Abstract
The insect integument is covered by cuticular hydrocarbons (CHCs) which provide protection against environmental stresses, but are also used for communication. Here we review current knowledge on environmental and insect-internal factors which shape phenotypic plasticity of solitary living insects, especially herbivorous ones. We address the dynamics of changes which may occur within minutes, but may also last weeks, depending on the species and conditions. Two different modes of changes are suggested, i.e. stepwise and gradual. A switch between two distinct environments (e.g. host plant switch by phytophagous insects) results in stepwise formation of two distinct adaptive phenotypes, while a gradual environmental change (e.g. temperature gradients) induces a gradual change of numerous adaptive CHC phenotypes. We further discuss the ecological and evolutionary consequences of phenotypic plasticity of insect CHC profiles by addressing the question at which conditions is CHC phenotypic plasticity beneficial. The high plasticity of CHC profiles might be a trade-off for insects using CHCs for communication. We discuss how insects cope with the challenge to produce and “understand” a highly plastic, environmentally dependent CHC pattern that conveys reliable and comprehensible information. Finally, we outline how phenotypic plasticity of CHC profiles may promote speciation in insects that rely on CHCs for mate recognition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organisms need to cope with a huge variety of environmental conditions that may change in space, time, intensity, and quality. The response of an individual to novel environmental conditions can take many forms, ranging from changes in physiology, alterations of morphology to shifts in behavioral responses (Schlichting and Pigliucci 1998; Wund 2012). This variation in phenotypic expression is defined as “phenotypic plasticity” – the ability of a single genotype to produce different phenotypes in response to different abiotic and biotic environmental conditions (Moczek et al. 2011; Pfennig et al. 2010; Pigliucci et al. 2006; Via et al. 1995). Phenotypic plasticity of a certain genotype plays a role in many evolutionary processes like selection within and between species (Salamin et al. 2010), formation of host races (Drès and Mallet 2002), or the establishment of reproductive isolation barriers between and within populations (Coyne and Orr 2004). Thus, phenotypic plasticity may promote speciation processes (Pfennig et al. 2010), and facilitate or even speed up the process of (adaptive) evolution (Ghalambor et al. 2007; West-Eberhard 2003). In order to understand the mechanisms and adaptive value of phenotypic plasticity, we need to investigate the responses of an organism to changing environmental factors and the costs and/or benefits of phenotypic changes (Moczek 2010; Snell-Rood 2012).
In this review, we focus on the phenotypic plasticity of cuticular hydrocarbons (CHCs) in solitary insects, with special emphasis to herbivorous species (Chung and Carroll 2015; Thomas and Simmons 2011). We do not consider CHCs of eusocial insects like termites, ants and bees because the chemistry and ecological relevance of their CHC profiles in social life have been excellently addressed in several recent reviews (Leonhardt et al. 2016; Oi et al. 2015; Smith and Liebig 2017).
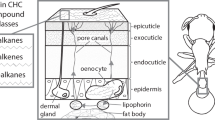
CHCs are of enormous functional significance in insects which have evolved a wide range of CHCs differing with respect to chain length (typically 20 to about 40 carbons), methyl branching pattern, and position and number of double bonds (Geiselhardt et al. 2011; Martin and Drijfhout 2009). CHCs are covering the insect’s integument and protect them from abiotic and biotic environmental stress, as will be outlined below. Insects can adjust the chemical composition of their CHC profiles to the environmental needs. CHCs do not only serve as protective device, but are also used by many insect species for intra- and interspecific communication (Blomquist and Bagnères 2010). When communicating by these highly variable chemical cues, insects need to be able to cope with this environmentally dependent, flexible chemical information.
A major issue of this review is to outline current knowledge and resulting ideas on the ecological processes that shape phenotypic plasticity of insect CHC profiles which may contribute to speciation of insects. We first provide a brief overview on factors influencing insect CHCs and address the dynamics of phenotypic changes. We further analyze two modes of phenotypic change and differentiate between saltatory and gradual adaptive changes. Finally, we critically discuss consequences of phenotypic plasticity in insect chemical communication systems, speciation processes and evolutionary biology. We address costs and benefits of the plasticity of insect CHC profiles with respect to the dynamics of changes of CHC patterns, the modes of changes, and the individual and evolutionary consequences of changes.
Phenotypic Plasticity of CHCs – Drivers and Temporal Dynamics
Several studies suggest that CHC profiles largely depend on the insect’s genetic background (e.g. Dembeck et al. 2015). In turn, numerous abiotic and biotic environmental factors (Ingleby 2015; Leonhardt et al. 2016) shape the internal status of an insect and expression of genes (Ferveur 2005; Martin and Drijfhout 2009; Menzel et al. 2017) (Fig. 1). The enormous impact of the environment on an insect’s CHC profile has been shown long ago by a study of Toolson and Kuper-Simbron (1989) who transferred Drosophila pseudoobscura from the field to the laboratory; the flies showed altered CHC profiles already in the first laboratory generation. However, the cause of this shift remained unclear. Below, we will outline the impact of several environmental and insect-internal factors on insect CHC patterns. Examples are listed in Table 1. Furthermore, the dynamics by which insect CHC phenotypes adapt to changing environmental conditions will be addressed and discussed.
Abiotic Environmental Factors as Drivers of Insect CHC Phenotypes
When insects are exposed to high temperature and/or low relative humidity, they face a high risk of desiccation because of their large surface-to-volume ratio. CHCs act as waterproofing agents and support prevention of dehydration in interaction with other cuticular compounds and respiratory regulation. The lipophilic, long-chained CHCs form a protective film on the insect’s integument which impairs permeation of water molecules to the outside. In addition, the hydrophobic CHCs prevent wetting and passage of water into the insect (Gibbs et al. 1998; Wang et al. 2016, and references therein). Insects can prevent critical water loss in response to high temperature and low humidity by a fast change of their CHC phenotype (Chown et al. 2011). They start to change their CHC phenotype in response to changing temperature or humidity within a day (Howard et al. 1995; Kwan and Rundle 2010; Savarit and Ferveur 2002; Stinziano et al. 2015). In general, desiccation stress leads to an adaptive shift towards increased levels of longer chain CHCs, a higher proportion of saturated CHCs, and/or greater proportions of straight- versus branched-chain CHCs.
Furthermore, CHCs can protect from harmful, cytotoxic sun radiation in combination with other cuticular compounds. Cockroaches are known to even increase their hydrocarbon content in the cuticle upon exposure to UV radiation (Gingrich 1975).
Also the photoperiod or time of day can significantly impact on the CHC phenotype of an insect. In male D. melanogaster, CHCs are dynamic traits that vary in response to time of day, and this diurnal pattern is sensitive to light (Gershman et al. 2014; Kent et al. 2007; Krupp et al. 2008). However, while trait values of CHC-based attractiveness in males are highest during day and low throughout the dark phase, females show exactly the opposite temporal pattern (Gershman et al. 2014). Jurenka et al. (1998) used short-day-conditions to induce reproductive diapause in face flies, Musca autumnalis. Diapausing flies had lower proportions of alkenes and higher proportions of methyl-branched alkanes compared to reproductive face flies reared under long-day conditions.
Biotic Environmental Factors as Drivers of Insect CHC Phenotypes
CHCs serve a crucial role in protection against pathogen infection. They contribute to formation of a physical barrier which prevents pathogens to penetrate into the insect. Especially fungal pathogens which require some water for germination are impaired by the hydrophobic interaction with CHCs (Hajek and St. Leger 1994). However, some entomopathogenic fungi can rapidly degrade insect CHCs (Napolitano and Juárez 1997) and thus, significantly alter the CHC phenotype of its host within a few hours after infection, depending on the insect species (Lecuona et al. 1991) and the age of the host (Zurek et al. 2002) (Table 2). In addition, other ecto- and endoparasites, (e.g. viruses, mites, tapeworms) or even an artificial immune stimulation may also elicit rapid modifications of CHC profiles (Beros et al. 2017 and references therein; Nielsen and Holman 2012).
Numerous studies have demonstrated a significant role of diet in phenotypic plasticity of CHCs in various insect orders (Table 2). For example, caterpillars of several lepidopteran species (Espelie and Bernays 1989) and grasshoppers (Blomquist and Jackson 1973) have been shown to incorporate dietary CHCs into their CHC profiles. Caterpillars of some polyphagous species use ingested dietary CHCs to mimic the cuticular chemistry of their actual host plant (chemical phytomimesis) to avoid predation by ants (Akino et al. 2004; Piskorski et al. 2010; Portugal and Trigo 2005;). Dietary effects on the CHC phenotype might also be triggered by varying compositions of CHC precursors in different diet, e.g. fatty acids (Otte et al. 2015; Pennanec'h et al. 1997; Steiger et al. 2007). When the mustard leaf beetle Phaedon cochleariae switches to a novel host species, it takes about two weeks until its novel CHC profile significantly differs from the former one (Geiselhardt et al. 2012). Not only herbivorous insects, but also parasitic and predatory insects have been shown to produce different CHC patterns when developing in different host species (e.g. Khidr et al. 2013; Kühbandner et al. 2012).
The type of diet affects the gut microbiome which may also impact on the insect’s CHC pattern. Sharon et al. (2010) have demonstrated that D. melanogaster flies (Oregon-R) reared on different diets show altered gut microbiomes and divergent CHC phenotypes, which in turn leads to diet-assortative mating because mating in these flies is mediated by their CHC patterns. However, follow-up studies led to inconsistent results about the role of the gut microbiome on mate preference and CHC phenotype (Leftwich et al. 2017; Ward 2017). Ward (2017) found a significant effect of diet on the CHC phenotype of Canton-S flies, but no effect of the gut microbiome on the CHC profile. Moreover, Leftwich et al. (2017) failed to show a significant role of diet or gut microbiome on mate preferences in two wild-type strains (Dahomey and Oregon-R). Thus, the significance of the gut microbiome as a trigger for CHC-mediated behavioral isolation remains ambiguous and needs further investigations.
Not just the type of diet and the depending microbiome, but also the quantity of diet significantly affects the insect’s CHC profile. Starvation leads to a significant change in insect CHC patterns (Peschke 1987a, b). In earwigs, which show parental care, the offspring CHC profile signals the nutritional need and thus, affects foraging and reproductive activities of the mother (Mas et al. 2009; Mas and Kölliker 2011). The mother’s CHC profile signals the nutritional provision, and thus, affects sibling cannibalism (Wong et al. 2014a).
The encounter of conspecifics can trigger rapid changes of CHC phenotypes. For example, males of D. serrata change their CHC profiles within a few minutes after exposure to female flies (Petfield et al. 2005). Moreover, in D. serrata, the attractiveness of male CHC profiles depends on the presence and absence of conspecific males and females, and their sex ratio (Gershman et al. 2014; Gershman and Rundle 2016, 2017). Similarly, the CHC profiles of male D. melanogaster are also affected upon encounter with conspecifics, and this in turn infers with the diurnal cycle of CHC expression (Kent et al. 2008; Krupp et al. 2008). Furthermore, in orthopteran species, the dominance of rivalling males (Thomas and Simmons 2011), experience of conspecific song (Thomas et al. 2011), and population density (Genin et al. 1986; Heifetz et al. 1998) affect the insect’s CHC profile. Biparental burying beetles are able to discriminate between breeding and non-breeding conspecifics based on the CHC profile of their counterpart (Scott et al. 2008; Steiger et al. 2007).
Mating is also known to trigger changes of the CHC phenotype in various insect groups (Table 2). Male rove beetles, Aleochara curtula, rapidly (within 30 min) change their CHC profile after onset of copulation (Peschke 1987a). In D. serrata, courtship results in a very rapid change in the CHC profile even without physical contact of the partners (Petfield et al. 2005). In addition, successful copulation with sperm transfer has been shown to induce the development of male CHC phenotypes associated with high mating success in this species (Gershman and Rundle 2016). Also both sexes of D. melanogaster show altered CHC profiles immediately after mating which is most likely caused by reciprocal physical transfer of CHCs between the sexes (Everaerts et al. 2010). In contrast, Farine et al. (2012) found only a marginal effect of sexual interactions on volatile CHCs (≤ n-C23) of D. melanogaster flies kept in heterosexual groups for 2 h. However, the sex peptide ACP70A, a component in the seminal fluid of D. melanogaster, is involved in down-regulation of the biosynthesis of female sex pheromones (Bontonou et al. 2015). So far, in many cases the effects on CHC phenotypes could not unambiguously be assigned to mating or insemination because differences in ovary development could not be ruled out as an alternative explanation for altered CHC profiles (Booksmythe et al. 2017; Mant et al. 2005; Polidori et al. 2017; Polerstock et al. 2002; Simmons et al. 2003).
Insect-Internal Factors
The composition of the CHC profile of an insect is subjected to dynamic changes during development and ageing of the insect (Moore et al. 2017, and references therein) (Table 1).
Interestingly, phenotypic changes in CHC profiles are not only obvious when comparing different developmental stages, but also occur within the first days after adult eclosion. In several flies (Jackson and Bartelt 1986; Mpuru et al. 2001), parasitic wasps (Ruther et al. 2011; Steiner et al. 2005; Steiner et al. 2007), and in rove beetles (Peschke 1985), newly emerged adults of both sexes have similar CHC phenotypes that diverge and become sex-specific within a week (Table 2). Hence, a change in CHC phenotype in adults is often correlated with sexual maturation and development of the gonads (Dillwith et al. 1983; Fedina et al. 2012, 2017; Schal et al. 1994; Wicker and Jallon 1995b).
Changes in the CHC profile during insect development may be ascribed to changing CHC biosynthesis by the insect (Howard and Blomquist 2005). However, also CHCs of empty dipteran puparia change over time, a process which is due to weathering (Moore et al. 2017). Changes of an insect’s CHC profile may be of quantitative nature when biosynthetic activities and thus quantities of CHC compounds vary (e.g. Krupp et al. 2008). But also qualitative changes over time are observed when novel CHC compounds are added to a CHC pattern by the insect’s CHC transport activity or CHC biosynthetic activity (e.g. Schal et al. 1994; Steiner et al. 2007). A qualitative change of a CHC pattern may also be due to environmentally dependent changes (weathering) of already produced compounds on the insect’s surface.
In summary, the switch from one CHC profile to a novel, adaptive one may take minutes to weeks (Table 2). The dynamics and type of changes might depend on the need of a new CHC phenotype for survival or successful reproduction, as well as on the mechanisms which mount the insect’s CHC pattern (Howard and Blomquist 2005). So far, it is difficult to predict which type of environmental stimulus induces a slow change of the CHC profile and which one triggers a highly rapid change.
Mechanisms of Phenotypic Plasticity of CHC Profiles
Rapid changes in insect CHC profiles are expected when an insect obtains CHCs from the environmental substrate and/or from other organisms by physical contact, e.g. during mating (e.g. Weddle et al. 2012). Nevertheless, fast changes in the CHC pattern may not only be possible by acquiring novel CHCs via contact, but may also depend on the dynamics of the transport of ingested CHCs to the cuticle via lipophorins (e.g. Schal et al. 1998).
If the change in an insect’s CHC pattern requires a change in CHC biosynthesis, the dynamics of this change will depend on the insect’s physiological state (e.g. the dynamics of changes in hormone levels) (Bagnères and Blomquist 2010), the availability of CHC precursors (Steiger et al. 2007; Otte et al. 2015), the uptake of compounds affecting biosynthesis and enzyme activities, and the expression activity of genes encoding the respective enzymes. Krupp et al. (2008) have demonstrated that oenocytes have functional molecular clocks that control the circadian rhythmicity of monoenes by controlling the expression of desat1, a gene that encodes a desaturase involved in pheromone biosynthesis of D. melanogaster. As mentioned above, several other studies show that abundance of CHCs on the insect’s cuticle is dependent on the daytime (or nighttime) when measurements are taken (Gershman et al. 2014; Kent et al. 2007). So far, it is unclear how much the 24-h-temporal pattern of CHC profiles depends on CHC biosynthesis (expression levels of genes, enzyme bioactivities), transport activity of CHCs to the cuticle and/or loss of once produced CHCs due to offprint on the substrate.
Modes of Phenotypic Changes of Insect CHC Profiles
Exposure to different environmental conditions is suggested to result in different modes of phenotypic changes. We distinguish between (A) a stepwise (saltatory) shift and (B) a continuous (gradual) one. The stepwise shift from one CHC pattern to another occurs upon a saltatory environmental change (e.g. switch from one host species to another) and is lacking an adaptive intermediate CHC profile. In contrast, the continuous change of a CHC profile occurs in response to gradual changes of environmental conditions (e.g. increasing temperature) and shows many intermediate, adaptive CHC profiles (West-Eberhard 2003) (Fig. 2).
Scheme of two modes of phenotypic plasticity: a stepwise (saltatory) shift from one CHC phenotype to another in individuals experiencing two distinct environments (e.g. different host species); no adaptive intermediate phenotype is formed (black lines) and b a continuous (gradual) change of a CHC phenotype in individuals experiencing a gradually changing environment (e.g. increasing temperature); many adaptive intermediate phenotypes are formed (grey line)
The stepwise (saltatory) type of plasticity (A) is characterized by a change in the mean CHC phenotype which is produced in (at least) two distinct environments. For example, when herbivorous beetles were exposed to a novel host plant species; they formed a different CHC pattern than conspecifics remaining on the original host plant species. The novel CHC phenotype enabled the beetles to distinguish between mates that had fed on the same plant species from mates feeding on the alternative plant species (Otte et al. 2016). This type-(A)-phenotypic plasticity may be quantified by the mean and variance of phenotypes within a population. A shift in a mean phenotype can occur when all individuals show a similar reaction norm, i.e. respond similarly to a change of an environmental cue. The variance can increase if different genotypes within a population respond differently to the same cue. Hence, type-(A)-phenotypes within a certain environment can show slight differences (but are mostly similar), but type-(A)-phenotypes occurring in two different environments are clearly different and separable (Ingleby et al. 2010).
The continuous (gradual) mode of phenotypic plasticity (B) focuses on a functional relationship between environmental conditions and phenotypes. Here organisms that are exposed to a continuously changing environmental gradient (e.g. increasing temperatures) express continuously different phenotypes (many intermediate phenotypes between different environments). For example, the chain length of CHCs may increase when temperature increases; the elongation of CHC chain length protects the organism from water loss (Geiselhardt et al. 2006). Such adaptation of the CHC pattern to temperature requires high plasticity because changes need to be reversible when temperature decreases again. Indeed, D. melanogaster shows reversible temperature-dependent cuticle permeability for an aqueous solution (Wang et al. 2016). However, whether this reversibility is due to a quick change in the chemical composition of the CHC profile has not been addressed in this study.
CHC Phenotypic Plasticity – A Challenge for Intraspecific Communication Via CHCs
Insects which use their CHCs for mediation of intra- and interspecific interactions are indeed challenged by high phenotypic plasticity of the CHC profile because intraspecific chemical communication requires high reliability and comprehensibility of the signals. If signals are not reliable, the receivers will be unable to gain fitness benefits. Moreover, if an expressed CHC phenotype does not match the receiver’s preferences or demands, the signal - perceiver system will collapse.
Thus, how can insects successfully use CHCs for intraspecific communication, although these compounds are phenotypically so plastic?
-
First, different CHCs or CHC classes might have differential importance for chemical communication (Dani et al. 2005). Thus, when insects change their behaviorally relevant CHC profiles due to different environmental conditions, these changes might be changes in compounds not relevant in intraspecific communication.
-
Second, when the alteration of a CHC phenotype is a change in quantities of all behaviorally relevant CHCs, their ratios may stay the same. Here quantities might change, but ratios of informative compounds might be kept the same. Ratios of compounds are well known to play a role in numerous chemically mediated intraspecific interactions (Geiselhardt et al. 2012; Weiss et al. 2013).
-
Finally, if qualitative and quantitative changes in the chemical composition of a CHC profile occur, a mechanism is required to recognize the new CHC phenotype. The insect may recognize a conspecific individual by comparing its own chemical phenotype with the profile of the counterpart (“self-referent phenotype matching”, Mateo 2010; Otte et al. 2016; Weddle et al. 2013). Assuming that insects use phenotype matching for recognition of conspecifics, the organisms exposed to the same environmental conditions match the chemical template more exactly than individuals from a different environment expressing an alternative CHC phenotype. This process corresponds with the signal matching process of the sensory drive hypothesis (Boughman 2002; Smadja and Butlin 2009). Thus, “self-referent phenotype matching” might help to cope with variable CHC phenotypes if these are important of intraspecific communication, but will also promote divergence in behavioral interactions (Geiselhardt et al. 2012; Otte et al. 2016).
Evolution of Phenotypic Plasticity of Insect CHC Profiles: Selective Advantages and Consequences
What are the benefits and costs of phenotypic plasticity of insect CHC patterns? An insect benefits from its phenotypic CHC plasticity when a certain phenotype induced by a specific environment has higher fitness in that environment than alternative phenotypes (DeWitt et al. 1998; Henneken et al. 2017; Snell-Rood et al. 2010). A CHC phenotype is expected to show higher fitness
-
(i)
when it has improved abilities to cope with the conditions in this specific environment. As outlined above, many insects can change their CHC phenotype in response to certain abiotic and biotic conditions and thus, improve their abilities to cope with this environment.
-
(ii)
when the costs for production and maintenance of the phenotype do not outweigh the benefit (Reylea 2002). These costs may especially occur when phenotypic plasticity requires continuous availability of the biosynthetic machinery and the maintenance of sensory and regulatory machinery to respond adequately to changing environmental conditions (DeWitt et al. 1998).
-
(iii)
when the dynamics of a change in a CHC profile is fast enough to follow the dynamics of environmental changes inducing a CHC profile. Otherwise costs arise from poor phenotype–environment matching resulting from the time lag between sensing and responding to environmental cues (lag-time limits) (DeWitt et al. 1998).
-
(iv)
when the phenotypic change of the CHC pattern is not outweighed by ecological costs. Which type of ecological costs might an insect need to “pay” when displaying phenotypic plasticity? For example, if an insect changes its CHC profile in response to changing temperatures, the novel profile might disrupt CHC-mediated communication systems (Geiselhardt et al. 2006; Peschke 1987b). If a novel CHC profile would no longer convey reliable and comprehensible information to those insects which communicate via CHCs, these costs would by far exceed the benefits of the adjustment of a CHC profile to a novel environment.
How can phenotypic divergence of CHC profiles affect speciation? If divergence of CHC profiles that affect sexual behavior leads to assortative mating, this might promote sexual isolation. For example, studies of the mustard leaf beetle P. cochleariae revealed that these beetles use their CHC profiles for mate recognition. They prefer partners with similar CHC profiles. The beetles show similar profiles when feeding on the same host plant species (Geiselhardt et al. 2009, 2012; Otte et al. 2016). Beetles of a given population of P. cochleariae may use different host plant species occurring in the population’s habitat; if the beetles show fidelity to a certain host species, their mating preferences for similar CHC phenotypes feeding on the same plant species is expected to lead to genetic divergence and thus, to promote ecological speciation. Such diet-induced changes in CHC profiles that affect mate references have also been observed in other beetle species (Fujiwara-Tsujii et al. 2013; Xue et al. 2016), in Drosophila flies (Etges et al. 2006; Havens and Etges 2013; Rundle et al. 2005), and in parasitoid wasps (Howard 2001; Kühbandner et al. 2012). Hence, in these cases phenotypic divergence might promote genetic divergence, and thus finally impact on insect speciation.
Conclusions
A plethora of studies demonstrated the immense plasticity of insect CHC profiles. Nevertheless, further studies are needed on the question why insects use so highly variable chemical signals for intraspecific communication although signal reliability is needed (Henneken et al. 2017; Ingleby 2015; Kather and Martin 2015). These studies will provide a deeper understanding on the evolution of CHC-based mating signals, the respective recognition systems as well as on ecological speciation processes and the impact of phenotype divergence on genetic divergence. Furthermore, even though much knowledge is available on how insects biosynthesize their CHCs, still many questions remain to be answered as to how environmental, especially nutritional factors, influence the biosynthesis of CHCs and its dynamics.
Phenotypic plasticity of insect CHC profiles is favored in changing environments because it allows an individual to adapt its phenotype to novel environmental conditions. Furthermore, plastic CHC phenotypes allow organisms to invade multiple, disparate ecological niches, thus extending the geographic range and decreasing the probability of extinction caused by habitat loss or environmental stochasticity (Snell-Rood et al. 2010). Moreover, if insects use their CHCs for intraspecific communication, phenotypic changes may promote divergence of individuals within a population. If the diverging phenotypes are maintained, ecological speciation will be promoted.
Hence, phenotypic plasticity of insect CHC profiles may greatly impact on the fitness of an insect species, its ecological niche and geographical distribution, and thus on the diversity of insect species that evolve.
References
Akino T, Knapp JJ, Thomas JA, Elmes GW (1999) Chemical mimicry and host specificity in the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc R Soc Lond B 266:1419–1426
Akino T, Nakamura K, Wakamura S (2004) Diet-induced chemical phytomimesis by twig-like caterpillars of Biston robustum Butler (Lepidoptera: Geometridae). Chemoecology 14:165–174
Armold MT, Regnier FE (1975) A developmental study of the cuticular hydrocarbons of Sarcophaga bullata. J Insect Physiol 21:1827–1833
Armold MT, Blomquist GJ, Jackson LL (1969) Cuticular lipids of insects—III. The surface lipids of the aquatic and terrestrial life forms of the big stonefly, Pteronarcys californica Newport. Comp Biochem Physiol 31:685–692
Bagnères A-G, Blomquist GJ (2010) Site of synthesis, mechanism of transport and selective deposition of hydrocarbons. In: Blomquist GJ, Bagnères, A-G (eds.). Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology. Cambridge University Press, Cambridge, pp 75–99.
Baker JE, Nelson DR, Fatland C (1979a) Developmental changes in cuticular lipids of the black carpet beetle, Attagenus megatoma. Insect Biochem 9:335–339
Baker JE, Sukkestad DR, Nelson DR, Fatland C (1979b) Cuticular lipids of larvae and adults of the cigarette beetle, Lasioderma serricorne. Insect Biochem 9:603–611
Beros S, Foitzik S, Menzel FJ (2017) What are the mechanisms behind a parasite-induced decline in nestmate recognition in ants? J Chem Ecol 43:869–880
Bilen J, Atallah J, Azanchi R, Levine JD, Riddiford LM (2013) Regulation of onset of female mating and sex pheromone production by juvenile hormone in Drosophila melanogaster. Proc Natl Acad Sci U S A 110:18321–18326
Blomquist GJ, Bagnères A-G (2010) Introduction: history and overview of insect hydrocarbons. In: Blomquist GJ, Bagnères A-G (eds) Insect hydrocarbons: biology, biochemistry, and chemical ecology. Cambridge University Press, Cambridge, pp 3–18
Blomquist GJ, Jackson LL (1973) Incorporation of labelled dietary n-alkanes into cuticular lipids of the grasshopper Melanoplus sanguinipes. J Insect Physiol 19:1639–1647
Bontonou G, Denis B, Wicker-Thomas C (2013) Interaction between temperature and male pheromone in sexual isolation in Drosophila melanogaster. J Evol Biol 26:2008–2020
Bontonou G, Shaik HA, Denis B, Wicker-Thomas C (2015) Acp70A regulates Drosophila pheromones through juvenile hormone induction. Insect Biochem Mol Biol 56:36–49
Booksmythe I, Rundle HD, Arnqvist G (2017) Sexual dimorphism in epicuticular compounds despite similar sexual selection in sex role-reversed seed beetles. J Evol Biol 30:2005–2016
Boughman JW (2002) How sensory drive can promote speciation. Trends Ecol Evol 17:571–577
Bousquet F, Chauvel I, Flaven-Pouchon J, Farine JP, Ferveur JF (2016) Dietary rescue of altered metabolism gene reveals unexpected Drosophila mating cues. J Lipid Res 57:443–450.
Braga M, Pinto Z, de Carvalho Queiroz MM, Blomquist G (2016) Effect of age on cuticular hydrocarbon profiles in adult Chrysomya putoria (Diptera: Calliphoridae). Forensic Sci Int 259:e37–e47
Chen N, Bai Y, Fan Y-L, Liu T-X (2017) Solid-phase microextraction-based cuticular hydrocarbon profiling for intraspecific delimitation in Acyrthosiphon pisum. PLoS One 12:e0184243
Chown SL, Sorensen JG, Terblanche JS (2011) Water loss in insects: an environmental change perspective. J Insect Physiol 57:1070–1084
Chung H, Carroll SB (2015) Wax, sex and the origin of species: dual roles of insect cuticular hydrocarbons in adaptation and mating. BioEssays 37:822–830
Coyne JA, Orr HA (2004) Speciation. Sinauer, Sunderland
Dani FR, Jones GR, Corsi S, Beard R, Pradella D, Turillazzi S (2005) Nestmate recognition cues in the honey bee: differential importance of cuticular alkanes and alkenes. Chem Senses 30:477–489
de Renobales M, Blomquist GJ (1983) A developmental study of the composition and biosynthesis of the cuticular hydrocarbons of Trichoplusia ni (Lepidoptera: Noctuidae). Insect Biochem 13:493–502
Delcourt M, Rundle HD (2011) Condition dependence of a multicomponent sexual display trait in Drosophila serrata. Am Nat 177:812–823
Dembeck LM, Böröczky K, Huang W, Schal C, Anholt RR, Mackay TF (2015) Genetic architecture of natural variation in cuticular hydrocarbon composition in Drosophila melanogaster. elife 4:e09861
DeWitt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13:77–81
Dillwith JW, Adams TS, Blomquist GJ (1983) Correlation of housefly sex pheromone production with ovarian development. J Insect Physiol 29:377–386
Drès M, Mallet J (2002) Host races in plant-feeding insects and their importance in sympatric speciation. Philos T Roy Soc B 357:471–492
Elmes GW, Akino T, Thomas JA, Clarke RT, Knapp JJ (2002) Interspecific differences in cuticular hydrocarbon profiles of Myrmica ants are sufficiently consistent to explain host specificity by Maculinea (large blue) butterflies. Oecologia 130:525–535
Espelie KE, Bernays EA (1989) Diet-related differences in the cuticular lipids of Manduca sexta larvae. J Chem Ecol 15:2003–2017
Espelie KE, Chapman RF, Sword GA (1994) Variation in the surface-lipids of the grasshopper, Schistocerca americana (Drury). Biochem Syst Ecol 22:563–575
Etges WJ, de Oliveira CC (2014) Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. X. Age-specific dynamics of adult epicuticular hydrocarbon expression in response to different host plants. Ecol Evol 4:2033–2045
Etges WJ, Veenstra CL, Jackson LL (2006) Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. VII. Effects of larval dietary fatty acids on adult epicuticular hydrocarbons. J Chem Ecol 32:2629–2646
Everaerts C, Farine J-P, Cobb M, Ferveur J-F (2010) Drosophila cuticular hydrocarbons revisited: mating status alters cuticular profiles. PLoS One 5:e9607
Farine J-P, Ferveur J-F, Everaerts C (2012) Volatile Drosophila cuticular pheromones are affected by social but not sexual experience. PLoS One 7:e40396
Fedina TY, Kuo T-H, Dreisewerd K, Dierick HA, Yew JY, Pletcher SD (2012) Dietary effects on cuticular hydrocarbons and sexual attractiveness in Drosophila. PLoS One 7:e49799
Fedina TY, Arbuthnott D, Rundle HD, Promislow DEL, Pletcher SD (2017) Tissue-specific insulin signaling mediates female sexual attractiveness. PLoS Genet 13:e1006935
Ferveur J (2005) Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav Genet 35:279–295
Fujiwara-Tsujii N, Yasui H, Wakamura S (2013) Population differences in male responses to chemical mating cues in the white-spotted longicorn beetle, Anoplophora malasiaca. Chemoecology 23:113–120
Geiselhardt SF, Geiselhardt S, Peschke K (2006) Chemical mimicry of cuticular hydrocarbons – how does Eremostibes opacus gain access to breeding burrows of its host Parastizopus armaticeps (Coleoptera, Tenebrionidae)? Chemoecology 16:59–68
Geiselhardt S, Otte T, Hilker M (2009) The role of cuticular hydrocarbons in male mating behavior of the mustard leaf beetle, Phaedon cochleariae (F.) J Chem Ecol 35:1162–1171
Geiselhardt SF, Geiselhardt S, Peschke K (2011) Congruence of epicuticular hydrocarbons and tarsal secretions as a principle in beetles. Chemoecology 21:181–186
Geiselhardt S, Otte T, Hilker M (2012) Looking for a similar partner: host plants shape mating preferences of herbivorous insects by altering their contact pheromones. Ecol Lett 15:971–977
Gemeno C, Laserna N, Riba M, Valls J, Castañé C, Alomar O (2012) Cuticular hydrocarbons discriminate cryptic Macrolophus species (Hemiptera: Miridae). Bull Entomol Res 102:624–631
Genin E, Jullien R, Perez F, Fuzeau-Braesch S (1986) Cuticular hydrocarbons of gregarious and solitary locusts Locusta migratoria cinerascens. J Chem Ecol 12:213–1238
Gershman SN, Rundle HD (2016) Level up: the expression of male sexually selected cuticular hydrocarbons is mediated by sexual experience. Anim Behav 112:69–177
Gershman SN, Rundle HD (2017) Crowd control: sex ratio affects sexually selected cuticular hydrocarbons in male Drosophila serrata. J Evol Biol 30:583–590
Gershman SN, Toumishey E, Rundle HD (2014) Time flies: time of day and social environment affect cuticular hydrocarbon sexual displays in Drosophila serrata. Proc R Soc B 281:20140821
Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21:394–407
Gibbs A, Mousseau TA (1994) Thermal acclimation and genetic variation in cuticular lipids of the lesser migratory grasshopper (Melanoplus sanguinipes) - effects of lipid-composition on biophysical properties. Physiol Zool 67:1523–1543
Gibbs A, Kuenzli M, Blomquist G (1995) Sex-related and age-related changes in the biophysical properties of cuticular lipids of the housefly, Musca domestica. Arch Insect Biochem 29:87–97
Gibbs AG, Louie AK, Ayala JA (1998) Effects of temperature on cuticular lipids and water balance in a desert Drosophila: is thermal acclimation beneficial? J Exp Biol 201:71–80
Gingrich JB (1975) Ultraviolet-induced changes in cuticular waxes of American cockroaches, Periplaneta americana (L.) (Dictyoptera, Blattaria, Blattidae). Can J Zool 53:1238–1240
Hadley NF (1977) Epicuticular lipids of desert tenebrionid beetle, Eleodes armata - seasonal and acclimatory effects on composition. Insect Biochem 7:277–283
Hajek AE, St. Leger RJ (1994) Interactions between fungal pathogens and insect hosts. Annu Rev Entomol 39:293–322
Havens JA, Etges WJ (2013) Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. IX. Host plant and population specific epicuticular hydrocarbon expression influences mate choice and sexual selection. J Evol Biol 26:562–576
Hebanowska E, Malinski E, Latowska A, Dubis E, Pihlaja K, Oksman P, Nawrot J, Szafranek J (1990) A comparison of cuticular hydrocarbons of larvae and beetles of the Tribolium destructor. Comp Biochem Physiol B 96:815–819
Heifetz Y, Miloslavski I, Aizenshtat Z, Applebaum SW (1998) Cuticular surface hydrocarbons of desert locust nymphs, Schistocerca gregaria, and their effect on phase behavior. J Chem Ecol 24:1033–1047
Henneken J, Goodger JQD, Jones TM, Elgar MA (2017) Diet-mediated pheromones and signature mixtures can enforce signal reliability. Front Ecol Evol 4:145
Howard RW (1998) Ontogenetic, reproductive, and nutritional effects on the cuticular hydrocarbons of the host-specific ectoparasitoid Cephalonomia tarsalis (hymenoptera: Bethylidae). Ann Entomol Soc Am 91:101–112
Howard RW (2001) Cuticular hydrocarbons of adult Pteromalus cerealellae (hymenoptera: Pteromalidae) and two larval hosts, Angoumois grain moth (Lepidoptera: Gelechiidae) and cowpea weevil (Coleptera: Bruchidae). Ann Entomol Soc Am 94:152–158
Howard RW, Baker JE (2003) Cuticular hydrocarbons and wax esters of the ectoparasitoid Habrobracon hebetor: ontogenetic, reproductive and nutritional effects. Arch Insect Biochem Physiol 53:1–18
Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol 50:371–393
Howard RW, Pérez-Lachaud G (2002) Cuticular hydrocarbons of the ectoparasitic wasp Cephalonomia hyalinipennis (hymenoptera: Bethylidae) and its alternative host, the stored product pest Caulophilus oryzae (Coleoptera: Curculionidae). Arch Insect Biochem Physiol 50:75–84
Howard RW, Akre RD, Garnett WB (1990) Chemical mimicry in an obligate predator of carpenter ants (hymenoptera: Formicidae). Ann Entomol Soc Am 83:607–616
Howard RW, Howard CD, Colquhoun S (1995) Ontogenic and environmentally-induced changes in cuticular hydrocarbons of Oryzaephilus surinamensis (Coleoptera: Cucujidae). Ann Entomol Soc Am 88:485–495
Ingleby FC (2015) Insect cuticular hydrocarbons as dynamic traits in sexual communication. Insects 6:732–742
Ingleby FC, Hunt J, Hosken DJ (2010) The role of genotype-by-environment interactions in sexual selection. J Evol Biol 23:2031–2045
Ingleby FC, Hosken DJ, Flowers K, Hawkes MF, Lane SM, Rapkin J, Dworkin I, Hunt J (2013) Genotype-by-environment interactions for cuticular hydrocarbon expression in Drosophila simulans. J Evol Biol 26:94–107
Ingleby FC, Hosken DJ, Flowers K, Hawkes MF, Lane SM, Rapkin J, House CM, Hunt J (2014) Environmental heterogeneity multivariate sexual selection and genetic constraints on cuticular hydrocarbons in Drosophila simulans. J Evol Biol 27:700–713
Jackson LL (1983) Cuticular hydrocarbons of the milkweed bug, Oncopeltus fasciatus by age and sex. Insect Biochem 13:19–25
Jackson LL, Bartelt RJ (1986) Cuticular hydrocarbons of Drosophila virilis: comparison by age and sex. Insect Biochem 16:433–439
Juárez MP, Brenner RR (1985) The epicuticular lipids of Triatoma infestans—II. Hydrocarbon dynamics. Comp Biochem Physiol 82B:93–803
Jurenka RA, Holland D, Krafsur ES (1998) Hydrocarbon profiles of diapausing and reproductive adult face flies (Musca autumnalis). Arch Insect Biochem Physiol 37:206–214
Kather R, Martin SJ (2015) Evolution of cuticular hydrocarbons in the hymenoptera: a meta-analysis. J Chem Ecol 41:871–883
Kent C, Azanchi R, Smith B, Chu A, Levine J (2007) A model-based analysis of chemical and temporal patterns of cuticular hydrocarbons in male Drosophila melanogaster. PLoS One 2:e962
Kent C, Azanchi R, Smith B, Formosa A, Levine JD (2008) Social context influences chemical communication in D. melanogaster males. Curr Biol 18:1384–1389
Khidr SK, Linforth RST, Hardy ICW (2013) Genetic and environmental influences on the cuticular hydrocarbon profiles of Goniozus wasps. Entomol Exp Appl 147:175–185
Krupp JJ, Kent C, Billeter JC, Azanchi R, So AKC, Schonfeld JA, Smith BP, Lucas C, Levine JD (2008) Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr Biol 18:1373–1383
Kühbandner S, Hacker N, Niedermayer S, Steidle JLM, Ruther J (2012) Composition of cuticular lipids in the pteromalid wasp Lariophagus distinguendus is host dependent. Bull Entomol Res 102:610–617
Kuo T-H, Yew JY, Fedina TY, Dreisewerd K, Dierick HA, Pletcher SD (2012) Aging modulates cuticular hydrocarbons and sexual attractiveness in Drosophila melanogaster. J Exp Biol 215:814–821
Kwan L, Rundle HD (2010) Adaptation to desiccation fails to generate pre- and postmating isolation in replicate Drosophila melanogaster laboratory populations. Evolution 64:710–723
Lebreton S, Mansourian S, Bigarreau J, Dekker T (2016) The adipokinetic hormone receptor modulates sexual behavior, pheromone perception and pheromone production in a sex-specific and starvation-dependent manner in Drosophila melanogaster. Front Ecol Evol 3:151
Lecuona R, Riba G, Cassier P, Clement JL (1991) Alterations of insect epicuticular hydrocarbons during infection with Beauveria bassiana or B. brongniartii. J Invertebr Pathol 58:10–18
Leftwich PT, Clarke NVE, Hutchings MI, Chapman T (2017) Gut microbiomes and reproductive isolation in Drosophila. P Natl Acad Sci USA 114:12767–12772
Leonhardt SD, Menzel F, Nehring V, Schmitt T (2016) Ecology and evolution of communication in social insects. Cell 164:1277–1287
Mant J, Brändli C, Vereecken NJ, Schultz CM, Francke W, Schiestl FP (2005) Cuticular hydrocarbons as sex pheromone of the bee Colletes cunicularis and the key to its mimicry by the sexually deceptive orchid, Ophrys exaltata. J Chem Ecol 31:1765–1787
Martin S, Drijfhout F (2009) A review of ant cuticular hydrocarbons. J Chem Ecol 35:1151–1161
Mas F, Kölliker M (2011) An offspring signal of quality affects the timing of future parental reproduction. Biol Lett 7:352–354
Mas F, Haynes KF, Kölliker M (2009) A chemical signal of offspring quality affects maternal care in a social insect. Proc R Soc B 276:2847–2853
Mateo JM (2010) Self-referent phenotype matching and long-term maintenance of kin recognition. Anim Behav 80:929–935
Menzel F, Blaimer BB, Schmitt T (2017) How do cuticular hydrocarbons evolve? Physiological constraints and climatic and biotic selection pressures act on a complex functional trait. P Roy Soc B-Biol Sci 284:20161727
Moczek AP (2010) Phenotypic plasticity and diversity in insects. Philos T Roy Soc B 365:593–603
Moczek AP, Sultan S, Foster S, Ledon-Rettig C, Dworkin I, Nijhout HF, Abouheif E, Pfennig DW (2011) The role of developmental plasticity in evolutionary innovation. P Roy Soc B 278:2705–2713
Mody NV, Hedin PA, Neel WW, Miles DH (1975) Hydrocarbons from males, females and larvae of pecan weevil: Curculio caryae (horn). Lipids 10:117–119
Moore HE, Butcher JB, Adam CD, Day CD, Drijfhout FP (2016) Age estimation of Calliphora (Diptera: Calliphoridae) larvae using cuticular hydrocarbon analysis and artificial neural networks. Forensic Sci Int 268:81–91
Moore HE, Pechal JL, Benbow ME, Drijfhout FP (2017) The potential use of cuticular hydrocarbons and multivariate analysis to age empty puparial cases of Calliphora vicina and Lucilia sericata. Sci Rep 7:1933
Mpuru S, Blomquist GJ, Schal C, Roux M, Kuenzli M, Dusticier G, Clement JL, Bagneres AG (2001) Effect of age and sex on the production of internal and external hydrocarbons and pheromones in the housefly, Musca domestica. Insect Biochem Mol Biol 31:139–155
Napolitano R, Juárez MP (1997) Entomopathogenous fungi degrade epicuticular hydrocarbons of Triatoma infestans. Arch Biochem Biophys 344:208–214
Nelson DR, Lee RE (2004) Cuticular lipids and desiccation resistance in overwintering larvae of the goldenrod gall fly, Eurosta solidaginis (Diptera: Tephritidae). Comp Biochem Physiol B 138:313–320
Nelson DR, Adams TS, Fatland CL (2003) Hydrocarbons in the surface wax of eggs and adults of the Colorado potato beetle, Leptinotarsa decemlineata. Comp Biochem Physiol B 134:447–466
Nielsen ML, Holman L (2012) Terminal investment in multiple sexual signals: immune-challenged males produce more attractive pheromones. Funct Ecol 26:20–28
Noorman N, Den Otter CJ (2002) Effects of relative humidity, temperature, and population density on production of cuticular hydrocarbons in housefly Musca domestica L. J Chem Ecol 28:1819–1829
Oi CA, van Zweden JS, Oliveira RC, Van Oystaeyen A, Nascimento FS, Wenseleers T (2015) The origin and evolution of social insect queen pheromones: novel hypotheses and outstanding problems. BioEssays 37:808–821
Otte T, Hilker M, Geiselhardt S (2015) The effect of dietary fatty acids on the cuticular hydrocarbon phenotype of an herbivorous insect and consequences for mate recognition. J Chem Ecol 41:32–43
Otte T, Hilker M, Geiselhardt S (2016) Phenotypic plasticity of mate recognition systems prevents sexual interference between two sympatric leaf beetle species. Evolution 70:1819–1828
Paulmier I, Bagneres AG, Afonso CMM, Dusticier G, Riviere G, Clement JL (1999) Alkenes as a sexual pheromone in the alfalfa leaf-cutter bee Megachile rotundata. J Chem Ecol 25:471–490
Pennanec'h M, Bricard L, Kunesch G, Jallon JM (1997) Incorporation of fatty acids into cuticular hydrocarbons of male and female Drosophila melanogaster. J Insect Physiol 43:1111–1116
Peschke K (1985) Immature males of Aleochara curtula avoid intrasexual aggression by producing the female sex pheromone. Naturwissenschaften 72:274–275
Peschke K (1987a) Male aggression, female mimicry and female choice in the rove beetle, Aleochara curtula (Coleoptera, Staphylinidae). Ethology 75:265–284
Peschke K (1987b) Cuticular hydrocarbons regulate mate recognition, male aggression, and female choice of the rove beetle, Aleochara curtula. J Chem Ecol 13:1993–2008
Petfield D, Chenoweth SF, Rundle HD, Blows MW (2005) Genetic variance in female condition predicts indirect genetic variance in male sexual display traits. P Natl Acad Sci USA 102:6045–6050
Pfennig DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP (2010) Phenotypic plasticity's impacts on diversification and speciation. Trends Ecol Evol 25:459–467
Pigliucci M, Murren CJ, Schlichting CD (2006) Phenotypic plasticity and evolution by genetic assimilation. J Exp Biol 209:2362–2367
Piskorski R, Trematerra P, Dorn S (2010) Cuticular hydrocarbon profiles of codling moth larvae, Cydia pomonella (Lepidoptera: Tortricidae), reflect those of their host plant species. Biol J Linn Soc 101:376–384
Polerstock AR, Eigenbrode SD, Klowden MJ (2002) Mating alters the cuticular hydrocarbons of female Anopheles gambiae Sensu stricto and Aedes aegypti (Diptera: Culicidae). J Med Entomol 39:545–552
Polidori C, Giordani I, Wurdack M, Tormos J, Asís JD, Schmitt T (2017) Post-mating shift towards longer-chain cuticular hydrocarbons drastically reduces female attractiveness to males in a digger wasp. J Insect Physiol 100:119–127
Pomonis JG (1989) Cuticular hydrocarbons of the screwworm, Cochliomyia hominivorax (Diptera: Calliphoridae). Isolation, identification, and quantification as a function of age, sex, and irradiation. J Chem Ecol 15:2301–2317
Portugal AHA, Trigo JR (2005) Similarity of cuticular lipids between a caterpillar and its host plant: a way to make prey undetectable for predatory ants? J Chem Ecol 31:2551–2261
Rajpurohit S, Hanus R, Vrkoslav V, Behrman EL, Bergland AO, Petrov D, Cvačka J, Schmidt PS (2017) Adaptive dynamics of cuticular hydrocarbons in Drosophila. J Evol Biol 30:66–80
Reylea RA (2002) Costs of phenotypic plasticity. Am Nat 159:272–282
Rouault JD, Marican C, Wicker-Thomas C, Jallon JM (2004) Relations between cuticular hydrocarbon (HC) polymorphism, resistance against desiccation and breeding temperature; a model for HC evolution in D. melanogaster and D. simulans. Genetica 120:195–212
Roux O, Gers C, Legal L (2008) Ontogenetic study of three Calliphoridae of forensic importance through cuticular hydrocarbon analysis. Med Vet Entomol 22:309–317
Rundle HD, Chenoweth SF, Doughty P, Blows MW (2005) Divergent selection and the evolution of signal traits and mating preferences. PLoS Biol 3:1988–1995
Ruther J, Döring M, Steiner S (2011) Cuticular hydrocarbons as contact sex pheromone in the parasitoid Dibrachys cavus. Entomol Exp Appl 140:59–68
Salamin N, Wuest RO, Lavergne S, Thuiller W, Pearman PB (2010) Assessing rapid evolution in a changing environment. Trends Ecol Evol 25:692–698
Sappington TW, Taylor OR (1990) Developmental and environmental sources of pheromone variation in Colias eurytheme butterflies. J Chem Ecol 16:2771–2786
Savarit F, Ferveur J-F (2002) Temperature affects the ontogeny of sexually dimorphic cuticular hydrocarbons in Drosophila melanogaster. J Exp Biol 205:3241–3249
Schal C, Gu X, Burns EL, Blomquist GJ (1994) Patterns of biosynthesis and accumulation of hydrocarbons and contact sex pheromone in the female German cockroach, Blattella germanica. Arch Insect Biochem Physiol 25:375–391
Schal C, Sevala VL, Young HP, Bachmann JAS (1998) Sites of synthesis and transport pathways of insect hydrocarbons: cuticle and ovary as target tissues. Am Zool 38:382–393
Schlichting CD, Pigliucci M (1998) Phenotypic evolution: a reaction norm perspective. Sinauer Associates, Sunderland
Schönrogge K, Wardlaw JC, Peters AJ, Everett S, Thomas A, Elmes GW (2004) Changes in chemical signature and host specificity from larval retrieval to full social integration in the myrmecophilous butterfly Maculinea rebeli. J Chem Ecol 30:91–107
Scott MP, Madjid K, Orians CM (2008) Breeding alters cuticular hydrocarbons and mediates partner recognition by burying beetles. Anim Behav 76:507–513
Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E (2010) Commensal bacteria play a role in mating preference of Drosophila melanogaster. P Natl Acad Sci USA 107:20051–20056
Simmons LW, Alcock J, Reeder A (2003) The role of cuticular hydrocarbons in male attraction and repulsion by female Dawson's burrowing bee, Amegilla dawsoni. Anim Behav 66:677–685
Smadja C, Butlin RK (2009) On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity 102:77–97
Smith AA, Liebig J (2017) The evolution of cuticular fertility signals in eusocial insects. Curr Opin Insect Sci 22:79–84
Snell-Rood EC (2012) Selective processes in development: implications for the costs and benefits of phenotypic plasticity. Integr Comp Biol 52:31–42
Snell-Rood EC, Van Dyken JD, Cruickshank T, Wade MJ, Moczek AP (2010) Toward a population genetic framework of developmental evolution: the costs, limits, and consequences of phenotypic plasticity. BioEssays 32:71–81
Steiger S, Peschke K, Francke W, Muller J (2007) The smell of parents: breeding status influences cuticular hydrocarbon pattern in the burying beetle Nicrophorus vespilloides. P Roy Soc B-Biol Sci 274:2211–2220
Steiger S, Peschke K, Muller JK (2008) Correlated changes in breeding status and polyunsaturated cuticular hydrocarbons: the chemical basis of nestmate recognition in the burying beetle Nicrophorus vespilloides? Behav Ecol Sociobiol 62:1053–1060
Steiner S, Steidle JLM, Ruther J (2005) Female sex pheromone in immature insect males: a case of pre-emergence chemical mimicry? Behav Ecol Sociobiol 58:111–120
Steiner S, Mumm R, Ruther J (2007) Courtship pheromones in parasitic wasps: comparison of bioactive and inactive hydrocarbon profiles by multivariate statistical methods. J Chem Ecol 33:825–838
Stennett MD, Etges WJ (1997) Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. III. Epicuticular hydrocarbon variation is determined by use of different host plants in Drosophila mojavensis and Drosophila arizonae. J Chem Ecol 23:2803–2824
Stinziano JR, Sové RJ, Rundle HD, Sinclair BJ (2015) Rapid desiccation hardening changes the cuticular hydrocarbon profile of Drosophila melanogaster. Comp Biochem Physiol A 180:38–42
Thomas ML, Simmons LW (2009) Male dominance influences pheromone expression, ejaculate quality, and fertilization success in the Australian field cricket, Teleogryllus oceanicus. Behav Ecol 20:1118–1124
Thomas ML, Simmons LW (2011) Short-term phenotypic plasticity in long-chain cuticular hydrocarbons. P Roy Soc B-Biol Sci 278:3123–3128
Thomas ML, Gray B, Simmons LW (2011) Male crickets alter the relative expression of cuticular hydrocarbons when exposed to different acoustic environments. Anim Behav 82:49–53
Thomas JA, Elmes GW, Sielezniew M, Stankiewicz-Fiedurek A, Simcox DJ, Settele J, Schönrogge K (2013) Mimetic host shifts in an endangered social parasite of ants. Proc R Soc B 280:20122336
Toolson EC (1982) Effects of rearing temperature on cuticle permeability and epicuticular lipid composition in Drosophila pseudoobscura. J Exp Zool 222:249–253
Toolson EC, Kuper-Simbron R (1989) Laboratory evolution of epicuticular hydrocarbon composition and cuticular permeability in Drosophila pseudoobscura: effects on sexual dimorphism and thermal-acclimation ability. Evolution 43:468–473
Toolson EC, Markow TA, Jackson LL, Howard RW (1990) Epicuticular hydrocarbon composition of wild and laboratory-reared Drosophila mojavensis Patterson and crow (Diptera: Drosophilidae). Ann Entomol Soc Am 83:1165–1176
Trabalon M, Campan M, Clémenl J-L, Thon B, Lange C, Lefevre J (1988) Changes in cuticular hydrocarbon composition in relation to age and sexual behavior in the female Calliphora vomitoria (Diptera). Behav Process 17:107–115
Tregenza T, Buckley S, Pritchard V, Butlin R (2000) Inter- and intrapopulation effects of sex and age on epicuticular composition of meadow grasshopper, Chorthippus parallelus. J Chem Ecol 26:257–278
Via S, Gomulkiewicz R, Dejong G, Scheiner SM, Schlichting CD, Vantienderen PH (1995) Adaptive phenotypic plasticity - consensus and controversy. Trends Ecol Evol 10:212–217
Wang Y, Yu Z, Zhang J, Moussian B (2016) Regionalization of surface lipids in insects. P Roy Soc B-Biol Sci 283:20152994
Ward HKE (2017) The genetic and environmental basis for CHC biosynthesis in Drosophila. Electronic Thesis and Dissertation Repository 4900 http://ir.lib.uwo.ca/etd/4900
Weddle CB, Mitchell C, Bay SK, Sakaluk SK, Hunt J (2012) Sex-specific genotype-by-environment interactions for cuticular hydrocarbon expression in decorated crickets Gryllodes sigillatus: implications for the evolution of signal reliability. J Evol Biol 25:2112−2125
Weddle CB, Steiger S, Hamaker CG, Ower GD, Mitchell C, Sakaluk SK, Hunt J (2013) Cuticular hydrocarbons as a basis for chemosensory self-referencing in crickets: a potentially universal mechanism facilitating polyandry in insects. Ecol Lett 16:346–353
Weiss I, Rössler T, Hofferberth J, Brummer M, Ruther J, Stökl J (2013) A nonspecific defensive compound evolves into a competition-avoidance cue and a female sex-pheromone. Nat Commun 4:2767
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, Oxford
Wicker C, Jallon J-M (1995a) Hormonal control of sex pheromone biosynthesis in Drosophila melanogaster. J Insect Physiol 41:65–70
Wicker C, Jallon J-M (1995b) Influence of ovary and ecdysteroids on pheromone biosynthesis in Drosophila melanogaster (Diptera: Drosophilidae). Eur J Entomol 92:197–202
Wong JW, Lucas C, Kölliker M (2014a) Cues of maternal condition influence offspring selfishness. PLoS One 9:e87214
Wong JW, Meunier J, Lucas C, Kolliker M (2014b) Paternal signature in kin recognition cues of a social insect: concealed in juveniles, revealed in adults. P Roy Soc B 281:20141236
Wund MA (2012) Assessing the impacts of phenotypic plasticity on evolution. Integr Comp Biol 52:5–15
Xue H-J, Wei J-N, Magalhães S, Zhang B, Song K-Q, Liu J, Li W-Z, Yang X-K (2016) Contact pheromones of 2 sympatric beetles are modified by the host plant and affect mate choice. Behav Ecol 27:895–902
Yocum GD, Buckner JS, Fatland CL (2011) A comparison of internal and external lipids of nondiapausing and diapause initiation phase adult Colorado potato beetles, Leptinotarsa decemlineata. Comp Biochem Physiol B 159:163–170
Yoon C, Yang J-O, Youn Y-N, Kim G-H (2012) Changes in cuticular hydrocarbons in different developmental stages of the bean bug, Riptortus pedestris (Hemiptera: Alydidae). J Asia Pac Entomol 15:579–587
Zhu GH, Ye GY, Hu C, Xu XH, Li K (2006) Development changes of cuticular hydrocarbons in Chrysomya rufifacies larvae: potential for determining larval age. Med Vet Entomol 20:438–444
Zurek L, Weston DW, Krasnoff SB, Schal C (2002) Effect of the entomopathogenic fungus, Entomophthora muscae (Zygomycetes: Entomophthoraceae), on sex pheromone and cuticular hydrocarbons of the house fly, Musca domestica. J Invertebr Pathol 80:171–176
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Otte, T., Hilker, M. & Geiselhardt, S. Phenotypic Plasticity of Cuticular Hydrocarbon Profiles in Insects. J Chem Ecol 44, 235–247 (2018). https://doi.org/10.1007/s10886-018-0934-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-018-0934-4