. Evaluation of antioxidant and antimicrobial activities of bergenin and its derivatives obtained by chemoenzymatic synthesis. European Journal of Medicinal Chemistry 46: 2415–2420.
Li, D., N. Zhang, Y. Cao, W. Zhang, G. Su, Y. Sun, et al. 2013. Emodin ameliorates lipopolysaccharide-induced mastitis in mice by inhibiting activation of NF-kappaB and MAPKs signal pathways. European Journal of Pharmacology 705: 79–85.
Boudjellab, N., H.S. Chan-Tang, and X. Zhao. 2000. Bovine interleukin-1 expression by cultured mammary epithelial cells (MAC-T) and its involvement in the release of MAC-T derived interleukin-8. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology 127: 191–199.
Li, B., J. Li, X. Pan, G. Ding, H. Cao, W. Jiang, J. Zheng, and H. Zhou. 2010. Artesunate protects sepsis model mice challenged with Staphylococcus aureus by decreasing TNF-alpha release via inhibition TLR2 and Nod2 mRNA expressions and transcription factor NF-kappaB activation. International Immunopharmacology 10: 344–350.
Lo Faro, M.L., B. Fox, J.L. Whatmore, P.G. Winyard, and M. Whiteman. 2014. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide: Biology and Chemistry/Official Journal of the Nitric Oxide Society 41C: 38–47.
Fu, Y.H., B. Liu, J.H. Liu, Z.C. Liu, D.J. Liang, F.Y. Li, et al. 2012. Geniposide, from Gardenia jasminoides Ellis, inhibits the inflammatory response in the primary mouse macrophages and mouse models. International Immunopharmacology 14: 792–798.
Dilshara, M.G., K.T. Lee, R.G.P.T. Jayasooriya, C.H. Kang, S.R. Park, Y.H. Choi, et al. 2014. Downregulation of NO and PGE(2) in LPS-stimulated BV2 microglial cells by trans-isoferulic acid via suppression of PI3K/Akt-dependent NF-kappa B and activation of Nrf2-mediated HO-1. International Immunopharmacology 18: 203–211.
Guo, M., N. Zhang, D. Li, D. Liang, Z. Liu, F. Li, et al. 2013. Baicalin plays an anti-inflammatory role through reducing nuclear factor-kappaB and p38 phosphorylation in S. aureus-induced mastitis. International Immunopharmacology 16: 125–130.
Miller, L.S., E.M. Pietras, L.H. Uricchio, K. Hirano, S. Rao, H. Lin, R.M. O’Connell, Y. Iwakura, A.L. Cheung, G. Cheng, and R.L. Modlin. 2007. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. Journal of Immunology 179: 6933–6942.
Boulanger, D., E. Brouillette, F. Jaspar, F. Malouin, J. Mainil, F. Bureau, and P. Lekeux. 2007. Helenalin reduces Staphylococcus aureus infection in vitro and in vivo. Veterinary Microbiology 119: 330–338.
Li, Q., and I.M. Verma. 2002. NF-kappaB regulation in the immune system. Nature Reviews Immunology 2: 725–734.
Godowski, P.J. 2005. A smooth operator for LPS responses. Nature Immunology 6: 544–546.
Liang, D.J., Y. Sun, Y.B. Shen, F.Y. Li, X.J. Song, E.S. Zhou, et al. 2013. Shikonin exerts anti-inflammatory effects in a murine model of lipopolysaccharide-induced acute lung injury by inhibiting the nuclear factor-kappaB signaling pathway. International Immunopharmacology 16: 475–480.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (Nos. 31272622, 31201925), the Research Fund for the Doctoral Program of Higher Education of China (Nos. 20110061130010, 20120061120098), and the Fundamental Research Funds for the Central Universities (No. 2662014BQ024)
Author information
Authors and Affiliations
Corresponding author
Additional information
Xue-jiao Gao and Meng-yao Guo contributed equally to this article.
Rights and permissions
About this article
Cite this article
Gao, Xj., Guo, My., Zhang, Zc. et al. Bergenin Plays an Anti-Inflammatory Role via the Modulation of MAPK and NF-κB Signaling Pathways in a Mouse Model of LPS-Induced Mastitis. Inflammation 38, 1142–1150 (2015). https://doi.org/10.1007/s10753-014-0079-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-0079-8