Abstract
Light spectrum of growing environment is a determinant factor for plant growth and photosynthesis. Plants under different light spectra exhibit different growth and photosynthetic behaviors. To unravel the effects of light spectra on plant growth, photosynthetic pigments and electron transport chain reactions, purple and green basil varieties were grown under five different light spectra including white (W: 400–730 nm), blue (B: 400–500 nm), red (R: 600–700 nm) and two combinations of R and B lights (R50B50 and R70B30), with same PPFD (photosynthetic photon flux density). Almost all values for shoot and root growth traits were higher in purple variety and were improved by combinational R and B lights (especially under R70B30), while they were negatively influenced by B monochromatic light when compared to growth traits of W-grown plants. Highest concentration of photosynthetic pigments was detected in R70B30. Biophysical properties of photosynthetic electron transport chain showed higher florescence intensity at all steps of OJIP kinetics in plants grown under R light in both varieties. Oxygen evolving complex activity (Fv/Fo) and PSII maximum quantum efficiency (Fv/Fm) in R-grown plants were lower than plants grown under other light spectra. Values for parameters related to specific energy fluxes per reaction center (ABS/RC, TRo/RC, ETo/RC and DIo/RC) were increased under R light (especially for purple variety). Performance index was significantly decreased under R light in both varieties. In conclusion, light spectra other than RB combination, induced various limitations on pigmentations, efficiency of electron transport and growth of basil plants and the responses were cultivar specific.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light is the main environmental cue that controls various aspects of plant growth and development ranging from seed germination to flowering (Carvalho et al. 2016). Different light attributes such as quality, quantity and duration affect plant growth and productivity (Colquhoun et al. 2013). Light sources such as metal-halide, fluorescent, high-pressure sodium, neon lamps and light-emiting diode (LED) can be used for production of plants in closed environments instead of sunlight (Lin et al. 2013). Most of these light sources contain wavelengths that are not important for plant growth or they are highly energy consumable. LEDs attracted lots of attentions due to their minimum heating, long lifetime, small mass and volume, solid state construction, and generation of specific wavelengths (Heo et al. 2002; Kim et al. 2004). With introduction of LEDs for plant production in closed environments, many studies have been conducted to find growth and physiological responses of plants under specific spectral compositions.
Plants dynamically respond to their surrounding light environments (Carvalho et al. 2016). Although the range of visible light (400–700 nm) is considered as the most important light spectrum for the photosynthesis, higher plants sense and respond to a range of light spectrum, from UV-C (260 nm) to the far red (720–780 nm) (Folta and Carvalho 2015). Exposure to each light spectrum triggers certain responses by plants. For instance, when plants are exposed to light environment with a low red/far red ratio or high in green light, they exhibit shade avoidance responses (Wang and Folta 2013; Carvalho et al. 2016). The exposure of plants to blue and UV wavelengths lead to carotenoids and anthocyanins accumulation in the leaves (Li and Kubota 2009; Carvalho et al. 2016). Blue light can limit stem elongation and induce leaf expansion (Neff and Chory 1998; Wang et al. 2015; Carvalho et al. 2016). Red and blue lights mainly contain range of wavelengths necessary for photosynthesis in plants (Cosgrove 1981; Kasajima et al. 2008). These studies highlight the importance of light spectra on regulation of different aspects of plant growth and physiology.
Excitation of electrons by intercepted light and transfer of electrons in the electron transport chain (ETC) of photosynthetic apparatus generate energy and reducing power for production of chemical compounds in plants (Jordan et al. 2001). Two photosystems (PSI and PSII) are involved in the ETC. It has been found that the PSII is sensitive to light spectra (Miao et al. 2016). Capturing light in the antenna complex of PSII by photosynthetic pigments (Chl a and b) triggers electron transport (Taiz and Zeiger 2002).
Patterns of electron transport under monochromatic and combinational light sources can facilitate understanding the physiological response of photosynthesis to light spectra. Fate of absorbed light energy and other information about structure and function of photosynthetic apparatus can be analyzed through Chl fluorescence signals by the so-called OJIP kinetics. This kinetics depends on energy flow in thylakoid membranes, which further gives insights into photosynthetic system by fluorescence signals measurement (Kalaji et al. 2017). Therefore, this test was mainly used in current study to investigate the effects of different light spectra on photosynthetic apparatus of basil plants. Pigments other than Chls such as carotenoids and anthocyanins are considered as accessory pigments involved mainly in photoprotection of plants under stressful conditions. However, little is known regarding the structure and function of photosynthetic apparatus under different light spectra in plants containing different leaf pigmentations. Therefore, in the current study, growth, development and photosynthetic performance of green and purple basil plants were investigated under different light spectra.
Sweet basil (Ocimum basilicum L.), a member of Labiatae family, is an annual and a tender summer herb, which grows in several regions around the world (Holm and Hiltunen 1999; Hussain et al. 2008; Chang et al. 2009). This plant was chosen for current experiment because it has cultivars with different pigmentations and there is a great interest to grow basil plants in closed environments under LEDs.
Materials and methods
Plant material and growth conditions
Seeds of two commercial basil plants (O. basilicum) with different leaf color [green (mobarake ecotype) and purple (ardestan ecotype)] were sown in cocopeat and perlite (1:1, V:V) in greenhouse. Following seven days (after germination) of plant growth in transplanting tray, the transplants were transferred to plastic pots (15 cm depth and 10 cm diameter) in the same culture medium. Plants were placed inside LED-illuminated chambers (1 × w × h = 1 m × 0.7 m × 1 m) with exactly same environmental conditions (25/20 °C day/night temperature and 40% relative humidity) except their light regimes. Plants were subjected to five different light spectra including: white (W), blue (B), red (R), 70% red + 30% blue (R70B30) and 50% red + 50% blue (R50B50) provided by LED modules (24 W, Iran Grow Light Co, Iran). The light intensity in all growth chambers was adjusted to photosynthetic photon flux density (PPFD) of 250 ± 10 µmol m−2 s−1. PPFD intensities and light spectra were monitored using a sekonic light meter (Sekonic C-7000, Japan). The relative spectra of the light treatments are shown in Fig. 1. The lighting cycles were 16/8 h day/night period. Seedlings were irrigated with half strength of Hoagland solution. One month following plant growth under different light spectra, the plants were evaluated for their photosynthetic pigments and biophysical parameters.
Plant growth and morphology
One month following plant growth under different light spectra, growth and morphological characteristics such as height, leaf number, root volume, root fresh and dry weights, shoot fresh and dry weights, stem diameter and node number were measured. To measure dry weight, the samples were dried in a drying oven for 3 days at 80 °C. Root volume was measured based on the method described by Aliniaeifard et al. (2016).
Determination of chlorophyll contents
One month following plant growth under different light spectra, young fully developed leaves (3rd to 5th leaves in acropetal order) were used for determination of pigments. Chlorophyll (Chl) contents in basil plants were determined using the method of Lichtenthaler and Wellburn (1983). The samples (250 mg) were extracted in 5 ml acetone (80%) and the extract was separated by centrifugation (SIGMA-3K30) at 7000 g for 5 min. Then the absorbance of supernatant was spectrophotometrically (Lambda 25- UV/VIS spectrometer) measured at 645, 663 nm and the content of Chl a, b and total Chl were calculated based on the Lichtenthaler and Wellburn method (1983).
Total anthocyanins
The amount of anthocyanin was determined by using the method of Mita et al. (1997). Frozen samples (500 mg) were extracted in 10 ml of 1% HCL in methanol for 48 h. The liquid extract was separated by centrifugation at 7000×g for 5 min. Thereafter, the absorbance of the supernatant was measured at 515 nm.
Chl a fluorescence measurements
One month following plant growth under different light spectra, youngest fully developed leaves (3rd to 5th leaves in acropetal order) were used for measuring maximum quantum efficiency of photosystem II (Fv/Fm) with a Handy FlourCam FC 1000-H (Photon System Instruments, PSI, Czech Republic). Plants were dark-adapted for 20 min and attached leaves were immediately used to measure Fv/Fm. The FluorCam consisting of a CCD camera and four fixed LED panels, one pair supplying the measuring pulse and the second pair providing actinic illumination and saturating flash was used. Fv/Fm was calculated using a custom-made protocol (Genty et al. 1989; Aliniaeifard et al. 2014; Aliniaeifard and van Meeteren 2014). At the end of the short flashes, the samples were exposed to a saturating light pulse (3900 µmol m−2 s−1) that resulted in a transitory saturation of photochemistry and reduction of primary quinone acceptor of photosystem II (Genty et al. 1989). After reaching steady state fluorescence, two successive series of fluorescence data were digitized and averaged, one during short measuring flashes in darkness (Fo), and the other during the saturating light flash (Fm). From this two parameters, Fv was calculated by the expression Fv = Fm − Fo. The Fv/Fm was calculated using the ratio (Fm − Fo)/Fm. The average values, and standard deviation of Fv/Fm were calculated by using version 7 FluorCam software.
The polyphasic Chl a fluorescence (OJIP) transients were measured by a FluorPen FP 100-MAX (photon system instruments, Drasov, Czech Republic) on young fully developed basil leaves following over-night dark adaptation. The fluorescence measurement was induced by a saturating light of approximately 3900 µmol m−2 s−1. The OJIP transients were analyzed according to the JIP test (Strasser et al. 2000). The following data from the original measurement were used after extraction by FluorPen software: fluorescence intensities at 50 µs (F 50 µs, considered as the minimum fluorescence F0), 2 ms (J-step, FJ), 60 ms (I-step, FI), and maximum fluorescence (Fm). Performance index was calculated on the absorption basis (PIABS) and densities of QA− reducing PSII reaction centers at time 0 and time to reach maximum fluorescence. The parameters related to the OJIP fluorescence transients including probability that a trapped exciton promote an electron in ETC beyond QA− (Ψ0), quantum yield of electron transport (φE0), quantum yield of energy dissipation (φD0), quantum yield for primary photochemistry (φPAV), maximum quantum yield of primary photochemistry (φP0), specific energy fluxes per reaction center (RC) for energy absorption (ABS/RC), trapped energy flux (TR0/RC), electron transport flux (ET0/RC) and dissipated energy flux (DI0/RC) were calculated according to Kalhor et al. (2018).
Statistical analysis
Ten plants from green and purple leaf basils were grown under each light treatment. All data are presented as the mean ± standard deviation (SD). Mean separations were conducted using a Duncan test protected by ANOVA at P ≤ 0.05.
Results
Light spectra induced changes in plant morphology
The morphology and growth of the two basil varieties were affected by the different light spectra. Tallest basil plants for the green variety were observed under R70B30 and for the purple variety, they were observed under R light. In both varieties, the shortest plants were detected under W light (Fig. 2a). In both varieties, the thickest stems were observed under R light and the thinnest stems were detected under B light (Fig. 2b). Highest shoot dry weight and root volume in both varieties were detected under R70B30 and lowest values of them were observed under B light (Fig. 2c). In both varieties, root dry weight was increased under R70B30 and decreased under B light when compared to root dry weight of plants grown under W light (Fig. 2d). In both varieties, highest and lowest numbers of leaves were observed under R50B50 and B lights, respectively (Fig. 2e). Number of nodes in purple variety was approximately two times higher than number of nodes in green variety. In purple variety, number of nodes was increased under R light and was decreased under B light, while in green variety, highest and lowest number of nodes were detected under R50B50 and W lights, respectively (Fig. 2F).
Plant height (a), stem diameter (b), Shoot dry weight (c), root dry weight (d), Leaf number (e) and node number (f) in the green (black bars) and purple (grey bars) varieties of basil plants grown under different light spectra [blue (B), red (R), white (W) and red and blue (RB)] with same intensity. Bars represent mean ± SD. In E interactions between light spectrum and cultivar were not significant
Light spectra altered leaf pigment concentrations
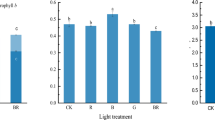
Concentrations of all photosynthetic pigments in the basil leaves were significantly influenced by the light spectra. Chl a (Fig. 3a) and b (Fig. 3b) were dramatically decreased by growing green basil variety under B light spectrum. Highest amounts of Chl a and Chl b in the leaves of green variety basils were detected under R50B50 and R70B30, respectively. In the purple variety, the highest Chl a concentration was observed under B light and highest Chl b concentration was detected under R70B30. Highest total Chl content was detected under R70B30 in both varieties, while the lowest total Chl content was detected under R light for green variety and W light for purple variety. In both varieties, total Chl content was almost doubled under R70B30 when compared to total Chl content of W light (Fig. 3c).
Under all light spectra, higher anthocyanin contents were detected in purple variety. Under R70B30 and B lights, anthocyanin content in purple variety was 30% higher than its content in green variety, while under R light there was only 3% difference between anthocyanin content of two varieties (Fig. 3d). The highest amount of anthocyanin (0.72 unit anthocyanin per g/fresh weight) was measured under R70B30 light in purple variety and the lowest anthocyanin content (0.29 unit anthocyanin per g/fresh weight) was detected under B light in green variety.
Polyphasic Chl a fluorescence (OJIP) kinetics
In our study, to assess the photosynthetic function under different light spectra, the nondestructive analysis of polyphasic fast Chl transient measurement was used in the leaves under dark-adapted state. In both varieties, growing plants under R light led to an induction in Chl fluorescence intensity during all steps of the OJIP graph (F0, FJ, FI and Fm), while lowest Chl fluorescence intensity during OJIP steps was detected under R50B50 (Fig. 4). In both varieties, highest differences between two steps (FV, VI and VJ) were observed under R light and the lowest FV (Fig. 5a), VI and VJ (data not shown) were detected under R50B50. In both varieties, Fv/Fm under R light was considerably decreased in comparison with Fv/Fm of plants grown under other light spectra. This decrease in Fv/Fm in the leaves of purple plants was considerably lower than its value in green variety (Fig. 5b). In both varieties, lowest Fv/F0 (Fig. 5c) and Fm/Fo (Fig. 5d) were detected under R light and their highest values were detected under W and R70B30 lights in green and purple varieties, respectively.
Intensity of chlorophyll a fluorescence during different steps of OJIP curve (vertical lines) exhibited by leaves of green (a) and purple (b) varieties of basil plants grown under different light spectra [blue (B), red (R), white (W) and red and blue (RB)] with same intensity. Bars represent mean ± SD
Chlorophyll a fluorescence of the OJIP-test including Fv (a), Fv/Fm (b), Fv/Fo (c) and Fm/Fo(D) from leaves of green (black bars) and purple (grey bars) varieties of basil plants grown under different light spectra [blue (B), red (R), white (W) and red and blue (RB)] with same intensity. Bars represent mean ± SD
In purple variety, under all light spectra, values for parameters related to specific energy fluxes per reaction (ABS/RC, TRo/RC, ETo/RC and DIo/RC) were higher than their values in green variety (Fig. 6a–d). In both varieties, ABS/RC, TRo/RC, ETo/RC and DIo/RC had highest values under R light and lowest values under R50B50. In purple varieties, all parameters related to specific energy fluxes per reaction center were higher in comparisons with their values in green variety. Plants grown under R light (especially purple variety) showed the highest φDo (Fig. 7a). In green variety, φPAV was highest under R70B30 and was lowest under B light. In purple variety, the highest and lowest φPAV values were detected under R and B lights, respectively (Fig. 7b). The highest φEo was detected under RB combinational lights in both varieties (Fig. 7c). ΦDo was dramatically increased in plants grown under R light (especially purple variety) and decreased in W light in both varieties (Fig. 7d). Among all light spectra, lowest Ψo value was detected under R light in both varieties; however, Ψo values in green variety were higher than their values in purple variety. This difference was more highlighted in plants grown under R and R70B30 lights (Fig. 7d). Under all light spectra, PIABS of green variety was higher than PIABS of purple variety (Fig. 8). In both varieties, PIABS was increased by growing plants under combinational lights, while its value was decreased in plants grown under R light.
Specific energy fluxes per reaction center (RC) for energy absorption (ABS/RC; a), trapped energy flux (TR0/RC; b), electron transport flux (ET0/RC; c) and dissipated energy flux (DIo/RC; d) from the fluorescence transient exhibited by leaves of green (black bars) and purple (grey bars) varieties of basil plants grown under different light spectra [blue (B), red (R), white (W) and red and blue (RB)] with same intensity. Bars represent mean ± SD
Quantum yield of energy dissipation (φD0; a), quantum yield for primary photochemistry (φPAV; b), quantum yield of electron transport (φE0; c) and probability that a trapped exciton promote an electron in ETC beyond QA− (Ψ0; d), from the fluorescence transient exhibited by leaves of green (black bars) and purple (grey bars) basil plants grown under different light spectra [blue (B), red (R), white (W) and red and blue (RB)] with same intensity. Bars represent mean ± SD
Analyzing of OJIP data in both varieties using spider plot diagram showed more variation in purple variety in comparison with green variety (Fig. 9a, b). In both varieties, growing plants under R light caused substantial changes in the fate of the absorbed light by PSII. In purple variety, involvement of R spectrum in the incident light (R and R70B30) resulted in substantial change in plant responses to the light, while in the green variety spectral range in only R region of visible light induced different responses of PSII biophysical parameters. PIABS in both varieties represent the most sensitive parameter for detection of photosynthetic performance under different light spectra.
Spider plot of the OJIP-test parameters from the fluorescence transient exhibited by leaves of purple (a) and green (b) basil plants grown under different light spectra [blue (B), red (R), white (W) and red and blue (RB)] with same intensity. The values of the calculated parameters were shown as relative to those of the control plants (W treatment)
Discussion
Growth and morphology of plants were considerably influenced by light spectra and variety in this study. Purple variety of basil plants had more vegetative growth in comparison with the growth parameters of green variety. Furthermore, RB combinational lights caused a better growth in comparison with monochromatic R and B lights. It has been frequently reported that R and B light spectra are the main light wavelengths for plant growth and development (Kozai 2016). The effects of R and B lights are mainly related to the absorption spectra of Chl a and b, which is in the range of wavelengths for R and B lights (Hopkins 1999; Son and Oh 2013). The use of both lights (R and B) together increases the Chl content of the plant (Wu et al. 2007). In accordance, in this study the highest concentrations for Chl pigments were detected in R and B combinational lights (Fig. 3).
It has been shown that R light generally increase plant growth by increasing fresh and dry weights, height and leaf area of the plants (Wang et al. 2009; Johkan et al. 2010; Heo et al. 2012; Son and Oh 2013), while, B light instead of directly affecting the biomass has an effect on photosynthetic functionality, formation of Chl and development of chloroplasts (Wang et al. 2009; Johkan et al. 2010; Savvides et al. 2011; Son and Oh 2013; He et al. 2017). However, in current study, B light resulted in declined growth in both basil varieties. In accordance, it has been shown that B light prevents cell growth, and can regulate expression of the genes that inhibit shoot elongation (Banerjee and Batschauer 2005; Shimazaki et al. 2007; Sabzalian et al. 2014). Furthermore, it has been reported that B light induces changes in development, density, opening and closure of stomata and reduce the synthesis of Chl (Kinoshita et al. 2001), while R light induce Chl synthesis and promote transcription of mRNAs to produce a set of light harvesting complexes in the PSII (Stutte 2009). Combination of R and B lights resulted in accumulation of anthocyanins in different plant species such as tomato (Giliberto et al. 2005) and lettuce plants (Samuolienė et al. 2012) which is in line with the result of current study. However, B light has been also reported as the most effective light spectrum for production of anthocyanin in plants (Crawford 1995). It seems the B light intensity varied among different species to trigger a specific response (Ouzounis et al. 2014).
Environmental conditions have a very important impact on the reactions involved in photosynthetic process of the plants. The photosynthetic reactions are directly effected by various light parameters including its spectrum and intensity (Chen et al. 2004; Zivcak et al. 2014). In our study, Chl fluorescence data were analyzed by the OJIP test. The OJIP test works based on the energy flow across the thylakoid membranes. Using this kinetics, we can investigate the efficiency of various biological phases of the electron transport system (Strasser et al. 2000; Rapacz et al. 2015; Kalaji et al. 2016). Through OJIP test, we can recognize the performance of PSII operating system. In the current study, the OJIP-derived biophysical characteristics confirmed the down-regulation of PSII operating system in green variety especially under R light. This result revealed that growing plants under combinational light spectra can help plant to develop a better photosynthetic apparatus. The high fluorescence intensity in Fo step is indicative of closure of reaction centers in the PSII. According to Zlatev and Yordanov (2004), the increase in Fo is related to damage to the protein D1 of the PSII structure. Fv/Fm decreased under R light and increased in white and blue light. Fv/Fm is related to photochemical quenching and the efficiency of the energy taken by PSII reaction centers. Decrease in Fv/Fm by R light can be related to the reduction of photochemical activity due to the inactivation of the PSII reaction centers and the damage to the D1 protein (Wu 2016). Parameters related to the energy dissipation in PSII such as φDo and DIo/RC were increased by growth of plants under R light (Figs. 7a, 6d). This parameter represents the rate of energy conversion to heat (Falqueto et al. 2017). Conversion of energy into heat is a response from the plant to protect cells against light-induced damage. In contrast to our result, Yang et al. (2018) reported that B light down-regulate the photosynthesis efficiency and increase non-photochemical quenching in tomato plants. However, in agreements with our finding, it has been reported that R light reduces Fv/Fm and increases energy dissipation from the plant (Aliniaeifard et al. 2018). Therefore, a certain unspecified intensities of B light is vital to keep proper functionality of photosynthetic system (Hogewoning et al. 2010). Otherwise occurrence of different abnormalities in photosynthesis and morphology (such as extreme leaf epinasty) would not be surprising (Aliniaeifard et al. 2018). ABS/RC increased under R light but photochemical quenching decreased. Increasing the rate of light absorption in R light may be as a result of inactivation of PSII response centers or an increase in the size of the receptors (Lu and Vonshak 1999; Parvanova et al. 2004). Although the maximum TRo/RC in basil plants was detected under R light, electron transfer beyond QA (ψ0) was restricted in plants exposed to R light, as a consequence down-regulation of efficiency of quantum yield occurred (Aliniaeifard et al. 2018). The ETo/RC is related to TRo/RC and active reaction centers. Increased TRo/RC when ETo/RC is low, indicate inactivity of PSII reaction centers (Ramalho et al. 2002).
In our study, PIABS represented the most sensitive parameters to light spectra (Fig. 8). More sensitivity of PIABS than Fv/Fm is because of this fact that PIABS responds to fluorescence changes between Fo and Fm, while Fv/Fm examines changes in Fo and Fm values (Van Heerden et al. 2007). PIABS is related to the energy fluxes from the early step of absorption process until reduction of plastoquinone (Strasser et al. 2000). It has been also reported that PIABS is a sensitive parameter to different environmental stresses and it has been used to measure photosynthetic and plant performance under abiotic stresses including high temperature (Martinazzo et al. 2012), salinity (Mathur et al. 2013), nutrient deficiency (Kalaji et al. 2014) and submergence (Sarkar and Ray 2016). This parameter represents the function of PSII, a decrease in PIABS rates could be due to suppression of electron transfer as a result of decrease in PSII functionality (Bukhov et al. 1992). In the current study, quantum yield of ETC system was decreased in purple variety. Decreased quantum yield in purple variety can be related to the limitation on electron transfer beyond QA. Presence of high concentration of anthocyanins in the purple variety caused a decline in the efficiency of biophysical parameters while resulted in more light energy dissipations as can be seen in higher φDo and DIo/RC in the leaves of purple varieties. In agreement with these findings, red leaf Coleus varieties which contained high concentrations of anthocyanins have lower quantum yield for the intercepted light, while their carbon fixation process is not different compared to green varieties (Burger and Edwards 1996). Therefore it seems in the current study, although anthocyanins resulted in decrease in quantum yield, but it does not necessarily mean negative impact on gas exchange responses and therefore negative impact on growth parameters.
Conclusion
Management of desirable light environment for basil plants can help to increase yield and quality of this herb. Currently, many research teams all over the world have targeted the issues related to the best light intensity and spectra for plants. In current study, we showed that monochromatic R and B lights are not suitable for growth, pigmentation and biophysical properties of electron transport chain of basil plants. In both purple and green basil varieties, combination of R and B lights (especially 70R30B) caused favorable growth, pigmentation and Chl fluorescence parameters of basil plants. Therefore, new approaches for cultivation of vegetables, medicinal plants and flowers under artificial light systems should consider combination of R and B lights to prevent problems for growth, morphology and photosynthesis during commercial production of plants.
Refrences
Aliniaeifard S, van Meeteren U (2014) Natural variation in stomatal response to closing stimuli among Arabidopsis thaliana accessions after exposure to low VPD as a tool to recognize the mechanism of disturbed stomatal functioning. J Exp Bot 65:6529–6542
Aliniaeifard S, Malcolm Matamoros P, van Meeteren U (2014) Stomatal malfunctioning under low VPD conditions: induced by alterations in stomatal morphology and leaf anatomy or in the ABA signaling. Physiol Plant 152:688–699
Aliniaeifard S, Hajilou J, Tabatabaei SJ (2016) Photosynthetic and growth responses of olive to proline and salicylic acid under salinity condition. Not Bot Horti Agrobot Cluj Napoca 44:579–585
Aliniaeifard S, Seif M, Arab M, Zare Mehrjerdi M, Li T, Lastochkina O (2018) Growth and photosynthetic performance of Calendula officinalis under monochromatic red light. Int J Hortic Sci Technol 5:123–132
Banerjee R, Batschauer A (2005) Plant blue-light receptors. Planta 220:498–502
Bukhov N, Drozdova I, Bondar V, Mokronosov A (1992) Blue, red and blue plus red light control of chlorophyll content and CO2 gas exchange in barley leaves: quantitative description of the effects of light quality and fluence rate. Physiol Plant 85:632–638
Burger J, Edwards GE (1996) Photosynthetic efficiency, and photodamage by UV and visible radiation, in red versus green leaf coleus varieties. Plant Cell Physiol 37:395–399
Carvalho SD, Schwieterman ML, Abrahan CE, Colquhoun TA, Folta KM (2016) Light quality dependent changes in morphology, antioxidant capacity, and volatile production in sweet basil (Ocimum basilicum). Front Plant Sci 7:1328
Chang X, Alderson PG, Wright CJ (2009) Enhanced UV-B radiation alters basil (Ocimum basilicum L.) growth and stimulates the synthesis of volatile oils. J Hortic For 1:27–31
Chen M, Chory J, Fankhauser C (2004) Light signal transduction in higher plants. Annu Rev Genet 38:87–117
Colquhoun TA, Schwieterman ML, Gilbert JL, Jaworski EA, Langer KM, Jones CR, Rushing GV, Hunter TM, Olmstead J, Clark DG (2013) Light modulation of volatile organic compounds from petunia flowers and select fruits. Postharvest Biol Technol 86:37–44
Cosgrove DJ (1981) Rapid suppression of growth by blue light occurrence, time course, and general characteristics. Plant Physiol 67:584–590
Crawford NM (1995) Nitrate: nutrient and signal for plant growth. Plant Cell 7:859
Falqueto AR, da Silva Júnior RA, Gomes MTG, Martins JPR, Silva DM, Partelli FL (2017) Effects of drought stress on chlorophyll a fluorescence in two rubber tree clones. Sci Hortic 224:238–243
Folta KM, Carvalho SD (2015) Photoreceptors and control of horticultural plant traits. HortSci 50:1274–1280
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta General Subj 990:87–92
Giliberto L, Perrotta G, Pallara P, Weller JL, Fraser PD, Bramley PM, Fiore A, Tavazza M, Giuliano G (2005) Manipulation of the blue light photoreceptor cryptochrome 2 in tomato affects vegetative development, flowering time, and fruit antioxidant content. Plant Physiol 137:199–208
He J, Qin L, Chong EL, Choong T-W, Lee SK (2017) Plant growth and photosynthetic characteristics of Mesembryanthemum crystallinum grown aeroponically under different blue-and red-LEDs. Front Plant Sci 8:361
Heo J, Lee C, Chakrabarty D, Paek K (2002) Growth responses of marigold and salvia bedding plants as affected by monochromic or mixture radiation provided by a light-emitting diode (LED). Plant Growth Regul 38:225–230
Heo JW, Kang DH, Bang HS, Hong SG, Chun CH, Kang KK (2012) Early growth, pigmentation, protein content, and phenylalanine ammonia-lyase activity of red curled lettuces grown under different lighting conditions. Korean J HorticSci Technol 30:6–12
Hogewoning SW, Trouwborst G, Maljaars H, Poorter H, van Ieperen W, Harbinson J (2010) Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J Exp Bot 61:3107–3117
Holm Y, Hiltunen R (1999) Basil: the genus Ocimum. Harwood Academic, Amsterdam
Hopkins WG (1999) Introduction to plant physiology. Wiley, Hoboken
Hussain AI, Anwar F, Sherazi STH, Przybylski R (2008) Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem 108:986–995
Johkan M, Shoji K, Goto F, Sn Hashida, Yoshihara T (2010) Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortSci 45:1809–1814
Jordan P, Fromme P, WittH T, Klukas O, Saenger W, Krauß N (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature 411:909–917
Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska IA, Cetner MD, Łukasik I, Goltsev V, Ladle RJ (2016) Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant 38:102
Kalaji MH, Goltsev VN, Zivcak M, Brestic M (2017) Chlorophyll fluorescence: understanding crop performance—basics and applications. CRC Press, Boca Raton
Kalaji HM, Oukarroum A, Alexandrov V, Kouzmanova M, Brestic M, Zivcak M, Samborska IA, Cetner MD, Allakhverdiev SI, Goltsev V (2014) Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol Biochem 81:16–25
Kalhor M, Aliniaeifard S, Seif M, Asayesh E, Bernard F, Hassani B, Li T (2018) Enhanced salt tolerance and photosynthetic performance: implication of γ-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol Biochem 130:157–172
Kasajima S, Inoue N, Mahmud R, Kato M (2008) Developmental responses of wheat cv. Norin 61 to fluence rate of green light. Plant Product Sci 11:76–81
Kim SJ, Hahn EJ, Heo JW, Paek KY (2004) Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci Hortic 101:143–151
Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki KI (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414:656–660
Kozai T (2016) LED lighting for urban agriculture. In: Kozai T (ed) LED lighting for urban agriculture. Springer, Singapore
Li Q, Kubota C (2009) Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ Exp Bot 67:59–64
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Portland Press Limited, London
Lin KH, Huang MY, Huang WD, Hsu MH, Yang ZW, Yang CM (2013) The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci Hortic 150:86–91
Lu C, Vonshak A (1999) Photoinhibition in outdoor Spirulina platensis cultures assessed by polyphasic chlorophyll fluorescence transients. J Appl Phycol 11:355
Martinazzo EG, Ramm A, Bacarin MA (2012) The chlorophyll a fluorescence as an indicator of the temperature stress in the leaves of Prunus persica. Braz J Plant Physiol 24:237–246
Mathur S, Mehta P, Jajoo A (2013) Effects of dual stress (high salt and high temperature) on the photochemical efficiency of wheat leaves (Triticum aestivum). Physiol Mol Biol Plants 19: 179–188
Miao Y, Wang X, Gao L, Chen Q-y QuM (2016) Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J Integr Agric 15:87–100
Mita S, Murano N, Akaike M, Nakamura K (1997) Mutants of Arabidopsis thaliana with pleiotropic effects on the expression of the gene for β-amylase and on the accumulation of anthocyanin that are inducible by sugars. Plant J 11:841–851
Neff MM, Chory J (1998) Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol 118:27–35
Ouzounis T, Fretté X, Rosenqvist E, Ottosen C-O (2014) Spectral effects of supplementary lighting on the secondary metabolites in roses, chrysanthemums, and campanulas. J Plant Physiol 171:1491–1499
Parvanova D, Popova A, Zaharieva I, Lambrev P, Konstantinova T, Taneva S, Atanassov A, Goltsev V, Djilianov D (2004) Low temperature tolerance of tobacco plants transformed to accumulate proline, fructans, or glycine betaine. Variable chlorophyll fluorescence evidence. Photosynthetica 42:179–185
Ramalho J, Marques N, Semedo J, Matos M, Quartin V (2002) Photosynthetic performance and pigment composition of leaves from two tropical species is determined by light quality. Plant Biol 4:112–120
Rapacz M, Sasal M, Kalaji HM, Kościelniak J (2015) Is the OJIP test a reliable indicator of winter hardiness and freezing tolerance of common wheat and triticale under variable winter environments. PLoS ONE 10:0134820
Sabzalian MR, Heydarizadeh P, Zahedi M, Boroomand A, Agharokh M, Sahba MR, Schoefs B (2014) High performance of vegetables, flowers, and medicinal plants in a red-blue LED incubator for indoor plant production. Agron Sustain Dev 34:879–886
Samuolienė G, Sirtautas R, Brazaitytė A, Duchovskis P (2012) LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem 134:1494–1499
Sarkar R, Ray A (2016) Submergence-tolerant rice withstands complete submergence even in saline water: probing through chlorophyll a fluorescence induction OJIP transients. Photosynthetica 54:275–287
Savvides A, Fanourakis D, van Ieperen W (2011) Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J Exp Bot 63:1135–1143
Shimazaki K, Doi M, Assmann SM, Kinoshita T (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58:219–247
Son KH, Oh MM (2013) Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortSci 48:988–995
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. Probing photosynthesis: mechanisms, regulation and adaptation, Martinus Nijhoff Publisher, Dordrecht, Netherlands, pp 445–483
Stutte GW (2009) Light-emitting diodes for manipulating the phytochrome apparatus. HortSci 44:231–234
Taiz L, Zeiger E (2002) Plant physiology. Sinauer Associates, Sunderland
Van Heerden P, Swanepoel J, Krüger G (2007) Modulation of photosynthesis by drought in two desert scrub species exhibiting C 3-mode CO 2 assimilation. Environ Exp Bot 61:124–136
Wang Y, Folta KM (2013) Contributions of green light to plant growth and development. Am J Bot 100:70–78
Wang H, Gu M, Cui J, Shi K, Zhou Y, Yu J (2009) Effects of light quality on CO 2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J Photochem Photobiol B Biol 96:30–37
Wang X, Xu X, Cui J (2015) The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 53:213–222
Wu H (2016) Effect of different light qualities on growth, pigment content, chlorophyll fluorescence, and antioxidant enzyme activity in the red alga Pyropia haitanensis (Bangiales, Rhodophyta). BioMed Res Int 2016:Article ID 7383918. https://doi.org/10.1155/2016/7383918
Wu MC, Hou CY, Jiang CM, Wang YT, Wang CY, Chen HH, Chang HM (2007) A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem 101:1753–1758
Yang X, Xu H, Shao L, Li T, Wang Y, Wang R (2018) Response of photosynthetic capacity of tomato leaves to different LED light wavelength. Environ Exp Bot 150:161–171
Zivcak M, Kalaji HM, Shao H-B, Olsovska K, Brestic M (2014) Photosynthetic proton and electron transport in wheat leaves under prolonged moderate drought stress. J Photochem Photobiol B 137:107–115
Zlatev ZS, Yordanov IT (2004) Effects of soil drought on photosynthesis and chlorophyll fluorescence in bean plants. Bulg J Plant Physiol 30:3–18
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hosseini, A., Zare Mehrjerdi, M., Aliniaeifard, S. et al. Photosynthetic and growth responses of green and purple basil plants under different spectral compositions. Physiol Mol Biol Plants 25, 741–752 (2019). https://doi.org/10.1007/s12298-019-00647-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-019-00647-7