Abstract
Objectives
Ankylosing spondylitis (AS) is a chronic inflammatory joint disease. The transporter associated with antigen processing (TAP) has been identified to play an important role in immune response as well as the HLA-associated diseases. The aim of our meta-analysis was to investigate the contribution of TAP (TAP1 and TAP2) polymorphisms to the risk of AS.
Methods
Meta-analyses were performed between 2 polymorphisms in TAP1 (TAP1-333, -637) and 3 polymorphisms in TAP2 (TAP2-379, -565, and -665) and AS.
Results
The meta-analyses were involved with 6 studies with 415 cases and 659 controls. Significant association was found between TAP1-333Val, TAP1-637Gly, and TAP2-565Thr and AS compared with combined control group (TAP1-333Val: p = 0.009, OR = 1.40, 95% CI 1.09–1.80; TAP1-637Gly: p = 0.002, OR = 1.48, 95% CI 1.15–1.91; p = 0.03, OR = 1.38, 95% CI 1.04–1.84). Subgroup analysis shown that significant association was only found in AS when compared with HLA-B27-negative controls (TAP1-333Val: p = 0.004, OR = 1.53, 95% CI 1.14–2.06; TAP1-637Gly: p = 0.004, OR = 1.52, 95% CI 1.15–2.02; p = 0.02, OR = 1.56, 95% CI 1.09–2.24), but not in AS when compared with HLA-B27-positive controls (p > 0.05). Moreover, no significant associations were found between haplotypes in TAP1 and TAP2 in both the combined and the subgroup analyses (p > 0.05).
Conclusions
TAP1-333Val, TAP1-637Gly, and TAP2-565Thr were likely to be associated with AS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory joint disease characterized by sacroiliitis, ankylosis of the spine, and enthesitis [1]. The etiology of AS is unclear yet. Family-based studies have demonstrated that genetic factors, as well as environmental factors, played important roles in the development of AS [2, 3]. HLA-B27 contributes to the strongest genetic association with AS. However, familial studies suggest that the HLA-B27 has an attributable risk of only 16–50% in AS [4, 5], and only 2% of B27-positive individuals develop AS [2]. Thus, additional genetic factors are involved in the pathogenesis of this disease.
Consistent association was found between human leukocyte antigen (HLA) loci and AS [6]. Potential candidates for these additional genetic susceptibility factors are the transporter associated with antigen-processing (TAP) genes: TAP1 and TAP2, which are located within the class II MHC region, between the HLA-DQB1 and HLA-DPA1 loci [7]. The TAP1 and TAP2 genes encode subunits of the functional TAP complex, which are thought to transport peptides that derived from the processed antigen from the cytosol into the endoplasmic reticulum (ER) [8]. Mutations in TAP1 or TAP2 result in structural changes that prevent TAP heterodimer formation and alter antigen recognition and presentation [9]. Thus, TAP genes are good candidate genes for autoimmune diseases including AS.
The previous findings of association between TAP1 and TAP2 polymorphisms and the occurrence and development of AS are well documented [10,11,12,13,14,15,16]. Among them, the TAP1-333, 637; TAP2-379, 565, and 665 are often studied. The TAPIC (333 Val-637 Asp) significantly increased in the AS group compared with random controls (p = 0.03) in British population [10]. Maksymowych et al. reported that the frequency of the TAP1B allele significantly increased in AS patients with extraspinal disease compared with AS patients without extraspinal disease (p = 0.005) or normal controls (p = 0.005) [14]. Otherwise, TAP1-637 and TAP2-565 were shown to be associated with AS compared with HLA-B27-negative control (p < 0.05) in a Chinese population [11]. However, no significant associations were found between TAP1 and TAP2 alleles in Spanish, Japanese, and Caucasian populations [12, 13, 15, 16].
Owing to the conflicting findings and limited availability of sample numbers in some studies, we aimed to perform a set of meta-analyses of the TAP1 and TAP2 polymorphisms by pooling up the data from individual association study. Our research is likely to provide a better evaluation of the contribution of TAP1 and TAP2 polymorphisms to the risk of AS.
Methods
Literature search
This study was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Literature works were searched on Pubmed, Embase, Web of Science, and the Cochrane Library databases and Chinese National Knowledge Infrastructure (CNKI) (last updated on January, 2017) by L. T. and Y. W. using the key words ‘’TAP1” or “transporter associated with antigen processing 1” and “TAP2” or “transporter associated with antigen processing 2” and “ankylosing spondylitis” or “AS” and “polymorphism” or “mutation” or “variant,” or “SNP” or “single nucleotide polymorphism”. Only data from the original publications were recruited. Meeting or conference abstracts were excluded. No particular language was restricted. Meanwhile, other potentially relevant literature studies were identified by cross-references within eligible studies. Related data were collected from the concerned authors when no data were available in the original publications.
Eligibility criteria
A study was included in the current meta-analysis if it conformed to the following criteria: (1) If it was unrelated case–control design study. (2) If it related to the TAP1 or TAP2 polymorphisms and AS. (3) If allele frequencies or genotypes were available in the study.
Data extraction
We extracted the information from each eligible publication manually by two investigators independently (L. T. and Y. F. Q.). Any disagreements were resolved by a consensus arrived at by G. L. W. Details of Information regarding the first author, year of publication, Assessment, ethnicity, the numbers of cases and controls (case/control (HLA-B27-positive and HLA-B27-negative)), variants, genotypes, haplotypes, and major findings were collected from each study.
Quality assessment
The study quality was assessed independently by G. L. W. and F. X. based on the following aspects: source of cases and controls, specimens, sample size, and evidence of HWE. The quality of each included article was assessed according to the Jadad scale [17]. The quality scores ranged from 0 to 5 (0 being the least and 5 being the highest). Any discrepancies in the assessment were resolved by discussion.
Statistical analysis
All the statistical analyses were conducted using the program RevMan 5 (Oxford, UK) and STATA12.0 (StataCorp, College Station, TX, USA). The odds ratio (OR) and the 95% confidence interval (95% CI) were calculated for TAP1 and TAP2 polymorphisms and AS for each study. The pooled ORs were calculated using genetic model of allelic model, recessive model, and dominant model. Furthermore, the statistical significance was determined by the Z test. Homogeneity was tested using Cochran’s Q-statistic and the I 2 statistic. If significant heterogeneity was observed across studies (I 2 > 50%), the random effects model (the DerSimonian and Laird method) was used for meta-analysis. Otherwise, the fixed effects model (Mantel–Haenszel method) was used. Sensitivity analysis was performed to assess the effects of individual study on pooled results and the stability of results. Funnel plots were used to assess publication bias by the method of Egger’s test. A p < 0.05 was considered to be statistically significant.
Results
Characteristics of eligible studies
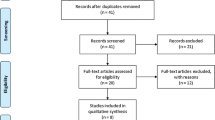
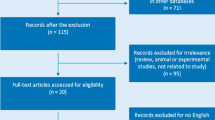
As shown in Fig. 1, a total of 273 relevant articles were initially collected from various databases. Of these, 234 were excluded for not being related to AS, 23 were removed for being only reviews, and 4 were excluded for having no available data of association between TAP1 and TAP2 polymorphisms and AS (Fig. 1). Altogether, 6 studies [10,11,12,13,14,15] with 415 AS and 659 controls meeting the criteria were retained for meta-analysis. The basic characteristics of enrolled patients are shown in Table 1.
Results of meta-analysis
In the present study, we tested the associations between two TAP1 and three TAP2 polymorphisms and AS disease. Allelic model was tested for all five polymorphisms. Different inheritable models, including dominant and recessive models, were also tested for two TAP1 polymorphisms, but not for three TAP2 polymorphisms for lack of available data. In addition, haplotypes containing two TAP1 and three TAP2 polymorphisms were investigated relatively. As shown in Table 2 and Figs. 2, 3, significant associations of TAP1-333Val, TAP1-637Gly, and TAP2-565Thr with increased risk of AS were found for the combined control groups (TAP1-333Val: p = 0.009, OR = 1.40, 95% CI 1.09–1.80; TAP1-637Gly: p = 0.002, OR = 1.48, 95% CI 1.15–1.91; p = 0.03, OR = 1.38, 95% CI 1.04–1.84). Subgroup analysis by HLA-B27 in control groups showed that the significant association was only found in AS when compared with HLA-B27-negative controls (TAP1-333Val: p = 0.004, OR = 1.53, 95% CI 1.14–2.06; TAP1-637Gly: p = 0.004, OR = 1.52, 95% CI 1.15–2.02; p = 0.02, OR = 1.56, 95% CI 1.09–2.24)(Table 2; Figs. 2, 3), but not in AS when compared with HLA-B27-positive controls (p > 0.05) (Table 2). On the other hand, significant contribution of TAP1-333Val to AS was found in the dominant model when compared with combined controls (p = 0.04, OR = 1.44, 95% CI 1.02–2.03), and significant contribution of TAP1-637Gly to AS was found in the dominant model when compared with both combined control groups (p = 0.003, OR = 1.72, 95% CI 1.20–2.47) and HLA-B27-negative controls (p = 0.01, OR = 1.68, 95% CI 1.13–2.48) (Table 2; Figs. 2, 3). For haplotypes in TAP1 (TAP1A, TAP1B, TAP1C, TAP1D) and TAP2 (TAP2A, TAP2B, TAP2C, TAP2D, TAP2E), no significant associations were found in both the combined and the subgroup meta-analyses (Table 2; Fig. 4).
Sources of heterogeneity
No significant heterogeneity was detected among all studies under allelic model (except for TAP1-333Val, p = 0.04, I 2 = 70%), recessive model, and dominant model (p > 0.05). The significant heterogeneity in TAP1-333Val under allelic model was contributed mainly by the study conducted by Burney et al. [10]. Removal of this study from meta-analysis gave 0% (p = 0.68) heterogeneity and showed that it had the highest effect on TAP1-333Val association with the risk effect of AS.
Sensitivity analysis
A sensitivity analysis which excluded the influence of a single study on the overall risk estimate by excluding one study at a time was performed. The ORs were not significantly altered in allelic model (Fig. 5).
Publication bias
Funnel plots and Egger’s test were performed to assess publication bias. The p values for Egger’s linear regression tests are shown in Table 3. Furthermore, no obvious publication bias was observed for all of the five polymorphisms in TAP1 and TAP2 (p > 0.05).
Discussion
To our knowledge, this study is the first meta-analysis investigating the associations between TAP1 and TAP2 polymorphisms and ankylosing spondylitis. Our meta-analysis demonstrated that TAP1-333Val, TAP1-637Gly, and TAP2-565Thr were more likely to be associated with AS compared with HLA-B27-negative controls. Our meta-analysis highlights several important unresolved issues in the genetics of TAP1 and TAP2 in AS. In our meta-analysis, the between-study heterogeneity was low and nonsignificant. There was no publication bias in the significant findings.
Although the role of TAP in the pathogenesis of autoimmune diseases such as AS is largely unknown, several studies have reported their direct or indirect participation in the development of autoimmunity [18, 19]. It is conceivable that TAP variants may influence the selection of epitopes which play a role in the pathogenesis of autoimmune diseases [20]. TAP alleles may be relevant for appropriate peptide selection in the initial antigen-processing event in autoimmune diseases’ (Ads’) pathophysiology. Peptide capture of class I molecules by immature heterodimers may be facilitated in their association with TAP [21]. In addition, it has been shown that polymorphisms in TAP1 or TAP2 may affect antigen recognition and presentation [22]. Specificity can be affected by variation in the structure and/or expression of these genes between individuals. Therefore, different sets of peptides can be derived from presentation of the same antigen to T cells in different people. Thus, TAP genes represent possible susceptibility factors for some autoimmune diseases [23]. Previously, TAP gene polymorphisms have been investigated in several MHC-associated diseases including viral infectious diseases (tuberculosis [24], leprosy [25]), allergic diseases (idiopathic bronchiectasis [26], psoriasis vulgaris [27], and autoimmune diseases (ankylosing spondylitis [10,11,12,13,14,15], Systemic lupus erythematosus [28], rheumatoid arthritis [19], multiple sclerosis [29], Grave’s diseases [30], and insulin-dependent diabetes mellitus [18]), among ethnic groups.
The TAP1 gene polymorphism was first discovered in the early 1990s. The major polymorphic sites are TAP1-333 and -637 (cDNA positions 1069 and 1982) [31]. The comparison of TAP1-333 and -637 allele frequencies in the combined AS cases and control subjects indicated a marked increase of the minor alleles due to high prevalence of heterozygote and recessive homozygote in patients. In a previous study, it was suggested that positive association of genotype TAP1-333 heterozygote (AG) exists with the risk of bronchiectasis in children [26]. Besides, recessive homozygote (GG) was suggested to be a risk factor to develop tuberculosis [32]. Investigations of TAP1-333 and -637 alleles were carried out also in other autoimmune disorders among ethnically different groups, but the changing nomenclatures of TAP1-333 and -637 render a comparison of these studies difficult. In previous studies, significant associations of TAP1-333 and -637 with Grave’s diseases were observed in Germany [33], but not in Taiwan [30]. No primary associations of TAP1-333 and -637 with SLE were observed in Colombians [34] and Taiwanese [28] as well as in Japanese populations [35]. In addition, negative results were also reported on the associations between TAP1-333, -637 and multiple sclerosis in Belgium [29], and rheumatoid arthritis in Eastern France [36] and Chinese populations [37]. As for ankylosing spondylitis (AS), the frequencies of TAP1-333 and -637 significantly increased in combined cases, but not in cases of any individual association study, which might indicate that the TAP1-333, -637 polymorphisms were the risk factors in AS, which seems to be reliable for the higher statistic power than that in the previous studies with moderate sample size.
Similar manifestation was found in the association between TAP2-565 and AS. No significant association was observed between TAP2-565 and AS in any individual study. However, the combined result has shown significant correlation between TAP2-565 and AS. Actually, the polymorphism TAP2-565 has been investigated in several autoimmune diseases with inconsistent results. Takeuchi et al. [35] and Zhang et al. [36] have reported significant association between TAP2-565 and RA in Japan and France. However, these positive results cannot be replicated in Grave’s diseases [30], SLE [34], RA [19], and T1D [38] in other ethnic groups. It may indicate that autoimmune diseases may share only part of susceptible genes. In addition, the interaction between genes and environmental factors may also be an important factor for the difference between the combined meta-analysis in AS and negative correlation in other autoimmune diseases.
Increased frequencies of TAP1 haplotypes B and C, as well as TAP2 haplotypes D and H were observed in cases compared with the controls in Caucasian and Japanese populations [10, 12,13,14]. However, the combined results have shown no significant association between TAP1 and TAP2 haplotypes and AS compared with the HLA-B27-negative controls, HLA-B27-positive controls, and combined controls. The positive results of TAP haplotypes may due to the various spectra of genetic pools among different ethnic groups. Other possible reasons for such discrepancies might be due to multiple factors such as different phenotypic characteristics, heterogeneity within groups of patients and controls, and different genotyping methods.
Interestingly, significant associations were detected between the TAP-333, -637 and TAP2-565 and AS compared with total controls and HLA-B27-negative controls, but not with HLA-B27-positive controls. The relatively limited numbers of studies and cases and controls, especially of HLA-B27-positive controls, included in the study may have contributed to a part of the difference (HLA-B27-positive cases vs. HLA-B27-positive controls: TAP1-333: 308 vs. 270; TAP1-637: 308 vs. 270; TAP2-565: 306 vs. 322).
The results of the present meta-analysis should be interpreted carefully because of the following limitations. First, most of the patients were Caucasians in the present study, and this limited the general application of the results to other populations. Second, AS is a multifactorial disease influenced by both genetic and environmental factors. Different clinical variables may influence the results of the current study. Hidden physiological factors may exist in the AS patients and affect the quality of the current meta-analysis. Third, although the number of current meta-analyses was much larger than that of the previous studies, more replicated studies with still larger number of patients are required to strengthen the stability of the association between TAP1 and TAP2 polymorphisms and AS. Fourth, according to the NCBI dbSNP database, there are thousands of polymorphisms for TAP1 and TAP2 genes. Only two and three polymorphisms in TAP1 and TAP2 were detected in our meta-analysis, which might make it hard to reach a full and firm conclusion regarding the contributions of TAP1 and TAP2 polymorphisms in AS.
In conclusion, TAP1-333Val, TAP1-637Gly, and TAP2-565Thr might be the risk factors for AS. Still more studies are warranted to validate the genetic association between these polymorphisms and AS.
References
Ward MM, Reveille JD, Learch TJ, Davis JC Jr, Weisman MH. Impact of ankylosing spondylitis on work and family life: comparisons with the US population. Arthritis Rheum. 2008;59:497–503.
Dakwar E, Reddy J, Vale FL, Uribe JS. A review of the pathogenesis of ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E2.
Brown MA. Breakthroughs in genetic studies of ankylosing spondylitis. Rheumatology. 2008;47(2):132–7.
Haroon N, Inman RD. Endoplasmic reticulum aminopeptidases: biology and pathogenic potential. Nat Rev Rheumatol. 2010;6:461–7.
Khan MA, Ball EJ. Genetic aspects of ankylosing spondylitis. Best Pract Res Clin Rheumatol. 2002;16:675–90.
Gonzalez-Roces S, Alvarez MV, Gonzalez S, Dieye A, Makni H, Woodfield DG, et al. Hla-b27 polymorphism and worldwide susceptibility to ankylosing spondylitis. Tissue Antigens. 1997;49(2):116.
Trowsdale J, Hanson J, Mockridge I, Beck S, Townsend A, Kelly A. Sequences encoded in the class II region of the MHC related to the ‘ABC’ superfamily of transporters. Nature. 1990;348:741–4.
York IA, Rock KL. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14:369–96.
Koch J, Guntrum R, Heintke S, Kyritsis C, TampÊ R. Functional dissection of the transmembrane domains of the transporter associated with antigen processing (TAP). J Biol Chem. 2004;279:10142–7.
Burney RO. Analysis of the MHC class II encoded components of the HLA class I antigen processing pathway in ankylosing spondylitis. Ann Rheum Dis. 1994;53(1):58–60.
Feng M, Yin B, Shen T, Ma Q, Liu L, Zheng J, et al. Tap1 and tap2 polymorphisms associated with ankylosing spondylitis in genetically homogenous chinese han population. Hum Immunol. 2009;70(4):257–61.
Fraile A, Collado MD, Matarán L, Martín J, Nieto A. Tap1 and tap2 polymorphism in spanish patients with ankylosing spondylitis. Exp Clin Immunogenet. 2000;17(4):199.
Konno Y, Numaga J, Mochizuki M, Mitsui H, Hirata R, Maeda H. Tap polymorphism is not associated with ankylosing spondylitis and complications with acute anterior uveitis in hla-b27-positive Japanese. Tissue Antigens. 1998;52(5):478–83.
Maksymowych WP, Tao S, Li Y, Wing M, Russell AS. Allelic variation at the tap 1 locus influences disease phenotype in hla-b27 positive individuals with ankylosing spondylitis. Tissue Antigens. 1995;45(5):328–32.
Westman P, Partanen J, Leirisalo-Repo M, Koskimies S. Tap1 and tap2 polymorphism in hla-b27-positive subpopulations: no allelic differences in ankylosing spondylitis and reactive arthritis. Hum Immunol. 1995;44(4):236–42.
Fraile A, Vinasco J, Nieto A, Matarán L, Martín J. LMP and TAP polymorphism in Ankylosing Spondylitis. Immunol Lett. 1997;56(56):311.
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12.
Li YY, Gao W, Pang SS, Min XY, Yang ZJ, Wang H, et al. Tap1 i333v gene polymorphism and type 1 diabetes mellitus: a meta-analysis of 2248 cases. J Cell Mol Med. 2014;18(5):929–37.
Dai D. Significant association between TAP2 polymorphisms and rheumatoid arthritis: a meta-analysis. Diagn Pathol. 2014;9(1):129.
Grandea AG, Androlewicz MJ, Athwal RS, Geraghty DE, Spies T. Dependence of peptide binding by MHC class I molecules on their interaction with tap. Science. 1995;270:105–8.
Holden C. Foreign competition. Science. 1995;270(5233):124.
Naderi M, Hashemi M, Amininia S. Association of tap1 and tap2 gene polymorphisms with susceptibility to pulmonary tuberculosis. Iran J Allergy Asthma Immunol. 2016;15(1):62.
Liu XQ, Lan LZ, Endocrinology DO. Research progress of the relationship between TAP, FOXJ1 gene polymorphism and autoimmune disease. China Mod Med. 2014;(14):195–7.
Roh EY, Yoon JH, Shin S, Song EY, Park MH. Association of tap1 and tap2 genes with susceptibility to pulmonary tuberculosis in Koreans. Apmis. 2015;123(6):457.
Shinde V, Marcinek P, Rani DS, Sunder SR, Arun S, Jain S, et al. Genetic evidence of tap1 gene variant as a susceptibility factor in indian leprosy patients. Hum Immunol. 2013;74(6):803.
Doğru D, Gerçeker FÖ, Yalçın E, Çobanoğlu N, Pekcan S, Özçelik U, et al. The role of tap1 and tap2 gene polymorphism in idiopathic bronchiectasis in children. Pediatr Pulmonol. 2007;42(3):237–41.
Witkowska-Toboła AM, Szczerkowska-Dobosz A, Nedoszytko B, Roszkiewicz J. Polymorphism of the tap1 gene in polish patients with psoriasis vulgaris. J Appl Genet. 2004;45(3):391–3.
Huang CM, Hang LW, Chen CL, Wu JY, Tsai FJ. Polymorphisms of tap1 transporter genes in chinese patients with systemic lupus erythematosus in Taiwan. Rheumatol Int. 2004;24(3):130–2.
Vandevyver C, Stinissen P, Cassiman JJ, Raus J. Tap 1 and tap 2 transporter gene polymorphisms in multiple sclerosis: no evidence for disease association with tap. J Neuroimmunol. 1994;54(1–2):35–40.
Chen RH, Wang TY, Chen WC, Tsai CH, Tsai FJ. Association between the tap2 gene codon 665 polymorphism and graves’ disease. J Clin Lab Anal. 2006;20(3):93–7.
Ozbasgerceker F, Bozman N, Gezici S, Pehlivan M, Yilmaz M, Pehlivan S, et al. Association of TAP1 and TAP2 gene polymorphisms with hematological malignancies. Asian Pac J Cancer Prev Apjcp. 2013;14(9):5213–7.
Sunder SR, Hanumanth SR, Gaddam S, Jonnalagada S, Valluri VL. Association of tap, 1 and 2 gene polymorphisms with human immunodeficiency virus-tuberculosis co-infection. Hum Immunol. 2011;72(10):908.
Rau H, Nicolay A, Usadel KH, Finke R, Donner H, Walfish PG, et al. Polymorphisms of tap1 and tap2 genes in graves’ disease. Tissue Antigens. 1997;49(1):16–22.
Correa PA, Molina JF, Pinto LF, Arcosburgos M, Herrera M, Anaya JM. Tap1 and tap2 polymorphisms analysis in northwestern colombian patients with systemic lupus erythematosus. Ann Rheum Dis. 2003;62(4):363–5.
Takeuchi F, Nakano K, Nabeta H, Hong GH, Kuwata S, Ito K. Polymorphisms of the tap1 and tap2 transporter genes in Japanese SLE. Ann Rheum Dis. 1996;55(12):924–6.
Zhang SL, Chabod J, Penfornis A, Reviron D, Tiberghien P, Wendling D, et al. Tap1 and tap2 gene polymorphism in rheumatoid arthritis in a population in eastern france. Eur J Immunogenet. 2002;29(3):241–9.
Yu MC, Huang CM, Wu MC, Wu JY, Tsai FJ. Association of, tap2, gene polymorphisms in chinese patients with rheumatoid arthritis. Clin Rheumatol. 2004;23(1):35.
Rau H, Nicolay A, Donner H, Usadel KH, Badenhoop K. Polymorphisms of tap1 and tap2 genes in german patients with type 1 diabetes mellitus. Eur J Immunogenet. 1997;24(3):229–36.
Acknowledgements
The work was funded by the Foundation of the Education Department of Hunan (11C0141, 15C0513, and 16C0153), the key Foundation of the Education Department of Hunan (16A027), and the Foundation of the health department of Hunan (B2016096).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Qian, Y., Wang, G., Xue, F. et al. Genetic association between TAP1 and TAP2 polymorphisms and ankylosing spondylitis: a systematic review and meta-analysis. Inflamm. Res. 66, 653–661 (2017). https://doi.org/10.1007/s00011-017-1047-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1047-1