Abstract
Introduction
Patients with persistent atrial fibrillation (AF) may additionally suffer from a concealed sinus node disease. We sought to determine the incidence, indications and predictors of acute pacemaker [PM] implantation within 1 week after the ablation of persistent AF.
Methods and results
We performed a retrospective analysis of patients, who had had an ablation of persistent AF at our center. Between 01/2011 and 08/2016, 1234 patients (mean age 65 ± 10 years, 66.7% male) without prior PM implantation underwent an ablation of persistent AF. Pulmonary vein isolation (PVI) was performed in 1158 (93.8%), the additional ablation of complex fractionated atrial electrograms (CFAE) in 1109 (89.9%) and linear ablation in 524 (42.5%) patients. Temporary cardiac pacing was necessary in 27 (2.2%) patients. The temporary PM was removed in 15 patients (1.2%) because sinus node recovered after a median of 1.0 (minimum 0.1−maximum 2.0) day. The remaining 12 (1.0%) patients required the implantation of a permanent PM. Another 13 (1.1%) patients required permanent PM implantation without prior temporary pacing. In a multivariable regression model, age [OR 1.07 (1.02–1.12), p = 0.006], sinus pauses prior to ablation [OR 7.97 (2.36–26.88), p = 0.001] and atria with low voltage [OR 2.83 (1.31–6.11), p = 0.008] were identified as significant predictors for acute cardiac pacing.
Conclusion
Acute cardiac pacing within 1 week after the ablation of persistent AF was necessary in 40 (3.2%) patients. Age, sinus pauses in history prior to ablation and the existence of low-voltage areas in the atria were identified as relevant risk factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Persistent atrial fibrillation (AF) is a common disease worldwide, accounting for an increased morbidity and mortality [1,2,3,4]. At present, mainly two different therapeutic options exist: the control of the heart rate in AF (frequency control strategy) and the restoration of sinus rhythm (rhythm control strategy). As several studies in the past have shown beneficial effects of a rhythm control strategy on several clinical factors, such as quality of life or left ventricular ejection fraction, the ablation of persistent AF has become a frequently used approach to treat this disease [5, 6]. The complete isolation of the pulmonary veins (PVI) is usually performed as a first step in patients suffering from persistent AF. Additional modification of substrate, such as complex fractionated atrial electrograms (CFAE), the ablation of rotors or the ablation of lines, has been described in several publications as a successful method to terminate AF during ablation [7,8,9,10].
Sinus node disease and AF have been shown to be associated with each other [11,12,13]. A preexisting sinus node disease in a patient suffering from persistent AF may only be unmasked after the termination of AF during the ablation procedure, necessitating temporary cardiac pacing and/or the implantation of a permanent PM. Some authors additionally presume the occurrence of a sinus node arrest or disease to be a complication of the ablation itself, due to ablation of the sinus node area or indirect damage of the sinus node artery [14, 15].
Aim of the study
The aim of the study was to retrospectively analyze the incidence and indications as well as the predictors of acute PM implantation within 1 week after ablation of persistent AF.
Methods
The study is a monocentric retrospective analysis, which included all patients who underwent an ablation of persistent and long-standing persistent AF at our institution between March 2011 and August 2016. Persistent AF was defined, according to the guidelines valid at that time, as an AF episode, which either lasts longer than 7 days or requires termination by cardioversion, either with drugs or by direct current cardioversion [16]. Long-standing persistent AF was defined, according to the guidelines valid at that time, as AF which has lasted for ≥ 1 year when it is decided to adopt a rhythm control strategy [16]. In patients undergoing several ablation procedures for AF within the study period, the last ablation procedure was defined as index procedure and was included in the analysis. Previous ablation procedures within the study period were defined as such and are incorporated in the baseline characteristics. Patients with chronically implanted PM or chronically implanted cardioverter defibrillators (ICD) were excluded from the analysis. The study protocol was approved by the ethics committee of the Technische Universität München, Munich, as the leading ethics committee for the German Heart Center Munich.
Ablation procedure
At our institution, the ablation procedure for AF is generally performed under conscious sedation and uninterrupted oral anticoagulation. A 3D-mapping system is used for every ablation of persistent AF, the manufacturer (NavX Ensite, Abbott Vascular; CARTO 3, Biosense Webster) is chosen by the physicians. Intracardiac electrograms are recorded on the BARD recording system (Boston Scientific Corporation). The procedure is undertaken in AF. After fluoroscopy-guided and pressure-guided transseptal puncture, heparin is administered, targeting a value of 300 s activated clotting time. A voltage map and a map of complex fractionated atrial electrograms of the left atrium and, if the ablation location, of the right atrium and coronary sinus are routinely performed at the beginning of every procedure using a multipolar catheter (Lasso Nav eco, Biosense Webster Inc.; Orbiter PV, Boston Scientific Corporation). The existence of low-voltage areas in the mapped anatomical structures is defined and notated by the operating physician on the basis of the 3D-voltage map and the corresponding electrograms. Low voltage is defined as atrial signal amplitude below 0.1 mV. Cycle length prior to ablation is measured in the left atrial appendage. A 4-mm irrigated tip catheter is used for radiofrequency ablation applying energies between 25 and 40 W, depending on the anatomical site and the performing physician. After circumferential pulmonary vein isolation, the ablation of complex fractionated atrial electrograms is performed until abolishment of all CFAE or until the lengthening of the atrial cycle length > 10% or until regularization to atrial flutter. Regarding the first two instances, external cardioversion is performed, pulmonary vein isolation is confirmed in sinus rhythm and the procedure is terminated. In the case of atrial macroreentrant flutter, suitable atrial lines are deployed to terminate the arrhythmia and bidirectional blockage is proven. In the case of a localized reentry, the origin of the arrhythmia is located and subsequently ablated. All antiarrhythmic drugs, except class II, are stopped after the ablation. During the entire hospital stay, the patient’s heart rhythm is continuously monitored.
Temporary cardiac pacing
In the case of sinus node or atrioventricular (AV) node dysfunction after the termination of AF, the insertion of a temporary PM wire is considered necessary, if the patient is hemodynamically compromised or unstable due to sinus arrest, complete AV block without escape rhythm or due to hemodynamically insufficient intrinsic rhythm. After the insertion of a temporary PM wire via femoral or jugular veins, the patient is transferred to the intensive care unit, where he is looked after as long as required. A continuous monitoring of the heart rhythm is ensured during the entire hospital stay.
Statistics
Data analysis was performed using the software package IBM SPSS Statistics for Windows, version 22 (IBM Corp., Armonk, N.Y., USA). For quantitative measures, means and standard deviations or medians and ranges (minimum to maximum) are shown. For categorical outcomes, absolute and relative frequencies are presented. For the assessment of the association between the need for acute pacing and recurrence of AF, chi-square test was used. Univariate and multivariable logistic regression models were fitted to the data to evaluate associations between baseline data and acute pacemaker implantation and to assess independent predictor variables. Variables with p < 0.05 in the univariate analyses were included in the multivariable regression model. Odds ratios (OR) are presented with 95% confidence intervals. All statistical tests were performed two sided on a significance level of α = 5%.
Results
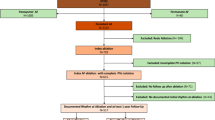
A total of 1363 patients underwent an ablation for persistent AF at our institution between March 2011 and August 2016. In 129 of these patients, a permanent PM or ICD was already chronically implanted, so that these patients were excluded from the analysis. The baseline characteristics of the remaining 1234 patients are demonstrated in Table 1; the procedural data are demonstrated in Table 2.
Incidence of acute cardiac pacing
The insertion of a temporary PM wire and/or the implantation of a permanent PM was necessary in 40 (3.2%) patients within 7 days after the ablation of persistent AF.
Of these, 27 patients (67.5% of patients in need of acute cardiac pacing and 2.2% of the overall study population) required temporary cardiac pacing during the ablation procedure or immediately thereafter due to acute sinus arrest (n = 10), severe sinus bradycardia (n = 16) or second-degree AV block type 2 (n = 1). After a median of 1.0 day (minimum 0.1−maximum 2.0 days; mean ± STD 0.9 ± 0.4 days), the temporary PM wire could be removed in 15 (37.5% of patients in need of acute cardiac pacing and 1.2% of the overall study population) of these patients because sinus node recovered from arrest (n = 4) or from sinus bradycardia (n = 11). The remaining 12 patients (30% of patients in need of acute cardiac pacing and 1.0% of the overall study population) required the implantation of a permanent pacemaker after a median of 1.0 day (min. 0.1−max. 5.0 days; mean ± STD 1.6 ± 1.6 days) due to persistent sinus arrest (n = 6), persistent sinus node bradycardia (n = 5) and second-degree AV block type 2 (n = 1). All 12 patients received a dual-chamber pacemaker.
Another 13 patients (32.5% of patients in need of acute cardiac pacing and 1.1% of the overall study population) required a permanent PM implantation within the first week after ablation without prior temporary cardiac pacing. The indications for permanent PM implantation in these patients, becoming evident during continuous monitoring of the heart rhythm after the ablation of persistent AF, were intermittent sinus arrest (n = 3), sustained symptomatic sinus bradycardia (n = 1), second-degree AV block type 2 (n = 3), third-degree AV block (n = 1) and binodal disease (n = 5). All 13 patients received a dual-chamber pacemaker after a median of 2.0 days (min 1.0 – max. 6.0 days; mean ± STD 2.7 ± 1.6 days) after the ablation procedure.
28 patients (28/40, 70.0%) with an indication for acute cardiac pacing were chronically treated with beta blocker prior to admission. Beta-blocker therapy was stopped in all patients as soon as there was an indication for acute or permanent pacing and resumed according to the treating physicians’ decision. One patient in need for temporary cardiac pacing received a single dose of intravenous Amiodarone during ablation to support the conversion to sinus rhythm.
Figure 1 shows a detailed overview of the incidence and indications for temporary cardiac pacing and permanent PM implantation within the first week after ablation of persistent AF.
Associations/predictors
Age [odds ratio 1.09 (95% confidence interval: 1.05–1.14), p < 0.0001], renal insufficiency [OR 2.99 (1.34–6.69), p = 0.008], mitral valve insufficiency [OR 2.12 (1.10–4.08), p = 0.025], beta-blocker therapy [OR 0.32 (0.16–0.65), p = 0.001], sinus bradycardia lower than 50 beats per minute in history prior to ablation [OR 4.29 (2.03–9.06), p = 0.0001], sinus pauses longer than 3 s in history prior to ablation [OR 10.34 (3.91–27.39), p < 0.0001], atria with low voltage [OR 4.33 (2.20–8.50), p < 0.0001] and cycle length prior to ablation [OR 1.01 (1.00–1.02), p = 0.006] were significantly associated with acute cardiac pacing after the ablation of persistent AF. In a multivariable regression model, age [OR 1.07 (1.02–1.12), p = 0.006], sinus pauses longer than 3 s in history prior to ablation [OR 7.97 (2.36–26.88), p = 0.001] and atria with low voltage [OR 2.83 (1.31–6.11), p = 0.008] were identified as significant independent variables. The complete results of the univariate and multivariable analyses are shown in Table 3.
Recurrence of atrial arrhythmias within 1 week after ablation
Appropriate electrocardiographic data were available for 1225 patients. Almost all patients (36/40, 90%) in need of temporary or permanent cardiac pacing within 1 week after ablation of persistent AF and 284 (284/1185, 24.0%) patients not in need of acute cardiac pacing suffered a recurrence of AF and/or atypical atrial flutter during the same hospital stay (p < 0.0001).
Discussion
Data concerning the incidence, indications and predictors for acute cardiac pacing after the ablation of persistent atrial fibrillation are scarce. The main findings of our study are (1) acute cardiac pacing was necessary in roughly 3% of patients in our study population within 1 week after the ablation of persistent AF. (2) More than two-third of these patients were in need of a temporary cardiac pacing wire immediately after the ablation procedure, which could be removed in more than half of the patients after the median of 1 day due to recovery of sinus node function. (3) In 2% of the study population, the implantation of a permanent PM was finally necessary within 1 week after the ablation procedure. Whereas the main indication for temporary cardiac pacing was sinus node disease, the main indication for the implantation of a permanent PM was binodal disease. (4) Most relevant risk factors for acute cardiac pacing were older age, sinus pauses longer than 3 s in history prior to ablation and the existence of areas of low voltage in any of the atria prior to ablation.
In contrast to other study populations, our population involved exclusively patients with persistent and long-standing persistent AF. The ablation procedure in almost all of the patients consisted of bilateral circumferential vein isolation and the ablation of CFAE. Half of the patients were additionally treated with the ablation of lines (anterior line/mitral isthmus line and/or roofline and/or right atrial isthmus line). The routine isolation of the vena cava superior was not performed. Our study confirms the rather low incidence of acute cardiac pacing after the ablation of persistent AF, following the above-mentioned ablation strategy. Previous studies have shown a 0.7–10% incidence of acute cardiac pacing after ablation in variant populations, however, considering diverse kinds of atrial arrhythmias and diverse kinds of ablation strategies [14, 17, 18].
In our study population, two-third of the 40 patients requiring cardiac pacing after the ablation of persistent AF were in need of temporary cardiac pacing immediately after termination of AF within the ablation procedure due to sinus node dysfunction. The association of sinus node disease and AF is a known entity though there may be different underlying mechanisms which lead to variable clinical manifestations. Persistent AF may lead to structural and electrical remodeling of the atria, involving apoptosis, fibrosis and dilatation and finally causing sinus node dysfunction. On the other hand, several factors such as ischemia, inflammation, genetic disposition, progressive fibrosis or even atrial myopathy with extensive scarring have been shown to cause sinus node dysfunction and secondary leading to persistent AF [11, 13, 19,20,21,22]. The existence of low-voltage areas in the atria as well as sinus pauses in history prior to the ablation were identified as relevant risk factors for acute cardiac pacing in our study population, both possibly acting as a surrogate for already severe pathologically remodeled atria. Some authors also claim a direct damage of the sinus node due to ablation in the right atrium and especially in the area of the sinus node or due to circumferential isolation of the superior vena cava to be a reason for the sinus arrest after termination of AF [14, 23]. Others hold the indirect, ablation-related damage of the sinus nodal artery responsible for this incidence, especially by performing PVI or by deployment of a roof line or mitral isthmus line in the left atrium [14, 15, 17]. However, after matching a cohort of patients with persistent AF treated by ablation to a cohort of patients being treated by external cardioversion, there was no relevant difference in the incidence of sinus node disease after the termination of persistent AF [17]. One may also speculate that dislodgment of the catheter or energy transfer towards the AV node when ablating complex fractionated electrograms at the ostium of the coronary sinus may be related to AV node dysfunction after ablation. However, in our study population, there was no statistically significant association of any procedural parameter with the need for acute cardiac pacing. Especially, neither the localization of the ablation in the atria (left atrium, right atrium and coronary sinus) nor the technique (ablation of CFAE or lines) was shown to be associated with the necessity of acute cardiac pacing in the multivariable analysis. Thus, the direct or indirect damage of the sinus node or AV node seems to be an existent but rare incidence.
In recent publications, the recovery of the sinus node after ablation of paroxysmal AF and atrial flutter due to reverse remodeling has been described [24,25,26], supporting previous animal and human studies showing the reversibility of atrial remodeling caused by long-lasting AF [11, 13, 21]. In our study population, the temporary pacing wire could be removed after 1 day due to the recovery of the sinus node in more than half of the patients in need of temporary cardiac pacing, and the implantation of a permanent PM was not necessary in these patients. This suggests that a watch-and-wait strategy with temporary cardiac pacing and continuous hemodynamic monitoring seems justified at first, even in cases with total sinus arrest at the end of the ablation procedure.
Whereas the main indication for temporary cardiac pacing was sinus node disease in our study, the main indication for the implantation of a permanent PM was dual node disease. Roughly two-third of the patients in need of cardiac pacing within 1 week after the ablation required the implantation of a permanent dual-chamber PM. Previous studies have shown the coexistence of sinus node disease and AV node disease in persistent AF patients, suggesting an advanced remodeling of the atria as causal for this entity [18, 27].
The association of cardiac pacing within 1 week after the ablation of persistent AF with older age, renal insufficiency, mitral valve insufficiency, sinus bradycardia in history prior to ablation, sinus pauses in history prior to ablation, atria with low voltage and longer cycle length prior to ablation was demonstrated in the univariable analysis. A beta blocker therapy seemed to be protective in the same analysis. Considering beta blocker therapy only in rhythmologically stable patients, who had not shown signs of sinus node disease or AV node disease in their medical history prior to ablation, might be a possible explanation for this phenomenon.
Age as a predictor of sinus arrest after the ablation of atrial fibrillation has been described earlier [17]. This was also confirmed in our population. In conformity with other publications, concomitant diseases were associated with acute cardiac pacing in the univariable analysis, as mentioned above. However, in our study, no baseline characteristic other than age was identified as a predictor for acute cardiac pacing within 1 week after ablation in the multivariable analysis.
Concerning electrocardiographic parameters, sinus pauses longer than 3 s in history prior to ablation were shown to be significantly predictive for acute cardiac pacing in the multivariable analysis. Whereas a low preprocedural ventricular heart rate during AF, which was shown to be associated with sinus node disease earlier [18], showed no relevant association with acute cardiac pacing in our population. A longer atrial fibrillation cycle length, measured in the left atrial appendage immediately before starting the ablation, was associated with acute cardiac pacing, although, in contrast to earlier published data [28], this was not identified as an independent variable in the multivariable analysis. The existence of low-voltage areas in the atria was significantly predictive for the necessity of acute cardiac pacing, which might be explained by progressive electroanatomical remodeling and concomitant sinus node disease as mentioned above.
Almost all patients in need of acute cardiac pacing within 1 week after the ablation procedure suffered an early recurrence of AF or atypical atrial flutter during the same hospital stay, whereas this could be observed only in roughly a quarter of the patients without acute pacing. Data concerning early recurrence of atrial arrhythmias after ablation in patients with persistent AF and sinus node disease are scarce. Recently published data showed that a certain type of sinus node disease and the duration of AF were independent predictors for the recurrence of AF after ablation [29], suggesting that advanced structural and electrical remodeling due to sinus node disease may favor the recurrence of AF.
Limitations
The study is a monocentric retrospective analysis including patients with persistent and long-standing persistent AF. No statement can be made about other kinds of atrial arrhythmias. Atrial voltage was only evaluated by the operating physician and no additional retrospective assessment was performed in this regard. The size of the low-voltage areas was not consistently determined and, therefore, was not part of the analysis. Considering the small number of patients in need of acute cardiac pacing after ablation of persistent AF, there might be a statistical bias influencing the results of the study.
Conclusion
Acute cardiac pacing within 1 week after the procedure was necessary in 40 (3.2%) patients undergoing an ablation of persistent AF. Age, sinus pauses longer than 3 s in history prior to ablation and the existence of areas of low voltage in any of the atria prior to ablation were identified as predictors for acute cardiac pacing. The vast majority of patients needed pacing because of sinus node dysfunction, which turned out to be only a temporary condition in approximately one-third of the patients. A watch-and-wait strategy with temporary cardiac pacing and continuous hemodynamic monitoring after ablation seems justified, even in cases with total sinus arrest at the end of the ablation procedure. Physicians should be aware of the rare incidence of sinus arrest after ablation of persistent AF and should appropriately inform their patients in advance.
References
Chugh SS, Roth GA, Gillum RF, Mensah GA (2014) Global burden of atrial fibrillation in developed and developing nations. Glob Heart 9:113–119
Freeman JV, Simon DN, Go AS, Spertus J, Fonarow GC, Gersh BJ, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Chang P, Peterson ED, Piccini JP (2015) Outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF) investigators and patients. Association between atrial fibrillation symptoms, quality of life, and patient outcomes: results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF). Circ Cardiovasc Qual Outcomes 8:393–402
Haim M, Hoshen M, Reges O, Rabi Y, Balicer R, Leibowitz M (2015) Prospective national study of the prevalence, incidence, management and outcome of a large contemporary cohort of patients with incident non-valvular atrial fibrillation. J Am Heart Assoc 4:e001486
Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, Seshadri S, Wolf PA, Vasan RS, Benjamin EJ, Levy D (2015) 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 386:154–162
Marrouche N, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bänsch D, for the CASTLE-AF Investigators (2018) Catheter ablation for atrial fibrillation with heart failure. NEJM 378:417–427
Mohanty S, Santangeli P, Mohanty P, Di Biase L, Holcomb S, Trivedi C, Bai R, Burkhardt D, Hongo R, Hao S, Beheiry S, Santoro F, Forleo G, Gallinghouse JG, Horton R, Sanchez JE, Bailey S, Hranitzky PM, Zagrodzky J, Natale A (2014) Catheter ablation of asymptomatic longstanding persistent atrial fibrillation: impact on quality of life, exercise performance, arrhythmia perception, and arrhythmia-free survival. J Cardiovasc Electrophysiol 25:1057–1064
Santangeli P, Di Biase L, Burkhardt DJ, Horton R, Sanchez J, Bai R, Pump A, Perez M, Wang PJ, Natale A, Al-Ahmad A (2012) Catheter ablation of atrial fibrillation: state-of-the-art techniques and future perspectives. J Cardiovasc Med (Hagerstown) 13:108–124
Steven D, Sultan A, Schäffer B, Servatius H, Hoffmann B, Lüker J, Willems S (2013) Catheter ablation of persistent and long-standing persistent atrial fibrillation. Strategies and results. Herzschrittmacherther Elektrophysiol 24(1):15–18
Tilz RR, Lin T, Rillig A, Heeger CH, Scholz L, Wohlmuth P, Bucur T, Metzner A, Mathew S, Wissner E, Ouyang F, Kuck KH (2017) Focal Impulse and rotor modulation for the treatment of atrial fibrillation: locations and 1 year outcomes of human rotors identified using a 64-electrode basket catheter. J Cardiovasc Electrophysiol 28:367–374
Wang YL, Liu X, Tan HW, Zhou L, Jiang WF, Gu J, Liu YG (2013) Evaluation of linear lesions in the left and right atrium in ablation of long-standing atrial fibrillation. Pacing Clin Electrophysiol 36:1202–1210
Jackson LR II, Rathakrishnan B, Campbell K, Thomas KL, Piccini JP, Bahnson T, Stiber JA, Daubert JP (2017) Sinus node dysfunction and atrial fibrillation: a reversible phenomenon? Pacing Clin Electrophysiol 40:442–450
Jackson LR II, Kim SH, Piccini JP Sr, Gersh BJ, Naccarelli GV, Reiffel JA, Freeman J, Thomas L, Chang P, Fonarow GC, Go AS, Mahaffey KW, Peterson ED, Kowey PR (2016) Sinus node dysfunction is associated with higher symptom burden and increased comorbid illness: results from the ORBIT-AF registry. Clin Cardiol 39:119–125
John RM, Kumar S (2016) Sinus node and atrial arrhythmias. Circulation 133:1892–1900
Killu AM, Fender EA, Deshmukh AJ, Munger TM, Araoz P, Brady PA, Cha YM, Packer DL, Friedman PA, Asirvatham SJ, Noseworthy PA, Mulpuru SK (2016) Acute sinus node dysfunction after atrial ablation: incidence, risk factors, and management. Pacing Clin Electrophysiol 39:1116–1125
Sohns C, Staab W, O´Neill M, Vollmann D (2016) Reversible sinus node injury during circumferential pulmonary vein ablation. Clin Res Cardiol 105:968–970
Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh H, Le Heuzey JY, Ponikowski P, Rutten FH (2010) Guidelines for the management of atrial fibrillation. Eur Heart J 31:2369–2429
Deshmukh AJ, Yao X, Schilz S, Van Houten H, Sangaralingham LR, Asirvatham SJ, Friedman PA, Packer DL, Noseworthy PA (2016) Pacemaker implantation after catheter ablation for atrial fibrillation. J Interv Card Electrophysiol 45:99–105
Masuda M, Inoue K, Iwakura K, Okamura A, Koyama Y, Kimura R, Toyoshima Y, Doi A, Sotomi Y, Komuro I, Fujii K (2012) Preprocedural ventricular rate predicts subsequent sick sinus syndrome after ablation for long-standing persistent atrial fibrillation. Pacing Clin Electrophysiol 35:1074–1080
Bao Z, Chen H, Yang B, Shehata M, Ju W, Zhang F, Yang G, Gu K, Li M, Cao K, Wang X, Chen M (2017) Prolonged sinus pauses upon termination of paroxysmal atrial fibrillation: abnormal right atrial electrophysiologic and electroanatomic findings. Tex Heart Inst J 44:107–114
De Sisti A, Leclercq JF, Fiorello P, Manot S, Halimi F, Attuel P (2000) Electrophysiologic characteristics of the atrium in sinus node dysfunction: atrial refractoriness and conduction. J Cardiovasc Electrophysiol 11:30–33
Weirich J (2017) Remodeling of the aging heart: sinus node dysfunction and atrial fibrillation. Herzschrittmacherther Elektrophysiol 28:29–38
Schumacher K, Dagres N, Hindricks G, Husser D, Bollmann A, Kornej J (2017) Characteristics of PR interval as predictor for atrial fibrillation: association with biomarkers and outcomes. Clin Res Cardiol 106:767–775
Chen G, Dong JZ, Liu XP, Zhang XY, Long DY, Sang CH, Ning M, Tang RB, Jiang CX, Ma CS (2011) Sinus node injury as a result of superior vena cava isolation during catheter ablation for atrial fibrillation and atrial flutter. Pacing Clin Electrophysiol 34:163–170
Inada K, Yamane T, Tokutake K, Yokoyama K, Mishima T, Hioki M, Narui R, Ito K, Tanigawa S, Yamashita S, Tokuda M, Matsuo S, Shibayama K, Miyanaga S, Date T, Sugimoto K, Yoshimura M (2014) The role of successful catheter ablation in patients with paroxysmal atrial fibrillation and prolonged sinus pauses: outcome during a 5-year follow-up. Europace 16:208–213
Hocini M, Sanders P, Deisenhofer I, Jaïs P, Hsu LF, Scavée C, Weerasoriya R, Raybaud F, Macle L, Shah DC, Garrigue S, Le Metayer P, Clémenty J, Haïssaguerre M (2003) Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation 108:1172–1175
Khaykin Y, Marrouche NF, Martin DO, Saliba W, Schweikert R, Wexman M, Strunk B, Beheiry S, Saad E, Bhargava M, Burkhardt JD, Joseph G, Tchou P, Natale A (2004) Pulmonary vein isolation for atrial fibrillation in patients with symptomatic sinus bradycardia or pauses. J Cardiovasc Electrophysiol 15:784–789
Rosen KM, Loeb HS, Sinno Z, Rahimtoola SH, Gunnar R (1971) Cardiac conduction in patients with symptomatic sinus node disease. Circulation 43:836–844
Sairaku A, Nakano Y, Oda N, Makita Y, Kajihara K, Tokuyama T, Motoda C, Fujiwara M, Kihara Y (2012) Prediction of sinus node dysfunction in patients with long-standing persistent atrial fibrillation using the atrial fibrillatory cycle length. J Electrocardiol 45:141–147
Chung H, Uhm JS, Sung JH, Kim JY, Pak HN, Lee MH, Joung B (2014) The type of sinus node dysfunction might predict the severity of atrial remodeling and clinical outcome after catheter ablation of atrial fibrillation. Int J Cardiol 172:487–489
Funding
No financial support of the study
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest concerning this study.
Rights and permissions
About this article
Cite this article
Semmler, V., von Krogh, F., Haller, B. et al. The incidence, indications and predictors of acute pacemaker implantation after ablation of persistent atrial fibrillation. Clin Res Cardiol 108, 651–659 (2019). https://doi.org/10.1007/s00392-018-1393-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1393-1