Abstract
Guanosine is a purine nucleoside that has been shown to exhibit antidepressant effects, but the mechanisms underlying its effect are not well established. We investigated if the antidepressant-like effect induced by guanosine in the tail suspension test (TST) in mice involves the modulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, voltage-dependent calcium channel (VDCC), and brain-derived neurotrophic factor (BDNF)/tropomyosin receptor kinase B (TrkB) pathway. We also evaluated if the antidepressant-like effect of guanosine is accompanied by an acute increase in hippocampal and prefrontocortical BDNF levels. Additionally, we investigated if the ability of guanosine to elicit a fast behavioral response in the novelty suppressed feeding (NSF) test is associated with morphological changes related to hippocampal synaptogenesis. The antidepressant-like effect of guanosine (0.05 mg/kg, p.o.) in the TST was prevented by DNQX (AMPA receptor antagonist), verapamil (VDCC blocker), K-252a (TrkBantagonist), or BDNF antibody. Increased P70S6K phosphorylation and higher synapsin I immunocontent in the hippocampus, but not in the prefrontal cortex, were observed 1 h after guanosine administration. Guanosine exerted an antidepressant-like effect 1, 6, and 24 h after its administration, an effect accompanied by increased hippocampal BDNF level. In the prefrontal cortex, BDNF level was increased only 1 h after guanosine treatment. Finally, guanosine was effective in the NSF test (after 1 h) but caused no alterations in dendritic spine density and remodeling in the ventral dentate gyrus (DG). Altogether, the results indicate that guanosine modulates targets known to be implicated in fast antidepressant behavioral responses (AMPA receptor, VDCC, and TrkB/BDNF pathway).
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major depressive disorder (MDD) is a prevalent psychiatric disorder with significant socioeconomic impact [1]. The pharmacotherapy for MDD has significant limitations, including low rates of remission [2, 3] and several side effects besides a long time to produce a therapeutic response [4, 5].
The glutamatergic system has been recognized as an important target for novel and rapid-acting antidepressants [6]. Agents that positively modulate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors have been used as cognitive enhancing drugs and are effective on cellular and behavioral models of learning and memory besides contributing to increase the expression of brain-derived neurotrophic factor (BDNF) [7, 8]. BDNF has a crucial role for the behavioral responses of conventional antidepressants following their chronic administration [9]. Moreover, the mechanisms underlying the effects of the fast-acting antidepressants like ketamine are dependent, at least in part, on the fast increase in neuronal BDNF [8]. The role of BDNF in the pathophysiology of MDD is also supported by postmortem studies that have indicated a reduction in mRNA and protein levels of BDNF and its receptor tropomyosin receptor kinase B (TrkB) in the hippocampus, prefrontal cortex (PFC), and locus coeruleus of depressed individuals when compared to controls [10, 11]. Additionally, an increase in BDNF may occur as a consequence of the activation of AMPA receptor that leads to the stimulation of L-type voltage-dependent calcium channels (VDCCs) [8, 12]. These events result in mechanistic target of rapamycin (mTOR) activation with the consequent translation of proteins required for the formation, maturation, and function of new dendritic spines [13]. Particularly, the activation of mTOR pathway has been functionally linked to the phosphorylation of P70S6K (Thr389) and synthesis of synaptic proteins such as PSD-95, and synapsin I, which are essential for the rapid antidepressant effect of ketamine [14]. Although ketamine promotes a rapid and persistent antidepressant effect in refractory depressive patients, it possesses psychomimetic properties, potential for abuse and may cause long-term neurotoxicity [15].
Considering the limitation of antidepressant therapy, our group has investigated the antidepressant properties of glutamatergic modulators, including guanosine [16]. It is a purine nucleoside released mainly by astrocytes under normally physiological conditions but mostly under pathological events [17, 18]. Guanosine has been reported to exhibit neuroprotective and trophic effects [18, 19]. Previous studies showed that guanosine exerts an antidepressant-like effect in the tail suspension test (TST) and forced swimming test (FST) by activating phosphatidylinositol 3-kinase (PI3K)/protein kinaseB (Akt) and mTOR signaling pathways [20]. Several studies have associated the activation of these pathways with synaptic plasticity, learning, memory, and antidepressant action [14, 21,22,23,24,25]. Taking into account that the antidepressant-like effect of guanosine involves the blockade of the responses mediated by N-methyl-D-aspartate (NMDA) receptors [20] and considering that enhancement of synaptic function and antidepressant responses involves AMPA and NMDA receptors [26, 27], the present study was carried out to investigate: (a) if the antidepressant-like effect of guanosine in the TST involves the modulation of AMPA receptors, VDCC, BDNF/TrkB signaling, and its downstream signaling targets: P70S6K (Thr389), PSD-95, and synapsin I; (b) if guanosine has a long-lasting antidepressant-like effect (up to 24 h) in the TST; (c) if guanosine is effective to acutely increase hippocampal and prefrontocortical BDNF levels; (d) if the effect of guanosine in the novelty suppressed feeding (NSF) test (a behavioral paradigm responsive only to chronic treatment with conventional antidepressants), is associated with alterations in the dendritic spines density and remodeling in the ventral dentate gyrus (DG).
Materials and methods
Animals
This study was performed in 207 adult female Swiss mice (2 months, 30–40 g) provided by the animal facility of the Federal University of Santa Catarina (Florianópolis, Brazil). The animals were maintained at 20–22 °C with free access to water and food, under a 12:12 h light/dark cycle, with lights on at 7:00 a.m. Mice were caged in groups of 12 in a 41 × 34 × 16 cm cage. The cages were placed in the experimental room for acclimatization 24 h before the tests, and manipulations were carried out between 9.00 a.m. and 5.00 p.m. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and experiments were performed after approval of the protocols by the Ethics Committee of the Institution (PP00795 and 7485180518 Protocol). All efforts were made to minimize animal suffering.
Drugs and treatment
The following drugs were used: guanosine, 6,7-dinitroquinoxaline-2,3-dione (DNQX) (AMPA receptor antagonist), verapamil (VDCC blocker), K-252a (TrkBantagonist), and BDNF antibody (rabbit polyclonal IgG). In all protocols, the appropriate vehicles were used in the control groups (distilled water to oral (p.o.) route, sterile saline (0.9% NaCl) to intraperitoneal (i.p.) route and 0.1% dimethyl sulfoxide to intracerebroventricular (i.c.v.) route). Guanosine was dissolved in distilled water and was given p.o. by gavage. Verapamil was dissolved in sterile saline (0.9% NaCl) and administered by i.p. route. DNQX, K-252a, and BDNF antibody were dissolved in a final concentration of 0.1% dimethyl sulfoxide and were administered by i.c.v. route. The i.c.v. injections were performed by employing a “free hand” method according to the procedure previously described [28]. Briefly, a 0.4-mm external diameter hypodermic needle attached to a 5 μL Hamilton syringe by polyethylene tubing was inserted perpendicularly through the skull. The drugs were then administered in a volume of 3 μL into the left lateral ventricle, according to the following coordinates from bregma: AP − 0.6 mm, ML + 1.1 mm, DV − 1.0 mm [29]. The injection was given over 30 s, and the needle remained in place for another 30 s in order to avoid the reflux of the substances injected. I.c.v. injections were performed by an experienced investigator, and after dissection of the brain of the animal, the success of the injection was examined, macroscopically, discarding results from mice presenting misplacement of the injection site or any sign of cerebral hemorrhage (< 5%).
Experimental protocol
In order to investigate the involvement of AMPA receptors in the antidepressant-like effect of guanosine in the TST, mice were treated with guanosine (0.05 mg/kg, p.o.) or vehicle (distilled water, p.o.), and after 45 min, they received DNQX (2.5 μg/site, i.c.v.) or vehicle (saline, i.c.v.). After 15 min, TST was carried out followed by OFT (Fig. 1a).
Antidepressant-like effect elicited by guanosine in the TST involves AMPA receptors, VDCCs, and BDNF signaling. Timeline of experimental protocols of administrations and behavioral tests (a, b, g, and h). Effect of the treatment of mice with DNQX on the guanosine-induced antidepressant-like effect in the TST (c) and locomotor activity in the OFT (d). Effect of verapamil on the guanosine-induced antidepressant-like effect in the TST (e) and locomotor activity in the OFT (f). The effect of the treatment of mice with K-252a on the antidepressant-like effect of guanosine in the TST (i) and locomotor activity in the OFT (j). Effect of BDNF antibody administration on the antidepressant-like effect elicited by guanosine in the TST (k) and locomotor activity in the OFT (l). Values are expressed as mean + SEM of 7–8 mice. * p < 0.05 and ** p < 0.01 compared with the vehicle-treated control group. # p < 0.05 and ## p < 0.01 compared with the guanosine-treated group.
To investigate if the antidepressant-like effect induced by guanosine involves VDCC, mice were treated orally with guanosine (0.05 mg/kg) or distilled water and after 30 min they received verapamil (10 mg/kg, i.p.) or vehicle (saline, i.p.). After 15 min, animals were submitted to the TST and OFT (Fig. 1b).
In another set of experiments, in order to examine the hypothesis that the antidepressant-like effect of guanosine is dependent on TrkB, mice were treated with guanosine (0.05 mg/kg, p.o.) or vehicle (distilled water, p.o.), and after 45 min, received K-252a (1 μg/site, i.c.v.) or vehicle (saline, i.c.v.). After 15 min, the TST was carried out, followed by OFT (Fig. 1g). To further evaluate the influence of BDNF in the antidepressant-like effect of guanosine, mice were treated with guanosine (0.05 mg/kg, p.o.) or vehicle (distilled water, p.o.) 45 min before i.c.v. administration of BDNF antibody (1 μg/site, i.c.v.) or vehicle (saline, i.c.v.). The TST was carried out 15 min after the last treatment followed by OFT (Fig. 1h).
The doses and administration route of DNQX, K-252a, and BDNF antibody were chosen based on previous studies [30,31,32].
In another set of experiments, guanosine was administered (0.05 mg/kg, p.o.), and after 60 min, the hippocampus and PFC were dissected and processed for Western blot analyses to verify phosphorylation of P70S6K (Thr389) and the immunocontent of synapsin I and PSD-95 in both structures (Fig. 2a).
Effect of guanosine treatment in P70S6K phosphorylation, synapsin I, and PSD-95 imunocontent in the hippocampus and PFC of mice. Timeline of experimental protocols of administrations and behavioral tests (a). P70S6K (Thr389) phosphorylation in the hippocampus (b) and PFC (e) of mice. Immunocontent of synapsin I in the hippocampus (c) and PFC (f). Immunocontent of PSD-95 in the hippocampus (d) and PFC (g). Results are presented as percentual of control (considered 100%) and are expressed as mean + SEM (n = 6). * p < 0.05 and ** p < 0.01 guanosine-treated groups compared with the vehicle-treated group
In order to evaluate if the effect of guanosine persists up to 24 h, mice were submitted to TST 1 h, 6 h, and 24 h after treatment with guanosine (0.05 mg/kg, p.o.). After the TST, animals were submitted to the OFT for evaluation of number of crossings, latency to exit the first quadrant, time and number of entries in center, number of groomings and rearings (Table 1). To evaluate BNDF levels in the hippocampus and PFC, right after the behavioral tests, mice were euthanized, and the brain structures were dissected (Fig. 3a).
Time-course of guanosine administration effects in the TST, OFT and BDNF levels. Timeline of experimental protocols of administrations and behavioral tests (a). The treatment with guanosine or vehicle 1 h, 6 h, and 24 h in the TST (b) and OFT (c). Effect of guanosine treatment or vehicle after 1 h, 6 h, and 24 h in BDNF levels in the hippocampus (d) and PFC (e). Values are expressed as mean + SEM (n = 7–8). * p < 0.05 and ** p < 0.01 compared with the respective vehicle-treated control group
To investigate the effect of guanosine in the NSF test, mice received an administration of guanosine (0.05 mg/kg, p.o.), ketamine (1 mg/kg, i.p., positive control), or vehicle (distilled water, p.o.), and after 1 h or 30 min, respectively, they were subjected to NSF test. After the test, the amount of food consumed by mice for 5 min was registered. Subsequently, mice were perfused to remove the hippocampus for morphological analyses (Fig. 4a).
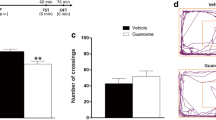
Effect of guanosine or ketamine treatment in the NSF test and dendritic spine density and morphology in the hippocampal ventral DG. Timeline of experimental protocols of administrations and behavioral tests (a). Representative draw of the morphology of dendritic spines subtypes (b). Effect of a single administration of guanosine or ketamine in the latency to feed (c) and food consumption (d). Representative images of different dendritic segments in the ventral DG area for each experimental group (e). Percentage of each type of dendritic spine in each experimental group (f). Cumulative probability of total spine density (g), stubby- (h), thin- (i), mushroom- (j), and filopodia-shaped (k) dendritic spine in ventral DG. The results are expressed as absolute values and expressed as mean + SEM (n = 7–8). ** p < 0.01 compared with the vehicle-treated groups
Behavioral tests
TST
The total duration of immobility induced by TST was measured according to the method described previously [33]. Animals were suspended 50 cm above the floor by adhesive tape placed approximately 1 cm from the tip of the tail. Mice were considered immobile only when they hung passively and completely motionless. Immobility time was manually recorded during a 6-min period by an experienced observer blinded to the experimental condition.
OFT
Locomotor activity was assessed in an open-field test (OFT) 10 min after TST, as previously described [34]. The apparatus consisted of a wooden box measuring 40 × 60 × 50 cm high. The floor of the arena was divided into 12 equal squares. The number of squares crossed with all paws (crossing) was counted during a 6-min session. The apparatus was cleaned with a solution of 10% ethanol between tests to hide animal clues.
NSF
First, mice were weighed, and food was removed from their cages, although water continued to be available to all animals. Approximately 24 h after the removal of the food, mice were placed in a wooden box (40 × 60 cm and 50 cm height) containing a small amount of food in its center. Each mouse was placed in the arena for 10 min, starting in the corner, and the time until the first feeding episode was recorded. Immediately after the mouse began to eat the chow, the tested animal was placed alone in a cage (41 × 34 × 16 cm) with a weighed piece of chow for 5 min. Subsequently, the amount of food consumed by each mouse was determined by weighing the chow before and after the test [35, 36].
Biochemical analyses
Western blot analysis
The western blot was performed in order to investigate the phosphorylation of P70S6K (Thr389) and the immunocontents of synapsin I and PSD-95 in the hippocampus and PFC of mice, 1 h after guanosine treatment (0.05 mg/kg, p.o.). Animals were previously submitted to TST followed by OFT. After behavioral tests, they were decapitated for quick dissection of PFC and hippocampus, which were placed in liquid nitrogen (4 °C) and stored at − 80 °C until use. Samples were mechanically homogenized in 400 μL of 50 mM TRIS pH 7.0, 1 mM EDTA, 100 mM NaF, 0.1 mM PMSF, 2 mM Na3VO4, 1% Triton X-100, 10% glycerol, and Sigma Protease Inhibitor Cocktail (P2714). Lysates were centrifuged (10,000g for 10 min, at 4 °C) to eliminate cellular debris. The supernatants were diluted 1/1 (v/v) in 100 mM Tris pH 6.8, 4 mM EDTA, 8% SDS, and boiled for 5 min. Thereafter, sample dilution (40% glycerol, 100 mM Tris, bromophenol blue, pH 6.8) in the ratio 25:100 (v/v) and β-mercaptoethanol (final concentration 8%) were added to the samples. Protein content was quantified by the method of (Peterson, 1977) using bovine serum albumin as a standard. The samples containing 80 μg protein/track) were separated by SDS-PAGE using 7%, 10%, or 12% gel, and the proteins were transferred to nitrocellulose membranes using a semi-dry blotting apparatus (1.2 mA/cm2; 1.5 h). To verify transfer efficiency process, membranes were stained with Ponceau. After the transfer process, membranes were blocked with 5% albumin in Tris-buffered saline for 60 min at room temperature and probed via incubation with P70S6K (Thr389), PSD-95 and synapsin I antibodies (obtained from Cell Signaling Technology—1:1000 dilution) diluted in a TRIS-buffered saline solution contained 0.1% Tween 20). Next, membranes were incubated with anti-mouse horseradish peroxidase-conjugated secondary anti-body (Cell Signaling, 1:2500) for 60 min, and the immunoreactive bands were developed using a chemiluminescence kit (Amersham ECL Prime Western Blotting Detection Reagent, GE Healthcare Life Sciences). After blocking and incubation steps, membranes were washed three times (5 min) with Tris-buffered saline solution containing 0.1% Tween 20. The expression level of a housekeeping protein β-actin was evaluated using a mouse anti-β-actin primary antibody (Santa Cruz, 1:2500) and an anti-mouse horseradish peroxidase-conjugated (Cell Signaling, 1:5000) secondary antibody. Optical density of the bands was quantified using Imagelab Software, and the phosphorylation of p-P70S6K (Thr389) was determined based on the ratio between optical density of the phosphorylated band and the optical density of the total P70S6K. Synapsin I and PSD-95 immunocontents were determined based on the ratio between the optical density of their bands and the optical density of the β-actin band.
BDNF ELISA immunoassay
Immediately after mice were subjected to TST for time-course evaluation, they were euthanized for quickly dissection of hippocampus and PFC. The structures were placed in liquid nitrogen (4 °C) and stored at − 80 °C until use. Samples were mechanically homogenized in 400 μL of 50 mM Tris pH 7.0, 1 mM EDTA, 100 mM NaF, 0.1 mM PMSF, 2 mM Na3VO4, 1% Triton X-100, 10% glycerol, and Sigma Protease Inhibitor Cocktail (P2714). Lysates were centrifuged (10,000g for 10 min, at 4 °C) to eliminate cellular debris. After centrifugation, supernatant was transferred to a new Eppendorf for the BDNF immunoassay. Protein content was quantified by the method of Peterson (1977) using bovine serum albumin as standard. Mature BDNF levels were measured by antigen-capture enzyme-linked immunosorbent assay (ELISA) using a Promega BDNF Emax® ImmunoAssay System ELISA kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions [37].
Morphological analyses
Golgi staining
Golgi staining is a classical technique based on the impregnation of neural tissue with heavy metal precipitate in a random set of neurons [38, 39]. After behavioral tests, mice were transcardially perfused with 0.9% saline (under deep anesthesia with isoflurane). Brains were immediately removed and deposited in vials containing 20 mL modified Golgi-Cox solution. The samples were stored at room temperature, in the dark for 4 days, and at the end of this period, the Golgi solution was replaced by 30% sucrose solution [40]. All procedures were performed without the presence of light.
Following saturation in sucrose, coronal sections (200 μm) of the hippocampus were obtained on a vibratome (Vibratome Series 1000, St Louis, MO, USA). Hippocampal sections were mounted onto 2% gelatin-coated microscope slides, and for 48 h, the samples were maintained in a humid chamber. After this period, slides were sequentially placed in distilled H2O (1 min), ammonium hydroxide (30 min), distilled H2O (1 min), Kodafix for film (30 min), distilled H2O (1 min), 50% ethanol (1 min), 70% ethanol (1 min), 95% ethanol (1 min), 100% ethanol (10 min), and 100% ethanol/xylene (1:1 for 20 min). Finally, a coverslipped was used to finalize the slide, which was fixed with Novo Entellan® (Merck). The finished slides were stored in the dark until analyzed [36].
Dendritic spine analysis
A Confocal Leica DMI6000 B microscope (Leica Microsystem, Wetzlar, Germany) was utilized to collect Z-sections of labeled neurons, dendritic segments were collected, and the images were processed using bath deconvolution to optimize the image quality. The following criteria were used to select the segments to analyze the Golgi-impregnated cells: had consistent impregnation throughout the extent of the cell body and dendrites, were able to be distinguished from neighboring impregnated cells, and had intact dendritic trees [36].
Only dendritic protrusions < 3 μm in length were manually counted using ImageJ software [41, 42]. The total number of dendritic spines as well as the number of each particular type of dendritic spine, normalized to 10 μm of the dendritic segment length, were counted (under × 100 magnification). From each dendritic segment, Z-stacks (stack depth varied depending on the dendrite segment) were obtained for each segment, and the number of spines per 10 μm was manually quantified. Dendritic spines were traced as a full dendrite in the z-plane and inspected in the x–y-plane for each individual z-step. Spines are categorized into morphological subtypes based on their size and the relative proportions of the spine head and neck. The spine types were classified based on the following dimensions: length, from the base of the dendrite to the tip of its head (L); the maximum neck diameter (nd); and the maximum head diameter (hd). Thus, individual spines were included in each category based on the specific ratios of L/nd and hd/nd [43]. Morphometric analyses were conducted for each dendritic spine, and measurements were used to categorize spines into stubby, mushroom, thin, and filopodia classes. Filopodium-shaped spines presented hd = nd with an L value < 3 μm, whereas thin-shaped spines presented hd = nd with an L value < 2 μm. Stubby-shaped spines presented an L similar to the nd and the hd values, which tended to be < 1 μm. Mushroom-shaped spines presented a hd value that was much larger than the nd value, with an L value < 1 μm [44].
Statistical analysis
All the statistical analyses were performed using STATISTICA 7.0 software (StatSoft Inc., Tulsa, OK, USA). The Kolmogorov-Smirnov test was used to assess data normality in all the experiments. Differences between the experimental groups in behavioral and biochemical analyses were determined by Student t test, one-way or two-way ANOVA, followed by Newman-Keuls post hoc test when appropriate. Data are expressed as mean + SEM.
Since dendritic spine data were not normally distributed, cumulative probability plots to measure shifts in the total number of dendritic spines, as well as filopodia, thin, stubby and mushroom dendritic spines per 10 μm dendritic segment in the different experimental groups were used. Cumulative distribution probabilities were compared by Kruskal-Wallis test followed by the Dunn’s multiple comparisons test. A value of p < 0.05 was considered significant.
Results
DNQX prevented the decreased immobility time in the TST induced by guanosine
Considering the proposed role of AMPA receptors for the rapid behavioral response of ketamine [14, 45], we tested the ability of the AMPA receptor antagonist DNQX to abolish the antidepressant-like effect of guanosine in the TST. Figure 1 c shows the influence of DNQX on the antidepressant-like effect of guanosine in the TST. Treatment with DNQX prevented the decreased immobility time in the TST induced by guanosine. The two-way ANOVA revealed significant differences for guanosine treatment [F(1,26) =15.95, p < 0.01], DNQX treatment [F(1,26) =4.942, p < 0.05] and guanosine treatment × DNQX treatment interaction [F(1,26) = 5.361, p < 0.05]. Post hoc analysis indicated that the treatment with DNQX prevented the reduction on the immobility time produced by guanosine in the TST. No alterations in the locomotor activity in the OFT were observed (Fig. 1d). A two-way ANOVA showed no significant differences of locomotion for guanosine treatment [F(1,26) = 0.028, p =0.866], DNQX treatment [F(1,26) = 1.092, p =0.305], and guanosine × DNQX interaction [F(1,26) = 0.531, p = 0.472].
VDCCs are required for the antidepressant-like effect of guanosine in the TST
Considering that the activation of AMPA receptors is associated with sodium influx that causes the stimulation of VDCC [12], in the next step of the study, we tested the influence of the blockade of these channels with verapamil on the effect of guanosine in the TST.
The effect of verapamil on the antidepressant-like effect induced by guanosine is illustrated in Fig. 1e. The two-way ANOVA revealed significant differences for verapamil treatment [F(1,24) = 12.463, p <0.05] and guanosine treatment × verapamil treatment interaction [F(1,24) = 6.653, p <0.05], but no significant main effect was observed for guanosine treatment [F(1,24) = 4.128, p = 0.05]. Post hoc analysis indicated that the treatment with verapamil prevented the decrease in immobility time in the TST produced by guanosine. Locomotor activity in the OFT was not altered by any treatment (Fig. 1f). A two-way ANOVA showed no significant differences of locomotor activity for guanosine treatment [F(1,24) = 0.232, p =0.633], verapamil treatment [F(1,24) = 2.694, p = 0.113], and guanosine × verapamil interaction [F(1,24) = 0.001, p = 0.968].
Involvement of BDNF/TrkB signaling pathway in the antidepressant-like effect of guanosine
To test the hypothesis that the antidepressant-like effect of guanosine in the TST is dependent on BDNF signaling pathway, in the next step of the study, mice were administered with the TrkBantagonist K-252a or with BDNF antibody. Figure 1 i demonstrates the effect of the treatment with K-252a on the antidepressant-like effect of guanosine in the TST. The two-way ANOVA revealed significant differences for guanosine treatment [F(1,25) = 26.115, p < 0.01], K-252a treatment [F(1,25) = 7.491, p < 0.05] and guanosine treatment × K-252a treatment interaction [F(1,25) = 6.936, p < 0.05]. Post hoc analysis indicated that administration of K-252a prevented the decrease in immobility time in the TST produced by guanosine (Fig. 1i). Locomotor activity in the OFT was not altered by any treatments (Fig. 1j). A two-way ANOVA showed no significant differences for guanosine treatment [F(1,25) = 0.994, p = 0.328], K-252a treatment [F(1,25) = 1.728, p = 0.200], and guanosine × K-252a interaction [F(1,25) = 1.728, p = 0.200].
Subsequently, it is possible to observe the influence of BDNF antibody administration on the antidepressant-like effect elicited by guanosine in the TST (Fig. 1k). The two-way ANOVA revealed significant differences for guanosine treatment [F(1,25) = 16.425, p < 0.01], BDNF antibody treatment [F(1,25) = 17.042, p < 0.01], and guanosine treatment × BDNF antibody treatment interaction [F(1,25) = 5.689, p < 0.05]. Post hoc analysis indicated that BDNF antibody abolished the antidepressant-like effect induced by guanosine in the TST (Fig. 1k). Figure 1 l shows the locomotor activity of mice in the OFT. A two-way ANOVA showed no significant differences for guanosine treatment [F(1,25) = 1.485, p = 0.234], BDNF antibody treatment [F(1,25) = 3.3320, p = 0.079], but showed significance difference for guanosine × BDNF antibody interaction [F(1,25) = 5.9140, p < 0.05]. Post hoc analysis showed that that the combined administration of BDNF antibody and guanosine decreased the number of crossings in the OFT.
Guanosine increased P70S6K (Thr389) phosphorylation and synapsin I immunocontent in the hippocampus, but did not alter PSD-95 in the hippocampus and PFC
Considering that the activation of BDNF signaling pathway leads to the activation of mTOR that in turn stimulates P70S6K (Thr389) phosphorylation and protein translation [13], we next examined the effect of a single administration of guanosine on P70S6K (Thr389) phosphorylation in the hippocampus and PFC as indicated in Fig. 2 b and e, respectively. Student’s t test revealed a significant increase on P70S6K (Thr389) phosphorylation in the hippocampus of mice treated with guanosine [t(14) = − 4.39, p < 0.01], but no difference was observed in the PFC [t(14) = 0.34, p = 0.74].
Guanosine was also able to increase the immunocontent of synapsin I in the hippocampus of mice 1 h after its administration, as shown in Fig. 2c [t(14) = − 2.35, p < 0.05]. However, no difference was observed in the PFC [t(14) = 0.06, p = 0.95] when compared to the control group (Fig. 2f).
Administration of guanosine produced no significant differences on PSD-95 immunocontent in the hippocampus and PFC of mice. Student’s t test revealed no significant differences on PSD-95 immunocontent in the hippocampus [t(14) = − 0.05, p = 0.96] (Fig. 2d) and PFC [t(14) = − 0.30, p = 0.77] (Fig. 2g) of mice compared to the control group.
Time course-response curve of guanosine in the TST
Considering that we showed herein that the antidepressant-like effect of guanosine is dependent on molecular targets related to rapid antidepressant response, we next aimed to evaluate if its antidepressant-like effect in the TST persists up to 24 h as previously reported for the fast-acting antidepressant ketamine [46,47,48]. Figure 3 b shows that guanosine decreased the immobility time of mice in the TST 1 h [t(13) = 3.35 p < 0.05], 6 h [t(13) = 2.26, p < 0.05] and 24 h after treatment [t(14) = 2.68, p < 0.05]. The administration of guanosine did not alter the number of crossings in the OFT as compared to the control group 1 h [t(13) = − 0.57, p = 0.58], 6 h [t(13) = − 1.48, p = 0.16], and 24 h [t(14) = 1.39, p = 0.19] after guanosine administration (Fig. 3c). No differences were observed in the latency to exit the first quadrant in the OFT, numbers of rearings, total time of grooming, total time in center of the OFT, and number of entries in center of the OFT as compared to the control group (Table 1).
Guanosine acutely increased BDNF levels in the hippocampus and PFC
BDNF has been reported to be increased in the hippocampus and PFC following chronic administration of conventional antidepressants, but it may be rapidly increased following a single administration of ketamine [8]. Therefore, we next examined if a single administration of guanosine is capable of increasing BDNF levels in the hippocampus and PFC 1 h, 6 h, and 24 h after its administration.
Student’s t test revealed a significant increase on BDNF level in the hippocampus of mice 1 h [t(13) = − 2.33, p < 0.05], 6 h [t(13) = − 2.42, p < 0.05], and 24 h [t(14) = − 3.48 p < 0.01] after guanosine treatment when compared to control group as indicated in Fig. 3d. In the PFC, Student’s t test revealed significant differences in BDNF levels 1 h after guanosine administration [t(13) = − 4.11, p < 0.01] but no differences were observed after 6 h [t(13) = − 0.31, p = 0.76], and 24 h [t(14) = − 1.35, p = 0.20] (Fig. 3e).
A single administration of guanosine, similar to ketamine, elicited rapid effect in the NSF, but did not alter total dendritic spine density or maturation in the ventral DG
Guanosine was able to reduce latency to feed in the NSF 1 h after its administration [F(1,27) = 9.14, p < 0.01]. A similar result was obtained when mice received a single administration of ketamine [F(1,27) = 9.14; p < 0.01] (Fig. 4c). There was no change in food consumption in the guanosine-treated group [F(1,27) = 0.43, p = 0.43] or in the ketamine-treated group [F(1,27) = 0.43, p = 1.00] when compared to control group (Fig. 4d). Finally, no differences were observed in loss of body weight both in the guanosine-treated group [F(1,27) = 0.68, p = 0.40] and in the ketamine-treated group [F(1,27) =0.68, p = 0.78] when compared to control group (data not shown). Also, no change in the latency to feed was observed in mice that received vehicle administered by i.p. route as compared with those that received distilled water orally (data not shown).
Figure 4 e shows representative images of the different dendritic segments in the ventral DG area for each group, and Fig. 4f shows the percentage of stubby-, mushroom-, thin-, and filopodia-shaped spines following the administration of guanosine or ketamine. No alteration was observed in total spine density in the ventral DG in any group (Fig. 4g) (Kruskal-Wallis test = 0.0033; p = 0.99). Analyses of immature spine morphology, namely stubby- (Kruskal-Wallis test = 0.003; p = 0.99), thin (Kruskal-Wallis test = 0.052; p = 0.97), and filopodia-shape (Kruskal-Wallis test = 0.137; p = 0.99) spines (Fig. 4, i, k), and mature spine morphology (mushroom-shaped spines) (Kruskal-Wallis test = 0.369; p = 0.83) (Fig. 4j) showed no significant alterations after treatments with ketamine or guanosine when compared to the control group.
Discussion
We provide evidence that the antidepressant-like effect of guanosine in the TST in female mice is dependent on the activation of AMPA receptors, VDCC, and BDNF/TrkB signaling pathway. Our data also indicate that guanosine induces a rapid increase in P70S6K (Thr389) phosphorylation and synapsin I immunocontent in the hippocampus, probably as a consequence of the activation of AMPA receptors and BDNF/TrkB pathway. Guanosine caused an antidepressant-like effect 1 h, 6 h, and 24 h after its administration accompanied by a rapid and sustained increase in BDNF levels in the hippocampus and by rapid but not sustained BDNF increment in the PFC of mice. Although a single administration of guanosine, like ketamine, was capable of causing the fast behavioral response in the NSF test, it was not accompanied by an increment on dendritic spines density and remodeling in the ventral DG.
The antidepressant properties of guanosine were shown for the first time in a study that indicated that this nucleoside decreased the immobility time in the TST and FST in mice of either sex [20]. In the same study, the activation of PI3K/Akt and mTOR pathway was reported to be crucial for the behavioral responses of guanosine in the TST. Guanosine also induced antidepressant-like effects in female mice subjected to acute restraint stress, an effect associated with its ability to reduce hippocampal oxidative damage [49]. In addition, the chronic administration of guanosine caused an antidepressant-like effect paralleled by the enhancement of hippocampal neuroblasts differentiation [50]. More recently, we showed that the antidepressant-like effect of guanosine in female mice also involves the inhibition of GSK-3β, as well as activation of MAPK/ERK and Nrf2/HO-1 signaling pathways [34]. To further give insight into the mechanisms underlying the antidepressant-like effect of guanosine, in the present study, we investigated the role of upstream and downstream targets of mTOR pathway in the behavioral response of female mice treated with guanosine and assessed in the TST. Female mice were chosen considering that the prevalence of depression is about twofold higher in women than in men, and also based on previous mentioned studies that investigated the antidepressant effects of guanosine in female mice [1].
In the first set of experiments, we found that the administration of DNQX, an AMPA receptor antagonist, abolished the anti-immobility effect of guanosine in the TST. This finding is suggestive that the antidepressant-like effect of guanosine is dependent on the activation of AMPA receptors. Accordingly, targeting AMPA receptors has been proposed as a therapeutic approach for the treatment of depression. AMPA activation is required for antidepressant activity, since mice with a deletion in AMPA receptor (Glu subunit A1) exhibited a depressive-like behavior [51]. Further supporting the role of AMPA receptors in the physiopathology of depression, it was reported that positive AMPA receptor modulators, including ampakines that potentiate currents mediated by AMPA receptors [52] have antidepressant-like properties in the TST and FST [53]. Of note, the activation of AMPA receptors is demonstrated to underlie the antidepressant effects of glutamatergic modulators, particularly the fast-acting antidepressant agent ketamine [14, 45]. Of particular interest, the administration of DNQX at the same dose used herein was previously demonstrated by our group to be able to completely prevent the antidepressant-like effect of ketamine in the TST, but failed to prevent the antidepressant-like effect of the conventional antidepressant fluoxetine in this test [30]. Several reports have indicated that AMPA receptor antagonists were also able to reverse the behavioral responses of other drugs, such as the mGlu group II receptor antagonist LY341495 [54]. Indeed, the antidepressant-like effect of LY341495 was also reversed by rapamycin [46, 55], and recently, LY341495 was reported to elicit fast-acting antidepressant response [56]. These results are similar to those reported for ketamine [14] and are in line with the assumption that the activation of AMPA receptors may be associated with fast antidepressant responses [57]. Therefore, these drugs and guanosine may share similar neuronal mechanisms that may be triggered by AMPA receptor stimulation to cause antidepressant effects. A recent study also showed that the antidepressant-like effects of three methoxetamine (MXE) analogs (that act as NMDA receptor antagonists) were blocked by the AMPA receptor antagonist NBQX, a finding that implicates AMPA receptor activation in their effects [58].
We also demonstrated that VDCCs are essential to antidepressant-like effect of guanosine in TST since verapamil treatment was able to abolish the anti-immobility effect of guanosine in the TST. This stimulation of VDCC plays a critical role for the rapid antidepressant effects of ketamine [8] and scopolamine [59]. Of note, the activation of these channels that occurs as a consequence of AMPA receptor activation is implicated with the release of BDNF [12, 60].
In the present study, we also demonstrate that the antidepressant-like effect of guanosine is dependent on TrkB/BDNF pathway. This conclusion is based on the fact that both the antagonist of TrkBK-252a and the BDNF antibody were effective to abolish the antidepressant-like effect of guanosine. Similar to these findings, the administration of these pharmacological tools was effective to counteract the effect agmatine [31], an endogenous compound that has been shown to exert fast antidepressant responses in animal models of depression in a way similar to ketamine [47, 48]. Moreover, the administration of the BDNF antibody into the mPFC was shown to abolish the behavioral effects of ketamine in the FST [8].
The activation of BDNF/TrkB signaling leads to the activation of PI3K/Akt pathway [61] which in turn leads to mTORC1 activation with the consequent increase in the translation of synaptic proteins implicated in synaptogenesis [14, 27]. The activation of mTOR is associated with increased phosphorylation of P70S6K and eukaryotic initiation factor 4E-binding protein 1 (4E-BP-1). P70S6K and 4E-BP-1 promote the initiation of protein translation for the synthesis of synaptic proteins such as synapsin I and PSD-95 [14]. Deficits in mTOR-dependent translation initiation, particularly via the P70S6K/eIF4B pathway, were found in the PFC of depressed individuals [62]. Moreover, a reduced expression PSD-95 in PFC of depressed subjects was observed in postmortem studies [63,64,65]. Importantly, antidepressant mechanism of ketamine and LY341495 involves the increase in phosphorylation of P70S6K and expression of synaptic protein PSD-95 [14, 55]. Here, we found that 60 min after guanosine administration, mice presented an increase in p70S6K phosphorylation at Thr389 and in the immunocontent of hippocampal synapsin I, a protein that contributes to the anchoring of synaptic vesicles [66], indicating the importance of these targets for the antidepressant-like effect of guanosine. However, no effect on these parameters were observed in the PFC. The phosphorylation of P70S6K is a downstream event to mTOR phosphorylation, therefore indicative of mTOR activation, in line with the fact that the inhibitor of mTOR rapamycin was effective to abolish the antidepressant-like effect of guanosine in the TST [20]. Regarding the differences observed in the neurochemical parameters analyzed in the hippocampus and prefrontal cortex, the result of P70S6K phosphorylation at Thr389 is in line with the reported ability of guanosine and ketamine administered in combination at subthreshold doses to stimulate P70S6K phosphorylation (Thr389) in the hippocampus, but not in the PFC [67]. A single dose of ketamine was also reported to increase the phosphorylation of this protein in the hippocampus [36], a result similar to that obtained with the administration of guanosine. Regarding the increase in hippocampal synapsin I elicited by the administration of guanosine, it is similar to the result obtained 1 h after the administration of ascorbic acid, a compound that shares with ketamine the ability to increase dendritic spine density in the ventral DG [36]. The absence of alteration of PSD-95 following guanosine administration observed in the present study suggests that this parameter was not sensitive to guanosine administration under the experimental conditions used and future investigation with different time periods of analysis and other guanosine doses should address this issue.
The results obtained in the TST observed 1 h, 6 h, and 24 h after treatment corroborate and extend previous findings performed by our group demonstrating that guanosine was able to reverse the increase in immobility time induced by acute restraint stress approximately 8 h after treatment and to elicit an antidepressant-like effect in control mice not submitted to stress (after 8 h of its administration) [49], but no previous evidence has indicated that the effect of guanosine persists 24 h after its administration. These results point to similarities between the behavioral profile of guanosine and ketamine since ketamine was capable of eliciting antidepressant-like effect in the TST 24 h after its administration [47, 48]. Considering the relatively short elimination half-life of either guanosine and ketamine administered to mice [68, 69], it is possible that the effects of guanosine and/or ketamine are a consequence of rapid onset mechanisms which persist during the time, even in the absence of appreciable plasma/brain levels of these drugs, and/or due to the presence of their metabolites.
The fact that guanosine treatment failed to induce alterations in the time and number of entries in the center of the apparatus and in the number of grooming in the OFT, anxiety-related parameters [70] in any of the time points analyzed, suggests that at the very low doses employed here (0.05 mg/kg, p.o.) guanosine does not exert anxiolytic effects in the OFT. It is interesting to note that the dose of guanosine used in the present study is lower than the one reported to cause anxiolytic-like effect in male rats (7.5 mg/kg, i.p.) in the OFT, and elevated plus maze and light/dark box [71]. An anxiolytic effect of guanosine was also observed following its chronic administration (2 weeks ad libitum consumption at 0.5 mg/ml) to mice [72]. These discrepancies raise the possibility that guanosine dose, protocol of administration, specie, and sex may account for the different results regarding its ability to produce anxiolytic effects.
Interestingly, a single administration of guanosine was capable of increasing BDNF levels in the hippocampus and PFC of mice just 1 h after guanosine treatment, an effect that persisted up to 24 h in the hippocampus but was transient in the PFC. MDD is associated with dysfunction and atrophy of hippocampus and PFC, brain regions involved in emotional processing, recompense systems, and executive functions [73,74,75]. The hippocampus plays a key role in learning, memory and regulation of mood [65, 76] and has high neuroplasticity capacity [77], whereas the PFC selects information and performs executive functions necessary to control the cognitive processing of the information [78]. It is well known that BDNF has an important role in the mechanism of action of chronically administered conventional antidepressants [79, 80]. It has been shown that chronic administration of several antidepressants increased BDNF mRNA and protein levels in the hippocampus and/or PFC of rodents [81]. Conversely, the acute administration of conventional antidepressants is not capable of increasing BDNF levels in the hippocampus and PFC of rodents [82]. Different from conventional antidepressants, previous studies have shown that ketamine was able to acutely increase hippocampal and prefrontocortical BDNF mRNA and protein levels [24, 37, 83]. Importantly, the expression of the BDNF Met allele in mice (a polymorphism that impairs trafficking of BDNF mRNA to dendrites) results in basal synaptic deficits and abrogates synaptogenic and antidepressant actions of ketamine in PFC, suggesting that BDNF also has an important role in its therapeutic effect [8, 84]. Similar to the result reported here with guanosine, the administration of the low-impact ampakine CX717 produced a rapid (up to 1 h) increase on BDNF on medial PFC [85], raising the hypothesis that BDNF may be acutely released following AMPA receptor activation. Indeed, ketamine was reported to be able to release BDNF in primary cortical neurons, an effect blocked by inhibition of AMPA receptors or blockade of L-type VDCCs [8]. Therefore, considering our results that indicate the participation of these targets in the effect of guanosine in the TST, the rapid increase on BDNF levels following the administration of guanosine is likely dependent on these events, similar to ketamine, but this hypothesis deserves further studies to be confirmed.
We also showed that a single administration of guanosine reduced the latency to feed in the NSF test, in agreement with a previous study that reported a similar result in male Swiss mice [86], indicating that guanosine is effective in this test either in female and male Swiss mice. Ketamine, used here as a positive control, also reduced the latency to feed in the NSF test, confirming previous studies and reinforcing the notion that this test is useful to detect fast-acting antidepressant agents [36, 86]. Accordingly, this test is sensitive to chronic but not acute administration of conventional antidepressants [87]. The latency to feed reflects how the animal copes with a behavioral conflict. Because the ability to solve conflicts is inversely related to anxiety and depression, and since NSF test assesses anhedonia in a situation where there is a conflict between food reward and novel open space, this test has been used for depression-related assessments [88]. Therefore, our results further corroborate with the hypothesis that guanosine may elicit fast antidepressant-related behavioral response. Interestingly, in line with this hypothesis, a recent study indicated that the acute administration of guanosine was effective to counteract the depressive-like behavior induced by olfactory bulbectomy in mice in a way similar to ketamine by a mechanism dependent on mTOR activation [89].
We investigated if the effects of guanosine and ketamine in the NSF test would be associated with an increase on dendritic spine density or maturation in the ventral DG of mice (evaluated 75 and 45 min after its administration, respectively). Ventral DG is recognized as related to stress, emotion, and affect [90]. Interestingly, chronically administered guanosine increased the number of immature neurons specifically in the ventral hippocampal DG [50]. However, neither guanosine nor ketamine altered dendritic spine density in this region. Indeed, ketamine was recently reported to increase the total dendritic spine density in the ventral DG when administered to female Swiss mice at the same dose used in the present study, but administered about 70 min (a time period longer than the one used here) before the morphological analyses. Therefore, future studies should investigate if a longer time period elapsed between guanosine administration and the morphological analyses is required to affect this parameter. No alterations were also observed in the subtypes of dendritic spines, either in filopodium-, stubby-, and thin-shaped dendritic spines that are considered immature spines or mushroom-shaped dendritic spines that mature spines [56, 91]. These results reinforce the notion that the experimental protocol used was not enough to alter dendritic maturation, despite being effective to cause behavioral effects in the NSF test. Altogether, these results suggest that the behavioral response of guanosine is accompanied by neurochemical alterations related to enhancement of hippocampal synaptic plasticity, that probably precedes any morphological alteration in dendritic spines.
Conclusions
Collectively, the results indicate that guanosine exerts antidepressant-like effects by acutely activating AMPA receptor, VDCC, and BDNF/TrkB signaling. Of note, its antidepressant-like effect is paralleled with increased levels of BDNF, especially in the hippocampus up to 24 h. In addition, guanosine increased the p70S6K (Thr389) phosphorylation and the immunocontent of the synaptic protein synapsin I in the hippocampus of mice. Figure 5 summarizes the effects of guanosine observed in the present study that is consistent with the hypothesis that it modulates critical targets for rapid antidepressant responses [16]. Despite these promising results, we should bear in mind that at the experimental conditions of the present study guanosine failed to increase the dendritic spine density and remodeling in ventral dentate gyrus, the p70S6K phosphorylation in PFC, and PDS-95 in the PFC and hippocampus.
Schematic diagram of the proposed mechanisms underlying the antidepressant-like effect of guanosine. The present study together with previous findings suggest that guanosine exerts antidepressant-like effect by a mechanism similar to ketamine. Particularly, present results suggest that this nucleoside causes AMPA receptor activation (1), which in turn results in the activation of VDCC (2) that induces release of BDNF (3), which subsequently stimulates TrkB (4). The stimulation of PI3K/Akt downstream signaling pathways (5) and mTOR (6), as reported previously [20], occurs as a consequence, leading to the phosphorylation of P70S6K (Thr389) and local translation of proteins (7), including expression of synaptic proteins such as synapsin I (8)
It is important to highlight that guanosine is an endogenous purine-based nucleoside that has been reported to exert neuroprotective effects [18, 68]. Besides this neuroprotective property, guanosine has a favorable safety profile [92], which warrant that it should be further investigated as an alternative therapeutic strategy that may replace or ameliorate the adverse effects of ketamine for treatment-resistant depression.
References
Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF (2016) Major depressive disorder. Nat Rev Dis Primers 2:16065. https://doi.org/10.1038/nrdp.2016.65
Berton O, Nestler EJ (2006) New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7(2):137–151. https://doi.org/10.1038/nrn1846
Masi G, Brovedani P (2011) The hippocampus, neurotrophic factors and depression: possible implications for the pharmacotherapy of depression. CNS Drugs 25(11):913–931. https://doi.org/10.2165/11595900-000000000-00000
Fava M (2003) Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 53(8):649–659. https://doi.org/10.1016/s0006-3223(03)00231-2
Papakostas GI, Ionescu DF (2015) Towards new mechanisms: an update on therapeutics for treatment-resistant major depressive disorder. Mol Psychiatry 20(10):1142–1150. https://doi.org/10.1038/mp.2015.92
Duman RS (2014) Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues Clin Neurosci 16(1):11–27. https://doi.org/10.1038/nrdp.2017.13
Arai AC, Kessler M, Rogers G, Lynch G (2000) Effects of the potent ampakine CX614 on hippocampal and recombinant AMPA receptors: interactions with cyclothiazide and GYKI 52466. Mol Pharmacol 58(4):802–813. https://doi.org/10.1124/mol.58.4.802
Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS (2014) BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 18(1). https://doi.org/10.1093/ijnp/pyu033
Castren E (2004) Neurotrophic effects of antidepressant drugs. Curr Opin Pharmacol 4(1):58–64. https://doi.org/10.1016/j.coph.2003.10.004
Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN (2003) Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry 60(8):804–815. https://doi.org/10.1001/archpsyc.60.8.804
Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ (2011) Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry 16(6):634–646. https://doi.org/10.1038/mp.2010.44
Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M (2009) Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci 29(27):8688–8697. https://doi.org/10.1523/JNEUROSCI.6078-08.2009
Hoeffer CA, Klann E (2010) mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33(2):67–75. https://doi.org/10.1016/j.tins.2009.11.003
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329(5994):959–964. https://doi.org/10.1126/science.1190287
Browne CA, Lucki I (2013) Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol 4:161. https://doi.org/10.3389/fphar.2013.00161
Camargo A, Rodrigues ALS (2019) Novel targets for fast antidepressant responses: possible role of endogenous neuromodulators. Chronic Stress 3:2470547019858083. https://doi.org/10.1177/2470547019858083
Ciccarelli R, Ballerini P, Sabatino G, Rathbone MP, D'Onofrio M, Caciagli F, Di Iorio P (2001) Involvement of astrocytes in purine-mediated reparative processes in the brain. Int J Dev Neurosci 19(4):395–414. https://doi.org/10.1016/s0736-5748(00)00084-8
Bettio LE, Gil-Mohapel J, Rodrigues AL (2016) Guanosine and its role in neuropathologies. Purinergic Signal 12(3):411–426. https://doi.org/10.1007/s11302-016-9509-4
Tasca CI, Lanznaster D, Oliveira KA, Fernandez-Duenas V, Ciruela F (2018) Neuromodulatory effects of guanine-based purines in health and disease. Front Cell Neurosci 12:376. https://doi.org/10.3389/fncel.2018.00376
Bettio LE, Cunha MP, Budni J, Pazini FL, Oliveira A, Colla AR, Rodrigues ALS (2012) Guanosine produces an antidepressant-like effect through the modulation of NMDA receptors, nitric oxide-cGMP and PI3K/mTOR pathways. Behav Brain Res 234(2):137–148. https://doi.org/10.1016/j.bbr.2012.06.021
Kelly A, Lynch MA (2000) Long-term potentiation in dentate gyrus of the rat is inhibited by the phosphoinositide 3-kinase inhibitor, wortmannin. Neuropharmacology 39(4):643–651. https://doi.org/10.1016/s0028-3908(99)00169-0
Dwivedi Y, Rizavi HS, Teppen T, Zhang H, Mondal A, Roberts RC, Conley RR, Pandey GN (2008) Lower phosphoinositide 3-kinase (PI 3-kinase) activity and differential expression levels of selective catalytic and regulatory PI 3-kinase subunit isoforms in prefrontal cortex and hippocampus of suicide subjects. Neuropsychopharmacology 33(10):2324–2340. https://doi.org/10.1038/sj.npp.1301641
Yang PC, Yang CH, Huang CC, Hsu KS (2008) Phosphatidylinositol 3-kinase activation is required for stress protocol-induced modification of hippocampal synaptic plasticity. J Biol Chem 283(5):2631–2643. https://doi.org/10.1074/jbc.M706954200
Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ (2014) Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 29(7):419–423. https://doi.org/10.1016/j.eurpsy.2013.10.005
Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E, Burgdorf J, Moskal J, Taylor J, Aghajanian G, Duman RS (2017) GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology 42(6):1231–1242. https://doi.org/10.1038/npp.2016.202
Maeng S, Zarate CA Jr (2007) The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep 9(6):467–474. https://doi.org/10.1007/s11920-007-0063-1
Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63(4):349–352. https://doi.org/10.1016/j.biopsych.2007.05.028
Kaster MP, Machado DG, Santos AR, Rodrigues ALS (2012) Involvement of NMDA receptors in the antidepressant-like action of adenosine. Pharmacol Rep 64(3):706–713. https://doi.org/10.1016/s1734-1140(12)70865-4
Paxinos, G., Franklin, K., 2004. The mouse brain in stereotaxic coordinates. Gulf Professional Publishing.
Cunha MP, Pazini FL, Ludka FK, Rosa JM, Oliveira A, Budni J, Ramos-Hryb AB, Lieberknecht V, Bettio LE, Martin-de-Saavedra MD, Lopez MG, Tasca CI, Rodrigues ALS (2015) The modulation of NMDA receptors and L-arginine/nitric oxide pathway is implicated in the anti-immobility effect of creatine in the tail suspension test. Amino Acids 47(4):795–811. https://doi.org/10.1007/s00726-014-1910-0
Neis VB, Moretti M, Bettio LE, Ribeiro CM, Rosa PB, Goncalves FM, Lopes MW, Leal RB, Rodrigues ALS (2016) Agmatine produces antidepressant-like effects by activating AMPA receptors and mTOR signaling. Eur Neuropsychopharmacol 26(6):959–971. https://doi.org/10.1016/j.euroneuro.2016.03.009
Manosso LM, Moretti M, Rosa JM, Cunha MP, Rodrigues ALS (2017) Evidence for the involvement of heme oxygenase-1 in the antidepressant-like effect of zinc. Pharmacol Rep 69(3):497–503. https://doi.org/10.1016/j.pharep.2017.01.010
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85(3):367–370. https://doi.org/10.3791/3769
Rosa PB, Bettio LEB, Neis VB, Moretti M, Werle I, Leal RB, Rodrigues ALS (2019) The antidepressant-like effect of guanosine is dependent on GSK-3beta inhibition and activation of MAPK/ERK and Nrf2/heme oxygenase-1 signaling pathways. Purinergic Signal 15(4):491–504. https://doi.org/10.1007/s11302-019-09681-2
Fukumoto K, Iijima M, Chaki S (2014) Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology (Berl) 231(11):2291–2298. https://doi.org/10.1007/s00213-013-3378-0
Fraga DB, Costa AP, Olescowicz G, Camargo A, Pazini FL, Freitas AE, Moretti M, Brocardo PS, ALS R (2020) Ascorbic acid presents rapid behavioral and hippocampal synaptic plasticity effects. Prog Neuropsychopharmacol Biol Psychiatry 96:109757. https://doi.org/10.1016/j.pnpbp.2019.109757
Pazini FL, Cunha MP, Rosa JM, Colla AR, Lieberknecht V, Oliveira A, Rodrigues ALS (2016) Creatine, similar to ketamine, counteracts depressive-like behavior induced by corticosterone via PI3K/Akt/mTOR pathway. Mol Neurobiol 53(10):6818–6834. https://doi.org/10.1007/s12035-015-9580-9
Spacek J (1992) Dynamics of Golgi impregnation in neurons. Microsc Res Tech 23(4):264–274. https://doi.org/10.1002/jemt.1070230403
Vints K, Vandael D, Baatsen P, Pavie B, Vernaillen F, Corthout N, Rybakin V, Munck S, Gounko NV (2019) Modernization of Golgi staining techniques for high-resolution, 3-dimensional imaging of individual neurons. Sci Rep 9(1):130. https://doi.org/10.1038/s41598-018-37377-x
Gibb R, Kolb B (1998) A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods 79(1):1–4. https://doi.org/10.1016/s0165-0270(97)00163-5
Chapleau CA, Calfa GD, Lane MC, Albertson AJ, Larimore JL, Kudo S, Armstrong DL, Percy AK, Pozzo-Miller L (2009) Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations. Neurobiol Dis 35(2):219–233. https://doi.org/10.1016/j.nbd.2009.05.001
Calfa G, Chapleau CA, Campbell S, Inoue T, Morse SJ, Lubin FD, Pozzo-Miller L (2012) HDAC activity is required for BDNF to increase quantal neurotransmitter release and dendritic spine density in CA1 pyramidal neurons. Hippocampus 22(7):1493–1500. https://doi.org/10.1002/hipo.20990
Koh IY, Lindquist WB, Zito K, Nimchinsky EA, Svoboda K (2002) An image analysis algorithm for dendritic spines. Neural Comput 14(6):1283–1310. https://doi.org/10.1162/089976602753712945
Giachero M, Calfa GD, Molina VA (2015) Hippocampal dendritic spines remodeling and fear memory are modulated by GABAergic signaling within the basolateral amygdala complex. Hippocampus 25(5):545–555. https://doi.org/10.1002/hipo.22409
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475(7354):91–95. https://doi.org/10.1038/nature10130
Koike H, Iijima M, Chaki S (2011) Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology 61(8):1419–1423. https://doi.org/10.1016/j.neuropharm.2011.08.034
Neis VB, Bettio LEB, Moretti M, Rosa PB, Ribeiro CM, Freitas AE, Goncalves FM, Leal RB, Rodrigues ALS (2016) Acute agmatine administration, similar to ketamine, reverses depressive-like behavior induced by chronic unpredictable stress in mice. Pharmacol Biochem Behav 150-151:108–114. https://doi.org/10.1016/j.pbb.2016.10.004
Neis VB, Bettio LB, Moretti M, Rosa PB, Olescowicz G, Fraga DB, Goncalves FM, Freitas AE, Heinrich IA, Lopes MW, Leal RB, Rodrigues ALS (2018) Single administration of agmatine reverses the depressive-like behavior induced by corticosterone in mice: Comparison with ketamine and fluoxetine. Pharmacol Biochem Behav 173:44–50. https://doi.org/10.1016/j.pbb.2018.08.005
Bettio LE, Freitas AE, Neis VB, Santos DB, Ribeiro CM, Rosa PB, Farina M, Rodrigues ALS (2014) Guanosine prevents behavioral alterations in the forced swimming test and hippocampal oxidative damage induced by acute restraint stress. Pharmacol Biochem Behav 127:7–14. https://doi.org/10.1016/j.pbb.2014.10.002
Bettio LE, Neis VB, Pazini FL, Brocardo PS, Patten AR, Gil-Mohapel J, Christie BR, Rodrigues ALS (2016) The antidepressant-like effect of chronic guanosine treatment is associated with increased hippocampal neuronal differentiation. Eur J Neurosci 43(8):1006–1015. https://doi.org/10.1111/ejn.13172
Chourbaji S, Vogt MA, Fumagalli F, Sohr R, Frasca A, Brandwein C, Hortnagl H, Riva MA, Sprengel R, Gass P (2008) AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. FASEB J 22(9):3129–3134. https://doi.org/10.1096/fj.08-106450
Arai AC, Kessler M (2007) Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets 8(5):583–602. https://doi.org/10.2174/138945007780618490
Alt A, Nisenbaum ES, Bleakman D, Witkin JM (2006) A role for AMPA receptors in mood disorders. Biochem Pharmacol 71(9):1273–1288. https://doi.org/10.1016/j.bcp.2005.12.022
Palucha-Poniewiera A, Wieronska JM, Branski P, Stachowicz K, Chaki S, Pilc A (2010) On the mechanism of the antidepressant-like action of group II mGlu receptor antagonist, MGS0039. Psychopharmacology (Berl) 212(4):523–535. https://doi.org/10.1007/s00213-010-1978-5
Dwyer JM, Lepack AE, Duman RS (2012) mTOR activation is required for the antidepressant effects of mGluR(2)/(3) blockade. Int J Neuropsychopharmacol 15(4):429–434. https://doi.org/10.1017/S1461145711001702
Duman RS, Li N (2012) A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci 367:2475–2484. https://doi.org/10.1098/rstb.2011.0357
Freudenberg F, Celikel T, Reif A (2015) The role of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in depression: central mediators of pathophysiology and antidepressant activity? Neurosci Biobehav Rev 52:193–206. https://doi.org/10.1016/j.neubiorev.2015.03.005
Sayson LV, Botanas CJ, Custodio RJP, Abiero A, Kim M, Lee HJ, Kim HJ, Yoo SY, Lee KW, Ryu HW, Acharya S, Kim KM, Lee YS, Cheong JH (2019) The novel methoxetamine analogs N-ethylnorketamine hydrochloride (NENK), 2-MeO-N-ethylketamine hydrochloride (2-MeO-NEK), and 4-MeO-N-ethylketamine hydrochloride (4-MeO-NEK) elicit rapid antidepressant effects via activation of AMPA and 5-HT2 receptors. Psychopharmacology (Berl). https://doi.org/10.1007/s00213-019-05219-x
Yu H, Li M, Shen X, Lv D, Sun X, Wang J, Gu X, Hu J, Wang C (2018) The Requirement of L-Type Voltage-Dependent Calcium Channel (L-VDCC) in the Rapid-Acting Antidepressant-Like Effects of Scopolamine in Mice. Int J Neuropsychopharmacol 21(2):175–186. https://doi.org/10.1093/ijnp/pyx080
Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D (1990) Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J 9(11):3545–3550 PMCID: PMC552104
Mai L, Jope RS, Li X (2002) BDNF-mediated signal transduction is modulated by GSK3beta and mood stabilizing agents. J Neurochem 82(1):75–83. https://doi.org/10.1046/j.1471-4159.2002.00939.x
Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B (2011) The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 35(7):1774–1779. https://doi.org/10.1016/j.pnpbp.2011.05.010
Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B (2009) Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 33(1):70–75. https://doi.org/10.1016/j.pnpbp.2008.10.005
Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, Burger C, Auberson YP, Sovago J, Stockmeier CA, Buck A, Hasler G (2011) Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psychiatry 168(7):727–734. https://doi.org/10.1176/appi.ajp.2011.09111607
Deng W, Aimone JB, Gage FH (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11(5):339–350. https://doi.org/10.1038/nrn2822
Coleman WL, Bykhovskaia M (2009) Synapsin I accelerates the kinetics of neurotransmitter release in mouse motor terminals. Synapse 63(6):531–533. https://doi.org/10.1002/syn.20635
Camargo A, Dalmagro AP, Zeni ALB, Rodrigues ALS (2020) Guanosine potentiates the antidepressant-like effect of subthreshold doses of ketamine: possible role of pro-synaptogenic signaling pathway. J Affect Disord 271:100–108. https://doi.org/10.1016/j.jad.2020.03.186
Lanznaster D, Dal-Cim T, Piermartiri TC, Tasca CI (2016) Guanosine: a neuromodulator with therapeutic potential in brain disorders. Aging Dis 7(5):657–679. https://doi.org/10.14336/AD.2016.0208
Pham TH, Gardier AM (2019) Fast-acting antidepressant activity of ketamine: highlights on brain serotonin, glutamate, and GABA neurotransmission in preclinical studies. Pharmacol Ther 199:58–90. https://doi.org/10.1016/j.pharmthera.2019.02.017
Fraga DB, Olescowicz G, Moretti M, Siteneski A, Tavares MK, Azevedo D, Colla ARS, Rodrigues ALS (2018) Anxiolytic effects of ascorbic acid and ketamine in mice. J Psychiatr Res 100:16–23. https://doi.org/10.1016/j.jpsychires.2018.02.006
Almeida RF, Comasseto DD, Ramos DB, Hansel G, Zimmer ER, Loureiro SO, Ganzella M, Souza DO (2017) Guanosine anxiolytic-like effect involves adenosinergic and glutamatergic neurotransmitter systems. Mol Neurobiol 54(1):423–436. https://doi.org/10.1007/s12035-015-9660-x
Vinade ER, Schmidt AP, Frizzo ME, Izquierdo I, Elisabetsky E, Souza DO (2003) Chronically administered guanosine is anticonvulsant, amnesic and anxiolytic in mice. Brain Res 977(1):97–102. https://doi.org/10.1016/s0006-8993(03)02769-0
Harrison PJ (2002) The neuropathology of primary mood disorder. Brain 125(7):1428–1449. https://doi.org/10.1093/brain/awf149
Krishnan V, Nestler EJ (2008) The molecular neurobiology of depression. Nature 455(7215):894–902. https://doi.org/10.1038/nature07455
Levy MJF, Boulle F, Steinbusch HW, DLA v d H, Kenis G, Lanfumey L (2018) Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology (Berl) 235(8):2195–2220. https://doi.org/10.1007/s00213-018-4950-4
Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J (2004) Regional dissociations within the hippocampus-memory and anxiety. Neurosci Biobehav Rev 28(3):273–283. https://doi.org/10.1016/j.neubiorev.2004.03.004
van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH (2002) Functional neurogenesis in the adult hippocampus. Nature 415(6875):1030–1034. https://doi.org/10.1038/4151030a
Lara AH, Wallis JD (2015) The role of prefrontal cortex in working memory: a mini review. Front Syst Neurosci 9:173. https://doi.org/10.3389/fnsys.2015.00173
Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT (2001) Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 50(4):260–265. https://doi.org/10.1038/npp.2008.107
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59(12):1116–1127. https://doi.org/10.1016/j.biopsych.2006.02.013
Mendez-David I, Tritschler L, Ali ZE, Damiens MH, Pallardy M, David DJ, Kerdine-Romer S, Gardier AM (2015) Nrf2-signaling and BDNF: A new target for the antidepressant-like activity of chronic fluoxetine treatment in a mouse model of anxiety/depression. Neurosci Lett 597:121–126. https://doi.org/10.1016/j.neulet.2015.04.036
Ludka FK, Zomkowski AD, Cunha MP, Dal-Cim T, Zeni AL, Rodrigues ALS, Tasca CI (2013) Acute atorvastatin treatment exerts antidepressant-like effect in mice via the L-arginine-nitric oxide-cyclic guanosine monophosphate pathway and increases BDNF levels. Eur Neuropsychopharmacol 23(5):400–412. https://doi.org/10.1016/j.euroneuro.2012.05.005
Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J (2008) Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 32(1):140–144. https://doi.org/10.1016/j.pnpbp.2007.07.027
Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK (2012) Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 71(11):996–1005. https://doi.org/10.1016/j.biopsych.2011.09.030
Gordillo-Salas M, Pascual-Anton R, Ren J, Greer J, Adell A (2020) Antidepressant-like effects of CX717, a positive allosteric modulator of AMPA receptors. Mol Neurobiol 57(8):3498–3507. https://doi.org/10.1007/s12035-020-01954-x
Camargo A, Pazini FL, Rosa JM, Wolin IAV, Moretti M, Rosa PB, Neis VB, Rodrigues ALS (2019) Augmentation effect of ketamine by guanosine in the novelty-suppressed feeding test is dependent on mTOR signaling pathway. J Psychiatr Res 115:103–112. https://doi.org/10.1016/j.jpsychires.2019.05.017
Blasco-Serra A, Gonzalez-Soler EM, Cervera-Ferri A, Teruel-Marti V, Valverde-Navarro AA (2017) A standardization of the Novelty-Suppressed Feeding Test protocol in rats. Neurosci Lett 658:73–78. https://doi.org/10.1016/j.neulet.2017.08.019
Dulawa SC, Holick KA, Gundersen B, Hen R (2004) Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 29(7):1321–1330. https://doi.org/10.1038/sj.npp.1300433
de Almeida RF, Pocharski CB, Rodrigues ALS, Elisabetsky E, Souza DO (2020) Guanosine fast onset antidepressant-like effects in the olfactory bulbectomy mice model. Sci Rep 10(1):8429. https://doi.org/10.1038/s41598-020-65300-w
Fanselow MS, Dong HW (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65(1):7–19. https://doi.org/10.1016/j.neuron.2009.11.031
Hering H, Sheng M (2001) Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci 2(12):880–888. https://doi.org/10.1038/35104061
Schmidt AP, Bohmer AE, Schallenberger C, Antunes C, Tavares RG, Wofchuk ST, Elisabetsky E, Souza DO (2010) Mechanisms involved in the antinociception induced by systemic administration of guanosine in mice. Br J Pharmacol 159(6):1247–1263. https://doi.org/10.1111/j.1476-5381.2009.00597.x
Acknowledgements
The authors would like to thank the Multiuser Laboratory for Biological Studies (LAMEB), Central Laboratory of Electron Microscopy (LCME) and the technical staff, UFSC for support. Bettio LE was supported by a Michael Smith Foundation for Health Research (MSFHR) fellowship.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This study was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) #449436/2014-4, #310113/2017-2, and Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES). RBL and ALSR are recipients of CNPq Research Productivity Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were performed in accordance with the Guidelines of Ethic Committee on Animal Use of the Federal University of Santa Catarina (CEUA/UFSC) the guidelines laid down by the NIH (NIH Guide for the Care and Use of Laboratory Animals) in the USA. The CEUA/UFSC has approved all experimental protocols (approval numbers PP00795 and 7485180518 protocol).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rosa, P.B., Bettio, L.E.B., Neis, V.B. et al. Antidepressant-like effect of guanosine involves activation of AMPA receptor and BDNF/TrkB signaling. Purinergic Signalling 17, 285–301 (2021). https://doi.org/10.1007/s11302-021-09779-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-021-09779-6