Abstract
Objective and design
Our study was designed to elucidate the precise molecular mechanisms by which sorbitol-modified hyaluronic acid (HA/sorbitol) exerts beneficial effects in osteoarthritis (OA).
Methods
Human OA chondrocytes were treated with increasing doses of HA/sorbitol ± anti-CD44 antibody or with sorbitol alone and thereafter with or without interleukin-1beta (IL-1β) or hydrogen peroxide (H2O2). Signal transduction pathways and parameters related to oxidative stress, apoptosis, inflammation, and catabolism were investigated.
Results
HA/sorbitol prevented IL-1β-induced oxidative stress, as measured by reactive oxygen species, p47-NADPH oxidase phosphorylation, 4-hydroxynonenal (HNE) production and HNE-metabolizing glutathione-S-transferase A4-4 expression. Moreover, HA/sorbitol stifled IL-1β-induced metalloproteinase-13, nitric oxide (NO) and prostaglandin E2 release as well as inducible NO synthase expression. Study of the apoptosis process revealed that this gel significantly attenuated cell death, caspase-3 activation and DNA fragmentation elicited by exposure to a cytotoxic H2O2 dose. Examination of signaling pathway components disclosed that HA/sorbitol prevented IL-1β-induced p38 mitogen-activated protein kinase and nuclear factor-kappa B activation, but not that of extracellular signal-regulated kinases 1 and 2. Interestingly, the antioxidant as well as the anti-inflammatory and anti-catabolic effects of HA/sorbitol were attributed to sorbitol and HA, respectively.
Conclusions
Altogether, our findings support a beneficial effect of HA/sorbitol in OA through the restoration of redox status and reduction of apoptosis, inflammation and catabolism involved in cartilage damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is a frequent musculoskeletal disease and a major source of disability in the elderly [1]. Knee OA is the foremost cause of consultations for OA-related symptoms [2]. It is well-established that during joint inflammation and degeneration, as in OA, important macromolecules are lost from the extracellular matrix (ECM), including type II collagen [3]. This phenomenon represents deregulation of chondrocyte metabolism due to the actions of inflammatory cytokines, such as interleukin-1beta (IL-1β), responsible for the downregulation of collagen [4] as well as proteoglycan biosynthesis [5] and stimulation of their degradation [6].
Current OA management combines pharmaceutical and non-pharmacological strategies [7, 8], including intraarticular injections of hyaluronic acid (HA), aimed at decreasing pain and improving joint function [9]. Despite the long history of this therapy, known as viscosupplementation, and numerous studies of its action, the mechanisms responsible for HA’s clinical outcome are not clearly recognized. Intraarticular HA injections may progress to the recovery of desirable viscoelastic behavior of OA-altered synovial fluid (SF) [10]. Other biological functions of exogenous HA in OA include analgesia via interaction with pain receptors [11], promotion of endogenous HA production and various anti-inflammatory effects [12–14]. Most of these effects could be mediated by interaction between HA and its receptors, CD44 and hyaluronan-mediated motility receptor [13, 14].
It has been reported that HA counteracts IL-β-induced inhibition of collagen biosynthesis at both the transcriptional and post-transcriptional levels [15]. The mechanism of HA’s post-transcriptional impact on collagen biosynthesis is seen in certain experimental conditions on analysis of prolidase activity. Intraarticular HA administration still offers potent and well-tolerated therapy to OA patients. In addition to its unique hygroscopic and rheological properties, HA binds to its cellular receptor CD44, initiating cell signaling. It is well-established that HA-dependent signaling affects chondrocyte proliferation and differentiation [16] as well as glycosaminoglycan and collagen synthesis [17].
The present study was undertaken to evaluate the influence and potential molecular mechanism of HA/sorbitol on IL-1β- and hydrogen peroxide (H2O2)-dependent stimulation of catabolic and inflammatory responses, oxidative stress and apoptosis in cultured human chondrocytes. Clinical investigation has disclosed that HA/sorbitol significantly reduces pain for at least 6 months after the first injection [18, 19]. HA/sorbitol is a commercially available product (Synolis V-A) which contains a unique combination of key ingredients: sodium hyaluronate and sorbitol. It provides lubrication and shock absorption to joints. In addition, sorbitol prevents tissue damage caused by inflammation and helps to protect sodium hyaluronate from degradation [20]. However, the mechanism of HA/sorbitol signaling is poorly defined.
Materials and methods
Specimen selection
Discarded human post-surgery OA articular cartilage was obtained from OA patients (n = 24, age 67 ± 9 years mean ± SD) who had undergone total knee arthroplasty. Informed consent was received from them to study their tissues for research purposes. All patients were evaluated by rheumatologists according to American College of Rheumatology criteria [21]. These specimens represented moderate to severe OA graded by the Mankin system [22]. Cartilage was mostly taken from femoral regions. Our experimental protocol for research into human tissues was approved by the Research Ethics Board of Hôpital du Sacré-Cœur de Montréal.
OA knee cartilage specimens were sliced and rinsed before chondrocyte extraction by sequential enzymatic digestion [23]. Cartilage samples were digested with 1 mg/ml of pronase (Sigma-Aldrich, Oakville, ON) for 1 h at 37 °C, and then with 2 mg/ml of type IV collagenase (Sigma-Aldrich) for 6 h in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Burlington, ON) supplemented with 10 % heat-inactivated fetal bovine serum (FBS, Invitrogen), 100 units/ml of penicillin, and 100 µg/ml of streptomycin (Invitrogen). Chondrocytes were seeded at high density in culture flasks at 37 °C in a humidified atmosphere of 5 % CO2/95 % air until they were confluent and ready for experimentation.
HA/sorbitol and experimental culture conditions
Synolis™ V-A (Anteis SA, Geneva, Switzerland), the specific HA/sorbitol formulation tested, is based on HA of high molecular weight (>2 MDa), of non-animal origin with a high HA concentration (20 mg/ml) combined with a high concentration of sorbitol (40 mg/ml), a free radical scavenger.
First-passage chondrocytes were prepared, as described previously [23]. Briefly, the cells were seeded in 24-well plates at high density (2 × 105 cells/cm2) and cultured in DMEM (Invitrogen) supplemented with 10 % FBS (Invitrogen), 100 units/ml of penicillin and 100 µg/ml of streptomycin (Invitrogen) at 37 °C in a humidified atmosphere. To ensure that isolated chondrocytes conserve their original phenotype, type II collagen was measured routinely by real-time polymerase chain reaction. In our experiments, the culture medium was replaced by DMEM containing 1 % FBS 24 h before treatment.
OA chondrocytes were pretreated with increasing HA/sorbitol concentrations (0, 20, 50, 100, 500 µg/ml) for 2 h, followed by incubation with or without 1 ng/ml of IL-1β (R&D Systems, Minneapolis, MN), or 0.5 mM H2O2 (Sigma-Aldrich) for 1 or 24 h in 1 % FBS-DMEM. In each experimental condition, the final sorbitol concentration was 0, 40, 100, 200 or 1,000 µg/ml. In another set of experiments, OA chondrocytes were pre-treated or not with 1 µg/ml anti-CD44 antibody and 50 µg/ml HA/sorbitol for 2 h, followed by another incubation for 24 h with or without 1 ng/ml IL-1β (R&D Systems). The cells were also treated with 100 µg/ml sorbitol or 50 µg/ml HA alone (Sigma-Aldrich) for 2 h, followed by another incubation for 24 h in the presence or absence of 1 ng/ml IL-1β (R&D Systems) or 0.5 mM H2O2 (Sigma-Aldrich).
Prostaglandin E2 (PGE2), metalloproteinase-13 (MMP-13), and nitric oxide (NO) measurement
Media were collected after chondrocyte incubation for 24 h, and PGE2 and MMP-13 levels were assessed by enzyme immunoassay (Cayman Chemical Company, Ann Arbor, MI) or enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems), respectively. Detection sensitivity was 9 and 8 pg/ml, respectively. All assays were performed in duplicate. Nitrite, a stable end-product of NO, was quantified in supernatants according to a spectrophotometric method based on the Griess reaction [24]. Absorbance was measured with a micro-ELISA Vmax photometer (Bio-Tek Instruments, Winooski, VT).
Cell viability
Chondrocyte viability was evaluated, as described previously [25], by 3-(4,5-dimethyl-thiazoyl)-2,5-diphenyl-SH-tetrazolium bromide (MTT) assay in 96-well plates (Fisher Scientific Company, Ottawa, ON), by incubating the cells with 0.5 mg/ml MTT reagent (Sigma-Aldrich) for 15 min at 37 °C. Then, 100 µl of solubilization solution (0.04 M HCl-isopropanol) was added, formazan salt was dissolved, and absorbance was read at 570 nm with a micro-ELISA Vmax photometer (Bio-Tek Instruments).
Protein detection by Western blotting
20 µg of total proteins of chondrocyte lysates, treated under the indicated conditions, were loaded for discontinuous 4–12 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis. They were then transferred electrophoretically onto nitrocellulose membranes (Bio-Rad Laboratories, Mississauga, ON) for protein immunodetection and semi-quantitative measurement [23]. The primary antibodies deployed were rabbit anti-phospho and total p47 NADPH oxidase (pp47-NOX, Sigma-Aldrich), anti-inducible NO synthase (iNOS, Cayman Chemical Company), anti-phospho and total p38 mitogen-activated protein kinase (p38 MAPK, Cell Signaling Technology, Inc., Danvers, MA), anti-phospho and total extracellular signal-regulated kinases 1 and 2 (ERK1/2, Cell Signaling Technology, Inc.), anti-phospho nuclear factor-kappaB/p65 (NF-κB/p65, Cell Signaling Technology, Inc.), anti-glutathione-s-transferase A4-4 (Gsta4-4, Sigma-Aldrich), and anti-human β-actin (Sigma-Aldrich). After serial washes, the primary antibodies were revealed by goat anti-mouse or anti-rabbit immunoglobulin G conjugated to horseradish peroxidase (Cell Signaling Technology, Inc.). Immunoreactive proteins were detected with SuperSignal blotting substrate (Pierce, Rockford, IL) and exposed to Kodak X-Omat film (Eastman Kodak Company, Rochester, NY).
Cellular level of 4-hydroxynonenal (HNE)-protein adducts
Total cellular levels of HNE-protein adducts were calculated in chondrocyte extracts by in-house ELISA under the conditions indicated [23]. HNE-modified bovine serum albumin served as standard for HNE-protein adduct assay.
Reactive oxygen species (ROS) measurement
Intracellular ROS formation was quantified with MitoSOX™ Red reagent (Invitrogen). Its oxidation by superoxide produces red fluorescence, as described by Bentz et al. [26]. Briefly, chondrocytes were seeded at a density of 2 × 104 cells/well in 96-well black plates (Becton–Dickinson, San Jose, CA). They were pretreated with increasing concentrations of HA/sorbitol 2 h before their exposure to IL-1β. Fluorescence was measured by a fluorescence plate reader at 510-nm absorption and 580-nm emission. The data were expressed as relative MitoSOX™ Red fluorescence.
DNA fragmentation
Cytoplasmic histone-associated DNA fragments were quantified with Cell Death Detection ELISAPLUS kit (Roche Applied Science, Laval, QC) according to the manufacturer’s recommendations. Briefly, chondrocytes (2 × 106 cells) were pretreated for 2 h with increasing HA/sorbitol concentrations (0–500 μg/ml) and then treated with or without 1 ng/ml IL-1β for 24 h. After incubation, the cells were lysed with lysis buffer for 30 min and centrifuged at 200g for 10 min. The supernatant and a mixture of anti-histone-biotin and anti-DNA-peroxidase were added to streptavidin-coated microplates and incubated for 2 h at room temperature. Absorbance was measured at 405 nm after addition of the substrate.
Statistical analysis
All values are expressed as mean ± SD unless indicated otherwise. Multiple comparisons were made by one-way analysis of variance, as required, followed by Bonferroni’s multiple-comparison post-test. Statistical analysis was undertaken with GraphPad Prism software, version 4b (GraphPad Software, San Diego, CA). In all tests, the criterion for statistical significance was P < 0.05.
Results
HA/sorbitol abolishes ROS generation and pp47-NOX
To test the hypothesis that HA/sorbitol scavenges ROS, OA chondrocytes were pretreated with HA/sorbitol (0–500 µg/ml) for 2 h, followed by another treatment with 1 ng/ml IL-1β for 24 h. ROS generation was quantified by commercial kit. Our data indicated that relative MitoSOX™ Red fluorescence was stronger in IL-1β-treated cells and reached 9.2 ± 1.7 × 103/2 × 104 cells (P < 0.001) (Fig. 1a). However, when the cells were treated with both 1 ng/ml IL-1β and 100 µg HA/sorbitol, relative MitoSOX™ Red fluorescence weakened significantly to 1.3 ± 0.33 × 103/2 × 104 cells (P < 0.001, vs. IL-1β) (Fig. 1a). Finally, an additional experiment was performed to determine the possible ability of HA/sorbitol to inhibit pp47-NOX, a ROS-generating enzyme. Western blotting analysis showed that HA/sorbitol, at a concentration of 20 µg/ml, prevented IL-1β-induced pp47-NOX (Fig. 1b). Collectively, our data indicated that suppression of ROS production and ROS-generating NOX confirmed the antioxidant properties of HA/sorbitol.
HA/sorbitol suppresses ROS generation and p47-NOX phosphorylation. Confluent human osteoarthritic (OA) chondrocytes were treated for 2 h with escalating doses of HA/sorbitol (0–500 μg/ml), followed by incubation for 24 h in the presence or absence of 1 ng/ml IL-1β. a ROS generation was quantified in cell extracts with MitoSOX™ Red reagent. ROS levels were expressed as relative MitoSOX™ Red fluorescence. b p47-NOX protein phosphorylation (pp47-NOX) was analyzed by Western blotting in extracts of human OA chondrocytes treated as described above. The data are mean ± SD of 4 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001: compared to untreated cells; # P < 0.05, & P < 0.01, @ P < 0.001: compared to IL-1β-treated cells (color figure online)
HA/sorbitol prevents HNE production and Gsta4-4 downregulation
It is well-documented that the generation of ROS, such as superoxide anion and hydroxyl radical, plays an important role in initiating lipid peroxidation (LPO). Thus, the purpose of this part of the present study was to investigate whether HA/sorbitol’s capacity to inhibit HNE production was attributed to its ability to also prevent ROS generation. To do so, OA chondrocytes were pretreated with HA/sorbitol (0–500 µg) for 2 h, followed by exposure to 1 ng/ml IL-1β for 24 h.
As illustrated in Fig. 2a, escalating HA/sorbitol doses blocked IL-1β-induced HNE production. HA/sorbitol at 50 µg reduced HNE level by 50 % (P < 0.01), compared to cells treated with IL-1β alone. These data suggest that inhibition of the LPO process by HA/sorbitol could be related to low ROS levels.
HA/sorbitol reduces IL-1β-induced HNE generation and Gsta4-4 downregulation in human osteoarthritic chondrocytes. a HNE-protein adducts and b Gsta4-4 protein expression were measured by ELISA and Western blotting, respectively, in cellular extracts of chondrocytes treated with various concentrations of HA/sorbitol (0–500 µg/ml) for 2 h in the presence or absence of 1 ng/ml IL-1β for 24 h. The data are mean ± SD of 4 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001: compared to untreated cells; # P < 0.05, & P < 0.01, @ P < 0.001: compared to IL-1β-treated cells
Gsta4-4 exhibits high catalytic efficiency in HNE metabolism. Here, we tested our hypothesis that HA/sorbitol prevents IL-1β-induced Gsta4-4 downregulation in human OA chondrocytes. Our results revealed that Gsta4-4 protein expression was lower in IL-1β-treated than in untreated cells (Fig. 2b). However, 100 µg HA/sorbitol significantly abolished IL-1β inhibition of Gsta4-4 expression in OA chondrocytes. Altogether, our findings confirm that HA/sorbitol restores redox status by preventing Gsta4-4 downregulation in OA chondrocytes.
HA/sorbitol prevents NO and iNOS production in OA chondrocytes
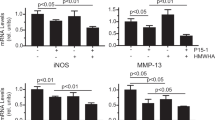
Next, we performed additional experiments to demonstrate that HA/sorbitol averts NO release and inhibits iNOS expression in isolated human OA chondrocytes. Cells were treated for 2 h with increasing doses of HA/sorbitol (0–500 µg) and thereafter with or without 1 ng/ml IL-1β for 24 h. As illustrated in Fig. 3, HA/sorbitol significantly reduced IL-1β-induced NO release (Fig. 3a) and iNOS protein levels (Fig. 3b) in a dose-dependent manner. At 100 µg, it suppressed NO release and iNOS expression by 95 % (P < 0.001). Collectively, these data confirm that HA/sorbitol is a potent iNOS inhibitor.
HA/sorbitol suppresses NO generation and iNOS expression. Confluent human osteoarthritic (OA) chondrocytes were treated for 2 h with 10 escalating doses of HA/sorbitol (0–500 μg/ml), followed by incubation for 24 h in the presence or absence of 1 ng/ml IL-1β. a NO levels were measured in culture medium by the Griess method. b iNOS protein expression was analyzed by Western blotting in extracts of human OA chondrocytes treated as described above. The data are mean ± SD of 4 independent experiments. **P < 0.01, ***P < 0.001: compared to untreated cells; # P < 0.05, & P < 0.01, @ P < 0.001: compared to IL-1β-treated cells
HA/sorbitol abrogates IL-1β-induced PGE2 and MMP-13 production
This part of our study was designed to verify HA/sorbitol’s ability to attenuate IL-1β-evoked production of inflammatory and catabolic mediators known to be involved in cartilage damage, such as PGE2 and MMP-13, respectively. When cells were treated with IL-1β, HA/sorbitol prevented PGE2 (Fig. 4a) and MMP-13 (Fig. 4b) release. At 100 µg, it reduced PGE2 and MMP-13 levels by 60 % (P < 0.001).
HA/sorbitol inhibits IL-1β-induced PGE2 and MMP-13 production in human osteoarthritic chondrocytes. Isolated cells were pretreated with increasing doses of HA/sorbitol (0–500 µg/ml) for 2 h, followed by incubation for 24 h in the presence of 1 ng/ml IL-1β. MMP-13 (a) and PGE2 (b) levels were measured in culture medium by commercial kits. The data are mean ± SD of 4 experiments. Student’s unpaired t test: *P < 0.05, **P < 0.01, ***P < 0.001: compared to untreated cells; # P < 0.05, & P < 0.01, @ P < 0.001: compared to IL-1β-treated cells
HA/sorbitol blocks H2O2-induced cell toxicity and death
In the next set of experiments, we evaluated the ability of HA/sorbitol to reduce H 2 O 2 cytotoxicity in cultured chondrocytes. Cell viability was assessed with MTT reagent. After 24 h of incubation, pretreatment with 20–500 µg HA/sorbitol for 2 h, before adding 0.5 mM H 2 O 2 to culture media, prevented H 2 O 2 -induced cell death (Fig. 5a) as well as markers of apoptosis, including caspase-3 activation (Fig. 5b) and DNA fragmentation (Fig. 5c). These data suggest that HA/sorbitol probably precludes H 2 O 2 ’s effects through direct hydroxyl radical- or HNE-quenching or p47-NOX inactivation.
HA/sorbitol suppresses H2O2-induced caspase-3 activation, cell death and DNA fragmentation. Confluent human osteoarthritic (OA) chondrocytes were treated for 2 h with 10 escalating doses of HA/sorbitol (0–500 μg/ml), followed by incubation for 24 h in the presence or absence of H2O2 (0.5 mM). a Caspase-3 activity was assessed in cellular extracts by commercial kit. b Cell viability was analyzed by MTT assay. c DNA was extracted after each treatment by commercial kit, and cytoplasmic histone-associated DNA fragments were quantified by kit. The data are mean ± SD of 3 independent experiments. *P < 0.05, **P < 0.01: compared to untreated cells; # P < 0.05, & P < 0.01: compared to H2O2-treated cells
HA/sorbitol inhibits p38 MAPK and NF-κB/p65 activation but not ERK1/2
To gain insights into the signaling pathways activated by IL-1β in isolated OA chondrocytes in the presence or absence of HA/sorbitol, we examined the phosphorylation patterns of p38 MAPK, ERK1/2 and NF-κB/p65. When cells were incubated with IL-1β and HA/sorbitol for 60 min, p38 MAPK and NF-κB/p65 phosphorylation levels were lower than in cells treated with IL-1β alone (Fig. 6). In contrast, HA/sorbitol had no effect on ERK1/2 protein activation evoked by IL-1β.
HA/sorbitol prevents p38 MAPK and p65-NF-κB but not ERK1/2 activation. OA chondrocytes were incubated with escalating doses of HA/sorbitol (0–500 µg/ml) for 2 h, followed by a second incubation for 24 h in the presence or absence of 1 ng/ml IL-1β. Cellular extracts were then subjected to Western blotting with specific polyclonal antibodies, anti-phospho and anti-total p38 MAPK, anti-phospho and total NF-κB/p65, and phospho and anti-total ERK1/2, as described in “Materials and methods.”
Distinct effects of sorbitol and HA
Finally, and to highlight the distinct effects of sorbitol and HA, OA chondrocytes were treated with 100 µg/ml sorbitol (~0.5 mM) or 50 µg/ml HA alone with or without 1 ng/ml IL-1β or 0.5 mM H2O2. As illustrated in Fig. 7a, b, sorbitol significantly reduced IL-1β-induced ROS generation and H2O2-induced cell death (P < 0.01). However, HA alone had a significant but moderate effect, compared to sorbitol (P < 0.05). To determine the effect of HA, cells were treated with 1 µg/ml anti-CD44 antibody and 50 µg/ml HA/sorbitol, followed by a second incubation with 1 ng/ml IL-1β for 24 h. As shown in Fig. 7c–e, the addition of anti-CD44 antibody blocked the biological effects of HA/sorbitol, as measured by NO, PGE2, and MMP-13 determination.
Distinct effects of sorbitol and HA. a, b Sorbitol suppresses ROS generation and prevents cell death. Confluent human osteoarthritic (OA) chondrocytes were treated for 2 h with 100 µg/ml sorbitol or 50 µg/ml HA, followed by a second incubation for 24 h in the presence or absence of 1 ng/ml IL-1β or 0.5 mM H2O2. ROS generation was quantified in cell extracts with MitoSOX™ Red reagent. Cell viability was measured by MTT assay. c, d HA/sorbitol blocks NO, PGE2, and MMP-13 production through the CD44 pathway. Cells were pretreated with 1 µg/ml anti-CD44 antibody and 50 µg/ml HA/sorbitol for 2 h, followed by another incubation for 24 h with 1 ng/ml IL-1β. NO level was assessed in culture media according to a spectrophotometric method based on the Griess reaction. PGE2 and MMP-13 levels were determined in culture media by EIA and ELISA kits, respectively. The data are mean ± SD of 4 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001: compared to untreated cells; # P < 0.05, & P < 0.01, @ P < 0.001: compared to H2O2- or IL-1β-treated cells
Discussion
HA is a widely distributed tissue ECM component characterized by a repeating disaccharide of N-acetylglucosamine and D-glucuronic acid and providing various structural and regulatory functions, including joint lubrication [27]. HA has been administered clinically to treat OA for more than 25 years. Although controversy still surrounds its clinical value [28, 29], numerous clinical trials and meta-analyses have reported durable benefits in knee OA [30–33]. In addition to OA, HA significantly alleviates pain and inflammation in some OA patients [34]. The rheological properties of SF are complex and depend on the molecular weight (MW) of HA and its concentration [35]. Both these HA parameters are significantly decreased in SF from OA and rheumatoid arthritis (RA) compared to normal knees. Low-MW fragments are biologically active in promoting inflammation [36, 37]. Emerging data suggest that high-MW HA has potential to suppress signaling activated by low-MW HA fragments in chondrocytes and various cells through different receptors, including the principal HA receptor CD44 [38].
In this study, we tested HA/sorbitol which contains a unique combination of key ingredients: sodium hyaluronate and sorbitol. Sodium hyaluronate is a natural component of joint SF that provides lubrication and shock absorption. Sorbitol prevents tissue damage caused by inflammation and helps to protect sodium hyaluronate from degradation [20]. It is considered to be a potent scavenger of hydroxyl radicals [39]. We have demonstrated that HA/sorbitol abrogates IL-1β-induced production of catabolic, inflammatory and oxidative stress mediators, such as MMP-13, NO, iNOS, PGE2, ROS and HNE as well as H2O2-induced markers of apoptosis, including caspase-3 activation and DNA fragmentation. Interestingly, our findings showed that the antioxidant as well as the anti-catabolic and anti-inflammatory properties of HA/sorbitol conjugate could be attributed to sorbitol and HA, respectively. All these processes (catabolism, inflammation, oxidative stress and apoptosis) are deemed to be hallmarks of cartilage degradation and synovial inflammation in OA. Upon examination of signaling pathways, we noted that HA/sorbitol prevented IL-1β-induced p38 MAPK and NF-κB/p65 activation, but not that of ERK1/2.
Our results are in concordance with the literature. In vitro and in vivo studies have demonstrated that HA reduces the expression of MMPs, iNOS and COX-2 [40, 41]. In particular, HA, via its CD44 receptor, suppresses MMP production through p38 MAPK downregulation in IL-1β-stimulated rheumatoid synovial fibroblasts [42] and chondrocytes [43]. Because p38 MAPK activation kindles the expression of various inflammatory genes that cause arthritis, its suppression could protect articular cartilage from destruction [44]. NF-κB is another important player as it initiates and sustains inflammatory reactions. It regulates many genes, including cytokines, chemokines, and adhesion molecules, that participate in the pathophysiology of synovial inflammation and bone and cartilage degradation [45]. HA can suppress NF-κB activation by fibronectin in RA chondrocytes [46]. With regard to ERK1/2 activation, our findings are in agreement with those of Julovi et al. [43], who reported that HA was ineffective in counteracting IL-1β-induced ERK1/2 phosphorylation in both OA and RA chondrocytes. In contrast to our study, Hashizume and Mihara showed that HA suppressed MMP induction by IL-6 in human chondrocytes via ERK1/2 inactivation through CD44 signaling [47]. CD44 inhibition, by blocking antibody, significantly reverses the inhibitory effect of HA on MMP-13 production. Taken together, HA is likely to suppress the intracellular pathways activated by catabolic stimulators in arthritic joints.
Our investigation into the effect of HA/sorbitol gel on redox status revealed a decrease in HNE and ROS generation as well as Gsta4-4 upregulation. The latter is a major HNE-metabolizing enzyme, and its inhibition induces chondrocyte apoptosis [48]. In particular, the antioxidant capacity of HA/sorbitol conjugate is mostly attributed to sorbitol rather than HA. The results of the present study are in contrast to those of others in that sorbitol can act as an oxidant agent [49, 50]. In those experiments, the authors used very high sorbitol concentrations (0.4–1 M). In regard to HA, our observations are in agreement with previous reports indicating the antioxidant properties of HA in different cell types. Ke et al. [51] demonstrated that HA reduces LPO products and ROS generation but, in contrast, induces the activity of superoxide dismutase, catalase and glutathione peroxidase. The antioxidant mechanisms of HA include also its capacity to inhibit the Fenton reaction via the entrapment of iron ions and to scavenge directly free radical [52]. On the other hand, the impact of extensive ROS and reactive nitrogen species accumulation on HA stability and properties has also been reported. Several authors have demonstrated that HA oxidation by these reactive species is linked with HA cleavage and fragmentation, resulting in reduced HA viscosity [53–55]. Monzon et al. [56] provided evidence that ROS evoke HA fragmentation in normal human bronchial epithelial cells via hyaluronidase 2 (Hyal2) upregulation. Hyal2, a HA-degrading enzyme, could be considered as a key candidate orchestrating inflammatory responses coupled with HA fragmentation, particularly in oxidative stress conditions.
In the present study, we also investigated the effect of HA/sorbitol on IL-1β-induced apoptosis of human OA chondrocytes. We found that HA reverses the process and inhibits apoptosis, as shown by caspase-3 assay and DNA fragmentation analysis. In a similar investigation conducted on isolated human chondrocytes, Grishko et al. [57] determined that HA has the capacity to protect mitochondria and its genome from the damaging effects of oxidative stress and preserve one of the most essential mitochondrial functions, energy production. Another study showed that HA inhibited IL-1β-induced chondrocyte apoptosis [58]. It has been established that adding HA to media dose dependently reduces the impairment of mitochondrial membrane potential and restores mitochondrial ATP production. In an experimental rabbit model of OA, HA administration is effective in ameliorating damage associated with the OA process, as evidenced by decreased apoptosis.
The chondrocyte death/apoptotic phenomenon in OA is complex and seems related to excessive synthesis of factors having pro-apoptotic activity in cartilage and synovium. Among them, NO and PGE2 seem predominant [59]. Peng et al. [60] demonstrated that HA blocks apoptosis and dedifferentiation of articular chondrocytes caused by NO production in a dose-dependent manner. The inhibitory effects of HA on apoptosis are derived from their ability to block NO-induced inhibition of protein kinase C alpha (PKCα). These results suggest that HA exerts a protective action on cartilage chondrocytes induced by NO, not only by reversing mitochondrial depolarization, but also by blocking PKCα inhibition. Altogether, suppression of NO and inflammatory cytokine activity within joints might be an important mechanism of the clinical action of intraarticular HA injection in OA treatment.
HA’s effect appears to be mediated through CD44 receptor binding. A large body of evidence indicates that CD44 receptors may play a critical role in the normal function and survival of many cell types. CD44 can promote resistance to apoptosis in the colonic epithelium via a mitochondria-controlled pathway [61]. In addition, it has been shown that CD44 expression in some cell types, such as stem cells, may provide the means to internalize HA by endocytosis, and one of the functions of internalized HA may be protection of DNA from oxidants [52]. In relation to cartilage biology, it has been determined that CD44 is important in both the normal and abnormal functions of cartilage through its adhesion to HA, which induces a variety of stimulatory signals that regulate chondrocyte proliferation as well as matrix synthesis in the cartilage microenvironment [62].
The present study shows that HA/sorbitol dose dependently suppresses catabolic and inflammatory responses as well as oxidative stress-induced chondrocyte apoptosis in isolated human OA chondrocytes. The suppression of these responses within joints might be a crucial mechanism of clinical HA/sorbitol action by intraarticular injection in OA treatment.
References
Blum MA, Ibrahim SA. Race/ethnicity and use of elective joint replacement in the management of end-stage knee/hip osteoarthritis: a review of the literature. Clin Geriatr Med. 2012;28:521–32.
Le Pen C, Reygrobellet C, Gerentes I. Financial cost of osteoarthritis in France. The “COART” France study. Joint Bone Spine. 2005;72(6):567–70.
Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteoarthr Cartil. 1998;6(6):374–6.
Pujol JP, Brisset M, Jourdan C, Bocquet J, Jouis V, Beliard R, Loyau G. Effect of a monocyte cell factor (MCF) on collagen production in cultured articular chondrocytes: role of prostaglandin E2. Biochem Biophys Res Commun. 1984;119:499–508.
Benton HP, Tyler JA. Inhibition of cartilage proteoglycan synthesis by interleukin I. Biochem Biophys Res Commun. 1988;154:421–8.
Gowen M, Wood DD, Ihrie EJ, Meats JE, Russell RG. Stimulation by human interleukin 1 of cartilage breakdown and production of collagenase and proteoglycanase by human chondrocytes but not by human osteoblasts in vitro. Biochim Biophys Acta. 1984;797:186–93.
Zhang W, Doherty M, Peat G, Bierma-Zeinstra MA, Arden NK, Bresnihan B, Herrero-Beaumont G, Kirschner S, Leeb BF, Lohmander LS, Mazieres B, Pavelka K, Punzi L, So AK, Tuncer T, Watt I, Bijlsma JW. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69:483–9.
Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr Cartil. 2010;18:476–99.
Waddell DD. Viscosupplementation with hyaluronans for osteoarthritis of the knee: clinical efficacy and economic implications. Drugs Aging. 2007;24:629–42.
Bagga H, Burkhardt D, Sambrook P, March L. Longterm effects of intraarticular hyaluronan on synovial fluid in osteoarthritis of the knee. J Rheumatol. 2006;33:946–50.
Gomis A, Miralles A, Schmidt RF, Belmonte C. Nociceptive nerve activity in an experimental model of knee joint osteoarthritis of the guinea pig: effect of intra-articular hyaluronan application. Pain. 2007;130:126–36.
Waddell DD, Kolomytkin OV, Dunn S, Marino AA. Hyaluronan suppresses IL-1beta-induced metalloproteinase activity from synovial tissue. Clin Orthop Relat Res. 2007;465:241–8.
Greenberg DD, Stoker A, Kane S, Cockrell M, Cook JL. Biochemical effects of two different hyaluronic acid products in a co-culture model of osteoarthritis. Osteoarthr Cartil. 2006;14:814–22.
Yatabe T, Mochizuki S, Takizawa M, Chijiiwa M, Okada A, Kimura T, Fujita Y, Matsumoto H, Toyama Y, Okada Y. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann Rheum Dis. 2009;68:1051–8.
Karna E, Miltyk W, Palka JA, Jarzabek K, Wolczynski S. Hyaluronic acid counteracts interleukin-1-induced inhibition of collagen biosynthesis in cultured human chondrocytes. Pharmacol Res. 2006;54:275–81.
Kawasaki K, Ochi M, Uchio Y, Adachi N, Matsusaki M. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J Cell Physiol. 1999;179:142–8.
Akmal M, Singh A, Anand A, Kesani A, Aslam N, Goodship A, Bentley G. The effects of hyaluronic acid on articular chondrocytes. J Bone Joint Surg Br. 2005;87:1143–9.
Heisel J, Kipshoven C. Hyaluronic acid with sorbitol-efficacy and tolerability of intra-articular treatment for osteoarthritis of the knee. Orthopädie und unfallchirurgie zeitschrift. 2012;1:1–7.
Heisel J, Kipshoven C. Safety and efficacy findings from a non-interventional study of a new hyaluronic acid/sorbitol formulation (GO-ON(R) matrix) for intra-articular injection to relieve pain and disability in osteoarthritis patients. Drug Res (Stuttg). 2013;63:445–9.
Bausani M. Assessing the efficacy of a viscosupplement combining hyaluronic acid and sorbitol (Synolis-VA) in patients with high grades knee osteoarthritis for whom corticotherapy is contraindicated. Osteoarthr Cartil. 2013;21:S270.
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49.
Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53(3):523–37.
Morquette B, Shi Q, Lavigne P, Ranger P, Fernandes JC, Benderdour M. Production of lipid peroxidation products in osteoarthritic tissues: new evidence linking 4-hydroxynonenal to cartilage degradation. Arthritis Rheum. 2006;54:271–81.
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15 N]nitrate in biological fluids. Anal Biochem. 1982;126:131–8.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Bentz M, Zaouter C, Shi Q, Fahmi H, Moldovan F, Fernandes JC, Benderdour M. Inhibition of inducible nitric oxide synthase prevents lipid peroxidation in osteoarthritic chondrocytes. J Cell Biochem. 2012;113:2256–67.
Conrozier T, Balblanc JC, Richette P, Mulleman D, Maillet B, Henrotin Y, Rannou F, Piroth C, Hilliquin P, Mathieu P, Walliser-Lohse A, Rousselot I, Plattner V, Maillefert JF, Vignon E, Chevalier X. Early effect of hyaluronic acid intra-articular injections on serum and urine biomarkers in patients with knee osteoarthritis: An open-label observational prospective study. J Orthop Res. 2012;30:679–85.
Rutjes AW, Juni P, da Costa BR, Trelle S, Nuesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157:180–91.
Lo GH, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA. 2003;290:3115–21.
Jazrawi LM, Rosen J. Intra-articular hyaluronic acid: potential treatment of younger patients with knee injury and/or post-traumatic arthritis. Phys Sportsmed. 2011;39:107–13.
Migliore A, Tormenta S, Massafra U, Bizzi E, Iannessi F, Alimonti A, Granata M. Intra-articular administration of hylan G-F 20 in patients with symptomatic hip osteoarthritis: tolerability and effectiveness in a large cohort study in clinical practice. Curr Med Res Opin. 2008;24:1309–16.
Mei-Dan O, Carmont M, Laver L, Mann G, Maffulli N, Nyska M. Intra-articular injections of hyaluronic acid in osteoarthritis of the subtalar joint: a pilot study. J Foot Ankle Surg. 2013;52:172–6.
Swiechowicz S, Ostalowska A, Kasperczyk A, Nowak D, Birkner E, Kasperczyk S. Evaluation of hyaluronic acid intra-articular injections in the treatment of primary and secondary osteoarthritis of the knee. Pol Orthop Traumatol. 2012;77:105–9.
Henrotin Y, Chevalier X, Deberg M, Balblanc JC, Richette P, Mulleman D, Maillet B, Rannou F, Piroth C, Mathieu P, Conrozier T. Early decrease of serum biomarkers of type II collagen degradation (Coll2-1) and joint inflammation (Coll2-1 NO(2)) by hyaluronic acid intra-articular injections in patients with knee osteoarthritis: A research study part of the Biovisco study. J Orthop Res. 2013;31:901–7.
Stafford CT, Niedermeier W, Holley HL, Pigman W. Studies on the concentration and intrinsic viscosity of hyaluronic acid in synovial fluids of patients with rheumatic diseases. Ann Rheum Dis. 1964;23:152–7.
Filion MC, Phillips NC. Pro-inflammatory activity of contaminating DNA in hyaluronic acid preparations. J Pharm Pharmacol. 2001;53:555–61.
Lyle DB, Breger JC, Baeva LF, Shallcross JC, Durfor CN, Wang NS, Langone JJ. Low molecular weight hyaluronic acid effects on murine macrophage nitric oxide production. J Biomed Mater Res A. 2010;94:893–904.
Campo GM, Avenoso A, Campo S, D’Ascola A, Traina P, Calatroni A. Differential effect of molecular size HA in mouse chondrocytes stimulated with PMA. Biochim Biophys Acta. 2009;1790:1353–67.
Joshi S, Husain MM, Chandra R, Hasan SK, Srivastava RC. Hydroxyl radical formation resulting from the interaction of nickel complexes of l-histidine, glutathione or l-cysteine and hydrogen peroxide. Hum Exp Toxicol. 2005;24:13–7.
Chou LW, Wang J, Chang PL, Hsieh YL. Hyaluronan modulates accumulation of hypoxia-inducible factor-1 alpha, inducible nitric oxide synthase, and matrix metalloproteinase-3 in the synovium of rat adjuvant-induced arthritis model. Arthritis Res Ther. 2011;13:R90.
Chang CC, Hsieh MS, Liao ST, Chen YH, Cheng CW, Huang PT, Lin YF, Chen CH. Hyaluronan regulates PPARgamma and inflammatory responses in IL-1beta-stimulated human chondrosarcoma cells, a model for osteoarthritis. Carbohydr Polym. 2012;90:1168–75.
Hiramitsu T, Yasuda T, Ito H, Shimizu M, Julovi SM, Kakinuma T, Akiyoshi M, Yoshida M, Nakamura T. Intercellular adhesion molecule-1 mediates the inhibitory effects of hyaluronan on interleukin-1beta-induced matrix metalloproteinase production in rheumatoid synovial fibroblasts via down-regulation of NF-kappaB and p38. Rheumatology (Oxford). 2006;45(7):824–32.
Julovi SM, Ito H, Nishitani K, Jackson CJ, Nakamura T. Hyaluronan inhibits matrix metalloproteinase-13 in human arthritic chondrocytes via CD44 and P38. J Orthop Res. 2011;29:258–64.
Chen WD, Jiang Q, Chen DY, Xu H, Zhang YF. Effects of intra-articular injection of p38 mitogen-activated protein kinase inhibitor on matrix metalloproteinase in articular cartilage of a rat model of osteoarthritis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2007;29:777–81.
Okamoto H, Cujec TP, Yamanaka H, Kamatani N. Molecular aspects of rheumatoid arthritis: role of transcription factors. FEBS J. 2008;275:4463–70.
Yasuda T, Nakamura T. Inhibition of nuclear factor-kappaB by hyaluronan in rheumatoid chondrocytes stimulated with COOH-terminal heparin-binding fibronectin fragment. Mod Rheumatol. 2007;17:391–7.
Hashizume M, Mihara M. High molecular weight hyaluronic acid inhibits IL-6-induced MMP production from human chondrocytes by up-regulating the ERK inhibitor, MKP-1. Biochem Biophys Res Commun. 2010;403:184–9.
Vaillancourt F, Fahmi H, Shi Q, Lavigne P, Ranger P, Fernandes JC, Benderdour M. 4-Hydroxynonenal induces apoptosis in human osteoarthritic chondrocytes: the protective role of glutathione-S-transferase. Arthritis Res Ther. 2008;10:R107.
Aquilano K, Filomeni G, Di RL, Vito M, Stefano C, Salimei PS, Ciriolo MR, Marfe G. Reactive oxygen and nitrogen species are involved in sorbitol-induced apoptosis of human erithroleukaemia cells K562. Free Radic Res. 2007;41:452–60.
Monetti E, Kadono T, Tran D, Azzarello E, rbelet-Bonnin D, Biligui B, Briand J, Kawano T, Mancuso S, Bouteau F. iphering early events involved in hyperosmotic stress-induced programmed cell death in tobacco BY-2 cells. J Exp Bot. 2014;65:1361–75.
Ke C, Sun L, Qiao D, Wang D, Zeng X. Antioxidant acitivity of low molecular weight hyaluronic acid. Food Chem Toxicol. 2011;49:2670–5.
Zhao H, Tanaka T, Mitlitski V, Heeter J, Balazs EA, Darzynkiewicz Z. Protective effect of hyaluronate on oxidative DNA damage in WI-38 and A549 cells. Int J Oncol. 2008;32:1159–67.
Al-Assaf S, Navaratnam S, Parsons BJ, Phillips GO. Chain scission of hyaluronan by peroxynitrite. Arch Biochem Biophys. 2003;411:73–82.
Li M, Rosenfeld L, Vilar RE, Cowman MK. Degradation of hyaluronan by peroxynitrite. Arch Biochem Biophys. 1997;341:245–50.
Stankovska M, Hrabarova E, Valachova K, Molnarova M, Gemeiner P, Soltes L. The degradative action of peroxynitrite on high-molecular-weight hyaluronan. Neuro Endocrinol Lett. 2006;27:31–4.
Monzon ME, Fregien N, Schmid N, Falcon NS, Campos M, Casalino-Matsuda SM, Forteza RM. Reactive oxygen species and hyaluronidase 2 regulate airway epithelial hyaluronan fragmentation. J Biol Chem. 2010;285:26126–34.
Grishko V, Xu M, Ho R, Mates A, Watson S, Kim JT, Wilson GL, Pearsall AW. Effects of hyaluronic acid on mitochondrial function and mitochondria-driven apoptosis following oxidative stress in human chondrocytes. J Biol Chem. 2009;284:9132–9.
Zhou PH, Liu SQ, Peng H. The effect of hyaluronic acid on IL-1beta-induced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop Res. 2008;26:1643–8.
Pelletier JP, Fernandes JC, Jovanovic DV, Reboul P, Martel-Pelletier J. Chondrocyte death in experimental osteoarthritis is mediated by MEK 1/2 and p38 pathways: role of cyclooxygenase-2 and inducible nitric oxide synthase. J Rheumatol. 2001;28:2509–19.
Peng H, Zhou JL, Liu SQ, Hu QJ, Ming JH, Qiu B. Hyaluronic acid inhibits nitric oxide-induced apoptosis and dedifferentiation of articular chondrocytes in vitro. Inflamm Res. 2010;59:519–30.
Lakshman M, Subramaniam V, Jothy S. CD44 negatively regulates apoptosis in murine colonic epithelium via the mitochondrial pathway. Exp Mol Pathol. 2004;76:196–204.
Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5:54–67.
Acknowledgments
This study was funded by Anteis S.A. (Geneva, Switzerland).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Mongkhon, JM., Thach, M., Shi, Q. et al. Sorbitol-modified hyaluronic acid reduces oxidative stress, apoptosis and mediators of inflammation and catabolism in human osteoarthritic chondrocytes. Inflamm. Res. 63, 691–701 (2014). https://doi.org/10.1007/s00011-014-0742-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-014-0742-4