Abstract
Objectives
Impact of ROS in development of hyperalgesia has recently motivated scientists to focus on ROS as novel target of anti-hyperalgesic interventions. However, role of ROS in molecular signaling of hyperalgesia is still poorly understood. The present study is aimed to analyze the effect of dietary antioxidant resveratrol on antioxidant defense system, ROS level and TNFR1–ERK signaling pathway during early and late phase of inflammatory hyperalgesia.
Methods and materials
Hyperalgesia was assessed by paw withdrawal latency test in complete Freund’s adjuvant-induced hyperalgesic rats. Activities of antioxidant enzymes were measured by in-gel assays, ROS level was measured by DCFH2DA, and expression of pERK, ERK and TNFR1 was estimated by Western blotting.
Results
Anti-hyperalgesic effect of resveratrol was observed by paw withdrawal latency test. ROS level was increased in paw skin as well as spinal cord during early phase which was further increased in paw skin, but remained constant in spinal cord up to late phase. Resveratrol differentially regulated the activities of SOD, catalase and GPx in paw skin as well as spinal cord of hyperalgesic rats in both phases. Activities were normalized back showing anti-hyperalgesic effect of resveratrol. Upregulated ERK signaling was modulated by resveratrol, whereas TNFR1 level remained unchanged.
Conclusion
Overall results suggest that resveratrol alleviates inflammatory hyperalgesia by downregulation of ERK activation, modulation of ROS and differential regulation of antioxidant enzymes during early and late phases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute pain acts as a warning system to detect noxious stimuli and to avoid potential tissue damage. Noxious stimulation of peripheral tissue leads to hypersensitivity of specialized peripheral nerves (nociceptors) causing acute inflammatory hyperalgesia via signaling through spinal cord and brain. The initiated hyperalgesia may lead to sensitization of central nerve terminals leading to chronic pain [1]. Presently, nonsteroidal anti inflammatory drugs (NSAID) are effective in treating acute inflammatory pain, although with serious side effects. Steroids are also used to treat inflammatory pain, but they have non-specific targets. Therefore, interest was focused to develop new approaches for treatment of pain.
ROS is known to modulate various signaling pathways in cell proliferation, apoptosis, necrosis, etc. Recent studies indicate ROS as key factor during initiation and maintenance of hyperalgesia. However, their exact mode of action still needs to be elucidated. Accordingly, ROS is considered as new target of anti-hyperalgesic interventions. Use of natural antioxidants as anti-hyperalgesic agents is being investigated as they may have the advantage of intervening hyperalgesia with fewer side effects. Resveratrol, a grape polyphenol has attracted attention for its potent and long-lasting anti-nociceptive effects [2, 3]. Inflammatory and opioidergic pathways are suggested to be involved in anti-hyperalgesic effect of resveratrol [2,3,4,5]. Interestingly, these pathways are linked with concomitant ROS generation [6,7,8]. Anti-nociceptive effect of resveratrol during inflammatory hyperalgesia has been reported by us via differential regulation of pro-inflammatory mediators [4]. Therefore, it was hypothesized that anti-nociceptive effect of resveratrol might be attributed to its antioxidant property.
Reactive oxygen species (ROS) are highly reactive oxygen-containing molecules. Oxidative burden imposed by ROS is balanced by antioxidant defense system of an organism. Accumulation of ROS leads to various diseases. ROS has been reported to induce neuropathic hyperalgesia [9,10,11,12,13]. Therefore, natural or synthetic antioxidants should be able to ameliorate hyperalgesia. We have reported the alleviation of thermal hyperalgesia to be associated with improvement in antioxidant defense system and reduction in oxidative stress by curcumin [14]. However, their specific role in initiation and maintenance of hyperalgesia is still not understood. Along with non-enzymatic components, antioxidant enzymes have a crucial role in antioxidant defense system. ROS may regulate these enzymes at transcriptional or posttranslational levels [15]. Further, various antioxidant enzymes may be modulated in different ways [16]. Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) are major constituent of antioxidant defense system. Both isozymes of SOD, Cu–Zn SOD and Mn-SOD protect cells from oxidative damage at the initial step by scavenging superoxide radicals. Hydrogen peroxide a byproduct of SOD may further be converted into water by either catalase or GPx [17, 18].
ROS may regulate a number of downstream signaling pathways leading to inflammation and hyperalgesia. Pro-inflammatory cytokine TNF-α is a major cytokine in inflammatory soup which has a lead role in activating a cascade of other cytokines in pain signaling [4, 19]. TNF-α increases the expression of transient receptor potential vanilloid 1 (TRPV1), which is a cation channel having a major role in nociceptive signaling. TNF-α receptor 1 (TNFR1) and TRPV1 are reciprocally activated in nociceptive neurons, mediated via ERK activation and ROS generation [20, 21]. Further, ROS may activate ERK and other MAPKs [22]. All the three MAPK pathways are reported to be involved in hyperalgesia [23]. Therefore, it may be hypothesized that ROS sensitize nociceptors by MAPK activation, and antioxidant resveratrol should be able to reduce the signaling pathway toward anti-hyperalgesic action.
Based on the hypothesis, we investigated the role of antioxidant enzymes, ROS and downstream targets TNFR1 and ERK in anti-nociceptive effect of resveratrol in initiation and maintenance of hyperalgesia. Early phase (6 h after CFA administration) and late phase (48 h after CFA administration) were selected to represent initiation and maintenance of hyperalgesia on the basis of our earlier report [4]. Paw skin and spinal cord were used to understand the molecular alteration at peripheral and central levels, respectively. Molecular analysis has been correlated with behavioral assessment of hyperalgesia during early as well as late phase.
Materials and methods
Materials
Analytical- and molecular biology-grade chemicals were used in the study. Complete Freund’s adjuvant (CFA) was purchased from Sigma-Aldrich (Saint Louis, USA). Resveratrol (purity ≥98%) was purchased from Cayman Chemicals (Ann Arbor, USA). Hydrogen peroxide, NBT (nitro blue tetrazolium), ferric chloride, potassium ferricyanide, riboflavin, PMS (phenazine methosulfate) and GSH (reduced glutathione) were purchased from SRL (Mumbai, India). Polyclonal anti-TNFR1, anti-ERK-1/2 and anti-pERK-1/2 rabbit antibodies were purchased from BioVision (Milpitas, CA, USA), and monoclonal anti-β-actin was purchased from Sigma-Aldrich (Saint Louis, USA). HRP-conjugated goat anti-rabbit secondary antibody was procured from Merck-Genei (Bangalore, India).
Animals and drug treatment
Charles Foster strain rats were bred and maintained under standard laboratory conditions with proper human care, as per guidelines of Institutional Animal Ethical Committee, Banaras Hindu University, Varanasi, at 25 ± 2 °C with 12-h light/dark schedules with ad libitum supply of standard animal food and drinking water. Normal adult (12–14 weeks old) male rats were used for induction of inflammatory hyperalgesia. All experiments were performed with the approval of Institutional Animal Ethical Committee, Banaras Hindu University (Letter No. Dean/10-11, dated 28.04.2011). Animals were categorized into four groups having 12 rats per group. Hyperalgesia was developed by intraplantar (i.pl.) injection of 100 μl CFA in three groups. Out of three CFA-induced groups, one group received saline (C), second group received DMSO as a vehicle control (CD), and third group received resveratrol dissolved in DMSO (CR) in 50 μl volume via i.p route. Control group received 100 μl of saline via i.pl. and 50 μl via i.p. route (N). Resveratrol was injected immediately after CFA administration. Resveratrol dose was selected as 20 mg kg/body wt/day on the basis of earlier reports [4]. Six animals of each group were killed after 6 h, and remaining animals were killed after 48 h of CFA administration.
Assessment of thermal hyperalgesia
Thermal hyperalgesia was measured by paw withdrawal latency test as described earlier [4]. Briefly, animals were placed on a hot plate (Eddy’s Hot-Plate, Orchid Scientific, India) with the temperature adjusted to 50 ± 0.5 °C. Hyperalgesia was assessed up to 48 h by measuring the latency period of paw licking or jump response. Three consecutive latencies measured at 10-min intervals were averaged. The cutoff time was 10 s in order to avoid tissue damage. The observer was blinded to all treatments until the analysis of results.
Preparation of tissue homogenate
Ipsilateral hind paw skin as well as lumbar spinal cord (L4–L6) were removed from all animals, washed with PBS and stored at −80 °C until use. Tissues were homogenized in 50 mM potassium phosphate buffer (pH 7.4), containing 1 mM PMSF, protease and phosphatase inhibitor cocktail and 0.1% Triton X-100 with a polytron homogenizer, and centrifuged at 14,000g for 20 min at 4 °C. The supernatant was used for in-gel activity assay of antioxidant enzymes and Western blot analysis.
Measurement of ROS
The ROS level was measured by using 2′,7′-dichlorofluorescein diacetate (H2DCFDA) as described previously [24]. Skin or spinal cord extracts were incubated at 37 °C for 60 min with equal volume of 2 mM H2DCFDA (Invitrogen). Fluorescence was recorded at 485 nm (excitation) and 527 nm (emission) with HITACHI F-3000 fluorescence spectrophotometer and presented in arbitrary units (AU) in terms of fluorescence intensity/mg protein.
In-gel activity assay of antioxidant enzymes
Catalase
Catalase activity of was analyzed by native polyacrylamide gel electrophoresis followed by ferricyanide method described by Woodbury et al. [25]. 30 μg of protein was resolved on 8% resolving gel, at 4 °C. After completion of electrophoresis, the gels were washed with distilled water and soaked in H2O2 (0.03%) for 5 min and rinsed with distilled water to remove excess H2O2. The gels were stained in a solution containing 1% ferric chloride and 1% potassium ferricyanide for 4–5 min at room temperature.
Superoxide dismutase (SOD)
The activity of SOD was analyzed by a procedure described by Beauchamp and Fridovich [26]. 30 μg of total protein from each sample was loaded and separated on 10% resolving gel by non-denaturing PAGE at 4 °C. After completion of electrophoresis, the gels were soaked in 1.23 mM NBT solution for 20 min at room temperature in dark. Gels were washed with distilled water and incubated in 100 mM phosphate buffer (pH 7.0) containing 28 mM TEMED and 0.28 mM riboflavin for 15–20 min at room temperature in dark. Thereafter, the gels were exposed to fluorescent light until achromatic bands appeared with purple-blue background.
Glutathione peroxidase (GPx)
The activity gel assay of GPx was performed according to the method described by Lin et al. [27]. 100 μg of protein from each sample was loaded and separated on 10% resolving gel by non-denaturing PAGE at 4 °C. After completion of electrophoresis, the gels were soaked in 50 mM tris–HCl (pH 7.9) containing 13 mM GSH and 0.004% H2O2 for 20 min in dark. Thereafter, gels were washed and developed in darkness at 30 °C containing 1.2 mM NBT and 1.6 mM PMS for 10 min and exposed to fluorescent light until appearance of clear zone of GPx band on purple background.
Western blotting
Levels of pERK, total ERK as well as TNFR1 were detected by Western blot analysis. Equal amount of protein extracts from each group were separated on 10% SDS-polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked with nonfat milk for 2 h. Blots were incubated overnight with primary antibodies (1:1000), washed in TBST for 5–10 min and incubated with HRP-conjugated anti-rabbit secondary antibody (1:2500) for 2 h. Bands were visualized using enhanced chemiluminescence (ECL) and detected on X-ray film.
Densitometric and statistical analysis
Data of the behavioral study were analyzed by repeated measure ANOVA. Group-wise comparisons were based on one-way ANOVA followed by Tukey’s post hoc test using SPSS (Statistical Package for the Social Sciences) software. ROS level was analyzed by two-way ANOVA. All other data were analyzed by one-way ANOVA followed by Tukey’s post hoc test. Densitometric analysis was performed by using Alpha Imager 2200 software (Alpha Innotech). Activity of antioxidant enzymes was measured on the basis of integrated densitometric value and presented in arbitrary units (AU). Increment or decrement was calculated by taking the activities of normal groups as 100%. Values were expressed as mean ± SEM. p < 0.05 was taken as statistically significant.
Results
Peripheral sensitization of nociceptive neurons takes place at the site of injury or inflammation (paw skin). Nociceptive neurons of hind paw innervate spinal cord in lumbar region (L4–L6). Therefore, all parameters were analyzed in paw skin and lumbar region of spinal cord. The initial hyperalgesic behavior is generated by peripheral sensitization of neurons; however, hyperalgesic response in late phase denotes central sensitization of neurons. Therefore, two time points (6 and 48 h) were selected for the study.
Effect of resveratrol on thermal hyperalgesia
CFA is conventionally used to develop inflammation and hyperalgesia [28]. The hyperalgesic effect of CFA administration was evidenced at different time intervals from 2 to 48 h. CFA-induced rats (C) showed significant decreases in paw withdrawal latency by approximately 35% (p < 0.05), 48% (p < 0.05), 50% (p < 0.05), 54% (p < 0.005) and 50% (p < 0.05) at 2, 6, 12, 24 and 48 h, respectively, as compared to control (N). DMSO did not show any significant difference.
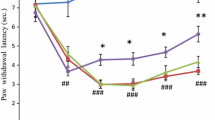
Resveratrol showed anti-hyperalgesic effect from 6 to 48 h (Fig. 1). The paw withdrawal latency was increased in resveratrol-treated rats (CR) by approximately 20% (p < 0.05), 38% (p < 0.05), 47% (p < 0.01) and 41% (p < 0.01) at 6, 12, 24 and 48 h, respectively, as compared to DMSO-injected rats (CD). The pattern of changes in behavioral hyperalgesia followed similar pattern as found during earlier work [4].
Effect of resveratrol on paw withdrawal latency in hyperalgesic rats. Anti-hyperalgesic activity in terms of paw withdrawal latency by hot plate test in normal (N), CFA-treated (C), CFA + DMSO-treated (CD) and CFA + resveratrol-treated (CR) rats at different time intervals after CFA injection. Each group included 6–9 rats. Results represent mean ± SEM obtained from three different sets of experiments. Hash denotes significant difference as compared with N group (# p < 0.05). Asterisk denotes significant difference as compared with CD group (*p < 0.05)
Comparison of ROS level during early and late phases
ROS level was found to be differentially altered in tissue specific manner. The level of ROS was progressively elevated from early phase (approximately by 129%) to late phase (approximately by 200%) in paw skin (Fig. 2a), whereas in spinal cord, ROS level was increased by approximately 33% in early phase and remained constant in late phase (Fig. 2b). Resveratrol treatment could bring down the ROS level up to almost normal level during early phase (6 h) in both tissues. Similarly, it was significantly decreased by resveratrol treatment during late phase. However, it remained approximately 37 and 17% higher than the normal level in paw skin and spinal cord, respectively.
Effect of resveratrol on ROS level in paw skin and spinal cord during early and late phases. A total of 48 rats were distributed into four groups with 12 rats in each group. First group of rats were injected with 100 µl normal saline via i.pl. route and 50 μl via i.p. route (N). For induction of hyperalgesia, 100 μl CFA was injected in remaining three groups via intraplantar (i.pl.) route. Out of three CFA-induced groups, one group received 50 μl of saline as control (C), second group received 50 μl DMSO as a vehicle control (CD), and third group received resveratrol dissolved in 50 μl DMSO (CR) via i.p route. Resveratrol (20 mg/kg body wt) was injected immediately after CFA administration. Six rats from each group were killed after 6 h (early phase). Remaining six rats were injected with additional dose of resveratrol at 24 h and killed at 48 h. Levels of ROS in a ipsilateral paw skin and b spinal cord were measured by oxidative conversion of H2DCFDA to fluorescent DCF. Results represent mean ± SEM. Asterisk represents significant difference at the level p < 0.05, and ‘ns’ denotes not significant difference
Effect of resveratrol treatment on antioxidant enzymes
Effect of resveratrol on antioxidant defense system was evaluated by monitoring antioxidant enzymes in hyperalgesic rats. The activities of primary antioxidant enzymes (CAT, SOD and GPX) were analyzed in paw skin and spinal cord of rats.
Catalase
In case of paw skin, the activity of catalase was found to be reduced after 6 h of CFA injection which was approximately 53% as compared to the normal rats; however, resveratrol treatment caused enhancement in the activity up to approximately normal level (Fig. 3a). However, in spinal cord, activity was increased by 27% as compared to normal rats, which was brought back to approximately normal level after resveratrol treatment (Fig. 4a). Catalase activity was found to be unaltered in paw skin of CFA-induced (C) and resveratrol-treated CFA-injected (CR) rats at 48-h time point (Fig. 5a). However, activity of catalase was higher in spinal cord of CFA-induced rats by approximately 58% which was normalized by resveratrol treatment (Fig. 5d).
Effect of resveratrol treatment on catalase, GPx and SOD activity in paw skin of hyperalgesic rats during early phase. A total of 24 rats were distributed into four groups with six rats in each group. First group of rats were injected with 100 µl normal saline via i.pl. route and 50 μl via i.p. route (N). For induction of hyperalgesia, 100 μl CFA was injected in remaining three groups via intraplantar (i.pl.) route. Out of three CFA-induced groups, one group received 50 μl of saline as control (C), second group received 50 μl DMSO as a vehicle control (CD), and third group received resveratrol dissolved in 50 μl DMSO (CR) via i.p route. Resveratrol (20 mg/kg body wt) was injected immediately after CFA administration. Rats were killed after 6 h (early phase). Activity of antioxidant enzymes a catalase, b SOD and c GPx was measured in paw skin. Results represent mean ± SEM. Hash denotes significant difference as compared with N group. Asterisk denotes significant difference as compared with CD group
Effect of resveratrol treatment on catalase, GPx and SOD activity in spinal cord of hyperalgesic rats during early phase. A total of 24 rats were distributed into four groups with six rats in each group. Rats of control group were injected with 100 µl normal saline via i.pl. route and 50 μl via i.p. route (N). For induction of hyperalgesia, 100 μl CFA was injected in remaining three groups via intraplantar (i.pl.) route. Out of three CFA-induced groups, one group received 50 μl of saline as control (C), second group received 50 μl DMSO as a vehicle control (CD), and third group received resveratrol dissolved in 50 μl DMSO (CR) via i.p route. Resveratrol (20 mg/kg body wt) was injected immediately after CFA administration. Rats were killed after 6 h (early phase). Activities of antioxidant enzymes a catalase, b SOD and c GPx were measured in spinal cord. Results represent mean ± SEM. Hash denotes significant difference as compared with N group. Asterisk denotes significant difference as compared with CD group
Effect of resveratrol treatment on catalase, GPx and SOD activity in paw skin and spinal cord of hyperalgesic rats during late phase. A total of 24 rats were distributed into four groups with six rats in each group. First group of rats were injected with 100 µl normal saline via i.pl. route and 50 μl via i.p. route (N). For induction of hyperalgesia, 100 μl CFA was injected in remaining three groups via intraplantar (i.pl.) route. Out of three CFA-induced groups, one group received 50 μl of saline as control (C), second group received 50 μl DMSO as a vehicle control (CD), and third group received resveratrol dissolved in 50 μl DMSO (CR) via i.p route. Resveratrol (20 mg/kg body wt) was injected immediately after CFA administration. Rats were killed after 48 h (late phase). The activities of antioxidant enzymes a catalase, b SOD and c GPx in paw skin and d catalase, e SOD and f GPx in spinal cord were measured by in-gel activity assay. Results represent mean ± SEM. Hash denotes significant difference as compared with N group. Asterisk denotes significant difference as compared with CD group
Superoxide dismutase (SOD)
The pattern of SOD activity in CFA-injected (C) as well as resveratrol-treated CFA-injected (CR) rats was similar to that of catalase activity in both tissues at 6 h. The activity of SOD was reduced in paw skin after 6 h of CFA injection which was approximately 63% as compared to normal, and was enhanced up to approximately 85% of normal after resveratrol treatment (Fig. 3b). SOD activity was increased by 120% as compared to normal rats in case of spinal cord. Resveratrol treatment brought the activity up to almost normal level (Fig. 4b). However, no changes were observed in the activity of SOD in skin and spinal cord after 48 h of CFA and resveratrol treatment (Fig. 5b, e).
Glutathione peroxidase (GPx)
Similar variation pattern of GPx activity in CFA-injected (C) as well as resveratrol-treated CFA-injected (CR) rats was found in both tissues at 6 h. The activity of GPx was reduced in paw skin after CFA injection which was approximately 69% as compared to normal, and was enhanced up to approximately 86% of normal after resveratrol treatment (Fig. 3c), whereas GPx activity was increased by 30% as compared to normal rats in case of spinal cord. Resveratrol treatment brought back the activity almost up to normal level (Fig. 4c). However, no change was observed in the activity of GPx in CFA-injected (C) as well as resveratrol-treated CFA-injected (CR) rats after 48 h, following the pattern of SOD activity (Fig. 5c, f).
All the three enzymes show changes in early phase in both the tissues in case of CFA-induced and resveratrol-treated CFA-induced rats. However, no such change in enzyme activities was observed in paw skin in the two groups of rats in late phase. In case of spinal cord, only catalase shows increase in activity in CFA-induced rats during late phase, which is decreased after resveratrol treatment.
Effect of resveratrol treatment on the level of pERK, ERK and TNFR1
ERK activation was measured indirectly by ratio of pERK/ERK. This ratio was found to be significantly high in CFA-injected rats (C). The pERK/ERK ratio was increased by approximately 51 and 49%, respectively, at 6 and 48 h in spinal cord (Fig. 6a, b). Resveratrol treatment caused a decrease in ERK phosphorylation up to approximately normal level at both the time points. Activation of early gene ERK has been recently considered as neuronal marker of pain [29]. Therefore, downregulation of pERK/ERK ratio by resveratrol indicated its anti-hyperalgesic potential.
Effect of resveratrol treatment on ERK phosphorylation and TNFR1 level in spinal cord. A total of 48 rats were distributed into four groups with 12 rats in each group. First group of rats were injected with 100 µl normal saline via i.pl. route and 50 μl via i.p. route (N). For induction of hyperalgesia, 100 μl CFA was injected in remaining three groups via intraplantar (i.pl.) route. Out of three CFA-induced groups, one group received 50 μl of saline as control (C), second group received 50 μl DMSO as a vehicle control (CD), and third group received resveratrol dissolved in 50 μl DMSO (CR) via i.p route. Resveratrol (20 mg/kg body wt) was injected immediately after CFA administration. Six rats from each group were killed after 6 h (early phase). Remaining six rats were injected with additional dose of resveratrol at 24 h and killed at 48 h. Levels of pERK and ERK at a 6 h and b 48 h and the level of TNFR1 at c 6 h and d 48 h were measured by Western blot analysis in spinal cord. Results represent mean ± SEM. Hash denotes significant difference as compared with N group. Asterisk denotes significant difference as compared with CD group
TNFR1-mediated ERK activation is involved in hyperalgesic signaling [20]. Therefore, we examined the effect of resveratrol treatment on TNFR1 level. However, no effect was found in CFA-injected (C) as well as resveratrol-treated CFA-injected (CR) rats in spinal cord at both time points (Fig. 6c, d). The results indicated TNFR1-independent action of ROS and resveratrol on ERK activation.
Discussion
Paw withdrawal latency is reciprocally related to extent of hyperalgesia. Paw withdrawal latency test showed that hyperalgesia was fully developed at 6 h and was maintained up to 48 h in CFA-induced rats. Therefore, hyperalgesia at 6 and 48 h is categorized as early and late phase, respectively. Anti-hyperalgesic effect of resveratrol was shown in both phases.
Generation of ROS has been earlier correlated with hyperalgesia development [30]. We have traced out the pattern of ROS generation during early and late phases at peripheral and central levels in CFA-induced hyperalgesia. Differential pattern of changes in ROS level was found at peripheral and central sites. The level of ROS was progressively elevated during early and late phases in paw skin (Fig. 2a). However, in spinal cord, the increase in early phase did not continue further in late phase (Fig. 2b). Development of hyperalgesic response is reported to be associated with modulation in activities of antioxidant enzymes; however, pattern of alteration varies with different antioxidant enzymes during neuropathic chronic pain. Naik et al. [31] have demonstrated decreased SOD activity and unaltered catalase activity in sciatic nerve in CCI model of neuropathic pain. Guedes et al. [32] have shown decreased activity of catalase and SOD in spinal cord of SNT model of neuropathic pain. In contrast, Varija et al. [33] have reported increased SOD and GPx activity and decreased catalase activity in different tissues of silver wire-ligated neuropathic pain model. We have earlier demonstrated a decreased activity of antioxidant enzymes in paw skin and increased activity in spinal cord in early phase of CFA-induced hyperalgesic rats [14]. Discrepancies in these reports might be a result of differences in animal models, tissues or time interval after induction of hyperalgesia. On the basis of these possibilities, we planned to analyze the activities of antioxidant enzymes at peripheral (paw skin) as well as central level (spinal cord) both at early and late phase of CFA-induced hyperalgesia. Differential pattern of changes in antioxidant enzymes in paw skin and spinal cord at early and late phases matches with changes in ROS level.
Synthetic antioxidant mimetic compounds or purified natural antioxidants are known to show anti-hyperalgesic effects [13, 34]. However, modulation of endogenous antioxidant enzymes is not well studied in inflammatory pain. In our previous study, modulation of antioxidant defense system was suggested to be an early event of CFA-induced hyperalgesia. In the present study, differential modulation of antioxidant enzymes was observed at peripheral and central level. The activities of antioxidant enzymes SOD, catalase and GPx were attenuated in paw skin and induced in spinal cord of hyperalgesic rats at 6 h. Decreased activity in paw skin and increased activity of enzymes in spinal cord are correlated with high level of ROS in paw skin as compared to spinal cord at early phase. The level of ROS was elevated by approximately 85% in paw skin and 33% in spinal cord during early phase (Fig. 2a, b). Opposite effects on activities of antioxidant enzymes in paw skin and spinal cord may be explained on the basis of differential response of these enzymes for difference in the level of ROS. Moderate level of ROS in spinal cord may lead to Nrf-2 mediated up regulation of antioxidant enzymes. However, high level of ROS is known to inactivate antioxidant enzymes by oxidative modification of active sites or by downregulation of Nrf-2 signaling [35]. The activities of these enzymes were brought back toward normal in both tissues after resveratrol treatment. Thus, anti-hyperalgesic effect of resveratrol might be correlated with antioxidant activity.
The in-gel activity assay showed only the activity of Cu–Zn SOD in both the tissues during both phases. Cu–Zn SOD was confirmed after comparison with previous in-gel activity assays in our laboratory [24], although the presence of Mn-SOD cannot be ruled out. Nonetheless, this result suggests a major role of Cu–Zn SOD in CFA-induced hyperalgesia at peripheral and central sites. Our results provide an evidence for involvement of Cu–Zn SOD in inflammatory hyperalgesia. Previously, Mn-SOD has been reported to be the main isoform involved in neurodegeneration [36] and its role has been emphasized in inflammatory hyperalgesia [13]. However, sufficient information is not available for Cu–Zn SOD. These results provide an evidence of involvement of Cu–Zn SOD in addition to Mn-SOD in inflammatory hyperalgesia.
In-gel activity staining of GPx indicated modulation in activity of GPx-1 isozyme in hyperalgesia. GPx-1 is a selenoprotein having substrate preference for hydrogen peroxide over other peroxides [37]. Therefore, modulation in GPx-1 along with catalase suggests an important role of hydrogen peroxide in paw skin and spinal cord during hyperalgesia.
The effect of ROS on activities of antioxidant enzymes was different in the late phase of hyperalgesia. Despite of high level of ROS, the activities of antioxidant enzymes were brought almost equal to that of normal rats in paw skin during late phase. In case of spinal cord, only catalase activity was increased in CFA-induced rats, which is decreased almost to normal level after resveratrol treatment. There is no report available about differential modulation of antioxidant enzymes during early and late phase of hyperalgesia. Only few reports are available about the regulation in antioxidant enzyme activities in spinal cord under different conditions of neuronal damage or oxidative stress. Kaynar et al. in 1994 have reported no change in level of SOD, catalase and GPX enzymes up to 24 h of spinal cord injury. However, when analyzed later beyond time limitation, catalase activity was found elevated significantly with no change in SOD and GPX activities [38]. Our result follows similar pattern of variation showing elevated activity in catalase during late phase of hyperalgesia with almost no change in other antioxidant enzymes. An alternative possibility might be the involvement of cysteine-based antioxidant enzymes peroxiredoxin and thioredoxin systems to bring down the ROS level in late phase apart from primary antioxidant enzymes. Modulation in the thioredoxin system in both, animal models and the postmortem brains of human patients is reported to be associated with the most common neurodegenerative disorders, showing its important role toward defense against oxidative stress [39]. Similarly peroxiredoxins are known to exert a neuroprotective effect against ROS in several models of neurodegeneration [40]. Similar mechanism might be involved in the present study. However, more definitive experiments are needed to understand the mechanism of differential response by antioxidant enzymes.
Pro-inflammatory cytokines are important mediators of hyperalgesia [4]. TNF-α has a lead role in activating a cascade of other cytokines in pain signaling [19]. We have earlier checked the level of spinal TNF-α, which was found to increase significantly in spinal cord during the late phase. Resveratrol treatment led to decrease in the level of TNF-α in spinal cord toward normal [4]. However, in the present study, there was no change in total TNFR1 level. The result suggests that resveratrol inhibits the TNF-α signaling in spinal cord during late phase without affecting TNFR1 level. TNFR1 is involved in TNF-α-mediated activation of TRPV1 channels in nociceptors [20]. Spinal ERK is a player between reciprocal regulation of TNFR1 and TRPV1, and its activation is now considered as a hallmark of hyperalgesia [20, 21, 29]. Our results suggest that ROS acts on downstream mediator ERK. Earlier report suggests that ROS may regulate TNF-α-mediated signaling and ERK activation [20].
ROS-mediated activation of ERK–MAPK pathway may be possible by inhibition of PTPs [41].
Since resveratrol treatment could decrease the ROS level without affecting antioxidant enzymes in late phase, it may be proposed that reversal of ERK phosphorylation by resveratrol might be due to direct scavenging of ROS, rather than involvement of antioxidant enzymes. Direct scavenging of hydroxyl and superoxide radicals by resveratrol is earlier reported [42]; however, more definitive experiments are needed to confirm this hypothesis.
In conclusion, antioxidant defense system has an important role in the initial events of hyperalgesia induction at peripheral as well as central level; however, only catalase activity is modulated at central level in late phase of hyperalgesia. Anti-hyperalgesic effect of resveratrol is exhibited by modulation of ROS and ERK activity in both phases; however, regulation of antioxidant defense system is limited to early phase of hyperalgesia.
References
Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84.
Pham-Marcou TA, Beloeil H, Sun X, Gentili M, Yaici D, Benoit G, Benhamou D, Mazoit JX. Anti-nociceptive effect of resveratrol in carrageenan-evoked hyperalgesia in rats: prolonged effect related to COX-2 expression impairment. Pain. 2008;140:274–83.
Torres-Lopez JE, Ortiz MI, Castaneda-Hernandez G, Alonso-Lopez R, Asomoza-Espinosa R, Granados-Soto V. Comparison of the antinociceptive effect of celecoxib, diclofenac and resveratrol in the formalin test. Life Sci. 2002;70:1669–76.
Singh AK, Vinayak M. Anti-nociceptive effect of resveratrol during inflammatory hyperalgesia via differential regulation of pro-inflammatory mediators. Phytother Res. 2016;30(7):1164–71.
Gupta YK, Sharma M, Briyal S. Antinociceptive effect of trans-resveratrol in rats: involvement of an opioidergic mechanism. Methods Find Exp Clin Pharmacol. 2004;26:667–72.
Martínez-Revelles S, Avendaño MS, García-Redondo AB, Alvarez Y, Aguado A, Pérez-Girón JV, García-Redondo L, Esteban V, Redondo JM, Alonso MJ, Briones AM, Salaices M. Reciprocal relationship between reactive oxygen species and cyclooxygenase-2 and vascular dysfunction in hypertension. Antioxid Redox Signal. 2013;18(1):51–65.
Doyle T, Bryant L, Muscoli C, Cuzzocrea S, Esposito E, Chen Z, Salvemini D. Spinal NADPH oxidase is a source of superoxide in the development of morphine-induced hyperalgesia and antinociceptive tolerance. Neurosci Lett. 2010;483(2):85–9.
Wartenberg M, Schallenberg M, Hescheler J, Sauer H. Reactive oxygen species-mediated regulation of eNOS and iNOS expression in multicellular prostate tumor spheroids. Int J Cancer. 2003;104(3):274–82.
Khalil Z, Khodr B. A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Radic Biol Med. 2001;31:430–9.
Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–24.
Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391:108–11.
Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17.
Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309(3):869–78.
Singh AK, Vinayak M. Curcumin attenuates CFA induced thermal hyperalgesia by modulation of antioxidant enzymes and down regulation of TNF-α, IL-1β and IL-6. Neurochem Res. 2015;40:463–72.
Harris ED. Regulation of antioxidant enzyes. FASEB J. 1992;06:2675–83.
Shull S, Henitz NH, Periasamy M, Manohar M, Janssen YMW, Marsh JP, Mossman BT. Differential regulation of antioxidant enzymes in response to oxidants. J Biol Chem. 1991;266(36):24398–403.
Gaetani GF, Ferraris AM, Rolfo M, Mangerini R, Arena S, Kirkman HN. Predominant role of catalase in the disposal of hydrogen peroxide within human erythrocytes. Blood. 1996;87(4):1595–9.
Mills GC. Hemoglobin catabolism. I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative damage. J Biol Chem. 1957;229(1):189–97.
Rittner HL, Machelska H, Stein C. Leuckocyte in the regulation of pain and analgesia. J Leukoc Biol. 2005;78:1215–22.
Hensellek S, Brell P, Schaible H, Bräuer R, Segond von Banchet G. The cytokine TNF-α increases the proportion of DRG neurons expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol Cell Neurosci. 2007;36:381–91.
Ma F, Zhang L, Westlund KN. Reactive oxygen species mediate TNFR1 increase after TRPV1 activation in mouse DRG neurons. Mol Pain. 2009;5:31.
Son Y, Kim S, Chung HT, Pae HO. Reactive oxygen species in the activation of MAP kinases. Methods Enzymol. 2013;528:27–48.
Ji RR, Gereau RW, Malcangioc M, Strichartza GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–48.
Das L, Vinayak M. Long term effect of curcumin in regulation of glycolytic pathway and angiogenesis via modulation of stress activated genes in prevention of cancer. PLoS One. 2014;9(6):e99583.
Woodbury W, Spencer AK, Stahman MA. An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem. 1971;44:301–5.
Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87.
Lin CL, Chen HJ, Hou WC. Activity staining of glutathione peroxidase after electrophoresis on native and sodium dodecylsulfate polyacrylamide gels. Electrophoresis. 2002;23:513–6.
Ren K, Dubner R. Inflammatory models of pain and hyperalgesia. ILAR J. 1999;40(3):111–8.
Gao YJ, Ji RR. c-Fos or pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009;2:11–7.
Viggiano A, Monda M, Viggiano A, Viggiano D, Viggiano E, Chiefari M, Aurilio C, De Luca B. Trigeminal pain transmission requires reactive oxygen species production. Brain Res. 2005;1050:72–8.
Naik AK, Tandan SK, Dudhgaonkar SP, Jadhav SH, Kataria M, Prakash VR, Kumar D. Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-l-cysteine in rats. Eur J Pain. 2006;10:573–9.
Guedes RP, Bosco LD, Teixeira CM, Araújo ASR, Llesuy S, Belló-Klein A, Ribeiro MF, Partata WA. Neuropathic pain modifies antioxidant activity in rat spinal cord. Neurochem Res. 2006;31:603–9.
Varija D, Kumar KP, Reddy KP, Reddy VK. Prolonged constriction of sciatic nerve affecting oxidative stressors and antioxidant enzymes in rat. Indian J Med Res. 2008;129:587–92.
Mittal N, Joshi R, Hota D, Chakrabarti A. Evaluation of antihyperalgesic effect of curcumin on formalin-induced orofacial pain in rat. Phytother Res. 2009;23:507–12.
Stefanson AL, Bakovic M. Dietary regulation of Keap1/Nrf2/ARE pathway: focus on plant-derived compounds and trace minerals. Nutrients. 2014;6:3777–801.
Noack H, Lindenau J, Rothe F, Asayama K, Wolf G. Differential expression of superoxide dismutase isoforms in neuronal and glial compartments in the course of excitotoxically mediated neurodegeneration: relation to oxidative and nitrergic stress. Glia. 1998;23(4):285–97.
Das L, Vinayak M. Anti-carcinogenic action of curcumin by activation of antioxidant defence system and inhibition of NF-kappaB signalling in lymphoma-bearing mice. Biosci Rep. 2012;32(2):161–70.
Kaynar MY, Hanci M, Kuday C, Belce A, Gumustas K, Kokoglu E. Changes in the activity of antioxidant enzymes (SOD, GPX, CAT) after experimental spinal cord injury. Tokushima J Exp Med. 1994;41(3–4):133–6.
Silva-Adaya D, Gonsebatt ME, Guevara J. Thioredoxin system regulation in the central nervous system: experimental models and clinical evidence. Oxid Med Cell Longev. 2014. doi:10.1155/2014/590808.
Soriano FX, Léveillé F, Papadia S, Higgins LG, Varley J, Baxter P, Hayes JD, Hardingham GE. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1,2-dithiole-3-thione. J Neurochem. 2008;107(2):533–43.
Lee K, Esselman WJ. Inhibition of PTPs by H2O2 regulates the activation of distinct MAPK pathways. Free Radic Biol Med. 2002;33(8):1121–32.
Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, Shi X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun. 2003;309(4):1017–26.
Acknowledgements
Authors are thankful to DRDO, India, for financial support (Grant No. ERIP/ER/1003851/M/01/1336). AKS thanks UGC, India, for JRF and SRF. Financial support by DST-FIST and UGC-CAS program to Department of Zoology, BHU, and UGC-UPE to BHU is also acknowledged.
Author information
Authors and Affiliations
Contributions
AKS has executed the experiments. MV and AKS have contributed in conception of the study, design of experiments, analysis and interpretation of data and finalizing the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, A.K., Vinayak, M. Resveratrol alleviates inflammatory hyperalgesia by modulation of reactive oxygen species (ROS), antioxidant enzymes and ERK activation. Inflamm. Res. 66, 911–921 (2017). https://doi.org/10.1007/s00011-017-1072-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1072-0