Abstract
Oxidative stress is an important pathophysiological mechanism of many neurological diseases. Reactive oxygen and nitrogen species have been cited as molecules involved in the nociceptive process. In this study, rats were submitted to sciatic nerve transection (SNT) for induction of neuropathic pain, and enzyme activities of SOD and catalase as well as lipid peroxidation (LPO) were measured in the lumbosacral spinal cord. The results show that LPO was not changed after SNT. SOD activity was reduced 7 days after SNT, while the change in catalase activity occurred on the third and seventh days in both sham and SNT animals. Hyperalgesia in SNT group was detected at the same points in time. These results suggest that SNT was not a strong enough stimulus to deplete all antioxidant content in the spinal cord, since increase in LPO was not detected. However, the role of oxidative stress in nociception can not be excluded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different methods have been employed in the study of pain, such as induced inflammation and neuropathic pain. The latter is evoked by insult to the peripheral or central nervous system. Manipulations of the peripheral nerves, such as axotomy or crush, are simple models that are widely used [1, 2]. The underlying pathophysiology is a complex mechanism involving both central and peripheral mechanisms, such as sensitization, which is described behaviorally as the sensory alterations of hyperalgesia—an increase in the pain elicited by a noxious stimulus—and allodynia—pain evoked by a normally innocuous stimulus [3, 4].
The spinal cord is the region where the nociceptive primary processing occurs, thus nerve injury causes a lot of neurochemical adaptations, up- or down-regulating a set of chemical compounds [2, 5, 6]. In the last years the involvement of reactive oxygen species (ROS) in this process has been reported [7, 8]. ROS act in normal cellular processes and the concentration of these compounds is controlled by the antioxidant system that involves numerous non-enzymatic molecules and enzymes such as superoxide dismutase (SOD) and catalase. Cells express two SOD isoforms, a cytoplasmic CuZnSOD and a mitochondrial MnSOD, and both isoforms convert superoxide, formed from the molecular oxygen, to hydrogen peroxide. Catalase is located in peroxisomes and converts hydrogen peroxide into water. When the ROS production is greater than the antioxidant system activity there is oxidative stress and cellular damage may occur [9, 10].
Oxidative stress has been implicated in several pathologies, including central nervous system (CNS) disorders. In the spinal cord, amyotrophic lateral sclerosis, a neurodegenerative disease affecting motor neurons, is associated with a mutation in the gene encoding CuZnSOD [11]. In spinal cord injury alterations in the antioxidant system have been detected [12], and the inflammatory process is related to oxidative stress at spinal cord [8]. Some works describe changes in antioxidant system after axotomy [13–15]. Peripheral nerve transection increases MnSOD in adult [13] but not in neonatal rats, which show decreased CuZnSOD [15]. However, the direct relationship among ROS, axotomy and neuropathic pain has not been studied. Therefore, the current study was designed to show the probable association between hyperalgesia evoked by neuropathic pain and alterations in activity and expression of antioxidant enzymes SOD and catalase, both involved in primary defense against oxidative damage.
Experimental procedure
Animals
Experiments were conducted in adult male Wistar rats weighing 200–250 g. All animal procedures were approved by the Ethics Committee of the Instituto de Ciências Básicas da Saúde of the Federal University of Rio Grande do Sul. Under anaesthesia (ketamine 80 mg/Kg and xylazine 2 mg/Kg) and sterile conditions, the right sciatic nerve was exposed and transected at mid thigh level. In order to expose the sciatic nerve in sham rats all surgical procedures involved in the experimental group were used except transection. For further comparisons a naïve group was included in which the animals did not undergo surgical manipulation. Groups of five animals were sacrificed after 0, 3, 7 and 15 days.
Hot plate test
Thermal hyperalgesia was measured by placing the rats on a hot plate maintained at 50°C (±2°C). The withdrawal latency was considered when the animal jumped or licked a hindpaw, independently of side. A cutoff time of 30s was employed to prevent tissue injury. The hot plate test was performed on different days using different animals. For each period (3, 7 and 15 days after injury) different naïve rats were tested.
Preparation of tissue samples
Rats were killed by decapitation and their lumbosacral spinal cords were promptly dissected out. The tissues were immediately cooled in ice and homogenized in 1.15% KCl diluted 1:5 (w/v) containing 1mmol/l PMSF. The homogenates were centrifuged at 800 x g for 20 min to discard nuclei and cell debris and the supernatant fraction obtained was frozen at −70°C for further measurements.
Antioxidant enzyme activities
Catalase activity was determined by following the decrease in absorption at 240 nm in a reaction medium containing 50 mmol/l phosphate buffer (pH 7.2) and 10 mmol/l hydrogen peroxide (H2O2) [16] and expressed as pmol of H2O2 reduced per minute per mg protein.
Superoxide dismutase (SOD) activity, expressed as U/mg protein, was based on the inhibition of superoxide radical reaction with pyrogallol [17]. The SOD activity was determined by measuring the velocity of oxidized pyrogallol formation at 420 nm for 2 min. The reaction medium contained tris buffer (50 mmol/l, pH = 8.2), pyrogallol (24 mmol/l) and catalase (30 mmol/l).
Lipid peroxidation measurement
Thiobarbituric acid reactive substances (TBARS) measurement was used to evaluate lipid peroxidation (LPO). For this assay, trichloroacetic acid (10%) was added to the homogenate to precipitate proteins and to acidify the sample [18]. This mixture was then centrifuged (3 min, 1000 g). The protein-free sample was extracted and thiobarbituric acid (0.67%) was added to the reaction medium. The samples and standards were placed in a water bath (100°C, 15 min). Malondialdehyde (MDA), an intermediate product of lipoperoxidation, was determined by the absorbance at 535 nm and the results are reported as nmol/mg protein.
Western blot
Sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (12%) was carried out using a miniprotein system (Bio-Rad, Hercules, CA) with full range rainbow (Amersham). Protein (16 μg) was loaded in each lane with loading buffer containing 0.375mol/l Tris (pH 6.8), 50% glycerol, 10% SDS, 0.5 mol/l mercaptoethanol, and 0.002% bromophenol blue. Samples were heated at 100°C for 2 min prior to gel loading. After electrophoresis, proteins were transferred to nitrocellulose membranes (Amersham) using an electrophoretic transfer system at 110V for 1 h. The membranes were then washed with TTBS (20 mmol/l Tris–HCl, pH 7.5; 150 mmol/l NaCl; 0.05% Tween-20, pH 7.4) and 8% nonfat dry milk for 1h. The membranes were incubated overnight at 4°C with the primary antibodies diluted in TTBS plus BSA. Rabbit polyclonal antibodies for CuZnSOD (1:1000, Chemicon International) and for catalase (1:1000, Chemicon International) were used as primary antibodies. After washing with TTBS, the membranes were incubated for 2h at room temperature with secondary antibody (1:1000, anti-rabbit IgG peroxidase conjugated; Santa Cruz), washed with TBS (20 mmol/l Tris–HCl; 150mM NaCl, pH 7.5) and revealed by chemiluminescence followed by apposition of the membranes to autoradiographic films. These films were analyzed using the Molecular Dynamics Image Quant software version 3.22 (computing densitometer model 300 A). The results were expressed as mean % of pixels. The correction of the amount of protein per lane transfer was made by Ponceau’s method [19].
Protein measurement
Protein was measured by the method of Bradford [20], using bovine serum albumin as standard.
Statistical analysis
Enzyme activities, TBARS and Western blot results were compared by one-way ANOVA followed by Student Newman Keuls post hoc multiple comparison test. The differences in latency measured in hot plate test were analyzed by Kruskal–Wallis nonparametric test followed by post hoc Dunn’s method. Differences were considered statistically significant when the P value was <0.05. All statistical analysis was carried out with Sigma Stat 2.0 software.
Results
Thermal hyperalgesia
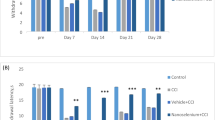
Three days after sciatic nerve transection (SNT) the animals showed thermal hyperalgesia as demonstrated by the hot plate test (Fig. 1A). At day 3 postoperatively, withdrawal latency was significantly decreased in SNT animals as compared to naïve ones but was similar as compared to sham-operated animals. At day 7 postoperatively there was a significant decrease in SNT animals as compared to both naïve and sham animals (Fig. 1B). Fifteen days after surgery there was no significant difference across the groups (Fig. 1C).
Thermal hyperalgesia measured by withdrawal latency (in seconds) in naïve, sham-operated and SNT rats at 3 (A), 7 (B) and 15 (C) days after surgery. Data are expressed as medians and errors are expressed as interquartile range 25–75% (n = 5 for each group). One asterisk indicates significant difference (p<0.05) between SNT and naïve animals, and two asterisks represent significant difference (p<0.05) between SNT and naïve and between SNT and sham-operated animals
Antioxidant enzyme activities and Western blot
There was no significant difference in SOD and catalase content as determined by the Western blot analysis (Fig. 2). The comparisons were made between naïve, sham-operated and SNT rats at the various postsurgical times. Representative western blots are shown in the top of histograms on Fig. 2. However, a significant difference in enzyme activities was detected (Fig. 3). Three days after surgery, SNT and sham-operated animals showed a reduction of around 30% in the catalase activity as compared to the naïve group. There was no significant difference between sham-operated and SNT groups, demonstrating that catalase activity is affected not only by neuropathic pain but also by surgical manipulation. Seven days after surgery the reduction in the catalase activity in the sham-operated and SNT groups as compared to naïve animals was more pronounced (approximately 40%). Finally, fifteen days after surgery catalase activity was still reduced in sham-operated and SNT rats, but these differences were not significant (P>0.05). SOD activity showed a reduction of around 30% but only seven days after SNT, demonstrating a specific effect related to axotomy.
Western blot analysis for catalase (A) and CuZnSOD (B) in spinal cord homogenates of naïve, sham-operated and SNT groups 3 (3D), 7 (7D) and 15 (15D) days after surgery. There was not any significant difference in these enzymes expression across all groups (p>0.05). Comparisons were drawn between the groups at the same postsurgical point in time. Representative Western blots are shown in the top of histograms. The results are expressed as percentages of pixels mean ± SEM (n = 5 for each group)
Antioxidant activity of catalase (A) and SOD (B) in spinal cord homogenates in sham-operated and SNT rats 3, 7 and 15 days after surgery (0 = naïve rats). Data are expressed as percentage of naïve group. Asterisks indicate significant difference (p<0.05) between naïve (100%) and sham or SNT. About 100% of catalase activity corresponds to 43 pmol/mg of protein, and 100% of SOD activity corresponds to 71.75 USOD/mg of protein. Bars represent SEM (n = 5 for each group)
TBARS measurement
There was not a significant difference in spinal cord LPO between naïve, sham-operated and SNT rats at any postsurgical time, as evaluated by TBARS. These results suggest that neuropathic pain induction does not cause lipid peroxidation changes in the spinal cord. The TBARS results are demonstrated as nmol/mg protein in Fig. 4.
Lipid peroxidation measured by thiobarbituric acid reactive species (TBARS) in spinal cord homogenates of naïve, sham-operated and SNT groups 3, 7 and 15 days after surgery. There was not any significant difference between the groups (p>0.05). The comparisons were made between the groups at the same postsurgical time. TBARS values are expressed as nmol/mg of protein. Data represent the means ± SEM (n = 5 for each group)
Discussion
The main finding in the present study was the reduction in SOD activity in the spinal cord at seven days after sciatic nerve transection. Moreover, catalase activity was also decreased at three and seven days, but this result was not specific to injury, since in sham animals the activity of this enzyme also decreased. Thus, catalase activity appears to be very susceptible to afferent stimulation. However, Western blot results showed that peripheral nerve injury do not alter the expression of these enzymes in the spinal cord.
Although the sham-operated group was not submitted to nerve transection, it suffered a slight injury due to skin cutting and connective tissue lesion, which also cause an increase in spinal cord sensory inputs as evidenced by higher sensitization detected by hot plate test three days after injury. At seven days the withdrawal latency of sham rats was similar to the naïve group. This result suggests that in the sham group the sensibility had already returned to basal levels. On the other hand, SNT rats showed hyperalgesia in this period. Withdrawal latency at fifteen days in SNT suggests reversion of hyperalgesia, but we do not believe that the total reversion of symptoms occurs within 2 weeks. However, using the hot plate test we did not find a significant statistical difference at this period in time. This could be a limitation of this test. One example of limitation of the hot plate test is that it is impossible to establish which side supports less weight. It is likely that the rats support less weight on the injured side, but this cannot be measured with the test employed. Despite these limitations, this method is widely used in pain studies. Therefore, the variation in the latency period shown in Fig. 1 may be due to application of the test on different days since the sensibility of animals may change.
The changes in catalase activity occurred mainly at the same times at which hyperalgesia was detected. This may suggest some connection between catalase activity and the development of hyperalgesia. In this case there is an apparent role of antioxidant protection in normal sensibility, since the reduced catalase activity is associated with sensory behavioral abnormality. Three days after axotomy the decrease in catalase activity may be related to a higher conversion of hydrogen peroxide to hydroxyl radical, a very cytotoxic ROS. However, astrocytes in the central nervous system have a high antioxidant content, especially glutathione [21], and glutathione efflux has been observed to occur following acute exposure to nitric oxide (NO). Thus, astrocytes have the ability to provide neurons with glutathione or their precursors, conferring protection against neuronal oxidant damage by elevated neuronal antioxidant levels [22]. Therefore, it may be suggested that astrocytes provide neuroprotection to the central nervous system, and peripheral nerve injury probably does not cause a strong enough stimulus to deplete the antioxidant reserves in the spinal cord, and thus LPO increase was not observed.
Changes in spinal cord SOD activity were more limited and specific to SNT. A significant decrease in SOD activity was detected only on day 7 after axotomy, and at this time the concomitant reduction in catalase activity may be explained by the low hydrogen peroxide content, since the low SOD activity reduces the superoxide conversion to hydrogen peroxide and consequently causes superoxide accumulation.
Superoxide is a highly reactive free radical and may react with nitric oxide leading to the formation of a highly toxic oxidant: peroxynitrite. Many studies have demonstrated that peripheral nerve injury causes up-regulation in both expression and activity of nitric oxide synthase (NOS), the enzyme involved in NO synthesis, in the spinal cord and dorsal root ganglia [23–25]. Zhang et al. [23] showed more marked NOS up-regulation seven days after injury. Therefore, during the same period of higher NO production there is a reduction in SOD activity and probably a higher concentration of peroxynitrite. The same events have been described in inflammatory pain: nociceptive inputs cause NMDA glutamate receptor activation and Ca++ influx in dorsal horn cells, leading to NOS activation; peroxynitrite formation causes nitration of proteins including MnSOD, which is inactivated and superoxide concentration becomes elevated maintaining hyperalgesia [8]. In neuropathic pain the same mechanisms may be occurring, since there is up-regulation in NOS [23–25] and SOD activity is reduced, as evidenced by the present study.
On the other hand, alteration in CuZnSOD protein content following SNT was not detected by Western blot, but previous studies have described an increase in MnSOD expression in the spinal cord after axotomy [13] and in the facial nucleus after facial nerve transection [26]. However, Yu [14] found a decrease in CuZnSOD immunoreactivity in the hypoglossal nucleus and spinal cord after injury to the hypoglossal and sciatic nerves, respectively, and this pattern occurred at the same time as an increase in NOS immunoreactivity in the same regions, demonstrating a close correlation between SOD and NO. In neonatal rats, sciatic nerve transection also causes a decrease in CuZnSOD in the lumbar spinal cord evidenced by Western blot [15]. Although the results of different studies are contradictory, it is likely that inactivated MnSOD has a more important role in hyperalgesia subsequent to peripheral nerve injury than the cytosolic isoform CuZnSOD does, since previous studies have demonstrated that the mitochondrial SOD has a neuroprotective effect against NO toxicity in vitro, and CuZnSOD does not present this effect [27].
However, antioxidant activity reduction is almost always accompanied by oxidative stress evidenced by damage to lipids, proteins and/or DNA. In the central nervous system damage to lipids is a very common event caused by oxidative stress due to the large amounts of polyunsaturated fatty acids. In this study, however, no alteration in lipid peroxidation was detected post axotomy as measured by the TBARS assay. In this situation other antioxidant defenses may protect lipids, and damage to other cellular constituents may be occurring. Neuropathic pain is related to an overexpression of many neurotransmitters, peptides and a large number of proteins [3], thus, in this case, the oxidative stress may involve proteins. Studies on damage to proteins and non-enzymatic antioxidant levels in the spinal cord after peripheral nerve lesion are currently being conducted in our laboratory.
The involvement of reactive oxygen and nitrogen species in neuropathic pain is accepted, as antioxidant injection exerts an analgesic effect [7, 28]. Nevertheless, the site of action of these compounds is not well-established. Intrathecal and systemic administration show similar effects, and therefore the spinal cord seems to be a major site of antioxidant action [7]. Possibly reactive oxygen and nitrogen species activate second messengers related to central sensitization, a mechanism involving hyperalgesia perpetuation based on neurochemical adaptations in the spinal cord.
Recently it was demonstrated that trigeminal transmission of facial pain is modulated by ROS such as superoxide and hydrogen peroxide, because treatments with N- acetylcysteine (an antioxidant) and 2-methoxyestradiol (an inhibitor of SOD) in the trigeminal nucleus after formalin injection in the upper lip modifies the behavior of facial grooming, suggesting the role of ROS in the mechanisms of synaptic plasticity underlying tonic pain. The authors describe an increase in hydrogen peroxide production measured by microdialysis, and therefore hydrogen peroxide may be an important modulator of pain transmission [29].
However, the participation of ROS in pain transmission may not really be related to cellular damage, as evidenced by the present study, where no increase in lipid peroxidation in spinal cord post SNT was detected. Hydrogen peroxide affects intracellular activity of signaling molecules such as kinase and phosphatase proteins [30] or may act directly on neurotransmitter receptors and alter ligand–receptor interactions [31]. Loss of inhibitory input was detected in thalamocortical circuitry after hydrogen peroxide application generating seizure activity [32]. Maybe in neuropathic pain, ROS like hydrogen peroxide act as second messenger modulating neurotransmitter systems associated with nociceptive processing. Thus it may be suggested that the role of ROS in this situation is not related to cellular damage to lipids, but these molecules can be acting as second messengers that maintain hyperalgesia through activation of pain-related neurotransmitters.
In conclusion, oxygen and nitrogen species may take part in neuropathic pain processing as evidenced by the present study by SOD activity reduction seven days after nerve transection. However, the precise mechanisms that involve oxidative stress in pain transmission have not yet been well-established. Hence, further research on these topics is crucial due to the possibility of developing new strategies to pain treatment.
References
Sommer C, Myers RR (1995) Neurotransmitters in the spinal cord dorsal horn in a model of painful neuropathy and in nerve crush. Acta Neuropathol 90:478–485
Zimmermann M (2001) Pathobiology of neuropathic pain. Eur J Pharmacol 429:23–37
Millan JM (1999) The induction of pain: an integrative review. Prog Neurobiol 57:1–164
Byers MR, Bonica JJ (2001) Peripheral pain mechanisms and nociceptor plasticity. In: Loeser JD, Butler SH, Chapman R, Turk DC (eds), Bonica’s management of pain, Lippincott Williams & Wilkins, Philadelphia, pp. 26–72
Rydh-Rinder M, Holmberg K, Elfvin LG, Wiesenfeld-Hallin Z, Hökfelt T (1996) Effects of peripheral axotomy on neuropeptides and nitric oxide synthase in dorsal root ganglia and spinal cord of the guinea pig: an immunohistochemical study. Brain Res 707:180–188
Ma W, Bisby MA (1998) Partial and complete sciatic nerve injuries induce similar increases of neuropeptide Y and vasoactive intestinal peptide immunoreactivities in primary sensory neurons and their central projections. Neuroscience 86:1217–1234
Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM (2004) Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 111:116–124
Wang Z-Q, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini DA (2004) Newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther 309:869–878
Yu BP (1994) Cellular defenses against damage from reactive oxygen species. Physiol Rev 74:139–162
Fridovich I (1998) Oxygen toxicity: a radical explanation. J Exp Biol 201:1203–1209
Liochev SI, Fridovich I (2003) Mutant Cu,Zn superoxide dismutases and familial amyotrophic lateral sclerosis: evaluation of oxidative hypotheses. Free Radic Biol Med 34:1383–1389
Lee YS, Sindhu RK, Lin CY, Ehdaie A, Lin VW, Vaziri ND (2004) Effects of nerve graft on nitric oxide synthase, NAD(P)H oxidase, and antioxidant enzymes in chronic spinal cord injury. Free Radic Biol Med 36:330–339
Rosenfeld J, Cook S, James R (1997) Expression of superoxide dismutase following axotomy. Exp Neurol 147:37–47
Yu WH (2002) Spatial and temporal correlation of nitric oxide synthase expression with CuZn-superoxide dismutase reduction in motor neurons following axotomy. Ann. N Y Acad Sci 962:111–121
Rogerio F, Teixeira SA, de Rezende AC, de Sa RC, Queiroz L, De Nucci G, Muscara MN, Langone F (2005) Superoxide dismutase isoforms 1 and 2 in lumbar spinal cord of neonatal rats after sciatic nerve transection and melatonin treatment. Brain Res Dev Brain Res 154:217–225
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126
Marklund SL (1985). Pyrogallol autooxidation. In: Greenwald RA (eds) Handbook of methods for oxygen radical research, CRC Press, Boca Raton, pp 243–247
Buege JA, Aust SD (1978) Microssomal lipid peroxidation. Meth Enzymol 52:302–309
Klein D, Kern RM, Sokol RZ (1995) A method for quantification and correction of proteins after transfer to immobilization membranes. Biochem Mol Biol Int 36:59–66
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Heales S, Lam A, Duncan A, Land J, (2004) Neurodegeneration or neuroprotection: the pivotal role of astrocytes. Neurochem Res 29:513–519
Gegg M, Beltran B, Salas-Pino S, Bolanos JP, Clark JB, Moncada S, Heales SJR (2003) Differential effect of nitric oxide on glutathione metabolism and mitochondrial function in astrocytes and neurones: implications for neuroprotection/neurodegeneration? J Neurochem 86:228–237
Zhang X, Verge V, Wiesenfeld-Hallin Z, Ju G, Bredt D, Synder SH, Hokfelt T (1993) Nitric oxide synthase-like immunoreactivity in lumbar dorsal root ganglia and spinal cord of rat and monkey and effect of peripheral axotomy. J Comp Neurol 335:563–575
Vizzard MA, Erdman SL, de Groat WC (1995) Increased expression of neuronal nitric oxide synthase (NOS) in visceral neurons after nerve injury. J Neurosci 15:4033–4045
Cizkova D, Lukacova N, Marsala M, Marsala J (2002) Neuropathic pain is associated with alterations of nitric oxide synthase immunoreactivity and catalytic activity in dorsal root ganglia and spinal dorsal horn. Brain Res Bull 58:161–171
Yoneda T, Inagaki S, Hayashi Y, Nombra T, Takagi H (1992) Differential regulation of manganese and copper/zinc superoxide dismutases by the facial nerve transection. Brain Res 582:342–345
Gonzalez-Zulueta M, Ensz L, Mukhina G, Lebovitz R, Zwacka R, Engelhardt J, Oberley L, Dawson V, Dawson T (1998) Manganese superoxide dismutase protects nNOS neurons from NMDA and nitric oxide-mediated neurotoxicity. J Neurosci 18:2040–2055
Khalil Z, Liu T, Helme RD (1999) Free radicals contribute to the reduction in peripheral vascular responses and the maintenance of thermal hyperalgesia in rats with chronic constriction injury. Pain 79:31–37
Viggiano A, Monda M, Viggiano A, Viggiano D, Viggiano E, Chiefari M, Aurilio C, De Luca B (2005) Trigeminal pain transmission requires reactive oxygen species production. Brain Res 1050:72–78
Rhee SG, Bae YS, Lee SR, Kwon J (2000) Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE 53: PE1
Sah R, Galeffi F, Ahrens R, Jordan G, Schwartz-Bloom RD (2002) Modulation of the GABA(A)-gated chloride channel by reactive oxygen species. J Neurochem 80:383–391
Frantseva MV, Perez Velazquez JL, Carlen PL (1998) Changes in membrane and synaptic properties of thalamocortical circuitry caused by hydrogen peroxide. J Neurophysiol 80:1317–1326
Acknowledgements
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guedes, R.P., Bosco, L.D., Teixeira, C.M. et al. Neuropathic Pain Modifies Antioxidant Activity in Rat Spinal Cord. Neurochem Res 31, 603–609 (2006). https://doi.org/10.1007/s11064-006-9058-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9058-2
Keywords
- Neuropathic pain
- Reactive oxygen species
- Sciatic nerve transection
- Superoxide dismutase
- Catalase
- Spinal cord
- Rat
- Lipid peroxidation
- Oxidative stress
- Antioxidant
- Axotomy
- Hyperalgesia
- Nociceptive processing
- Western blot
- Hot plate test
- Hydrogen peroxide
- Superoxide
- Nervous system
- Pain
- Sciatic nerve
- Nitric oxide
- Nitric oxide synthase
- Peroxynitrite
- Inflammation
- Peripheral nerve lesion
- Neurons
- Astrocytes
- Malondialdeyde
- TBARS
- Bradford method
- Thermal hyperalgesia
- Sensitization
- Free radicals
- Nerve injury
- Nociception
- Gluthatione
- SOD
- CuZnSOD
- MnSOD
- Neurological disorders
- Withdrawal latency
- Electrophoresis
- LPO
- Polyclonal antibody
- Sodium dodecyl sulfate
- Chemiluminescence