Abstract
A green chromatographic method for the successful separation and determination of eight synthetic food colorants (Tartrazine E 102, Quinoline Yellow E 104, Sunset Yellow E 110, Carmoisine E 122, Ponceau 4R E 124, Allura Red E 129, Indigo Carmine E 132 and Brilliant Blue E 133) was developed. A C8 stationary phase was used and the mobile phase was a mixture of 50 mM phosphate buffer at pH 7 containing triton X-100 (0.25% v/v). The method was validated as regards its selectivity, linearity, precision, accuracy, limit of detection (LOD) and quantification (LOQ). LOD of colorants varied between 0.17 μg mL−1 in Allura Red and 1.91 μg mL−1 in Quinoline Yellow. In the case of LOQ, it was ranged from 0.52 in the Allura Red to 5.79 in the Quinoline Yellow. The method applicability was verified by the determination of colorants present in 22 samples. The 15 samples were only unicolor and the color concentration in these samples varied from 18.426 ± 0.100 to 610.390 ± 4.711 ppm. The method can be used successfully to the determination of binary and ternary color food and drug samples too. This method provides substantial green benefits without using organic solvents in extraction procedure and in both liquid and paper chromatographic methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Synthetic colorants are used worldwide in food, medicine, textiles, etc. Although the amount of synthetic colorants permitted has been reduced in recent years, many of their kind are widely used because of their low price, high effectivity, and good stability (Sadecka and Polonsky 2000). Some of these substances pose a potential risk to human health and, in contact with some drugs, they can cause allergic and asthmatic reactions. Some evidence also suggests that synthetic precursors, intermediates, and degradation products of colorants could be harmful due to their toxicity and carcinogenic effects (Cunha and Alpendurada 2002).
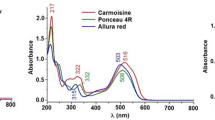
Since colorant intake is evaluated as large as about 10 g year−1, control of synthetic colorants in food should be stressed (Gianotti et al. 2005). Many analytical techniques have been developed to identify and determine the amount of various synthetic colorants such as thin layer chromatography (Baranowska et al. 2007), voltametry (Florian et al. 2002; Alghamdi 2005), differentia1 pulse polarography (Chanlon et al. 2005), derivative spectrometry (Gianotti et al. 2005; Vidotti et al. 2005), and spectrophotometric methods in combination with chemometrics (Dinc et al. 2002; Oveisi et al. 2003; Hajimahmoodi et al. 2008). Capillary electrophoresis (Cunha and Alpendurada 2002; Huang et al. 2002; Jager et al. 2005; Cifuentes 2006; Prado et al. 2006) and micellar electrokinetic capillary chromatography (Chou et al. 2002) have also been used for these applications but they have sensitivity problems as a result of their small injection volume. High-performance ion chromatography (Chen et al. 1998), reversed-phase liquid chromatography (Yoshioka and Ichihashi 2008), and ion pair liquid chromatography coupled with UV or diode-array detectors (Gianotti et al. 2005; Fuh and Chia 2002; Kiseleva et al. 2003; Vaněrková et al. 2007) are still the preferred methods because they provide unrivaled resolution, sensitivity, and selectivity. Both isocratic (Kiseleva et al. 2003) and gradient (Fuh and Chia 2002; Sharma et al. 2003; Garcia-Falcon and Simal-Gandara 2005) systems are used and the latter one is preferred to separate complex mixture of colorants. Nowadays, modern analytical methods need to be green (Sharma et al. 2003; Vidotti et al. 2004) and different analytical procedures have been mentioned as green methods (Song et al. 2002; Melchert and Rocha 2005; Vidotti et al. 2006; Kruanetr et al. 2007; March and Simonet 2007; Teixeira and Rocha 2007; Li et al. 2009). According to these methods, chemists should develop analytical procedures that do not use hazardous reagents and minimize the amount of chemical waste that is generated during the analysis. Seven standard FD and C colorants are used in Iran. These colorants are divided into four groups: Azo compounds (Sunset Yellow E110, Carmoisine E122, Ponceau 4R E124, and Allura Red E129), Triaryl methane group (Brilliant Blue E133), Chinophthalon derivative of Quinoline Yellow (Quinoline Yellow E104), and Indigo colorant (Indigo Carmine E132; Minioti et al. 2007). Their chemical structures, common names, European Community numbers (E numbers), and Color Index dominations are reported in Fig. 1. This study has developed a green extraction procedure and chromatographic method that use no organic solvent to identify and quantify seven commonly used food colorants in some foodstuffs and medicines in Iran.
Materials and Methods
Reagents and Solutions
Deionized double-distilled water was used to prepare the solutions and for mobile phases. The colorants were donated by Iran’s Institute of Standards and Industrial Research, and were dried at 65 °C for 6 h. Stock solutions of each colorant were prepared at a concentration of 1 mg mL−1 and diluted to 100 μg mL−1 for use. All the chemicals were of analytical grade and were purchased form Merck (Darmstadt, Germany). The samples were collected from Tehran malls and pharmacies.
Equipment
The colorants were separated with a high-performance liquid chromatography (HPLC) system equipped with a degasser, a mixing chamber, an auto injector, a column oven, a D-4163 interface, a K-1000 pump and a K-2500 UV detector from Knauer (Berlin, Germany). A Eurospher-100 C8 (5 μm, 4.6 × 250 mm) column and Eurochrom 2000 software were used throughout. A UV visible Cintra 40 double-beam spectrophotometer connected to an IBM Pentium 100 computer was also used.
Sample Preparation
Treatment of Drink and Syrup
Twenty-five milliliter of the sample was transferred to a 50-mL volumetric flask and diluted to the volume with deionized water.
Treatment of Candy and Jelly Gum
The candy was ground in a mortar and dissolved 5 g of it in deionized distilled water and volumed to 25 mL. The procedure was the same for jelly gum without grounding.
Treatment of Chocolate in Crisp Sugar Shell
Five grams of the sample was weighted and transferred into the baker. To prevent dispersion of titanium dioxide (the shell), 20 mL NH3 (0.25% w/v) was added and shaked slowly to remove dyes and then the uncolored residue was separated.
Treatment of Belowgun (Pofak)
Five grams of the sample was ground in a mortar and 20 mL NH3 (0.25% w/v) was added. The dye was separated from the samples using 20 mL of NH3 (0.25% w/v), ultrasonic wave and filtration. The procedure was repeated to dissolve remaining dye from texture and the solutions were mixed.
Treatment of Gum
Five grams of the sample was sliced, transferred into a 200 mL baker and then 20 mL NH3 (0.25% w/v) was added. The gum texture was destroyed using microwave oven (30 s, 60% power) and the dissolved dye was filtered.
Further Treatment of Food Samples
The pH of the above solutions were adjusted to 3 with HCl (7% w/v), 0.5 g of polyamide adsorbent were added and then stirred the mixture vigorously to adsorb all the colorants in the solution (if the solution still was colored, a small further amount of polyamide adsorbent should be added). The mixture was filtered and the adsorbent was washed with 20 mL distilled deionized water thrice. The prepared adsorbent was transferred to a 100 mL baker, 20 mL solution of alkaline–ammonia (NH3, 25% w/v) was added and the solution was then filtered. The procedure was repeated twice to remove all the dyes from the polyamide. The collected solution was dried out on a boiling water bath and the residue was transferred to a 25-mL volumetric flask using mobile phases. Then it was filtered through a sample filter (0.45 μm) to analyze by HPLC.
Paper Chromatography
Whatman chromatographic paper no. 3 was used to separate colorants. The mobile phase consisted of an equal volume of NH3 (0.25% w/v) and NaCl (1% w/v).
Liquid Chromatography and Separation Conditions
The mobile phase was a mixture of 50 mM phosphate buffer at pH 7 containing triton X-100 (0.25% v/v). The details of the HPLC separation system are shown in Table 1.
Before sample injection, the column was flushed with 300 mL mobile phase at a 0.2 mL min−1 flow rate. The volume of injection was 100 μL.
Validation
Validation was performed following the International Conference on Harmonization (ICH) guidelines (Walfish 2006). The method was validated as regards its selectivity, linearity, precision (within and between days), accuracy, and limit of detection (LOD) and quantification (LOQ). The validation results are shown in Tables 2 and 3.
Selectivity
To determine selectivity, the chromatogram of spiked samples and blank ones were compared and no interferences were observed in corresponding retention times.
Linearity
Linearity was studied for each colorant concentration ranged in Table 2. To establish linearity, six concentrations of each colorant were injected three times and the correlation coefficient, y-intercept, slope of the regression line, residual sum of squares, and relative standard deviation (RSD%) for slope and intercept were calculated. Following ICH recommendations the correlation coefficient calculation and the regression analysis were performed without applying any type of mathematical transformation or data weighting.
Precision
To evaluate the intra-day precision (as RSDr), each sample was extracted twice as described in “Sample Preparation” section and each extracted sample was analyzed two times in the same day. Three replicates of each sample preparation and analysis were done to evaluate inter-day precision (as RSDR) in three different days (Table 4).
Accuracy
To check the accuracy of the method, different kinds of clean matrices were spiked with the analyte of interest at low, intermediate, and high concentrations of the each calibration curve that is mentioned in Table 4. The concentrations were recalculated from the corresponding calibration straight line (experimental concentration) and were compared with the theoretical concentrations. Recovery was estimated as the relationship between the experimental and the theoretical concentration expressed as a percentage (C exp/C theo) × 100; Table 3).
Limit of Detection and Quantification
LOD and LOQ for each colorant were set as:
-
LOD = 3.3 σ/S
-
LOQ = 10 σ/S
where, σ is the standard deviation of regression line y-intercept and S is the slope of the calibration curve (Table 2).
Results and Discussion
System Optimization
Currently, seven synthetic colorants are permitted to use in food and pharmaceuticals in Iran. This work aimed to develop a practical and safe method to monitor colorants in laboratories. Therefore, a green chromatographic method adopted from Vidotti et al. (2006) was used with some modification. A C18 column was applied as a stationary phase in Vidotti et al.’s study to separate Tartrazine, Brilliant Blue, and Sunset Yellow, which are highly water soluble. Except these colorants, Indigo Carmine, Quinoline Yellow, and Poncea 4R are the other highly water soluble colorants whose separation of them was proposed in this research. Due to the C18 column’s nonpolar characteristics, it seems that using a more polar column leads to better separation. So a C8 column was used in this study as a stationary phase and more retention time was observed for Brilliant Blue (8.63 min) and Sunset Yellow (9.51 min) than the previous study (Vidotti et al. 2006). Allura Red and Carmoisine are the other two colorants whose separation is proposed. Because of naphthalene rings, they are more hydrophobic than the others (Vidotti et al. 2006) and when C8 column is used, the elution of these two colorants is time consuming which decreases the analytical frequency. To improve retention time, analytical frequency and sensitivity, the column’s temperature was set at 35 °C and the flow rate was increased from 1 to 1.5 mL min−1 after 15 min (Table 1). In the case of Briliant Blue and Sunset Yellow, the monitoring wavelengths were different for them and there was insufficient time to switch wavelengths under these conditions. Therefore, it was preferred to separate the colorants into two different analytical runs (Table 1). Quinoline Yellow, Ponceau 4R, Brilliant Blue, Allura Red, and Cormoisine were separated into one analytical run (Fig. 2a); and Indigo Carmine, Ponceau 4R, Sunset Yellow, Allura Red, and Carmoisine into another run (Fig. 2b). Probably the use of photodiode array detector can solve this problem and all colorant can be analyzed in one run.
a Chromatogram of the mixed colorant in the first analytical run. The chromatogram was obtained using a mobile phase solution containing Triton X-100 0.25% (v/v) plus 50 mmol l−1 phosphate buffer at pH 7 and a flow rate of 1 ml min−1. The colorants E104, E124, E133, E129, and E122 were eluted at 4.551, 6.068, 8.918, 17.230, and 27.387 min, respectively. b Chromatogram of the mixed colorant in second analytical run. The chromatogram was obtained using a mobile phase solution containing Triton X-100 0.25% (v/v) plus 50 mmol l−1 phosphate buffer at pH 7 and a flow rate of 1 ml min−1. The colorants E132, E124, E110, E129, and E122 were eluted at 3.834, 6.001, 9.535, 17.150, and 27.370 min, respectively
As phosphate concentration increase, the retention time would be raised. This phenomenon is probably due to stability in the charge of colorant molecules as a result of interaction with buffer. In 50 mM buffer concentration, the resolution is well while in higher concentration the frequency would decrease as a result of long retention time. The pH of mobile phase was adjusted to 7 since this pH is not harmful to column and the resolution was well. As organic solvents are not allowed in this method, selecting the appropriate mobile phase is very important. Triton x-100 as a nonionic surfactant which has both hydrophilic and hydrophobic groups was selected among other surfactant. The mechanism of triton x100 is micelle making and its critical micelle concentration is 0.25%. Although Tartrazine is a prohibited colorant in Iran, it is used in some products. So it can be added to the first analytical run for monitoring (r.t. = 2.2). As Fig. 2a and b show, there were three common colorants in the two runs and depending on the other colorants in the product, each of the methods can be used. To select a suitable method, prior paper chromatography can be used before liquid chromatography to detect the food colorant type. The paper chromatography which is used in this work is also green with NH3 and NaCl solutions as mobile phase. The food colorants’ Rf values were 0.67, 0.30, 0.074, 0.44, 0.25, 0.125, and 0.878 for Tartrazine, Sunset Yellow, Carmoisine, Ponceau 4R, Allura Red, Indigocarmine, and Brilliant Blue, respectively. In the case of Quinoline Yellow, the color divided into two parts with different Rf values (0.08 and 0.32) because of its impurity.
If one colorant type is used in a sample product, a simple spectrophotometric method at the maximum wavelength absorbance can be used for determination of colorant. To compare spectrophotometry with the HPLC results, the concentration of one colorant’s samples was spectrophotometrically determined at the optimized HPLC wavelength. The results show that they are comparable (Table 4).
Method Validation
In the case of selectivity, the results show good selectivity because of no interference observation in the corresponding retention times. Calibration equations for the mixed standard solutions, coefficients of determination (R 2), linear ranges, LOD, and LOQ are presented in Table 2. Calibration equations were calculated using the area under the curves and in the case of Quinoline Yellow, the sum of peak area under the curves was used for calibration. The analyte’s linear ranges were differed according to their absorbencies. LOD for all colorants varied between 0.17 μg mL−1 in Allore Red and 1.91 μg mL−1 in Quinoline Yellow. In the case of LOQ it was ranged from 0.52 in the Allora Red to 5.79 in the Quinoline Yellow. The experiment resulted in good RSD for both intra- and inter-day precision. The results of recovery are presented as the percentage ± standard deviation in Table 3. According to the results, recovery is ranged from 87.9 ± 1.3% in high concentration of gum to 103.5 ± 0.9% in low concentration of Smartiz. The results propose extraction is less efficient in the case of solid samples and when color is merged with the texture. The intra-day precision (as RSDr) ranged from 0.066% for Tartrazine in Smartiz at the concentration of 158 μg g−1 to 5.362% for Allura Red in drink 1 at the concentration of 10 μg mL−1. The inter-day precision (as RSDR) ranged from 0.132% to 9.588% for the same samples. The results obtained from this study are in general agreement with those obtained from other studies, where the maximum RSDs ranged between 2.1% (Angelino et al. 1998) and 6.4% (Ma et al. 2006).
Under the wavelength scan condition, the commonly used additives such as saccharin, citrate, benzoate, and sorbic acid cannot interfere with the detection of colorants because they are not colorful and the absorption was done in visible range.
Real Samples
The method was applied to food samples from market control. Each sample was analyzed as described in “Sample Preparation” and “Precision” sections. Data from Table 4 show that 15 of 22 samples contain only one colorant type and can be easily analyzed with a spectrophotometer. One of the other seven samples has three food colorants and can be analyzed in the first analytical run. One of the Belladonna–Pb syrup colorants could not be recognized by this method because its retention time did not comply with any of the eight standard retention times. Four of the others can be analyzed in the first analytical run and only drink 1 should be analyzed with the second run. The concentration of the colorants in analyzed samples ranged from 9.91 μg mL−1 (Allura Red in Drink 1) to 610.39 μg mL−1 (Quinoline Yellow in Smartiz 3).
Conclusion
This study has developed and optimized an efficient and accurate analytical green method for simultaneous determination of eight market food colorants permitted in Iran. This method provides substantial green benefits without using organic solvents in extraction procedure and in chromatographic methods. The proposed method includes a simple pretreatment procedure to extract colorants from food and drugs and offers a combination of sensitivity, selectivity, and simplicity. This method permits the detection of colorants at low concentrations. Its applicability was verified by the determination of colorants present in various foodstuffs. But it should be stressed that column flushing is time consuming and analytical run time is not ideal. Also, if all colorants were separated in one run, the method became more user friendly. To sum up, the best advantage of this valid method is using no organic solvent in extraction, paper chromatography, and HPLC system.
References
Alghamdi AH (2005) J AOAC Int 88:1387
Angelino S, Fell AF, Gennaro MC (1998) J Chromatogr A 797:65
Baranowska I, Zydroń M, Szczepanik K (2007) JPC-J Planar Chromat 17:54
Chanlon S, Joly-Pottuz L, Chatelut M, Vittori O, Cretier JL (2005) J Food Comp Anal 18:503
Chen QC, Mou SF, Hou XP, Riviello JM, Ni ZM (1998) J Chromatogr A 827:73
Chou SS, Lin YH, Cheng CC, Hwang DF (2002) J Food Sci 67:1314
Cifuentes A (2006) Electrophoresis 27:283
Cunha ER, Alpendurada MF (2002) J Liq Chromatogr Relat Technol 25:1835
Dinc E, Baydan E, Kanbur M, Onur F (2002) Talanta 58:579
Florian M, Yamanaka H, Carneiro PA, Zanoni MVB (2002) Food Addit Contam 19:803
Fuh MR, Chia KJ (2002) Talanta 56:663
Garcia-Falcon MS, Simal-Gandara J (2005) Food Control 16:293
Gianotti V, Angioi S, Gosetti F, Marengo E, Gennaro MC (2005) J Liq Chromatogr Relat Technol 28:923
Hajimahmoodi M, Oveisi MR, Sadeghi N, Jannat B, Nilfroush E (2008) Food Anal Methods 1:214
Huang HY, Shih YC, Chen YC (2002) J Chromatogr A 959:317
Jager AV, Tonin FG, Tavares MF (2005) J Sep Sci 28:957
Kiseleva MG, Pimenova VV, Eller KI (2003) J Anal Chem 58:685
Kruanetr S, Liawruangrath S, Youngvises N (2007) Talanta 73:46
Li R, Jiang ZT, Wang RX (2009) Food Anal Method 2:264
Ma M, Luo X, Chen B, Su S, Yao S (2006) J Chromatogr A 1103:170
March JG, Simonet BM (2007) Talanta 73:232
Melchert WR, Rocha FRP (2005) Talanta 65:461
Minioti ES, Sakellariou CF, Thomaidis NS (2007) Anal Chim Acta 583:103
Oveisi MR, Hajimahmoodi M, Davami F (2003) Daru 11:1
Prado MA, Boas LFV, Bronze MR, Godoy HT (2006) J Chromatogr A 1136:231
Sadecka J, Polonsky J (2000) J Chromatogr A 880:243
Sharma RK, Mittal S, Koel M (2003) Crit Rev Anal Chem 33:183
Song Z, Hou S, Zhang N (2002) J Agric Food Chem 50:4468
Teixeira LSG, Rocha FRP (2007) Talanta 71:1507
Vaněrková D, Jandera P, Hrabica J (2007) J Chromatogr A 1143:112
Vidotti EC, Almeida VC, Oliveira CC (2004) Talanta 64:993
Vidotti EC, Cancino JC, Oliveira CC, Rollemberg MDCE (2005) Anal Sci 21:149
Vidotti EC, Costa WF, Oliveira CC (2006) Talanta 68:516
Walfish S (2006) Bio Pharm 19:28
Yoshioka N, Ichihashi K (2008) Talanta 74:1408
Acknowledgments
The authors acknowledge Tehran University of Medical Sciences and Health Services for its support with a grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khanavi, M., Hajimahmoodi, M., Ranjbar, A.M. et al. Development of a Green Chromatographic Method for Simultaneous Determination of Food Colorants. Food Anal. Methods 5, 408–415 (2012). https://doi.org/10.1007/s12161-011-9259-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-011-9259-4