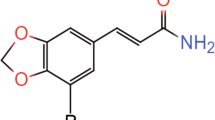
Abstract
Conventional antidepressant drugs elevate the availability of monoamine neurotransmitters. However, these pharmacological therapies have limited efficacy and a slow onset of action as main limitations. New glutamatergic drugs such as ketamine have shown promise as a rapid-acting antidepressant drugs although with adverse effects. The mechanism of action of ketamine is hypothesized to involve a dis-inhibition of cortical pyramidal neurons produced by an stimulation of AMPA receptors by glutamate. In this context, low-impact positive allosteric modulators of the AMPA receptors (a.k.a. ampakines) have been regarded as potential antidepressant drugs. Here, we have examined the behavioral, biochemical, and molecular effects of a low-impact ampakine, CX717. Our results show that CX717 has a rapid (30 min) but short-lasting (up to 24 h) antidepressant-like effect in the forced swim test. Intra-cortical infusion of CX717 increases the efflux of noradrenaline, dopamine, and serotonin, but not glutamate. However, systemic CX717 does not alter these neurotransmitters. CX717 also produced a rapid (up to 1 h) increase of brain-derived neurotrophic factor (BDNF) and a more sustained (up to 6 h) increase of p11. Overall, CX717 appears to possess a rapid but not sustained antidepressant action possibly caused by rapid increases of BDNF and p11.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major depressive disorder (MDD) is the most prevalent of psychiatric diseases, being one of the leading causes of disability with enormous personal and societal costs. Conventional antidepressant drugs are still based on the classic monoaminergic hypothesis, thus elevating the availability of monoamine neurotransmitters such as serotonin, noradrenaline, and/or dopamine in the brain. However, these therapies exhibit limited efficacy (about one third of patients are resistant to pharmacological treatment) and a slow onset of action. Therefore, there is an urgent need to develop new pharmacological approaches that lead to more rapid and effective clinical therapies.
The AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor is a subtype of ionotropic glutamate receptor that mediate most of the fast excitatory neurotransmission [1] and is ubiquitously distributed throughout the central nervous system, particularly in brain regions known to be involved in mood regulation such as the prefrontal cortex and hippocampus [2,3,4]. In fact, decreased AMPA receptor subunit levels have been reported in the prefrontal cortex of depressive patients [5] and chronic antidepressant treatments increased the protein levels of some AMPA receptor subunits [6].
Recent research has shown that systemic administration of AMPA evoked dose-dependent antidepressant-like effects in both the forced swim test (FST) and sucrose preference test [7]. Furthermore, we have demonstrated that the direct infusion of the full receptor agonist (S)-AMPA in the medial prefrontal cortex (mPFC) elicits an antidepressant-like action in the FST [8], thus underscoring the importance of AMPA receptors of the mPFC in antidepressant effects. Interestingly, the antidepressant-like behavior of ketamine is dependent on the activation of AMPA receptors [9,10,11]. Other glutamatergic drugs such as mGluR2/3 receptor antagonists also increase the efflux of gutamate in the brain [12, 13] and exhibit antidepressant-like effects in the FST. Thus, it is likely that this mGluR2/3 receptor antagonist-induced glutamate release activates postsynaptic AMPA receptors. As a matter of fact, AMPA receptor antagonists attenuated the antidepressant-like effects of mGluR2/3 receptor antagonists [14, 15]. In addition, activation of AMPA receptors show positive effects on synaptic plasticity [16] and cognitive performance [17, 18], two domains impaired in depression. Taken together, these findings suggest that the stimulation of AMPA receptors may be a beneficial strategy to treat MDD [19]. However, excessive AMPA receptor stimulation by full AMPA receptor agonists can lead to psychostimulant and seizure activity [20]. Instead, positive allosteric modulators (PAMs) -also known as ampakines- bind allosterically to AMPA receptors and slow the rate of their deactivation or desensitization [21], thus prolonging the duration of AMPA receptor-mediated responses [22]. Because ampakines do not activate directly AMPA receptors, the drugs are only effective with a underlying glutamatergic transmission [23]. Hence, it was postulated that ampakines would exert rapid antidepressant effects. This was first suggested from rodent studies that examined the positive response to behavioral despair (FST and tail suspension test) or assessed submissive behavior in an animal model [24, 25]. Collectively, these findings demonstrate that ampakines are active in animal models relevant to procedures probing clinically effective antidepressants although the exact mechanism involved is not fully understood [26]. Ampakines can be subdivided in high impact ampakines that reduced latency to deactivation of AMPA receptors, and low impact ampakines, which preferentially accelerated channel opening, thereby enhancing the amplitude of the AMPA-mediated current, but without affecting the deactivation process.
The aim of the present study was to investigate the antidepressant-like effects of CX717, a low impact ampakine in the FST, one of the most commonly used assays to examine depressive-like behaviors in rodents. Unlike high impact ampakines, low impact ampakines do not seem to produce adverse, pro-convulsant effects even after administration of high doses. Therefore, low impact ampakines are potentially safe and well tolerated in humans. Indeed, CX717 has passed through initial clinical studies in humans [27,28,29]. Further, previous positron emission tomography (PET) studies in nonhuman primates have revealed increased activity in prefrontal cortex, dorsal striatum and hippocampus following administration of CX717, which has been associated to improvement in learning and memory, two behaviors impaired in depression [30]. Therefore, in the present work the effects of CX717 on intracellular signaling pathways and extracellular levels of noradrenaline (NA), dopamine (DA), serotonin (5-HT) and glutamate in the mPFC were also examined and associated with behavioral responses. In addition, because PAMs of AMPA receptors potentiate the antidepressant-like response of monoamine-based antidepressants [31, 32], in the present study, we also sought to examine whether CX717 was able to enhance the antidepressant-like effects of two treatments that we previously demonstrated that reduced immobility in the FST, i.e. deep brain stimulation (DBS) and local administration of (S)-AMPA in the mPFC [8].
Materials and Methods
Animals
Male Sprague-Dawley rats (Envigo, Sant Feliu de Codines, Spain) weighing 280–350 g were used. The rats were group-housed (four per cage) in a controled environment (12 h light/dark, 22 ± 1 °C) with food and water available ad libitum. All the experimental procedures were conducted in accordance with national (RD 53/2013) and European legislation (Directive 2010/63/EU, on the Protection of Animals Used for Scientific Purposes, 22 September 2010), and were approved by the Animal Care and Use Committee of the University of Cantabria.
Drugs and Reagents
Serotonin hydrochloride (5-hydroxytryptamine, 5-HT), noradrenaline (NA), dopamine hydrochloride (DA), and glutamate were purchased from Sigma-Aldrich (Tres Cantos, Spain). CX717 was kindly provided by RespireRx Pharmaceuticals Inc. (Glen Rock, NJ). For intraperitoneal (i.p.) injection, CX717 was dissolved in 33% 2-hydroxypropyl-β-cyclodextrin (HPCD, Sigma-Aldrich) to a concentration of 20 mg/ml by gently warming. The dose of 20 mg/kg (administered at 1 ml/kg) of CX717 was chosen because it was reported to alleviate drug-induced respiratory depression without evoking seizure activity [33, 34]. This near maximal dose for rodents is similar to the upper end dose used in human clinical trials. Going beyond that dose could result in behavioral effects (e.g. animal lethargy). For intracortical infusion, a solution of 85 mM of CX717 in HPCD was further diluted with artificial cerebrospinal fluid (aCSF, see below for composition) to a concentration of 300 μM.
Forced Swim Test
In naïve animals, the FST was conducted as previously described [35]. Rats were handled daily for 1 week before FST for habituation. On day 1 (pretest), rats were placed in a clear plexyglas cylinder (46 cm height, 20 cm diameter) filled with 24 ± 1°C water to a height of 30 cm, for 15 min. After this pretest, animals were returned to their home cages and dried under a lamp for 30 min. It is established that this lapse is sufficient for healing of damage caused by surgery but not long enough for gliosis to alter the environment of the probe, which could interfere with results [36, 37]. Twenty-four hours after the pretest, rats received an i.p. injection of 20 mg/kg of CX717. Three tests were conducted 30 min, 24 h and 7 days after drug administration in the same cylinder for 5 min. The test sessions were videotaped (ANY-maze, Stoelting Europe, Dublin, Ireland) and scored for immobility, climbing and swimming by an experimenter blind to the treatment, as previously defined [38].
Locomotor Activity Test
Locomotor activity was recorded for 15 min in an open field arena (100 cm × 100 cm × 40 cm) with black plastic walls dimly lighted. Ambulatory behavior was video-tracked by a computerized system (Any-maze) and total distance travelled was measured.
Intracerebral Microdialysis and Biochemical Analysis
Concentric dialysis probes with a 4-mm membrane length were implanted under sodium pentobarbital anesthesia (60 mg/kg, i.p.) in the mPFC (AP +3.2 mm, L ±0.6 mm, DV -6.0 mm; from bregma), according to Paxinos and Watson [39]. In all cases, microdialysis experiments were conducted 24 h after surgery in freely moving rats by continuously perfusing probes with aCSF (147 mM NaCl, 3 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2) at a rate of 1.5 μl/min. Dialysate samples of 30 μl were collected every 20 min, and monoamines and glutamate were determined using an Alexys® Analyzer (Antec Scientific, Leiden, The Netherlands) following its published methods. At the completion of dialysis experiments, rats were given an overdose of sodium pentobarbital and processed for cresyl violet staining to assess the correct location of the probes.
Deep Brain Stimulation
The experimental timeline of the concomitant behavioral effects of CX717 and deep brain stimulation (DBS) are depicted in Figure S1a. Given the short half-life of CX717, an additional dose of 10 mg/kg was administered 30 min after DBS onset. Bipolar stimulating electrodes were implanted bilaterally under sodium pentobarbital anesthesia (60 mg/kg, i.p.) in the infralimbic area of the medial prefrontal cortex (mPFC) using the coordinates from the Paxinos and Watson [39] (AP + 3.2 mm, L ± 0.6 mm, DV -5.4 mm from bregma). Electrodes consisted of two stainless steel enamel-coated wires (California Fine Wire, Grover Beach, CA) with a diameter of 150 μm and a tip separation of ~100 μm and in vitro impedances of 10-30 kΩ. The stimulation settings were similar to those used in depressive states [40]. Stimulation lasted for 1 h and its parameters were: frequency, 130 Hz; current intensity, 200 μA and pulse width, 90 μs. Continuous alternating current with monophasic square pulses was delivered with a CS-20 Stimulator (Cibertec, Madrid, Spain) attached to an overhead electrical swivel (Plastics One Inc, Roanoke, VA, USA). In the control (sham) groups, all rats had the two electrodes implanted, but no current was delivered.
(S)-AMPA Perfusion
The experimental timeline of the concomitant behavioral effects of CX717 and intra-prefrontal (S)-AMPA infusion were previously described [8] and depicted in Figure S2a. Concentric dialysis probes with a 4-mm membrane length were implanted bilaterally under sodium pentobarbital anesthesia (60 mg/kg i.p.) in the mPFC (AP +3.2 mm, L ±0.6 mm, DV −6.0 mm; from bregma), according to Paxinos and Watson atlas [39]. Experiments were conducted 24 h after surgery in freely moving rats by continuously perfusing probes with aCSF at a rate of 1 μl/min. After three basal samples the aCSF was changed to an aCSF solution containing 100 μM of (S)- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [(S)-AMPA], which was perfused for 1 h. Given the short half-life of CX717, an additional dose of 10 mg/kg was administered 30 min after the onset of (S)-AMPA perfusion.
Protein Extraction and Western Blotting
In a separate set of experiments, animals were killed by decapitation thirty min, 1 h, 2 h and 6 h after the administration of 20 mg/kg of CX717, their brains removed from the skulls, and mPFCs dissected out on ice and stored at -80 ̊C. Samples of mPFC were homogenized (1:15) in a solution containing 10 mM HEPES–HCl (pH 7.9), 1.5 mM MgCl2, 100 mM KCl, and the following protease and phosphatase inhibitors: 1 mM phenylmethylsulfonyl fluoride (PMSF), 0.2 mg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, 10 μg/ml antipain, 10 μg/ml chymostatin, 1 mM Na3VO4, 1 mM NaF, 1 mM cantharidin and 1 μM E-64. Homogenates were next sonicated on ice-cold protein lysis buffer (homogenization buffer containing 1% Igepal®, 0.5% sodium deoxycholate, 0.1% SDS and 2.5 mM CHAPS) for 30 min. Solubilized proteins were recovered in the supernatant after centrifugation at 14,000 x g for 10 min at 4 ̊C. Protein quantification was performed according to the Lowry method.
Fifty micrograms of protein for each sample (in duplicate) were loaded into 8.5-15% SDS-PAGE gel and transferred to nitrocellulose membranes and incubated with primary antibodies overnight at 4 ̊C. The sources and dilution of primary antibodies used were: mouse anti glial fibrillary acidic protein [anti-GFAP (1:1000)], mouse anti- glyceraldehyde-3-phosphate dehydrogenase [anti-GAPDH (1:2000)], rabbit anti-brain-derived neurotrophic factor [anti-BDNF (1:200)], goat anti-PSD95 (1:500), and rabbit anti-excitatory amino acid transporter 1 [anti-EAAT1 (1:500)] from Santa Cruz Biotechnology (Santa Cruz, CA, USA); goat anti-p11 (1:200) from R&D Systems (Bio-Techne, Abingdon, UK); and rabbit anti-GluA1 (1:700) from Abcam (Cambridge, UK). The next day, membranes were washed with a mixture of Tris-buffered saline and 0.05% Tween 20 (TBST) and incubated with fluorochrome conjugated anti-rabbit, anti-mouse or anti-goat secondary antibodies from Li-Cor Biosciences (Lincoln, NE, USA). Secondary antibodies were detected with Odyssey CLx Scanner, also from Li-Cor Biosciences (Lincoln, NE, USA). Blot quantitation was performed by using Image Studio Lite software from Li-Cor Biosciences (Lincoln, NE, USA), and densitometry values were normalized with respect to the values obtained with anti-GAPDH antibody.
Statistics
Data are expressed as mean ± SEM. Differences between two groups were assessed by two-tailed Student’s t-test. One or two-way analysis of variance (ANOVA) followed by post-hoc Fisher’s least significant difference (LSD) multiple comparisons test was used to analyze differences among three or more independent groups. For microdialysis experiments, changes in monoamines and glutamate concentrations were assessed by repeated measures ANOVA with drug and time as factors, followed by post-hoc Fisher’s least significant difference (LSD) multiple comparisons test. In all cases the level of significance was set at p < 0.05.
Results
Antidepressant-like Effects of CX717
As shown in Fig. 1a, 30 min after a single injection of 20 mg/kg CX717 immobility in the FST was significantly reduced (t = 2.799, df = 8; P < 0.03, Student’s t-test), an effect that persisted 24 h later (t = 4.058, df = 10; P < 0.003, Student’s t-test), but disappeared 7 days later. The decrease in immobility was associated with an increase in swimming 30 min (t = 3.202, df = 9; P < 0.02, Student’s t-test) and 24 h (t = 2.636, df = 10; P < 0.03, Student’s t-test) later (Fig. 1b). Further, climbing was also increased (Fig. 1c) 24 h after CX717 administration (t = 2.327, df = 10; P < 0.05, Student’s t-test). In contrast, no significant differences were found in immobility, climbing and swimming 7 days after the injection of CX717. The CX717-induced decrease in immobility was not caused by increased locomotor activity (Fig. 1d). The administration of CX717 was not able to potentiate the antidepressant-like effects of 1 h DBS, (Supplemental Fig. S1) and the intracortical perfusion of 100 μM (S)-AMPA for 1 h (Supplemental Fig. S2) in the FST. A histological section showing the location of the two dialysis probes in the mPFC is depicted in Figure 2.
Behavioral changes in immobility (a), swimming (b), climbing (c) in the forced swim test (FST) and locomotor activity (d) in the open-field test, 30 min, 24 h and 7 days after the administration of 20 mg/kg of CX717. Locomotor (Loc.) activity (d) is expressed as distance traveled in meters during 15 min. Results expressed as mean ± SEM of n?=?5–6 rats per group, *p?<?0.05 at least, two-tailed Student’s t test.
Effects of CX717 on Prefrontal Monoamines and Glutamate
The same dose of CX717 that evoked antidepressant-like effects (20 mg/kg, i.p.) did not alter the extracellular concentrations of NA (Fig. 3a), DA (Fig. 3b), 5-HT (Fig. 3c) and glutamate (Fig. 3d) in the mPFC. However, repeated measures two-way ANOVA showed that the direct perfusion of 300 μM of CX717 into the mPFC enhanced dialysate NA (Fig. 4a) as measured by significant effects of drug (F1,21 = 13.982, P < 0.002), time (F9,189 = 7.879; P < 0.0001) and the drug x time interaction (F9,189 = 8.055; P < 0.0001). In a similar way, intracortical CX717 increased dialysate DA (Fig. 4b) as measured by significant effects of drug (F1,24 = 16.165; P < 0.0001), time (F9,216 = 2.793; P < 0.05) and time x treatment interaction (F9,216 = 6.117; P < 0.0001). Finally, intracortical CX717 also increased dialysate 5-HT (Fig. 4c) as measured by significant effects of drug (F1,24 = 5.458; P < 0.03), time (F9,216 = 3.384; P < 0.001) and time x treatment interaction (F9,216 = 4.334; P < 00005). In contrast, the direct perfusion of 300 μM of CX717 into the mPFC did not alter the extracellular concentration of glutamate (Fig. 4d).
Lack of effect of a single intraperitoneal injection of 20 mg/kg of CX717 or vehicle (arrow) on the extracellular concentration of noradrenaline (a), dopamine (b), 5-HT (c) and glutamate (d) in the mPFC. Data (mean ± SEM) are expressed as percentage changes of the four basal pretreatment values. Number of animals is given in parentheses.
Effects of 2-h intra-mPFC perfusion of CX717 or aCSF (black bar) on the extracellular levels of noradrenaline (a), dopamine (b), 5-HT (c) and glutamate (d) in the right and left mPFC. Data (mean ± SEM) are expressed as percentage changes of the four basal pretreatment values. Number of animals is given in parentheses. *p < 0.05 at least, different from the corresponding vehicle group, Fisher’s LSD test following significant two-way repeated measures ANOVA.
Effects of CX717 on Prefrontal Protein Expression
As shown in Fig. 5a, CX717 evoked a rapid increase in mPFC BDNF determined at 30 min (t = 2.942, df = 5; P < 0.05, Student’s t-test) and 1 h (t = 2.364, df = 7; P < 0.05, Student’s t-test) after drug administration. CX717 also increased PSD95, but only 2 h after its administration (t = 3.091, df = 9; P < 0.02, Student’s t-test), as shown in Fig. 5b. On the other hand, the synthesis of p11 in the mPFC was enhanced 30 min (t = 2.992, df = 6; P < 0.03, Student’s t-test), 1 h (t = 3.913, df = 5; P < 0.02, Student’s t-test), 2 h (t = 4.426, df = 5; P < 0.01, Student’s t-test) and 6 h (t = 3.373, df = 5; P < 0.02, Student’s t-test) after CX717 administration (Fig. 5c). The same injection of CX717 did not change the concentration of the GluA1 subunit in any of the time points tested (Fig. 5d).
Effects of CX717 (CX, 20 mg/kg) and vehicle (Veh) on the concentration of BDNF (a), PSD95 (b), p11 (c) and GluA1 (d) in the mPFC at 30 min, 1 h, 2 h and 6 h after its intraperitoneal administration. Results expressed as mean ± SEM of n = 4–6 rats per group, *p < 0.05 at least, different from the corresponding vehicle group, two-tailed Student’s t-test.
Discussion
Low impact ampakines such as CX717 are characterized by keeping the AMPA receptor channel open without desensitizing the receptor or alter its affinity for agonists [41]. The main finding of the present study is that the single administration of CX717 evoked an antidepressant response in the FST, which is in line with previous work with other ampakines [18, 32]. In contrast to what has been observed after repeated FST in mice (i.e. progressive increase in immobility counts over time [42]), the present results shown that immobility counts are constant over the different tests, which is in line with previous results in rats [43, 44] and suggests that this behavior may truly reflect a depressive-like state instead of a sign of learning after repeated tests. Importantly, this antidepressant-like effect persisted 24 h later although was no longer observed 7 days after drug administration. Because CX717 has a short half-life in rodents (~45 min), this finding suggests that CX717 can induce plastic changes of a relatively short duration (24 h), but perhaps an extended treatment may be needed to achieve longer-lasting effects. It is important to note herein that the present study has two main limitations. First, the use of only one test (FST) to examine depression-related behaviors. However, an established strength of the FST is its predictive validity, which suggests a potential of CX717 to treat depression. Moreover, it has been argued that FST is particularly predictive of non-monoaminergic antidepressant efficacy [9, 10]. Second, the absence of examining the effects of CX717 in a rat model relevant for depression. Further research using other behavioral tests and model(s) for depressive states is needed to further support the potential of CX717 as a novel antidepressant. CX717 was not able to potentiate the antidepressant-like effects of two treatments, i.e. deep brain stimulation (DBS) of the infralimbic prefrontal cortex and intra-mPFC infusion of the full agonist (S)-AMPA [8]. The reduction of immobility behavior in the FST after these procedures is so substantial that cannot be incremented by CX717 (ceiling effect). Alternatively, there is evidence in mice that some pharmacological treatments (such as 5-HT4 receptor agonists [45]) and genetic manipulations such as constitutive 5-HT1A receptor knockout [46] and conditional ablation of the expression of 5-HT1A autoreceptors in the raphe nuclei [47, 48] also reduces immobility in the FST. However, there is no evidence of changes in such serotonergic receptors after CX717.
The rapid antidepressant-like effects of systemic CX717 did not appear to be associated with increased output of monoamines and glutamate in the mPFC because no change of the extracellular concentrations of these transmitters was observed under the same experimental conditions. As aforementioned, the short half-life of CX717 in the rat makes it hardly likely that the lack of biochemical effects after systemic CX717 would result from adaptive mechanisms such as reuptake, degradation or autoreceptor-induced inhibitory feedback. Interestingly, after 2h of continuous perfusion into the mPFC, CX717 was indeed able to enhance locally the extracellular concentration of NA, DA and 5-HT, but not that of glutamate. In light of previous results [35], our experiments suggest that elevated swimming and climbing reflects activation of brain 5-HT and NA systems, respectively, although not to an extension to be reflected in dialysate levels. Given that CX717 readily penetrates the brain, it is conceivable that an opposing mechanism could operate under systemic conditions or that, alternatively, the antidepressant-like effects of CX717 could be related to changes in monoamines in other brain area(s). Alternatively, a more parsimonious explanation could be that, under condition of systemic administration the brain concentration of CX717 is sufficient for reducing immobility in the FST, but not for altering prefrontal monoamine transmitters. Further, unlike the perfusion of a full agonist, which increases the output of 5-HT, DA and glutamate in the mPFC [8, 49], CX717, increases monoamine, but not glutamate output. We postulate that the increase in prefrontal monoamines can be accounted for by the stimulation of AMPA receptors localized to layer V pyramidal neurons that project to brainstem monoaminergic nuclei. The findings that stimulation of prefrontal cortex projections to brainstem monoaminergic nuclei, evoked antidepressant responses through the activation of monoaminergic neurons [8, 50,51,52] gives support to our view. Further research is currently underway to examine the effects of ampakines on the firing rate of serotonergic, noradrenergic and dopaminergic neurons. There is no information about the effects of AMPA receptor antagonists on the prefrontal release of monoamines elicited by intra-cortical perfusion of CX717. However, the AMPA receptor antagonist, NBQX, attenuated the antidepressant-like action and the increase in 5-HT and glutamate induced by DBS of the infralimbic prefrontal cortex [8] and the ampakine-induced synthesis of BDNF (see below), which again suggests that CX717 would support a similar mechanism of action. Moreover, ampakines do not activate the AMPA receptor without glutamate being bound. Thus, presumably, CX717 would not function with a bound AMPA receptor antagonist. The absence of increased glutamate release could be responsible for the lack of seizure activity after administration of CX717 [34]. Although the precise mechanism responsible for this different effect is not known, it is possible that the stimulation of AMPA receptors by full agonists is more potent and effective for evoking glutamate release whereas PAMs would exert a more efficient utilization of pre-existing synaptic glutamate (by keeping the AMPA receptor channel open).
Our results also show that acute CX717 produced a rapid increase in the expression of BDNF in the mPFC although this effect lasted only for 1 h. This is coincident to what occurs with other ampakines [53,54,55]. The formation of BDNF (a trophic factor involved in neurogenesis, and synaptic plasticity is one of the well-established molecular changes related to the onset of antidepressant effects [56, 57]. Interestingly, the transient increase in BDNF protein synthesis was no longer present beyond 2 h after CX717 administration, which suggests that intracellular signaling and possibly synaptic plasticity effects were required for the antidepressant response [10, 57, 58]. Since the BDNF-induced proliferation of new-born neurons and their differentiation into adult mature neurons requires weeks to promote antidepressant response, we could likely rule out neurogenesis as being responsible for the antidepressant effects of CX717. Instead, we postulate that the rapid stimulation of AMPA synaptic activity by CX717 would result in a rapid Ca2+ influx and subsequent release of BDNF, as reported for other ampakines [26, 59]. This would lead to a depletion of internal BDNF sources with the concomitant induction of its new synthesis. Eventually, the increased synthesis of BDNF protein would activate mTOR signaling and evoke formation of new synaptic proteins. Indeed, it has been shown that ampakines stimulate mTOR activation, which induces a rapid stimulation of translation machinery, a process prevented by an AMPA receptor blocker [59]. Thus, the elaboration of new synapses in the prefrontal cortex is likely important for the behavioral effects observed in the time frame of the present study. Furthermore, the increase of PSD95 2 h after CX717 might be indicative of the formation of new synaptic contacts at this time point [60]. CX717 also increased the concentration of p11 in the mPFC. It has been shown that the protein p11 (also known as S100A10) plays an important role in depression as well as in the responses to antidepressant treatment [61]. Thus, the absence of p11 from birth induced a depressive-like behavior in mice, an effect abrogated by the overexpression of p11 in the nucleus accumbens [62]. Also, p11 is upregulated by antidepressant drugs [63]. It is known that BDNF stimulates the formation of p11 in primary neuronal cultures [64], and the present work also suggests that the synthesis of p11 persisted after BNDF returned to basal levels. On the other hand, p11 has a bidirectional relationship with 5-HT transmission in that p11 potentiates 5-HT neurotransmission [61] and 5-HT controls p11 expression [63, 64]. It is important to note herein that the expression of p11 is enriched in prefrontal cortex, DRN, ventral tegmental area and locus coeruleus [65], thus underscoring the relevance of p11 expression in the brain circuitry implicated in mood regulation. In fact, layer V projection pyramidal cells of the mPFC that express p11 are particularly responsive to effective antidepressant treatment, and this action requires p11 [66].
Among the four subunits of AMPA receptors, the GluA1 subunit appears to play a role in the response to stress, depression and neuroplasticity [67]. In fact, membrane expression of GluA1 is found consistently enhanced after chronic antidepressant treatment [6, 68] and rapidly after NMDA receptor antagonists [44, 69]. In the present work, however, CX717 did not stimulate glutamate release nor did it alter GluA1 synthesis, possibly because only the stimulation of AMPA receptors by full agonists such as glutamate or (S)-AMPA is able to elevate the formation of GluA1. Thus, the view that increased levels of GluA1 is a common feature of rapid-acting antidepressant drugs [70] does not seem to adhere to the effects of PAMs of AMPA receptors. It is possible that CX717 is able to increase the biological activity of the GluA1 subunit without changing its level of expression. Further research is needed to unveil the mechanism(s) responsible for this process.
In summary, CX717 shows future promise as a novel antidepressant drug. Our findings suggest a key role of BDNF and p11 in the mPFC in the antidepressant-like effects of CX717. Elevations of prefrontal monoamines might be responsible for more protracted effects of CX717.
References
Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51:7–61
Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH (2007) Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology 32:1888–1902
Duric V, Banasr M, Stockmeier CA, Simen AA, Newton SS, Overholser JC, Jurjus GJ, Dieter L et al (2013) Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int J Neuropsychopharmacol 16:69–82
Wisden W, Seeburg PH, Monyer H (2000) AMPA, kainate and NMDA ionotropic glutamate receptor expression – an in situ hybridization atlas. In: Ottersen OP, Storm-Mathisen J (eds) Handbook of Chemical Neuroanatomy, Glutamate, vol 18. Elsevier, Amsterdam, pp. 99–143
Beneyto M, Meador-Woodruff JH (2008) Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology 33:2175–2186
Barbon A, Caracciolo L, Orlandi C, Musazzi L, Mallei A, La Via L, Bonini D, Mora C et al (2011) Chronic antidepressant treatments induce a time-dependent up-regulation of AMPA receptor subunit protein levels. Neurochem Int 59:896–905
Akinfiresoye L, Tizabi Y (2013) Antidepressant effects of AMPA and ketamine combination: role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacology 230:291–298
Jiménez-Sánchez L, Castañé A, Pérez-Caballero L, Grifoll-Escoda M, López-Gil X, Campa L, Galofré M, Berrocoso E et al (2016) Activation of AMPA receptors mediates the antidepressant action of deep brain stimulation of the infralimbic prefrontal cortex. Cereb Cortex 26:2778–2789
Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: Role of a-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63:349–352
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95
Koike H, Iijima M, Chaki S (2011) Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res 224:107–111
Melendez RI, Kalivas PW (2003) Metabotropic glutamate receptor regulation of extracellular glutamate levels in the prefrontal cortex. Ann NY Acad. Sci 1003:443–444
Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW (2002) Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther 300:162–171
Karasawa J, Shimazaki T, Kawashima N, Chaki S (2005) AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res 1042:92–98
Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN, Carter G, Johnson B et al (2016) The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. J Pharmacol Exp Ther 358:71–82
Derkach VA, Oh MC, Guire ES, Soderling TR (2007) Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci 8:101–113
O'Neill MJ, Dix S (2007) AMPA receptor potentiators as cognitive enhancers. IDrugs 10:185–192
Hampson RE, España RA, Rogers GA, Porrino LJ, Deadwyler SA (2009) Mechanisms underlying cognitive enhancement and reversal of cognitive deficits in nonhuman primates by the ampakine CX717. Psychopharmacology 202:355–369
Kunugi A, Tanaka M, Suzuki A, Tajima Y, Suzuki N, Suzuki M, Nakamura S, Kuno H et al (2019) TAK-137, an AMPA-R potentiator with little agonistic effect, has a wide therapeutic window. Neuropsychopharmacology 44:961–970
Meldrum BS (1994) The role of glutamate in epilepsy and other CNS disorders. Neurology 44(Suppl 8):S14–S23
Lynch G (2006) Glutamate-based therapeutic approaches: ampakines. Curr Opin Pharmacol 6:82–88
Lapidus KA, Soleimani L, Murrough JW (2013) Novel glutamatergic drugs for the treatment of mood disorders. Neuropsychiatr Dis Treat 9:1101–1112
Arai A, Guidotti A, Costa E, Lynch G (1996) Effect of the AMPA receptor modulator IDRA 21 on LTP in hippocampal slices. Neuroreport 7:2211–2215
Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P (2001) Antidepressant-like actions of an AMPA receptor potentiator (LY392098). Neuropharmacology 40:1028–1033
Knapp RJ, Goldenberg R, Shuck C, Cecil A, Watkins J, Miller C, Crites G, Malatynska E (2002) Antidepressant activity of memory-enhancing drugs in the reduction of submissive behavior model. Eur J Pharmacol 440:27–35
Alt A, Nisenbaum ES, Bleakman D, Witkin JM (2006) A role for AMPA receptors in mood disorders. Biochem Pharmacol 71:1273–1288
Boyle J, Stanley N, James LM, Wright N, Johnsen S, Arbon EL, Dijk DJ (2012) Acute sleep deprivation: the effects of the AMPAKINE compound CX717 on human cognitive performance, alertness and recovery sleep. J Psychopharmacol 26:1047–1057
Oertel BG, Felden L, Tran PV, Bradshaw MH, Angst MS, Schmidt H, Johnson S, Greer JJ et al (2010) Selective antagonism of opioid-induced ventilatory depression by an ampakine molecule in humans without loss of opioid analgesia. Clin Pharmacol Ther 87:204–211
Wesensten NJ, Reichardt RM, Balkin TJ (2007) Ampakine (CX717) effects on performance and alertness during simulated night shift work. Aviat Space Environ Med 78:937–943
Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA (2005) Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol 3:e299
Andreasen JT, Gynther M, Rygaard A, Bøgelund T, Nielsen SD, Clausen RP, Mogensen J, Pickering DS (2013) Does increasing the ratio of AMPA-to-NMDA receptor mediated neurotransmission engender antidepressant action? Studies in the mouse forced swim and tail suspension tests. Neurosci Lett 546:6–10
Li X, Witkin JM, Need AB, Skolnick P (2003) Enhancement of antidepressant potency by a potentiator of AMPA receptors. Cell Mol Neurobiol 23:419–430
Ren J, Lenal F, Yang M, Ding X, Greer JJ (2013) Coadministration of the AMPAKINE CX717 with propofol reduces respiratory depression and fatal apneas. Anesthesiology 118:1437–1445
Ren J, Ding X, Greer JJ (2012) Respiratory depression in rats induced by alcohol and barbiturate and rescue by ampakine CX717. J Appl Physiol 113:1004–1011
Cryan JF, Valentino RJ, Lucki I (2005) Assessing substrates underlying the behavioral effects of antidepressants using the modi?ed rat forced swimming test. Neurosci Biobehav Rev 29:547–569
Benveniste H, Diemer NH (1987) Cellular reactions to implantation of a microdialysis tube in the rat hippocampus. Acta Neuropathol 74:234–238
Benveniste H, Drejer J, Schousboe A, Diemer NH (1987) Regional cerebral glucose phosphorylation and blood flow after insertion of a microdialysis fiber through the dorsal hippocampus in the rat. J Neurochem 49:729–734
Detke MJ, Rickels M, Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 121:66–72
Paxinos C, Watson D (2005) The rat brain in stereotaxic coordinates. Elsevier/Academic Press, Amsterdam
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45:651–660
Arai AC, Xia YF, Rogers G, Lynch G, Kessler M (2002) Benzamide-type AMPA receptor modulators form two subfamilies with distinct modes of action. J Pharmacol Exp Ther 303:1075–1085
Mul JD, Zheng J, Goodyear LJ (2016) Validity assessment of 5 day repeated forced-swim stress to model human depression in young-adult C57BL/6J and BALB/cJ mice. eNeuro 29:3
Mezadri TJ, Batista GM, Portes AC, Marino-Neto J, Lino-de-Oliveira C (2011) Repeated rat-forced swim test: reducing the number of animals to evaluate gradual effects of antidepressants. J Neurosci Methods 195:200–205
Gordillo-Salas M, Pilar-Cuéllar F, Auberson YP, Adell A (2018) Signaling pathways responsible for the rapid antidepressant-like effects of a GluN2A-preferring NMDA receptor antagonist. Transl Psychiatry 8:84
Lucas G, Rymar VV, Du J, Mnie-Filali O, Bisgaard C, Manta S, Lambas-Senas L, Wiborg O et al (2007) Serotonin4 (5-HT4) receptor agonists are putative antidepressants with a rapid onset of action. Neuron 55:712–725
Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D et al (1998) Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA 95:14476–14481
Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A et al (2010) 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 65:40–52
Bortolozzi A, Castañé A, Semakova J, Santana N, Alvarado G, Cortés R, Ferrés-Coy A, Fernández G et al (2012) Selective siRNA-mediated suppression of 5-HT1A autoreceptors evokes strong anti-depressant-like effects. Mol Psychiatry 17:612–623
Jedema HP, Moghaddam B (1994) Glutamatergic control of dopamine release during stress in the rat prefrontal cortex. J Neurochem 63:785–788
Covington HE 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E et al (2010) Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci 30:16082–16090
Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM et al (2012) A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 492:428–432
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC et al (2013) Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493:532–536
Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM (2000) Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J Neurosci 20:8–21
Mackowiak M, O'Neill MJ, Hicks CA, Bleakman D, Skolnick P (2002) An AMPA receptor potentiator modulates hippocampal expression of BDNF: an in vivo study. Neuropharmacology 43:1–10
Radin DP, Johnson S, Purcell R, Lippa AS (2018) Effects of chronic systemic low-impact ampakine treatment on neurotrophin expression in rat brain. Biomed Pharmacother 105:540–544
Nibuya M, Morinobu S, Duman RS (1995) Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 15:7539–7547
Björkholm C, Monteggia LM (2016) BDNF - A key transducer of antidepressant effects. Neuropharmacology 102:72–79
Ramaker MJ, Dulawa SC (2017) Identifying fast-onset antidepressants using rodent models. Mol Psychiatry 22:656–665
Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M (2009) Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci 29:8688–8697
Nikonenko I, Boda B, Steen S, Knott G, Welker E, Muller D (2008) PSD-95 promotes synaptogenesis and multiinnervated spine formation through nitric oxide signaling. J Cell Biol 183:1115–1127
Svenningsson P, Kim Y, Warner-Schmidt J, Oh YS, Greengard P (2013) p11 and its role in depression and therapeutic responses to antidepressants. Nat Rev Neurosci 14:673–680
Warner-Schmidt JL, Schmidt EF, Marshall JJ, Rubin AJ, Arango-Lievano M, Kaplitt MG, Ibañez-Tallon I, Heintz N et al (2012) Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci USA 109:11360–11365
Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG et al (2006) Alterations in 5-HT1B receptor function by p11 in depression-like states. Science 311:77–80
Warner-Schmidt JL, Chen EY, Zhang X, Marshall JJ, Morozov A, Svenningsson P, Greengard P (2010) A role for p11 in the antidepressant action of brain-derived neurotrophic factor. Biol Psychiatry 68:528–535
®2015 Allen Institute for Brain Science. Allen Brain Atlas API. Available from: http://brain-map.org/api/index.html.
Schmidt EF, Warner-Schmidt JL, Otopalik BG, Pickett SB, Greengard P, Heintz N (2012) Identification of the cortical neurons that mediate antidepressant responses. Cell 149:1152–1163
Freudenberg F, Celikel T, Reif A (2015) The role of a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in depression: central mediators of pathophysiology and antidepressant activity? Neurosci Biobehav Rev 52:193–206
Martínez-Turrillas R, Del Río J, Frechilla D (2005) Sequential changes in BDNF mRNA expression and synaptic levels of AMPA receptor subunits in rat hippocampus after chronic antidepressant treatment. Neuropharmacology 49:1178–1188
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G et al (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964
Duman RS, Shinohara R, Fogaça MV, Hare B (2019) Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function. Mol Psychiatry 24:1816–1832
Acknowledgement
We thank RespireRX Pharmaceuticals for the generous gift of CX717.
Funding
This work was supported by the Instituto de Salud Carlos III, Subdirección General de Evaluación y Fomento de la Investigación (FIS Grant PI16/00217) that was co-funded by the European Regional Development Fund (‘A way to build Europe’). Funding from the Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Instituto de Salud Carlos III is also acknowledged. The funding agencies had no role in the design and conduct of the study, collection, management, analyses, and interpretation of the data; and preparation, review, or approval of the manuscript and the decision to submit it for publication. MG-S and RP-A were recipients of contracts from the Sistema Nacional de Garantía Juvenil co-funded by the European Social Fund..
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 234 kb)
Rights and permissions
About this article
Cite this article
Gordillo-Salas, M., Pascual-Antón, R., Ren, J. et al. Antidepressant-Like Effects of CX717, a Positive Allosteric Modulator of AMPA Receptors. Mol Neurobiol 57, 3498–3507 (2020). https://doi.org/10.1007/s12035-020-01954-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01954-x