Abstract
Background
The second-generation cryoballoon ablation (CB-A) has been proven to be safe and effective for pulmonary vein (PV) isolation. Little is known regarding the long-term outcome following CB-A ablation for paroxysmal atrial fibrillation (AF). The aim of the study was to evaluate the freedom from atrial arrhythmias during a 5-year follow-up period among consecutive patients having undergone PV isolation with the CB-A for paroxysmal AF
Methods and results
A total of 208 consecutive patients having undergone index PV isolation using CB-A (138 males, 66%; mean age 59.0 ± 12.6 years) were included in our retrospective analysis. Follow-up was based on outpatient clinic visits including Holter electrocardiograms. Recurrence of atrial tachyarrhythmias was defined as a symptomatic or documented episode of > 30 s. At a median follow-up of 62 months, freedom from atrial arrhythmias after a single procedure was achieved in 57.2% of patients. Multivariate analysis demonstrated that obesity, left atrial diameter, and duration of symptoms before AF ablation were independent predictors of ATas recurrences. Major complications occurred in 2.4% of patients.
Conclusions
The present study found a 5-year single-procedure success rate of 57.2% following CB-A ablation procedure. Obesity, higher LA dimensions, and longer duration of symptoms before ablation independently predicted the outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pulmonary vein (PV) isolation is currently the cornerstone of percutaneous transcatheter ablation for drug-resistant paroxysmal atrial fibrillation (AF) [1]. In the last years, cryoballoon (CB) ablation has emerged as a valid alternative to radiofrequency (RF) ablation [2, 3]. The second-generation cryoballoon (CB-A; Arctic Front Advance, Medtronic) has been released with technical developments resulting in a larger and more homogeneous zone of freezing on the balloon surface, leading to significant improvements in procedural and clinical outcomes as compared with the first-generation device [4].
To the best of our knowledge, data on long-term follow-up following PV isolation with the CB-A technology is sparse [5]. The present study sought to analyse the clinical results of patients having undergone ablation with CB-A in our centre for paroxysmal AF over a 5-year follow-up period.
2 Methods
2.1 Patient population
All patients referred our electrophysiology centre of UZ Brussel (Brussels, Belgium) from October 2012 were retrospectively analysed. Consecutive patients with drug-refractory paroxysmal AF scheduled for second-generation CB ablation were included in our analysis. Atrial fibrillation was defined as paroxysmal if recurrent AF (≥ 2 episodes) spontaneously interrupted within 7 days or AF episodes were terminated by electrical or pharmacological cardioversion within 48 h after AF onset.
The protocol was carried out in accordance with the ethical principles for medical research involving human subjects established by Helsinki’s declaration, protecting the privacy of all the participants as well as the confidentiality of their personal information.
2.2 Pre-procedural management
All patients provided written informed consent prior to the procedure. To exclude the presence of thrombi in the left atrial appendage, all patients underwent trans-oesophageal echocardiography (TEE) the day before the procedure, along with trans-thoracic examination (TTE) enabling assessment of left atrial (LA), left ventricle, and valvular dimensions/function. Also, patients underwent a pre-procedural computed tomography (CT) to assess left atrium and PV anatomy. The pre-procedural imaging was used as a roadmap for the procedure and for the detection of eventual unconventional venous drainage patterns such as adjunctive veins or early branching. The exclusion criteria were any contraindication for the procedure including the presence of an intracavitary thrombus, uncontrolled heart failure, and contraindications to general anaesthesia.
2.3 Ablation procedure
All procedures were performed under general anaesthesia and using short-acting neuromuscular blocking drugs for endotracheal intubation. After having achieved left atrial access with a single transseptal puncture, a 100 IU/kg heparin IV bolus was given. A 0.32 French Emerald exchange wire (Cordis, Johnson and Johnson, Diamond Bar, CA, USA) was advanced in the left superior PV, and a steerable 15 French over-the-wire sheath (FlexCath, Cryocath, Medtronic, USA) was positioned in the left atrium (LA). A 20-mm diameter Achieve inner lumen mapping catheter (ILMC) (Medtronic, Minnesota, USA) was sequentially positioned in each PV ostium to obtain baseline electrical information. Then a 28-mm double-walled cryoballoon (Artic Front or Arctic Front Advance, Cryocath) was advanced over the ILMC up to the LA, inflated, and positioned in the PV ostium of each vein. Optimal vessel occlusion was considered to have been achieved when selective dye injection showed total contrast retention with no backflow to the atrium. Once occlusion is documented, cryothermal energy commenced. A single 3-min freeze was applied for each vein and a second freeze was delivered in the case of failure to isolate the PV after the first cycle, in the case of nadir temperature greater than − 35 °C, and in the occurrence of early PV reconnection. The left-sided veins were treated first, with left superior pulmonary vein (LSPV) first and then left inferior pulmonary vein (LIPV).
In the case of phrenic nerve palsy (PNP), cryoenergy application was aborted and recovery of diaphragmatic contraction was carefully monitored for 30 min. Pulmonary vein isolation was documented with the help of a dedicated ILMC (Achieve, Medtronic, USA), evaluating the electrical activity in the PVs. During the whole procedure, activated clotting time was maintained over 250 s by supplementing heparin infusion as required.
2.4 Phrenic nerve monitoring
In order to avoid PNP, a standard decapolar catheter was placed in the superior vena cava in order to pace the right phrenic nerve (PN) (at 20 mA/1.0-ms pulse width at a cycle length of 1200 ms) during ablation of the right-sided PVs. The reason to pace at such a slow rate is to prevent catheter displacement, due to diaphragmatic contraction in the early phases of cryoenergy application. Phrenic nerve capture was achieved when contraction of the right hemidiaphragm could be observed under fluoroscopic imaging and by manual palpation of the abdomen. If PN contraction resumed during the procedure, the latter was defined as transient PNP. Conversely, if PNP persisted at the end of the procedure, this complication was defined as persistent.
2.5 Assessment of electrical isolation
Pulmonary vein activity was recorded with the ILMC at a proximal site in the ostium prior to ablation in each vein. If pulmonary vein potentials (PVPs) were visible during cryoenergy application, time to isolation was recorded when PVPs completely disappeared or were dissociated from LA activity. If PVPs were not visible during ablation due to a distal positioning of the ILMC, the latter was immediately retracted after completion of the freeze–thaw cycle to a more proximal position in which PVPs had been recorded prior to ablation to record their presence or absence. If needed, pacing from the distal or proximal coronary sinus catheter was performed to distinguish far-field atrial signals from PVP recorded on the mapping catheter, for left- and right-sided veins, respectively.
2.6 Post-ablation management
All patients were discharged the day following ablation. A pre-discharge TTE was performed in all individuals to exclude pericardial effusion. Oral anticoagulation was started the same evening of ablation and continued for at least 3 months. Anti-arrhythmic therapy was administered for 3 months following the procedure and discontinued if the patient was free of AF relapse.
2.7 Follow-up
After discharge from the hospital, patients were scheduled for follow-up visits at 1, 3, 6, 12 months and biannually thereafter. A 12-lead ECG and 24-h-Holter monitoring were reviewed during the clinical evaluations; previously implanted pacemakers and cardioverter-defibrillators were interrogated during the visits.
All reports of Holter monitoring or ECG recordings having been performed in referring centres were sent to the Heart Rhythm Management Centre (UZ Brussels, Brussels, Belgium) for diagnosis confirmation during the follow-up. Furthermore, patients were also contacted by telephone during the follow-up. For patients reporting symptoms outside of the scheduled routine follow-up, a 12-lead ECG and/or 24-h-Holter or interrogation of implanted devices were performed for diagnosis confirmation. Recurrences were defined as symptomatic and/or documented episodes of atrial tachyarrhythmias lasting more than 30 s.
2.8 Statistical analysis
Categorical variables are expressed as absolute and relative frequencies. Continuous variables are expressed as mean ± SD or median and range as appropriate. Event-free survival was estimated by the method of Kaplan–Meier and compared by the log-rank test. Predictors of arrhythmia recurrence were assessed using the Cox proportional hazards regression models. The multivariate prediction models for time to recurrence after the final ablation procedure were performed by stepwise regression based on likelihood ratios. Variables which showed a significant association of p < 0.1 were included into the multivariate prediction model. For each variable, hazard ratio (HR), 95% confidence interval (CI), and Wald test p values of the final model are displayed. A two-tailed probability value ≤ 0.05 was deemed significant. Statistical analyses were conducted using the SPSS software (SPSS v20, Chicago, IL, USA).
3 Results
3.1 Baseline characteristics
A total of 208 consecutive patients (138 males, 66%; mean age 59.0 ± 12.6 years) having undergone PV isolation with CB-A were included. The patients’ baseline characteristics are listed in Table 1.
3.2 Procedural characteristics
All CB-A procedures were performed with a large 28-mm balloon and PV isolation was successfully achieved in all veins without the need of additional focal catheter applications. In particular, mean procedural time and mean fluoroscopy time were 64.9 ± 17.7 and 16.8 ± 8.3 min, respectively. The mean number of freeze-thaw cycles was 1.3 ± 0.4 for the LSPV, 1.3 ± 0.3 for the LIPV, 1.2 ± 0.3 for the right inferior pulmonary vein (RIPV), and 1.2 ± 0.3 for the right superior pulmonary vein (RSPV). The mean minimal temperature achieved was − 52.9 ± 5.3 °C during LPSV ablation, − 49.3 ± 5.5 °C during LIPV ablation, − 50.2 ± 4.7 °C during RIPV ablation, and − 53.9 ± 5.4 °C during RSPV ablation.
3.3 Complications
Five patients (2.4%) experienced procedure-related major complications. A detailed list of complications is shown in Table 2. One cardiac tamponade occurred (0.48%) successfully treated with pericardiocentesis; 1 transient ischemic attack (0.48%) was diagnosed a few hours after an ablation procedure; 2 femoral pseudoaneurysms (0.96%) were found both requiring the surgical closure. A total amount of 17 PNPs (8.1%) were observed; among them, all were transient (94.1%) except 1 (5.9%) that completely recovered in 1 month.
3.4 Follow-up
A total of 808 PVs were found: 184 left superior PVs, 184 left inferior PVs, 24 left common PVs, 208 right inferior PVs, 208 right superior PVs. Ninety-nine percent of all PVs (802/808) were successfully isolated during the first ablation procedure. Because of PNP during the right inferior PV ablation, 6/208 (2.9%) ipsilateral right superior PVs were not targeted for ablation. Clinical follow-up could be obtained in all patients. All patients completed at least two Holter ECG recordings during the follow-up, and more than 80% completed all the scheduled 24-h ECG monitorings.
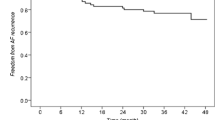
After a median follow-up of 62 months (mean duration 60.9 ± 6.7 months) including a 3-month blanking period, 119/208 (57.2%) patients remained in stable sinus rhythm after a single index procedure of CB-A ablation, off antiarrhythmic drugs (Fig. 1).
Repeat ablation was offered to all patients with symptomatic recurrence of atrial tachyarrhythmias (n = 89) and eventually performed in 46/89 (51.7%) patients after a median of 10 (2–34) months following index procedure. Among 178 PVs, including 6 left common PVs, 51 (28.7%) showed a late PV reconnection in 33 patients (1.55 per patient), at the time of repeat ablation procedure. Overall, persistent PV isolation could be documented in 127 of 178 PVs (71.3%). In 13 of 46 patients (28%), persistent isolation could be demonstrated in all PVs, whereas PV reconnection could be documented in 33 of 46 patients (72%). None of the patients exhibited reconnection in all 4 veins. According to the PV location, different rates of persistent isolation could be documented: 31 LSPVs (77.5%), 30 left inferior PVs (75%), 34 right superior PV (73.9%), 32 right inferior PVs (69.6%). Re-isolation of all PVs was successfully performed in 36/36 (100%) patients using the radiofrequency technology. Linear lesions for the treatment of left atrial flutters and tachycardias were deployed in 7/46 (15.2%) patients (n = 3 mitral isthmus line, n = 3 roof line, n = 1 anterior line). In 3 patients (6.5%), ablation of complex fractionated atriale electrogram (CFAE) was performed. After a median follow-up duration of 21 months (11–30 months) including a 3-month blanking period, 30/46 (65.2%) still showed recurrences of atrial tachyarrhythmias following repeat ablation; therefore, after a repeat procedure, the freedom from recurrence of atrial tachyarrhythmias accounted for 64.9% (135 of 208 patients).
A third procedure was offered to those patients who still had recurrences of atrial arrhythmias after the second procedure (n = 30) and performed in 4/30 (13.3%) patients. In 2 patients (50%), resumption of PV conduction was found and re-isolation of all PVs was successfully performed using irrigated radiofrequency technology. In the remaining 2 patients (50%), left atrial flutters were still detected and underwent successful roof line ablation.
3.5 Predictors of arrhythmia recurrence
Analysing the patients’ baseline characteristics, body mass index (hazard ratio, 1.068; 95% CI 1.019–1.118; p = 0.006), duration of symptoms (hazard ratio, 1.004; 95% CI 1.001–1.008; p = 0.016), and left atrial diameter (hazard ratio, 1.055; 95% CI 1.022–1.088; p = 0.001) were identified as predictors for AF recurrence at the univariate analysis (Table 3). Multivariate analysis confirmed body mass index (hazard ratio, 1.067; 95% CI 1.010–1.127; p = 0.021), duration of symptoms (hazard ratio, 1.004; 95% CI 1.001–1.007; p = 0.047), and left atrial diameter (hazard ratio, 1.049; 95% CI 1.012–1.086; p = 0.008) as all independent predictors for AF recurrence (Table 3).
4 Discussion
The main findings of the present study are that (1) the 5-year single-procedure success rate in patients having undergone CB-A ablation for paroxysmal AF was 57.2% off antiarrhythmic drugs; (2) the incidence of major complications is relatively low (2.4%); (3) obesity, duration of symptoms, and left atrial diameter were independently associated with higher risk of AF recurrence.
Several previous studies have already demonstrated the safety and efficacy of CB-A in the setting of paroxysmal AF ablation [1,2,3,4,5]. The main randomized trials have already shown that CB ablation is non-inferior to RF ablation in terms of safety and efficacy in patients with paroxysmal AF [2, 3]. Previous 3-year follow-up analyses showed a freedom from AF after a single CB-A ablation procedure ranging from 60.2 to 71.5% without antiarrhythmic drugs which might be explained by high rate of durability following PV isolation [6,7,8]. In detail, a recent, prospective, multicentre, nonrandomized study conducted on 344 patients having undergone PV isolation using CB-A technology found a freedom from all atrial arrhythmias of 64% over a 3-year follow-up [8].
In a prospective, observational study of 178 patients, Akkaya et al. [5] reported the first 5-year follow-up results following CB-A ablation procedures. The authors demonstrated a single-procedure success rate after CB-A in paroxysmal AF of 61% adopting a 4-min, double-freeze strategy in most of patients (the first 168 treated with a double freeze strategy and the last 11 patients with a single-freeze approach). They also found through the Cox regression analysis that left atrial area and the absence of diabetes independently predicted AF recurrences [5]. Although no studies having addressed a direct comparison between CB-A and RF on a long-term follow-up are available, however, long-term studies following RF ablation in paroxysmal AF showed single-procedure success rate ranging from 46.6 to 58.7% [9,10,11], thus demonstrating comparable efficacy to CB-A. Our findings over a median follow-up of 62 months showed a single-procedure success rate of 57.2% without antiarrhythmic drugs, using a 3-min single-freeze approach. This long-term success rate increased to 65.9% following a mean of 1.26 procedures.
The most frequent periprocedural complication was represented by PNP, detected in 8.1% of cases, a slightly higher rate than previously reported [6, 7], but comparable with initial experiences after marked release of CB-A. All the other complications were comparable with those previously reported in literature [12, 13]. No late or unexpected complications occurred at the 5-year follow-up.
The identification of simple, reliable predictors of clinical outcomes after PVI with the CB-A is challenging because of the complexity of AF and independent predictors of 5-year outcomes after PV isolation have not been still reported for the CB-A. In a meta-analysis, D’Ascenzo et al. [14] reported that persistent AF, higher LA diameter, and early AF recurrence within 1 month following the procedure were the most powerful predictors of AF ablation failure. Our findings showed that obesity, duration of symptoms before ablation, and higher LA diameter were independent predictors of AF recurrence over our long-term follow-up. Left atrial dilatation is already known as one of the most important predictors of AF recurrence following PV isolation [15]. Increased LA dimensions might be already associated with atrial remodelling and interstitial fibrosis, which may also contribute to the increased incidence of AF recurrence [15]. Higher LA dimensions may result in marked atrial heterogeneity allowing propagation and development of reentry circuits.
Our results also found that obesity represents an independent predictor of AF recurrence following PV isolation. In animal models, obesity is associated with the presence of inflammation infiltrates in the left atrium along with lipidosis, increased conduction velocity and conduction dispersion, increased expression of endothelin A and B, endothelin 1, and platelet growth factor [16]. However, the literature did not find consistent findings about this relationship and this still remains a controversial issue [16]. Two recent studies respectively including 774 and 474 patients with a mean follow-up of 3.0 ± 1.9 years and 30 ± 13 months, respectively, which performed atrial fibrillation ablation pursuing the complete elimination of all non-PV triggers, did not find any significant association between body mass index and recurrences of atrial tachyarrhythmias [17, 18].
A more prolonged duration of AF before the ablation has been also found as a significant, independent predictor of recurrence post-PV isolation. This might probably explained by the important modifications occurring both in the structure and in the electrical properties of the atrial tissue; these electrical alterations together with LA dilatation lead to shortened atrial effective refractory periods (ERPs), increased spatial heterogeneity of ERPs, and loss of normal ERP rate adaptation resulting in non-uniform conduction slowing and increased dispersion in refractoriness in the atrial tissue [19].
4.1 Limitations
The present study includes data from a single centre enrolling only a limited amount of patients in a retrospective fashion. Patients were followed up by clinical consultations and 24-h Holter monitoring. The latter might have overestimated the overall success rate and a closer follow-up using inner loop recorders might hypothetically have found higher rates of recurrences. Moreover, redo procedures did not routinely include the search of triggers from extra-PV sites and isoproterenol or adenosine was not generally used to unmask the extra-PV foci. Targeting non-PV foci might have led to more favourable results following the repeat procedures.
5 Conclusions
The present study found a 5-year single-procedure success rate of 57.2% following CB-A ablation procedure. Major complication occurred in 2.4% of patients. Obesity, higher LA dimensions, and longer duration of symptoms before ablation were independent predictors of AF recurrence after the ablation procedure.
References
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–78.
Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR, et al. Tondo C; FIRE AND ICE Investigators. N Engl J Med. 2016;374:2235–45.
Hoffmann E, Straube F, Wegscheider K, Kuniss M, Andresen D, Wu LQ, et al. Europace. 2019;21:1313–24.
Giovanni GD, Wauters K, Chierchia GB, Sieira J, Levinstein M, Conte G, et al. One-year follow-up after single procedure cryoballoon ablation: a comparison between the first and second generation balloon. J Cardiovasc Electrophysiol. 2014;25:834–9.
Akkaya E, Berkowitsch A, Zaltsberg S, Greiss H, Hamm CW, Sperzel J, et al. Five-year outcome and predictors of success after second-generation cryoballoon ablation for treatment of symptomatic atrial fibrillation. Int J Cardiol. 2018;266:106–11.
Takarada K, Overeinder I, de Asmundis C, Stroker E, Mugnai G, de Regibus V, et al. Long-term outcome after second-generation cryoballoon ablation for paroxysmal atrial fibrillation – a 3-years follow-up. J Interv Card Electrophysiol. 2017;49:93–100.
Heeger CH, Wissner E, Knoll M, Knoop B, Reissmann B, Mathew S, et al. Three-year clinical outcome after 2nd-generation cryoballoon-based pulmonary vein isolation for the treatment of paroxysmal and persistent atrial fibrillation- a 2-center experience. Circ J. 2017;81:974–80.
Knight BP, Novak PG, Sangrigoli R, Champagne J, Dubuc M, Adler SW, et al. Long-term outcomes after ablation for paroxysmal atrial fibrillation using the second-generation cryoballoon: final results from STOP AF post-approval study. JACC Clin Electrophysiol. 2019;5:306–14.
Gokoglan Y, Mohanty S, Gunes MF, Trivedi C, Santangeli P, Gianni C, Asfour IK, Bai R, Burkhardt JD, Horton R, Sanchez J, Hao S, Hongo R, Beheiry S, Di Biase L, Natale A. Pulmonary vein antrum isolation in patients with paroxysmal atrial fibrillation: more than a decade of follow-up. Circ Arrhythm Electrophysiol 2016: 9(5).
Ouyang F, Tilz R, Chun J, Schmidt B, Wissner E, Zerm T, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368–77.
Tilz RR, Heeger CH, Wick A, Saguner AM, Metzner A, Rillig A, et al. Ten-year clinical outcome after circumferential pulmonary vein isolation utilizing the Hamburg approach in patients with symptomatic drug-refractory paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2018;11(2):e005250.
Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–8.
Mugnai G, Irfan G, de Asmundis C, Ciconte G, Saitoh Y, Hunuk B, et al. Complications in the setting of percutaneous atrial fibrillation ablation using radiofrequency and cryoballoon techniques: a single-center study in a large cohort of patients. Int J Cardiol. 2015;196:42–9.
D’Ascenzo F, Corleto A, Biondi-Zoccai G, Anselmino M, Ferraris F, Di Biase L, et al. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation?: a meta-analysis. Int J Cardiol. 2013;167:1984–9.
Jalife J, Kaur K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc Med. 2015;25:475–84.
Winkle RA, Mead RH, Engel G, Kong MH, Fleming W, Salcedo J, et al. Impact of obesity on atrial fibrillation ablation: patient characteristics, long-term outcomes, and complications. Heart Rhythm. 2017;14:819–27.
Wokhlu A, Hodge DO, Monahan KH, Asirvatham SJ, Friedman PA, Munger TM, et al. Long-term outcome of atrial fibrillation ablation: impact and predictors of very late recurrence. J Cardiovasc Electrophysiol. 2010;21:1071–8.
Miyazaki S, Kuwahara T, Kobori A, Takahashi Y, Takei A, Sato A, et al. Preprocedural predictors of atrial fibrillation recurrence following pulmonary vein antrum isolation in patients with paroxysmal atrial fibrillation: long-term follow-up results. J Cardiovasc Electrophysiol. 2011;22:621–5.
Mugnai G, Chierchia GB, de Asmundis C, Julia J, Conte G, Sieira-Moret J, et al. P-wave indice sas predictors of atrial fibrillation recurrence after pulmonary vein isolation in normal left atrial size. J Cardiovasc Med (Hagerstown).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The protocol was carried out in accordance with the ethical principles for medical research involving human subjects established by Helsinki’s declaration, protecting the privacy of all the participants as well as the confidentiality of their personal information.
Conflict of interest
GBC and CdA receive compensation for teaching purposes and proctoring from AF solutions, Medtronic. PB receives research grants on behalf of the centre from Biotronik, Medtronic, St Jude Medical, Sorin, Boston Scientific and speaker’s fees from Biosense-Webster, Biotronik, and Medtronic. CdA is consultant for Daiichi Sankyo. SB: consultant fees from Medtronic, Boston Scientific, Microport, and Zoll. GM received an educational grant from Medtronic for Postgraduate in Cardiac Electrophysiology and Pacing academic course.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mugnai, G., Paparella, G., Overeinder, I. et al. Long-term clinical outcomes after single freeze cryoballoon ablation for paroxysmal atrial fibrillation: a 5-year follow-up. J Interv Card Electrophysiol 61, 87–93 (2021). https://doi.org/10.1007/s10840-020-00788-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-020-00788-w