Abstract
Carbon-ceramic electrode modified with multi-walled carbon nanotubes–ionic liquid (MWCNTs–IL) nanocomposite was constructed. This electrode was used for electrochemical determination of food dyes Sunset Yellow (SY) and tartrazine (Tz). The modified electrode based on high surface area and high ionic conductivity of nanocomposite exhibited electrocatalytic effect for oxidation of SY and Tz; also, oxidation peak potentials of SY and Tz effectively separated on modified electrode, and their simultaneous determination was possible. Operational parameters, such as the amount of MWCNTs in suspension, IL volume, solution pH, and scan rate, which affect the analytical performance of determination, were optimized. The present electrode behaved linearly to Sunset Yellow and tartrazine in the concentration range of 4 × 10−7 to 1.1 × 10−4 M and 3 × 10−6 to 0.7 × 10−4 M with a detection limit of 10−7 M (0.045 mg L−1) and 1.1 × 10−6 M (0.59 mg L−1), respectively. The proposed method was successfully utilized for simultaneous determination of SY and Tz in different food samples, and the obtained results were in good agreement to those obtained by HPLC method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic dyes are usually added to foodstuffs and soft drinks not only to improve appearance, color, and texture but also to maintain the natural color during process or storage. Compared to natural dyes, synthetic dyes show several advantages such as high stability to light, oxygen and pH, color uniformity, low microbiological contamination, relatively lower production costs, etc (Ghoreishi et al. 2012).
Sunset Yellow and tartrazine are synthetic organic food dyes that can be found in common food products such as beverages, soft drinks, candies, dairy products, and bakery products. The presence and content of these dyes must be controlled due to their potential harmfulness to human beings. For example, recent studies show that tartrazine has significant adverse effects on neurobehavioral parameters (Tanaka 2006); so sensitive and selective determination of Sunset Yellow (SY) and tartrazine (Tz) in food product is necessary. On the other hand, simultaneous determination of SY and Tz is very important if their binary mixture is present in real samples. Various methods such as high-performance liquid chromatography (HPLC) (Khanavi et al. 2011), spectrophotometry (Sayar and Ozdemir 1998), and spectrophotometry with chemometrics analysis (Dinc et al. 2002; Llamas et al. 2009) have been reported for the determination of SY and Tz. Also, voltammetric methods (Ghoreishi et al. 2012; Zhang et al. 2009; Lopez-de-Alba et al. 2002; Silva et al. 2007) have been used for their determination based on electrochemical activity of SY and Tz.
Carbon nanotubes (CNTs) were discovered by Iijima in 1991 (Iijima 1991). CNTs can be described as a graphite sheet rolled up into a nanoscale tube (which are single-wall carbon nanotubes (SWCNTs)), or with additional graphene tubes around the core of an SWCNT (which are multi-wall CNTs (MWCNTs)). These CNTs have diameters in the range between fractions of nanometers and tens of nanometers and lengths up to several centimeters with both their ends normally capped by fullerene-like structures (Trojanowicz 2006). Carbon nanotubes, because of their unique properties such as high electrical conductivity, high surface area, chemical stability, and significant mechanical strength have attracted much attention in electrochemistry. They can be used as electrode materials to promote electron transfer reactions in electrochemical devices (Babaei et al. 2010).
Ionic liquids (ILs) are either organic salts or mixtures of salts that are fluid at room or near-room temperature. Typically, ILs is composed of bulky 1,3-dialkylimidazolium, alkylammonium, alkylphosphonium, or alkylpyridinium organic cations and inorganic anions such as AlCl4 −, BF4−, PF6 −, NO3 −, ClO4 −, CF3COO−, CF3SO3 −, and CH3COO−. The most commonly used neutral ILs include 1-butyl-3-methylimidazolium hexafluorophospate or tetrafluoroborate abbreviated as [bmim][PF6] and [bmim][BF4] correspondingly (Kubisa 2004).
Recently, room temperature ionic liquids (RTILs) have been utilized in the fields of electrochemistry and electroanalysis due to their unique physicochemical properties including higher ionic conductivity, wider electrochemical windows, and good solubility. RTILs can be used as not only the supporting electrolyte for the electrochemical process but also the modifier for the chemically modified electrodes (Zhu et al. 2010).
At the first time, Fukushima (Fukushima et al. 2003) reported that carbon nanotubes (CNTs) could form gels with excellent electrocatalytic properties by RTILs. They claimed that single-wall carbon nanotubes–ionic liquid gels were easily constructed based on the high viscosity of the IL and the “cation-π” interactions of RTILs and CNTs. A nanocomposite of CNTs and ILs would be expected to display excellent and useful properties.
In sol-gel technology, the three-step process of construction of materials involves the low-temperature hydrolysis of a monomeric precursor of organometalic alkoxide, its condensation being followed by polycondensation to yield a polymeric oxo-bridged network. Low preparation temperatures and inorganic supports offer some advantages to this process over other methods, among which are chemical inertness, physical strength, and good surface renewability. Additional advantages come from the fact that sol-gel derived materials are porous, and, thus, mass transport is relatively easy through pores (Yong Ha et al. 1999). Carbon-ceramic composite electrodes (CCEs) are comprised of a dispersion of carbon powder that is held together by sol-gel derived ceramic binder (Rabinovich and Lev 2001). However, bare carbon-ceramic electrodes have some drawbacks such as low detection sensitivity and poor resolution to specific analytes; therefore, their modification is necessary.
In the present work, for the first time, SY and Tz were simultaneously determined on the carbon-ceramic electrode modified with carbon nanotube–ionic liquid (CNT–IL) nanocamposite. Based on electrocatalytic and surface-enhanced effect of CNT–IL nanocamposite, sensitivity of the modified electrode were improved for the determination of SY and Tz. Therefore, simultaneous determination of low levels of SY and Tz in food samples was done. This method was successfully used for the determination of SY and Tz in different food samples.
Experimental
Chemicals
Methyltrimethoxy silane (MTMOS) and graphite powder were purchased from Merck and used without any further purification.
Sunset Yellow, tartrazine, and other reagents were of analytical grade from Merck. 1-Butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) as room temperature ionic liquid was obtained from Aldrich. Multi-walled carbon nanotubes with wall number of 3–15 and length of 1–10 μm was purchased from Aldrich. The fruit juice powder was obtained from Bolexo Company of Germany. Beverage of Zam zam Company and fruit juice of mikhak from Urmai Zarnoosh Company were purchased from a local market. The pH of solutions was adjusted to 7.0 with phosphate buffer. The distilled, deionized, and sterilized water was used in all solution preparation.
Apparatus
Electrochemical experiments were performed using AUTOLAB PGSTAT 30 electrochemical analysis system and GPES 4.9 software package (Eco Chemie, The Netherlands). The utilized three-electrode system was composed of a modified carbon-ceramic electrode with 3-mm diameter as the working electrode, a saturated calomel electrode as the reference electrode, and a platinum wire as the auxiliary electrode. All experiments were performed at room temperature, without removing the dissolved oxygen. An electrochemical glass vessel was employed throughout the experiments. The HPLC determinations of SY and Tz were carried out using WATER Product liquid chromatograph (USA), equipped with a WATERS 515 pump, and a WATERS 2489 UV–Vis detector set at 484 and 454 nm, respectively. A Microsorb C18 250-mm × 4.6-mm column with particles of 5-μm chromatographic column was used. The mobile phase consisted of a mixture of an aqueous (0.08 mol L−1) ammonium acetate solution (55 %) and methanol (45 %) at a flow rate of 1.0 mL min−1, while the injection volume was 20 mL (Pereira Alves et al. 2008).
Preparation of Modified Carbon-Ceramic Electrode
The two-step modification of the carbon-ceramic electrode involves the following: first, the silica sol solution was prepared by mixing 0.6 ml of MTMOS, 0.9 mL methanol, and 0.1 mL hydrochloric acid (0.1 M) and then stirred for 5 min until a homogeneous gel solution resulted. This ormosil was mixed well with 300-mg graphite powder. The mixture was added to Teflon tube (with 3-mm id and 3-cm length, and the length of composite in the tube was about 8 mm) and dried for 48 h at room temperature (Majidi et al. 2010). The surface of all electrodes was removed by mechanical polishing with 800, 2,000, 2,500, and 3,000 grit polishing papers, respectively. The electrodes were rinsed thoroughly with water to yield shiny surfaces. Copper wire contacted to the other end, providing the electrical contact. The scanning electron microscopy (SEM) experiment was made on a Hitachi Japan S4160 SEM.
MWCNTs–IL composite was prepared by mixing 10 mg purified MWCNTs, 0.3 mL IL and 4.7 mL ethanol. Then they were stirred for 30 min to obtain black homogeneous suspension. After that, 10 μL of freshly prepared suspension was dropped on the polished surface of carbon-ceramic electrode and allowed to dry at room temperature for 12 h.
Analysis of Soft Drink
An accurate soft drink sample volume of real samples was transferred to a 50-mL flask and adjusted to volume with doubly distilled water. A 0.15-mL portion of the solution was subjected for differential pulse voltammetry (DPV) measurements as described in the “Application” section.
Results and Discussion
Surface Morphology of Modified Electrode
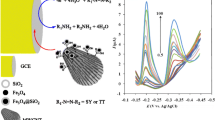
Scanning electron microscopy was used to characterize the top views of the modified electrode and bare electrode as shown in Fig. 1. On bare carbon-ceramic electrode (Fig. 1a, b), a dense, scaly, and porous surface appeared with separated carbon layer, which was due to the heterogeneous dispersion of the carbon powder. While on the MWCNTs–IL/carbon-ceramic electrode (Fig. 1c, d), MWCNTs are untangled after being sonicated in the BMIMBF4 with average diameter of 16 nm. Based on these observations, there were plenty of tubes dispersed on the surface of carbon-ceramic electrode, indicating that MWCNTs were successfully immobilized on the surface of carbon-ceramic electrode but with good individualization of nanotubes, and carbon nanotubes distributed homogeneously on the surface of electrode. These observations revealed that the MWCNTs–IL nanocomposite was immobilized on the surface of carbon-ceramic electrode, and we could conclude that RTILs plays an important role in the modification process.
Cyclic Voltammetry
The cyclic voltammograms of bare and modified carbon-ceramic electrode were recorded between 0.3 and 1.1 V using the scan rate of 50 mV s−1 in phosphate buffer at pH 5 containing 2-mM SY and Tz. As seen in Fig. 2a, on the bare carbon-ceramic electrode, one anodic peak appeared with peak potential of 0.817 V by low peak current height for SY; whereas a well-defined, sharp redox pair with obviously increased current response was obtained for SY on the modified electrode with peak potential separation of 0.25 V. Also, one anodic peak appeared with peak potential of 1.01 V by low peak current height for Tz on the bare carbon-ceramic electrode (Fig. 2b), whereas a sharp peak with obviously increased current response was obtained for Tz on the modified electrode. These results indicated that the modified electrode exhibited excellent electrocatalytic activity toward electrooxidation of SY and Tz and accelerated electron transfer rate between them and the electrode. Therefore, in the latter experiments, operational conditions such as MWCNTs value, IL volume, pH, and scan rate which affect performance of the modified electrode were optimized.
Optimization of Experimental Conditions
Effect of Amount of MWCNTs in Nanocomposite Suspension
Figure 3 illustrates the effect of MWCNT values in nanocomposite suspension on the oxidation peak current of SY and Tz, as gradually increasing the amount of MWCNTs in suspension from 1 to 10 mg/5 mL, the oxidation peak currents was obviously improved. This behavior attributed to the increase of surface area of MWCNTs on the electrode substrate which caused to improve diffusion of SY and Tz to the electrode surface and enhancement of electron transfer rate between them and the electrode. The electrocatalytic behavior of MWCNTs on SY and Tz electrooxidation contributed to the increase of surface area of the electrode due to their high surface-area-to-volume ratio and high value of edge plane-like defect sites on the carbon nanotubes. When increasing the MWCNTs value from 10 to 12 mg/5 mL, the oxidation peak currents were leveled, which indicated that maximum surface area was achieved. Considering these findings, a value of 10 mg/5 ml of MWCNTs in the suspension was chosen to modify the carbon-ceramic electrode surface.
Effect of IL Volume
Effect of IL volume on the performance of the modified electrode was investigated in the constant value of 10 mg/5 mL MWCNTs. Experimental results showed that the 0.3 mL/5 mL IL represented the highest catalytic peak currents for both analytes, but increasing IL volume was not promoted in the peak current (figure not shown here). These results proved that ionic liquid itself improve electrochemical responses toward SY and Tz oxidation. Due to higher ionic conductivity and wide electrochemical stability, the presence of ionic liquid on the electrode surface could act as suitable charge transfer bridge to facilitate the electron transfer efficiency by reducing charge transfer resistance (Maleki et al. 2007). In fact, the IL has large quantities of caves within its molecular structure which was facile to hold more charges (Liu et al. 2005). This property with inherent catalytic activity of ionic liquids has led to the improvement of the electrochemical responses obtained in MWCNT–IL/carbon-ceramic electrode. Because of these findings, an IL volume of 0.3 mL was chosen for the next experiments.
Effect of pH
The electrochemical behavior of SY and Tz was investigated in phosphate buffer (0.1 M with different pH values) using cyclic voltammetry (Fig. 4). By increasing pH, the catalytic peaks of analytes shifted toward a less positive potential. This behavior suggests that the redox system obeys the involvement of proton transfer mechanism in the overall electrode reaction. The slope of peak potential curves versus pH for SY and Tz were 26 and 28 mV/pH, which is very close to Nernstian value of 29 mV/pH for two proton, one electron participating process of electrochemical reactions.
The anodic peak currents of SY and Tz in pH 2 were the highest value, but the analyte peak potential separation was not high enough to simultaneously determine possible analytes. While in pH 7, the anodic peak currents of SY and Tz were slightly smaller than pH 2, but the peak potential separation was high enough to simultaneously determine that SY and Tz were possible. Therefore, one compromise was done, so pH 7 was chosen for latter experiments.
Effects of Scan Rate
The influences of the scan rate was studied on the voltammetric responses of the MWCNTs–IL/carbon-ceramic electrode in the range of 10–600 mV s−1 for SY and 10–500 mV s−1 for Tz, respectively (Fig. 5a, b). As seen, the anodic currents of SY and Tz increased with increasing the scan rate, and the peak potentials shifted to more positive potentials. The plots of the anodic peak currents of SY and Tz versus the square root of scan rate show a linear relationship (inset of Fig. 5a, b). This behavior suggests that the oxidation process of SY and Tz is diffusion-limited.
Interference Study
The influence of various cations and anions, such as Mg2+, Ca2+, Fe3+, Zn2+, K+, and NO2 −, on the determination of 5.0 × 10−6 M SY and 7.0 × 10−6 M Tz was studied by DPV under optimized conditions. The results showed that the mentioned species have no significant influence on the height of the peak currents of SY and Tz. To assess the selectivity of modified electrode toward Sy and Tz, effect of some food colorants such as Allura Red, Brilliant Blue, and Quinoline Yellow were investigated by voltammetric determinations. Based on experimental results, these colorants were not interfering in SY and Tz voltammetric response.
Effect of Sunset Yellow and Tartrazine Concentration
As the charging current contribution on the background current was a limiting factor in the analytical determination, experiments were carried out using the DPV mode (Zhang et al. 2008). A typical differential pulse voltammograms (DPVs) obtained in the presence of different concentrations of SY and Tz in the range of 4 × 10−7 to 1.1 × 10−4 and 3 × 10−6 to 0.7 × 10−4 M on the modified electrode are presented in Figs. 6 and 7, respectively. As can be seen in the figures, currents of anodic peaks increase by increasing the concentration of SY and Tz. The calibration graph, shown as the inset in Figs. 6 and 7, was linear with the SY and Tz concentration with correlation coefficient of 0.999 and 0.997, respectively. The detection limits were 10−7 M (0.045 mg L−1) and 1.1 × 10−6 M (0.59 mg L−1) for SY and Tz, calculated by means of the following equation: yLOD = yB + 3Sy/x and regression equations of y = 0.323 + 0.386C (in micromolar) and y = 0.344 + 0.216C (in micromolar), respectively, where yB is the signal of the blank (here intercept of calibration graph) and Sy/x is the standard deviation of the blank (here standard deviation of the calibration graph) (Miller and Miller 2010). A solution of 3 × 10−5 M SY and Tz was chosen to examine the repeatability of the differential pulse voltammetry measurement of the modified electrode. It was found that the mean value of current for five determinations of SY and Tz was 0.5 and 0.4 μA with relative standard deviation of 1.3 and 1.5 %, respectively.
Differential pulse voltammogram for increasing amount of SY from (a) 0.4, (b) 0.6, (c) 0.8, (d) 1, (e) 3, (f) 5, (g) 7, (h) 10, (i) 15, (j) 20, (k) 25, (l) 30, (m) 40, (n) 50, (o) 70, (p) 90, and (q) 110 μM in buffer solution (pH = 7.0) on MWCNTs–IL nanocomposite modified carbon-ceramic electrode. Inset: corresponding calibration graph
Table 1 compares the response characteristics for the determination of SY and Tz at various modified electrodes. In comparison with other modified electrodes reported previously (Ghoreishi et al. 2012; Zhang et al. 2009; Lopez-de-Alba et al. 2002; Medeiros et al. 2012a, b), the proposed modified electrode possesses its unique properties.
Firstly, it has all the advantages of CCE and benefitted from the advantages of the carbon nanotubes and ionic liquid. The proposed modified electrode can be easily constructed in a simple procedure, and the surface of electrode is also easily renewable. Also, the modified electrode shows high conductivity, promotion of fast electron transfer, and antifouling properties. Secondly, SY and Tz detection limits on MWCNTs–IL/CCE are comparable with majority of values reported by other research groups, and, in the case of vast linear range, the MWCNTs–IL/CCE are better than others. Finally, in this method, we used a low-cost and nontoxic modified electrode instead of expensive and, in some cases, toxic electrode, such as mercury electrode for the determination of SY and Tz, and some of the previously reported electrodes are time-consuming and use complicated methods for SY and Tz assay.
Differential Pulse Voltammograms of Sunset Yellow and Tartrazine in the Presence of Excess Amount of Each Other
Typical DPVs of different concentrations of SY in the presence of 100 μM Tz on the modified electrode was presented in Fig. 8a. Also, DPVs of Tz in the presence of 100 μM SY was shown in Fig. 8b. As can be seen, the presence of excess amount of the second analyte exhibited no tangible effect on the calibration curve regression of the desired analyte. Therefore, simultaneous determination of SY and Tz was possible on the modified electrode. To examine this idea, DPVs of SY and Tz in their binary mixture were done by increasing their concentrations. The typical voltammograms were shown in Fig. 9. Similar regression equations for the calibration curves of SY and Tz compared with their individual calibration curve regression equations were resulted (not presented in figure). Based on these observations, we could conclude that SY and Tz have no obvious interference in the determination of the others.
Typical differential pulse voltammogram for increasing amount of a SY in the presence of 100 μM Tz from (a) 0.4, (b) 0.6, (c) 0.8, (d) 1, (e) 3, (f) 5, (g) 7, (h) 10, (i) 15, (j) 20, (k) 25, (l) 30, (m) 40, (n) 50, (o) 70, (p) 90, and (q) 110 μM and b Tz in the presence of 100 μM Sy from (a) 3, (b) 5, (c) 7, (d) 10, (e) 15, (f) 20, (g) 25, (h) 30, (i) 40, (j) 50, and (k) 70 μM at the MWCNTs–IL nanocomposite modified carbon-ceramic electrode. Insets: corresponding calibration graphs
Typical differential pulse voltammograms for simultaneous increasing amount of SY from (a) 0.4, (b) 1, (c) 5, (d) 10, (e) 20, (f) 30, (g) 50, (h) 70, (i) 90, and (j) 110 μM and Tz from (a) 3, (b) 5, (c) 10, (d) 15, (e) 20, (f) 25, (g) 30, (h) 40, (i) 50, and (j) 70 μM on MWCNTs-IL nanocomposite modified carbon-ceramic electrode
Application
The modified carbon-ceramic electrode was used for the determination of SY and Tz in soft drinks under optimized experimental conditions by the standard addition method. The recovery experiments were performed to evaluate matrix effects, after the standard-solution additions yielded a good average recovery for food colorants (94.4–106 % for SY and 93.2–102 % for Tz), indicating that there were no important matrix interferences for the samples analyzed by the proposed method. Table 2 presents the SY and Tz concentrations simultaneously determined in the analyzed food products, employing the proposed method and an HPLC method (Medeiros et al. 2012b). As can be seen in Table 2, the results obtained by the proposed method agree quite well with those obtained by the reference HPLC method. Also, the paired t test was applied for the results obtained by both methods, and the resulting t values (0.063 for SY and 0.818 for Tz at a confidence level of 95 %) are much smaller than the critical one (4.30, α = 0.05), revealing that there is no difference between the obtained results.
In the present work, unlike the other reports which employed mechanical rubbing, the MWCNTs–IL nanocompsite gel on the surface of electrode for modifying the MWCNTs, IL, and ethanol suspension was dropped on the carbon-ceramic electrode surface, then ethanol evaporated, and MWCNTs–IL nanocomposite remained. This procedure is simple and produces stable and homogeneous surface of modified electrode which ensures repeatable results in analytical procedure.
Conclusions
A novel carbon-ceramic modified electrode has been developed for the simultaneous determination of SY and Tz by using MWCNTs–IL nanocomposite. MWCNTs have high surface area, and their edge plane-like defects are the main factor in increasing the modified electrode response to SY and Tz and high conductivity of IL responsible for high electron transfer rate between the modified electrode and analytes. High enough anodic peak potential separation of SY and Tz at MWCNTs–IL nanocomposite modified carbon-ceramic electrode provided their simultaneous determination with good selectivity and venial interaction effects of analytes. SY and Tz were determined by the standard addition method in some soft drink samples, and the obtained values for both analytes were in good agreement with those obtained by HPLC method and the proposed method applicable to the analysis of SY and Tz in different matrixes. Based on this method, a new sensor was successfully developed for the simultaneous determination of SY and Tz in different food samples.
References
Babaei A, Afrasiabi M, Babazadeh M (2010) Electroanalysis 22:1743
Dinc E, Baydan E, Kanbur M, Onur F (2002) Talanta 58:579
Fukushima T, Kosaka A, Lshimura Y, Yamamoto T, Takigawa T, Ishii N, Aida T (2003) Science 300:2072
Ghoreishi SM, Behpour M, Golestaneh M (2012) Food Chem 132:637
Iijima S (1991) Nature 354:56
Khanavi M, Hajimahmoodi M, Ranjbar AM, Oveisi MR, Shams Ardekani MR, Mogaddam G (2011) Food Anal Method 5:408
Kubisa P (2004) Prog Polym Sci 29:3
Liu H, He P, Li Z, Sun C, Shi L, Liu Y, Zhu G, Li J (2005) Electrochem Commun 7:1357
Llamas NE, Garrido M, Nezio MSD, Band BSF (2009) Anal Chim Acta 655:38
Lopez-de-Alba PL, Martinez LL, De-Leon-Rodriguez LM (2002) Electroanalysis 14:197
Majidi MR, Asadpour-Zeynali K, Gholizadeh S (2010) Electroanalysis 22:1772
Maleki N, Safavi A, Tajabadi F (2007) Electroanalysis 19:2247
Medeiros RA, Lourencao BC, Rocha-Filho RC, Fatibello-Filho O (2012a) Talanta 99:883
Medeiros RA, Lourencao BC, Rocha-Filho RC, Fatibello-Filho O (2012b) Talanta 97:291
Miller JC, Miller JN (2010) Statistics and chemometrics for analytical chemistry, Edinburgh Gate Harlow: Pearson Education Limited. Sixth edition, pp 118-121
Pereira Alves S, Mares Brum D, Castello Branco de Andrade E, Duarte Pereira Netto A (2008) Food Chem 107:489
Rabinovich L, Lev O (2001) Electroanalysis 13:265
Sayar S, Ozdemir Y (1998) Food Chem 61:367
Silva MLS, Garcia MBQ, Lima JLFC, Barrado E (2007) Talanta 72:282
Tanaka T (2006) Food Chem Toxicol 44:179
Trojanowicz M (2006) Trends Anal Chem 25:480
Yong Ha S, Kim S, Electroanal J (1999) Chem 468:131
Zhang X, Jiao K, Wang X (2008) Electroanalysis 20:1361
Zhang W, Liu T, Zheng X, Huang W, Wan C (2009) Colloid Surface B Bioint 74:28
Zhu Z, Qu L, Li X, Zeng Y, Sun W, Huang X (2010) Electrochim Acta 55:5959
Acknowledgments
We gratefully acknowledge the financial support from the Post Graduate Office of the University of Tabriz. The authors wish to thank Prof. Mir Ali Farajzadeh from the University of Tabriz and Mrs. Rasoolzadeh from the Drug Applied Research Center of Tabriz University of Medical Sciences for their valuable technical support in the determination of dyes by HPLC method.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Majidi, M.R., Fadakar Bajeh Baj, R. & Naseri, A. Carbon Nanotube–Ionic Liquid (CNT–IL) Nanocamposite Modified Sol-Gel Derived Carbon-Ceramic Electrode for Simultaneous Determination of Sunset Yellow and Tartrazine in Food Samples. Food Anal. Methods 6, 1388–1397 (2013). https://doi.org/10.1007/s12161-012-9556-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-012-9556-6