Abstract
Impact of reactive oxygen species (ROS) in development of hyperalgesia has recently motivated scientists to focus on ROS as novel target of anti-hyperalgesic interventions. Studies have indicated the usefulness of ROS scavengers and exogenous antioxidants as anti-nociceptive agents in animal models of neuropathic and inflammatory hyperalgesia. In present study, we suggest the anti-hyperalgesic potential of the dietary antioxidant quercetin on chronic inflammatory hyperalgesia induced by Complete Freund’s Adjuvant (CFA). Three doses of quercetin (25, 50 and 75 mg/kg body weight) for consecutive 7 days were used for the study. Thermal hyperalgesia was assessed by paw withdrawal latency (PWL) test and inflammation was checked in terms of changes in paw edema. The insight of molecular signaling during chronic hyperalgesia was analyzed by TNF-α–TNFR1–ERK1/2 pathway in relation to change in ROS level in DRG and spinal cord. CFA-induced hyperalgesia was confirmed by decreased PWL and increased c-Fos activity in dorsal horn of spinal cord, determined by immunohistochemical analysis. It was characterized with elevated level of ROS and TNF-α estimated by ELISA. The activation of ERK1/2 and NF-κB in DRG and spinal cord and over-expression of TNFR1 in DRG were analyzed by Western blotting. Up-regulation of Iba1 and GFAP indicates glial activation in spinal cord. Expression of GFAP and its co-localization with NF-κB were examined by immunofluorescence. All the molecular modulators of hyperalgesia were brought towards normal after quercetin treatment showing its anti-hyperalgesic activity, indicating that repeated quercetin treatment is able to alleviate chronic inflammatory hyperalgesia by attenuating TNF-α-TNFR1–ERK1/2 signaling pathway via modulation of ROS and by suppression of central sensitization via inhibition of spinal glial activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Any noxious stimulus or tissue injury causes sensitization of nociceptors which leads to hyperalgesia, a condition in which the increased response to a normal painful stimulus is observed (Costigan et al. 2009). An immediate nociceptive response following tissue injury results in the hypersensitivity of peripheral nociceptors that elicit a sharp, localized and short lived pain referred as acute hyperalgesia. Sustained nociceptive signals from the peripheral organ relentlessly sensitize and induce changes in the properties of neurons in the dorsal horn of the spinal cord (central sensitization) marking chronic hyperalgesia. Chronic hyperalgesia is a major clinical problem. Opioids and non-steroidal anti-inflammatory drugs (NSAIDs) are most preferred and effective for the treatment of chronic inflammation and the associated pain. However, these drugs are of great concern because of their serious side effects and non-specific targets. Therefore, the interest was focused to explore alternate approaches for treatment of pain posing minimal side effect.
Recent studies indicate ROS as key factor during inflammation, and initiation and maintenance of hyperalgesia. Various pro-inflammatory cytokines are released by neutrophils and macrophages in the close vicinity of issue injury as well as by nociceptive afferent neurons, which leads to inflammation. Pro-inflammatory cytokine TNF-α is the major cytokine in inflammatory soup which initiates a cascade of other pro-inflammatory cytokines in the pain signaling (Huang et al. 2017) and regulates a number of downstream signaling pathways. Activation of ERK and other MAPKs is believed to play a central role in the development of inflammation and hyperalgesia. Recent study reveals the role of ERK signaling in the periphery as it influences the transition from acute to chronic pain (Skopelja-Gardner et al. 2017). Activation of ERK1/2 has been recently considered as a neuronal marker of pain (Gao and Ji 2009). Reports demonstrate the critical roles of glial cells, such as microglia, astrocytes and satellite glial cells (SGCs) in the genesis of persistent pain. Further, inflammatory cytokines and mediators released from glial cells participate in peripheral (Gold and Gebhart 2010; Basbaum et al. 2009) and central sensitization (Kuner 2010). Activation of glial cells and neuro-glial interactions are emerging as key mechanisms underlying chronic pain via central sensitization.
Reactive oxygen species (ROS) are implicated in development and maintenance of inflammatory pain (Schwartz et al. 2009; Ibi et al. 2008) and neuropathic pain (Fidanboylu et al. 2011) primarily through central sensitization. ROS scavengers and exogenous antioxidants have been implicated as anti-nociceptives in several studies, in various animal models of neuropathic as well as in chronic inflammatory hyperalgesia (Bernardy et al. 2017; Kim et al. 2017; Singh and Vinayak 2016, 2017a). Earlier reports from our group have attributed the antioxidant property of resveratrol and curcumin to be responsible for its anti-nociceptive effect (Singh and Vinayak 2015, 2016, 2017a). Quercetin is a ubiquitous dietary flavonoid, rich in vegetables and beverages. It is shown to be a potent antioxidant. Pain ameliorating effect of quercetin is previously reported; however, its regulatory mechanism is poorly understood. Based on the literature, it is hypothesized that phytoflavonoid quercetin should modulate ROS-mediated signaling pathways towards anti-hyperalgesic action. To validate the hypothesis, the present study is planned to evaluate the role of quercetin in regulation of ROS-mediated ERK1/2 signaling pathway and glial activation in complete Freund’s adjuvant (CFA)-induced chronic hyperalgesia.
Materials and Methods
Drugs and Reagents
Complete Freund’s adjuvant (CFA) and general chemicals were purchased from Sigma-Aldrich (Saint Louis, USA). Quercetin was purchased from Sigma-Aldrich (Saint Louis, USA). Polyclonal anti-c-Fos rabbit antibody (cat# ab7963) was purchased from Abcam (Cambridge, UK), monoclonal rabbit anti-NF-κB (cat# 3033S) and monoclonal mouse anti-GFAP (cat# 3670P) antibody from Cell Signaling Technology (Danvers, Massachusetts, USA), monoclonal mouse anti-pERK1/2 (cat# SC7383) and goat anti-Iba1 (cat# SC28528) antibody from Santa-Cruz Biotech (USA), polyclonal anti-TNFR1 (cat# 3125-100) and anti-ERK1/2 (cat# 3085-100) antibodies from BioVision (Milpitas, CA, USA), and monoclonal mouse anti-β-actin antibody (cat# A3854) from Sigma-Aldrich (Saint Louis, USA). TRITC (red)-conjugated anti-mouse antibody (cat# A16071) was procured from Novex, Life Biotechnologies, USA; HRP-conjugated rabbit anti-goat secondary antibody (cat# HPO4), FITC (green)-conjugated anti-rabbit (cat# FTC2), HRP-conjugated anti-rabbit (cat# HPO3), and HRP-conjugated anti-mouse (cat# HPO5) antibodies were purchased from Merk-Genei (Bangalore, India).
Animals and Treatments
Healthy male rats of Charles Foster strain (12–14 weeks and 160–180 gm) were used for the experiments. Charles Foster rats have been most extensively used in the pain research and it is a well-established animal model to study various types of neuropathic as well as inflammatory pain. All animal experiments were carried out in accordance with the EU Directive 2010/63/EU for animal experiments. Rats were bred and maintained under standard laboratory conditions with the approval of Committee for the Purpose of Control and Supervision of Experiments on Animals (License: 1802/GO/Re/S/15/CPCSEA) at 22 ± 2 °C with 12-h light/dark schedule with ad libitum supply of standard animal feed and drinking water. All the experiments were performed with the approval of Central Animal Ethical Committee, Banaras Hindu University.
Experimental Plan and Treatment Schedule
Each rat was acclimatized for at least 5 days by keeping it for 5 min on unheated plate in the Eddy’s analgesiometer. Total 54 normal adult male rats (12–14 weeks) were distributed randomly in six groups (Fig. 1a). First group (N) was given intraplantar (i.pl.) injection of 100 µl normal saline, while other groups were injected with 100 µl CFA (i.pl.) in right hind paw. After 48 h of saline/CFA injection, rats of first (N) and second (C) groups received 150 µl NS intraperitoneally (i.p.); third group received 150 µl DMSO (CD) and fourth (CQ25), fifth (CQ50) and sixth (CQ75) groups received i.p. 25, 50 and 75 mg/kg BW dissolved in 150 µl DMSO, respectively. Figure 1b depicts schematic representation of the schedule for CFA injection and chronic treatment of quercetin. Briefly, baseline paw withdrawal latency was recorded for animals of each group just before i.pl. injection of saline/ CFA to respective groups. Quercetin/DMSO/saline was injected intraperitoneally to the corresponding group daily up to 7 days.
a Schematic diagram showing distribution of rats in different experimental groups and the treatment given. Male adult rats of Charles Foster strain of age 12–14 weeks weighing ~160–180 g were used for the study. Rats were randomly distributed in six groups (n = 9). Saline in control rats (N) and CFA in rest of five groups into the plantar surface of right hind paw of rats. Treatment with intraperitoneal (i.p.) administration of saline in control rats (N) and one group of CFA-injected rats (C), vehicle DMSO in second group of CFA-injected rats (CD), and quercetin dissolved in DMSO at dose of 25 mg/kg BW (CQ25), 50 mg/kg BW (CQ50) and 75 mg/kg BW (CQ75) was given to other three groups of CFA-injected hyperalgesic rats. b Treatment schedule of CFA and quercetin. Baseline readings were taken of naive animals of all the groups. Immediately after recording the baseline data, intraplantar (i.pl.) injection of CFA/saline was given to rats of respective groups. After 48 h, treatment with quercetin or saline to corresponding groups was done for 7 days. Data for PWL and paw edema were obtained on next day of each treatment. Rats were sacrificed on day 9 and tissues (DRG and spinal cord) of lumbar region (L4–L6) were dissected out
Assessment of Thermal Hyperalgesia and Measurement of Paw Edema
Thermal hyperalgesia in terms paw withdrawal latency (PWL) was measured by previously described method (Singh and Vinayak 2016). PWL was measured by Eddy’s hot plate analgesiometer (Orchid Scientific, India) maintained at 48 ± 0.5 °C. A cut-off time of 15 s was set in order to avoid tissue damage. Paw edema was checked by Vernier caliper. PWL and paw edema were recorded just before CFA injection (baseline) and then after every 24 h. Further, PWL and paw edema were recorded just before giving subsequent quercetin/DMSO/NS to the respective groups. Three consecutive readings were taken at an interval of 10 min and were averaged. The observer was blinded to any type of treatments given to the different groups.
Collection of Tissues
CFA-induced rat model is considered as a relevant model for chronic pain. CFA induces reflexive pain (Gregory et al. 2013), i.e., exaggerated nociception in response to an external noxious stimulus like heat or pressure (hyperalgesia). 48-h post-CFA injection time was selected for our study as model of chronic pain as per the earlier reports showing hyperalgesia peak within 6 to 8 h of CFA injection which is maintained up to 48 h (Singh and Vinayak 2016; Wilson et al. 2006). DRG and spinal cord of lumbar region (L4–L6) were used for biochemical analysis as they constitute 98–99% of the total innervations from hind paw (Puigdellívol‐Sánchez et al. 2000; Rigaud et al. 2008).
For dissection of DRG and spinal cord, rats were anesthetized with diethyl ether and decapitated. Then, the skin from posterior-dorsal side of the animal was cut; vertebral column of lumbar region (L4–L6) was localized and dissected out. Using sharp fine scissors, vertebral bones were cut and spinal cord and underlying DRGs (located in small pits in vertebrae on each lateral sides) were collected carefully, washed in 10 mM ice-cold PBS and stored in − 80 °C until further processing.
Immunohistochemistry (IHC) of c-Fos
Animals were perfused and tissues were collected after the schedule of quercetin treatment was over and processed as described earlier (Singh and Vinayak 2016). Rats were deeply anaesthetized by sodium pentobarbital (65 mg/kg i.p.) and were transcardially perfused with 0.6 M phosphate-buffered saline (PBS), followed by ice-cold fixative (4% paraformaldehyde in 0.6 M phosphate buffer). Spinal cord of lumbar region (L4–L6) was dissected out and was post-fixed in 4% formaldehyde for 6 h and cryoprotected overnight in 20% and 30% sucrose solution at 4 °C. 12-µm-thick sections were collected on poly-l-lysine-coated clean glass slides using a cryo-microtome (Microm HM525, Thermo Scientific). Immunohistochemical staining of c-Fos was performed in spinal cord sections using standard protocol. Sections were rinsed in 10 mM PBS (pH 7.4) for three times (10 min each). Endogenous peroxidase activity was quenched with 0.3% H2O2 for 30 min. Non-specific binding was blocked with 5% normal goat serum in 10 mM PBS for 2 h at the room temperature (RT). The sections were incubated overnight at 4 °C with rabbit anti c-Fos antibody (1:200) overnight at 4 °C, washed with 10 mM PBS once and then incubated with HRP-conjugated goat anti-rabbit secondary antibodies (1:500) in 5% normal goat serum for 2 h at RT. c-Fos-positive cells were detected by DAB (di-amino benzidine) staining. Stained sections were observed under a light microscope (Leitz “laburlux S” microscope, Earnst Leitz GmbH, Wetzlar, Germany) and images were taken with Leica DCF290 camera (Leica Microsystems Ltd., Germany).
Immunofluorescence
DRG and spinal cord were dissected out and processed as described above (IHC of c-fos). Sections were rinsed in 10 mM PBS (pH 7.4) for three times (10 min each), blocked with 5% goat serum in 10 mM PBS for 2 h at RT and then used for immunofluorescent staining. The sections were incubated overnight at 4 °C with the primary antibodies: Mouse anti-GFAP (1:250) only or mixed with rabbit anti-NF-κB (1:100) for co-localization. The sections were then washed for three times in 10 mM PBS (5 min each) and incubated for 2 h at RT with the corresponding secondary antibody: FITC-conjugated goat anti-rabbit antibody (1:500) and TRITC-conjugated goat anti-mouse antibody (1:500). Images were obtained using a florescence microscope (Motic BA410, Japan) and images were captured with Moticam-5 (Motic, Japan) camera.
Preparation of Homogenates
DRG and spinal cord (L4–L6) were homogenized in 50 mM Tris–Cl (pH 7.6) containing 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 0.1% SDS, 0.5% sodium deoxycholate, 0.1% Triton X-100, 1 mM PMSF and protease inhibitor cocktail with a polytron homogenizer on ice and centrifuged at 14,000xg for 30 min at 4 °C. The supernatant was collected, and total protein content in each sample was determined using the method of Bradford by using bovine serum albumin (BSA) as standard. Protein samples were diluted to 100× in autoclaved triple distilled water. Diluted protein sample (100 µl) and Bradford regent (900 µl) were mixed well and incubated for 1 min at RT. Absorbance was measured at 595 nm. Protein samples were stored in aliquots at − 80 °C for further use or used directly.
Determination of ROS Level
ROS level was measured by fluorometric method using 2′,7′-dichlorofluorescein diacetate (H2DCFDA) as previously reported (Das and Vinayak 2014). DRG or spinal cord extracts containing equal amount of protein were incubated with equal volume of 50 µM H2DCFDA (Invitrogen) at 370C for 60 min. Fluorescence was recorded at 485 nm (excitation) and 530 nm (emission) with Biotek Synergy H1 Hybrid Multi-Mode Reader and presented in arbitrary units (AU) in terms of fluorescence intensity/mg protein.
Estimation of Cytokine TNF-α
Level of TNF-α was measured by kit (cat# KB3145; Krishgen Biosystems, Mumbai, India) based on sandwich ELISA method following manufacturer’s instruction. Briefly, 100 μl of antigen standard solutions and tissue samples were added to capture antibody-coated ELISA plate wells. Captured antigens were allowed to bind with biotin-conjugated detection antibodies. Detection was done by Avidin-HRP conjugate. 3,3′,5,5′-Tetramethylbenzidine (TMB) was used as color development reagent. Reaction was stopped by stop solution. Color intensity was measured by recording absorbance at 450 nm in ELISA reader (micro scan, ECIL, India). Concentration of cytokines was calculated by standard curve generated by serial dilutions of antigen standard.
Western Blotting
DRG and spinal cord (L4–L6) were homogenized as stated above (under “Preparation of Homogenates” section). The supernatant was used for Western blot analysis of pERK1/2, ERK1/2, TNFR1, NF-κB and Iba1. Equal amount of total protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked with 5% non-fat milk in 1X TBST (20 mM TBS + 0.1% Tween-20) for 2 h to prevent non-specific binding. Blots were incubated overnight at 4 °C with anti-pERK1/2 (1:1000), anti-ERK1/2 (1:1500), anti-TNFR1 (1:1200), anti-Iba1 (1:1000); and anti-NF-κB (1:800) antibodies in 1% BSA or 5% non-fat milk in 1X TBST; washed in 1X TBST three times for 5–10 min each and incubated with HRP-conjugated secondary antibodies (1:2500) in 5% non-fat milk in 1X TBST for 2 h at RT. Bands were detected by enhanced chemiluminescence (ECL) on X-ray film. Alpha Imager 2200 software (Alpha Innotech) was used for densitometric analysis. β-Actin served as a loading control.
Statistical Analysis
Results were analyzed by one-way ANOVA followed by Tukey post hoc test. Paw withdrawal latency and paw edema test were analyzed by repeated measure ANOVA followed by Tukey post hoc test using Statistical Package for the Social Sciences, SPSS software (IBM corporation, New York, USA). Values were expressed as mean ± S.E.M. obtained from three different sets of experiments; p < 0.05 was taken as statistically significant.
Results
Chronic Treatment with Quercetin Reduces Thermal Hyperalgesia in CFA-Induced Inflammation
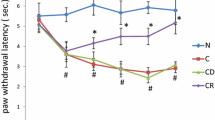
Thermal hyperalgesia was measured as paw withdrawal latency (PWL) in CFA-induced rats. Hyperalgesia is inversely related to PWL, i.e., lower the PWL, higher the thermal hyperalgesia and vice versa. When compared between control rats (N), CFA-induced hyperalgesic (C and CD) and quercetin-treated hyperalgesic (CQ25, CQ50 and CQ75) rats, there was no significant difference in the baseline latency of different groups (Fig. 2a). Further, saline injection (i.pl.) in control rats did not cause any significant hyperalgesic response when compared to baseline latency. PWL in CFA-induced rats was approximately 3.45 s and 3.46 s as compared to 7.77 s and 8.25 s in rats of group N at 24 h and 48 h, respectively, which indicated significant increase (p < 0.05) in the hyperalgesic response in CFA-induced rats. All the three doses of quercetin (25, 50 and 75 mg/kg of BW) led to significant attenuation (p < 0.05) in hyperalgesia, which was consistent throughout the treatment schedule (Table 1).
Effect of quercetin on a CFA-induced thermal hyperalgesia. PWL was measured everyday on Eddy’s hot plate maintained at 48 °C ± 0.5 °C. Graph is plotted as PWL (sec.) versus time (days), and b paw edema. Edema was measured by Vernier caliper in millimeters. Graph is plotted for paw edema (% change) against time (days). Rats were distributed in six groups (n = 9) as N, C, CD, CQ25, CQ50 and CQ75 as mentioned earlier (Fig. 1a). Results were analyzed by repeated measure ANOVA followed by Tukey post hoc test. Results represent mean ± S.E.M. a,b,f,g,hDenote significant difference of groups C, CD, Q25, CQ50 and CQ75, respectively, with N group (p < 0.05). c,d,eDenote significant difference of groups Q25, CQ50 and CQ75, respectively, with CD group (p < 0.05)
Recurrent Doses Of Quercetin Exhibit Ameliorative Effect On Paw Inflammation
Index of paw inflammation was represented by paw edema which was measured in terms of paw thickness. With the help of Vernier caliper, paw thickness was recorded before and at various time intervals after saline or CFA injection. A transient swelling was observed in the paw after saline injection in CFA-induced group C, which started regressing after 1 h and completely disappeared after 4–6 h of the injection, indicating that the resulting edema was probably due to the volume of saline and not a consequence of inflammatory response. On the other hand, edema caused just after CFA injection was similar to saline injection. However, it was increased to approximately 125–150% (p < 0.05) after 24 h (day1) and was maintained up to 48 h (day2), after which it began to recede gradually in time-dependent manner. DMSO did not show any significant effect in CFA-treated groups.
Quercetin treatment showed anti-inflammatory response (Fig. 2b). When compared with CFA-induced group C rats, the paw edema was regressed after quercetin treatment in a dose-dependent manner. The dose of 75 mg/kg BW of quercetin was found to cause significant decrease in paw edema approximately by 27.49%, 28.64%, 42.22% (p < 0.05) on 2nd, 6th and 7th day, respectively, whereas 50 mg/kg BW repeated quercetin treatment showed significant decrease of approximately 27.53% and 40.76% (p < 0.05) on day 6th and 7th day of quercetin treatment, respectively (Fig. 2b, Table 2). Chronic treatment of 25 mg/kg BW quercetin showed its effect on paw edema only on day 7, a decrease in paw edema of approximately by 28.46% (p < 0.05).
Repetitive Quercetin Treatment Reduces ROS Level
Quercetin is known for its potent antioxidant property. Our result shows that quercetin strongly reduces ROS level. CFA administration resulted in substantial increase in generation and accumulation of ROS in DRG (approximately 2.36-fold in group C; p < 0.01 and 2.27-fold in group CD; p < 0.01) and spinal cord (approximately 21.84-fold in C; p < 0.01 and 1.78-fold in CD; p < 0.01) as compared to control (N) group.
Regular quercetin treatment strongly reduced ROS level in a dose-dependent manner. 25, 50 and 75 mg/kg BW of quercetin caused approximately 23% (p < 0.01), 31.6% (p < 0.01) and 32.9% (p < 0.01) decrease in level of ROS in DRG and 20.3% (p < 0.01), 31.8% (p < 0.01) and 28.2% (p < 0.01) decrease in spinal cord, respectively, as compared to CD group (Fig. 3a, b).
Effect of quercetin on ROS level. Rats were distributed in six groups (n = 5) as N, C, CD, CQ25, CQ50 and CQ75 as mentioned earlier (Fig. 1a). a DRG. b Spinal cord. ROS level was measured by oxidative conversion of H2DCFDA to fluorescent DCF. Data are presented in terms of fluorescence intensity. Results were analyzed by one-way ANOVA followed by Tukey post hoc test. Results represent mean ± S.E.M. *Denotes significant difference as compared with normal (N) group (*p < 0.05, **p < 0.01 and ***p < 0.001). #Denotes significant difference as compared with CD group (#p < 0.05, ##p < 0.01 and ###p < 0.001)
Quercetin Mitigates Increased c-Fos Expression in SCDH
The anti-hyperalgesic effect of quercetin was further confirmed by expression of c-Fos detected by immunohistochemistry (IHC) in the ipsilateral dorsal horn of the spinal cord of lumbar region (L4–L6). c-Fos is a well-accepted neuronal marker of pain (Harris, 1998). CFA administration up-regulated the expression of c-Fos in the ipsilateral side in C and CD group as compared to the contralateral side in control group (Fig. 4a, b). The number of c-Fos positive cells was high as compared to control (N) rats. It was 51 ± 1.5 in control (N) rats which increased to 90 ± 3.6 (p < 0.01) in CFA-induced group C and 83 ± 3.6 (p < 0.01) in group CD. There was no significant difference between C and CD groups. The number of c-Fos-positive cells was found to decrease after quercetin treatment in a dose-dependent manner, i.e., 64 ± 4.4 (p < 0.01), 36 ± 2.3 (p < 0.001) and 28 ± 3.7 (p < 0.001) in CQ25, CQ50 and CQ75 groups, respectively, as compared to CD group (Fig. 4a, b). Result supports the anti-hyperalgesic effect of quercetin in CFA-induced hyperalgesia.
Effect of quercetin on c-Fos expression in spinal cord dorsal horn. a Anti-nociceptive activity in terms of c-Fos expression in ipsilateral dorsal horn in group N, C, CD, CQ25, CQ50 and CQ75. Scale bar represents 500 µm. b Number of c-Fos-positive cells. c-Fos-positive cells were counted manually in 4–6 sections for each group irrespective of the intensity of stain. Results were analyzed by one-way ANOVA followed by Tukey post hoc test. Results represents mean ± S.E.M. *Denotes significant difference as compared with normal (N) group (*p < 0.05, **p < 0.01 and ***p < 0.001). #Denotes significant difference as compared with CD group (#p < 0.05, ##p < 0.01 and ###p < 0.001)
Anti-hyperalgesic Effect of Quercetin by Down-Regulating the Expression of TNF-α
Inflammation is associated with rapid increase in the level of pro-inflammatory cytokines. TNF-α is reported to be the most important pro-inflammatory cytokine (Rittner and Stein 2005). Level of TNF-α was measured in DRG and spinal cord of lumbar region (L4–L6). Hyperalgesic rats of groups C and CD showed increase in level of TNF-α approximately up to 2.4- (p < 0.001) and 2.15-fold (p < 0.001) in DRG, and 2.1- (p < 0.01) and 2.0-fold (p < 0.001) in spinal cord, respectively, as compared to control (N) group.
Different doses of quercetin were found to significantly reduce TNF-α level towards normal at both peripheral as well as central sites. CQ25, CQ50 and CQ75 caused decrease of approximately 21.4% (p > 0.05), 43% (p < 0.01), 61% (p < 0.001) in DRG (Fig. 5a) and 22% (p < 0.05), 47.1% (p < 0.001) and 53.7% (p < 0.001) in spinal cord (Fig. 5b) as compared to CD group.
Effect of quercetin on a and b TNF-α level. Level of cytokine TNF-α was checked using kit in the six groups (n = 5) as N, C, CD, CQ25, CQ50 and CQ75 as mentioned earlier (Fig. 1a). a DRG. b Spinal cord. Data are presented in terms of level of TNF-α (pg/mg protein), (c & d) protein level of TNFR1. c DRG, d spinal cord; c-i and d-i protein level measured by Western blot analysis; c-ii and d-ii densitometric analysis normalized by β-actin. Results were analyzed by one-way ANOVA followed by Tukey post hoc test. Results represent mean ± S.E.M. *Denotes significant difference as compared with normal (N) group (*p < 0.05, **p < 0.01 and ***p < 0.001). #Denotes significant difference as compared with CD group (#p < 0.05, ##p < 0.01 and ###p < 0.001)
TNFR1 Is Differentially Involved In DRG And Spinal Cord
Release of tumor necrosis factor-alpha (TNF-α) following inflammation sensitizes nociceptive neurons directly via TNFR1 (Wheeler et al. 2014; Zhang et al. 2011). Our results showed differential expression pattern of TNFR1 in a tissue-dependent manner (Fig. 5c, d). CFA injection caused significant elevation in the protein expression of TNFR1 at peripheral site (DRG) of approximately 1.7-fold (p < 0.01) as compared to control group N (Fig. 5c-i, c-ii), which was significantly inhibited by chronic treatment with 25, 50 and 75 mg/kg BW quercetin by approximately 16.9% (p < 0.05), 49.3% (p < 0.01) and 50% (p < 0.01), respectively. However, no significant change was obtained in the expression of TNFR1 at central site (spinal cord) between all the six groups (Fig. 5d-i, d-ii).
Quercetin Inhibits ERK Phosphorylation at Both Peripheral as Well as Central Sites
ERK activation was measured in terms of ratio of pERK/ERK. Hyperalgesic status in CFA-induced rats is correlated with the higher ratio of pERK/ERK approximately to 1.62-fold (p < 0.001) in C and 1.28-fold (p < 0.05) in CD group in DRG (Fig. 6a) and 1.25-fold (p < 0.05) in C and 1.23-fold (p < 0.05) in CD group in spinal cord (Fig. 6b), with respect to the control (N). Multiple administration of quercetin for 1 week was able to attenuate phosphorylation of ERK1/2 and thus the activation. We observed decrease in pERK/ERK ratio approximately by 49.6% (p < 0.01), 35.5% (p < 0.01) and 22.3% (p < 0.05) in DRG, and 34.1% (p < 0.01), 37.2% (p < 0.01) and 34.4% (p < 0.01) in spinal cord with 25, 50 and 75 mg quercetin/kg BW treatment, respectively, as compared to vehicle group (CD). However, the level of total ERK1/2 remained unchanged in all the groups. The result demonstrates ERK1/2 inactivation by chronic treatment of quercetin.
Effect of quercetin on a and b Level of phosphorylated ERK1/2. a DRG, b spinal cord a-i and b-i protein level measured by Western blot analysis; a-ii and b-ii densitometry of pERK1/2/ total ERK1/2 ratio. c and d The activation of NF-κB. c DRG, d spinal cord; c-i and d-i protein level measured by Western blot analysis; c-ii and d-ii densitometric analysis normalized by β-actin. e co-localization of NF-κB with astroglial marker GFAP in the spinal cord. Enlarged image of astrocyte is shown in inset. Scale bar represents 500 µm. Rats were distributed in six groups (n = 5) as N, C, CD, CQ25, CQ50 and CQ75 as mentioned earlier (Fig. 1a). Results were analyzed by one-way ANOVA followed by Tukey post hoc test. Results represent mean ± S.E.M. *denotes significant difference as compared with normal (N) group (*p < 0.05, **p < 0.01 and ***p < 0.001). #denotes significant difference as compared with CD group (#p < 0.05, ##p < 0.01 and ###p < 0.001)
CFA-Induced Activation Of NF-Κb Is Inhibited By Quercetin Treatment
Activation of transcription factor NF-κB is known to be involved in regulation of inflammatory hyperalgesia (Noort et al. 2015). CFA injection to rats establishes inflammatory hyperalgesia as shown by induced activation of NF-κB in DRG (group C; approximately 1.95 fold, p < 0.001 and group CD; 1.64 fold, p < 0.001) and spinal cord (group C; approximately 2.8 fold, p < 0.001 and group CD; 3.12 fold, p < 0.001) compared to control rats (Fig. 6c, d). Inhibition of the elevated activation of NF-κB at both peripheral (DRG) as well as central (spinal cord) sites demonstrates anti-inflammatory action of quercetin. As compared to CD group, there was a significant attenuation in quercetin-treated groups approximately by 50.3% (p < 0.001), 77.9% (p < 0.001) and 85.3% (p < 0.001) in DRG and 41.6% (p < 0.001), 70.1% (p < 0.001) and 55.2% (p < 0.001) in spinal cord, in groups CQ25, CQ50 and CQ75, respectively.
Further, the immunofluorescence study of NF-κB with astrocyte marker GFAP in the spinal cord demonstrates its co-localization with astrocytes, suggesting that astrocytic NF-κB is involved in CFA-induced hyperalgesia (Fig. 6e).
The anti-inflammatory and anti-hyperalgesic activity of quercetin was evident with change in all the molecular modulators of hyperalgesia towards normal.
Quercetin Alleviates Central Sensitization by Inhibition of Spinal Glial Activation
Glial cells (microglia and astrocytes) within the SCDH respond to the enhanced nociceptive input following peripheral injury, and contribute to the development and maintenance of chronic inflammatory pain states (Carniglia et al. 2017; Okada-Ogawa et al. 2009). Accordingly the expression of GFAP, an astrocytes specific protein was found elevated in spinal cord in groups C (approximately 1.83 times, p < 0.001) and CD (2.06 times, p < 0.001) as compared to the control group N. Quercetin treatment resulted in significant decrease in GFAP expression by approximately 38.5% (p < 0.001), 55.4% (p < 0.001) and 47.2% (p < 0.001) with 25, 50 and 75 mg/kg BW quercetin treatment, respectively (Fig. 7a-i, ii). Similar pattern was evidenced in expression of Iba1, a microglia specific protein in the spinal cord. Iba1 expression was up-regulated in spinal cord up to approximately 2.23-fold (p < 0.001) and 2.3-fold (p < 0.001) in groups C and CD, respectively, as compared to the control (N). Further, quercetin was found to be effective in decreasing the enhanced level of microglia marker protein Iba1 in a dose-dependent manner, by approximately 12.2% (p > 0.05); 65.6% (p < 0.001) and 67.5% (p < 0.001) in CQ25, CQ50 and CQ75, respectively (Fig. 7b-i, b-ii).
Effect of quercetin on glial activation. a-i Anti-hyperalgesic activity of quercetin is represented in terms of GFAP expression (astrocyte marker) in ipsilateral dorsal horn in group N, C, CD, CQ25, CQ50 and CQ75. Scale bar represents 500 µm. Enlarged image of astrocyte is shown in inset. a-ii Densitometric analysis of GFAP expression in SCDH; b-i protein level of Iba1 (microglial marker) measured by Western blot analysis and b-ii densitometric analysis as bar graph. Results were analyzed by one-way ANOVA followed by Tukey post hoc test. Results represents mean ± S.E.M. *denotes significant difference as compared with normal (N) group (*p < 0.05, **p < 0.01 and ***p < 0.001). #denotes significant difference as compared with CD group (#p < 0.05, ##p < 0.01 and ###p < 0.001)
Discussion
Noxious stimulation results in the release of inflammatory mediators that activate nociceptors and modulate signaling pathways leading to pain sensation. Accumulation of ROS helps to sustain the hyperalgesic state, and antioxidants are reported to reduce pain sensation by decreasing ROS. The present study investigated the effect of quercetin on chronic inflammatory pain induced by CFA, and the signaling mechanism involved therein. Quercetin, a polyphenol, is one of the most abundant dietary flavonoids and has been identified as potent antioxidant. Preliminary results established that CFA-induced inflammation and behavioral hyperalgesic response was reversed by chronic quercetin administration. Accumulating literatures suggest that quercetin has several pharmacological properties, including anti-inflammatory and anticancer (Maurya and Vinayak 2017), anti-hepatic fibrosis (Lee et al. 2003), anti-hyperalgesic (Ji et al. 2017; Nie and Liu 2017; Valério et al. 2009) and neuroprotective in different animal models of neuropathy (Azevedo et al. 2013; Raygude et al. 2012; Narenjkar et al. 2011). However, the reports about anti-hyperalgesic and neuroprotective effects of quercetin are scarce, and the mechanistic insight of the ameliorative effect of quercetin in inflammatory pain remains insufficiently explored. Therefore, the present study was undertaken to evaluate the role of quercetin in regulation of inflammatory chronic hyperalgesia and its signaling mechanism.
Attenuation of CFA-induced thermal hyperalgesia throughout the duration of experiment by chronic administration of quercetin and decrease in the intense edema in the ipsilateral foot to about half in CFA-induced rats indicate ameliorating effect of quercetin in inflammatory pain. This is in accordance with earlier reports showing chronic treatment or high dose of quercetin to be effective in different animal models of inflammatory and neuropathic pain (Çivi et al. 2016; Raygude et al. 2012; Narenjkar et al. 2011). c-Fos is considered as the neuronal marker of pain since long, emphasizing its role in the development of a pain state. c-fos is the early gene reported to be highly expressed following peripheral noxious stimulation (Ahmad and Ismail 2002). It is expressed in the nuclei of postsynaptic neurons (mainly Aδ and C-fibers) of ipsilateral dorsal horn of spinal cord, largely in regions demarcated as laminae I–IV. The rapid transcriptional activation of c-fos gene peaks at 30–40 min and protein level approximately 1–2 h after peripheral noxious stimulation (Harris, 1998; Morgan and Curran, 1991). Our findings showing decrease in the c-Fos expression in ipsilateral dorsal horn by quercetin in a dose-dependent manner validates its role in pain relief. The result is in line with earlier reports of anti-inflammatory agents (Singh and Vinayak 2016). The overall findings support anti-inflammatory and anti-hyperalgesic role of quercetin.
Generation and accumulation of ROS has been earlier correlated with development and pathogenesis of hyperalgesia (Singh and Vinayak 2017b; Khattab 2006). ROS generated via NADPH oxidase complex (Kumar et al. 2019; Hackel et al. 2013; Ibi et al. 2011, 2008) and mitochondrial profiles (Lu et al. 2011; Schwartz et al. 2008, 2009) primarily contribute to pain hypersensitivity, following an inflammatory stimulus or peripheral nerve injury. Further, blockage of ROS generation by inhibition/ablation of NADPH oxidase (Hackel et al. 2013; Ibi et al. 2008, 2011) or removal of ROS using ROS scavengers (Bernardy et al. 2017; Kim et al. 2017) and antioxidants (Singh and Vinayak 2016, 2017a) is reported to cause inhibition of inflammatory pain. Our results demonstrate rise in ROS level in DRG and spinal cord following inflammation caused by CFA, suggesting that ROS plays crucial role in both peripheral as well as central sensitization. Quercetin being a potent antioxidant successfully reduced ROS level possibly because of its strong radical scavenging property. Alternatively, quercetin has been reported to exert its analgesic effect by inhibiting pro-nociceptive cytokine production (Valério et al. 2009). Similar analgesic effect of quercetin was demonstrated in the present study by suppression of CFA-induced elevated TNF-α secretion at both the peripheral (DRG) and central (spinal cord) levels. TNF-α secretion by nociceptors and glial cell in response to inflammatory insult is well known to modulate hyperalgesia by initiating and regulating the cascade of other inflammatory cytokines like interleukins: IL-1β and IL-6 (Huang et al. 2017; McInnes and Schett 2017; Ferraz et al. 2015; Wang et al. 2015; Lima et al. 2014). These pro-inflammatory cytokines act via cell surface receptors of nociceptor. TNF-α-mediated regulation mainly operates via TNFR1 to activate stress-activated protein kinase (SAPK) and mitogen-activated protein kinase (MAPK) signaling pathways, resulting into excitability and hypersensitivity of nociceptors (Xu et al. 2017). Our result showing differential involvement of TNFR1 during hyperalgesia in a tissue-dependent manner is in line with previous reports (Wheeler et al. 2014; Zhang et al. 2011). Up-regulated level of TNFR1 in DRG following CFA stimulation reveals that ROS-mediated TNF-α-TNFR1 is mainly operative at peripheral site, whereas unaltered TNFR1 level in spinal cord may be due to involvement of an alternate signaling as reported to be mediated via Src family kinases at central site (Singh and Vinayak 2017b). Zhang et al. (2011) have suggested that unaltered TNFR1 level at central site may be due to high copy number of TNFR1 mRNA that suffice the elevated TNF-α signal transduction. However, there are other reports suggesting that expression of TNFR1 in spinal cord is apparently dependent on the type of noxious stimulation (Del Rivero et al. 2019; Yamacita-Borin et al. 2015; Ma et al. 2009). Suppression of elevated level of TNFR1 in DRG by quercetin further supports its pain relieving potential.
Increasing body of evidence demonstrates critical role of ERK1/2 activation in peripheral and central sensitization of inflammatory responses (Kumar and Vinayak 2020; Kumar et al. 2019; Uttam et al. 2018; Singh and Vinayak 2017b; Zhang et al. 2014). Downstream signaling of activated pERK1/2 leads to transcriptional activation of several genes like c-fos, Cox-2, and NK-1, involved in synaptic plasticity in response to tissue injury and hypersensitivity (Ji 2004). Activation of ERK1/2 in CFA-induced rats and subsequent deactivation by quercetin treatment in the present study supports the efficacy of antioxidant quercetin in decreasing pain hypersensitivity. We have earlier reported such attenuation of ROS-mediated ERK1/2 activation by antioxidant resveratrol (Singh and Vinayak 2017a).
Involvement of nuclear factor-κB (NF-κB) in various inflammatory and immune responses is well known (Noort et al. 2015; Sun 2011). Increased NF-κB activity is linked to different chronic pain conditions (Hartung et al. 2015; Luo et al. 2014). The present finding of up-regulated NF-κB in astrocytes is supported by the previous reports (Hartung et al. 2015). While NF-κB is ubiquitously expressed in a variety of cell types, its contribution is driven largely by signaling in the dorsal root ganglia and in astrocytes of spinal cord during inflammatory and neuropathic pain (Lee et al. 2011). The present study exhibits the impact of quercetin in mitigating inflammatory hyperalgesia by inhibiting NF-κB activation in both DRG and spinal cord. The activated spinal glial cells have important role in the development and maintenance of persistent pain states (Old et al. 2015; Ikeda et al. 2012). Microglia and astrocytes are reported to have distinct role in different pain etiologies where microglial activation precedes astrocytic activation, suggesting the role of microglia in the initiation phase and astrocytes in maintenance of hypersensitivity following peripheral noxious stimulation (Mika et al. 2009; Fu et al. 2007). Recently the ameliorative effect of quercetin in mouse model of intense acute swimming-induced muscle pain was correlated with inhibition of spinal glial mRNA over-expression (Borghi et al. 2016). CFA-induced hyperalgesia was associated with activation of both microglia and astrocytes, and the activated astroglia was found to exhibit hypertrophy. Anti-hyperalgesic effect of quercetin is confirmed by inactivation of astrocytes and microglia (as indicated by decreased level of marker protein Iba1). Deactivation of spinal glia (microglia and astrocytes) by chronic treatment of quercetin was functionally correlated with inhibition of NF-κB activation in spinal cord.
In summary, the results indicate up-regulation of ROS-TNF-α-ERK1/2 signaling pathway and spinal glial (astrocytes and microglia) activation in CFA-induced hyperalgesia. Repeated treatment with quercetin exhibits anti-hyperalgesic potential by regulating the molecular mediators involved in the aforesaid pathway. The study provides first molecular insight on quercetin-mediated anti-hyperalgesic effect via attenuation of ROS-mediated ERK1/2 signaling and glial activation.
The overall molecular mechanism of hyperalgesia ameliorating effect of quercetin on CFA-induced chronic pain is graphically demonstrated below (Fig. 8).
Abbreviations
- ANOVA:
-
Analysis of variance
- CFA:
-
Complete Freund’s Adjuvant
- DMSO:
-
Dimethyl sulfoxide
- DRG:
-
Dorsal root ganglion
- ELISA:
-
Enzyme-linked immunosorbent assay
- ERK:
-
Extracellular signal-regulated kinase
- FITC:
-
Fluorescein isothiocyanate
- GFAP:
-
Glial fibrillary acidic protein
- HRP:
-
Horseradish peroxidase
- MAPK:
-
Mitogen-activated protein kinases
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- PBS:
-
Phosphate buffer saline
- PWL:
-
Paw withdrawal latency
- SCDH:
-
Spinal cord dorsal horn
- TNF-α:
-
Tumor necrosis factor-alpha
- TNFR1:
-
Tumor necrosis factor receptor 1
- TRITC:
-
Tetramethylrhodamine
References
Ahmad, A. H., & Ismail, Z. (2002). c-fos and its Consequences in Pain. Malaysian Journal of Medical Science, 9(1), 3–8.
Azevedo, M. I., Pereira, A. F., Nogueira, R. B., Rolim, F. E., Brito, G. A., Wong, D. V., et al. (2013). The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Molecular Pain, 9, 53. https://doi.org/10.1186/1744-8069-9-53.
Basbaum, A. I., Bautista, D. M., Scherrer, G., & Julius, D. (2009). Cellular and molecular mechanisms of pain. Cell, 139(2), 267–284. https://doi.org/10.1016/j.cell.2009.09.028.
Bernardy, C. C. F., Zarpelon, A. C., Pinho-Ribeiro, F. A., Calixto-Campos, C., Carvalho, T. T., Fattori, V., et al. (2017). Tempol, a superoxide dismutase mimetic agent, inhibits superoxide anion-induced inflammatory pain in mice. Biomed Research International, 2017, 9584819. https://doi.org/10.1155/2017/9584819.
Borghi, S. M., Pinho-Ribeiro, F. A., Fattori, V., Bussmann, A. J., Vignoli, J. A., Camilios-Neto, D., et al. (2016). Quercetin inhibits peripheral and spinal cord nociceptive mechanisms to reduce intense acute swimming-induced muscle pain in mice. PLoS ONE, 11(9), e0162267. https://doi.org/10.1371/journal.pone.0162267.
Carniglia, L., Ramírez, D., Durand, D., Saba, J., Turati, J., Caruso, C., et al. (2017). Neuropeptides and microglial activation in inflammation, pain, and neurodegenerative diseases. Mediators of Inflammation, 2017, 5048616. https://doi.org/10.1155/2017/5048616.
Çivi, S., Emmez, G., Dere, Ü. A., Börcek, A. Ö., & Emmez, H. (2016). Effects of quercetin on chronic constriction nerve injury in an experimental rat model. Acta Neurochirurgica (Wien), 158(5), 959–965. https://doi.org/10.1007/s00701-016-2761-0.
Costigan, M., Scholz, J., & Woolf, C. J. (2009). Neuropathic pain, a maladaptive response of the nervous system to damage. Annual Review of Neuroscience, 32, 1–32. https://doi.org/10.1146/annurev.neuro.051508.135531.
Das, L., & Vinayak, M. (2014). Long term effect of curcumin in regulation of glycolytic pathway and angiogenesis via modulation of stress activated genes in prevention of cancer. PLoS ONE, 9(6), e99583.
Del Rivero, T., Fischer, R., Yang, F., Swanson, K. A., & Bethea, J. R. (2019). Tumor necrosis factor receptor 1 inhibition is therapeutic for neuropathic pain in males but not in females. Pain, 160(4), 922–931. https://doi.org/10.1097/j.pain.0000000000001470.
Ferraz, C. R., Calixto-Campos, C., Manchope, M. F., Casagrande, R., Clissa, P. B., Baldo, C., et al. (2015). Jararhagin-induced mechanical hyperalgesia depends on TNF-α, IL-1β and NF-κB in mice. Toxicon, 103, 119–128. https://doi.org/10.1016/j.toxicon.2015.06.024.
Fidanboylu, M., Griffiths, L. A., & Flatters, S. J. (2011). Global inhibition of reactive oxygen species (ROS) inhibits paclitaxel-induced painful peripheral neuropathy. PLoS ONE, 6, e25212. https://doi.org/10.1371/journal.pone.0025212.
Fu, X., Zhu, Z. H., Wang, Y. Q., & Wu, G. C. (2007). Regulation of proinflammatory cytokines gene expression by nociceptin/orphanin FQ in the spinal cord and the cultured astrocytes. Neuroscience, 144(1), 275–285. https://doi.org/10.1016/j.neuroscience.2006.09.016.
Gao, Y. J., & Ji, R. R. (2009). c-Fos and p-ERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain Journal, 2, 11–17. https://doi.org/10.2174/1876386300902010011.
Gold, M. S., & Gebhart, G. F. (2010). Nociceptor sensitization in pain pathogenesis. Nature Medicine, 16, 1248–1257. https://doi.org/10.1038/nm.2235.
Gregory, N. S., Harris, A. L., Robinson, C. R., Dougherty, P. M., Fuchs, P. N., & Sluka, K. A. (2013). An overview of animal models of pain, disease models and outcome measures. Journal of Pain, 14(11), 1255–1269. https://doi.org/10.1016/j.jpain.2013.06.008.
Hackel, D., Pflücke, D., Neumann, A., Viebahn, J., Mousa, S., Wischmeyer, E., et al. (2013). The connection of monocytes and reactive oxygen species in pain. PLoS ONE, 8(5), e63564. https://doi.org/10.1371/journal.pone.0063564.
Harris, J. A. (1998). Using c-fos as a neural marker of pain. Brain Research Bulletin, 45(1), 1–8.
Hartung, J. E., Eskew, O., Wong, T., Tchivileva, I. E., Oladosu, F. A., O'Buckley, S. C., et al. (2015). Nuclear factor-kappa B regulates pain and COMT expression in a rodent model of inflammation. Brain Behavior and Immunity, 50, 196–202. https://doi.org/10.1016/j.bbi.2015.07.014.
Huang, P. C., Tsai, K. L., Chen, Y. W., Lin, H. T., & Hun, C. H. (2017). Exercise combined with ultrasound attenuates neuropathic pain in rats associated with downregulation of IL-6 and TNF-α, but with upregulation of IL-10. Anesthesia and Analgesia, 124(6), 2038–2044. https://doi.org/10.1213/ANE.0000000000001600.
Ibi, M., Matsuno, K., Matsumoto, M., Sasaki, M., Nakagawa, T., Katsuyama, M., et al. (2011). Involvement of NOX1/NADPH oxidase in morphine-induced analgesia and tolerance. Journal of Neuroscience, 31(49), 18094–18103. https://doi.org/10.1523/JNEUROSCI.4136-11.2011.
Ibi, M., Matsuno, K., Shiba, D., Katsuyama, M., Iwata, K., Kakehi, T., et al. (2008). Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. Journal of Neuroscience, 28, 9486–9494. https://doi.org/10.1523/JNEUROSCI.1857-08.2008.
Ikeda, H., Kiritoshi, T., & Murase, K. (2012). Contribution of microglia and astrocytes to the central sensitization, inflammatory and neuropathic pain in the juvenile rat. Molecular Pain, 8, 43–53. https://doi.org/10.1186/1744-8069-8-43.
Ji, C., Xu, Y., Han, F., Sun, D., Zhang, H., Li, X., et al. (2017). Quercetin alleviates thermal and cold hyperalgesia in a rat neuropathic pain model by inhibiting toll-like receptor signaling. Biomedicine & Pharmacotherapy, 94, 652–658. https://doi.org/10.1016/j.biopha.2017.07.145.
Ji, R. R. (2004). Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases. Current Drug Targets, 3, 299–303. https://doi.org/10.2174/1568010043343804.
Khattab, M. M. (2006). TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia, a key role for superoxide anion. European Journal of Pharmacology, 548, 167–173. https://doi.org/10.1016/j.ejphar.2006.08.007.
Kim, H. K., Hwang, S. H., & Abdi, S. (2017). Tempol ameliorates and prevents mechanical hyperalgesia in a rat model of chemotherapy-induced neuropathic pain. Frontiers in Pharmacology, 7, 532. https://doi.org/10.3389/fphar.2016.00532.
Kumar, S., & Vinayak, M. (2020). NADPH oxidase1 inhibition leads to regression of central sensitization during formalin induced acute nociception via attenuation of ERK1/2-NFκB signaling and glial activation. Neurochemistry International, 134, 104652. https://doi.org/10.1016/j.neuint.2019.104652.
Kumar, S., Singh, A. K., & Vinayak, M. (2019). ML171, a specific inhibitor of NOX1 attenuates formalin induced nociceptive sensitization by inhibition of ROS mediated ERK1/2 signaling. Neurochemistry International, 129, 104466. https://doi.org/10.1016/j.neuint.2019.104466.
Kuner, R. (2010). Central mechanisms of pathological pain. Nature Medicine, 16, 1258–1266.
Lee, E. S., Lee, H. E., Shin, J. Y., Yoon, S., & Moon, J. O. (2003). The flavonoid quercetin inhibits dimethylnitrosamine-induced liver damage in rats. Journal of Pharmacy and Pharmacology, 55, 1169–1174. https://doi.org/10.1211/0022357021396.
Lee, M. K., Han, S. R., Park, M. K., Kim, M. J., Bae, Y. C., Kim, S. K., et al. (2011). Behavioral evidence for the differential regulation of p-p38 MAPK and p-NF-kappaB in rats with trigeminal neuropathic pain. Molecular Pain, 7, 57. https://doi.org/10.1186/1744-8069-7-57.
Lima, C. K., Silva, R. M., Lacerda, R. B., Santos, B. L., Silva, R. V., Amaral, L. S., et al. (2014). LASSBio-1135, a dual TRPV1 antagonist and anti-TNFalpha compound orally effective in models of inflammatory and neuropathic pain. PLoS ONE, 9(6), e99510. https://doi.org/10.1371/journal.pone.0099510.
Lu, R., Kallenborn-Gerhardt, W., Geisslinger, G., & Schmidtko, A. (2011). Additive antinociceptive effects of a combination of vitamin C and vitamin E after peripheral nerve injury. PLoS ONE, 6(12), e29240. https://doi.org/10.1371/journal.pone.0029240.
Luo, J. G., Zhao, X. L., Xu, W. C., Zhao, X. J., Wang, J. N., Lin, X. W., et al. (2014). Activation of spinal NF-kappaB/p65 contributes to peripheral inflammation and hyperalgesia in rat adjuvantinduced arthritis. Arthritis & Rheumatology, 66, 896–906. https://doi.org/10.1002/art.38328.
Ma, F., Zhang, L., & Westlund, K. N. (2009). Reactive oxygen species mediate TNFR1 increase after TRPV1 activation in mouse DRG neurons. Molecular Pain, 5, 31. https://doi.org/10.1186/1744-8069-5-31.
Maurya, A. K., & Vinayak, M. (2017). Quercetin attenuates cell survival, inflammation, and angiogenesis via modulation of AKT signaling in murine T-Cell lymphoma. Nutrition and Cancer, 69(3), 470–480. https://doi.org/10.1080/01635581.2017.1267775.
McInnes, I. B., & Schett, G. (2017). Pathogenetic insights from the treatment of rheumatoid arthritis, targeted treatments for rheumatoid arthritis 1. Lancet, 389, 2328–2337. https://doi.org/10.1016/S0140-6736(17)31472-1.
Mika, J., Osikowicz, M., Rojewska, E., Korostynski, M., Wawrzczak-Bargiela, A., Przewlocki, R., et al. (2009). Differential activation of spinal microglial and astroglial cells in a mouse model of peripheral neuropathic pain. European Journal of Pharmacology, 623(1–3), 65–72. https://doi.org/10.1016/j.ejphar.2009.09.030.
Morgan, J. I., & Curran, T. (1991). Stimulus-transcription coupling in the nervous system, involvement of the inducible proto-oncogenes fos and jun. Annual Review of Neuroscience, 14, 421–451. https://doi.org/10.1146/annurev.ne.14.030191.002225.
Narenjkar, J., Roghani, M., Alambeygi, H., & Sedaghati, F. (2011). The effect of the flavonoid quercetin on pain sensation in diabetic rats. Basic and Clinical Neuroscience, 2(3), 51–57.
Nie, J., & Liu, X. (2017). Quercetin alleviates generalized hyperalgesia in mice with induced adenomyosis. Molecular Medicine Reports, 16(4), 5370–5376. https://doi.org/10.3892/mmr.2017.7238.
Noort, A. R., Tak, P. P., & Tas, S. W. (2015). Non-canonical NF-κB signaling in rheumatoid arthritis, Dr Jekyll and Mr Hyde? Arthritis Research & Therapy, 17(1), 15. https://doi.org/10.1186/s13075-015-0527-3.
Okada-Ogawa, A., Suzuki, I., Sessle, B. J., Chiang, C. Y., Salter, M. W., Dostrovsky, J. O., et al. (2009). Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. Journal of Neuroscience, 29(36), 11161–11171. https://doi.org/10.1523/JNEUROSCI.3365-09.2009.
Old, E. A., Clark, A. K., & Malcangio, M. (2015). The role of glia in the spinal cord in neuropathic and inflammatory pain. Handbook of Experimental Pharmacology, 227, 145–170. https://doi.org/10.1007/978-3-662-46450-2_8.
Puigdellívol-Sánchez, A., Forcada-Calvet, P., Prats-Galino, A., & Molander, C. (2000). Contribution of femoral and proximal sciatic nerve branches to the sensory innervation of hindlimb digits in the rat. Anatomical Record, 260, 180–188. https://doi.org/10.1002/1097-0185(20001001)260,2<180,AID-AR70>3.0.CO;2-E.
Raygude, K. S., Kandhare, A. D., Ghosh, P., Ghule, A. E., & Bodhankar, S. L. (2012). Evaluation of ameliorative effect of quercetin in experimental model of alcoholic neuropathy in rats. Inflammopharmacology, 20, 331–341. https://doi.org/10.1007/s10787-012-0122-z.
Rigaud, M., Gemes, G., Barabas, M. E., Chernoff, D. I., Abram, S. E., Stucky, C. L., et al. (2008). Species and strain differences in rodent sciatic nerve anatomy, implications for studies of neuropathic pain. Pain, 136(1–2), 188–201. https://doi.org/10.1016/j.pain.2008.01.016.
Rittner, H. L., & Stein, C. (2005). Involvement of cytokines, chemokines and adhesion molecules in opioid analgesia. European Journal of Pain, 9(2), 109–112. https://doi.org/10.1016/j.ejpain.2004.05.009.
Schwartz, E. S., Kim, H. Y., Wang, J., Lee, I., Klann, E., Chung, J. M., et al. (2009). Persistent pain is dependent on spinal mitochondrial antioxidant levels. Journal of Neuroscience, 29(1), 159–168. https://doi.org/10.1523/JNEUROSCI.3792-08.2009.
Schwartz, E. S., Lee, I., Chung, K., & Chung, J. M. (2008). Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain, 138(3), 514–524. https://doi.org/10.1016/j.pain.2008.01.029.
Skopelja-Gardner, S., Saha, M., Alvarado-Vazquez, P. A., Liponis, B. S., Martinez, E., & Romero-Sandoval, E. A. (2017). Mitogen-activated protein kinase phosphatase-3 (MKP-3) in the surgical wound is necessary for the resolution of postoperative pain in mice. Journal of Pain Research, 10(763–774), 2017. https://doi.org/10.2147/JPR.S129826.eCollection.
Singh, A. K., & Vinayak, M. (2017a). Resveratrol alleviates inflammatory hyperalgesia by modulation of reactive oxygen species (ROS), antioxidant enzymes and ERK1/2 activation. Inflammation Research, 66(10), 911–921. https://doi.org/10.1007/s00011-017-1072-0.
Singh, A. K., & Vinayak, M. (2017b). Activation of ERK1/2 signaling by Src family kinases (SFKs) in DRG neurons contribute to hydrogen peroxide (H2O2) induced thermal hyperalgesia. Free Radical Research, 51(9–10), 838–850. https://doi.org/10.1080/10715762.2017.
Singh, A. K., & Vinayak, M. (2016). Anti-nociceptive effect of resveratrol during inflammatory hyperalgesia via differential regulation of pro-inflammatory mediators. Phytotherapy Research, 30(7), 1164–1171. https://doi.org/10.1002/ptr.5624.
Singh, A. K., & Vinayak, M. (2015). Curcumin attenuates CFA induced thermal hyperalgesia by modulation of antioxidant enzymes and down regulation of TNF-α, IL-1β and IL-6. Neurochemical Research, 40(3), 463–472. https://doi.org/10.1007/s11064-014-1489-6.
Sun, S. C. (2011). Non-canonical NF-kappaB signaling pathway. Cell Research, 21, 71–85. https://doi.org/10.1038/cr.2010.177.
Uttam, S., Wong, C., Amorim, I. S., Jafarnejad, S. M., Tansley, S. N., Yang, J., et al. (2018). Translational profiling of dorsal root ganglia and spinal cord in a mouse model of neuropathic pain. Neurobiology of Pain, 4, 35–44. https://doi.org/10.1016/j.ynpai.2018.04.001.
Valério, D. A., Georgetti, S. R., Magro, D. A., Casagrande, R., Cunha, T. M., Vicentini, F. T., et al. (2009). Quercetin reduces inflammatory pain, inhibition of oxidative stress and cytokine production. Journal of Natural Products, 72, 1975–1979. https://doi.org/10.1021/np900259y.
Wang, C., Song, S., Zhang, Y., Ge, Y., Fang, X., Huang, T., et al. (2015). Inhibition of the Rho/Rho kinase pathway prevents lipopolysaccharide-induced hyperalgesia and the release of TNF-α and IL-1β in the mouse spinal cord. Scientific Reports, 5, 145–153. https://doi.org/10.1038/srep14553.
Wheeler, M. A., Heffner, D. L., Kim, S., Espy, S. M., Spano, A. J., Cleland, C. L., et al. (2014). TNF-α/TNFR1 signaling is required for the development and function of primary nociceptors. Neuron, 82(3), 587–602. https://doi.org/10.1016/j.neuron.2014.04.009.
Wilson, A. W., Medhurst, S. J., Dixon, C. I., Bontoft, N. C., Winyard, L. A., Brackenborough, K. T., et al. (2006). An animal model of chronic inflammatory pain: Pharmacological and temporal differentiation from acute models. European Journal of Pain, 10, 537–537. https://doi.org/10.1016/j.ejpain.2005.08.003.
Xu, T., Li, D., Zhou, X., Ouyang, H. D., Zhou, L. J., Zhou, H., et al. (2017). Oral application of magnesium-l-threonate attenuates vincristine-induced allodynia and hyperalgesia by normalization of tumor necrosis factor-α/nuclear factor-κB signaling. Anesthesiology, 126(6), 1151–1168. https://doi.org/10.1097/ALN.0000000000001601.
Yamacita-Borin, F. Y., Zarpelon, A. C., Pinho-Ribeiro, F. A., Fattori, V., Alves-Filho, J. C., Cunha, F. Q., et al. (2015). Superoxide anion-induced pain and inflammation depends on TNFα/TNFR1 signaling in mice. Neuroscience Letters, 605, 53–58. https://doi.org/10.1016/j.neulet.2015.08.015.
Zhang, L., Berta, T., Xu, Z. Z., Liu, T., Park, J. Y., & Ji, R. R. (2011). TNF-α contributes to spinal cord synaptic plasticity and inflammatory pain, distinct role of TNF receptor subtypes 1 and 2. Pain, 152(2), 419–427. https://doi.org/10.1016/j.pain.2010.11.014.
Zhang, X., Zhang, H., Shao, H., Xue, Q., & Yu, B. (2014). ERK MAPK activation in spinal cord regulates phosphorylation of Cdk5 at Serine 159 and contributes to peripheral inflammation induced pain hypersensitivity. PLoS ONE, 9(1), e87788. https://doi.org/10.1371/journal.pone.0087788.
Acknowledgments
The authors are thankful to DRDO, India for financial support (Grant No. ERIP/ER/1003851/M/01/1336). Sanjay Kumar thanks Council of Scientific & Industrial Research (CSIR), India for Junior Research Fellowship (JRF) and Senior Research Fellowship (SRF). Financial support by DST-FIST, UGC-UPE and UGC-CAS to Department of Zoology, BHU is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, S., Vinayak, M. Quercetin Ameliorates CFA-Induced Chronic Inflammatory Hyperalgesia via Modulation of ROS-Mediated ERK1/2 Signaling and Inhibition of Spinal Glial Activation In Vivo. Neuromol Med 22, 517–533 (2020). https://doi.org/10.1007/s12017-020-08609-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-020-08609-z