Abstract
Low concentrations of dietary triacylglycerols including tristearin, triolein, and tripalmitolein, were assessed to determine their effects during egg to pupal stages on adult epicuticular hydrocarbon (EHC) variation in cactophilic Drosophila mojavensis. Triacylglycerols were added singly and in combination at concentrations of 1%, 3%, and 9% to a lipid-free culture medium. Control diets included Carolina Drosophila and lipid-free media. Egg to adult viability was reduced at triacylglycerol concentrations greater than 1%, except for tristearin. Both triolein and tripalmitolein increased EHC amounts to levels similar to those in combination and control diets. Tristearin caused significantly lower quantities of EHCs in adult flies than triolein or tripalmitolein, consistent with previous studies on reduced tristearin assimilation into adult EHCs. We rejected the hypothesis that unsaturated and saturated triacylglycerols were assimilated into unsaturated and saturated adult EHCs, respectively. Since these triacylglycerols comprise a fraction of known lipids in the columnar cacti used for breeding in nature, and EHCs serve as contact pheromones in D. mojavensis, these and other naturally occurring triacylglycerols may provide a direct causal link between host plant use and patterns of chemically mediated mate choice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acoustic and chemical signals can play important roles during mate choice in many insects, including Drosophila species (Gerhardt and Huber, 2002). Pheromonal signaling systems can influence both reproductive isolation and speciation, in addition to influencing proximate decisions during courtship (Greenfield, 2002). It is of considerable interest to identify particular mate–mate signals that influence mating success in local populations and those that may determine isolation between populations or species. In some cases, mate recognition systems can influence both sexual selection and sexual isolation (Boake et al., 1997; Carson, 2000). Understanding both the genetic and environmental influences on expression of these signaling systems should help to reveal the nature of genetic differences in mate recognition systems and their evolution (Etges, 2002).
In many Drosophila species, epicuticular hydrocarbons (EHC) are thought to serve as contact pheromones as well as to enhance desiccation resistance (Blomquist et al., 1987). Although the genetic basis of some hydrocarbon components is partially understood in some drosophilids (reviewed in Gleason et al., 2005), genetic and environmental influences on production and deposition (Wigglesworth, 1988) are poorly known for most species. Once revealed, the structure and function of individual hydrocarbon genes alone will be insufficient without knowledge of how EHCs are expressed in natural or experimental populations subject to potential environmental modification. We seek to gain a further understanding of the role of lifetime environmental influences on genetic variation in EHC production in insects and how this EHC variation influences behavior, mate choice, sexual selection, and sexual isolation in nature (e.g., Toolson and Kuper-Simbron, 1989; Howard et al., 1995; Howard, 1998; Liang and Silverman, 2000; Woodrow et al., 2000; Savarit and Ferveur, 2002; Buczkowski et al., 2005).
Here, we investigate the role of dietary triacylglycerols in larval Drosophila mojavensis on subsequent adult EHC profiles. Differences in natural larval feeding substrates vs. synthetic laboratory media influence EHC abundance in this species (Stennett and Etges, 1997). These differences also influence adult mating preferences (Etges, 1992, 1998; Brazner and Etges, 1993). In D. mojavensis, the function of cuticular lipids seems unrelated to desiccation resistance by reducing water loss (Gibbs et al., 1998), underscoring their role, and probable maintenance, as contact pheromones (Etges and Ahrens, 2001). We hypothesize that identifying substrate-specific precursors that influence adult EHC profiles will help to resolve host plant influences on mating behavior, as well as identify ecological sources of variation on patterns of reproductive isolation in nature.
Fatty acid metabolism and EHC biosynthesis have been well studied in D. melanogaster and should help in understanding lipid metabolism in D. mojavensis. Adult EHC production involves modification of fatty acid precursors, but little is known about the influences of fatty acids in larval diets on adult EHCs. Production of adult D. melanogaster sex pheromone (Z,Z)-7,11-heptacosadiene starts from either acetate or from medium-sized fatty acids (Chan Yong and Jallon, 1986; Jallon et al., 1986). From preformed fatty acids produced by fatty acid synthetase (de Renobales and Blomquist, 1984), palmitate is a better substrate than stearate and is desaturated into palmitoleate (ω-7) (Ferveur et al., 1989) by the desat1 gene product (Wicker-Thomas et al., 1997; Dallerac et al., 2000; Labeur et al., 2002). From palmitoleate there is elongation to cis-vaccenate and subsequently several elongations, a second desaturation (the substrate of which has not yet been characterized) by another desaturase (Wicker-Thomas and Jallon, 2001; Jallon and Wicker-Thomas, 2003), and a subsequent decarboxylation (Jallon, 1984; Pennanec’h et al., 1997). Fatty acid analysis of sexually mature D. melanogaster indicates high percentages of palmitoleate, but lesser quantities of oleate and cis-vaccenate, with oleate and linoleate decreasing as (Z,Z)-7,11-heptacosadiene synthesis occurs (Pennanec’h et al., 1997). Very long chain (C20, C22, C24, and C26) fatty acids with apparent ω-7 unsaturation and female dienoic acids with 22 and 24 carbons have also been detected (Pennanec’h et al., 1997). Together, these experiments and those of other groups (Keith, 1967a,b; Pennanec’h et al., 1991) suggest that fatty acids, either from biosynthesis or the diet, impact the biosynthesis of pheromones and other dipteran EHCs.
We hypothesize that different fatty acids in the cactus diets of D. mojavensis can change pheromone and other adult EHC profiles. A number of different hosts are used by D. mojavensis, including agria cactus, Stenocereus gummosus, in Baja California and organ pipe cactus, S. thurberi, in mainland Mexico and Arizona (Heed and Mangan, 1986). Fatty acid profiles of both agria and organ pipe cactus are mainly characterized by caproic (C10) and lauric (C12) acids in concentrations of 34–45%, with much lower concentrations of caprylic acid (C8) and others in sizes up to C18 (Fogleman and Kircher, 1986). There are no overall significant differences between cactus species with respect to fatty acid composition (Fogleman and Kircher, 1986). However, cactus fatty acid analysis shows greater quantities of unsaturated fatty acids (12:1, 12:2, and 18:1) in agria vs. organ pipe cactus, but the location of double bonds has not been determined. It may be that minor quantities of unsaturated fatty acids in the diet of D. mojavensis influence biosynthesis of unsaturated EHCs, so we employed both saturated and unsaturated triacylglycerols to determine whether they influence EHC profiles of adult D. mojavensis.
EHCs of adult D. mojavensis are composed of C28 to C40 alkanes, 2-methylalkanes, alkenes, methyl-branched alkenes, and multimethylene interrupted alkadienes, with the largest fraction of adult hydrocarbons composed of C35 alkadienes (Toolson et al., 1990; Stennett and Etges, 1997; Etges and Jackson, 2001). We hypothesize that the EHCs of D. mojavensis are derived from three fatty acid pools: ω-9, ω-7, and saturated fatty acids. The ω-9 fatty acids are precursors of the 9-alkenes and the 8, X-alkadienes (e.g., C8 = C16 = C9, C8 = C18 = C9, C8 = C20 = C9, etc.) since they are really 9, X-alkadienes counting from the other end. The ω-7 fatty acids are precursors to the 7-alkenes and the 7,X-alkadienes (e.g., C7 = C16 = C10, C7 = C18 = C10, C7 = C20 = C10, etc.). The saturated fatty acids are precursors to the alkanes, and the 10-, 12-, and 8-alkenes also result from saturated fatty acids when desaturation occurs late in the biosynthetic pathway.
Given the previously described fatty acid profiles in agria and organ pipe cactus, and the substrates known to be involved in EHC biosynthesis, we used tripalmitolein (C16:1, ω-7), tristearin (C18), and triolein (C18:1, ω-9) in differing concentrations in larval diets to assess their influences on egg to adult viability and on adult EHC composition in two populations of D. mojavensis.
Methods and Materials
Fly Stocks
Populations of D. mojavensis were collected in 1996 from south of Santa Rosalia, Baja California Sur (stock no. A975, 15 adults), and El Fuerte, Sinaloa (stock no. A991, 185 adults), over baits or by collecting adults emerging from fermenting cactus tissues, “rots,” returned to the laboratory. All flies were maintained on banana–malt–yeast–agar food at room temperature in mass cultures (Brazner and Etges, 1993). Several hundred adults from each population were placed in separate oviposition chambers and kept in an incubator programmed for a 14:10 L/D photoperiod and a temperature cycle of 27:17°C. Eggs were collected daily on oviposition media from 8:00 am to 6:00 pm in removable Petri dishes attached to each chamber. Eggs were washed with deionized water, 70% ethanol, and surface sterilized for 10 min in 70% ethanol. Eggs were then counted in sterile, deionized water in groups of 100 onto 1-cm2 pieces of sterile filter paper and placed into vials containing Drosophila medium (see below). Filter paper was removed after 24–48 hr, and numbers of unhatched eggs were counted in order to calculate egg to adult viability.
Food vials were prepared with standard Instant Drosophila Medium® formula 4–24 (Carolina Biological Supply Co., Burlington, NC, USA); or with “defatted” medium (both controls), as well as by adding different concentrations of triacylglycerols (TAGS) to the dry, homogeneous defatted diet. For the defatted medium, lipids were removed from the standard medium by Soxhlet extraction with hexane. We prepared 9% stock solutions of each triacylglycerol. Triolein (ω-9), tripalmitolein (ω-7), and tristearin in pentane were added to 2 g of dry defatted diet in final concentrations of 1%, 3%, and 9%. Each 2-g aliquot of food was completely dried under a fume hood, added to a sterile 8 dram shell vial, and rehydrated with an equal volume of sterile, deionized water. Combinations of equal amounts of all three TAGs were also prepared so that the final concentrations of the three combined would be 1%, 3,% and 9%. These latter treatments were prepared to test the hypothesis that all three TAGS should produce EHC profiles more like the standard or defatted diets, but at comparable concentrations to the single TAG treatments. Two replicate cultures of each TAG concentration were created for each population of D. mojavensis, and six replicates were included for both the standard and defatted diets. Live baker’s yeast was added to each vial ad libidum to insure larval survivorship.
Epicuticular Hydrocarbon Analyses
All eclosed adults were aspirated from each culture vial daily, sexed under light CO2, and placed into separate vials containing banana–yeast–malt–agar food for at least 14 d and then frozen in hexane-rinsed glass vials at −20°C. EHCs were extracted from groups of males and females in Biosil minicolumns. Each column consisted of a Pasteur pipette containing packed glass wool and Biosil (silica gel, Sigma™ S-4133, Sigma-Aldrich, St. Louis, MO, USA) flushed several times with HPLC-grade hexane. Flies were added to the top of the column, washed in 8 ml of hexane, and the hydrocarbons were collected in hexane-rinsed vials. After the hexane was evaporated with nitrogen, each sample was sealed and stored at −20°C. When only one or two flies were available from some replicates, we used hydrocarbon extraction procedures described by Savarit et al. (1999). Flies were placed in conical glass microvials and immersed in 50 μl hexane per fly. The vials were agitated, and after 20 min, flies were removed, the hexane was evaporated off, and each vial was frozen.
Each hydrocarbon sample was redissolved in hexane (2 μl/fly) containing 382 ng of docosane (C22) per μl as an internal standard with the exception of samples containing only one or two flies, which were redissolved in 5 μl hexane with the same concentration of internal standard. One μl of each sample was analyzed by capillary gas–liquid chromatography using a Shimadzu GC14 (Shimadzu Scientific Instruments, Columbia, MD, USA) fitted with a 30 m DB-1 fused silica column (Agilent Technologies, Palo Alto, CA, USA). Injector and detector temperatures were set at 345°C with the injector port in split mode (split ratio = 100:1) and the column was heated from 200°C to 345°C at 10°C/min holding at 345°C for 5 min (Stennett and Etges, 1997).
Statistical Analyses
Egg to adult viability was calculated as the number of eclosed adults divided by the number of hatched eggs. Variation in viability due to substrate and TAG concentration was assessed with analysis of variance (ANOVA; SAS Institute, 2004). Viability data were arcsin transformed prior to analysis.
EHC amounts were estimated by analysis of peak integrations using EZCHROM software (ver. 2.1) provided by Shimadzu. Each sample amount was normalized by the amount of internal standard. Replicate groups of flies were analyzed together. All data were expressed as ng/fly of EHCs and were analyzed by ANOVA and multivariate analysis of variance (MANOVA) using PROC GLM in SAS (SAS Institute, 2004) with population, TAG, TAG concentration, and sex as main effects and all interactions between main effects. Population and all interactions with population were considered random effects.
Post-hoc multiple comparisons were evaluated, and canonical correlation analysis in PROC GLM and CANDISC was employed to reveal EHC responses to different TAGs. Contrasts among diet treatments were also carried out using EHCs grouped by chemical structure according to Etges and Jackson (2001).
Results
Effects of Triacyglycerols on Egg to Adult Viability
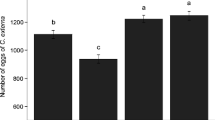
Type of TAG, concentration of TAG, and their interactions had significant effects on egg to adult viability in these two populations of D. mojavensis (Fig. 1, Table 1). Overall, flies reared on standard medium, defatted medium, or defatted medium with tristearin had equivalent viabilities that were significantly higher than those from flies reared on defatted medium with triolein, tripalmitolein, or combinations of all three TAGs (Tukey’s multiple range test, P < 0.05). Except for tristearin, concentrations of TAGs exceeding 1% drastically reduced viability: egg to adult viability of controls (mean ± 1 SD on standard diet = 0.47 ± 0.21; on defatted diet = 0.57 ± 0.30) and of defatted diet with all three TAGs at 1% (0.24 ± 0.10) were higher than viabilities for diets with TAGs at 3% and 9% (both 0.18; Tukey’s multiple range test, P < 0.05). Egg to adult viability of laboratory populations of D. mojavensis reared on fermenting cactus or banana food tend to be much higher (>0.80, see Etges and Ahrens, 2001) than on standard diet, suggesting that the latter medium reduced preadult fitness in these experiments. Similar viabilities of flies reared on standard and defatted diets were surprising given the removal of lipids in the latter (Fig. 1), but may have been mediated by the addition of live baker’s yeast.
At triolein and tripalmitolein concentrations of 3% and 9%, very few flies survived to eclosion (Fig. 1), with many never making it to the pupal stage. At these higher concentrations, flies that did eclose seemed to be unable to initiate cuticle hardening and died before wing expansion.
Effects of Triacylglycerols on Adult Epicuticular Hydrocarbons
Differences in EHC amounts due to TAG type were apparent for all but six of the 25 EHC components assayed (Table 2 for quantities and Appendix for ANOVA of the 20 most abundant EHCs). The ANOVA results were based on all TAGs employed, plus combinations of them at each concentration for which flies eclosed. The two control media were not included in these ANOVAs so we could focus on TAG differences. In most cases, EHC amounts were lowest for flies reared on defatted media, but similar among flies reared on standard medium and defatted media supplemented with TAGs (Table 2). Missing data due to high or total egg to adult mortality, particularly in the 3% and 9% tripalmitolein-containing cultures, meant that TAG type and concentration were somewhat confounded in the ANOVAs. This may partially explain the lack of a concentration effect in these analyses, except in a few cases.
EHC differences due to TAG type were overlaid on the known population differences that in some cases resulted in Population × TAG interactions (Appendix). Consistent with most previous studies, 13 of the EHC components differed between the Baja California and Sinaloa (mainland) populations, especially 2-methyloctacosane (C28.65), 8,24-tricontadiene (C33.63), 9,25-pentatricontadiene (C34.59), and 9,27-heptatricontadiene (C36.5). The latter three alkadienes showed characteristic regional differentiation in amounts, where mainland populations all expressed much greater amounts of these EHCs than Baja California populations (Stennett and Etges, 1997; Etges and Ahrens, 2001). Two of these EHCs, C33.63 and C34.59, exhibited Population × TAG interactions suggesting that even though amounts differed between populations, Sinaloa EHCs responded to the three TAGs far differently than Baja California EHCs (Fig. 2a). In both cases, combinations of the three TAGs increased EHC amounts in the Sinaloa population far more than in the Baja population. For the large C34.59 diene component, 9% TAG concentrations vastly reduced the amounts of this EHC as compared to 1% and 3% concentrations (Tukey’s multiple range test, P < 0.05).
Canonical discriminant function plot (a) showing the effects of triacylglycerols for each population (EF: El Fuerte, Sinaloa, mainland; SR: Santa Rosalia, Baja California) with the control diet data excluded to better illustrate the effects of the three triacylglycerols on epicuticular hydrocarbon variation. Canonical discriminant function plot (b) showing the effects of the three triacylglycerols alone and in combination, plus the two control diets on variation in epicuticular hydrocarbon profiles for all Drosophila mojavensis in this study grouped by population, sex, and triacylglycerol concentration
Multivariate ANOVA of EHC differences revealed significant variation due to all treatments except for the Population × Sex interaction (Table 3). Here, Roy’s Greatest Root is the largest characteristic root or eigenvalue that explains the greatest proportion of variation (percent) in each factor of the MANOVA. The largest eigenvalues for population and sex accounted for 100% of the total variation in EHC abundance, with more complex sources of variation explaining TAG differences given that the first eigenvalue accounted for approximately half of the variation (Table 3). Males had more EHCs than females (likelihood ratio = 0.3331, F = 4.76, df = 24/57, P < 0.001). Because the data were unbalanced by the absence of flies in the 3% and 9% triolein and tripalmitolein treatments (Fig. 1), concentration effects were confounded with TAG type, and may have spuriously resulted in a significant TAG X concentration interaction term.
The overall pattern of EHC variation due to control and TAG treatments was assessed by calculating Mahalanobis distances between groups in PROC CANDISC (Table 4). Significance of these differences indicated that these dietary treatments almost always caused significantly different EHC profiles, except for a number of comparisons involving control diets (overall variation indicated by Roy’s Greatest Root = 3.33, F = 7.91, df = 24/57, P < 0.001). EHC profiles were different between triolein and tripalmitolein treatments, and among these two diets and all others. Both control diets produced similar EHC profiles, but only the defatted control diet resulted in similar EHC profiles as the combination treatment (P = 0.159, Table 4).
Loadings for each diet on the first three canonical variables, accounting for 89.8% of TAG variation, showed clearer similarities between tristearin and control diet treatments (Fig. 2b). The overall effects of tripalmitolein and triolein strongly influenced the effects of the combination diets, i.e., EHC differences due to the three TAGs in combination were more “similar” to the tripalmitolein and triolein centroids than to the tristearin centroid (Fig. 2b). Removing the control diets from this analysis made this tripalmitolein-combination comparison somewhat more apparent (Fig. 2a). Here, population differences were included, revealing some overlap between EHC differences from tristearin-treated Baja California files and those due to the combination diet. Qualitatively, these results suggest that there may have been a greater influence of this unsaturated TAG in the combination diets for this Baja California population than for the Sinaloa population. However, replicate numbers were limited for the Sinaloa population.
Pairwise post-hoc comparisons revealed the effects of dietary treatments on EHC profiles (Table 5). Missing data due to the absence of flies in the 3% and 9% triolein and tripalmitolein treatments did not permit the use of the CONTRAST option in the MANOVA for all EHCs together except for one case, so pairwise contrasts between treatments were performed, despite a loss of power (Scheiner, 1993). Only one post-hoc comparison was possible, tristearin vs. TAG combination, revealing the nature of this unsaturated TAG on EHC profiles (F = 3.62, df = 24/7, P = 0.035). Least square means were greater for a majority of the EHCs assayed due to the TAG combination diets than tristearin, including C28.65, C30.78, C32.63, C32.70, C32.79, all C34 components, C34.59, C34.66, and C36.5. Thus, although not toxic even at higher concentrations (Fig. 1), tristearin is insufficient alone as a substrate for EHC biosynthesis vs. the combination of the unsaturated and saturated TAGs used here.
The remaining pairwise TAG comparisons for EHC profiles caused a reduction in the degrees of freedom for the error term required for significance testing, and this resulted in undefined eigenvalues. To increase degrees of freedom, EHC components were combined into hydrocarbon classes, i.e., alkanes, methylalkanes, alkenes, methylalkenes, and alkadienes (Etges and Jackson, 2001). Grouping the EHCs together by chemical structure reduced the dimensionality and perhaps some of the variation in the data (Table 5). Several of the comparisons were not significantly different despite the significance of the Mahalanobis’ distances (Table 4). Pairwise post-hoc comparisons again showed that most EHC differences were caused by tristearin vs. the unsaturated TAGs (Fig. 2b), suggesting that TAG substrate-induced shifts in adult EHCs may not have necessarily influenced specific classes of hydrocarbons, but caused overall shifts in most types of hydrocarbons (Table 5). Triolein caused greater amounts of all EHCs than tristearin, particularly in 2-methyloctacosane (C28.65), 2-methyltricontane (C30.65), 7- and 9-hentricontene (C30.78), 8,24-tricontadiene (C32.63), 9,25-pentatricontadiene (C34.59), 8,26- and 7,27-pentatricontadiene (C34.66), and all three major C37 components (Table 2 and Tukey’s multiple range tests, all P < 0.05 based on ANOVAs in Appendix). Similarly, tripalmitolein caused greater amounts of all EHCs than tristearin in many of the same EHCs including 2-methyloctacosane (C28.65), 2-methyltricontane (C30.65), 7, 25-tricontadiene (C32.70), 10-, 12-, and 14-tritricontene (C32.79), 10-, 12-, and 14 tetratricontene (C34ene), 9,25-pentatricontadiene (C34.59), 8,26- and 7,27-pentatricontadiene (C34.66), 9,27-heptatricontadiene (C36.5), and 8,28-heptatricontadiene (C36.6) (Table 2 and Appendix, Tukey’s multiple range tests, P < 0.05). Thus, we tentatively rejected the hypothesis that particular unsaturated or saturated TAGs in larval diets induced variation in specific saturated or unsaturated EHCs in the adults. Both unsaturated TAGs caused higher levels of all adult EHCs, suggesting they are more easily incorporated into EHC biosynthesis than tristearin despite the higher toxicity of the unsaturated TAGs during larval development (Fig. 1).
Discussion
Low larval diet concentrations of some triacylglycerols present in the fermenting cactus tissues used by D. mojavensis as breeding sites caused significant shifts in amounts of EHCs expressed in mature adults. The hypothesis that different fatty acid pools from larval diets would be preferentially used as substrates for different saturated and unsaturated adult EHCs was rejected. Unsaturated triacylglycerols caused increases in a wide range of adult EHCs in D. mojavensis (Tables 1, 2, and 5), and although not toxic at higher concentrations, tristearin diets were consistently associated with lower quantities of most adult EHCs. Thus, tristearin is not likely a suitable substrate for lipid biosynthesis in D. mojavensis, consistent with Pennanec’h et al. (1997), who found that stearic acid was not a preferred precursor for hydrocarbon production in adult D. melanogaster, yet palmitic acid was readily incorporated. The chemical cues that determine mate recognition and sexual isolation in D. mojavensis are therefore sensitive to some of the triacylglycerols present in natural rearing substrates.
The observed variation in TAG response shown by the Population × TAG interactions (Appendix) in EHC abundance suggests these genetically differentiated populations differ in ability to synthesize adult EHCs from fatty acid precursors. The large geographic differences in EHC profiles, particularly due to differences between Baja California and mainland Mexico and Arizona populations (Stennett and Etges, 1997; Etges and Ahrens, 2001) in amounts of 8,24-tricontadiene (C32.63), 9,25-pentatricontadiene (C34.59), and 9,27-heptatricontadiene (C36.5) are thus sensitive to TAG variation. Overall, variation in amounts of EHCs was more influenced by population differences than by the types of TAGs used in this study (Fig. 2a, Table 1), but the mainland population here (Sinaloa) was more sensitive to TAG amounts than the Baja California population. Such geographical variation is consistent with previous observations that mainland populations of D. mojavensis are more sensitive to available nutrients in rearing substrates than Baja populations (Etges and Heed, 1987). At higher larval densities in cactus cultures where competition for food is increased, mainland populations express reduced viability and longer development times than Baja California populations, suggesting lower genetic homeostasis in mainland populations for these components of fitness (Etges, 1989).
Even though all larval cultures were supplemented with live baker’s yeast and adults were aged to maturity on banana media, EHC profiles from triolein- and tripalmitolein-treated flies were significantly different among other treatment groups, with few differences due to the control diets (Table 4). Flies were aged at least 12–14 d on lab food in order to ensure that they were sexually mature (ca. 8–10 d for males at 25°C; Markow, 1982). This may have influenced adult EHC profiles despite the effects of these TAGs during larval stages, along with the effects of including live yeast to insure survivorship on the diet. Supplemental yeast provides larvae with nucleic acids, sterols, and essential vitamins and nutrients, including fatty acids. However, cultures from all treatment groups included the yeast, so if the additional yeast affected fatty acid metabolism in the flies, all treatment groups should have been influenced to the same degree. Because EHC amounts increase in abundance from eclosion to sexual maturity in D. mojavensis (Toolson et al., 1990), we wanted to be sure that our assays were performed with adults that had been reared in the same way as in previous experiments that had shown the consequences of preadult rearing environments on adult mating behavior.
EHC production has been intensively studied in adult insects (reviewed in Blomquist, 2003), but the influence of metabolism of lipids during larval stages on adult EHC profiles is not well understood. Biosynthesis of adult EHCs has been intensively studied, particularly in immature males and females in D. melanogaster, D. simulans, and D. erecta. EHC sexual dimorphism increases with age until sexual maturity is attained (Jallon and Wicker-Thomas, 2003). In D. mojavensis, rearing larvae on fermenting cactus lowers premating isolation compared to laboratory media (Etges, 1992; Brazner and Etges, 1993) despite a maturation period of up to 2 wk on banana food. We used this protocol to directly compare the effects of preadult rearing conditions without changing adult rearing procedures used in previous experiments. Thus, the rearing substrates experienced by preadult D. mojavensis cause a “carryover” effect that influences adult EHC composition and mating behavior past the age of sexual maturity.
The effects of tristearin in larval diets on adult D. mojavensis EHCs were consistent with earlier observations of its role as a lesser used substrate for adult EHC biosynthesis (Pennanec’h et al., 1997). Tristearin’s effects on larval viability suggest it is either not metabolized effectively in larvae, or once assimilated, it has negligible effects even at higher concentrations (Fig. 1). Since the effects of tristearin on EHC profiles were not significantly different from either of the control diets, but very different from both triolein and tripalmitolein (Table 4), it may not be assimilated into adult hydrocarbons. More data will be needed to confirm this. Tripalmitolein, tristearin, and triolein are present in much lower concentrations (0–2.3%) in agria and organ pipe cacti than the most abundant saturated fatty acids, capric (C10) and lauric (C12) acid, which together account for 30–45% of total fatty acids (Fogleman and Kircher, 1986). Capric and lauric acids have been shown to have negligible effects on larval viability in D. mojavensis at 0.5% concentrations, but lauric acid significantly depressed viability at 1%. Thus, saturated fatty acids per se are not poor substrates for lipid and hydrocarbon metabolism: some are assimilated during larval growth and development and have variable effects depending on concentration.
Direct chemical links between host substrates and the resulting pheromonal cues used as both mate recognition signals and part of species recognition systems have broad significance to studies on ecological speciation (Funk, 1998; Nosil et al., 2002; Sandoval and Nosil, 2005). In general, host plant adaptation typically involves host chemistry to some degree (Harborne, 1982; Bernays and Chapman, 1994; Thompson, 1994; Becerra, 1997; Funk et al., 2002), so host plant fatty acid or TAG determinants of EHC abundance in adult D. mojavensis provide direct evidence for how differential host use might determine reproductive isolation among insects. Although future studies will be necessary to show that TAG-induced shifts in adult EHC abundance directly influence patterns of mate choice, these diet-induced pheromonal shifts need to be considered in studies on reproductive isolation, particularly in Drosophila species. Enough is known about cactus stem chemistry that we can now design further experiments to examine the effects of these secondary compounds on the behavior and physiology of the insects that use these host plants.
Perhaps half of the species in the large D. repleta group (ca. 100 species) use cacti to carry out their life cycles. Along with a few other drosophilids that are cactus specialists, e.g., species in the nannoptera group (Heed, 1982), there are likely other species in which adult physiology and behavior has been shaped by the host plants used for feeding and breeding. In the Sonoran Desert, cactophilic Drosophila use different species of columnar cacti as breeding sites (Heed and Mangan, 1986). Host chemistry has been studied in detail in order to understand why certain host cacti are used preferentially by particular Drosophila species (Fogleman and Abril, 1990; Fogleman and Danielson, 2001). For D. mojavensis, crude lipid extracts from its major host plants, agria and organ pipe cactus, are not toxic because fatty acids and sterol diols are esterified to either neutral triterpenes, betulin and calenduladiol, or di-hydroxysterols (Fogleman et al., 1986). These neutral triterpenes are not toxic, but low concentrations of medium chain fatty acids and sterol diols can significantly depress larval viability. However, D. mojavensis is more tolerant of free fatty acids during larval growth and development than other desert drosophilids (Fogleman and Kircher, 1986). Agria and organ pipe cacti contain higher concentration of these fatty acids than other host cacti, so this may help to explain why D. mojavensis, but not other Drosophila species, tend to specialize on these cacti.
Throughout its evolutionary history, D. mojavensis has expanded its range by switching to different host cacti. Originating in Baja California, D. mojavensis colonized mainland Mexico, Arizona, and southern California by switching host plants. Use of agria cactus in Baja California is considered ancestral, with derived mainland populations using organ pipe cactus in Sonora and Sinaloa, Mexico, as well as southern Arizona (Etges et al., 1999). Sina cactus, S. alamosensis, is occasionally used in Sonora and northern Sinaloa, and in southern California, California barrel cactus, Ferocactus cylindraceous, is a major host. The colonization of mainland Mexico from Baja California by switching to organ pipe cactus has caused widespread genetic differences in life history traits, chromosome and genic frequency shifts, and physiological adaptation to these secondary hosts that has also resulted in the evolution of altered mating preferences in mainland populations (Etges, 1998; Etges and Ahrens, 2001, 2002). The evolutionary relationships between D. mojavensis and its host cacti suggest that this is an ideal system with which to pursue the molecular and chemical causes of ecological speciation, particularly if the mechanisms underlying these evolutionary shifts are related to host plant chemistry. These host plants have shaped the development and physiology of the insects that use them, causing not only adaptive responses in terms of host use and specialization, but also potential reproductive isolation among species that use different hosts.
References
Becerra, J. X. 1997. Insects on plants: Macroevolutionary chemical trends in host use. Science 276:253–256.
Bernays, E. A. and Chapman, R. F. 1994. Host-Plant Selection by Phytophagous Insects. Chapman and Hall, London.
Blomquist, G. J. 2003. Biosynthesis and ecdysteroid regulation of housefly sex pheromone production, pp. 231–252, in G. J. Blomquist and R. C. Vogt (eds.). Insect Pheromone Biochemistry and Molecular Biology. Elsevier, San Diego.
Blomquist, G. J., Dillworth, J. W., and Adams, T. S. 1987. Biosynthesis and endocrine regulation of sex pheromone production in Diptera, pp. 217–250, in G. D. Prestwitch and G. J. Blomquist (eds.). Pheromone Biochemistry. Academic Press, London.
Boake, C. R. B., Deangelis, M. P., and Andreadis, D. K. 1997. Is sexual selection and species recognition a continuum? Mating behavior of the stalk-eyed fly Drosophila heteroneura. Proc. Natl. Acad. Sci. USA 94:12442–12445.
Brazner, J. C. and Etges, W. J. 1993. Pre-mating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. II. Effects of larval substrates on time to copulation, mate choice, and mating propensity. Evol. Ecol. 7:605–624.
Buczkowski, G., Kumar, R., Suib, S. L., and Silverman, J. 2005. Diet-related modification of cuticular hydrocarbon profiles of the Argentine ant, Linepithema humile, diminishes intercolony aggression. J. Chem. Ecol. 31:829–843.
Carson, H. L. 2000. Sexual selection in populations: the facts require a change in the genetic definition of the species, pp. 495–512, in R. S. Singh and C. Krimbas (eds.). Evolutionary Genetics: From Molecules to Morphology. Cambridge University Press, New York.
Chan Yong, T. P. and Jallon, J. M. 1986. Synthese de novo d’hydrocarbures potentiellement aphrodisiaques chez les Drosophiles. C. R. Acad. Sci. Paris 303:197–202.
Dallerac, R., Labeur, C., and Wicker-Thomas, C. 2000. A Delta-9 desaturase gene with a different substrate specificity is responsible for cuticular diene hydrocarbon polymorphism in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 97:9449–9454.
De Renobales, M. and Blomquist, G. J. 1984. Biosynthesis of medium chain fatty acids in Drosophila melanogaster. Arch. Biochem. Biophy. 228:407–414.
Etges, W. J. 1989. Evolution of developmental homeostasis in Drosophila mojavensis. Evol. Ecol. 3:189–201.
Etges, W. J. 1992. Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. Evolution 46:1945–1950.
Etges, W. J. 1998. Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. IV. Correlated responses in behavioral isolation to artificial selection on a life history trait. Am. Nat. 152:129–144.
Etges, W. J. 2002. Divergence in mate choice systems: Does evolution play by rules? Genetica 116:151–166.
Etges, W. J. and Ahrens, M. A. 2001. Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. V. Deep geographic variation in epicuticular hydrocarbons among isolated populations. Am. Nat. 158:585–598.
Etges, W. J. and Heed, W. B. 1987. Sensitivity to larval density in populations of Drosophila mojavensis: Influences of host plant variation on components of fitness. Oecologia 71:375–381.
Etges, W. J. and Jackson, L. L. 2001. Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. VI. Epicuticular hydrocarbon variation in Drosophila mojavensis cluster species. J. Chem. Ecol. 27:2125–2149.
Etges, W. J., Johnson, W. R., Duncan, G. A., Huckins, G., and Heed, W. B. 1999. Ecological genetics of cactophilic Drosophila, pp. 164–214, in R. Robichaux (ed.). Ecology of Sonoran Desert Plants and Plant Communities. University of Arizona Press, Tucson.
Ferveur, J. F., Cobb, M., and Jallon, J. M. 1989. Complex chemical messages in Drosophila, pp. 397–409, in N. Singh and N. Strausfeld (eds.). Neurobiology of Sensory Systems. Plenum Press, New York.
Fogleman, J. C. and Abril, J. R. 1990. Ecological and evolutionary importance of host plant chemistry, pp. 121–141, in J. S. F. Barker, W. T. Starmer, and R. J. MacIntyre (eds.). Ecological and Evolutionary Genetics of Drosophila. Plenum, New York.
Fogleman, J. C. and Danielson, P. B. 2001. Chemical interactions in the cactus–microorganism–Drosophila model system of the Sonoran Desert. Am. Zool. 41:877–889.
Fogleman, J. C. and Kircher, H. W. 1986. Differential effects of fatty acid chain length on the viability of two species of cactophilic Drosophila. Comp. Biochem. Physiol. 83A:761–764.
Fogleman, J. C., Duperret, S. M., and Kircher, H. W. 1986. The role of phytosterols in host plant utilization by cactophilic Drosophila. Lipids 21:92–96.
Funk, D. J. 1998. Isolating a role for natural selection in speciation: Host adaptation and sexual isolation in Neochlamisus bebbianae leaf beetles. Evolution 52:1744–1759.
Funk, D. J., Filchak, K. E., and Feder, J. L. 2002. Herbivorous insects: Model systems for the comparative study of speciation ecology. Genetica 116:251–267.
Gerhardt, H. C. and Huber, F. 2002. Acoustic Communication in Insects and Anurans. Univ. Chicago Press, Chicago.
Gibbs, A. G., Louie, A. K., and Ayala, J. A. 1998. Effects of temperature on cuticular lipids and water balance in a desert Drosophila: Is thermal acclimation beneficial? J. Exp. Biol. 201:71–80.
Gleason, J. M., Jallon, J.-M., Rouault, J.-D., and Ritchie, M. G. 2005. Quantitative trait loci for cuticular hydrocarbons associated with sexual isolation between Drosophila simulans and D. sechellia. Genetics 171:1789–1798.
Greenfield, M. D. 2002. Signalers and Receivers: Mechanisms and Evolution of Arthropod Communication. Oxford Univ. Press, New York.
Harborne, J. B. 1982. Introduction to Ecological Biochemistry. Academic Press, New York.
Heed, W. B. 1982. The origin of Drosophila in the Sonoran Desert, pp. 65–80, in J. S. F. Barker and W. T. Starmer (eds.). Ecological Genetics and Evolution: The Cactus–Yeast–Drosophila Model System. Academic Press, Sydney.
Heed, W. B. and Mangan, R. L. 1986. Community ecology of the Sonoran Desert Drosophila, pp. 311–345, in M. Ashburner, H. L. Carson, and J. N. Thompson (eds.). The Genetics and Biology of Drosophila. Academic Press, New York.
Howard, R. W. 1998. Ontogenetic, reproductive, and nutritional effects on the cuticular hydrocarbons of the host-specific ectoparasitoid Cephalonomia tarsalis (Hymenoptera: Bethylidae). Ann. Entomol. Soc. Am. 91:101–112.
Howard, R. W., Howard, C. D., and Colquhoun, S. 1995. Ontogenetic and environmentally induced changes in cuticular hydrocarbons of Oryzaephilus surinamensis (Coleoptera: Cucujidae). Ann. Entomol. Soc. Am. 88:485–495.
Jallon, J.-M. 1984. A few chemical words exchanged during courtship and mating of Drosophila melanogaster. Behav. Gen. 14:441–478.
Jallon, J.-M., Antony, C., Chang Yong, T. P., and Maniar, S. 1986. Genetic factors controlling the production of aphrodisiac substance in Drosophila, pp. 445–452, in M. Porchet, J. C. Andries, and A. Dhainaut (eds.). Advances in Invertebrate Reproduction. Elsevier, Amsterdam.
Jallon, J.-M. and Wicker-Thomas, C. 2003. Genetic studies on pheromone production in Drosophila, pp. 253–281, in G. J. Blomquist and R. C. Vogt (eds.). Insect Pheromone Biochemistry and Molecular Biology. Elsevier, San Diego.
Keith, A. D. 1967a. Fatty acid metabolism in D. melanogaster: Formation of palmitoleate. Life Sci. 6:213–218.
Keith, A. D. 1967b. Fatty acid metabolism in Drosophila melanogaster: Interaction between dietary fatty acids and de novo synthesis. Comp. Biochem. Physiol. 21:587–600.
Labeur, C., Dallerac, R., and Wicker-Thomas, C. 2002. Involvement of Desat1 gene in the control of Drosophila melanogaster pheromone biosynthesis. Genetica 114:269–274.
Liang, D. and Silverman, J. 2000. “You are what you eat”: Diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften 87:412–416.
Markow, T. A. 1982. Mating systems of cactophilic Drosophila, pp. 273–287, in J.\ S. F. Barker and W. T. Starmer (eds.). Ecological Genetics and Evolution: The Cactus–Yeast–Drosophila Model System. Academic Press, Sydney.
Nosil, P., Crespi, B. J., and Sandoval, C. P. 2002. Host-plant adaptation drives the parallel evolution of reproductive isolation. Nature 417:441–443.
Pennanec’h, M., Bricard, L., Kunesch, G., and Jallon, J.-M. 1997. Incorporation of fatty acids into cuticular hydrocarbons of male and female Drosophila melanogaster. J. Insect Physiol. 43:1111–1116.
Pennanec’h, M., Ferveur, J. F., Pho, D. B., and Jallon, J.-M. 1991. Insect fatty acid related pheromones: A review of their biosynthesis, hormonal regulation and genetic control. Ann. Soc. Entomol. Fr. 27:245–263.
Sandoval, C. P. and Nosil, P. 2005. Counteracting selective regimes and host preference evolution in ecotypes of two species of walking-sticks. Evolution 59:2405–2413.
SAS Institute, I. 2004. SAS/STAT 9.1.2. SAS Institute, Cary, NC.
Savarit, F. and Ferveur, J.-F. 2002. Temperature affects the ontogeny of sexually dimorphic cuticular hydrocarbons in Drosophila melanogaster. J. Exp. Biol. 205:3241–3249.
Savarit, F., Sureau, G., Cobb, M., and Ferveur, J.-F. 1999. Genetic elimination of known pheromones reveals the fundamental chemical bases of mating and isolation in Drosophila. Proc. Nat. Acad. Sci. USA 96:9015–9020.
Scheiner, S. M. 1993. Manova: Multiple response variables and multispecies interactions, pp. 94–112, in S. M. Scheiner and J. Gurevitch (eds.). Design and Analysis of Ecological Experiments. Chapman and Hall, New York.
Stennett, M. D. and Etges, W. J. 1997. Pre-mating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. III. Epicuticular hydrocarbon variation is determined by use of different host plants in Drosophila mojavensis and Drosophila arizonae. J. Chem. Ecol. 23:2803–2824.
Thompson, J. N. 1994. The Coevolutionary Process. University of Chicago Press, Chicago.
Toolson, E. C. and Kuper-Simbron, R. 1989. Laboratory evolution of epicuticular hydrocarbon composition and cuticular permeability in Drosophila pseudoobscura: Effects of sexual dimorphism and thermal-acclimation ability. Evolution 43:468–472.
Toolson, E. C., Markow, T. A., Jackson, L. L., and Howard, R. W. 1990. Epicuticular hydrocarbon composition of wild and laboratory-reared Drosophila mojavensis Patterson and Crow (Diptera: Drosophilidae). Ann. Entomol. Soc. Am. 83:1165–1176.
Wicker-Thomas, C., Henriet, C., and Dallerac, R. 1997. Partial characterization of a fatty acid desaturase gene in Drosophila melanogaster. Insect Biochem. Mol. Biol. 27:963–972.
Wicker-Thomas, C. and Jallon, J.-M. 2001. Control of female pheromones in Drosophila melanogaster by homeotic genes. Genet. Res. (Camb) 78:235–242.
Wigglesworth, V. B. 1988. The source of lipids and polyphenols for the insect cuticle: The role of fat body, oenocytes and oenocytoids. Tissue Cell 20:919–932.
Woodrow, R. J., Grace, J. K., Nelson, L. J., and Haverty, M. I. 2000. Modification of cuticular hydrocarbons of Cryptotermes brevis (Isoptera: Kalotermitidae) in response to temperature and relative humidity. Environ. Entomol. 29:1100–1107.
Acknowledgments
We thank J. C. Fogleman for information about host cactus chemistry, B. Durham for access to the GC, and S.J. Seybold and two anonymous reviewers for constructive comments. This work was partially supported by an REU supplement to NSF INT-9724790 (to W. J. Etges and W. B. Heed), NSF DEB-0211125 (to W.J.E.), a SILO Undergraduate Research Fellowship (SURF) grant from the Arkansas Science Information Liaison Office, and the Sturgis Fellowship program at the University of Arkansas.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplemetary material.
Rights and permissions
About this article
Cite this article
Etges, W.J., Veenstra, C.L. & Jackson, L.L. Premating Isolation is Determined by Larval Rearing Substrates in Cactophilic Drosophila mojavensis. VII. Effects of Larval Dietary Fatty Acids on Adult Epicuticular Hydrocarbons. J Chem Ecol 32, 2629–2646 (2006). https://doi.org/10.1007/s10886-006-9187-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9187-8