Abstract
Background
The second-generation cryoballoon (cryoballoon Advance; CB-A) offers excellent outcomes on the mid-term follow-up. To the best of our knowledge, little is known regarding the long-term outcome after CB-A ablation for paroxysmal atrial fibrillation (AF).
Objective
The aim of the study was to evaluate the freedom from recurrence of AF during a 3-year follow-up period, among consecutive patients having undergone pulmonary vein isolation (PVI) with the CB-A for paroxysmal AF (PAF).
Methods
Consecutive patients with drug-resistant PAF who underwent PVI using CB-A as an index procedure formed the study group. Patients were evaluated with holter ECG recordings at 1, 3, 6 and 12 months and subsequent follow-up was biannual or based on the clinical status and at the physician discretion.
Results
Seventy-six consecutive patients were enrolled. Of these patients, 6 were excluded because of lack of long-term follow-up. A total of 70 patients [44 male (63%); mean age 57.9 ± 14.5 years] with a mean follow-up of 38.0 ± 7.4 months were finally included. In total, 278 PVs were depicted on the pre-procedural CT scan. All PVs (100%) could be isolated with the CB-A only. The freedom from AF without antiarrhythmic drug (AADs) after a single procedure was 71.5% of patients at a mean 38.0 ± 7.4 months follow-up. If including repeat procedures, 80% of the patients were free from AF recurrence after 1.11 ± 0.32 procedures without AADs.
Conclusion
The second-generation cryoballoon offers long-term freedom from PAF in 71.5% of treated patients with a single procedure without AADs on a 3-year follow-up period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pulmonary vein isolation (PVI) has become the standard of care for drug refractory atrial fibrillation (AF) [1–4]. The second-generation cryoballoon (CB-A, Arctic Front, Medtronic, Minnesota, USA) carries an enhanced procedural efficacy of PVI compared to the first-generation device [5–9], and exhibits similar efficacy and safety to RF ablation with contact force-sensing [10, 11]. The recent guidelines regarding AF ablation define a long-term follow-up as lasting longer than 3 years from the procedure. To the best of our knowledge, data on long-term follow-up after PVI with the CB-A is sparse if not missing in today’s literature. Therefore, in the present study, we analyzed the clinical results of patients having undergone ablation with CB-A in our center for paroxysmal AF over a 3-year follow-up period.
2 Methods
2.1 Patient characteristics
This single-center, retrospective observational study consisted of consecutive patients who underwent ablation with CB-A as the first modality of PVI at our center for drug resistant paroxysmal atrial fibrillation (PAF) from June 2012 to March 2013. Only patients having undergone the index ablation procedure at least 2 ½ years before were considered in order to facilitate long-term follow-up. The protocol was carried out in accordance with the ethical principles for medical research involving human subjects established by Helsinki’s declaration, protecting the privacy of all the participants as well as the confidentiality of their personal information.
2.1.1 Pre-procedural management
All patients provided written informed consent prior to the procedure. To exclude the presence of thrombi in the left atrial appendage, all patients underwent trans-esophageal echocardiography (TEE) the day before the procedure, along with trans-thoracic examination (TTE) enabling assessment of left atrial (LA), LV, and valvular dimensions/function. Also, patients underwent a pre-procedural CT-scan to assess LA and PV anatomy. The pre-procedural imaging was used as a roadmap for the procedure and for the detection of eventual unconventional venous drainage patterns such as adjunctive veins or early branching. Exclusion criteria: Patients in whom cryoballoon ablation was not the index modality of PVI and those with contraindications for the procedure including, presence of a left atrial thrombus, uncontrolled heart failure and contraindications to general anesthesia. The study was approved by our local ethics committee.
2.1.2 Ablation procedure
In our center, all patients indicated for a PVI for drug resistant PAF undergo the index procedure with the CB-A. These procedures are all performed by 2 experienced operators with the help of junior physicians in training. The cryoballoon ablation procedure was carried out as previously described in detail [12]. Ablation was performed under general anesthesia. Briefly, after gaining LA access, through a steerable 15 Fr sheath (FlexCath Advance®, Medtronic), an inner lumen mapping catheter (ILMC) (Achieve®, Medtronic) was advanced into each PV ostium. Then a 28 mm CB-A (Arctic Front AdvanceTM, Medtronic) was advanced inflated and positioned sequentially in the PV ostium of each vein. Optimal vessel occlusion was considered as achieved upon selective contrast injection. Cryothermal applications lasted 4 min, and a bonus freeze following isolation was systematically performed in the initial procedures; later on, all the procedures were performed with a single 3 min application for each vein. Due to the high rate of PV isolation in the early phases of the first cryoenergy application we decided to adopt a single 3 min freeze per vein after our initial strategy with 2 cryo-applications of 240 s per vein. The single shot isolation was in line with the findings described by Fürnkranz A et al. [5]. The decision to reduce the freeze duration to 3 min was taken because of the very short time to isolation occurring in high proportion of PVs. In addition, experimental studies found that the size of cryo-lesions plateau within the 3 min of ablation and that lesion depth measured by ICE grows progressively during this freezing time in atrial myocardium and that an additional 1 min prolongation of the freeze does not determine further benefit since adequate and transmural damage is already achieved [13, 14]. And a second freeze was delivered in the case of failure to isolate the PV after the first cycle, in the case of nadir temperature greater than −35 degrees Celsius, and in the occurrence of early PV reconnection. To monitor right phrenic nerve (PN) activity during cryoablation in the right sided veins, a quadripolar catheter was placed in the superior vena cava and paced at high output at 1200 ms cycle length to capture the phrenic nerve. During the whole procedure activated clotting time was maintained >250 s with boluses of heparin as required. PVI was documented at the end of the procedure using ILMC and was defined as absence of PV potentials (PVP) or dissociation of the latter from LA activity. The sheaths were removed once the ACT was <200 s.
2.1.3 Post-procedural management
All patients were discharged the day following ablation. A pre-discharge TTE was performed in all individuals to exclude pericardial effusion. LMWH was started 6 h after the ablation. Oral anticoagulation was started the same evening of ablation and continued for at least 3 months. Anti-arrhythmic therapy was administered for 3 months following the procedure and discontinued if the patient was free of AF relapse.
2.1.4 Follow-up
After the procedure, all patients underwent clinical evaluation, an ECG and a 24-h holter recording at 1, 3, 6, 12 months. A clinical evaluation and an ECG were performed biannually during the subsequent years. As the majority of patients was symptomatic before ablation, any symptoms were deemed as deserving a Holter recording, unless symptom correlating arrhythmia was documented on an ECG during hospital visit. We did not use trans-telephonic monitoring or implantable loop recorders for monitoring purposes. Nine patients had implanted pacemakers which were also utilized for monitoring purposes. Finally, all patients were contacted at our institution between April 2016 and July 2016, for the final follow-up which consisted of a clinical evaluation, ECG and a 24-h Holter recording. As previously mentioned all individuals completed at least 2 ½ years of follow-up. All required information on clinical findings and Holter recordings after ablation was sent by the referring physicians and contributed to complete the follow-up of each patient successfully. A 24-h holter was performed in all the patients in the month prior to analysis. In case of repeat ablation, the follow-up schedule was the same as for the index procedure. A blanking period of 3 months was considered for the study. AF recurrence during the blanking period was taken into consideration for final analysis.
2.1.5 Repeat procedures
Repeat ablations were performed in those who had documented AF recurrence beyond the blanking period. In all repeat procedures persistence of PVI was checked and re-isolation undertaken. Repeat procedures were usually performed using RF energy with 4 mm irrigated tip bi-directional ablation catheters and 3D mapping. Further ablation was performed in case of persistent AF and of organized atrial tachy-arrhythmias.
2.1.6 Endpoints
The primary endpoints were: absence of symptomatic recurrence of AF and absence of AF or any other LA arrhythmia off AADs, lasting for >30s documented by either 12-lead ECG or a Holter recording after the blanking period as per guidelines [1].
2.2 Statistical analysis
Continuous valuables with the normal distribution were described with mean ± SD and the variables with an abnormal distribution were described with median and interquartile range. The assumption of normal distribution of our sample was tested with Kolmogolov-Smirnov test. Statistical differences were calculated using chi-square test for discrete variables and t-test for continuous variables (expressed as mean values and standard deviation). All tests were two-sided. Although this study was exploratory in nature, Logistic regression and Cox analysis were conducted to identify any predictors for AF recurrence. All statistics were performed with the use of the SPSS software (SPSS v20, IBM, USA). A p value ≤0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics
No patient was excluded due to anatomical reasons based on the pre-procedural CT scan. Seventy-six consecutive patients who underwent ablation with CB-A in the first year of its inception in our institute (from June 2012) were considered and complete follow up was available in 91% of individuals (70/76 patients). In the remaining 6 patients, after a short-term follow-up period of 30 days no periprocedural complications such as stroke or atrioesophageal fistula occurred. Thus, 70 patients (44 male; mean age 57.9 ± 15 years) formed the study group (Table 1).
3.2 Procedural data
The mean procedural time (from groin puncture to removal of catheters from the LA) of the second-generation cryoballoon ablation was 65.8 ± 20 min, while the mean fluoroscopy time was 16.0 ± 7 min. The first 52 patients were treated with 4 min freeze cycles with a systematic bonus freeze. The following 18 individuals were treated with a single 3 min freeze cycle strategy. The mean minimal achieved temperatures were −52.9 ± 5.2 degrees in the LSPV, −49.6 ± 5.6 degrees in the LIPV, −52.1 ± 5.7 degrees in the RSPV and −50.2 ± 6.8 degrees in the RIPV (p = ns). The Median time to PV isolation was 38.5 s [interquartile range, 30.0 to 46.0 s] in the LSPV, 29.0 s [interquartile range, 18.0 to 35.0 s] in the LIPV, 27.0 [interquartile range, 17.3 to 44.3 s] in the RSPV, 36.0 [interquartile range, 21.5 to 70.8 s] seconds in the RIPV. Real-time recordings could be observed in 56% of PV during cryo-applications. After a mean of 1.5 (range 1–3) applications, isolation could be demonstrated in 278/278 (100%) PVs and in 70/70 patients (100%). Six underwent electrical cardioversion at the end of the procedure.
3.3 Complications
The most frequent complication observed was transient PNP which occurred in 11 (16%) of 70 cases. In 73% (8/11 patients), PNP resolved completely before discharge. In three cases, this complication persisted after discharge. In 2 (3%) patients, the sniff test performed 2 months after the procedure showed a total recovery of the diaphragmatic function. In one patient, the recovery of PNP occurred after 10 months following ablation. At the time of PNP the PVs were all isolated. Of note, virtually all PNPs occurred in the first 20 patients (7/8). The rate of this complication was dramatically reduced in the following patients in whom more proximal applications with less vigorous wedging were performed in the right-sided veins. This maneuver has been described in detail previously [15]. One (2.2%) patient experienced a pseudoaneurysm which required surgical treatment. In 1 patient a TIA was observed the day after ablation with no permanent neurological sequelae. Neither pericardial tamponade nor clinically significant pericardial effusion occurred in our study group.
3.4 Follow-up
3.4.1 Clinical outcome
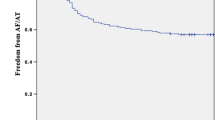
Thirty-six percent of our patients (25/70) were being followed up by the referring physicians after the first year. In the overall population at a mean follow-up of 38.0 ± 7.4 months, the success rate after a single procedure was 71.5% (50/70). Freedom from recurrence increased to 80% (56/70) of patients after a mean 1.11 ± 0.3 procedures without AADs. Finally the overall success rate was 84% (59/70) if patients on AADs were also considered. Seven patients (10%) presented with recurrence during the blanking period. Of these, 4 presented with further recurrence after the blanking period. If recurrences during the blanking period where were included the success rate decreased to 76%. The success rate without AADs at 12 and 24 months was 84% (59/70) and 74% (52/70) respectively. Eight patients (11%) underwent repeat ablations. Mean time to first recurrence of AF was 12.2 ± 8.5 months. A total of 8 patients in this group were still on class I or III AADs at final follow-up. Five among these continued to have atrial fibrillation and 3 patients were AF free on AADs. Kaplan-Meier curve of the survivor free of atrial fibrillation recurrence during the whole follow-up is shown in Fig. 1. Finally, no baseline characteristics were found to predict AF recurrence (Table 2).
3.4.2 Repeat ablation
Eight patients underwent repeat ablation 464 ± 323 days after the index procedure. All repeat ablation procedures were performed in our center, with open irrigated tip RF catheters under electroanatomical guidance. Repeat procedures were performed for AF in 4 patients and for atrial tachycardia or atrial flutter in the other individuals. (Fig. 2.) Twenty-six out of 32 (81%) PVs showed permanent electric isolation. The mean number of reconnected PVs per patient was 0.8 (range 1–3). Specifically, 2 LSPV, 2 LIPV, 1 RSPV and 1 RIPV. In the patients in whom the repeat procedures were performed, in only one PV, RT recordings could be observed during the index procedure. Specifically a LSPV, in which isolation could be observed at 38 s after the beginning of the freeze. In all the other veins RT recordings were not observed during cryo-applications. In the other reconnected PVs specifically, 2 LSPV, 2LIPV and 1 RIPV received 240 s freeze with an additional bonus freeze and 1 RSPV was treated with a 180 s single freeze.
3.4.3 - atrial fibrillation
Three patients presented with PAF, while the other exhibited persistent AF at the moment of the repeat procedure. Interestingly, in the PAF cases LA-PV reconnections could be documented. One individual presented with 3 PV reconnections (LSPV, LIPV and RIPV) simultaneously. In one patient, additional isolation of the SVC was performed because of documented frequent ectopic activity. The patient with persistent AF presented with absence of LA-PVs reconnection and underwent posterior box isolation.
3.4.4 - atrial tachycardia or atrial flutter
Two cases of left atrial flutter, 1 case of left atrial tachycardia and 1 case of right atrial tachycardia underwent repeat procedures. A left atrial roof dependent flutter and a mitral isthmus dependent flutter were documented and treated accordingly. The left atrial tachycardia originated from a reconnected LIPV. The right AT originated from the inferior lateral region of the RA.
3.4.5 Drugs
All patients had failed a class I or III AAD before considering CB-A. OAC was discontinued uniformly in all cases that remained in SR and had no evidence of AF after 3 months from the index procedures. Patients with AF recurrences continued OAC even after 3 months according to the guidelines. Among patients with CHADS2 score of ≥2, OAC was prescribed continuously. At the final follow-up, 2 patients continued taking class I AAD, 5 patients for class III AAD, and 1 patient for both AADs without interruption during the follow-up after the blanking period because of the fact or the risk of AF recurrences (Table 3).
4 Discussion
To the best of our knowledge, this is the first study evaluating the clinical results following second-generation cryoballoon ablation after a long-term follow-up of 3 years. The main findings of our study are: 1) the long-term single procedural success rate without AADs is 71.5%, 2) this success rate increases to 80% with a mean of 1.1 procedures, 3) the LA-PV reconnection rate was 0.8 PVs per patient during the repeat procedures and 4) predictors of recurrence could not be identified.
4.1 Long-term success rate of the second-generation cryoballoon
In our study, the long-term single procedure outcome as AF free survival was 71.5%. We observed most of the recurrences in the first 2 years following the index procedure and a small decrease between the second to third year from 74% to 71.5%. Previous studies have described the long-term outcome following PVI with the first-generation CB. Neumann et al. reported their findings on a 5-year follow-up period in a relatively large population. If analyzing the data from the abovementioned article approximately 57% of patients were free of AF at 3 years [16]. Similar findings were published by our group. In fact, we observed a success rate of 57.5% on a long-term follow-up after first-generation CB ablation. In the same period, Vogt J. et al. reported single procedural freedom from AF in 62% of patients at a mean follow-up period of 33 months [17]. The latter might be explained by the use of either balloon diameters (23 mm or 28 mm) based on venous dimensions. Furthermore, in a non-negligible amount of patients, both balloon sizes were used in the same patient in order to optimize occlusion in all PVs. Therefore, the CB-A seems to yield better outcomes if compared to its predecessor. The CB-A has been launched on the market with technical modifications designed to significantly improve procedural outcome with respect to the first-generation balloon. The number of injection ports has been doubled, from 4 to 8, and these have been positioned more distally on the catheters shaft resulting in a larger and more uniform zone of freezing on the balloons surface if compared with the previous version. Therefore, this might translate in better long-term outcomes due to an increased rate of permanency of PV isolation. In addition, our study showed that the success rate rose up to 80% with repeat procedures. Higher success rates following repeat procedures are well documented in today’s literature. In fact, in patients relapsing with AF re-isolation of the reconnected veins yields good clinical outcome [18]. In addition, 4 patients recurred with organized atrial arrhythmias such as atrial flutters or focal tachycardias. Ablation of the critical isthmus on the one hand and targeting of the focus in another led to termination of these tachycardias. Finally, we performed a posterior box lesion in a patient presenting with persistent AF after the index procedure. Although, ablation approaches are less standardized when dealing with persistent forms of AF, substrate modification (such as ablation on the posterior wall) in the latter might hypothetically yield beneficial effects [19].
4.2 LA-PV reconnections
In our observation, we documented a mean of 0.8 reconnected PVs per patient during the repeat procedures. The rate of permanent PVI was high and could be observed in 26 out of 32 (81%) PVs at 464 days after the index procedure. A high rate of chronic persistence of LA-PV isolation following CB-A has already been reported in previous publications [20–22]. In our study, all patients with PAF have LA-PV reconnection. Pulmonary vein reconnection is known to be strongly correlated to AF recurrence after the index procedure. Furthermore, during repeat procedures re-isolation of the PVs is usually associated with better outcomes [23]. This was confirmed in our study by the freedom from AF following the repeat procedure in the 3 patients with PAF. Interestingly, in patients presenting with persistent AF or organized atrial arrhythmias the rate of LA-PV reconnection was very low. In the latter only one LIPV was found to be reconnected out of 16 PVs. Second-generation cryoballoon ablation offers large and homogeneous lesions extending into the PV antra [24]. These might lead to the creation of larger and more proximal lesions resulting in the occurrence of slow conduction areas, especially on the LA roof, which might lead to macro reentrant left atrial flutters. Typically, this might occur on the LA roof if the lesions between both superior veins are close enough. In our study, in both patients presenting with LA flutters, sinus rhythm was readily resumed once the isthmus was closed. One patient presented with persistent AF at the repeat procedure with all PVs still isolated. A previous study [25] comparing the incidence of ATs following traditional point by point RF ablation and CB ablation showed that post-ablation flutters were significantly rarer after PVI with the second generation CB. This might explained by various factors. First, balloon-based technology allows the creation of circumferential lesions around the PVs with a lower number of applications. On the other hand, point by point RF ablation carries the obvious difficulty of creating continuous circumferential lines. In addition, cryothermal energy ensures increased catheter stability due to freeze-mediated catheter adhesion. This might result in creation of more demarcated and homogeneous lesions with potentially less proarrhythmic effect if compared with RF [26].
4.3 Identification of predictors of AF recurrence
We could not identify any predictor of AF recurrence following ablation. Previous studies show that the size of LA is an independent predictor of AF recurrence for persistent AF patients [27]. In our study the mean LA size was normal. In fact, only patients with PAF were analyzed. Paroxysmal AF represents an earlier stage of the disease with a lower amount of electroanatomical modification of the LA substrate if compared to more persistent forms of AF. Another explanation might lie in the fact that this study was clearly underpowered to find predictors for recurrence.
4.3.1 Limitations
The study was a retrospective single-center trial conducted in a small number of patients. Further larger studies are necessary to confirm our findings. The rate of completed follow up was limited to 91%. Several patients received a bonus freeze which might have overestimated the results. We did not systematically monitor possible procedural complications (for example cardiac CT scan for PV stenosis, esophagoscopy in order to verify the presence of esophageal damage or brain MRI for silent cerebral infarctions), that could have caused underestimation of the complication rate. Most importantly, only 10% of patients had implanted devices such as PM, loop-recorders or ICD, therefore, although all patients suffered from symptomatic PAF prior to ablation, asymptomatic episodes might have gone undiagnosed and the overall success rate overrated.
5 Conclusions
The second-generation cryoballoon offers long-term freedom from PAF in 71.5% of treated patients with a single procedure off AADs on a 3-year follow-up period. The success rate increases to 80% after a mean 1.1 of procedures off AADs. Larger studies are required to confirm our findings.
References
Calkins H, Kuck KH, Cappato R, Brugada J, John Camm A, Chen S-A, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Patient Selection, Procedural Techniques, Patient Management and Follow-up, Definitions, Endpoints, and Research Trial Design. Heart Rhythm. 2012;9:632–96.
Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66.
Pappone C, Rosanio S, Oreto G, et al. Circumferential RF ablation of pulmonary vein ostia: a new anatomic approach for curing atrial fibrillation. Circulation. 2000;102:2619–28.
Riccardo Cappato, Hugh Calkins, Shih-Ann Chen, Wyn Davies, Yoshito Iesaka, Jonathan Kalman, et al. Updated Worldwide Survey on the Methods, Efficacy, and Safety of Catheter Ablation for Human Atrial Fibrillation Circ Arrhythm Electrophysiol. 2010;3:32–38
Fürnkranz A, Bordignon S, Schmidt B, Gunawardene M, Schulte-Hahn B, Urban V, Bode F, Nowak B, Chun JK. Improved procedural efficacy of pulmonary vein isolation using the novel second-generation cryoballoon. J Cardiovasc Electrophysiol. 2013;24:492–7.
Martins RP, Hamon D, Césari O, Behaghel A, Behar N, Sellal JM, Daubert JC, Mabo P, Pavin D. Safety and efficacy of a second-generation cryoballoon in the ablation of paroxysmal atrial fibrillation. Heart Rhythm. 2014;11:386–93.
Straube F, Dorwarth U, Vogt J, Kuniss M, Heinz Kuck K, Tebbenjohanns J, Garcia Alberola A, Chun KR, Souza JJ, Ouarrak T, Senges J, Brachmann J, Lewalter T, Hoffmann E. Differences of two cryoballoon generations: insights from the prospective multicentre, multinational FREEZE cohort Substudy. Europace. 2014;16:1434–42.
Aytemir K, Gurses KM, Yalcin MU, Kocyigit D, Dural M, Evranos B, Yorgun H, Ates AH, Sahiner ML, Kaya EB, Oto MA. Safety and efficacy outcomes in patients undergoing pulmonary vein isolation with second-generation cryoballoon†. Europace. 2015;17:379–87.
Liu J, Kaufmann J, Kriatselis C, Fleck E, Gerds-Li JH. Second generation of cryoballoons can improve efficiency of cryoablation for atrial fibrillation. Pacing Clin Electrophysiol. 2015;38:129–35.
Jourda F, Providencia R, Marijon E, Bouzeman A, Hireche H, Khoueiry Z, Cardin C, Combes N, Combes S, Boveda S, Albenque JP. Contact-force guided radiofrequency vs. second-generation balloon cryotherapy for pulmonary vein isolation in patients with paroxysmal atrial fibrillation-a prospective evaluation. Europace. 2015;17:225–31.
Squara F, Zhao A, Marijon E, Latcu DG, Providencia R, Di Giovanni G, Jauvert G, Jourda F, Chierchia GB, De Asmundis C, Ciconte G, Alonso C, Grimard C, Boveda S, Cauchemez B, Saoudi N, Brugada P, Albenque JP, Thomas O. Comparison between radiofrequency with contact force-sensing and second-generation cryoballoon for paroxysmal atrial fibrillation catheter ablation: a multicentre European evaluation. Europace. 2015;17:718–24.
Irfan G, de Asmundis C, Mugnai G, Poelaert J, Verborgh C, Umbrain V, Beckers S, Hacioglu E, Hunuk B, Velagic V, Stroker E, Brugada P, Chierchia G-B. One-year follow-up after second-generation cryoballoon ablation for atrial fibrillation in a large cohort of patients: a single-Centre experience. Europace. 2016;18:987–93.
Chierchia GB, Di Giovanni G, Sieira-Moret J, de Asmundis C, Conte G, Rodriguez-Mañero M, et al. Initial experience of three-minute freeze cycles using the second-generation cryoballoon ablation: acute and short-term procedural outcomes. J Interv Card Electrophysiol. 2014;39:145–51.
Ciconte G, Sieira-Moret J, Hacioglu E, Mugnai G, DI Giovanni G, Velagic V, Saitoh Y, Conte G, Irfan G, Baltogiannis G, Hunuk B, Stroker E, Brugada P, DE Asmundis C, Chierchia GB. Single 3-minute versus double 4-minute freeze strategy for second-generation cryoballoon ablation: a single-center experience. J Cardiovasc Electrophysiol. 2016;27:796–803.
Casado-Arroyo R, Chierchia GB, Conte G, Levinstein M, Sieira J, Rodriguez-Mañero M, di Giovanni G, Baltogiannis Y, Wauters K, de Asmundis C, Sarkozy A, Brugada P. Phrenic nerve paralysis during cryoballoon ablation for atrial fibrillation: a comparison between the first- and second-generation balloon. Heart Rhythm. 2013;10:1318–24.
Neumann T, Wójcik M, Berkowitsch A, Erkapic D, Zaltsberg S, Greiss H, Pajitnev D, Lehinant S, Schmitt J, Hamm CW, Pitschner HF, Kuniss M. Cryoballoon ablation of paroxysmal atrial fibrillation: 5-year outcome after single procedure and predictors of success. Europace. 2013;15:1143–9.
Vogt J, Heintze J, Gutleben KJ, Muntean B, Horstkotte D, Nölker G. Long-term outcomes after cryoballoon pulmonary vein isolation: results from a prospective study in 605 patients. J Am Coll Cardiol. 2013;61:1707–12.
Conte G, Chierchia GB, Sieira J, Levinstein M, Casado-Arroyo R, De Asmundis C, Sarkozy A, Rodriguez-Manero M, Di Giovanni G, Baltogiannis G, Wauters K, Brugada P. Repeat procedure using radiofrequency energy for recurrence of atrial fibrillation after initial cryoballoon ablation: a 2-year follow-up. Europace. 2013;15:1421–5.
Kottkamp H, Berg J, Bender R, Rieger A, Schreiber D. Box isolation of fibrotic areas (BIFA): a patient-tailored substrate modification approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:22–30.
Heeger CH, Wissner E, Mathew S, Deiss S, Lemes C, Rillig A, Wohlmuth P, Reissmann B, Tilz RR, Ouyang F, Kuck KH, Metzner A. Once isolated, always isolated? Incidence and characteristics of pulmonary vein Reconduction after second-generation cryoballoon-based pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2015;8:1088–94.
DE Regibus V, Mugnai G, Moran D, Hünük B, Ströker E, Hacioglu E, Ruggiero D, Coutiño-Moreno HE, Takarada K, Brugada P, DE Asmundis C, Chierchia GB. Second-generation cryoballoon ablation in the setting of lone paroxysmal atrial fibrillation: single procedural outcome at 12 months. J Cardiovasc Electrophysiol. 2016;27:677–82.
Reddy VY, Neuzil P, d'Avila A, Laragy M, Malchano ZJ, Kralovec S, Kim SJ, Ruskin JN. Balloon catheter ablation to treat paroxysmal atrial fibrillation: what is the level of pulmonary venous isolation? Heart Rhythm. 2008;5:353–60.
Ouyang F, Tilz R, Chun J, Schmidt B, Wissner E, Zerm T, Neven K, Köktürk B, Konstantinidou M, Metzner A, Fuernkranz A, Kuck KH. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368–77.
Ciconte G, Mugnai G, Sieira J, Velagić V, Saitoh Y, Irfan G, Hunuk B, Ströker E, Conte G, Di Giovanni G, Baltogiannis G, Wauters K, Brugada P, de Asmundis C, Chierchia GB. On the quest for the best freeze: predictors of late pulmonary vein reconnections after second-generation cryoballoon ablation. Circ Arrhythm Electrophysiol. 2015;8:1359–65.
Juliá J, Chierchia GB, de Asmundis C, Mugnai G, Sieira J, Ciconte G, Di Giovanni G, Conte G, Baltogiannis G, Saitoh Y, Wauters K, Irfan G, Brugada P. Regular atrial tachycardias following pulmonary vein isolation for Paro-xysmal atrial fibrillation: a retrospective comparison between the cryoballoon and conventional focal tip radiofrequency techniques. J Interv Card Electrophysiol. 2015;42:161–9.
Andrade JG, Dubuc M, Guerra PG, Macle L, Mondésert B, Rivard L, et al. The biophysics and biomechanics of cryoballoon ablation. Pacing Clin Electrophysiol. 2012;35:1162–8.
McCready JW, Smedley T, Lambiase PD, Ahsan SY, Segal OR, Rowland E, Lowe MD, Chow AW. Predictors of recurrence following radiofrequency ablation for persistent atrial fibrillation. Europace. 2011;13:355–61.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was used for this study.
Conflict of interests
Gian-Battista Chierchia, Carlo de Asmundis MD receive compensation for teaching purposes from AF Solutions, Medtronic. Gian-Battista Chierchia receives compensation for proctoring purposes from AF Solutions, Medtronic. Pedro Brugada has received speaker fees from Medtronic.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Ken Takarada and Ingrid Overeinder contributed equally to this work.
Rights and permissions
About this article
Cite this article
Takarada, K., Overeinder, I., de Asmundis, C. et al. Long-term outcome after second-generation cryoballoon ablation for paroxysmal atrial fibrillation - a 3-years follow-up. J Interv Card Electrophysiol 49, 93–100 (2017). https://doi.org/10.1007/s10840-017-0237-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-017-0237-7