Abstract
The depletion of fossil fuel reserves, environmental pollution, and climate change are driving the search for clean carbon-neutral fuels. Lignocellulosic biomass is considered as a promising feedstock for production of bioethanol and biochemicals. The overall potency or utilization depends on the effectual hydrolysis of lignocellulose; however, removal or deconstruction of the lignin polymer could be a key step in the process of biomass to monomeric fermentation sugars but remains challenging. Laccases (EC 1.10.3.2) are copper-containing oxidoreductases that have been investigated for use as a pretreatment of lignocellulose and might have a potential to remove phenolic compounds derived from lignin. This chapter focuse on recent trends in ligninolytic green biotechnology and major advances within the application of laccases as a possible pretreatment strategy. Also, it discuss the negative roles of lignin within the processes of converting biomass to biofuels. Views and future directions to boost the biomass conversion process are also mentioned.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
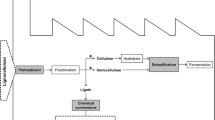
In the twenty-first century, the demand for fossil fuels is increasing progressively beside improvements within the quality of life, inauguration of the economic revolution, and increase of the global population. It’s long been recognized that the rise in the rate of fossil fuel consumption not solely ends up in decreasing fuel reserves; however, it conjointly includes an important adverse impact on the surroundings, leading to raised health risks and the threat of worldwide climate change (Panwar et al. 2011). Hence, there is a need to develop a sustainable alternative to fossil fuels. The usage of potential food resources in first-generation biorefineries has sparked intensive dialogue, due to the lack of land for food cultivation and large commitment of land required for biofuel production (Bugg et al. 2011). The food vs fuel dialogue encouraged the event of second-generation biofuel industry that uses lignocellulose biomass as feedstock. Lignocellulose biomass is one among the foremost rich sources of renewable energy and is principally composed of polysaccharide (40–50%), hemicellulose (20–40%), and polymer (20–30%).
Enzymatic hydrolysis reaction of lignocellulosic biomass is one of the key steps for sugar production and a requirement for succeeding conversion of sugars to biofuels and chemicals.
However, the presence of lignin in biomass forms a major obstacle for efficient utilization of cellulose and hemicellulose in biofuel production. The inhibition impact of lignin on enzymatic hydrolysis is classified into three classes: (1) Enzymes are adsorbed on lignin polymer through hydrophobic, static, and hydrogen bonding interactions. (2) Lignin blocks the accessible surface of carbohydrate polysaccharides through the physical and chemical blockage. (3) Soluble lignin derivatives such as phenolic compound can deactivate enzymes.
An appropriate pretreatment method is required to overcome this obstacle and makes polysaccharides easily available for enzymatic hydrolysis. To date, various pretreatment methods such as physical, chemical, and physicochemical are used to remove this obstacle (Sun et al. 2016). However, these pretreatment methods may generate inhibitory compounds that interfere with the enzymatic hydrolysis and succeeding fermentation processes.
There is an increasing interest to use biological pretreatments as an alternative physicochemical treatment (Kuijk et al. 2015; Lee et al. 2012). For the last decades, white-rot fungi have received a lot of interest because of their potential to reduce lignin content in wood and other lignocellulosics (Dashtban et al. 2010). Through an extracellular ligninolytic system, comprising a complex cocktail of oxidative enzymes, the substrate is oxidized by single electron withdrawals to create reactive radical intermediates. The substrate is then modified through continuous nonspecific and enzyme-independent chain reactions to produce various free radicals (Zhou et al. 2013). The enzymes responsible for degradation of lignin in white-rot fungi belong to four major groups: lignin peroxidases (LiP) (EC 1.11.1.14), manganese-dependent peroxidases (MnP) (EC 1.11.1.13), versatile peroxidases (VP) (EC 1.11.1.16), and laccases (Lac) (EC 1.10.3.2). In addition to LiP, MnP, VP, and Lac, other types of oxidative enzymes, together denominated auxiliary enzymes, have been reported to assist the lignin oxidation. These include aryl alcohol dehydrogenase, glyoxal oxidase, and pyranose oxidase, which have been reported to enhance the process through their peroxidase generating activity (Martinez et al. 2005; Ruiz-Duenas and Martinez 2009). The impact of the individual oxidases, as well as their possible interactions in the white-rot fungi degrading system, has not been fully elucidated. Published literature tend to deal with either single enzymes or a broth produced by specific fungal strains. The peroxidases (LiP, MnP, VP) are all dependent on hydrogen peroxide in order to be efficient enzyme catalysts in lignin oxidation. The hazardous effect of hydrogen peroxide prohibits the use of peroxidases in the food industry and severely complicates the utilization in other industrial applications. Besides being an additional financial expense, the corrosive action of hydrogen peroxidase is problematic in large scale, and locally high concentrations may inhibit or inactivate the enzymes.
However, the aim of this chapter is to provide a compressive review of the application potential of laccases in lignocellulose processing, such as delignification and detoxification of biomass by removal of phenolic compounds from hydrolysates and highlighting future prospects of the laccases in biofuel production (Table 1).
2 Laccases (Lac)
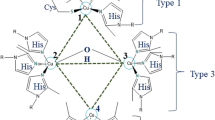
Laccases (EC 1.10.3.2, benzenediol-oxygen oxidoreductases) are polyphenol oxidases that belong to the multicopper oxidase family and the superfamily of cupredoxins. Laccases (EC 1.10.3.2, benzenediol-oxygen oxidoreductases) are polyphenol oxidases that belong to the multicopper oxidase family and the superfamily of cupredoxins. Laccase catalyzes oxidization of various substrates like diphenols, methoxy-substituted monophenols, as well as aliphatic and aromatic amines (Kudanga and Le Roes-Hill 2014). The fact that these polyphenol oxidases use O2 as the final electron acceptor rather than H2O2, differentiate them from other ligninolytic enzymes. Moreover, they are cofactor independent and produce water as a sole by-product; they are very attractive biocatalysts for a variety of industrial applications. Laccases incorporate three copper atoms: one paramagnetic type 1 copper (T1 Cu), this is responsible for their characteristic blue color, wherein the oxidation of the substrate proceeds, one type 2 copper (T2 Cu), and an antiferromagnetically coupled binuclear copper pair of type 3 coppers (T3 Cu) that conform a trinuclear cluster wherein molecular O2 is decreased to two molecules of H2O (Mate and Alcalde 2015). During substrate oxidation, laccases receive electrons at the T1 copper sites from electron-donating substrate and then transfer the electrons to the trinuclear center (T2/T3) (Shleev et al. 2005). The substrate specificity and catalytic efficiency of the enzymes depend on the redox potential of the T1 Cu center, which is used to categorize the enzymes as low (0.35–0.5 V) or medium to high (0.5–0.8 V) redox laccases (Gutierrez et al. 2009). The main structural determinant believed to cause changes in the redox potential is the presence of an axial ligand at the T1 Cu center. The low-redox potential laccase from ascomycete fungus Melanocarpus albomyces (E 0 = 0.46 V) has a Met residue as an axial ligand, while high-redox potential laccases from basidiomycete fungi Trametes hirsuta (E 0 = 0.78 V) and Trametes versicolor (E 0 = 0.80 V) have a Phe residue as an axial ligand (Frasconi et al. 2010). It has been proposed that such an axial ligand helps to stabilize the center, thereby lowering the redox potential. If the redox potential of the phenolic substrate is higher than that of the enzyme, it needs a small compound called a mediator to be able to abstract an electron (see next section). Thus, to evaluate potential applications of a laccase, it is important to know its redox potential.
Regardless of an increase in the number of reports exploring the enzymatic activities of laccases, the role of laccases in lignocellulose or lignin processing remains unclear. Earlier studies showed the prominent involvement of laccases in lignin synthesis, and the consensus was that laccases do not take part in lignin degradation (Lundell et al. 2010). This is supported by studies using P. chrysosporium, which can degrade lignin but lack laccase activity. However, on the contrary, Eggert et al. (1997) identified laccase as the only ligninolytic enzyme predominantly secreted by the lignin-degrading fungi Pycnoporus cinnabarinus. Moreover, Xie et al. (2014) and Ryu et al. (2013) provided evidence toward the involvement of laccase in several stages of wood degradation by applying systematic gene deletion in the filamentous fungus Podospora anserina and overexpression of laccase in Polyporus brumalis. Also, Ander and Eriksson (1976) have shown possible involvement of laccase in lignin degradation by demonstrating that a laccase-deficient Sporotrichum pulverulentum mutant (obtained by UV mutagenesis) did not change lignin polymer; however, the wild type transforms the polymer easily.
3 Lignin
Lignin is a very composite biopolymer within the plant cell wall and is usually taken into consideration as a contaminant or glue in industrial applications which includes pulp/paper and biofuel production (Ko et al. 2015). It comprises approximately 20–30% of the lignocellulose, wherein it forms a matrix in close association with the cellulose and hemicellulose (Bugg et al. 2011).The lignin content varies between different types of plants, e.g., softwood contains 30%, hardwood 20–25%, and grass lignin only 10–15% of the total plant biomass. Due to the distinctly high-lignin content material in biomass, lignin removal is a crucial technical issue both for paper and bioethanol production (Chen and Dixon 2007). However, the lignin polymer is highly resistant to breakdown, and it is, therefore, considerable interest in methods to break down the lignin. Much attention has been drawn to the improvement of eco-friendly technologies for treating lignin with the aid of ligninolytic enzymes. Even though a number of the study’s results are encouraging, there is still a far way to go, and numerous hurdles need to be addressed.
Structurally lignin is an amorphous tridimensional polymer of three different cinnamyl alcohol monomers: p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol (Fig. 1a–c). The three monolignols differ in the degree of methoxylation and are catalyzed by an oxidative enzyme (e.g., peroxidases or laccases) to form the corresponding p-hydroxyphenol (H), guaiacyl (G), and syringyl (S) lignin components, respectively. As part of the increased interest in the utilization of lignin, a lot of effort has been put into studies of lignin structure and mechanisms involved in the synthesis. The three monomeric precursors are predominately linked either by ether or C–C bonds. In native lignin, two-thirds or more of the total linkages are ether bonds, and the rest are C–C bonds. During the radical polymerization process, the complexity of lignin rises due to resonance delocalization of electrons, forming a three-dimensional, irregular matrix (Vanholme et al. 2010; Wong 2009). This results in a range of different ether and C–C linkages between the three major precursors, including β-O-4, β-β, α-O-4, 4-O-5, 5–5, α-O-γ, and β-1 (Li et al. 2015) (Fig. 1d–i). The binding types are, to some extent, affected by the content of the H-, G-, and S-units in the lignin (Djikanovic et al. 2012). Softwood lignins are often made out of G-units and minor quantities of H-units, whereas hard wood lignins are made out of G- and S-units in roughly equal ratios (Espineira et al. 2011). Softwoods are known to have a more branched structure compared to hardwood, which demonstrates a linear structure due to the higher amounts of S-units. Grass lignins contain significant amounts of G-, S-, and H-units, but the ratios between the three units and the resulting types of bindings vary within this group of plants (Buranov and Mazza 2008). The composition of natural lignin is influenced by the plant’s exposure to stress during synthesis (Moura et al. 2010), and it differs among cell kinds and may even range at the extent of individual cell wall layers (Vanholme et al. 2010). Moreover, lignin is covalently bound to the carbohydrate networks in the plant, which altogether impede a consistent determination of native lignin structures. Besides the variation in native structure, lignin-containing biomasses are exposed to different industrial processing which often implies the structural modification of the lignin (Zakzeski et al. 2010). Lignin-containing biomasses with native or industrially modified structures are frequently used in scientific studies to explore new and more sustainable conversion methods and applications (Munk et al. 2015).
The synthetic low molecular weight compound known as lignin model substrate has 1–3 aromatic rings that represent substructures and linkages similar to those observed in natural lignin (Rochefort et al. 2004). The most important compounds are those with β-aryl ether linkages (β-O-4) as they represent the majority of lignin bounds (Majumdar et al. 2014). Aromatic alcohols have also been used as model compounds. Phenolic subunits affect the redox potential (Polak and Jarosz-Wilkolazka 2012), which is closely related to the ability of the enzyme to catalyze oxidation reactions of the substrate. In native lignin, nonphenolic subunits comprise 80–90%, while the remaining 10–20% is phenolic subunits. A distinction between phenolic and nonphenolic lignin model compounds is therefore often used in studies of enzymatic lignin modification.
4 Laccase-Mediator System (LMS)
Mediators are small molecules (synthetic or natural) that help to carry electron in between laccase and normal laccase substrate (higher-redox potential than laccase) consisting of lignin. Overall, the laccase-mediator system helps to oxidize compound by relocating electrons from its phenoxy sites to oxygen. In this way, the mediator expands the oxidation ability of the enzyme. The function of mediators in laccase oxidations is outlined in Fig. 2a. The primary synthetic mediator to be used within the laccase-mediator approach for pulp oxidation/modification was ABTS (2,2′-Azino-bis(three-ethylbenzothiazoline-6-sulfonic acid), which used to be introduced in 1990 (Bourbonnais et al. 1995). They followed that laccase used in combination with a mediator (ABTS) was capable of extending the oxidation of lignin. Considering this discovery, numerous different compounds were delivered for use in the LMS centered for nonphenolic lignin oxidation. Among those are the –NOH mediators (1-hydroxybenzotriazole (HBT), N-hydroxyphthalimide (HPI), violuric acid (VLA), and N-hydroxyacetanilide (NHA)) (Shumakovich et al. 2006; Couto and Sanromán 2007; Moldes and Vidal 2008; Fillat et al. 2010).
(a) The overall oxidation and reduction reactions of the laccase and LMS to delignification. (b) The ET and radical HAT route, (c) suggested mechanism of oxidation by TEMPO (Astolfi et al. 2005)
The more effective oxidation and a broader range of substrates make the laccase-mediator system interesting for industrial application. It has been verified that depending on their chemical structure, mediators follow three different kinds of oxidation mechanism: (I) electron transfer (ET) in the case of ABTS radicals (ABTS•+or ABTS2+), where the mediator abstracts an electron from the substrate.
The particular generating pressure intended for electron abstraction is the distinction in redox potential between the mediator and substrate (Fig. 2b). (II) Hydrogen atom transfer (HAT) for nitroxyl radicals (N–O•) 1-hydroxybenzotriazole (HBT) and violuric acid (VLA). The oxidation of mediators through laccase generates an especially reactive radical (> N–O•), because of the enzymatic elimination of an electron followed by the release of a proton (Fig. 2b). (III).The ionic mechanism is observed for mediators such as TEMPO (Astolfi et al. 2005) (Fig. 2c). LMS were utilized to numerous procedures and exhaustively reviewed several occasions in contemporary years (Riva 2006; Morozova et al. 2007; Husain and Husain 2008; Kunamneni et al. 2008; Widsten and Kandelbauer 2008; Canas and Camarero 2010; Kudanga et al. 2011; Christopher et al. 2014; Kudanga and Le Roes-Hill 2014; Forootanfar and Faramarzi 2015; Munk et al. 2015; Roth and Spiess 2015; Singh et al. 2015). From the documented studies and reviews, it is possible to outline or define the required characteristics for effective mediators. A mediator must be a good laccase substrate, implying that its oxidized and reduced forms must be steady without inactivating the enzymatic reaction and that its reactivity must permit recycling without degeneration.
From an industrial application and environmental point of view, laccase mediators must be eco-friendly and cost-effective. Despite promising results associated with synthetic mediators in LMS (Martin-Sampedro et al. 2011; Gutierrez et al. 2012), there are few drawbacks interfering with the effective use of mediators: they are expensive and they can create toxic compounds. Furthermore, in some reaction, even as oxidizing the mediator, the laccases are deactivated via radicals, or the mediators may be loss mediating capability by transforming into inactive compounds (Kunamneni et al. 2008). Some of these problems could be solved by utilizing the natural mediators and have come around with an increasing interest in the recent years. The important advantage of utilizing natural phenols as mediators is that they are easily obtained from plant materials. Additionally, their low price and toxicity can offer economic and environmental benefits (Canas and Camarero 2010). Lignin-derived phenols used as laccase mediators have been found to perform in a similar way to or even better than the artificial mediator compounds, with elevated reactivity (Fillat et al. 2010).
5 Laccases in Lignocellulose Processing
Laccases have recently attracted attention as candidate enzymes for the biological pretreatment of biomass. Due to their likely role as lignin-degrading enzymes, they offer the potential for a highly specific and environmentally friendly method for removing lignin.
Improved knowledge of laccases/laccase-mediator system and their role in lignin degradation will have a significant impact on a wide range of applications focused on lignin degradation. Even though the effect of laccases on lignin has been intensively studied, the only consensus in the literature is that laccases oxidize subunits of lignin into reactive radical intermediates, which lead to lignin modifications.
Earlier studies have investigated the reactivity of laccases by using lignin model compounds. Both phenolic and nonphenolic dimeric β-O-4 lignin model compounds (as more than 50% of lignin structure is composed of β-O-4 bonds) are widely used to study Cα-Cβ bond cleavage by the formation of phenoxy radicals. This leads to α-oxidations, Cα-Cβ, alkyl-aryl, or alkyl-phenyl cleavages that form a range of products (Higuchi 2004; Wong 2009). The phenolic monomers present on the surface of the lignin polymer can be easily oxidized by laccase alone. In studies related to the role of LMS in degradation of lignin or lignin model compounds, it has been found that mediators facilitate a strong lignin oxidation capability in combination with laccases. Several of these studies show a decrease in lignin molecular weight suggesting depolymerization (Martin-Sampedro et al. 2011; Zheng et al. 2012; Rico et al. 2015). However, both depolymerization and polymerization reactions may take place depending on whether the treatment is with laccases alone or with a laccase-mediator system (Shleev et al. 2005). A range of studies where laccases were used in the presence of synthetic mediators like HBT and proven to effectively depolymerize different forms of biomasses(Chen et al. 2012; Gutierrez et al. 2012; Rico et al. 2014). The elucidation of the recently discovered LMS (Pycnoporus cinnabarinus laccase-HBT) has extended the substrate activity of laccase to include oxidation of nonphenolic lignin model compounds (Du et al. 2013). Figure 3 illustrates the oxidation mechanism of nonphenolic substrate (β-O-4 compound). During the oxidation process, LMS is able to form carbon-centered radicals by oxidation of both S- and G-units of nonphenolic lignin units (structure I). In the next step, O2 attacks the carbon-centered radical intermediate, forming unstable structure (structure IIa, β-aryl, or benzylic radical) in a nonenzymatic reaction. The consecutive reactions lead to additional Cα oxidation (structure III), β-ether cleavage, and aromatic ring cleavage. The alternative route involves suggesting radical structure IIa abstract proton from a substrate to form nonradical structure IIb. This can be followed by the cleavage between Cα and Cβ forming structures IV and V (Kawai et al. 2002; Du et al. 2013). Recently, Rico et al. (2014, 2015) showed a decrease in lignin molecular weight with shortened side chains and increased syringyl-to-guaiacyl ratio by applying LMS with laccase from Myceliophthora thermophila and natural mediator methyl syringate. Contradictory, application of LMS has also shown that the oxidizing capability of some mediators leads to polymerization of lignin (Moya et al. 2011), and this has also been demonstrated for ABTS and HBT (Prasetyo et al. 2012).
Systematic delignification mechanism of the lignocellulose substrate by laccase or laccase-mediator system. The LMS-catalyzed nonphenolic oxidation (β-O-4) occurs in four types of reactions such as Cα-oxidation reactions (structure III), followed by β-ether cleavage and aromatic ring cleavage, further Cα-Cβ cleavage (structure IV and V) led decrease in lignin molecular weight (Kawai et al. 2002; Du et al. 2013; Kudanga and Roes-Hill 2014). However, in case of phenolic monomers, laccases subtract electron from –OH of phenolic monomer to form phenoxy radicals; further it undergoes polymerization or condensation via radical coupling (Kudanga and Roes-Hill 2014)
The choice of mediator is an important factor for boosting the effectivity of laccases. Beside mediators, several other factors such as biomass processing, reaction condition in the form of pH, type of solvent, temperature, and other small compounds present may influence the reaction outcome. Shleev et al. (2006) observed different molecular weight in oxidative compounds in response to two of most commonly applied mediators, ABTS and HBT, which support the fact that these two mediators act through different mechanisms. This finding is supported by another study by Hernández Fernaud et al. (2006), who reported similar results using the same mediators but different laccase and substrate. Thus, the type of mediators seems to be important for which reactions laccases may catalyze. However, this topic is rarely addressed in the literature (Moldes and Vidal 2012). In spite of the enormous information availability on the laccase reaction with lignin model compounds and different varieties of natural lignins, a comprehensive knowledge of the LMS is still lacking. With an appropriate mediator at hand, the LMS be examined both for an evaluation of the overall performance of synthetic and natural mediator or to clarify their mechanism of action. Nevertheless, it is also important to use real lignin polymers in experiments. It is also important to be aware of the presence of possible natural mediators in the substrates, which may also influence the balance between polymerization and depolymerization.
The cost of enzyme is one of the fundamental challenges in present-day large-scale biofuel production process. However, several strategies for cost reduction are investigated. For instance, enzyme co-expression or chimeras between laccases and cellulolytic enzymes could help to reduce cost and restrictions of conventional workflow for biofuel production from lignocellulosic biomass (Fonseca-Maldonado et al. 2014). The co-expression of laccases with xylanases or endoglucanases helps to improve catalytic activity, resulting in higher glucose yields (Ribeiro et al. 2011; Furtado et al. 2013; Fonseca-Maldonado et al. 2014). Recently, Zhao et al. (2016) have combined the laccase and Fenton reaction system to assessing the synergism in lignin depolymerization. Apparently, bacterial fermentation (Rhodococcus opacus) in the presence of laccase led to significant improvements in hydroxyl group degradation, lignin molecular weight, cell growth, and overall higher lignin consummation and improved lipid production (17-fold). However, immobilization of laccase on Sepa beads carriers has been shown to polymerize toxic phenolics from hydrolysate by precipitation onto the carrier surface. Incorporation of anion exchange as a subsequent step led to the reduction of HMF, formic acid, acetic acid, and levulinic acid and helped to improve the ferment ability of an organosolv wheat straw hydrolysate (Ludwig et al. 2013). Immobilization of enzymes is advantageous for commercial application due to convenience in handling and recycling and improves stability. These capabilities can be exploited for designing eco-friendly conversion of lignin toward numerous useful products or third generation of biorefineries.
Enzymatic/LMS delignification followed by alkali pretreatments can increase the enzymatic hydrolysis yields despite the fact that removal of lignin does not drastically improve (Gutierrez et al. 2012; Li et al. 2012). However, Li et al. (2012) reported combination of alkaline and laccase treatment significantly increases porosity and surface area of corn straw, which result in noteworthy saccharification yield as compared to alkaline treatment alone. Gutiérrez et al. (2012) observed the same effect for wood (Eucalyptus globulus) and non-wood (Pennisetum purpureum) biomass using a Trametes villosa laccase, in combination with alkaline extraction and HBT.
6 Techniques for Identifying Enzymatic Lignin Degradation
The use of established tools and techniques and the improvement of novel assays are essential to progress research on lignocellulosic biofuels. Such tools and techniques additionally inform us on the structure and conformation of lignin and enable us to assess the various strategies for biomass processing. To evaluate or elucidate the mechanism of biological degradation or modification of the lignin is a challenging task, because of its complex macromolecular structure and intimate association to cellulose and hemicellulose (Gellerstedt and Henriksson 2008). Analytical methods exist as either nondestructive (mostly spectroscopic) or destructive (use chemical or thermal degradation and fragment analysis) techniques. Nonetheless, some cutting-edge analytical techniques such as two-dimensional (2D) or three-dimensional (3D) nuclear magnetic resonance (NMR) can be used for the evaluation of complicated macromolecules like lignin (Gutierrez et al. 2012; Nugroho Prasetyo et al. 2010; Salanti et al. 2010; Rico et al. 2015). The combination of quantitative 2D or 3D NMR with 1H and 13C NMR spectroscopic techniques represents an extraordinary advance in the structural analysis of this complex polymer (Balakshin et al. 2011; Liu et al. 2014; Mori et al. 2015; Rico et al. 2015). Moreover, fluorescence monitoring, Fourier transform-infrared (FTIR) spectroscopy, and size exclusion chromatography (SEC) are also used to study chemical changes in lignin (Ibarra et al. 2007; Maijala et al. 2012; Sun et al. 2013; Majumdar et al. 2014; Oliva-Taravilla et al. 2015; Rajak and Banerjee 2015). The usage of pyrolysis technique in combination with mass spectrometry (Py-GC/MS) has proved to be of specific interest within the look at of lignocellulosic macromolecules (Rio et al. 2002; Dey Laskar et al. 2013; Du et al. 2013; Heap et al. 2014).
7 Conclusion
Laccases are potent enzymes for industrial applications in sustainable biomass conversion, but some hurdles need to be overcome before this can be realized. In spite of intensive studies in the field, it is still not clear how to control and most efficiently utilize the laccases. The type of mediator and biomass appear to influence the resulting lignin oxidation, but it is likely that the importance of other factors may be discovered. Accordingly, laccase-catalyzed detoxification or depolymerization needs to be properly incorporated into relevant process steps of the biorefinery. By exploring greater diversity of the laccase-producing organisms (which can react with and metabolize lignin) and discovering new natural mediators (which can be applied in an LMS system for lignin depolymerization), we may be able to gain new insight into strategies for biomass deconstruction. A more thorough interpretation of analysis methodologies along with application of representative reference enzymes, mediators, and model substrates may facilitate the increased understanding of the mechanisms that occur when laccases interact with lignocellulosic biomass.
References
Alvira P, Moreno AD, Ibarra D, Saez F, Ballesteros M (2013) Improving the fermentation performance of saccharomyces cerevisiae by laccase during ethanol production from steam-exploded wheat straw at high-substrate loadings. Biotechnol Prog 29:74–82. doi:10.1002/btpr.1666
Ander P, Eriksson K-E (1976) The importance of phenol oxidase activity in lignin degradation by the white-rot fungus Sporotrichum pulverulentum. Arch Microbiol 109:1–8. doi:10.1007/BF00425105
Astolfi P, Brandi P, Galli C, Gentili P, Gerini MF, Greci L, Lanzalunga O (2005) New mediators for the enzyme laccase: mechanistic features and selectivity in the oxidation of non-phenolic substrates. New J Chem 29:1308–1317. doi:10.1039/b507657a
Balakshin M, Capanema E, Gracz H, Chang HM, Jameel H (2011) Quantification of lignin-carbohydrate linkages with high-resolution NMR spectroscopy. Planta 233:1097–1110. doi:10.1007/s00425-011-1359-2
Bourbonnais R, Paice MG, Reid ID, Lanthier P, Yaguchi M (1995) Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,2′-Azinobis(3-Ethylbenzthiazoline-6-Sulfonate) in kraft lignin depolymerization. Appl Environ Microbiol 61:1876–1880
Bugg TD, Ahmad M, Hardiman EM, Rahmanpour R (2011) Pathways for degradation of lignin in bacteria and fungi. Nat Prod Rep 28:1883–1896. doi:10.1039/c1np00042j
Buranov AU, Mazza G (2008) Lignin in straw of herbaceous crops. Ind Crop Prod 28:237–259. doi:10.1016/j.indcrop.2008.03.008
Canas AI, Camarero S (2010) Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol Adv 28:694–705. doi:10.1016/j.biotechadv.2010.05.002
Chen F, Dixon RA (2007) Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol 25:759–761. doi:10.1038/nbt1316
Chen Q, Marshall MN, Geib SM, Tien M, Richard TL (2012) Effects of laccase on lignin depolymerization and enzymatic hydrolysis of ensiled corn stover. Bioresour Technol 117:186–192. doi:10.1016/j.biortech.2012.04.085
Christopher LP, Yao B, Ji Y (2014) Lignin biodegradation with laccase-mediator systems. Front Energy Res 2:12. doi:10.3389/fenrg.2014.00012
Couto SR, Sanromán MÁ (2007) The effect of violuric acid on the decolourization of recalcitrant dyes by laccase from Trametes hirsuta. Dyes Pigments 74:123–126. doi:10.1016/j.dyepig.2006.01.021
Dashtban M, Schraft H, Syed TA, Qin W (2010) Fungal biodegradation and enzymatic modification of lignin. Int J Biochem Mol Biol 1:36–50
del Rio JC, Speranza M, Gutierrez A, Martinez MJ, Martinez AT (2002) Lignin attack during eucalypt wood decay by selected basidiomycetes: a Py-GC/MS study. J Anal Appl Pyrolysis 64:421–431. doi:10.1016/S0165-2370(02)00043-8
Dey Laskar D, Ke J, Zeng J, Gao X, Chen S (2013) Py-GC/MS as a powerful and rapid tool for Determining lignin compositional and structural changes in biological processes. Curr Anal Chem 9:335–351. doi:10.2174/1573411011309030003
Du XY, Li JB, Gellerstedt G, Rencoret J, Del Rio JC, Martinez AT, Gutierrez A (2013) Understanding pulp delignification by laccase-mediator systems through isolation and characterization of lignin-carbohydrate complexes. Biomacromolecules 14:3073–3080. doi:10.1021/bm4006936
Djikanovic D, Simonovic J, Savic A, Ristic I, Bajuk-Bogdanovic D, Kalauzi A, Cakic S, Budinski-Simendic J, Jeremic M, Radotic K (2012) Structural differences between lignin model polymers synthesized from various monomers. J Polym Environ 20(2):607–617. doi:10.1007/s10924-012-0422-9
Eggert C, Temp U, Eriksson KEL (1997) Laccase is essential for lignin degradation by the white-rot fungus Pycnoporus cinnabarinus. FEBS Lett 407:89–92. doi:10.1016/S0014-5793(97)00301-3
Espineira JM, Uzal EN, Ros LVG, Carrion JS, Merino F, Barcelo AR, Pomar F (2011) Distribution of lignin monomers and the evolution of lignification among lower plants. Plant Biol 13:59–68. doi:10.1111/j.1438-8677.2010.00345.x
Fang ZM, Liu XM, Chen LY, Shen Y, Zhang XC, Fang W, Wang XT, Bao XM, Xiao YZ (2015) Identification of a laccase Glac15 from Ganoderma lucidum 77002 and its application in bioethanol production. Biotechnol Biofuels 8:54. doi:10.1186/s13068-015-0235-x
Fillat A, Colom JF, Vidal T (2010) A new approach to the biobleaching of flax pulp with laccase using natural mediators. Bioresour Technol 101:4104–4110. doi:10.1016/j.biortech.2010.01.057
Fonseca-Maldonado R, Ribeiro LF, Furtado GP, Arruda LM, Meleiro LP, Alponti JS, Botelho-Machado C, Vieira DS, Bonneil E, Furriel RDM, Thibault P, Ward RJ (2014) Synergistic action of co-expressed xylanase/laccase mixtures against milled sugar cane bagasse. Process Biochem 49:1152–1161. doi:10.1016/j.procbio.2014.03.027
Forootanfar H, Faramarzi MA (2015) Insights into laccase producing organisms, fermentation states, purification strategies, and biotechnological applications. Biotechnol Prog 31:1443–1463. doi:10.1002/btpr.2173
Frasconi M, Favero G, Boer H, Koivula A, Mazzei F (2010) Kinetic and biochemical properties of high and low redox potential laccases from fungal and plant origin. Biochim Biophys Acta 1804(4):899–908. doi:10.1016/j.bbapap.2009.12.018
Furtado GP, Ribeiro LF, Lourenzoni MR, Ward RJ (2013) A designed bifunctional laccase/beta-1,3-1,4-glucanase enzyme shows synergistic sugar release from milled sugarcane bagasse. Protein Eng Des Sel 26:15–23. doi:10.1093/protein/gzs057
Gellerstedt G, Henriksson G (2008) Chapter 9 – Lignins: major sources, structure and properties. In: Gandini MNB (ed) Monomers polymers and composites from renewable resources. Elsevier, Amsterdam, pp 201–224
Gutierrez A, del Rio JC, Martinez AT (2009) Microbial and enzymatic control of pitch in the pulp and paper industry. Appl Microbiol Biotechnol 82:1005–1018. doi:10.1007/s00253-009-1905-z
Gutierrez A, Rencoret J, Cadena EM, Rico A, Barth D, del Rio JC, Martinez AT (2012) Demonstration of laccase-based removal of lignin from wood and non-wood plant feedstocks. Bioresour Technol 119:114–122. doi:10.1016/j.biortech.2012.05.112
Heap L, Green A, Brown D, van Dongen B, Turner N (2014) Role of laccase as an enzymatic pretreatment method to improve lignocellulosic saccharification. Cat Sci Technol 4:2251–2259. doi:10.1039/c4cy00046c
Hernández Fernaud JR, Carnicero A, Perestelo F, Hernández Cutuli M, Arias E, Falcón MA (2006) Upgrading of an industrial lignin by using laccase produced by Fusarium proliferatum and different laccase-mediator systems. Enzym Microb Technol 38:40–48. doi:10.1016/j.enzmictec.2005.01.043
Higuchi T (2004) Microbial degradation of lignin: role of lignin peroxidase, manganese peroxidase, and laccase. Proc Jpn Acad Ser B Phys Biol Sci 80:204–214. doi:10.2183/pjab.80.204
Husain M, Husain Q (2008) Applications of redox mediators in the treatment of organic pollutants by using oxidoreductive enzymes: a review. Crit Rev Environ Sci Technol 38:1–42. doi:10.1080/10643380701501213
Hyeon JE, You SK, Kang DH, Ryu SH, Kim M, Lee SS, Han SO (2014) Enzymatic degradation of lignocellulosic biomass by continuous process using laccase and cellulases with the aid of scaffoldin for ethanol production. Process Biochem 49:1266–1273. doi:10.1016/j.procbio.2014.05.004
Ibarra D, Chavez MI, Rencoret J, del Rio JC, Gutierrez A, Romero J, Camarero S, Martinez MJ, Jimenez-Barbero J, Martinez AT (2007) Structural modification of eucalypt pulp lignin in a totally chlorine-free bleaching sequence including a laccase-mediator stage. Holzforschung 61:634–646. doi:10.1515/Hf.2007.096
Kalyani D, Tiwari MK, Li JL, Kim SC, Kalia VC, Kang YC, Lee JK (2015) A highly efficient recombinant laccase from the yeast Yarrowia lipolytica and its application in the hydrolysis of biomass. PLoS One 10:e0120156. doi:10.1371/journal.pone.0120156
Kapoor RK, Rajan K, Carrier DJ (2015) Applications of Trametes versicolor crude culture filtrates in detoxification of biomass pretreatment hydrolyzates. Bioresour Technol 189:99–106. doi:10.1016/j.biortech.2015.03.100
Kawai S, Nakagawa M, Ohashi H (2002) Degradation mechanisms of a nonphenolic β-O-4 lignin model dimer by Trametes versicolor laccase in the presence of 1-hydroxybenzotriazole. Enzym Microb Technol 30:482–489. doi:10.1016/S0141-0229(01)00523-3
Ko JK, Um Y, Park YC, Seo JH, Kim KH (2015) Compounds inhibiting the bioconversion of hydrothermally pretreated lignocellulose. Appl Microbiol Biotechnol 99:4201–4212. doi:10.1007/s00253-015-6595-0
Kudanga T, Le Roes-Hill M (2014) Laccase applications in biofuels production: current status and future prospects. Appl Microbiol Biotechnol 98:6525–6542. doi:10.1007/s00253-014-5810-8
Kudanga T, Nyanhongo GS, Guebitz GM, Burton S (2011) Potential applications of laccase-mediated coupling and grafting reactions: a review. Enzym Microb Technol 48:195–208. doi:10.1016/j.enzmictec.2010.11.007
Kunamneni A, Camarero S, Garcia-Burgos C, Plou FJ, Ballesteros A, Alcalde M (2008) Engineering and applications of fungal laccases for organic synthesis. Microb Cell Factories 7:32. doi:10.1186/1475-2859-7-32
Lee KM, Kalyani D, Tiwari MK, Kim TS, Dhiman SS, Lee JK, Kim IW (2012) Enhanced enzymatic hydrolysis of rice straw by removal of phenolic compounds using a novel laccase from yeast Yarrowia lipolytica. Bioresour Technol 123:636–645. doi:10.1016/j.biortech.2012.07.066
Li J, Sun FH, Li XZ, Yan ZY, Yuan YX, Liu XF (2012) Enhanced saccharification of corn straw pretreated by alkali combining crude ligninolytic enzymes. J Chem Technol Biotechnol 87:1687–1693. doi:10.1002/jctb.3818
Li C, Zhao X, Wang A, Huber GW, Zhang T (2015) Catalytic transformation of lignin for the production of chemicals and fuels. Chem Rev 115:11559–11624. doi:10.1021/acs.chemrev.5b00155
Liu L, Qian C, Jiang L, Yu HQ (2014) Direct three-dimensional characterization and multiscale visualization of wheat straw deconstruction by white rot fungus. Environ Sci Technol 48:9819–9825. doi:10.1021/es5020983
Ludwig D, Amann M, Hirth T, Rupp S, Zibek S (2013) Development and optimization of single and combined detoxification processes to improve the fermentability of lignocellulose hydrolyzates. Bioresour Technol 133:455–461. doi:10.1016/j.biortech.2013.01.053
Lundell TK, Makela MR, Hilden K (2010) Lignin-modifying enzymes in filamentous basidiomycetes – ecological, functional and phylogenetic review. J Basic Microbiol 50:5–20. doi:10.1002/jobm.200900338
Maijala P, Mattinen ML, Nousiainen P, Kontro J, Asikkala J, Sipila J, Viikari L (2012) Action of fungal laccases on lignin model compounds in organic solvents. J Mol Catal B Enzym 76:59–67. doi:10.1016/j.molcatb.2011.12.009
Majumdar S, Lukk T, Solbiati JO, Bauer S, Nair SK, Cronan JE, Gerlt JA (2014) Roles of small laccases from Streptomyces in lignin degradation. Biochemistry 53:4047–4058. doi:10.1021/bi500285t
Martinez AT, Speranza M, Ruiz-Duenas FJ, Ferreira P, Camarero S, Guillen F, Martinez MJ, Gutierrez A, del Rio JC (2005) Biodegradation of lignocellulosics: microbial chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol 8:195–204
Martin-Sampedro R, Capanema EA, Hoeger I, Villar JC, Rojas OJ (2011) Lignin changes after steam explosion and laccase-mediator treatment of eucalyptus wood chips. J Agric Food Chem 59:8761–8769. doi:10.1021/jf201605f
Mate DM, Alcalde M (2015) Laccase engineering: from rational design to directed evolution. Biotechnol Adv 33:25–40. doi:10.1016/j.biotechadv.2014.12.007
Matsakas L, Rova U, Christakopoulos P (2015) Sequential parametric optimization of methane production from different sources of forest raw material. Front Microbiol 6(1163). doi:10.3389/fmicb.2015.01163
Moilanen U, Kellock M, Vamai A, Andberg M, Viikari L (2014) Mechanisms of laccase-mediator treatments improving the enzymatic hydrolysis of pre-treated spruce. Biotechnol Biofuels 7:177. doi:10.1186/S13068-014-0177-8
Moldes D, Vidal T (2008) Laccase-HBT bleaching of eucalyptus kraft pulp: influence of the operating conditions. Bioresour Technol 99:8565–8570. doi:10.1016/j.biortech.2008.04.008
Moldes D, Vidal T (2012) Laccase for biobleaching of eucalypt kraft pulp by means of a modified industrial bleaching sequence. Biotechnol Prog 28:1225–1231. doi:10.1002/btpr.1594
Moreno AD, Ibarra D, Ballesteros I, Gonzalez A, Ballesteros M (2013a) Comparing cell viability and ethanol fermentation of the thermotolerant yeast Kluyveromyces marxianus and Saccharomyces cerevisiae on steam-exploded biomass treated with laccase. Bioresour Technol 135:239–245. doi:10.1016/j.biortech.2012.11.095
Moreno AD, Tomas-Pejo E, Ibarra D, Ballesteros M, Olsson L (2013b) Fed-batch SSCF using steam-exploded wheat straw at high dry matter consistencies and a xylose-fermenting Saccharomyces cerevisiae strain: effect of laccase supplementation. Biotechnol Biofuels 6:160. doi:10.1186/1754-6834-6-160
Moreno AD, Ibarra D, Alvira P, Tomás-Pejó E, Ballesteros M (2015) Exploring laccase and mediators behavior during saccharification and fermentation of steam-exploded wheat straw for bioethanol production. J Chem Technol Biotechnol 91:1816–1825. doi:10.1002/jctb.4774
Mori T, Tsuboi Y, Ishida N, Nishikubo N, Demura T, Kikuchi J (2015) Multidimensional high-resolution magic angle spinning and solution-state NMR characterization of (13)C-labeled plant metabolites and lignocellulose. Sci Rep 5:11848. doi:10.1038/srep11848
Morozova OV, Shumakovich GP, Shleev SV, Yaropolov YI (2007) Laccase-mediator systems and their applications: a review. Appl Biochem Microbiol 43:523–535. doi:10.1134/S0003683807050055
Moura JCMS, Bonine CAV, Viana JDF, Dornelas MC, Mazzafera P (2010) Abiotic and biotic stresses and changes in the lignin content and composition in plants. J Integr Plant Biol 52:360–376. doi:10.1111/j.1744-7909.2010.00892.x
Moya R, Saastamoinen P, Hernandez M, Suurnakki A, Arias E, Mattinen ML (2011) Reactivity of bacterial and fungal laccases with lignin under alkaline conditions. Bioresour Technol 102:10006–10012. doi:10.1016/j.biortech.2011.08.046
Munk L, Sitarz AK, Kalyani DC, Mikkelsen JD, Meyer AS (2015) Can laccases catalyze bond cleavage in lignin? Biotechnol Adv 33:13–24. doi:10.1016/j.biotechadv.2014.12.008
Nugroho Prasetyo E, Kudanga T, Ostergaard L, Rencoret J, Gutierrez A, del Rio JC, Ignacio Santos J, Nieto L, Jimenez-Barbero J, Martinez AT, Li J, Gellerstedt G, Lepifre S, Silva C, Kim SY, Cavaco-Paulo A, Seljebakken Klausen B, Lutnaes BF, Nyanhongo GS, Guebitz GM (2010) Polymerization of lignosulfonates by the laccase-HBT (1-hydroxybenzotriazole) system improves dispersibility. Bioresour Technol 101:5054–5062. doi:10.1016/j.biortech.2010.01.048
Oliva-Taravilla A, Moreno AD, Demuez M, Ibarra D, Tomas-Pejo E, Gonzalez-Fernandez C, Ballesteros M (2015) Unraveling the effects of laccase treatment on enzymatic hydrolysis of steam-exploded wheat straw. Bioresour Technol 175:209–215. doi:10.1016/j.biortech.2014.10.086
Panwar NL, Kaushik SC, Kothari S (2011) Role of renewable energy sources in environmental protection: a review. Renew Sust Energ Rev 15:1513–1524. doi:10.1016/j.rser.2010.11.037
Polak J, Jarosz-Wilkolazka A (2012) Fungal laccases as green catalysts for dye synthesis. Process Biochem 47:1295–1307. doi:10.1016/j.procbio.2012.05.006
Prasetyo EN, Kudanga T, Fischer R, Eichinger R, Nyanhongo GS, Guebitz GM (2012) Enzymatic synthesis of lignin-siloxane hybrid functional polymers. Biotechnol J 7:284–292. doi:10.1002/biot.201100106
Rajak RC, Banerjee R (2015) Enzymatic delignification: an attempt for lignin degradation from lignocellulosic feedstock. RSC Adv 5:75281–75291. doi:10.1039/c5ra09667g
Ribeiro LF, Furtado GP, Lourenzoni MR, Costa AJ, Santos CR, Nogueira SCP, Betini JA, Polizeli MDTM, Murakami MT, Ward RJ (2011) Engineering bifunctional laccase-xylanase chimeras for improved catalytic performance. J Biol Chem 286:43026–43038. doi:10.1074/jbc.M111.253419
Rico A, Rencoret J, Del Rio JC, Martinez AT, Gutierrez A (2014) Pretreatment with laccase and a phenolic mediator degrades lignin and enhances saccharification of Eucalyptus feedstock. Biotechnol Biofuels 7:6. doi:10.1186/1754-6834-7-6
Rico A, Rencoret J, del Rio JC, Martinez AT, Gutierrez A (2015) In-depth 2D NMR study of lignin modification during pretreatment of eucalyptus wood with laccase and mediators. Bioenergy Res 8:211–230. doi:10.1007/s12155-014-9505-x
Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 24:219–226. doi:10.1016/j.tibtech.2006.03.006
Rochefort D, Leech D, Bourbonnais R (2004) Electron transfer mediator systems for bleaching of paper pulp. Green Chem 6:14–24. doi:10.1039/b311898n
Roth S, Spiess AC (2015) Laccases for biorefinery applications: a critical review on challenges and perspectives. Bioprocess Biosyst Eng 38:2285–2313. doi:10.1007/s00449-015-1475-7
Ruiz-Duenas FJ, Martinez AT (2009) Microbial degradation of lignin: how a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb Biotechnol 2:164–177. doi:10.1111/j.1751-7915.2008.00078.x
Ryu SH, Cho MK, Kim M, Jung SM, Seo JH (2013) Enhanced lignin biodegradation by a laccase-overexpressed white-rot fungus polyporus brumalis in the pretreatment of wood chips. Appl Biochem Biotechnol 171:1525–1534. doi:10.1007/s12010-013-0412-y
Salanti A, Zoia L, Tolppa EL, Giachi G, Orlandi M (2010) Characterization of waterlogged wood by NMR and GPC techniques. Microchem J 95:345–352. doi:10.1016/j.microc.2010.02.009
Schroyen M, Vervaeren H, Van Hulle SWH, Raes K (2014) Impact of enzymatic pretreatment on corn stover degradation and biogas production. Bioresour Technol 173:59–66. doi:10.1016/j.biortech.2014.09.030
Shleev S, Tkac J, Christenson A, Ruzgas T, Yaropolov AI, Whittaker JW, Gorton L (2005) Direct electron transfer between copper-containing proteins and electrodes. Biosens Bioelectron 20:2517–2554. doi:10.1016/j.bios.2004.10.003
Shleev S, Persson P, Shumakovich G, Mazhugo Y, Yaropolov A, Ruzgas T, Gorton L (2006) Interaction of fungal laccases and laccase-mediator systems with lignin. Enzyme Microb Technol 39(4):841–847. doi:10.1016/j.enzmictec.2006.01.010
Shumakovich GP, Shleev SV, Morozova OV, Khohlov PS, Gazaryan IG, Yaropolov AI (2006) Electrochemistry and kinetics of fungal laccase mediators. Bioelectrochemistry 69:16–24. doi:10.1016/j.bioelechem.2005.10.001
Singh G, Kaur K, Puri S, Sharma P (2015) Critical factors affecting laccase-mediated biobleaching of pulp in paper industry. Appl Microbiol Biotechnol 99:155–164. doi:10.1007/s00253-014-6219-0
Sitarz A, Mikkelsen JD, Meyer A, Lezyk M (2013) Novel laccase from Ganoderma lucidum capable of enhancing enzymatic degradation of lignocellulolytic biomass. Patent No. WO2014041030, IPC No. C12N9/00
Sun Y, Qiu XQ, Liu YQ (2013) Chemical reactivity of alkali lignin modified with laccase. Biomass Bioenergy 55:198–204. doi:10.1016/j.biombioe.2013.02.006
Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol 199:49–58. doi:10.1016/j.biortech.2015.08.061
van Kuijk SJA, Sonnenberg ASM, Baars JJP, Hendriks WH, Cone JW (2015) Fungal treated lignocellulosic biomass as ruminant feed ingredient: a review. Biotechnol Adv 33:191–202. doi:10.1016/j.biotechadv.2014.10.014
Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W (2010) Lignin biosynthesis and structure. Plant Physiol 153:895–905. doi:10.1104/pp.110.155119
Widsten P, Kandelbauer A (2008) Laccase applications in the forest products industry: a review. Enzym Microb Technol 42:293–307. doi:10.1016/j.enzmictec.2007.12.003
Wong DW (2009) Structure and action mechanism of ligninolytic enzymes. Appl Biochem Biotechnol 157:174–209. doi:10.1007/s12010-008-8279-z
Xie N, Chapeland-Leclerc F, Silar P, Ruprich-Robert G (2014) Systematic gene deletions evidences that laccases are involved in several stages of wood degradation in the filamentous fungus Podospora anserina. Environ Microbiol 16:141–161. doi:10.1111/1462-2920.12253
Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM (2010) The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev 110:3552–3599. doi:10.1021/cr900354u
Zanirun Z, Bahrin EK, Lai-Yee P, Hassan MA, Abd-Aziz S (2015) Enhancement of fermentable sugars production from oil palm empty fruit bunch by ligninolytic enzymes mediator system. Int Biodeterior Biodegrad 105:13–20. doi:10.1016/j.ibiod.2015.08.010
Zhao C, Xie S, Pu Y, Zhang R, Huang F, Ragauskas AJ, Yuan JS (2016) Synergistic enzymatic and microbial lignin conversion. Green Chem 18:1306–1312. doi:10.1039/C5GC01955A
Zheng Z, Li H, Li L, Shao W (2012) Biobleaching of wheat straw pulp with recombinant laccase from the hyperthermophilic Thermus thermophilus. Biotechnol Lett 34:541–547. doi:10.1007/s10529-011-0796-0
Zhou XW, Cong WR, Su KQ, Zhang YM (2013) Ligninolytic enzymes from Ganoderma spp: current status and potential applications. Crit Rev Microbiol 39:416–426. doi:10.3109/1040841X.2012.722606
Acknowledgment
This work was supported by the Research Council of Norway, grant number 238850.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Kalyani, D.C., Madhuprakash, J., Horn, S.J. (2017). Laccases: Blue Copper Oxidase in Lignocellulose Processing. In: Kalia, V. (eds) Microbial Applications Vol.2. Springer, Cham. https://doi.org/10.1007/978-3-319-52669-0_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-52669-0_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52668-3
Online ISBN: 978-3-319-52669-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)