Abstract
Background
The 12-month follow-up (F/U) efficacy of CBA PVI performed at community hospitals for treatment of symptomatic paroxysmal and persistent atrial fibrillation (AF) is unknown. This study determined the 12-month efficacy of pulmonary vein isolation (PVI) using cryoballoon ablation (CBA) performed at community hospitals with limited annual case numbers.
Methods
This registry study included 983 consecutive patients (pts) from 19 hospitals, each with an annual procedural volume of < 100 PVI procedures/year. Pts underwent CBA PVI for paroxysmal AF (n = 520), persistent AF (n = 423), or redo PVI (n = 40). The primary endpoint was frequency of documented recurrent AF, the occurrence of atrial flutter or tachycardia following a 90-day period after the index ablation and up to 12 months. The frequency of repeat ablation was determined.
Results
Isolation of all PVs was documented in 98% of pts at the end of the procedure. Twelve-month F/U data could be obtained in 916 pts. A 24-h ECG registration was performed in 641 pts (70.0%); in 107 pts (16.7%) of them, recurrent AF was documented. The primary endpoint was met in 193 F/U pts (21.1%). It occurred in 80/486 F/U pts with paroxysmal AF (16.4%), and in 107/390 F/U pts with persistent AF (27.4%). Redo PVI was performed in 71 pts (7.8%), and atrial flutter ablation was performed in 12 pts (1.4%).
Conclusions
CBA PVI for paroxysmal or persistent AF can be performed at community hospitals with adequate rates of 12-month symptom freedom and arrhythmia recurrence. The study was registered at the German register of clinical studies (DRKS00016504).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pulmonary vein isolation (PVI) using cryoballoon ablation (CBA) has become an established treatment for symptomatic paroxysmal and persistent atrial fibrillation (AF), with procedural success and complication rates comparable to those of radiofrequency ablation [1,2,3,4,5]. CBA PVI has been considered to be less complex compared to PVI using radiofrequency ablation with 3D mapping systems [6, 7], potentially allowing its application at less specialized centers. Twelve months freedom from recurrent AF after second-generation CBA has been reported to be in the range of 80–84% for paroxysmal AF and in the range of 59–69% for persistent AF [8,9,10]. Previous studies on the recurrence of AF 12 months after CBA PVI for paroxysmal and persistent AF as well as after redo PVI using CBA have included only limited patient numbers and have been reported from large referral centers [4, 11,12,13]. To our knowledge, there are no data on freedom from recurrent arrhythmia after implementation of CBA PVI at community hospitals with limited procedure volumes. We documented a high acute procedural success rate and a low complication rate in a prospective registry on PVI using second-generation CBA performed at 20 local hospitals [14].
The aim of this prospective registry study was to define the 12-month efficacy of CBA PVI performed at community hospitals with limited case numbers on patients with paroxysmal and persistent AF as well as on patients undergoing CBA for redo PVI. The impact of the board certification status of the operator as invasive electrophysiologist and hospital case load on the 12-month efficacy of PVI was also evaluated.
2 Methods
We performed a prospective German multicenter registry on the efficacy and safety of CBA PVI at community hospitals. Details of this registry study have been previously published [14]. CBA for AF was performed on patients with paroxysmal or persistent AF and on patients with recurrence of AF after previous AF ablation. Categorization of patients as having paroxysmal or persistent AF considered the definition of the 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement [15].
Nineteen of the community hospitals participated in the collection of follow-up data for this researcher-initiated study, which did not have external financial support. One of the initial 20 centers could not provide follow-up data due to a lack of staff able to collect data and was thus omitted from the database. A total of 983 consecutive patients were included at the 19 hospitals into the registry. The participating hospitals had at least 1 year of experience performing CBA PVI, and each had a maximum caseload of 100 CBA procedures annually.
At 7 centers, CBA was the only method used for PVI; at the other 12 centers, CBA was the predominantly used PVI technique. On average, the number of operators performing the procedures was 1.1 per center (± 0.0). In all but one center, procedures were performed by only one operator. Thus, the number of CBA procedures performed per year by each operator was consistent with the numbers reported for the centers in all but one center, as was the number of all PVI procedures per year per operator. Only one center met the previously described criteria [16] required for qualified AF PVI center designation. Ten study centers performed ≥ 70 PVI procedures per year using either CBA or RFA and > 60 CBA procedures per year. The other 9 study centers performed < 70 PVI procedures per year and ≤ 60 CBA procedures per year.
No guidelines for the performance of the CBA procedure were formulated. Each participating center performed the procedure according to local standards. Procedures were carried out considering the standards and recommendations of the cryoballoon manufacturer. The Medtronic Arctic Front Advance™ Cardiac Cryoablation Catheter (Medtronic, Minneapolis, Minnesota, USA) was used for all procedures. Extensive stimulation maneuvers were required to document proof of effective PVI. The complete isolation of all veins was documented in 98% of patients.
Phrenic nerve stimulation combined with the continuous monitoring of compound motor action potentials was recommended to allow for the early recognition of phrenic nerve palsy.
For all patients included in the registry, the local hospitals aimed at obtaining 12-month follow-up data. To obtain follow-up data, patients were actively contacted by the local hospital. It was left to the discretion of the local hospitals to obtain follow-up data via personal or telephone contact. A structured questionnaire was used by all hospitals to obtain data on follow-up events. It was stipulated, but not mandatory, to obtain a 24-h ECG at follow-up contact. As all patients were symptomatic before ablation, any symptom indicating possible recurrence of AF was deemed deserving the recording of an ECG or 24-h ECG.
The frequency of documented recurrent AF (> 30 s), the occurrence of atrial flutter or atrial tachycardia, the use of antiarrhythmic drug therapy (ADT; class I or III antiarrhythmic agent), electrical/pharmacologic cardioversion, and repeat ablation following a 90-day period after the index ablation and up to 12 months post-procedure were determined. The primary 12-month follow-up endpoint was determined as (I) documented recurrent AF, atrial flutter, or atrial tachycardia irrespective of the use of ADT, and (II) documented recurrent AF, atrial flutter, or atrial tachycardia or the use of ADT more than 90 days after the procedure.
The 12-month follow-up evaluated also persistent complications of the procedure defined as residual of a stroke, vascular or gastrointestinal complication, or a persistent phrenic nerve palsy. A persistent phrenic nerve palsy had to be confirmed by fluoroscopy. To ensure that all persistent complications were defined, a questionnaire was used at follow-up which actively addressed all potential long-term complications of the procedure. In addition, the patient could describe issues potentially related to the procedure.
The approval to perform the registry study was initially given by the ethical committee of the Ärztekammer Niedersachsen and subsequently by the local ethical committees of each federal state that participated in the study. Each patient had to provide written informed consent regarding the inclusion of his or her patient data in the study prior to the procedure. All patient data were pseudonymized and transferred to a centralized database at the Bonifatius Hospital Lingen. The study was registered in the German Register of Clinical Studies (DRKS00016504) and displayed on http://apps.who.int/trialsearch/.
2.1 Statistical analysis
All data were collected in a centralized database at the Bonifatius Hospital Lingen. Statistical analysis was performed using Medcalc statistical software (Version 4.2, Ostend, Belgium). Continuous data are expressed as mean (± SD). Comparisons of categorical variables were performed using χ2 tests. To evaluate the impact of the body mass index (BMI) on the frequency of the primary endpoint, four BMI groups were formed, (1) BMI < 25 kg/m2, (2) BMI ≥ 25–30 kg/m2, (3) BMI ≥ 30–35 kg/m2, and (4) BMI ≥ 35 kg/m2. Univariate and multivariate logistic regression analysis was performed to determine predictors of the primary endpoint at 12-month follow-up which did not consider ADT use. Parameters included into the analysis were patient characteristics, procedural characteristics, type of atrial fibrillation, number of PVI procedures per year performed at a hospital (≥ 70 or < 70), and board certification status of the operator. The odds ratio (OR) and its confidence interval were calculated for each parameter. A p < 0.05 was considered statistically significant.
3 Results
3.1 Patients
A total of 983 patients were prospectively included into this study at 19 German community hospitals. 520 patients (53%) presented with paroxysmal AF and 423 patients (43%) with persistent AF. In 40 patients (4%), CBA PVI was performed as a redo procedure. Patient characteristics are shown in Table 1.
3.2 Study centers
The mean number of PVI procedures performed using CBA at each study center was 62 per year (± 25), and the total number of PVI procedures performed using either technique at each study center was 73 per year (± 26).
In total, 557 procedures (57%) were performed by a board-certified electrophysiologist and 426 procedures (43%) were performed by a non-board-certified operator. The 10 centers with higher procedural numbers per year contributed a total of 623 procedures to the study, whereas the nine centers with lower procedural numbers per year contributed a total of 360 procedures.
3.3 Procedural technique
Procedural characteristics have been reported previously [14].
The mean number of CBA procedures per vein was 1.6 ± 0.8, and the lowest balloon temperature reached during cryoablation was − 48.1 ± 7.3 °C.
Overall, PV isolation was documented in 99.0% of the veins. The procedural efficacy endpoint “all PV isolated at the end of the procedure” was achieved in 964 patients (98.1%). Occurrence of phrenic nerve palsy was the main reason that isolation of all PV could not be achieved at the end of the procedure.
3.4 Complications
The occurrence of one or several of the following complications defined a major complication: death, stroke or TIA, esophageal-atrial fistula, pericardial effusion treated by drainage or by surgery, vascular complications that required intervention or surgery, and phrenic nerve palsy not resolved until discharge. Major complications occurred in 12 patients (1.2%). The details of the major complications until hospital discharge after PVI have been reported [14].
3.5 Twelve-month efficacy
Twelve-month follow-up data could be obtained in 916 patients (93%). Sixty-seven patients (7%) refused to provide follow-up data, withdrew consent to the study, or could not be contacted. Of the 916 follow-up patients, 263 (28.6%) complained of recurrent palpitations. In 160 of these patients (61%), AF was documented. 641 patients (70.0%) underwent 24-h ECG registration, with the documentation of AF in 107 patients (16.7%). The primary endpoint I of documented recurrent AF, atrial flutter, and atrial tachycardia was met in 193 patients (21.1%). Redo PVI for AF was performed in 71 patients (7.8%) and atrial flutter ablation in 12 patients (1.4%). In 186 patients (21.0%), ADT was administered later than 90 days after CBA. The primary endpoint II including documented recurrent AF, atrial flutter, or atrial tachycardia, as well as the administration of ADT later than 90 days after CBA as an endpoint, was documented in 305 patients (33.3%).
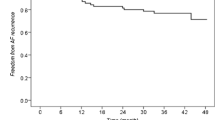
The primary endpoint I (not considering ADT administration as endpoint) occurred in 80/486 follow-up patients with paroxysmal AF (16.4%), in 107/390 follow-up patients with persistent AF (27.4%) (p < 0.001 vs. paroxysmal) (Fig. 1), and in 6/40 patients with redo PVI (15.0%). The primary endpoint II (considering ADT as an endpoint) occurred in 143/486 follow-up patients with paroxysmal AF (29.4%), in 148/390 follow-up patients with persistent AF (37.9%) (p = 0.010 vs. paroxysmal) (Fig. 1), and in 14/40 patients with a redo PVI (35.0%) (Table 2).
Frequency of primary endpoint at 12-month follow-up defined as combination of documented recurrent atrial fibrillation, occurrence of atrial flutter or atrial tachycardia either (endpoint I) excluding the administration of ADT as endpoint (left panel) or (endpoint II) including the administration of ADT as endpoint (right panel). Frequencies are provided for all patients with follow-up data included into the study, and separately only for patients with paroxysmal (paroxys) atrial fibrillation, patients with persistent (persist) atrial fibrillation, and patients with redo PVI. F/U, follow-up
The primary endpoint I occurred in 21.8% of the 641 patients with 24-h ECG monitoring and in 19.4% of the 275 patients without 24-h ECG monitoring (p = 0.479). Considering the BMI, there was a strong trend towards higher occurrence of the primary endpoint not considering ADT with higher BMI group. The primary endpoint not considering ADT occurred in 18.1% of patients with a BMI < 25 kg/m2, in 19.8% with a BMI ≥ 25–30 kg/m2, in 23.9% with a BMI ≥ 30–35 kg/m2, and in 26.3% of patients with a BMI ≥ 35 kg/m2.
Out of the 916 follow-up patients, 409 patients were treated by a non-board-certified operator, and 507 patients were treated by a board-certified electrophysiologist. The primary endpoint I was met in 87 (21.3%) patients treated by a non-board-certified operator and in 106 (20.9%) patients treated by a board-certified operator (p = 0.959). The primary endpoint II was met in 146 (35.7%) patients treated by a non-board-certified operator and in 159 (31.4%) patients treated by a board-certified operator (p = 0.1921). Of the follow-up patients, 291 patients were treated at a center with < 70 PVI/year, and 625 patients were treated at a center with ≥ 70 PVI/year. The primary endpoint I was met in 69 (23.4%) patients treated at a center with < 70 PVI/year and in 124 (19.8%) patients treated by a center with ≥ 70 PVI/year (p = 0.2137). The primary endpoint II was met in 86 (29.6%) patients treated by a center with < 70 PVI/year and in 219 (35.0%) patients treated by a center with ≥ 70 PVI/year (p = 0.1194).
3.6 Predictors of the primary outcome
If the primary endpoint I at the 12-month follow-up was considered, the following univariate predictors could be defined: persistent versus paroxysmal AF OR 1.138 (1.101–1.164), p = 0.001, lowest freeze temperature of the right inferior pulmonary vein (RIPV), OR per 1 °C increase: 1.003 (1.001–1.004), p = 0.048, and body mass index, OR per 1 kg/m2 increase: 1.006 (1.001–1.011), p = 0.0336. In the multivariate logistic regression analysis, persistent versus paroxysmal AF OR 1.132 (1.100–1.162), p = 0.001, lowest freeze temperature of the RIVP, OR per 1 °C increase: 1.003 (1.001–1.004), p = 0.048, and body mass index, OR per 1 kg/m2 increase 1.005 (1.001–1.011), p = 0.038, remained predictors of the primary endpoint of recurrent AF at 12-month follow-up.
3.7 Persistent complications at 12-month follow-up
There were three patients in whom symptomatic complications persisted at the 12-month follow-up. These complications consisted of phrenic nerve palsy in two patients and hoarseness due to transesophageal echocardiography in one patient.
4 Discussion
This study documents: (1) rates of symptomatic recurrent AF at 12-month follow-up after PVI performed at community hospitals using CBA are adequate, (2) recurrent AF is more frequent after PVI for persistent AF than for paroxysmal AF, (3) board certification status of the operator as an invasive electrophysiologist and a center procedure volume ≥ 70 PVI/year are no predictors of fewer recurrences of AF at 12-month follow-up, (4) persistent AF, the lowest freeze temperature in the RIPV, and the BMI are predictors of recurrent AF, and (5) phrenic nerve palsy persists at 12-month follow-up in 0.2% of patients.
CBA PVI has become a cornerstone for successful ablation in AF [1,2,3,4,5]. The recurrence of AF is a limitation. Pulmonary vein reconnection is assumed to be the reason for AF recurrence, as studies have shown that late-term recurrence between 3 and 12 months post-PVI is predominantly linked to the recovery of electrical conduction between the PVs and the left atrium, irrespective of the type of AF [17, 18]. While freedom from AF at 12 months has been found to be about 70% after the ablation of paroxysmal AF using first-generation cryoballoon, it has been shown to be substantially higher with second-generation cryoballoon [19]. After first-generation CBA, a typical reconduction gap pattern has been described that predominantly affected the inferior PV. Using second-generation CBA, freedom from recurrent AF rates of 80–84% have been found for paroxysmal AF [8, 9]. Metzner et al. [9] reported AF recurrence after PVI using second-generation CBA in 50 patients with paroxysmal or short-standing persistent AF. Metzner et al. defined recurrence as a symptomatic or documented arrhythmia episode of > 30 s, excluding a 3-month blanking period. A total of 80% of patients remained in stable sinus rhythm during a mean follow-up duration of 440 days. In a study on 42 patients, Chierchia et al. [8] reported freedom from AF at a 12-month follow-up in 83% of patients affected by drug-resistant paroxysmal AF following a 3-month blanking period. PVI alone has also become an accepted technique for treatment of persistent AF as well as for recurrent AF after PVI. The basis for the use of PVI alone in persistent AF is the STAR-AF II study [20], which demonstrated that PVI alone was not inferior to PVI plus linear ablation or PVI plus the ablation of complex fractionated electrocardiograms in preventing the recurrence of any AF lasting longer than 30 s. In patients with recurrent AF after PVI, for paroxysmal AF, Fichtner et al. [21] demonstrated that repeat PVI was not inferior to repeat PVI plus one left atrial line in ensuring freedom from AF at 12-month follow-up. Ciconte et al. [10] reported a 1-year freedom of recurrence of atrial tachyarrhythmias, defined as a symptomatic or documented episode > 30 s in 60.3% of patients after CBA for drug-refractory persistent AF. In a review, Tzeis et al. [22] described the experience of 5 previous studies on second-generation CBA for persistent AF that included between 49 and 100 patients. A 12-month freedom from all documented atrial tachyarrhythmias lasting more than 30 s without antiarrhythmic drugs and following a single ablation procedure was used as endpoint, considering a 3-month blanking period. It was reported to have occurred in 59–69% of patients. The chronic PVI rate post redo CBA for recurrent AF after a PVI procedure was evaluated by Bordignon et al. [23]. They reported a chronic PVI rate of 77% after second-generation CBA for redo PVI.
4.1 Impact of endpoint
We applied two definitions when evaluating freedom from recurrent AF at 12 months. One excluded the administration of ADT as an endpoint, while the other did consider it. The application of ADT increased the overall endpoint rate by more than 10%, from 21 to 33%, which similarly affected patients treated for paroxysmal AF, persistent AF, and patients with redo PVI for the recurrence of AF after PVI. Thus, in a significant proportion of patients, no freedom from recurrent AF was obtained if continued ADT was considered as an endpoint, although recurrence of AF could not be objectified. The administration of ADT has been considered differently in previous endpoint definitions of freedom from recurrent AF. Varma et al. [20] defined the primary study endpoint as freedom from any documented episode of AF lasting longer than 30 s and occurring after the performance of a single ablation procedure, with or without the use of ADT. The fire-and-ice study by Kuck et al. [4] considered freedom from recurrent AF only if ADT was not administered. In an expert consensus statement on AF [15], the non-administration of ADT was not explicitly mentioned as a requirement to define freedom from AF after PVI. While the impact of the continued use of ADT after PVI is not well proven and is thus not generally recommended, in real-life practice, ADT is continued beyond the blanking period in up to 50% of patients [24]. In the POWDER AF trial [25], a reduced rate of atrial tachyarrhythmia lasting > 30 s during the 12-month post-procedure was documented if ADT was given beyond 90 days after PVI.
4.2 Predictors of recurrent AF at 12-month follow-up
We found that persistent AF, the lowest temperature in the RIPV, and the BMI are predictors of the endpoint. Persistent AF and BMI as predictors of recurrent AF had to be expected.
This study did not include an analysis of the reconnection site in patients with recurrent AF at follow-up and reablation during follow-up. Nonetheless, the results indicate that the RIPV is sensitive to reconnection. There are varying reports on the most frequent sites of reconnection. The right inferior and superior PV have been previously reported [23, 26]. Heeger et al. [26] found in a study on 66 patients with CBA for reablation that the highest electrical reconduction rates were in the RIPV, while Bordignon et al. [23] reported the highest rate of reconduction to be in the right superior PV. There was a trend towards lower CB temperatures in chronically isolated PVs. The time to isolation tended to be higher in PVs with reconnection. It is noteworthy that age was not a predictor of recurrent AF in a univariate and multivariate analysis of predictors for AF recurrence, indicating that elderly patients may well be considered for PVI if symptoms are dominant.
4.3 Impact of hospital procedure volume and board certification status
An association between operator and hospital procedure volume and outcome has been found for several conditions and procedures. However, the volume threshold required for high procedural success is often not well defined; in fact, the volume thresholds varied significantly in a study of 25 procedures and medical conditions [27]. We recently demonstrated that high acute procedural success rates and low complication rates can be achieved with CBA PVI performed at community hospitals with limited case numbers [14]. This study provides an important extension of this observation, as CBA PVI performed at local hospitals is also associated with freedom from symptomatic recurrent AF at a 12-month follow-up at a rate comparable to that of previous reports.
Thus, the results of this study indicate that CBA procedures can be performed with adequate freedom from recurrent AF at a 12-month follow-up at community hospitals that have low to moderate case volumes. Board certification as invasive electrophysiologist was not associated with higher rates of CBA effectiveness regarding freedom from AF at 12-month follow-up compared to non-board certification status.
4.4 Twelve-month complications after CBA PVI
Phrenic nerve palsy is the most frequently observed complication using CBA. The rate of persistent phrenic nerve palsy 12 months or longer after CBA PVI has been described to be between 0 and 6% [24, 28], but there is a lack of data from large studies on the frequency of persistent phrenic nerve palsy at a 12-month follow-up. In a study of 511 consecutive patients, Takuda et al. [29] reported a 0.6% risk of persistent phrenic nerve palsy even at 4 years, which was only related to phrenic nerve palsy occurring during the PVI of the right superior vein. We reported a 4.8% rate of acute phrenic nerve palsy during the intervention and a 0.6% rate of persistent phrenic nerve palsy at the time of hospital discharge in 1004 patients [14]. In this follow-up study, phrenic nerve palsy persisted at the 12-month follow-up in two patients (0.2%). Thus, persistent, or even permanent, phrenic nerve palsy appears to be a potential risk after CBA.
4.5 Limitations
Routine 24-h ECG monitoring was not required as part of the follow-up and has been performed in only 70% of patients at the 12-month follow-up. This distinguishes the study from those with structured follow-up requiring 24-h ECG monitoring and may have resulted in an under detection of recurrent AF in patients without 24-h ECG monitoring at follow-up. Thus, the reported rates of recurrent arrhythmia may be compared with caution to previous studies requiring structured follow-up with requested Holter monitoring. However, the rate of Holter monitoring is similar to that of a large European ESC-EHRA long-term registry, which reported a Holter monitoring rate of 64.5% [24]. Furthermore, there was no significant difference between patients with and without 24-h ECG monitoring in terms of the primary endpoint (21.8% vs. 19.4%). This may be due to the limited sensitivity of 24-h ECG recordings to detect AF [30] and the inclusion of only symptomatic AF patients into the study which might have lowered the rate of clinically undetected asymptomatic AF recurrence to be detected only by Holter monitoring.
Data were entered into the database as they were reported by the centers, and no independent audit was conducted to confirm procedural and outcome data. Data were analyzed for consistency and validity at the data collection center prior to being entered into the database, but there was no independent safety or data monitoring board due to the limited financial resources of this researcher-initiated study. The administration of antiarrhythmic drugs later than 90 days after CBA was documented. No distinction was made between their ongoing administration beyond 90 days after CBA and their new administration for clinical reasons.
4.6 Clinical competencies
The study findings indicate that CBA PVI for paroxysmal or persistent AF can be provided at community hospitals with high 12-month efficacy.
References
Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498.
Van Belle Y, Janse P, Rivero-Ayerza MJ, et al. Pulmonary vein isolation using an occluding cryoballoon for circumferential ablation: feasibility, complications, and short-term outcome. Eur Heart J. 2007;28:2231–7.
Packer DL, Kowal RC, Wheelan KR, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Artic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61:1713–23.
Kuck KH, Brugada J, Fürnkranz A, et al. FIRE and ICE Investigators Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–45.
Lemes C, Wissneer E, Lin T, et al. One-year clinical outcome after pulmonary vein isolation in persistent atrial fibrillation using the second-generation 28 mm cryoballoon: a retrospective analysis. Europace. 2016;18:201–5.
Providencia R, Defaye P, Lambiase PD, et al. Results from a multicentre comparison of cryoballoon vs radiofrequency ablation for paroxysmal atrial fibrillation: is cryoablation more reproducible? Europace. 2017;19:48–57.
Hoffmann E, Straube F, Wegscheider K, et al. Outcomes of cryoballoon or radiofrequency ablation in symptomatic paroxysmal or persistent atrial fibrillation. Europace. 2019;21:1313–24.
Chierchia GB, Di Giovanni G, Ciconte G, et al. Second-generation cryoballoon ablation for paroxysmal atrial fibrillation: 1-year follow-up. Europace. 2014;16:639–44.
Metzner A, Reissmann B, Rausch P, et al. One-year clinical outcome after pulmonary vein isolation using the second-generation 28 mm cryoballoon. Circ Arrhythm Electrophysiol. 2014;7:288–92.
Ciconte G, Ottaviano L, de Asmundis C, et al. Pulmonary vein isolation as index procedure for persistent atrial fibrillation: one-year clinical outcome after ablation using the second-generation cryoballoon. Heart Rhythm. 2015;12:60–6.
Reddy VY, Sediva L, Petru J, et al. Durability of pulmonary vein isolation with cryoballoon ablation: results from the Sustained PV Isolation with Arctic Front Advance (SUPIR) study. J Cardiovasc Electrophysiol. 2015;26:493–500.
Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384:305–15.
Heeger CH, Wissner E, Knoll M, et al. Three-year clinical outcome after 2nd-generation cryoballoon-based pulmonary vein isolation for the treatment of paroxysmal and persistent atrial fibrillation — a 2-center experience. Circ J. 2017;81:974–80.
Hoffmann R, Parade U, Bauerle HJ, et al. Safety and acute efficacy of cryoballoon ablation for atrial fibrillation at community hospitals. Europace. 2021;21:1744–50.
Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–444.
Kuck K, Böcker D, Chun J, et al. Qualitätskriterien zur Durchführung der Katheterablation von Vorhofflimmern. Kardiologe. 2017;11:161–82.
Verma A, Kilicaslan F, Pisano E, et al. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation. 2005;112:627–35.
Nery P, Belliveau D, Nair GM, et al. Relationship between pulmonary vein reconnection and atrial fibrillation recurrence: a systematic review and meta-analysis. J Am Coll Cardiol EP. 2016;2:474–83.
Martins RP, Galand V, Cesari O, et al. The second generation cryoballoon has improved durable isolation of left but not right pulmonary veins: new insights from a multicenter study. Europace. 2018;20:1115–21.
Verma A, Jiang C, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–22.
Fichtner S, Sparn K, Reents T, et al. Recurrence of paroxysmal atrial fibrillation after pulmonary vein isolation: is repeat pulmonary vein isolation enough? A prospective, randomized trial. Europace. 2017;17:1371–5.
Tilz RR, Heeger CH, Wick A, et al. Ten-year clinical outcome after circumferential pulmonary vein isolation utilizing the Hamburg approach in patients with symptomatic drug-refractory paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2018;11:e005250.
Bordignon S, Furnkranz A, Perrotta L, et al. High rate of durable pulmonary vein isolation after second-generation cryoballoon ablation: analysis of repeat procedures. Europace. 2015;17:725–31.
Arbelo E, Brugada J, Blomström-Lundqvist C, et al. Contemporary management of patients undergoing atrial fibrillation ablation: in-hospital and 1-year follow-up findings from the ESC-EHRA atrial fibrillation long-term registry. Eur Heart J. 2017;38:1303–16.
Duytschaever M, Demolder A, Phlips T, et al. Pulmonary vein isolation with vs without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER EF) results from a multicenter randomized trial. Eur Heart J. 2018;39:1429–37.
Heeger CH, Wissner E, Mathew S, et al. Once isolated, always isolated? Incidence and characteristics of pulmonary vein reconduction after second-generation cryoballoon-based pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2015;8:1088–94.
Nimptsch U, Mansky T. Hospital volume and mortality for 25 types of inpatient treatment in German hospitals: observational study using complete national data from 2009 to 2014. BMJ Open. 2017;7:e016184. https://doi.org/10.1136/bmjopen-2017-016184.
Okishige K, Aoyagi H, Nishimura T, et al. Left phrenic nerve injury during electrical isolation of left-sided pulmonary veins with the second-generation cryoballoon. Pacing Clin Electrophysiol. 2017;40:1426–31.
Tokuda M, Yamashita S, Sato H, et al. Long-term course of phrenic nerve injury after cryoballoon ablation of atrial fibrillation. Sci Rep. 2021;11:6226. https://doi.org/10.1038/s41598-021-85618-3.
Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–86.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was obtained by the Ärztekammer Niedersachsen and subsequently by the local ethical committees of each federal state that participated in the study.
Conflict of interest
Dr. Bauerle reports speaker fees and consulting fees by Medtronic and Boston Scientific, both companies providing medical supply for pulmonary vein ablation procedures. All other authors do not have any conflicts of interest to be disclosed.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Michaelsen, J., Parade, U., Bauerle, H. et al. Twelve-month efficacy of second-generation cryoballoon ablation for atrial fibrillation performed at community hospitals: results of the German register on cryoballoon ablation in local hospitals (regional). J Interv Card Electrophysiol 66, 417–425 (2023). https://doi.org/10.1007/s10840-022-01331-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-022-01331-9