Abstract
The rising global population and worldwide industrialization have led to unprecedented energy demand that is causing fast depletion of fossil reserves. This has led to search for alternative energy sources that are renewable and environment friendly. Use of lignocellulosic biomass for energy generation is considered a promising approach as it does not compete with food supply. However, the lignin component of the biomass acts as a natural barrier that prevents its efficient utilization. In order to remove the lignin and increase the amount of fermentable sugars, the lignocellulosic biomass is pretreated using physical and chemical methods which are costly and hazardous for environment. Moreover, during the traditional pretreatment process, numerous inhibitory compounds are generated that adversely affect the growth of fermentative microbes. Alternatively, biological methods that use microbes and their enzymes disrupt lignin polymers and increase the accessibility of the carbohydrates for the sugar generation. Microbial laccases have been considered as an efficient biocatalyst for delignification and detoxification offering a green initiative for energy generation process. The present review aims to bring together recent studies in bioenergy generation using laccase biocatalyst in the pretreatment processes. The work provides an overview of the sustainable and eco-friendly approach of biological delignification and detoxification through whole-cell and enzymatic methods, use of laccase-mediator system, and immobilized laccases for this purpose. It also summarizes the advantages, associated challenges, and potential prospects to overcome the limitations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lignocellulosic biomass generated through photosynthesis is considered a valuable renewable source of organic carbon that can be utilized for energy production. Lignocellulose biomass consists mainly of cellulose (20–50%), hemicellulose (15–35%), and lignin (18–35%) along with pectin, ash, minerals, and salts (Wang et al. 2017; Kumar and Chandra 2020).Cellulose and hemicellulose are considered the sugar component in biomass, while lignin is a non-sugar component, and it is the second abundant amorphous biopolymer besides cellulose. Lignin is composed of phenylpropane units of p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S), originating from three hydroxycinnamyl alcohols, namely p-coumaryl, coniferyl, and sinapyl alcohols (Li et al. 2015; Wang et al. 2017). However, the composition of lignin varies with different biomass and environmental conditions. Agricultural by-products, perennial grasses, agro-industrial by-products, wood, and vegetable residues are examples of lignocellulosic feedstock. These carbon-rich waste biomasses can be effectively utilized for biofuel production without affecting the food supplies. The microbes utilize cellulosic and hemicellulosic components of biomass as raw material for the energy generation. The release of sugars and its use in microbial fermentation for the generation of biofuel and bio-based chemicals depend on the access of cellulose from lignocellulosic biomass. The major impediment in utilizing lignocellulosic biomass is the presence of lignin that acts as a barrier, restricting chemical or enzymatic digestion of cellulose and hemicelluloses (Ying et al. 2018). Moreover, lignin unspecifically adsorbs hydrolytic enzymes, decreasing the enzyme concentration in reaction (Fillat et al. 2017). Hence, the removal of lignin is essential for the complete utilization of cellulose for further valorization (Kalyani et al. 2015; Koklukaya et al. 2016). Although several methods have been developed to remove lignin from lignocellulosic biomass, they suffer from challenges arising from the use of solvent, high energy expenditure, and generation of toxic inhibitors (Sindhu et al. 2016). With the rising concern for environment, there has been mounting interest in the search of biocatalyst that can play an important role in carrying out the catalytic transformation of lignin with the advantages of high substrate specificity, selectivity, and efficiency (Naghdi et al. 2018). Additionally, biocatalytic processes are environment-friendly, cost-effective, and sustainable (Tu and Hallett 2019). The biological methods utilize microorganisms and their enzymes for the removal of lignin from the biomass. These enzymes are called ligninolytic enzymes. The major ligninolytic enzymes are lignin peroxidase (LiP, EC 1.11.1.14), manganese peroxidase (MnP, EC 1.11.1.13), versatile peroxidase EC 1.11.1.16), and dye decolorizing peroxidase (DyP, EC 1.11.1.19) (Vrsanska et al. 2015; Datta et al. 2017). Lignin peroxidase has the ability to catalyze the oxidation of phenolic and aromatic compounds of high redox potential up to 1.2V (Datta et al. 2017). Manganese peroxidase has specificity similar to lignin peroxidases and also oxidizes Mn2+ to form diffusible oxidants (Mn3+) that are capable of penetrating the cell wall matrix and oxidize the phenolic substrates (Cragg et al. 2015; Andlar et al. 2018). The versatile peroxidase is considered a hybrid between MnP and LiP capable of oxidizing Mn2+ and veratryl alcohol and phenolic aromatic compounds in manganese-independent reactions (Andlar et al. 2018). DyP has ability to oxidize high redox potential dyes such as anthraquinone and active at lower pH than the other peroxides. It has shown to oxidize phenolic and non-phenolic β-O-4 dimeric lignin model compound at low pH, indicating its lignin-degrading activity. All these ligninolytic enzymes require H2O2 as a co-factor to start the action. In addition to the above, there are some lignin-degrading auxiliary (LDA) enzymes like Aryl-alcohol oxidase (EC 1.1.3.7), quinone reductases (EC 1.6.5.5), Glyoxal oxidase (EC 1.2.3.5), and Glucose dehydrogenase (EC 1.1.99.10) produced by fungi (Pollegioni et al. 2015). These enzymes are unable to degrade lignin on their own; however, they support the degradation process by providing the H2O2 to another peroxidase. Apart from these enzymes, there is another ligninolytic enzyme known as laccase that has a proven role in lignin degradation/modification and is independent of H2O2.
Laccases (p-diphenoloxidase, EC 1.10.3.2) are multi-copper-containing enzymes that belong to the polyphenol oxidase group. They catalyze a range of organic and inorganic substrates, including mono-, di- and polyphenols, amino phenols, methoxy phenols, aromatic amines, diamines, and ascorbate, and require molecular oxygen as a co-factor instead of H2O2. Laccases are widespread in nature and can be obtained from various sources like plants, bacteria, and fungi. Plants laccases are monomeric proteins produced extracellularly that mainly participate in plant lignin biosynthesis and are readily found in xylem tissues (Wang et al. 2015). Apart from lignin synthesis, plant laccases play an essential role in wound healing and defense against external conditions (Wang et al. 2019). Fungal laccases are both extracellular and intracellular and play various roles, including morphogenesis, plant pathogen (host) interaction, protection against stress, and degradation of lignin (Forootanfar and Faramarzi 2015). Fungal species like ascomycetes, deuteromycetes, and basidiomycetes are known as laccase producers (Rodríguez-Couto 2018). Most of the laccases reported so far are of fungal origin, especially from white-rot fungi that produce high percentages of fungal laccase as extracellular enzymes such as T. versicolor, Trametes hirsute, Coriolus versicolor, Trametes pubescens, Abortiporus biennis, Cerrena unicolor, and Phlebia radiate (Forootanfar and Faramarzi 2015; Yang et al. 2017a). Very limited bacterial laccases have been reported so far, although these are more stable at high pH and temperatures (Chandra and Chowdhary 2015; Forootanfar and Faramarzi 2015). The presence of laccase has also been reported in insects and crustacean (Cannatelli and Ragauskas 2017; Asano et al. 2019; Chukwuma et al. 2020). Laccases have been used in a variety of industrial and biotechnological processes as biological catalysts due to their broad substrate specificity. Industries such as pulp and paper textile, cosmetics, fuel, and food have been using laccases for pulping, deinking, dyeing/finishing, pigmentation, and as a flavor agent (Agrawal et al. 2018; Rodríguez-Couto 2019). Laccases have also find application in the field of nanotechnology such as use in biosensors (Mate and Alcalde 2017). Applications of laccases in the above-mentioned area have been discussed in the published review articles (Prasad 2016; Mate and Alcalde 2017; Agrawal et al. 2018).
Use of laccases in the field of biomass utilization and sustainable energy generation has gained momentum in recent past. This review focuses on the laccase structure, mechanism, and potential in energy generation through biocatalytic delignification of biomass and detoxification of pretreated biomass hydrolysate for enhancement of fermentation yield.
Laccase structure and mechanism of action
Laccases are a multi-copper-containing enzyme, i.e., its active site usually contains four copper atoms, which are classified into three types type I (T1), type II (T2), and type III (T3) based on spectroscopic and electronic paramagnetic resonance spectra (Fig. 1). Type 1(T1) copper center has an intense absorption at 600–610 nm, which is caused by the covalent copper-cysteine bond and is responsible for the typical blue color. Due to its high redox potential, T1 copper is the site of substrate oxidation. It is connected trigonally with two histidine and two sulfur, one of methionine and the other of cysteine in bacterial laccases; however, fungal laccases have leucine and phenylalanine (Agrawal et al. 2018). The type II (T2) copper center is the mononuclear center coordinated by two histidine residues. The T2 copper is spectrophotometrically undetectable and has no visible absorption spectrum; however, it generates a characteristic electron paramagnetic resonance (EPR) signal. Type III (T3) is a diamagnetic coupled binuclear copper center, i.e., it has two copper ions coordinated by six histidine residues. The two copper ions are anti-ferromagnetically coupled by a hydroxyl bridge, which is the reason for its diamagnetic properties. Because of diamagnetic property, this center is not detected by an electron paramagnetic spectrum, but its characterization can be done spectrophotometrically. It exhibits a characteristic absorbance at 330 nm and displays a characteristic spectrum (Agrawal et al. 2018).
Laccases catalyze the oxidation of substrates via two different reaction mechanisms, i.e., direct and indirect as shown in Fig. 2. In the direct mechanism of oxidation, the substrate interacts with the copper center of laccase resulting in radical formation (Agrawal et al. 2018). The direct oxidation mechanism occurs only when the substrate’s ionization potential is inferior to the redox potential of the T1 copper ion of laccases. The phenolic fragments have lower ionization potential than that of the redox potential of T1 copper ion in laccase; hence, laccases are observed to attack the phenolic compounds forming phenoxy radicals, which are further rearranged by Cα-oxidation, cleavage of Cα-Cβ bonds, benzyl hydroxyl oxidation, and aryl-alkyl bond cleavage (Longe et al. 2018).
However, direct oxidation is not feasible when substrates have an ionization potential higher than the redox potential of the T1 copper ion (Agrawal et al. 2018). Substrates such as non-phenolic compounds having higher ionization potential than the redox potential of T1 copper cannot be oxidized by laccase alone and require low molecular weight compounds called mediators to accelerate the catalytic activity. The mechanism involved in the process is indirect oxidation, which is carried out in two steps, as shown in Fig. 2b. In the first step, the laccase carries out the oxidation of the mediator. In the next step, the oxidized mediator leads to the oxidation of substrate by itself, getting reduced to its initial form (Agrawal et al. 2018). After getting oxidized by laccase, a mediator can non-enzymatically oxidize the non-phenolic compounds (Chaurasia et al. 2013; Komal et al. 2018).
Potential of laccases in energy generation
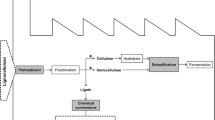
Laccases are considered as a powerful tool in the utilization of lignocellulosic biomass for production of biofuels. Deconstruction of lignocellulose to sugars includes two major steps: pretreatment and enzymatic hydrolysis. The pretreatment process plays a crucial role in assisting the enzymatic hydrolysis of lignocellulosic biomass by removal of hemicellulose, lignin, and reduction in the degree of polymerization and crystallinity of cellulose, by increasing the porosity of pretreated materials (De La Torre et al. 2017). The enzymatic hydrolysis processes break down the cellulosic polymers into fermentable sugars. Furthermore, microorganisms utilize the fermentable sugars and produce valuable products such as alcohols, lipids, organic acids, and other platform chemicals. This removal of lignin during pretreatment is termed delignification, enhancing the carbohydrate’s accessibility for the subsequent saccharification step. The extent of delignification depends on the laccase’s redox potential and the lignin composition, which varies in different biomass. Additionally, lignocellulose pretreatment process often involves side reactions that result in release of products that act as inhibitors of downstream biochemical processes (Suman et al. 2018a). These mainly include aromatic compounds like extractives such as terpenes, fats, waxes, phenolics, and inorganic compounds that inhibit the successive enzymatic steps (De La Torre et al. 2017). Removal of these inhibitors from the pretreated biomass is termed as detoxification that results in enhanced saccharification and conversion yields. Biological methods of delignification and detoxification involve the use of microorganisms and/or their enzymes. Laccases can work as a delignifying and detoxifying agent and thus improve the saccharification and fermentation of pretreated lignocellulosic biomass.
Role of laccases in delignification
Laccase-mediated delignification is an effective and eco-friendly method that can be used to remove lignin and improve the accessibility of cellulose and thereafter enhancement of fermentable sugar production from lignocellulosic biomass. While physical methods such as steam explosion, liquid hot water, ammonia fiber explosion/expansion, wet oxidation, and thermal pretreatment require large infrastructure, chemical methods such as use of alkali- and acid-based pretreatments are fast and more effective but have a negative impact on environment (Karimi and Taherzadeh 2016). Use of organic solvents and ionic liquids has also been reported for this purpose but suffers from high-cost limitations. Use of laccases as a biocatalyst offers advantages of low-energy requirements, milder reaction conditions, fewer side-reactions, and environmentally safe (Rastogi and Shrivastava 2017). Diverse methods of delignification and the advantages of laccase-based delignification over other methods are presented in Fig. 3.
Delignification has been studied in two different ways. If a microorganism is used directly for the purpose of delignification, it is termed microbial delignification. Alternatively, if the enzyme is directly used for lignin removal, it is called enzymatic delignification.
Microbial (whole-cell) delignification
In microbial delignification, microbes are allowed to grow on lignocellulosic biomass in solid state fermentation process. This increases the porosity of the biomass and significantly improves the subsequent hydrolysis. Various bacterial and fungal strains have been utilized for delignificanation. Bacterial strains such as Streptomyces cyaneus, Acinetobacter, Thermomonospora mesophila, Pseudomonas sp., Streptomyces coelicolor, Streptomyces lividans, and Streptomyces viridosporus have shown lignin degradation ability (Majumdar et al. 2014; Guo et al. 2019; Tsegaye et al. 2019). In one study, Lysinibacillus sps. isolated from termite gut was used for the degradation of the lignin component from oil palm residues. The residues were biotreated for 7days, and the result was compared with untreated control. The maximum laccase activity of 405.4 U/L was recorded on the 5th day of the treatment. The percentage loss in lignin was highest in empty fruit bunch (6.6%), followed by oil palm trunk 5.7% and oil palm leaf by 4.5%.The removal of lignin was also supported by structural changes in the cell wall of the residues after SEM images analysis (Ayeronfe et al. 2019). Laccase secreted by Pseudomonas sp. AS1 during a 5-day culture was able to remove up to 50.1% lignin of Miscanthus in presence of ABTS as a mediator resulting in a 2.2-fold glucose yield in comparison of the untreated biomass (Guo et al. 2019). Wheat straw treated with Ochrobactrum oryzae bacteria strain was able to degrade 44.47% lignin after 16 days of biotreatment while increasing the cellulose content by 22.38% and hemicellulose content by 18.64% (Tsegaye et al. 2019). Scanning electron microscope, mid-infrared analysis by Fourier transform infrared spectroscopy (FTIR), and X-ray diffraction analysis confirmed the change in composition due to removal of lignin (Tsegaye et al. 2018). In comparison to bacterial laccases, fungal laccases have superior lignin degradation capabilities. Three groups of fungi, namely soft-rot, brown-rot, and white-rot, are majorly involved in biomass degradation by different mechanisms. The soft-rot fungi belonging to ascomycetes preferably degrade polysaccharide part of the biomass. The presence of ligninolytic enzymes in few ascomycetes has been observed and reported for degradation of a range of phenolic and lignin related compounds; however, lignin degradation in wood has not been confirmed (Haider and Trojanowski 1975; Bugos et al. 1988). Thermophilic ascomycetes, Talaromycetes termophilus, and Thielavia terrestris have also been reported as weak ligninolytic enzyme producer but their true effect on delignification is limited (Daniel 2016). Brown-rot fungi are basidiomycetes that rapidly attack cellulose and hemicellulose compared to lignin. They use a non-enzymatic mechanism of depolymerization of lignin to allow access of the cellulosic components (Goodell 2003). The most widely used fungi are white-rot fungi, which are able to decompose all three major constituents (lignin, cellulose, and hemicellulose) of lignocellulose. However, they preferentially attack lignin in biomass and leave behind the cellulose and hemicellulose, although some of them break down both lignin and cellulose. Degradation of lignin by white rot fungi is more efficient than the brown-rot and soft-rot fungi because they possess ligninolytic extracellular oxidative enzymes and have a unique ability to complete mineralization to CO2. White-rot fungus Trametes versicolor, Pleurotus ostreatus, Cyathus stercoreus, Ceriporiopsis subvermispora, I. lacteus, P. chrysosporium, Pycnoporu scinnabarinus, Gloeophyllum trabeum, Cadophora spp. etc. have been widely explored for microbial delignification. Rice straw treated with Trichoderma viridae was able to reduce the lignin content by 56% (Ghorbani et al. 2015). In a comparative study of wheat straw delignification by different laccase-producing fungus, 30.6% delignification was observed with Phlebia brevispora while Trametes trogii resulted in 21.9% delignification (Tian et al. 2018). Although fungus has good delignification capabilities, they usually grow slowly and require a long time to produce ligninolytic enzymes, thereby increasing the pretreatment time and also results in loss of cellulosic components during long pretreatments (Madadi and Abbas 2017). Hence, co-culture of ligninolytic and cellulolytic fungi has been studied to minimize the loss of sugars and reduce the pretreatment time. Co-culturing of Coprinus comatus and Trichoderma reesei was performed for simultaneous delignification and enhanced saccharification of corn stover. Up to 66.5% of delignification and 82% of saccharification yield were observed by simultaneous biodelignification and saccharification process. A 2.6-fold increase in the laccase activity over the monoculture was observed due to synergistic interaction between two fungi (Ma and Ruan 2015). Rice straw was treated with fungal strains Pleurotus ostreatus and Trichoderma reesei to improve the biodegradability of the biomass. Pretreatment with Pleurotus ostreatus resulted in 33.4% lignin removal in 20 days of incubation with selective removal of lignin. The selectivity ratio of lignin/cellulose removal was found to 4.2. Moreover, in case of Trichoderma reesei, 23.6% lignin removal was achieved with a lignin/cellulose removal ratio of 2.88 (Mustafa et al. 2016). However, there is possibility that co-culturing method may lead to the unfavorable interaction. This can occur due to the different optimum conditions for biodelignification and enzymatic saccharification that adversely affect the overall efficiency of the simultaneous process. Combination of physicochemical processes along with biological treatment has also been studied. The steam exploded wheat straw was treated with T. versicolor for 40 days, and it was found that 55.4% of lignin was degraded in T. versicolor-treated wheat straw while only 20% of lignin reduction was obtained in the only steam-exploded treatment (Nadir et al. 2019). Microbial delignification, combined with mild alkali extraction, has been used for lignin content removal from agro-waste. Treatment of wheat straw with two different fungal strains C. subvermispora and I. lacteus for 21 days resulted in lowering of lignin content by 30% and 34% respectively (Nadir et al. 2019). Besides fungal strain and treatment method, incubation time is also key factor in microbial delignification and has therefore been considered for enhancement of delignification. Notably, the incubation time for efficient delignification is strain-dependent and can vary from days to weeks. In a study with P. anustigrinus, wheat straw delignification was increased from 17 to 47% when the incubation time changed from 1 to 3 weeks. After 2 weeks of treatment, Phanerochaete chrysosporium removed 28% of lignin from cotton stalks, and the removal of lignin from cotton stalks was further increased to 60% when treatment time increased to 30 days (Shi et al. 2008). In another study by Bari et al. (2016), Trametes versicolor was used to treat beech wood; the result showed the 57.4% of lignin was removed within 120 days of pretreatment (Bari et al. 2016). In addition to white-rot basidiomycetes, certain endophytic fungi have been recently reported for the production of laccases. These fungi have advantage over the saprophytes; they inhabit asymptomatically in plant tissues, living in association with their host plants. Once the plant dies, these endophytes change from an inactive state to active and are involved in the decomposition of plant tissues (Martín-Sampedro et al. 2015). The endophytic fungi isolated from the eucalyptus trees were assessed for the pretreatment of Eucalyptus globulus wood. Biomass pretreated with endophytic fungi Ulocladium sp. and Hormonema sp. exhibited enhanced saccharification up to 2.7-fold compared to non-pretreated wood. Furthermore, in combined pretreatment process (fungal and autohydrolysis), synergistic effect was observed with increased sugar yields demonstrating the high potential of these new endophytic fungi for saccharification enhancement (Martín-Sampedro et al. 2015). In the other study, endophytic fungi used for biological pretreatment of olive tree pruning biomass increased glucose digestibility from 4.9 to 12% compared to the control (Martín-Sampedro et al. 2017). Although the microbial delignification process occurs under mild conditions, the drawbacks associated with them are longer processing time, space for large-scale microbial growth, and continuous monitoring of microbes (Sindhu et al. 2016). These problems can be overcome up to a certain extent by optimizing influential factors (Ghorbani et al. 2015). Separation of fungi from the system after the delignification is also a challenge. Immobilization is a frequently reported method for separating the microbial cell/enzyme from the system and is widely used in industrial applications. However, very limited work has been reported for delignification using an immobilized cell system. Immobilization through entrapment consists of a porous polymeric matrix enclosing the microbial cells but allows diffusion of substrates and products. Karimi et al. (2017) has demonstrated delignification of rice straw using immobilized Trichoderma viride species with lignin removal efficiency of up to 71% (Karimi et al. 2017). Overall, microbial delignification is an energy-saving approach that can enhance the yield of fermentable sugars. Novel laccase-producing strains with fast growth and high delignification capabilities can help to overcome the current limitations of this method.
Some recent delignification studies using laccase-producing whole-cell biocatalysts are presented in Table 1.
Enzymatic (laccase) delignification
Direct use of laccase is another practical strategy for the delignification of lignocellulosic biomass. It reduces the processing time from weeks to hours without consuming the carbohydrate, which is a possible drawback of microbial delignification. Wide optimal pH and temperature range and non-requirement of additional nutrient supplementation are other advantages of enzymatic delignification over microbial delignification (Mukhopadhyay et al. 2011). Both crude culture supernatant and the purified enzyme have been used for ligninolytic activity (Lu et al. 2010; Moilanen et al. 2011). The delignification studies have been performed with different ratios of the solid and liquid with varying concentrations of the laccase (Mukhopadhyay et al. 2011; Deng et al. 2019). Laccases can be used individually or in combination with specific molecules called mediators for the delignification purpose as described below:
Only laccase-based delignification
The direct use of laccases for delignification is limited to the phenolic unit, which contributes a small percentage of the total polymer. Several studies have shown the delignifying ability of laccase in different pretreated materials and improved enzymatic hydrolysis. Fungal laccases have been most commonly used enzymes for delignification, although the use of bacterial laccases has also been reported. Laccase from Pleurotus sps. was used for the delignification of grass species Saacharum spontaneum, and a maximum delignification of 84.67% was obtained after 6 h of incubation (Rajak and Banerjee 2015). The same biomass, when treated with laccase from Lentinus squarrosulus, displayed 81.67% delignification, along with a 7.03-fold increase in sugar production (Rajak and Banerjee 2016). It has been reported that treatment of biomass with laccase and subsequent extraction with alkaline peroxidase helps in removal of the partially degraded lignin by laccase and increases the released glucose concentration. Rencoret, J. carried out the delignification of wheat straw with Pycnoporus cinnabarinus laccase followed by an alkaline peroxide extraction, and 18% of lignin loss was recorded (Rencoret et al. 2016). In a similar study, the efficacy of laccase from M. thermophila and P. Cinnabarinus was compared for the delignification of milled eucalyptus wood followed by an alkaline peroxide extraction. The result showed M. thermophila laccase decreased the lignin content by 20%; however, no lignin reduction was observed in P. cinnabarinus laccase (Rico et al. 2015). Pleurotus djamor laccase 430.3 IU/mL was used for the delignification of sugarcane tops and resulted in 79.1% delignification and improved reducing sugar yield (Sherpa et al. 2018). Biomass of sweet sorghum stover treated with laccase from the fungus Trichoderma asperellum could achieve 76.93% delignification, and the delignified biomass was used for the biohydrogen production resulting in 3.26-fold improved biohydrogen yield (Shanmugam et al. 2018). Apart from the lignin removal, structural changes in microfiber by laccase-treated biomass have been reported. Properties of microfibers such as surface area, porosity, and hydrophobicity had been altered resulting in reduction in the unproductive binding of hydrolases (Banerjee et al. 2019). Delignification of multiple feedstocks was attempted with laccase from Pleurotus djamor, showed 80% delignification and 587 mg/g of reducing sugar yield. The presence of various lignin-derived compounds such as carboxylic acids, phenolic acids, and N-heterocyclic in GC–MS analysis confirmed the delignification process (Avanthi and Banerjee 2016). Laccase from a similar source has been used for the pretreatment of pineapple leaf, resulted in 78.57% delignification of biomass and released of 492.33 ± 3.1 mg/g of sugar from the biomass (Banerjee et al. 2019). Delignification of Aloe vera leaf rind white-rot fungal laccase resulted in 76.67% delignification, causing a 2-fold increase in the saccharification compared to untreated biomass (Rajeswari and Jacob 2020). Lignin removal from ginseng residues performed using laccase from Aspergillus sp. displayed 40.11 % of lignin removal while 59.89% of sugar including 12.04% water soluble polysaccharides, 16.24% water-insoluble polysaccharides and 5.08% reducing sugar was obtained from delignified substance (Zhang et al. 2020). Contrarily, loss in saccharification yields has been reported when pretreatment and saccharification were performed simultaneously due to reduction in cellulase and β-glucosidase activity. This is attributed to the coupling reactions between phenoxyl radicals generated during the laccase pretreatment and saccharification enzymes (Oliva-Taravilla et al. 2015). Laccase has also been used to remove lignin from agro-food wastes such as apple pomace, potato peels, and coffee silverskin. Pleurotus ostreatus laccase pretreatment of agro-waste was able to remove the lignin content up to 50% of the initial lignin present (Giacobbe et al. 2018).
Bacterial laccases are less explored when compared to their fungal counterparts. Delignification of woody biomass using small bacterial laccase (sLac) from Amycolatopsis sp. enhanced the acid-precipitable polymeric lignin by ~6-fold (Singh et al. 2017). Use of halophilic bacterial laccase for the delignification displayed up to 39.3% delignification of sugar beet pulp and up to 45% delignification of a peanut shell. Moreover, in the presence of ionic liquid, the delignification was enhanced to 78.4%.and 87%, respectively (Ahmed et al 2018). Delignification of almond shell waste using bacterial laccase from Chromohalobacter salexigens resulted in the reduction of kappa number of the almond shell lignin to 27%, and delignification efficiency was raised up to 58% (Jafari et al. 2017). The potential of bacterial laccase from Streptomyces ipomoeae for delignification of steam-exploded wheat straw was tested, and reduction in lignin content was detected. Furthermore, the delignified biomass was saccharified and resulted in both glucose and xylose production by 16 and 6%, respectively (De La Torre et al. 2017). In another study of sugar beet pulp delignification, a halophilic bacterium laccase was tested with varying laccase concentration, and a maximum delignification of 39.3% was observed after 12 h of treatment. The same laccase was tested in the presence of ionic liquid for the delignification of sugar beet pulp, and up to 78.4%, delignification was observed, showing that the enzyme activity improved using ionic liquid (Rezaei et al. 2017). In addition to these, commercial bacterial laccases have been used satisfactorily for delignification and detoxification of steam-exploded biomass in bioethanol production. Commercial bacterial laccase (MetZyme) has been reported for enhancing the hydrolysability and fermentability of steam-exploded wheat straw. It was observed that the laccase treatment of steam-exploded wheat straw in combination with mild alkali increased the glucose concentration in the hydrolysate by 21%. Additionally, the reduction in phenolic content by 21% in pretreated slurry was reported that allowed to improve the fermentation performance of the thermotolerant yeast Kluyveromyces marxianus (Moreno et al. 2016b). Similar to microbial immobilization, laccase immobilization has also been studied in order to make the process economical, more comfortable, and reusable. Different methods such as adsorption, covalent bonding, and self-immobilization have been tested to achieve the maximum immobilization yield and efficiency (Bilal et al. 2019). Among the immobilization methods, adsorption is the widely used method since it is relatively inexpensive and simple. Covalent binding is another method that exploits functional groups of the support material for modification and chemical reaction with amino groups present on the laccase surface. Laccase immobilization through covalent binding has been reported on many different supports, including silica-based supports, alumina, epoxy-activated resins, polymers, carbon materials, and natural fibers (Suman et al. 2018b; Huang et al. 2019b; Guardado et al. 2021). The covalent immobilization results showed that the laccase has improved temperature, pH, and storage stability compared to free enzyme. Also, laccase could be reused for several cycles without loss of activity (Taheran et al. 2017; Lonappan et al. 2018). However, the major drawbacks of covalent binding may be the possible modification of the laccase structure in the active site and lower turnover number compared to the free enzyme (Yang et al. 2017b).
In a self-immobilization method, cross-linked enzyme crystals (CLECs) exhibit excellent activities and operational stability. Still, their major drawback is the high purity required for the enzyme’s crystallization (Matijošytė et al. 2010). Cross-linked enzyme aggregates (CLEAs) involve the precipitation of the laccase, combining the purification and immobilization into a single operation. The CLEA technology is cost-effective and straightforward and overcomes the need for solid support or dedicated specialized equipment (Zerva et al. 2020). Several studies have been reported where laccase CLEAs exhibited increased stability and reusability compared to the free enzyme (Vršanská et al. 2018; Primožič et al. 2020)
Use of immobilized laccase for de-lignification studies has been very limited. The magnetic nanocomposite of Fe3O4@SiO2@KIT-6 was used to immobilize laccase supported for the delignification and degradation of phenolic extracts from olive pomace. Treatment with immobilized laccase resulted in 77.3% and 76.5% degradation of lignin and phenol after 6-h incubation (Amin et al. 2018). A significant reduction in lignin content of different lignocellulosic wastes was observed using laccase from Trametes versicolor immobilized on alginate–chitosan. After 15 h, a maximum delignification of 78.29% was achieved in sugarcane bagasse, followed by wheat bran and corn stover (Asgher et al. 2018).
Laccase-mediator-based delignification
Mediators are compounds that enhance the laccase operation. The mediator maintains the cyclic redox conversion after getting oxidized by the laccase and subsequently reduced by the substrate acting as an electron carrier between the enzyme and the substrate. Compounds such as 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) (ABTS) and 1-hydroxybenzotriazole (HBT) have been successfully used as laccase mediator enabling oxidation of both phenolic and non-phenolic components of lignin polymer (Mate and Alcalde 2017). Different cellulosic materials have been treated with laccase in the presence of mediators for extensive cleavage of covalent bonds in lignin and simultaneous improvement of enzymatic hydrolysis. Crude laccase from P. sanguineus used along with mediators HBT and ABTS for the delignification of milled oil palm empty fruit bunch resulted in 8% and 8.7% lignin removal respectively. As a consequence, the mediator treated biomass resulted in 30 g L−1 of fermentable sugars in saccharification, while a yield of 19.1 g L−1 of maximum fermentable sugars was achieved without a mediator (Zanirun et al. 2015). T. versicolor laccase and HBT as mediator were used for the treatment of palm trees fronds and seaweed; the result showed 9% lignin reduction from palm tree and 24% from the seaweed. Furthermore, a combination of laccase-HBT with ionic liquid increased the saccharification yield from 3.1 to 13% (Al-Zuhair et al. 2015). In another study, the reactivity of T. versicolor laccase was assessed with and without mediators towards acid-pretreated wheat straw. The result showed that the combination of laccase with mediator increased the released glucose concentration (Heap et al. 2014). Deng et al. (2019) investigated the effects of laccase pretreatments on wheat straw showing the changes in chemical groups of lignin and lignocellulose. Additionally, the adsorption ability of the enzyme to lignin was also reduced after laccase mediator treatment (Deng et al. 2019). Effectiveness of P. ostreatus laccases was tested with mediators to remove lignin from agro-food waste such as apple pomace and coffee silverskin. A delignification yield of around 50% and the saccharification yields of 83% and 73% were observed for apple pomace and coffee silverskin, respectively (Giacobbe et al. 2018). Laccase from P. cinnabarinus was used along with hydroxybenzotriazole as a mediator to remove lignin in sugarcane bagasse and straw residues. Lignin content was decreased by up to 27% and 31%, and the concentration of released glucose was increased by around 39% and 46% in bagasse and straw, respectively. Additionally, lignin depolymerization was also confirmed by 2D-NMR spectra by a significant reduction in the number of aliphatic side chains involved in the main β-O-4′ and β-5′ interunit linkages. Removal of p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) lignin units, as well as the associated p-coumarates and ferulates, was confirmed with compared untreated biomass (Rencoret et al. 2017). In another study by the same group, methyl syringate was used as a mediator with laccase from Myceliophthora thermophila to delignify the fast-growing paulownia species. Lignin removal up to 24% was observed, and glucose and xylose yield increased by 38% and 34%. The delignification was confirmed by structural modification in lignin by 2D-NMR spectroscopy and the presence of oxidized lignin compounds (vanillin, vanillic acid, syringaldehyde, and syringic acid) in enzyme-treated filtrate concluded the role of laccase mediator system delignification (Rencoret et al. 2018a). The incubation of steam-exploded eucalyptus biomass with laccase and HBT as mediator changed the lignocellulosic structural properties, as evidenced by Fourier transform infrared spectroscopy and thermo-gravimetric analysis. However, this did not increase the yield of sugars released by enzymatic saccharification (Navas et al. 2019). In a recent study by Hilger et al. (2020), delignification of wheat straw and corn stover by laccase/HBT system revealed up to 28–51% lignin removal efficiency (w/w). The py-GC-MS study showed that delignification occurred via cleavage of both Cα–Cβ and ether cleavage of β-O-4′ aryl ethers, and further through cleavage of the β-O bond (Hilgers et al. 2020). Although various engineering approaches such as directed evolution, rational designing, and heterologous expression have been attempted to improve the catalytic performance of the laccase, very few studies describe the use of engineered laccase for the delignification purpose. In one of the studies, the recombinant strain of Phanerochaete chrysosporium enhanced the delignification by 25% compared to the wild-type strain. Moreover, the recombinant laccase expression was 4-fold higher, and sugar release from the treated biomass was increased up to the 6-fold compared to the wild type (Linares et al. 2018). Recombinant laccase from Lentinula edodes was expressed in Pichia pastoris, and it was demonstrated the enzyme was resistant to temperature, but thermal stability was compromised. However, the enzyme degraded the lignin and improved the utilization of the rape straw (Liu et al. 2020). In another study, loss of lignin up to 24.61% from various common crops has been reported after treatment with recombinant laccase expressed in P. pastoris under the control of an alcohol oxidase promoter (Kumar et al. 2018). Recombinant laccase has also been used for delignification of crop residues of sorghum stover, maize straw, finger millet, and finger millet straw. Finger millet reported a maximum loss of 24.61% while sorghum stover recorded the lowest lignin removal of 1.64% after treatment with laccase-mediator system (Kumar et al. 2018).
To sum up, enzymatic catalysts can significantly reduce the time and increase the efficiency of delignification process. The limitation caused due to the low-redox potential of laccase can be conquered by novel laccase with high redox potential or use of mediator molecules.
Some other delignification studies using laccase as an enzymatic biocatalyst are presented in Table 2.
Role in detoxification
In the pretreatment process, C5 sugar (pentoses) and some extent of hexose sugar (glucose) are released. However, due to the harsh pretreatment conditions, inhibitory compounds are generated that are the major challenges in producing fermentable sugars from the lignocellulosic biomass. Inhibitors such as weak acids, furfural, 5-hydroxymethylfurfural (HMF), and phenolic compounds are generated due to the decomposition of sugars and lignin during thermo-chemical, acid, and steam explosion processes for pretreatment. Furfural and 5-HMF are derived from the decomposition of pentoses sugar (xylose) and hexoses, respectively. The pretreated hydrolysate also contains aliphatic acids, such as acetic acid, formic acid, and levulinic acid. Acetic acid in pre-hydrolysate is generated from the acetyl group of hemicellulose, while formic acid and levulinic acid are the degraded product of furfural and HMF, respectively (Fillat et al. 2017). Also, a large number of phenolic compounds, such as vanillin, syringaldehyde, syringic acid, ferulic acid, p-coumaric acid dihydro coniferyl alcohol, and coniferyl aldehyde, are derived from the lignin. These compounds are toxic to fermenting microorganisms and promote the inhibition of enzymes; presence of these inhibitory compounds is strongly dependent on the feedstock and method selected for pretreatment, as shown in Fig. 4.
In addition to the inhibitors discussed above, the residual lignin is also present in the pretreated materials that limit the enzymatic hydrolysis of polymeric carbohydrates. Lignin physically adsorbs the hydrolytic enzymes and reduces the enzyme concentration during the biomass’s saccharification (Fillat et al. 2017). Physical methods like filtration and washing have been widely used for removal of inhibitors but have associated disadvantages of loss of soluble sugars (Pan et al. 2019). Vacuum evaporation has also been studied for the removal of organic solvents (Wang et al. 2020). Use of chemical agents like dithionite and sulfite has also been reported for the chemical transformation of inhibitors (Zhong et al. 2019). However, drawbacks associated with these methods are the wastage of huge amounts of water and loss of soluble sugars. Furfural, acetic acid, and vanillin can be reduced from the pretreated material by the vacuum evaporation method. Solvents such as ethyl acetate, activated carbon, and ion exchange resins have been used to remove inhibitory compounds either by extraction or adsorption (Fillat et al. 2017). The addition of reducing agents (dithionite and sulfite) has been tested for the chemical transformation of inhibitors. Treatment with Ca(OH)2 is one of the efficient methods of detoxification, phenolic compounds, and furan derivative removal which has been reported by over liming of pre-hydrolysate (Li et al. 2018). Overall, the physical and chemical methods are expensive, require high energy, and are not eco-friendly (Guo et al. 2017; Rastogi and Shrivastava 2017; Silva-Fernandes et al. 2017). Hence, there is an inclination to research biological methods, which involves treatment with fungal and bacterial strains and the enzymes produced by them (Masran et al. 2016). Biocatalyst-based detoxifications of lignocellulosic hydrolysate are mild and more specific in their action, and these methods are quite comparable to other physical and chemical methods. Use of whole-cell laccase producers for the purpose of detoxification is not well established due to the requirement of a long time for microbial growth and the utilization of available sugars by these microbes. Although few reports have used whole-cell biocatalyst for detoxification, there is no confirmation of laccase secretion by these microorganisms (Yu et al. 2011). Laccase-based enzymatic detoxification of inhibitory compounds has been well studied; however, very few studies have been reported recently.
Enzymatic detoxification
Enzymatic detoxification is employed to overcome the inhibition problem and enhance detoxification (Jönsson and Martín 2016). It is faster and catalytically efficient than microbial detoxification. Laccases are the most commonly used enzymes for the detoxification of hydrolysate. Laccases catalyze phenolic compounds’ oxidation and generate unstable phenoxy radicals that lead to polymerization into less toxic aromatic compounds (Su et al. 2018a). The substrate specificity towards phenols showed by laccases offers advantages such as mild reaction conditions and low energy requirements for detoxification compared with chemical and physical methods (De La Torre et al. 2017). These enzymes are mainly present in white-rot fungi such as Coriolopsisrigida, Coltriciaperennis, Cyathusstercoreus, P. cinnabarinus, T. versicolor, and T. villosa while it is also produced by few bacterial species like Streptomyces ipomoeae (De La Torre et al. 2017). Variations have been observed in the enzyme’s behavior towards the detoxification of phenolic compounds, which can be explained by the physicochemical characteristics of these enzymes. The higher redox potential (around +730 mV to +800 mV) of fungal laccase compared to the bacterial laccases (<500 mV) increases their capability to act towards a wider range of soluble phenolic compounds. The fungal laccase from T. villosa was able to oxidize majority of the phenolic inhibitors such as vanillin, syringaldehyde, and p-coumaric and ferulic acids identified in steam-exploded wheat straw, while bacterial laccase from Streptomyces ipomoeae only acted on syringaldehyde and ferulic acid. The reduction in total phenolic content in steam-exploded whole slurry was up to 35% and 71% with bacterial and fungal laccases, respectively. Furthermore, the reduction of phenolic inhibitors resulted in improved performance of Saccharomyces cerevisiae during fermentation and enhanced ethanol production rate (De La Torre et al. 2017). Rice straw is another agricultural residue and source of biomass for energy generation. Treatment of rice straw hydrolysate with T. versicolor laccase demonstrated 76% removal of phenolic compounds after 12 of treatment (Kapoor et al. 2015). Laccase from P. cinnabarinus was used to detoxify steam-exploded wheat straw for increasing the enzymatic saccharification and ethanol production. Total phenolic compounds up to 95% were removed, resulting in enhanced fermentation and ethanol yields (Moreno et al. 2016a). In another study, the laccase treated liquid fraction from sugarcane bagasse acid hydrolysate was used for the ethanol fermentation by Candida shehatae. The ethanol yield obtained was competitive to activated carbon-treated acid hydrolysate (Chandel et al. 2007). Apart from the agricultural waste, laccase has also been used for detoxification of agroforestry residues after pretreatment of the biomass (Sharma et al. 2016). Eucalyptus globulus, an agroforesterial biomass, was steam exploded to remove lignin and detoxify by laccase obtained from the secretome of Marasmiellus palmivorus. It was found that 70% of phenolic compounds were reduced and resulted in 25% increase in sugar and 10% increase in ethanol (Schneider et al. 2020). Poplar, another agroforestrial biomass, was pretreated with dilute acid to remove lignin from the biomass. The hydrolysate containing phenolic inhibitors was treated with T. versicolor laccase for 12 h at room temperature, resulting in a 94% reduction in inhibitors (Kapoor et al. 2015). In a study, laccase obtained from T. maxima removed 66% of lignin-derived soluble phenolic compounds from sugarcane bagasse within half hour of incubation period (Suman et al. 2018a). Moreno et al. (2012) compared the detoxification of steam-exploded wheat straw slurry by treating laccase before enzymatic hydrolysis and after enzymatic hydrolysis. Results showed that both treatment strategies could remove 88–92% of the phenolic compounds, resulting in higher ethanol concentration and shorter lag phase of the fermenting yeast (Moreno et al. 2012). Additionally, utilization of laccases as detoxifying agents would allow the use of higher substrate loadings or even the use of whole pretreated slurries (Oliva-Taravilla et al. 2016). Despite the advantages of using laccases for detoxification in free form, their high production cost, low operational stability, and failure of recovery throw challenges in their utilization at an industrial scale (Gonçalves et al. 2019). These limitations can be overcome by binding the enzyme to an appropriate carrier substrate that increases the stability and reusability of enzymes and eases the product recovery, resulting in lower operational costs (Liu et al. 2018). Immobilization also improves the enzyme’s chemical and thermal resistance (Bernal et al. 2018; Drozd et al. 2018). Various inorganic, organic, and synthetic polymers have been used for enzyme immobilization, such as epoxy-functionalized silica (gel) (Mohammadi et al. 2018), iron magnetic nanoparticle (Chen et al. 2020), titania nanoparticles (García-Morales et al. 2018), alginate-gelatin (Reda et al. 2018), and poliurea microsphere (Jiang et al. 2017). Laccase from Gluconacetobacter xylinus was immobilized on cellulose nanofibers for detoxification of three different lignocellulosic derivatives, namely furfural, acetosyringone, and coniferyl aldehyde that cause inhibition of microbial fermentation. Complete degradation of these derivatives was obtained after the incubation of 36 h, where furfural and coniferyl alcohol was detoxified by laccase alone, but acetosyringone required a redox mediator, hydroxybenzotriazole, along with laccase for its detoxification (Saravanakumar et al. 2016). In another study, an immobilized enzyme cocktail containing laccase was evaluated for its detoxification ability and enhancement of saccharification yield. Results showed that the laccase-supplemented immobilized enzyme cocktail reduced phenolic compounds by 73.8%, resulting in 84.6% of saccharification yield (Kumar et al. 2019). Conclusively, enzymatic detoxification is a promising approach for removal of toxic inhibitors from the pre-hydrolysate. Laccases immobilization and ability for detoxification of multiple inhibitors can prove to be a key success for use of this process in future.
Challenges and future prospects
The rate-limiting step in converting abundantly available lignocellulosic biomass to green fuels and other value-added products is the removal of the lignin barrier. Biological pretreatment methods offer sustainable and environment-friendly alternatives to traditional physico-chemical technologies, which are high in cost and have a large carbon footprint. Laccase can serve as an environment friendly tool for detoxification and delignification of lignocellulosic biomass offering higher saccharification and fermentation yields. However, there are challenges of laccase stability and functionality like reduction in activity or complete deactivation due to harsh conditions in processes. These challenges can be addressed by making the enzyme robust by using the directed mutation, semi-rational design, and rational designing approach. The search for novel laccase using function-based metagenomic approaches can be exploited for the generation of potential laccase. Furthermore, the use of new bioinformatics tools such as Laccase Engineering Database (Sirim et al. 2011) can be useful to design a better laccase enzyme system for biomass pretreatment, and shall be beneficial for the production of biofuels.
Use of mediators in the enzyme-based process increased the delignification of woody and non-woody lignocellulose materials along with enhanced detoxification; however, there is also a continued need for low-cost mediator search to make the enzymatic delignification and detoxification process economically viable. The search for natural lignin-derived mediators can be a promising alternative solution to synthetic mediators. Furthermore, the synthetic mediators used in the process are associated with disadvantages, e.g., the generation of by-products that can lead to laccases’ inactivation. The second disadvantage is the loss of mediator functionality due to the side reactions. The third is repolymerization in the laccase mediator-based process, which can be avoided by developing a quick separation method of potential degradation products and eliminating reactive species like radicals or quinones from the reaction medium.
Whole-cell delignification and detoxification involve time and monitoring, and there is also a chance of loss of fermentative sugar yields. Moreover, the whole cell-based processes are challenging to scale up. Use of laccases directly in the enzymatic processes overcomes these difficulties, but the high costs for enzyme production and mediators prevent the successful implementation of laccases. Additionally, not all the inhibitory compounds can be removed by the enzymatic detoxification process. Nevertheless, the biocatalytic approach for delignification and detoxification is a promising area that needs further research. The use of immobilized system needs to be explored to minimize the cost and increase the process’s reproducibility. Integration of biocatalytic approach with other methods can help in conversion of waste lignocellulosic biomass into energy generation in a greener way.
Conclusion
In view of the new concept of biorefinery and circular economy, lignocellulose has become a promising feedstock for biofuel and platform chemicals production. However, their recalcitrant nature prevents them from being fully utilized; the utilization of carbohydrates requires the removal of strongly bonded lignin from the lignocellulose. The exposure of carbohydrate for enzymatic hydrolysis depends on the pretreatment process for removing and modifying the lignin. Laccases have been identified as one of the key ligninolytic enzyme that not only has the potential to be used as a pretreatment agent but can also be used as a detoxifying agent to remove the inhibitors generated during pretreatment processes. With the growing environmental concern and stringent regulations, use of chemicals in pretreatment process is bound to be minimized. Laccase-catalyzed oxidation of lignin can promote biomass delignification in an environment-friendly and cost-effective manner. Use of lacasses can increase the energy efficiency of the process, yet the research is still in a nascent stage in terms of large-scale application of laccases for biomass de-lignification. Further research is required to overcome the existing limitations associated with laccases and laccase-mediator systems to use these multi-purpose biocatalysts at an industrial scale.
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Adekunle AE, Zhang C, Guo C, Liu C-Z (2017) Laccase production from Trametes versicolor in solid-state fermentation of steam-exploded pretreated cornstalk. Waste Biomass Valorization 8:153–159
Agrawal K, Chaturvedi V, Verma P (2018) Fungal laccase discovered but yet undiscovered. Bioresour Bioprocess 5:4
Agrawal K, Bhardwaj N, Kumar B, Chaturvedi V, Verma P (2019) Process optimization, purification and characterization of alkaline stable white laccase from Myrothecium verrucaria ITCC-8447 and its application in delignification of agroresidues. Int J Biol Macromol 125:1042–1055
Al-Zuhair S, Abualreesh M, Ahmed K, Abdul Razak A (2015) Enzymatic delignification of biomass for enhanced fermentable sugars production. Energy Technol 3:121–127
Amin R, Khorshidi A, Shojaei AF, Rezaei S, Faramarzi MA (2018) Immobilization of laccase on modified Fe3O4@ SiO2@ Kit-6 magnetite nanoparticles for enhanced delignification of olive pomace bio-waste. Int J Biol Macromol 114:106–113
Andlar M, Rezić T, Marđetko N, Kracher D, Ludwig R, Šantek B (2018) Lignocellulose degradation: an overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng Life Sci 18:768–778
Asano T, Seto Y, Hashimoto K, Kurushima H (2019) Mini-review an insect-specific system for terrestrialization: laccase-mediated cuticle formation. Insect Biochem Mol Biol 108:61–70
Asgher M, Wahab A, Bilal M, Iqbal HMN (2018) Delignification of lignocellulose biomasses by alginate–chitosan immobilized laccase produced from Trametes versicolor IBL-04. Waste Biomass Valorization 9:2071–2079
Avanthi A, Banerjee R (2016) A strategic laccase mediated lignin degradation of lignocellulosic feedstocks for ethanol production. Ind Crop Prod 92:174–185
Ayeronfe F, Kassim A, Hung P, Zainulabidin H, Ishak N, Syarifah S, Aripin A (2019) Evaluation of Lysinibacillus SP, isolated from Coptotermes curvignathus Gut, for the delignification of oil palm residues. Biosci J 35
Banerjee R, Chintagunta AD, Ray S (2019) Laccase mediated delignification of pineapple leaf waste: an ecofriendly sustainable attempt towards valorization. BMC Chem 13:58
Bari E, Taghiyari HR, Naji HR, Schmidt O, Ohno KM, Clausen CA, Bakar ES (2016) Assessing the destructive behaviors of two white-rot fungi on beech wood. Int Biodeterior Biodegradation 114:129–140
Bernal C, Rodriguez K, Martinez R (2018) Integrating enzyme immobilization and protein engineering: an alternative path for the development of novel and improved industrial biocatalysts. Biotechnol Adv 36:1470–1480
Bilal M, Rasheed T, Nabeel F, Iqbal HM, Zhao Y (2019) Hazardous contaminants in the environment and their laccase-assisted degradation–a review. J Environ Manag 234:253–264
Bugos RC, Sutherland JB, Adler JH (1988) Phenolic compound utilization by the soft rot fungus Lecythophora hoffmannii. Appl Environ Microbiol 54:1882–1885
Bule MV, Chaudhary I, Gao AH, Chen S (2016) Effects of extracellular proteome on wheat straw pretreatment during solid-state fermentation of Phlebia radiata ATCC 64658. Int Biodeterior Biodegradation 109:36–44
Cannatelli MD, Ragauskas AJ (2017) Two decades of laccases: advancing sustainability in the chemical industry. Chem Rec 17:122–140
Chandel AK, Kapoor RK, Singh A, Kuhad RC (2007) Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour Technol 98:1947–1950
Chandra R, Chowdhary P (2015) Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environ Sci Process Impacts 17:326–342
Chaurasia PK, Yadav RSS, Yadava S (2013) A review on mechanism of laccase action. Res Rev Biosci 7:66–71
Chen X, He B, Feng M, Zhao D, Sun J (2020) Immobilized Laccase on magnetic nanoparticles for enhanced lignin model compounds degradation. Chin J Chem Eng
Chukwuma OB, Rafatullah M, Tajarudin HA, Ismail N (2020) Lignocellulolytic enzymes in biotechnological and industrial processes: a review. Sustainability 12:7282
Ćilerdžić J, Stajić M, Vukojević J (2016) Degradation of wheat straw and oak sawdust by Ganoderma applanatum. Int Biodeterior Biodegradation 114:39–44
Cragg SM, Beckham GT, Bruce NC, Bugg TD, Distel DL, Dupree P, Etxabe AG, Goodell BS, Jellison J, McGeehan JE, McQueen-Mason SJ (2015) Lignocellulose degradation mechanisms across the Tree of Life. Curr Opin Chem Biol 29:108–119
da Silva MA, Ferraz A (2017) Biological pretreatment of sugarcane bagasse with basidiomycetes producing varied patterns of biodegradation. Bioresour Technol 225:17–22
Daniel G (2016) Fungal degradation of wood cell walls. In: Secondary xylem biology. Elsevier, pp 131–167
Datta R, Kelkar A, Baraniya D, Molaei A, Moulick A (2017) Enzymatic degradation of lignin in soil: a review. Sustainability 9:1163
De La Torre M, Martín-Sampedro R, Fillat Ú, Eugenio AE, Blánquez A, Hernández M, Arias ME, Ibarra D (2017) Comparison of the efficiency of bacterial and fungal laccases in delignification and detoxification of steam-pretreated lignocellulosic biomass for bioethanol production. J Ind Microbiol Biotechnol 44:1561–1573
Deng Z, Xia A, Liao Q, Zhu X, Huang Y, Fu Q (2019) Laccase pretreatment of wheat straw: effects of the physicochemical characteristics and the kinetics of enzymatic hydrolysis. Biotechnol Biofuels 12:159
Drozd R, Rakoczy R, Wasak A, Junkac A, Fijałkowskia K (2018) The application of magnetically modified bacterial cellulose for immobilization of laccase. Int J Biol Macromol 108:462–470
Fillat Ú, Ibarra D, Eugenio M, Moreno AD, Tomás-Pejó E, Sampedro RM (2017) Laccases as a potential tool for the efficient conversion of lignocellulosic biomass: a review. Fermentation 3:17
Forootanfar H, Faramarzi MA (2015) Insights into laccase producing organisms, fermentation states, purification strategies, and biotechnological applications. Biotechnol Prog 31:1443–1463
García-Morales R, García-García A, Orona-Navar C, Osma JF, Nigam KDP, Ornelas-Sotoa N (2018) Biotransformation of emerging pollutants in groundwater by laccase from P. sanguineus CS43 immobilized onto titania nanoparticles. J Environ Chem Eng 6:710–717
Ghorbani F, Karimi M, Biria D, Kariminia HR, Jeihanipoura A (2015) Enhancement of fungal delignification of rice straw by Trichoderma viride sp. to improve its saccharification. Biochem Eng J 101:77–84
Giacobbe S, Pezzella C, Lettera V, Sannia G, Piscitelli A (2018) Laccase pretreatment for agrofood wastes valorization. Bioresour Technol 265:59–65
Gonçalves MCP, Kieckbusch TG, Perna RF, Fujimoto JT, Morales SAV, Romanellic JP (2019) Trends on enzyme immobilization researches based on bibliometric analysis. Process Biochem 76:95–110
Goodell B (2003) Brown-rot fungal degradation of wood: our evolving view. ACS Publications
Guardado ALP, Druon-Bocquet S, Belleville M-P, Sanchez-Marcano J (2021) A novel process for the covalent immobilization of laccases on silica gel and its application for the elimination of pharmaceutical micropollutants. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-12394-y
Guo H, Wu Y, Hong C, Chen H, Chen X, Zheng B, Jiang D, Qin W (2017) Enhancing digestibility of Miscanthus using lignocellulolytic enzyme produced by Bacillus. Bioresour Technol 245:1008–1015
Guo H, Zhao Y, Chen X, Shao Q, Qin W (2019) Pretreatment of Miscanthus with biomass-degrading bacteria for increasing delignification and enzymatic hydrolysability. Microb Biotechnol 12:787–798
Haider K, Trojanowski J (1975) Decomposition of specifically 14 C-labelled phenols and dehydropolymers of coniferyl alcohol as models for lignin degradation by soft and white rot fungi. Arch Microbiol 105:33–41
Heap L, Green A, Brown D, Van DB, Turner N (2014) Role of laccase as an enzymatic pretreatment method to improve lignocellulosic saccharification. Catal Sci Technol 4:2251–2259
Hilgers R, van Erven G, Boerkamp V, Sulaeva I, Potthast A, Kabel MA, Vincken JP (2020) Understanding laccase/HBT-catalyzed grass delignification at the molecular level. Green Chem 22:1735–1746
Huang W, Wachemo AC, Yuan H, Li X (2019a) Modification of corn stover for improving biodegradability and anaerobic digestion performance by Ceriporiopsis subvermispora. Bioresour Technol 283:76–85
Huang Y, Li J, Yang Y, Yuan H, Wei Q, Liu X, Zhao Y, Ni C (2019b) Characterization of enzyme-immobilized catalytic support and its exploitation for the degradation of methoxychlor in simulated polluted soils. Environ Sci Pollut Res 26:28328–28340
Jafari N, Rezaei S, Rezaie R, Dilmaghani H, Khoshayand MR, Faramarzi MA (2017) Improved production and characterization of a highly stable laccase from the halophilic bacterium Chromohalobacter salexigens for the efficient delignification of almond shell bio-waste. Int J Biol Macromol 105:489–498
Jiang X, Yu Y, Li X, Kong XZ (2017) High yield preparation of uniform polyurea microspheres through precipitation polymerization and their application as laccase immobilization support. Chem Eng J 328:1043–1050
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112
Jović J, Buntić A, Radovanović N, Petrović B, Mojović L (2018) Lignin-degrading abilities of novel autochthonous fungal isolates Trametes hirsuta F13 and Stereum gausapatum F28. Food Technol Biotechnol 56:354–365
Kalyani D, Tiwari MK, Li J, Kim SC, Kalia VC, Kang YC, Lee JK (2015) A highly efficient recombinant laccase from the yeast Yarrowia lipolytica and its application in the hydrolysis of biomass. PLoS One 10:e0120156
Kapoor RK, Rajan K, Carrier DJ (2015) Applications of Trametes versicolor crude culture filtrates in detoxification of biomass pretreatment hydrolyzates. Bioresour Technol 189:99–106
Karimi K, Taherzadeh MJ (2016) A critical review of analytical methods in pretreatment of lignocelluloses: composition, imaging, and crystallinity. Bioresour Technol 200:1008–1018
Karimi M, Esfandiar R, Biria D (2017) Simultaneous delignification and saccharification of rice straw as a lignocellulosic biomass by immobilized Thrichoderma viride sp. to enhance enzymatic sugar production. Renew Energy 104:88–95
Koklukaya SZ, Sezer S, Aksoy S, Hasirci N (2016) Polyacrylamide-based semi-interpenetrating networks for entrapment of laccase and their use in azo dye decolorization. Biotechnol Appl Biochem 63:699–707
Komal A, Venkatesh C, Pradeep V (2018) Fungal laccase discovered but yet undiscovered. Bioresour Bioprocess 5:4
Kumar A, Chandra R (2020) Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 6:e03170
Kumar VP, Kolte AP, Dhali A, Naik C, Sridhar M (2018) Enhanced delignification of lignocellulosic substrates by Pichia GS115 expressed recombinant laccase. J Gen Appl Microbiol 2011–2017 64(4):180–189
Kumar V, Patel SKS, Gupta RK, Otari SV, Gao H, Lee JK, Zhang L (2019) Enhanced saccharification and fermentation of rice straw by reducing the concentration of phenolic compounds using an immobilized enzyme cocktail. Biotechnol J 14:1800468
Li C, Zhao X, Wang A, Huber GW, Zhang T (2015) Catalytic transformation of lignin for the production of chemicals and fuels. Chem Rev 115:11559–11624
Li J, Shi S, Tu M, Via B, Sun FF, Adhikari S (2018) Detoxification of organosolv-pretreated pine prehydrolysates with anion resin and cysteine for butanol fermentation. Appl Biochem Biotechnol 186:662–680
Linares NC, Fernández F, Loske AM, Gómez-Lim MA (2018) Enhanced delignification of lignocellulosic biomass by recombinant fungus phanerochaete chrysosporium overexpressing laccases and peroxidases. J Mol Microbiol Biotechnol 28:1–13
Liu D-M, Chen J, Shi Y-P (2018) Advances on methods and easy separated support materials for enzymes immobilization. TrAC Trends Anal Chem 102:332–342
Liu C, Zhang W, Qu M, Pan K, Zhao X (2020) Heterologous expression of laccase from Lentinula edodes in Pichia pastoris and its application in degrading rape straw. Front Microbiol 11:1086
Lonappan L, Liu Y, Rouissi T, Pourcel F, Brar SK, Verma M, Surampalli RY (2018) Covalent immobilization of laccase on citric acid functionalized micro-biochars derived from different feedstock and removal of diclofenac. Chem Eng J 351:985–994
Longe LF, Couvreur J, Leriche Grandchamp M, Garnier G, Allais F, Saito K (2018) Importance of mediators for lignin degradation by fungal laccase. ACS Sustain Chem Eng 6:10097–10107
Lu C, Wang H, Luo Y, Guo L (2010) An efficient system for pre-delignification of gramineous biofuel feedstock in vitro: application of a laccase from Pycnoporus sanguineus H275. Process Biochem 45:1141–1147
Ma K, Ruan Z (2015) Production of a lignocellulolytic enzyme system for simultaneous bio-delignification and saccharification of corn stover employing co-culture of fungi. Bioresour Technol 175:586–593
Ma F, Huang X, Ke M, Shi Q, Chen Q, Shi C, Zhang J, Zhang X, Yu H (2017) Role of selective fungal delignification in overcoming the saccharification recalcitrance of bamboo culms. ACS Sustain Chem Eng 5:8884–8894
Madadi M, Abbas A (2017) Lignin degradation by fungal pretreatment: a review. J Plant Pathol Microbiol 8:1–6
Majumdar S, Lukk T, Solbiati JO, Bauer S, Nair SK, Cronan JE, Gerlt JA (2014) Roles of Small Laccases from in Lignin Degradation. Biochemistry 53(24):4047–4058
Martín-Sampedro R, Fillat Ú, Ibarra D, Eugenio ME (2015) Use of new endophytic fungi as pretreatment to enhance enzymatic saccharification of Eucalyptus globulus. Bioresour Technol 196:383–390
Martín-Sampedro R, López-Linares JC, Fillat Ú, Gea-Izquierdo G, Ibarra D, Castro E, Eugenio ME (2017) Endophytic fungi as pretreatment to enhance enzymatic hydrolysis of Olive tree pruning. Biomed Res Int 2017:9727581
Masran R, Zanirun Z, Bahrin EK, Ibrahim MF, Yee PL, Abd-Aziz S (2016) Harnessing the potential of ligninolytic enzymes for lignocellulosic biomass pretreatment. Appl Microbiol Biotechnol 100:5231–5246
Mate DM, Alcalde M (2017) Laccase: a multi-purpose biocatalyst at the forefront of biotechnology. Microb Biotechnol 10:1457–1467
Matijošytė I, Arends IWCE, de Vries S, Sheldon RA (2010) Preparation and use of cross-linked enzyme aggregates (CLEAs) of laccases. J Mol Catal B Enzym 62:142–148
Mohammadi M, As’habi MA, Salehi P, Yousefi M, Nazari M, Brask J (2018) Immobilization of laccase on epoxy-functionalized silica and its application in biodegradation of phenolic compounds. Int J Biol Macromol 109:443–447
Moilanen U, Kellock M, Galkin S, Viikari L (2011) The laccase-catalyzed modification of lignin for enzymatic hydrolysis. Enzym Microb Technol 49:492–498
Moreno AD, Ibarra D, Fernández JL, Ballesteros M (2012) Different laccase detoxification strategies for ethanol production from lignocellulosic biomass by the thermotolerant yeast Kluyveromyces marxianus CECT 10875. Bioresour Technol 106:101–109
Moreno AD, Ibarra D, Alvira P, Tomás-Pejó E, Ballesteros M (2016a) Exploring laccase and mediators behavior during saccharification and fermentation of steam-exploded wheat straw for bioethanol production. J Chem Technol Biotechnol 91:1816–1825
Moreno AD, Ibarra D, Mialon A, Ballesteros M (2016b) A bacterial laccase for enhancing saccharification and ethanol fermentation of steam-pretreated biomass. Fermentation 2:11
Mukhopadhyay M, Kuila A, Tuli DK, Banerjee R (2011) Enzymatic depolymerization of Ricinus communis, a potential lignocellulosic for improved saccharification. Biomass Bioenergy 35:3584–3591
Mustafa AM, Poulsen TG, Sheng K (2016) Fungal pretreatment of rice straw with Pleurotus ostreatus and Trichoderma reesei to enhance methane production under solid-state anaerobic digestion. Appl Energy 180:661–671
Nadir N, Ismail NL, Hussain AS (2019) Fungal pretreatment of lignocellulosic materials. In: Biomass for bioenergy-recent trends and future challenges. IntechOpen
Naghdi M, Taheran M, Brar SK, Kermanshahi-pour A, Verma M, Surampalli RY (2018) Biotransformation of carbamazepine by laccase-mediator system: kinetics, by-products and toxicity assessment. Process Biochem 67:147–154
Navas LE, Martínez FD, Taverna ME, Fetherolf MM, Eltis LD, Nicolau V, Estenoz D, Campos E, Benintende GB, Berretta MF (2019) A thermostable laccase from Thermus sp. 2.9 and its potential for delignification of Eucalyptus biomass. AMB Express 9:1–10
Oliva-Taravilla A, Tomás-Pejó E, Demuez M, González-Fernández C, Ballesteros M (2015) Inhibition of cellulose enzymatic hydrolysis by laccase-derived compounds from phenols. Biotechnol Prog 31:700–706
Oliva-Taravilla A, Tomas-Pejo E, Demuez M, Gonzalez-Fernandez C, Ballesteros M (2016) Effect of laccase dosage on enzymatic hydrolysis of steam-exploded wheat straw. Cellul Chem Technol 50:391–395
Pan L, He M, Wu B, Wang Y, Hu G, Ma K (2019) Simultaneous concentration and detoxification of lignocellulosic hydrolysates by novel membrane filtration system for bioethanol production. J Clean Prod 227:1185–1194
Pollegioni L, Tonin F, Rosini E (2015) Lignin-degrading enzymes. FEBS J 282:1190–1213
Prasad R (2016) Advances and applications through fungal nanobiotechnology. Springer
Primožič M, Kravanja G, Knez Ž, Crnjac A, Leitgeb M (2020) Immobilized laccase in the form of (magnetic) cross-linked enzyme aggregates for sustainable diclofenac (bio) degradation. J Clean Prod 275:124121
Rajak RC, Banerjee R (2015) Enzymatic delignification: an attempt for lignin degradation from lignocellulosic feedstock. RSC Adv 5:75281–75291
Rajak RC, Banerjee R (2016) Enzyme mediated biomass pretreatment and hydrolysis: a biotechnological venture towards bioethanol production. RSC Adv 6:61301–61311
Rajeswari G, Jacob S (2020) Deciphering the aloe vera leaf rind as potent feedstock for bioethanol through enzymatic delignification and its enhanced saccharification. Ind Crop Prod 143:111876
Rastogi M, Shrivastava S (2017) Recent advances in second generation bioethanol production: an insight to pretreatment, saccharification and fermentation processes. Renew Sust Energ Rev 80:330–340
Reda FM, Hassan NS, El-Moghazy A-N (2018) Decolorization of synthetic dyes by free and immobilized laccases from newly isolated strain Brevibacterium halotolerans N11 (KY883983). Biocatal Agric Biotechnol 15:138–145
Rencoret J, Pereira A, José C, Del Río JC, Martínez ÁT, Gutiérrez A (2016) Laccase-mediator pretreatment of wheat straw degrades lignin and improves saccharification. BioEnergy Res 9:917–930
Rencoret J, Pereira A, del Río JC, Martínez AT, Gutiérrez A (2017) Delignification and saccharification enhancement of sugarcane byproducts by a laccase-based pretreatment. ACS Sustain Chem Eng 5:7145–7154
Rencoret J, Pereira A, Marques G, del Río JC, Martínez ÁT, Gutiérrez A (2018a) A commercial laccase-mediator system to delignify and improve saccharification of the fast-growing Paulownia fortunei (Seem.) Hemsl. Holzforschung 73:45–54
Rencoret J, Pereira González A, Marques G, Río Andrade JC, Martínez ÁT, Gutiérrez SA (2018b) Laccase-based pretreatment to delignify and improve the saccharification of sugarcane bagasse and straw
Rezaei S, Shahverdi AR, Faramarzi MA (2017) Isolation, one-step affinity purification, and characterization of a polyextremotolerant laccase from the halophilic bacterium Aquisalibacillus elongatus and its application in the delignification of sugar beet pulp. Bioresour Technol 230:67–75
Rico A, Rencoret J, José C, Martínez AT, Gutiérrez A (2015) In-depth 2D NMR study of lignin modification during pretreatment of Eucalyptus wood with laccase and mediators. BioEnergy Res 8:211–230
Rodríguez-Couto S (2018) Solid-State Fermentation for laccases production and their applications. In: Current developments in biotechnology and bioengineering. Elsevier, pp 211–234
Rodríguez-Couto S (2019) Fungal laccase: a versatile enzyme for biotechnological applications. In: Recent advancement in white biotechnology through fungi. Springer, pp 429–457
Saravanakumar T, Park H-S, Mo A-Y, Choi MS, Kim DH, Park SM (2016) Detoxification of furanic and phenolic lignocellulose derived inhibitors of yeast using laccase immobilized on bacterial cellulosic nanofibers. J Mol Catal B Enzym 134:196–205
Schneider WD, Fontana RC, Baudel HM, Siqueira FG, Rencoret J, Gutiérrez A, Eugenio LI, Prieto A, Martínez MJ, Martínez AT, Dillon AJ, Camassola M (2020) Lignin degradation and detoxification of eucalyptus wastes by on-site manufacturing fungal enzymes to enhance second-generation ethanol yield. Applied Energy 262:114493
Shanmugam S, Hari A, Ulaganathan P, Yang F, Krishnaswamy S, Wu YR (2018) Potential of biohydrogen generation using the delignified lignocellulosic biomass by a newly identified thermostable laccase from Trichoderma asperellum strain BPLMBT1. Int J Hydrog Energy 43:3618–3628
Sharma N, Bohra B, Pragya N, Ciannella R, Dobie P, Lehmann S (2016) Bioenergy from agroforestry can lead to improved food security, climate change, soil quality, and rural development. Food Energy Secur 5:165–183
Sherpa KC, Ghangrekar MM, Banerjee R (2018) A green and sustainable approach on statistical optimization of laccase mediated delignification of sugarcane tops for enhanced saccharification. J Environ Manag 217:700–709
Shi J, Chinn MS, Sharma-Shivappa RR (2008) Microbial pretreatment of cotton stalks by solid state cultivation of Phanerochaete chrysosporium. Bioresour Technol 99:6556–6564
Shirkavand E, Baroutian S, Gapes DJ, Young BR (2017) Pretreatment of radiata pine using two white rot fungal strains Stereum hirsutum and Trametes versicolor. Energy Convers Manag 142:13–19
Silva-Fernandes T, Santos JC, Hasmann F, Rodrigues RC, Izario Filho HJ, Felipe MG (2017) Biodegradable alternative for removing toxic compounds from sugarcane bagasse hemicellulosic hydrolysates for valorization in biorefineries. Bioresour Technol 243:384–392
Sindhu R, Binod P, Pandey A (2016) Biological pretreatment of lignocellulosic biomass–an overview. Bioresour Technol 199:76–82
Singh R, Hu J, Regner MR, Round JW, Ralph J, Saddler JN, Eltis LD (2017) Enhanced delignification of steam-pretreated poplar by a bacterial laccase. Sci Rep 7:42121
Sirim D, Wagner F, Wang L, Schmid RD, Pleiss J (2011) The laccase engineering database: a classification and analysis system for laccases and related multicopper oxidases. Database 2011:bar006
Su J, Fu J, Wang Q, Silva C, Cavaco-Paulo A (2018a) Laccase: a green catalyst for the biosynthesis of poly-phenols. Crit Rev Biotechnol 38:294–307
Su Y, Yu X, Sun Y, Wang G, Chen H, Chen G (2018b) Evaluation of screened lignin-degrading fungi for the biological pretreatment of corn stover. Sci Rep 8:1–11
Suman SK, Khatri M, Dhawaria M, Kurmi A, Pandey D, Ghosh S, Jain SL (2018a) Potential of Trametes maxima IIPLC-32 derived laccase for the detoxification of phenolic inhibitors in lignocellulosic biomass prehydrolysate. Int Biodeterior Biodegradation 133:1–8
Suman SK, Patnam PL, Ghosh S, Jain SL (2018b) Chicken feather derived novel support material for immobilization of laccase and its application in oxidation of veratryl alcohol. ACS Sustain Chem Eng 7:3464–3474
Taheran M, Naghdi M, Brar SK, Knystautas EJ, Verma M, Surampalli RY (2017) Covalent immobilization of laccase onto nanofibrous membrane for degradation of pharmaceutical residues in water. ACS Sustain Chem Eng 5:10430–10438
Tian S-Q, Zhao R-Y, Chen Z-C (2018) Review of the pretreatment and bioconversion of lignocellulosic biomass from wheat straw materials. Renew Sust Energ Rev 91:483–489
Tsegaye B, Balomajumder C, Roy P (2018) Biodegradation of wheat straw by Ochrobactrum oryzae BMP03 and Bacillus sp. BMP01 bacteria to enhance biofuel production by increasing total reducing sugars yield. Environ Sci Pollut Res 25:30585–30596
Tsegaye B, Balomajumder C, Roy P (2019) Microbial delignification and hydrolysis of lignocellulosic biomass to enhance biofuel production: an overview and future prospect. Bull Natl Res Cent 43:51
Tu W-C, Hallett JP (2019) Recent advances in the pretreatment of lignocellulosic biomass. Curr Opin Green Sustain Chem 20:11–17
Vrsanska M, Buresova A, Damborsky P, Adam V (2015) Influence of different inducers on ligninolytic enzyme activities. J Met Nanotechnol 3:64–70
Vršanská M, Voběrková S, Jimenez Jimenez AM, Strmiska V, Adam V (2018) Preparation and optimisation of cross-linked enzyme aggregates using native isolate white rot fungi Trametes versicolor and Fomes fomentarius for the decolourisation of synthetic dyes. Int J Environ Res Public Health 15:23
Wang J, Feng J, Jia W, Chang S, Li S, Li Y (2015) Lignin engineering through laccase modification: a promising field for energy plant improvement. Biotechnol Biofuels 8:1–11
Wang S, Dai G, Yang H, Luo Z (2017) Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog Energy Combust Sci 62:33–86
Wang Q, Li G, Zheng K, Wang D, Tang K, Feng X, Leng J, Yu H, Yang S (2019) The soybean laccase gene family: evolution and possible roles in plant defense and stem strength selection. Genes (Basel) 10:701
Wang Y, Yan J, Wang J, Zhang X, Wei L, Du Y, Yu B, Ye S (2020) Superhydrophobic metal organic framework doped polycarbonate porous monolith for efficient selective removal oil from water. Chemosphere 260:127583
Yang J, Li W, Ng TB, Deng X, Lin J, Ye X (2017a) Laccases: production, expression regulation, and applications in pharmaceutical biodegradation. Front Microbiol 8:832
Yang J, Wang Z, Lin Y et al (2017b) Immobilized Cerrena sp. laccase: preparation, thermal inactivation, and operational stability in malachite green decolorization. Sci Rep 7:1–9
Ying W, Shi Z, Yang H, Xu G, Zheng Z, Yang J (2018) Effect of alkaline lignin modification on cellulase–lignin interactions and enzymatic saccharification yield. Biotechnol Biofuels 11:214
Yu Y, Feng Y, Xu C, Liu J, Li D (2011) Onsite bio-detoxification of steam-exploded corn stover for cellulosic ethanol production. Bioresour Technol 102 (8):5123–5128
Zanirun Z, Bahrin EK, Lai-Yee P, Hassan HA, Aziz SA (2015) Enhancement of fermentable sugars production from oil palm empty fruit bunch by ligninolytic enzymes mediator system. Int Biodeterior Biodegradation 105:13–20
Zerva A, Pentari C, Topakas E (2020) Crosslinked enzyme aggregates (CLEAs) of laccases from Pleurotus citrinopileatus induced in olive oil mill wastewater (OOMW). Molecules 25:2221
Zhang R, Lv C, Lu J (2020) Studies on laccase mediated conversion of lignin from ginseng residues for the production of sugars. Bioresour Technol 317:123945
Zhong N, Chandra R, Saddler JJ (2019) Sulfite post-treatment to simultaneously detoxify and improve the enzymatic hydrolysis and fermentation of a steam-pretreated softwood lodgepole pine whole slurry. ACS Sustainable Chemistry & Engineering 7(5): 5192–5199
Acknowledgements
Authors are thankful to Director Dr. Anjan Ray, CSIR-Indian Institute of Petroleum, for providing the necessary facilities to complete this work and constant encouragement.
Funding
This research was funded by CSIR-Indian Institute of Petroleum, Dehradun, India, as in-house project under OLP-981 and MLP 1099.
Author information
Authors and Affiliations
Contributions
MM collected research data and prepared the arts. SKS conceptualized, wrote, and edited the manuscript. Both the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malhotra, M., Suman, S.K. Laccase-mediated delignification and detoxification of lignocellulosic biomass: removing obstacles in energy generation. Environ Sci Pollut Res 28, 58929–58944 (2021). https://doi.org/10.1007/s11356-021-13283-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13283-0