Abstract
Objective
To investigate the inhibitory effect of hyaluronan (HA) on mechanical stress- induced expression of a disintegrin and metalloproteinase with thrombospondin type1 motifs (ADAMTS)-4, -5 and matrix metalloproteinase (MMP)-13 by human chondrocytes.
Materials and methods
Normal human articular chondrocytes were pre-incubated with or without 1.0 mg/mL HA (2700 kDa) for 12 h at 37 °C in stretch chambers, then they were exposed to uni-axial cyclic tensile strain (CTS, 0.5 Hz, 10 % elongation). The expression of ADAMTS-4, -5, and MMP-13 were analyzed by real-time polymerase chain reaction and Immunocytochemistry. The concentration of IL-1β in the supernatant was measured using enzyme-linked immunosorbent assay (ELISA). The nuclear translocation of runt-related transcription factor 2 (RUNX-2) and nuclear factor-κB (NF-κB) was examined by ELISA and immunocytochemistry, and phosphorylation of NF-κB was examined by western blotting.
Results
HA inhibited mRNA expression of ADAMTS-4, -5, and MMP13 after 24 h CTS via inhibition of IL-1β secretion and NF-κB activation. However, HA failed to inhibit CTS-induced RUNX-2 expression and subsequent expression of ADAMTS-5 and MMP-13 1 h after CTS.
Conclusions
Our results demonstrated that HA significantly suppressed mechanical stress-induced expression of catabolic proteases by inhibition of the NF-κB–IL-1β pathway, but did not suppress mechanical stress-induced RUNX-2 signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is one of the most common joint diseases, and the number of patients with OA is continuously increasing [1]. Hyaluronan (HA), a polysaccharide consisting of a long chain of disaccharides, is a natural component of cartilage and synovial fluid, and is known to act as a shock absorber in the articular environment [2, 3]. HA is commonly used to treat OA of joints such as the knee and shoulder by intra-articular injection [4–6]. It has been shown to relieve joint pain by masking free nerve ending organelles in animal experiments [7, 8]. Moreover, it is considered not only a joint lubricant but also a physiological factor related to intracellular signaling via specific receptors such as cluster determinant 44 (CD44) and intracellular adhesion molecule-1 (ICAM-1) [9, 10].
A disintegrin and metalloproteinase with thrombospondin type1 motifs (ADAMTS) and matrix metalloproteinases (MMPs) are known as catabolic proteases (aggrecanases) which play significant roles in aggrecan depletion that lead to cartilage destruction [11–13]. In particular, ADAMTS-4 and ADAMTS-5 are considered to be important key factors among the ADAMTS family in the pathogenesis of OA [12, 14, 15]. Among the MMPs, MMP-13 is also known to play an important role in cartilage destruction in OA [13, 16]. Intracellular signaling leading to cartilage destruction is mediated by catabolic pathways including mitogen-activated protein kinases (MAPKs) and nuclear factor-κB (NF-κB) [17].
Recent studies have demonstrated that HA suppresses inflammatory cytokine-induced catabolic proteases such as members of the ADAMTS and/or MMP families [18–20]. It is also reported that HA suppresses MAPK and/or NF-κB activation further upstream and suppress production of catabolic proteases by chondrocytes [19, 21].
We previously reported that runt-related transcription factor 2 (RUNX-2) plays an important role in the regulation of mechanical stress-induced expression of ADAMTSs and MMPs. In addition, it has been suggested that inflammatory cytokines such as IL-1β also play an important role in regulating the expression of ADAMTSs or MMPs [22]. In the current study, we used human chondrocytes to examine whether HA affects the mechanical stress-induced gene expression of ADAMTS-4, ADAMTS-5, and MMP-13 in vitro. Our findings may help to clarify the favorable effect and mechanism of HA on mechanical stress-induced cartilage destruction.
Materials and methods
Cells and cell culture
Normal human articular chondrocytes from knee cells (NHAC-kn) obtained from a 15-year-old male, a 34-year-old male, and a 38-year-old male were purchased from Lonza (Walkersville, MD). Cells were cultured at 37 °C in chondrocyte basal medium (CBM, Lonza) containing chondrocyte growth medium, fetal bovine serum (FBS), transforming growth factor-beta (TGF-β), R3 insulin-like growth factor (R3-IGF), transferrin, insulin, gentamicin, and amphotericin-B (CDM™ BulletKit®, Lonza). The medium was changed every 3 days, and NHAC-kn was used at passage 3.
Stretching experiments
For all experiments, chondrocytes were transferred to serum-free medium with or without HA for 12 h before exposure to cyclic tensile strain (CTS). HA (2700 kDa) was purchased from Chugai Pharmaceutical Co., Ltd. (Tokyo, Japan), and 800 kDa HA was purchased from Kaken Pharmaceutical Co., Ltd. (Tokyo, Japan). All experiments except for the experiments shown in the Fig. 1e were performed using 2700 kDa HA. The concentration of HA was adjusted to 1.0 mg/mL for all experiments. NHAC-kn were seeded onto stretch chambers, each having a type I collagen-coated culture surface of 2 × 2 cm for isolating RNA and collecting cell culture supernatant or 3 × 3 cm for immunocytochemistry, isolating nuclear extracts, and isolating proteins. CTS was applied using the ST-140-10 mechanical stretch system (STREX, Osaka, Japan). In this system, the chamber is attached to the stretching apparatus, which has a fixed side opposite a movable side that can be driven by a computer-controlled motor. Using this apparatus, the entire silicon membrane can be stretched uniformly [23, 24]. In the current study, a CTS (0.5 Hz, 10 % elongation) was applied for 30 min as described in our previous study [22, 25]. Cells incubated without mechanical stress were used as control.
Reverse transcription polymerase chain reaction (RT-PCR) and real-time PCR analysis
At 1, 6, 12, and 24 h after CTS, the cells were washed three times with phosphate buffered saline (PBS), and total RNA was extracted using ISOGEN reagent (Nippon Gene, Toyama, Japan). RNA samples (400 ng) were reverse-transcribed with ReverTra Ace (Toyobo, Osaka, Japan). The resulting cDNAs were used for PCR amplification in the presence of 10 pmol of specific primers using ExTaq DNA polymerase (TaKaRa, Ohtsu, Japan). Each RT-PCR reaction was allowed to proceed for 32–42 cycles. The specific primers used are described in Table 1 [ADAMTS-4, ADAMTS-5, MMP-13 and glyceraldehyde-3-phosphate dehydrogenase (G3PDH)].
Real-time PCR was performed using an Mx3000P QPCR System (Agilent Technologies, Santa Clara, CA, USA) with Brilliant III Ultra-Fast SYBR Green QPCR Master Mix. The PCR reaction was performed in a total volume of 20 μL of 1× SYBR Green PCR Master Mix, which included DNA polymerase, SYBR Green dye, dNTPs (including dUTP), PCR buffer, 10 pmol each of the forward and reverse primers, and cDNA of the samples. Amplification of a housekeeping gene, G3PDH, was used to normalize the efficiency of cDNA synthesis and the amount of RNA. We calculated the final expression levels by dividing the expression levels of ADAMTS-4, ADAMTS-5 and MMP-13 by the expression level of G3PDH. Each value obtained for the control cells (un-stretched cells without HA) was set to 1.
Immunocytochemistry
Immunocytochemistry was used to observe the mechanical stress-induced expression and localization of the protein expression of ADAMTS-4, ADAMTS-5, MMP13, RUNX-2 and NF-κB p65. Cells were stretched with or without HA according to the protocols described above, and fixed with 1 % paraformaldehyde solution at 24 h after CTS for ADAMTS-4, ADAMTS-5, and MMP13, and at 30 min after CTS for RUNX-2 and NF-κB. The membranes of the culture chambers were then removed and incubated with anti-ADAMTS-4 antibody (1:100, ab89112, Abcam, Cambridge, UK), anti-ADAMTS-5 antibody (1:100, ab45042, Abcam), anti-MMP-13 antibody (1:100, ab39012, Abcam), anti-RUNX-2 antibody (1:100, ab76956, Abcam), and anti-NF-κB p65 antibody (1:100, C22B4, Cell Signaling, Danvers, MA, USA), for 120 min at room temperature. Bovine serum albumin-containing solutions without primary antibodies were used as negative controls. We used Alexa Fluor 488-conjugated antibody (10 mg/mL, anti-mouse/rabbit) as secondary antibodies, Alexa Fluor 568-conjugated phalloidin (2 mg/mL, Molecular Probes, Eugene, OR, USA) for actin staining, and Hoechst 33342 (1 mg/mL, ICN Biomedicals, Aurora, OH, USA) for nuclear staining. Cells were observed under a fluorescence microscope (Leica, Wetzlar, Germany), and protein expression was evaluated by the positive cell ratio of ADAMTS-4, ADAMTS-5, MMP-13, RUNX-2, and NF-κB p65 (number of positive cells/all cells). The cell number was counted in four fields, at 100× magnification, and the mean was calculated.
Treatment with anti-CD44 antibodies and anti-ICAM-1 antibodies
Anti-CD44 neutralizing antibodies (αCD44) were purchased from BD Biosciences (San Diego, CA, USA), and anti-ICAM-1 neutralizing antibodies (αICAM-1) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Cells were incubated for 12 h before exposure to CTS with or without HA in the presence or absence of αCD44 (20 µg/mL) and/or αICAM-1 (20 µg/mL). Cell stretching protocols were the same as above. After application of CTS, real-time PCR analysis was performed, and the expression levels of ADAMTS-4, ADAMTS-5, and MMP-13 24 h after CTS were evaluated.
Treatment with IL-1 antagonist
We used the IL-1 receptor antagonist (IL-1ra) (ProSpec-Tany TechnoGene, Rehovot, Israel) to examine the influence of IL-1 on the CTS-induced expression of aggrecanases. IL-1ra at 100 ng/mL was added to the culture medium and cells were incubated for 12 h before exposure to CTS. Cell stretching protocols were the same as described above. After application of CTS, the cells were harvested for real-time PCR analysis, and the expression levels of ADAMTS-4, ADAMTS-5, and MMP-13 at 24 h after CTS were evaluated.
ELISA for IL-1β in the culture medium
Cell culture supernatants were collected at 1, 6, 12, and 24 h after CTS. The concentration of IL-1β in the supernatant was measured using a high sensitivity IL-1β enzyme-linked immunosorbent assay (ELISA) kit (Quantikine® HS ELISA Human IL-1β/IL-1F2 Immunoassay, R&D Systems, Inc., Minneapolis, MN, USA), according to the manufacturer’s protocol.
Western blot analysis
At 30 min after CTS, cells were resuspended using Mammalian Protein Extraction Buffer (GE Healthcare, Piscataway, NJ, USA). Cell lysates (10 μg of total protein/lane) were loaded onto SDS–polyacrylamide gels using the BioRad Any kDTM Mini-PROTEAN® TGXTM Gels (BioRad, Munchen, Germany) and run for 1 h at 150 V. Proteins were transferred to PVDF membranes using a Trans-Blot® Turbo™ Blotting System (BioRad). The membranes were blocked with Blocking reagent (TOYOBO) and incubated overnight at 4 °C with anti-NF-κB p65 antibody (1:2000, Cell Signaling) or anti-phospho-NF-κB p65 antibody (1:1000, Ser536, Cell Signaling). After washing with washing buffer, the membranes were incubated with IRDye Goat Anti-Rabbit IgG (LI-COR Biosciences, Lincoln, NE) or IRDye Goat Anti-Mouse IgG (LI-COR Biosciences) as secondary antibodies at room temperature for 1 h. Immunoreactive proteins were detected using the OdysseyFc Imaging System (LI-COR Biosciences). We also analyzed the densities of the obtained western blotting fragments using the OdysseyFc Imaging System. The volume of phosphorylated NF-κB fragments is indicated as a ratio, which was normalized to the density of NF-κB fragments.
Nuclear extract preparation and ELISA for NF-κB p65
At 30 min after CTS, the cells were washed with PBS three times. The nuclear extracts were collected using a nuclear extract kit (Active Motif, Carlsbad, CA, USA), according to the manufacturer’s protocol. The supernatant was frozen at −80 °C until use.
The nuclear extracts prepared as above were used to detect endogenous levels of NF-κB p65 by ELISA. ELISA of nuclear extracts for NF-κB p65 was performed using a TransAM® NF-κB p65 Kit (Active Motif), according to the manufacturer’s protocols. The absorbance value obtained for the control cells (unstretched cells without HA) was set to 1.
Statistical analysis
All data are expressed as means with 95 % confidence interval (CI). All experiments were repeated at least three times and similar results were obtained. Differences among groups were compared using one- or two-way analysis of variance (ANOVA) with the Bonferroni post hoc test. All differences were considered statistically significant at a P value <0.05.
Results
HA inhibits CTS-induced up-regulation of aggrecanases at 24 h after CTS (late phase), but not at 1 h after CTS (early phase)
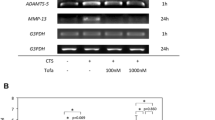
In samples without HA, CTS induced the expression of ADAMTS-4 at 24 h after CTS. In contrast, the expression of ADAMTS-4 in HA-treated samples was significantly down-regulated (Fig. 1a, b). Induction of ADAMTS-5 and MMP-13 gene expression was biphasic (at 1 and 12–24 h after CTS) in samples without HA. In HA-treated samples, the first peak (early phase) was not affected by treatment with HA, and only the second peak (late phase) was down-regulated (Fig. 1a, c, d). We found no difference between 2700 and 800 kDa HA with regard to its down-regulatory effect on ADAMTS-4, ADAMTS-5, and MMP-13 (Fig. 1e). The effects of HA (2700 kDa) on CTS-induced expressions of ADAMTS-4, ADAMTS-5, and MMP-13 were examined by immunocytochemistry. The protein expression level of ADAMTS-4, ADAMTS-5, and MMP-13 in the cytoplasm after 24 h CTS was inhibited by HA (2700 kDa) (Fig. 2). Treatment with αCD44 or αICAM-1 suppressed the effect of HA on the expression of ADAMTS-4, -5, and MMP-13 at 24 h after CTS. Furthermore, the combination treatment of αCD44 and αICAM-1 canceled the effect of HA almost completely (Fig. 3).
RT-PCR (a) and real-time PCR results (b–d) show the effects of HA on expression of ADAMTS-4, -5, and MMP-13. CTS induced the expression of ADAMTS-4 at 24 h after CTS, and the expression of ADAMTS-5 and MMP-13 at 1 h and again at 12–24 h after CTS (biphasic) in control samples. HA almost completely down-regulated ADAMTS-4 gene expression, but on ADAMTS-5 and MMP-13 gene expression, HA down-regulated only the late phase, and not the early phase. Experiments using 800 kDa HA in comparison to 2700 kDa HA revealed that both produced equivalent down-regulation of late phase ADAMTS-4, ADAMTS-5, and MMP-13 (24 h after CTS). We also found that 800 kDa HA did not affect early phase up-regulation of ADAMTS-5 and MMP-13 (e). *P < 0.01 relative to 0 h, § P < 0.01
Immunocytochemistry shows that HA inhibited the protein expression of ADAMTS-4, ADAMTS-5, and MMP-13 in the cytoplasm after 24 h CTS. In non-HA treated samples, these proteins were expressed in the cytoplasm following CTS (green signals), while they were suppressed in HA-treated samples. Merged images showing staining by phalloidin and Hoechst 33342 are shown in the right side panels. *P < 0.01 relative to control (CTS-, HA-), § P < 0.01
Involvement of IL-1β in the inhibitory effect of HA on CTS-induced proteases
The concentration of IL-1β in the supernatant increased in a time-dependent manner in samples without HA, and there were significant differences at 12–24 h in the CTS group compared with non-CTS (not shown). In contrast, the concentration of IL-1β in HA-treated samples did not increase (Fig. 4a). The up-regulation of ADAMTS-5 and MMP-13 in the early phase (1 h after CTS) was not affected by IL-1 antagonist, but the up-regulation of ADAMTS-4, -5 and MMP-13 in the late phase (24 h after CTS) was inhibited by IL-1 antagonist. These data suggest that HA suppression of ADAMTS-5 and MMP-13 at least partly involves IL-1β (Fig. 4b).
a ELISA for IL-1β in the culture medium shows that HA suppressed IL-1β production. However, the concentrations of IL-1β were very low, barely above the lower limit of detection. This seemed to be because the cell density of the cultures used for the ELISA was relatively low. *P < 0.01 relative to 0 h, § P < 0.001. b The results of real-time PCR in the experiments using IL-1 antagonist. IL-1 antagonist inhibited the late-phase up-regulation of ADAMTS-4, -5 and MMP-13, but did not affect the early-phase up-regulation of ADAMTS-5 and MMP-13. These data are similar to the results of the experiments using HA. *P < 0.01 relative to 0 h
HA inhibits CTS-induced nuclear translocation of NF-κB p65, but does not affect nuclear translocation of RUNX-2
The results of immunocytochemistry showed that CTS induced RUNX-2 translocation to the nucleus, and HA did not affect the RUNX-2 translocation. CTS also induced NF-κB p65 translocation to the nucleus, but this was inhibited in the cells treated with HA (Fig. 5). The results of western blot analysis showed that HA inhibited CTS-induced phosphorylation of NF-κB p65 (Fig. 6a, b). These results were further confirmed by ELISA for the NF-κB p65 subunit in nuclear extracts of the cells (Fig. 6c).
Immunocytochemistry shows that HA inhibited NF-κB localization to the nucleus, but did not affect RUNX-2 localization to the nucleus. In non-HA treated samples, both RUNX-2 and NF-κB localized to the nucleus following CTS (green signals), while in HA-treated samples, only the green signals of NF-κB were suppressed. Merged images showing staining by phalloidin and Hoechst 33342 are shown in the right side panels. *P < 0.01 relative to control (CTS-, HA-), § P < 0.01
Western blot analysis of NF-κB p65 and phospho-NF-κB p-65 (a, b) showed that CTS induced the protein phosphorylation of NF-κB, and that HA inhibited this phosphorylation. ELISA of nuclear extracts for p65 NF-κB (c) also showed that HA inhibited p65 NF-κB localization to the nucleus induced by CTS. *P < 0.01 relative to control (CTS-, HA-), § P < 0.01
Discussion
Protein catabolic enzymes such as ADAMTSs and MMPs play important roles in cartilage degradation. HA has been reported to suppress the catabolic behavior of chondrocytes by suppression of these proteolytic enzymes [18–20, 26].
A recent report suggests that HA suppresses NF-κB activation of MMP production [21] and that HA suppresses aggrecan degradation by down-regulating IL-1α-induced expression of ADAMTS-4, ADAMTS-5, and ADAMTS-9 [19]. However, most reports on the inhibitory effects of HA tested its effects using cytokines as OA models, and the mechanism underlying the chondroprotective role of HA against mechanical stress has not been fully elucidated.
It is known that the ADAMTS-5 promoter has a RUNX-2 binding site, suggesting that ADAMTS-5 is a potential downstream target of RUNX-2 [27]. It is also reported that the expression of RUNX-2 contributes to increased expression of MMP-13 [16]. Our previous report demonstrated that RUNX-2 is an upstream regulator of the mechanical stress-induced ADAMTS-5 and MMP-13 [22, 25].
NF-κB transcription also plays an important role in the production of ADAMTSs and MMPs [28–30]. The NF-κB pathway is known to be activated upon stimulation by various factors including mechanical stress or some cytokines [17, 31]. It is also known that NF-κB induces IL-1 production, and that IL-1 also induces NF-κB [32]. Both IL-1 and NF-κB are known to lead ADAMTS-5 up-regulation [28, 33].
We previously reported that CTS-induced up-regulation of ADAMTS-5 is biphasic in human chondrosarcoma cells [22]. In this study, CTS-induced up-regulation of both ADAMTS-5 and MMP-13 was found to be biphasic. This suggests that early phase up-regulation is dependent on transcription of RUNX-2, while late phase up-regulation is dependent on IL-1β production which is induced by NF-κB transcription and nuclear translocation under mechanical stress. We examined the expression of NF-kB in several time points, and found that the expression was most increased at 30 min. We guess a signaling from NF-kB to downstream may require several hours to produce IL-1 protein.
These results suggest that expression of ADAMTS-5 and MMP-13 are subject to dual control by both RUNX-2 and NF-κB in this experimental system. On the other hand, CTS-induced up-regulation of ADAMTS-4 in the early phase was not observed. ADAMTS-4 is suggested to be subject to control by NF-κB, but not RUNX-2.
The up-regulation of ADAMTS-4, -5 and MMP-13 in the late phase was also inhibited by IL-1 antagonist, suggesting that HA might act as an inhibitor of IL-1 production by inhibiting the nuclear translocation of NF-κB. In addition, NF-κB translocation to the nucleus was observed at 30 min after application of CTS, which is too early for induction by IL-1. Thus, HA might inhibit mechanical stress-induced nuclear translocation of NF-κB which leads to IL-1β production, resulting in the inhibition of late phase up-regulation of aggrecanases such as ADAMTS-4, ADAMTS-5, and MMP-13. HA almost completely inhibited mechanical stress-induced up-regulation of ADAMTS4, while partially inhibiting mechanical stress-induced up-regulation of ADAMTS5 and MMP-13. These results might be reasonably explained by the hypothesis that RUNX-2, which plays an important role in early phase up-regulation of aggrecanases, is not affected by HA.
There have been several studies on differences in the effects of HA due to differences in its molecular weight. Some studies conclude that the effect of HA on chondrocyte degeneration tends to depend on molecular size [19, 34]. In this study, we found no difference between 2700 and 800 kDa HA with regard to its down-regulatory effect on aggrecanases. In clinical practice, it is reported that difference in the molecular weight of HA used does not affect clinical outcomes [35], and the results of this study appear to support these facts.
The MAPK pathway, which involves extracellular signal-regulated kinase (ERK), p38 MAPK (p38), and c-Jun N-terminal kinase (JNK), is known to be modulated by various external stimuli [36, 37], and specific MAPK is thought to regulate RUNX-2 transcription under mechanical stress [22]. We previously reported that ERK1/2, p38, and JNK were phosphorylated by mechanical stress in human chondrocytes, and that activation of these MAPKs lead to transcription of RUNX-2 [25]. While HA is reported to suppress phosphorylation of ERK1/2 in a cytokine treatment OA chondrocyte model [19], the combination of MEK–ERK inhibitor and HA has a synergistic effect in vivo [38]. These results suggest that HA cannot completely inhibit the MAPK pathway in the physiological environment (i.e., under mechanical stress), and support the results of the current study. As HA did not inhibit mechanical stress-induced RUNX-2 activation, a combination of HA and agents that can inhibit RUNX-2 transcription may be necessary to completely inhibit the expression of aggrecanases.
There are several limitations to this study. First, the stretching system used in this study was a simple model because cells were cultured in monolayer. Second, the way that HA is taken up by chondrocytes is unknown, and it may be difficult to claim that our OA model completely reproduces the pharmacokinetics of HA in vivo. Third, NF-κB-induced cytokines other than IL-1β were not examined.
In conclusion, the results of the current study demonstrate that HA suppresses the expression of ADAMTS-4 almost completely, and partially suppress the expressions of ADAMTS-5 and MMP-13 via inhibition of NF-κB activation in mechanical stress-loaded human chondrocytes.
References
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96. doi:10.1016/S0140-6736(12)61729-2.
Rydell N, Balazs EA. Effect of intra-articular injection of hyaluronic acid on the clinical symptoms of osteoarthritis and on granulation tissue formation. Clin Orthop Relat Res. 1971;80:25–32.
Uebelhart D, Williams JM. Effects of hyaluronic acid on cartilage degradation. Curr Opin Rheumatol. 1999;11(5):427–35.
Goldberg VM, Buckwalter JA. Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthr Cartil. 2005;13(3):216–24. doi:10.1016/j.joca.2004.11.010.
Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5(2):54–67.
Gigante A, Callegari L. The role of intra-articular hyaluronan (Sinovial) in the treatment of osteoarthritis. Rheumatol Int. 2011;31(4):427–44. doi:10.1007/s00296-010-1660-6.
Gotoh S, Onaya J, Abe M, Miyazaki K, Hamai A, Horie K, et al. Effects of the molecular weight of hyaluronic acid and its action mechanisms on experimental joint pain in rats. Ann Rheum Dis. 1993;52(11):817–22.
Yoshida M, Sai S, Marumo K, Tanaka T, Itano N, Kimata K, et al. Expression analysis of three isoforms of hyaluronan synthase and hyaluronidase in the synovium of knees in osteoarthritis and rheumatoid arthritis by quantitative real-time reverse transcriptase polymerase chain reaction. Arthritis Res Ther. 2004;6(6):R514–20. doi:10.1186/ar1223.
Migliore A, Granata M. Intra-articular use of hyaluronic acid in the treatment of osteoarthritis. Clin Interv Aging. 2008;3(2):365–9.
Lisignoli G, Grassi F, Zini N, Toneguzzi S, Piacentini A, Guidolin D, et al. Anti-Fas-induced apoptosis in chondrocytes reduced by hyaluronan: evidence for CD44 and CD54 (intercellular adhesion molecule 1) invovement. Arthritis Rheum. 2001;44(8):1800–7. doi:10.1002/1529-0131(200108)44:8<1800:AID-ART317>3.0.CO;2-1.
Huang K, Wu LD. Aggrecanase and aggrecan degradation in osteoarthritis: a review. J Int Med Res. 2008;36(6):1149–60.
Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–8. doi:10.1038/nature03369.
Wang M, Sampson ER, Jin H, Li J, Ke QH, Im HJ, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15(1):R5. doi:10.1186/ar4133.
Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434(7033):648–52. doi:10.1038/nature03417.
Glasson SS, Askew R, Sheppard B, Carito BA, Blanchet T, Ma HL, et al. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 2004;50(8):2547–58. doi:10.1002/art.20558.
Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthr Cartil. 2004;12(12):963–73. doi:10.1016/j.joca.2004.08.008.
Rigoglou S, Papavassiliou AG. The NF-kappaB signalling pathway in osteoarthritis. Int J Biochem Cell Biol. 2013;45(11):2580–4. doi:10.1016/j.biocel.2013.08.018.
Julovi SM, Yasuda T, Shimizu M, Hiramitsu T, Nakamura T. Inhibition of interleukin-1beta-stimulated production of matrix metalloproteinases by hyaluronan via CD44 in human articular cartilage. Arthritis Rheum. 2004;50(2):516–25. doi:10.1002/art.20004.
Yatabe T, Mochizuki S, Takizawa M, Chijiiwa M, Okada A, Kimura T, et al. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann Rheum Dis. 2009;68(6):1051–8. doi:10.1136/ard.2007.086884.
Hashizume M, Mihara M. High molecular weight hyaluronic acid inhibits IL-6-induced MMP production from human chondrocytes by up-regulating the ERK inhibitor, MKP-1. Biochem Biophys Res Commun. 2010;403(2):184–9. doi:10.1016/j.bbrc.2010.10.135.
Yasuda T. Nuclear factor-kappaB activation by type II collagen peptide in articular chondrocytes: its inhibition by hyaluronan via the receptors. Mod Rheumatol. 2012;. doi:10.1007/s10165-012-0804-9.
Tetsunaga T, Nishida K, Furumatsu T, Naruse K, Hirohata S, Yoshida A, et al. Regulation of mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2 transcriptional factor in SW1353 chondrocyte-like cells. Osteoarthr Cartil. 2011;19(2):222–32. doi:10.1016/j.joca.2010.11.004.
Naruse K, Yamada T, Sokabe M. Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am J Physiol. 1998;274(5 Pt 2):H1532–8.
Hirano Y, Ishiguro N, Sokabe M, Takigawa M, Naruse K. Effects of tensile and compressive strains on response of a chondrocytic cell line embedded in type I collagen gel. J Biotechnol. 2008;133(2):245–52. doi:10.1016/j.jbiotec.2007.07.955.
Saito T, Nishida K, Furumatsu T, Yoshida A, Ozawa M, Ozaki T. Histone deacetylase inhibitors suppress mechanical stress-induced expression of RUNX-2 and ADAMTS-5 through the inhibition of the MAPK signaling pathway in cultured human chondrocytes. Osteoarthr Cartil. 2013;21(1):165–74. doi:10.1016/j.joca.2012.09.003.
Hashizume M, Mihara M. Desirable effect of combination therapy with high molecular weight hyaluronate and NSAIDs on MMP production. Osteoarthr Cartil. 2009;17(11):1513–8. doi:10.1016/j.joca.2009.04.018.
Thirunavukkarasu K, Pei Y, Wei T. Characterization of the human ADAMTS-5 (aggrecanase-2) gene promoter. Mol Biol Rep. 2007;34(4):225–31. doi:10.1007/s11033-006-9037-3.
Kobayashi H, Hirata M, Saito T, Itoh S, Chung UI, Kawaguchi H. Transcriptional induction of ADAMTS5 protein by nuclear factor-kappaB (NF-kappaB) family member RelA/p65 in chondrocytes during osteoarthritis development. J Biol Chem. 2013;288(40):28620–9. doi:10.1074/jbc.M113.452169.
Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43(4):801–11. doi:10.1002/1529-0131(200004)43:4<801:aid-anr10>3.0.co;2-4.
Tian Y, Yuan W, Fujita N, Wang J, Wang H, Shapiro IM, et al. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-kappaB. Am J Pathol. 2013;182(6):2310–21. doi:10.1016/j.ajpath.2013.02.037.
Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1(4):a000034. doi:10.1101/cshperspect.a000034.
Sylvester J, El Mabrouk M, Ahmad R, Chaudry A, Zafarullah M. Interleukin-1 induction of aggrecanase gene expression in human articular chondrocytes is mediated by mitogen-activated protein kinases. Cell Physiol Biochem. 2012;30(3):563–74. doi:10.1159/000341438.
Wang J, Markova D, Anderson DG, Zheng Z, Shapiro IM, Risbud MV. TNF-alpha and IL-1beta promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286(46):39738–49. doi:10.1074/jbc.M111.264549.
Campo GM, Avenoso A, Campo S, D’Ascola A, Traina P, Calatroni A. Differential effect of molecular size HA in mouse chondrocytes stimulated with PMA. Biochim Biophys Acta. 2009;1790(10):1353–67. doi:10.1016/j.bbagen.2009.07.003.
Lee PB, Kim YC, Lim YJ, Lee CJ, Sim WS, Ha CW, et al. Comparison between high and low molecular weight hyaluronates in knee osteoarthritis patients: open-label, randomized, multicentre clinical trial. J Int Med Res. 2006;34(1):77–87.
Fan X, Rahnert JA, Murphy TC, Nanes MS, Greenfield EM, Rubin J. Response to mechanical strain in an immortalized pre-osteoblast cell is dependent on ERK1/2. J Cell Physiol. 2006;207(2):454–60. doi:10.1002/jcp.20581.
Katz S, Boland R, Santillan G. Modulation of ERK 1/2 and p38 MAPK signaling pathways by ATP in osteoblasts: involvement of mechanical stress-activated calcium influx, PKC and Src activation. Int J Biochem Cell Biol. 2006;38(12):2082–91. doi:10.1016/j.biocel.2006.05.018.
Prasadam I, Mao X, Shi W, Crawford R, Xiao Y. Combination of MEK-ERK inhibitor and hyaluronic acid has a synergistic effect on anti-hypertrophic and pro-chondrogenic activities in osteoarthritis treatment. J Mol Med (Berl). 2013;91(3):369–80. doi:10.1007/s00109-012-0953-5.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yoshiya Tanaka.
Rights and permissions
About this article
Cite this article
Ozawa, M., Nishida, K., Yoshida, A. et al. Hyaluronan suppresses mechanical stress-induced expression of catabolic enzymes by human chondrocytes via inhibition of IL-1β production and subsequent NF-κB activation. Inflamm. Res. 64, 243–252 (2015). https://doi.org/10.1007/s00011-015-0804-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0804-2