Abstract
The aim of this study was to evaluate the effects of different light quality of light emitting diode (LED) on the growth, concentration of chlorophyll and chlorophyll biosynthesis precursors of non-heading Chinese cabbage (Brassica campestris L.). Seedlings of the cultivar Te Ai Qing were cultured for 28 days under 6 treatments: red light (R), blue light (B), green light (G), yellow light (Y), red plus blue light (RB) and dysprosium lamp (CK). Lighting experiments were performed under controlled conditions (photon flux density 150 μmol m−2 s−1; 12 h photoperiod; 18–20 °C). The fresh and dry mass were the greatest under RB, which were significantly higher than other light treatments. The fresh mass under RB was almost twice higher compared to other light treatments. Plant height was highest under R treatment and was lowest under B. RB treatment also lowered the plant height significantly. The highest soluble sugar concentration was observed under B. The soluble protein concentration was the greatest under RB. The R treatment was adverse to pigment accumulation. The concentration of photosynthetic pigments and chlorophyll biosynthesis precursors were higher under RB. The RB treatment was beneficial to pigment accumulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among various environmental factors, light is one of the most important variables affecting the photo-morphogenesis and photosynthesis of plants (Avercheva et al. 2009). Light is also an essential energy source for plant photosynthesis and an important signal for plant growth and development. Changes in light quality have a profound effect on plant growth, particularly on photosynthesis (Neff et al. 2000; Fukuda et al. 2008).
Chlorophyll is the material base of plant photosynthesis. Its concentration and composition directly influence the photosynthetic rate of the leaves. Chlorophyll is synthesized via a complex biosynthetic pathway: glutamate is converted to 5-aminolevulinic acid (ALA), protoporphyrin IX (Proto IX) is biosynthesized, magnesium is inserted into Proto IX to form Mg-proto IX, proto chlorophyll (Pchlide) is turned into Pchlide a, and chlorophyllide a is esterified into chlorophyll a (Porra 1997; Tanaka and Tanaka 2007).
Chlorophyll biosynthesis requires light (Jilani et al. 1996; Hoober and Eggink 1999). The formation of photosynthetic pigment is controlled by different light qualities. At the same time, different photosynthetic pigments absorb different light spectrum. Blue light is generally considered to be beneficial for the formation of Chl a. Higher Chl a/b ratios are observed under blue light and lower Chl a/b ratios under red light (Rivkin et al. 1989; Marsac and Houmard 1993). Blue light improves gene expression of MgCH, GluTR and FeCH which regulates synthesis of chlorophyll (Wang et al. 2009) and promotes chlorophyll synthesis (Poudel et al. 2008; Kurilcik et al. 2008). Red light is not conducive to the formation of chlorophyll, because of the reduction in tetrapyrrole precursor 5-aminolevulinic acid (Tanaka et al. 1998; Sood et al. 2005).
However, previous reports have scarcely described the effects of LED light sources on the biosynthesis of chlorophyll in plant. In this paper, the chlorophyll biosynthesis of non-heading Chinese cabbage cultured under different light treatments was investigated to examine how different light qualities affect chlorophyll biosynthesis in the plant.
Materials and methods
Light treatments
All the LEDs were designed by College of Agriculture, Nanjing Agricultural University, China. The light treatments were designated as red LEDs, blue LEDs, green LEDs, yellow LEDs, a mixture of red plus blue LEDs (R:B = 6:1) and dysprosium lamp(CK). The spectral distributions of the yellow (peak at 590 nm), green (peak at 520 nm), red (peak at 658 nm) and blue (peak at 460 nm) lights were measured using a spectroradiometer (OPT-2000, ABDPE CO., Beijing, China). All the treatments were irradiated with the same photon flux density (PFD) of 150 μmol m−2 s−1. PFD was measured using a quantum sensor (LI-250, LI-COR, USA) and was separately controlled by adjusting both the electric currents and numbers of light bulbs. The parameters of the light in each treatment are shown in Table 1.
Plant materials
Seedlings of non-heading Chinese cabbage (Brassica campestris L. Te’aiqing) were germinated and were planted in plastic pots containing a mixture of peat, vermiculite and perlite (1:1:1, v/v/v). When the second leaf was fully expanded, 120 seedlings were selected, randomized into six groups and were cultured under six light quality treatments for 28 days. Plants were fertigated using Garden Test Standard nutrient solution. Since there was ventilation in the controlled environment, the CO2 level was the same as the CO2 level of atmosphere. The relative humidity (RH) was maintained at 70 ± 10 %, with a 12-h photoperiod, and the growth temperature was at 18–20 °C.
Biomass and growth parameters analysis
A total of 20 seedlings for each treatment were selected for biomass analysis. To determine the dry mass, the seedlings were dried at 85 °C until a constant mass was reached. The fresh mass and dry mass of the seedlings were measured using an electronic balance. Plant height was measured from main stem base to top of the plant by a ruler.
Soluble protein measurement
Leaves (1.0 g of fresh weight, W) were ground in a mortar with liquid nitrogen, to which 5 ml (V1) of 0.067 mol l−1 potassium phosphate buffer (PBS) was added, and were then filtered through filter paper. The extract was centrifuged at 12,000g for 10 min, and the supernatant was removed. The extract (1 ml, V2) and Coomassie brilliant blue G-250 (5 ml) was thoroughly mixed. The optical density was measured using a UV-1200 spectrophotometer (Jin Peng, Shanghai, China) at 595 nm. The optical density was measured by a UV-1200 spectrophotometer at 595 nm (ρ). The concentration of soluble protein was determined using the following equation: soluble protein (mg g−1) = ρ V1/W V2 (Li et al. 2010).
Soluble sugar measurement
The sampling timing was at 10 a.m. after the plants were irradiated for 3 h. Leaves (0.5 g, dry weight) were ground in a mortar with liquid nitrogen. Then 1 ml of 80 % ethanol was added, and the mixture was filtered through filter paper. The filtrates were recovered, and the residues were washed again with 70 % ethanol and filtered. Both filtrates were mixed, and 3 ml of distilled water was added. The extract was centrifuged at 12,000g for 15 min, and 1 ml of supernatant was collected. Soluble sugar concentration was determined by the sulfuric acid anthrone method and measured at 620 nm (Fairbairn 1953).
Pigment measurement
0.1 g of leaves (fresh mass, W) was submerged in 10 ml (V) of 80 % acetone. The pigment was extracted until the leaf turned white. Optical density (OD) was measured with a UV-1200 spectrophotometer at 470 nm for carotenoid (OD470), at 663 nm for chlorophyll a (OD663), and at 645 nm for chlorophyll b (OD645), and calculated by the following equations by Holm (1954):
Chl a (mg g−1) = (12.72 OD663 − 2.59 OD645) V/1,000 W
Chl b (mg g−1) = (22.88 OD645 − 4.67 OD663) V/1,000 W
Chl (a + b) (mg g−1) = (8.05 OD663 + 20.29 OD645) V/1,000 W
Carotenoid (mg g−1) = (1,000 OD470 − 3.27 Chl a − 104 Chl b) V/(229 × 1,000 W)
where V is the total volume of acetone extract (ml), and W is the fresh weight (g) of the sample.
Assessment of chlorophyll biosynthetic precursors
The concentration of 5-aminolevulinic acid (ALA) was determined according to Dei (1985). Plant culture solution (200 ml) was centrifuged at 1,000×g for 5 min. The plant pellet was collected. 200 ml of buffer solution with 10 mmol l−1 of levulinic acid was used to suspend plant cells, which were then cultured in an incubator under the original culture conditions but under light for 12 h. ALA was extracted using 4 % trichloroacetic acid. The extracted solution (5 ml) was added with 2.35 ml of 1 mol l−1 sodium acetate, 0.15 ml of acetylacetonate and 2.5 ml of 1 mol l−1 acetate buffer (pH 4.6), and was heated in boiling water for 10 min. Finally, the solution was cooled down and added with Ehrlich reagent. The concentration of ALA was analyzed by UV-1200 spectrophotometer at 553 nm.
The concentration of protoporphyrin IX (Proto IX), Mg-proporphyrin IX (Mg-Proto IX) and protochlorophyllide (Pchlide) were measured according to Rebeiz et al. (1975) and Lee et al. (1992). The plant was cultured in the dark for 12 h, and was then homogenized by ultrasonication with 8 ml of extraction solution (acetone: 0.1 mol l−1 NH3·H2O = 9:1, V/V) in an ice bath. The extracted solutions were then re-extracted by equal volume and 1/3 volume n-hexane in turn. Finally, the acetone phase was taken to measure fluorescence intensities at 633, 622, 640 and 595 nm with 400 nm of excitation light, by utilizing the LS 55 fluorescence/phosphorescence/luminescence spectrophotometer (PerkinElmer USA). The relative concentration of Mg-Proto IX was directly represented by 595 nm (Lee et al. 1992). The relative concentration of Proto IX and Pchlide was calculated according to following equations by Rebeiz et al. (1975):
Statistical analyses
Statistical analyses were conducted using SPSS 16.0 (SPSS, version 16.0 Inc., USA). The experiment was repeated six times with five plants in each treatment. The data were analyzed by ANOVA and the differences between the means were tested using Duncan’s multiple range test (P < 0.05).
Results
Growth, biomass and concentration of soluble sugar and soluble protein
Different light quality had variable effects on the growth of non-heading Chinese cabbage (Table 2). In general, the fresh and dry mass under LEDs were greater than those under CK treatment. There were no significant differences in fresh mass between CK and Y treatment. Under RB, the fresh and dry mass were the greatest in the seedlings, meanwhile the fresh mass was almost twice higher compared to other light treatments. The fresh mass was the lowest under CK and the dry mass was the lowest under Y. Plant height of non-heading Chinese cabbage was highest under R treatment and was lowest under B. RB treatment also inhibited plant height significantly.
Under B, the highest concentration of the soluble sugar was observed, and the lowest under Y. Under RB, the soluble protein concentration was the greatest in seedlings and the lowest under Y. These results showed that the B treatment might be suitable for accumulation of soluble sugars, and the RB treatment seemed to promote the accumulation of soluble protein.
Concentration of photosynthetic pigments
The concentration of photosynthetic pigments of non-heading Chinese cabbage varied in response to different light quality (Table 3). The concentration of chlorophyll a and chlorophyll b and the total concentration of chlorophyll (a + b) were higher in seedlings under RB and the lowest under R light. The concentration of carotenoid presented the same trend. The largest value of chlorophyll a/b was found in the B treatment, and the lowest in the G treatment. There were no significant differences between RB and CK in the concentration of pigments, and RB was the same effective as CK.
Biosynthesis precursors of chlorophyll
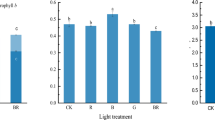
The variation in the chlorophyll concentration detected in different light treatments prompted us to further test changes in the concentration of chlorophyll biosynthesis precursors including ALA, Proto IX, Mg-proto IX and Pchlide. We found that all the chlorophyll biosynthesis precursors in non-heading Chinese cabbage were the lowest under R treatment. There were no significant differences between RB and CK treatments in the concentration of chlorophyll biosynthesis precursors in seedlings (Fig. 1).
Discussion
The results of the present study demonstrated that under LEDs, the fresh mass and dry mass in seedlings were significantly higher than under CK, which agreed with those observed by Went (1957) and Li et al. (2010). Under the RB treatment, the fresh mass and dry mass were the greatest in the seedlings, since blue and red lights promote development and drive photosynthesis. In our study, the fresh mass and dry mass of plants under RB were the greatest in seedlings, and the fresh mass was almost twice higher comparing to other light treatments. Previous study also found that the combination of red and blue light might promote fresh weight and dry weight in many plant species such as chrysanthemum, upland cotton and tomato (Kim et al. 2004; Li et al. 2010; Liu et al. 2011).
The spectra of red and blue light are in accordance with absorption spectrum peak area of chlorophyll, and the percentage absorption of blue and red light by plant leaves is about 90 % (Terashima et al. 2009). Therefore, the red and blue light strongly influence the development and physiology of plant and have the best effective photosynthesis rate (Hogewoning et al. 2010). While white light contains a green component which consumes part of the red and blue lights (Went 1957), and the effects of green light may possibly be reconciled as the negative cry-dependent or cry-independent effects on seedling development and growth (Folta and Maruhnich 2007). Consequently, the RB treatment had better effect on biomass accumulation of non-heading Chinese cabbage.
Our study showed that plant height was highest under R, and was inhibited significantly under B and RB treatment. Previous study also indicated that red light promoted stem elongation while blue light had inhibition effect on stem elongation (Nhut et al. 2003; Li et al. 2013). The photoinhibitions of elongation by blue light are achieved through changes in cell wall properties. Blue light reduces relaxation mainly by increasing in the yield threshold (Kigel and Cosgrove 1991). The current results suggested blue light seemed to be an important factor for inducing the compact shape of plant; therefore, under RB treatment the plant height was lower. Generally speaking, plants with restricted growth or even more compact shape have higher level of chlorophyll. This may one of the reasons why RB treatment caused the highest concentration of chlorophyll.
Light quality regulates the carbohydrate metabolism of plants. Compared to the control, the R, B and RB treatments contributed to the accumulation of soluble sugar in leaf, with the B treatment being the most effective in increasing the soluble sugar concentration (Table 1). Blue light effectively promoted soluble sugar accumulation and was considered a means of studying the regulation of photosynthetic carbon metabolism in relation to plant growth (Britz and Sager 1990; Shin et al. 2008; Chang et al. 2010). Saebo et al. (1995) and Li et al. (2013) found that photosynthetic capacity was highest when the plants were exposed to blue light, which enhanced carbohydrate metabolism of higher plants, while single yellow light caused weak photosynthesis of plants, resulting in reduction of carbohydrate synthesis (Liu et al. 2011). Changes in carbohydrate concentration under different light qualities are also controlled by key enzyme activities related to soluble sugar metabolism in several species (Heo et al. 2006). Under Y treatment, the concentration of soluble protein was the lowest; hence, we speculated that the concentration and activity of enzyme are relatively low.
Chlorophyll plays an important role in photosynthesis, and light is the essential condition for chlorophyll formation. Light quality also plays an important role in chlorophyll biosynthesis (Jilani et al. 1996). Recent studies show that red light was not conducive to the formation of chlorophyll, because of the reduction in tetrapyrrole precursor 5-aminolevulinic acid (Tanaka et al. 1998; Sood et al. 2005). We made the same observation that the chlorophyll concentration was the lowest under the R treatment, and so were the concentrations of ALA, Proto IX, Mg-proto IX and Pchlide which are important biosynthetic intermediates of chlorophyll. We found that red light reduced chlorophyll biosynthesis because less ALA was available for further synthesis.
Blue light was reported to be beneficial to pigment accumulation (Tanaka et al. 1998; Poudel et al. 2008; Kurilcik et al. 2008). It could reverse red light-induced inhibition response (Sood et al. 2005). Long-term exposure to blue light enhanced the ALA-synthesizing activity (Kamiya et al. 1981). The present results were consistent with previous studies. However, added with blue light, the RB treatment promoted chlorophyll biosynthesis even more significantly in this experiment.
We found that, under the G treatment chlorophyll biosynthesis was decreased with less ALA. The Y treatment induced higher concentration of chlorophyll biosynthesis precursors, but lower concentration of chlorophyll. The biosynthesis of chlorophyll could be blocked at any step. We speculated that low protein concentration led to low availability of enzymes which were responsible for the metabolism of chlorophyllide esterification to chlorophyll a (Stobart et al. 1985). This phenomenon requires further research.
Our study showed that red plus blue LEDs (R:B = 6:1) increased the biomass of non-heading Chinese cabbage. Red light alone was unprofitable for chlorophyll biosynthesis. There were no significant differences between RB and CK treatment in the concentration of photosynthetic pigments and of chlorophyll biosynthesis precursors. The RB light might be propitious to non-heading Chinese cabbage cultivation.
Author contribution
XiaoXue Fan and Jie Zang designed and conducted the experiments, collected and analyzed data, and wrote the manuscript. ZhiGang Xu and ShiRong Guo designed, conducted the experiments, and assisted in writing the manuscript. Xuelei Jiao participated in the LED devices. XiaoYing Liu and Gao Ying helped in the biochemical analysis.
Abbreviations
- ALA:
-
5-Aminolevulinic acid
- B:
-
Blue LED
- CK:
-
Dysprosium lamp
- G:
-
Green LED
- LED:
-
Light emitting diode
- Mg-Proto IX:
-
Mg-proporphyrin IX
- Pchlide:
-
Protochlorophyllide
- PFD:
-
Photon flux density
- Proto IX:
-
Protoporphyrin IX
- R:
-
Red LED
- RB:
-
Red plus blue LED
- Y:
-
Yellow LED
References
Avercheva OV, Berkovich YA, Erokhin AN, Zhigalova TV, Pogosyan SI, Smolyanina SO (2009) Growth and photosynthesis of Chinese cabbage plants grown under light-emitting diode-based light source. Russ J Plant Physiol 56:14–21
Britz SJ, Sager JS (1990) Photomorphogenesis and photoassimilation in soybean and sorghum grown under broad spectrum or blue-deficient light sources. Plant Physiol 125:448–454
Chang TT, Liu XY, Xu ZG, Yang Y (2010) Effects of light spectral energy distribution on growth and development of tomato seedlings. Scientia Agric Sinica 43:1748–1756 (in Chinese)
Dei M (1985) Benzyladenine-induced stimulation of 5-aminolevulinic acid accumulation under various light intensities in levulinic acid-treated cotyledons of etiolated cucumber. Physiol Plantarum 6:153–160
Fairbairn NJ (1953) A modified anthrone reagent. Chem Ind 31:86
Folta KM, Maruhnich SA (2007) Green light: a signal to slow down or stop. J Exp Bot 58:3099–3111
Fukuda N, Fujitan M, Ohta Y, Sase S, Nishimura S, Ezura H (2008) Directional blue light irradiation triggers epidermal cell elongation of abaxial side resulting in inhibition of leaf epinasty in geranium under red light condition. Sci Hortic 115:176–182
Heo JW, Shin KS, Kim SK, Paek KY (2006) Light quality affect sin vitro growth of grape ‘Teleki 5BB’. J Plant Biol 49:276–280
Hogewoning SW, Trouwborst G, Maljaars H, Poorter H, Ieperen WV, Harbinson J (2010) Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J Exp Bot 61:3107–3117
Holm G (1954) Chlorophyll mutation in barley. Acta Agric Scandinavica 1:457–471
Hoober JK, Eggink LL (1999) Assembly of light-harvesting complex II and biogenesis of thylakoid membranes in chloroplasts. Photosynth Res 61:197–215
Jilani A, Kar S, Bose S, Tripathy BC (1996) Regulation of the carotenoid content and chloroplast development by levulinic acid. Physiol Plantarum 96:139–145
Kamiya A, Ikegami I, Hase E (1981) Effects of light on chlorophyll formation in cultured tobacco cells I. chlorophyll accumulation and phototransformation of protochlorophyll(ide) in callus cells under blue and red light. Plant Cell Physiol 8:1385–1396
Kigel J, Cosgrove DJ (1991) Photoinhibition of stem elongation by blue and red light. Plant Physiol 95:1049–1056
Kim SJ, Hahn EJ, Heo JW, Paek KY (2004) Effects of LEDs on net photosynthetic rate, growth and leaf stomata of Chrysanthemum plantlets in vitro. Sci Hortic 101:143–151
Kurilcik A, Canova MR, Dapkuniene S, Zilinskaite S, Kurilcik G (2008) In vitro culture of Chrysanthemum plantlets using light-emitting diodes. Cent Eur J Biol 2:161–167
Lee HJ, Ball MD, Parham R, Rebeiz CA (1992) Enzymic conversion of protoporphyrin IX to Mg-protoporphyrin IX in a subplastidic membrane fraction of cucumber etiochloroplasts. Plant Physiol 3:1131–1140
Li HM, Xu ZG, Tang CM (2010) Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell Tiss Org 103:155–163
Li HM, Xu ZG, Tang CM (2013) Effects of different light resources on growth of upland cotton (Gossypium hirsutum L.) seedlings. Crop Sci Accept
Liu XY, Guo SR, Xu ZG, Jiao XL, Takafumi T (2011) Regulation of chloroplast ultrastructure, cross-section anatomy of leaves and morphology of stomata of cherry tomato by different light irradiations of LEDs. Hortscience 46:217–221
Marsac NT, Houmard J (1993) Adaptation of cyanobacteria to environmental stimuli: new steps towards molecular mechanisms. FEMS Microbiol Rev 1:119–189
Neff MM, Fankhauser C, Chory J (2000) Light: an indicator of time and place. Genes Dev 14:257–271
Nhut DT, Takamura T, Watanabe H, Okamoto K, Tanaka M (2003) Responses of strawberry plantlets cultured in vitro under superbright red and blue light-emitting diodes (LEDs). Plant Cell Tiss Org Cult 73:43–52
Porra RJ (1997) Recent process in porphyrin and chlorophyll biosynthesis. Photochem Photobiol B 65:492–516
Poudel PR, Kataoka I, Mochioka R (2008) Effect of red-and blue-light-emitting diodes on growth and morphogenesis of grapes. Plant Cell Tiss Org 2:147–153
Rebeiz CA, Mattheis JR, Smith BB, Rebeiz CC, Dayton DF (1975) Chloroplast biogenesis and accumulation of protochlorophyll by isolaced etioplasts and developing chloroplasts. Arch Biochem Biophys 171:549–567
Rivkin RB (1989) Influence of irradiance and spectral quality on the carbon metabolism of phytoplankton. I. Photosynthesis, chemical composition and growth. Mar Ecol Prog Ser 55:291–304
Saebo A, Krekling T, Appelgren M (1995) Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tiss Org Cult 41:177–185
Shin KS, Murthy HN, Heo JW, Hahn EJ, Paek KY (2008) The effect of light quality on the growth and development of in vitro cultured Doritaenopsis plants. Acta Physiol Plant 30:339–343
Sood S, Gupta V, Tripathy BC (2005) Photoregulation of the greening process of wheat seedlings grown in red light. Plant Mol Biol 59:269–287
Stobart AK, Griffiths WT, Bukhari AI, Sherwood RP (1985) The effect of Cd2+ on the biosynthesis of chlorophyll in leaves of barley. Physiol Plantarum 3:293–298
Tanaka R, Tanaka A (2007) Tetrapyrrole biosynthesis in higher plants. Plant Biol 58:321–346
Tanaka A, Ito H, Tanaka R, Tanaka NK, Yoshida K, Okada K (1998) Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proc Natl Acad Sci USA 21:12719–12723
Terashima I, Fujita T, Inoue T, Chow WS, Oguchi R (2009) Green light drives leaf photosynthesis more efficiently than red light in strong white light: revisiting the enigmatic question of why leaves are green. Plant Cell Physiol 50:684–697
Wang H, Gu M, Cui JX, Shi K (2009) Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J Photochem Photobiol B 96:30–37
Went FW (1957) The experimental control of plant growth. Chronica Botanica Co, Waltham Mass, p 343
Acknowledgments
This research was partially supported by the National Natural Science Foundation of China (30972035), the National 863 High Technology Program of China (2011AA03A114, 2013AA103003), the National Science and Technology Support Project of China (2011BAE01B01), Agricultural research special funds for public welfare projects (201303108), and Jiangsu Science and Technology Key Program (BE2011197). Prof. Feirong Gu of the College of Foreign Studies helped with the language editing of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by W. Filek.
Rights and permissions
About this article
Cite this article
Fan, X., Zang, J., Xu, Z. et al. Effects of different light quality on growth, chlorophyll concentration and chlorophyll biosynthesis precursors of non-heading Chinese cabbage (Brassica campestris L.). Acta Physiol Plant 35, 2721–2726 (2013). https://doi.org/10.1007/s11738-013-1304-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1304-z