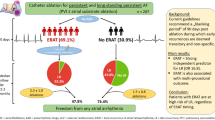
Abstract
Catheter ablation of complex fractionated atrial electrograms (CFAE), also known as defragmentation ablation, may be considered for the treatment of persistent atrial fibrillation (AF) beyond pulmonary vein isolation (PVI). Concomitant antiarrhythmic drug (AAD) therapy is common, but the relevance of AAD administration and its optimal timing during ablation remain unclear. Therefore, we investigated the use and timing of AADs during defragmentation ablation and their possible implications for AF termination and ablation success in a large cohort of patients. Retrospectively, we included 200 consecutive patients (age: 61 ± 12 years, LA diameter: 47 ± 8 mm) with persistent AF (episode duration 47 ± 72 weeks) who underwent de novo ablation including CFAE ablation. In all patients, PVI was performed prior to CFAE ablation. The use and timing of AADs were registered. The follow-ups consisted of Holter ECGs and clinical visits. Termination of AF was achieved in 132 patients (66 %). Intraprocedural AADs were administered in 168/200 patients (84 %) 45 ± 27 min after completion of PVI. Amiodarone was used in the majority of the patients (160/168). The timing of AAD administration was predicted by the atrial fibrillation cycle length (AFCL). At follow-up, 88 patients (46 %) were free from atrial arrhythmia. Multivariate logistic regression analysis revealed that administration of AAD early after PVI, LA size, duration of AF history, sex and AFCL were predictors of AF termination. The administration of AAD and its timing were not predictive of outcome, and age was the sole independent predictor of AF recurrence. The administration of AAD during ablation was common in this large cohort of persistent AF patients. The choice to administer AAD therapy and the timing of the administration during ablation were influenced by AFCL, and these factors did not significantly influence the moderate single procedure success rate in this retrospective analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Catheter ablation (CA) has become an established therapy for persistent atrial fibrillation (AF) [1–4]. The ablation of complex fractionated atrial electrograms was recently challenged by observations of modest long-term success rates [5, 6], and a randomized trial questioned its benefit [7]. However, solid evidence supporting the role of CFAE ablation in conjunction with PVI exists [2, 8–11]. Ablation approaches that have focused on PVI rather than CFAE ablation have achieved lower success rates at long-term follow-up [12] compared to the stepwise ablation approach [5, 8, 13]. The ideal protocol for ablation in patients with persistent AF remains unclear.
Antiarrhythmic drug (AAD) administration prior to or during CFAE ablation is controversial. While this treatment may suppress possible bystander and passively activated areas of CFAEs [14, 15], the subsequent unmasking of culprit regions that sustain atrial fibrillation for ablation may thereby facilitate the achievement of the endpoint of AF termination. It is also conceivable that the use of AADs may mask important CFAE areas or trigger regions and therefore diminish the procedural success rates, as has been indicated recently [16]. The aims of this retrospective analysis of a large cohort of patients undergoing ablation of persistent AF were to examine the use of AADs in a clinical setting and to investigate possible implications of AAD administration and the timing of the administration during the course of CFAE ablation.
Methods
Study population
This study is a retrospective analysis of 200 consecutive patients (pts) who underwent de novo PVI and CFAE ablation for symptomatic persistent AF at the University Heart Center of Hamburg. AF was considered persistent if the episodes lasted longer than 48 h without spontaneous conversion and/or electrical cardioversion was necessary to restore sinus rhythm (SR), and AF did not terminate after the completion of PVI. Our analysis comprised all pts who met these criteria and were treated at the University Heart Center in Hamburg, Germany between January 2010 and December 2011. Patients with clinically persistent AF that terminated to SR after PVI were not included in this study.
Electrophysiological study
Informed written consent was obtained at least 1 day prior to the procedure. The electrophysiological studies were performed under Propofol sedation via femoral access and a single transseptal puncture with non-steerable sheaths. Open-irrigation 3.5-mm tip ablation catheters with a 30 W power setting were used (Thermocool D-curve, Biosense Webster, Diamond Bar, CA, USA). Oral anticoagulation was paused 2–3 days prior to the ablation to achieve an INR of <2. Further details have been described previously [11]. A three-dimensional mapping system (Ensite NavX, St. Jude Medical, St.Paul, MN, USA) was utilized in all pts.
Pulmonary vein isolation and CFAE ablation
In all pts, pulmonary vein isolation (PVI) was defined as the elimination of all PV signals or the documentation of the dissociation of the PV signals in the circumferential mapping catheter and was performed using irrigated-tip RF energy. In pts who presented in sinus rhythm, AF was induced using burst pacing from the coronary sinus (CS) catheter prior to PVI. According to standard practice, in all pts who remained in AF for a minimum of 10 min after PVI was completed and who met the clinical definition of persistent AF, CFAE ablation was undertaken with the goal of AF termination according to established protocol [9, 17]. CFAE ablation was first performed in the LA with additional RF application in the CS and the right atrium (RA) as necessary to achieve termination. In accordance with the previously established modified stepwise ablation approach [11, 18], linear ablation was limited to macro-reentrant arrhythmias that occurred during the course of the procedure.
Periprocedural AAD treatment
Prior AAD treatment was not discontinued before the ablation procedure. The administration of antiarrhythmic drugs during the procedure (i.e., the infusion of 300 mg of Amiodarone or 1 mg/kg of Flecainide over 15 min) and the timing of the administration were documented on the procedural data sheet. No AAD administrations were allowed prior to the completion of PVI. The patients were otherwise excluded.
Follow-up
All pts were seen at 3, 6 and 9 months after the index procedure and at 12 months if they were available. Follow-up ECGs were recorded, the recent clinical history since the AF ablation was assessed, and all available Holter ECGs were analysed. Holter monitoring was available for all 3-month visits either via our own outpatient clinic or from an external referring physician.
Ablation success was defined as the absence of atrial arrhythmia for >30 s in all available 12-lead ECG and Holter recordings and the absence of symptoms. As previously established, a 12-week blanking period was applied [4]. We advised the patients to discontinue AAD use after the blanking period.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation. Student’s t tests were utilized to compare the continuous variables. The categorical variables were compared using the Chi-square test.
Logistic regressions were used to analyse the effects on AF termination and the outcomes after ablation. The multiple regression model included the following parameters: age, sex, duration of the longest AF episode, history of AF in years, LA size, baseline AFCL, timing of the AAD administration, amount of energy for CFAE ablation, prior AAD medication and AF termination. Regression analyses were utilized to examine factors that influenced the timing of the AAD administration. A p value <0.05 was considered statistically significant. The tests were performed using Stata Statistical Software (StataCorp. 2015, Release 14. StataCorp LP, College Station, TX, USA).
Results
Procedural data and intraprocedural administration of AADs
Termination of AF during ablation was achieved in 132/200 pts (66 %) at a procedure duration of 219 ± 55 min. The study population primarily consisted of long-standing persistent AF pts. The patient characteristics are shown in Table 1. CFAE ablation was performed in all of the patients as required by the inclusion criteria. Additional applications of RF in the coronary sinus were performed in 169/200 pts (84.5 %) and in the RA in 146/200 pts (73 %). AADs were administered to 168 pts (84 %) 45 ± 27 min after completion of PVI. Amiodarone (300 mg) was used in the majority of the pts (160/168). Additional data are provided in Table 2. The baseline AFCL was the sole independent predictor of AAD administration (odds ratio 0.96 per ms, 95 % CI 0.93–1.00, p = 0.031) and the early administration of an AAD (odds ratio 0.40 per ms, 95 % CI 0.10–0.69, p = 0.01).
AF termination and the timing of AADs
The late administration of an AAD was associated with higher rates of AF termination, and 43 min was chosen as a cutoff value because this value divided the population into as close to even groups and discriminated between the “early” and “late” administration of an AAD. The resulting groups exhibited significant differences in termination rates without significant differences in any of the established predictors of AF termination (Table 3). While a shorter AFCL was predictive of AAD administration in general, the mean AFCL did not differ significantly between the early and late administration groups.
Follow-up
At follow-up (313 ± 176 days), 94 pts (46 %) were free from atrial arrhythmia after a single procedure. Of those, 68 (77 %) were off AADs. There was no statistically significant difference in the recurrence rate between the early and late AAD administration groups.
Multivariate analysis
Logistic regression analysis identified larger LA size (odds ratio 0.87, 95 % CI 0.76–0.99, p = 0.042), longer AF history (per twofold increase. Odds ratio 0.54, 95 % CI 0.30–0.94, p = 0.03) and male sex (odds ratio 0.04, 95 % CI 0.01–0.35, p = 0.003) as negative predictors of AF termination during the ablation procedure. A longer AFCL (odds ratio 1.05 per ms, 95 % CI 1.01–1.10, p 0.026) was a positive predictor of AF termination (Table 4).
In the logistic regression models that included all known influencing factors and possible confounders, early administration of AADs was found to be an independent predictor of AF termination (odds ratio 0.88 per min, 95 % CI 0.80–0.97, p = 0.008). Antiarrhythmic medication prior to ablation was not a significant predictor of termination (Table 4).
The administration of AAD and its timing and the amount of ablation without AAD were not found to be independent predictors of recurrence after ablation (Tables 4, 5). Age was the sole independent predictor of AF recurrence (odds ratio 1.07 per year, 95 % CI 1.01–1.14, p = 0.015).
Discussion
The administration of an AAD during CFAE ablation was common in this large cohort of patients who underwent persistent AF ablation. Amiodarone was the most commonly used AAD during CFAE ablation. Early administration of AAD during the course of CFAE ablation was an independent predictor of termination.
The administration and timing of the AADs did not affect arrhythmia-free survival after ablation in this retrospective analysis. A shorter AFCL, which is a known predictor of adverse short-term outcomes, was the only independent predictor of AAD administration. The single procedure success rates were modest as could be expected in a long-standing persistent AF population [1, 5, 11, 13, 19, 20].
Antiarrhythmic drug administration during catheter ablation of persistent AF is a common practice used by many ablation centres to facilitate persistent AF ablation. The intravenous administration of Amiodarone or Flecainide results in the prolongation of the effective refractory period of atrial myocytes and, depending on the dose and AF duration, in the potential termination to sinus rhythm [21–24]. In addition, alteration of refractory periods has effects on areas of CFAE [25]. Thus, concomitant antiarrhythmic drugs may confound CFAE ablation.
The available data in the published literature regarding the implications of AAD administration in the context of AF ablation are limited. In theory, two effects of AAD administration prior to and during CFAE ablation can be discussed. The first is the unmasking of the sites that perpetuate AF, which thereby facilitates CFAE ablation and prevents RF application to atrial tissues that are not involved in the AF maintenance process. The second is that on-going treatment with AADs prior to an ablation procedure or the early administration of AADs during the procedure may mask important CFAE areas that contribute to AF and therefore diminish the likelihood of successfully terminating AF during ablation.
Evidence for the latter effect was recently published by Mohanty and colleagues who investigated the effects of Amiodarone treatment prior to CA in a prospective randomized trial [16]. In 112 pts who underwent CA for long-standing persistent AF, the discontinuation of Amiodarone 4 months prior to ablation was associated with a lower termination rate during CA but also with a lower recurrence rate during the follow-up compared with the control group that was kept on Amiodarone for the ablation procedure.
Regarding the acute effects of AADs during CA, a small pilot study by Singh et al. is notable. This study analysed the effects of Ibutilide during the ablation of persistent AF in a small cohort of pts. Building on prior work in a goat model, the study by Singh hypothesized that the culprit regions of the CFAEs may be identified through AAD administration prior to defragmentation [26, 27], which thus reduces the extent of the ablation and the procedural time necessary to accomplish AF termination by excluding the passively activated CFAEs. The results at follow-up in the small group of 11 pts (i.e., freedom from arrhythmia in 7/11 (64 %) after 1.3 procedures) are comparable to our success rate, but the termination rate was much higher (10/11; 91 %); keeping the small number of pts. in mind, this finding may indicate that AF termination is not a positive predictor of long-term success when it is not achieved through defragmentation but is rather achieved through the administration of AADs. This study observed the expected significant reduction in the areas of the CFAEs, but because it was feasibility pilot study, no comparison to patients who did not receive AAD administration was possible, and it remains unclear whether the reduction in the CFAEs actually represented a focus on the culprit regions responsible for AF. A randomized, multi-centre controlled trial (MAGIC-AF, Clinicaltrials.gov ID: NCT01014741) based on this pilot study has been initiated and will hopefully provide further insight into this matter.
Data on the effect of the timing of AAD administration during defragmentation ablation have not yet been published. Based on the findings of Mohanty et al. [16], it is conceivable that intraprocedural administration of AAD in general and early administration in particular may conceal areas of CFAE and short AFCL and thereby obstruct the identification and subsequent ablation of these sites. If this is the case, the early administration of an AAD might hinder AF termination and could have negative effects on acute and long-term outcomes following the ablation of persistent AF.
In multivariate analysis of our data, earlier administration of AAD was revealed as a predictor of AF termination. This was independent of the amount of energy spent on CFAE ablation and other known influencing factors (Table 4). Shorter AFCL was a predictor of AAD administration, and of earlier timing. This was most likely the result of the bias of the operator in the form of the assumption of a low chance of termination without the use of AAD. Hence, the AFCL was included in the multivariate analysis to adjust for this confounding factor.
As neither termination nor timing of AAD administration, nor amount of energy spent on CFAE ablation were predictive of a recurrence at follow-up, the significant impact of early AAD administration on AF termination may most likely be regarded as a result of pharmacologic cardioversion rather than a more appropriately targeted ablation.
Limitations
The procedures were analysed retrospectively. The timing of AAD administration was not blinded or randomized, which resulted in a possible bias towards the earlier administration of AAD for the group of patients for which the operator suspected a lower likelihood of termination as indicated by the regression analysis. The multivariate logistic regression model included the AFCL to adjust for this bias.
Conclusion
The administration of AADs, predominantly Amiodarone, during CFAE ablation was common in this cohort that consisted of mostly long-standing persistent AF patients. The operators were more likely to administer AADs to patients with shorter AFCLs. Early administration of AAD was an independent predictor of AF termination during ablation. Overall, we did not observe significant independent influences of AAD administration or the timing of that administration on the outcome following catheter ablation of persistent AF. The single procedure success rate following CFAE ablation of persistent AF was moderate, and age was the sole independent predictor of AF recurrence.
Abbreviations
- AADs:
-
Antiarrhythmic drugs
- AF:
-
Atrial fibrillation
- AFCL:
-
Atrial fibrillation cycle length
- CA:
-
Catheter ablation
- CFAE:
-
Complex fractionated atrial electrograms
- PVI:
-
Pulmonary vein isolation
- SR:
-
Sinus rhythm
References
Brooks AG, Stiles MK, Laborderie J, Lau DH, Kuklik P, Shipp NJ, Hsu LF, Sanders P (2010) Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 7:835–846
Hayward RM, Upadhyay GA, Mela T, Ellinor PT, Barrett CD, Heist EK, Verma A, Choudhry NK, Singh JP (2011) Pulmonary vein isolation with complex fractionated atrial electrogram ablation for paroxysmal and nonparoxysmal atrial fibrillation: a meta-analysis. Heart Rhythm 8:994–1000
January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW (2014) 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the american college of cardiology/american heart association task force on practice guidelines and the heart rhythm society. Circulation 130:2071–2104
Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D, Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation (2012) 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the heart rhythm society (HRS) task force on catheter and surgical ablation of atrial fibrillation. Developed in partnership with the european heart rhythm association (EHRA), a registered branch of the european society of cardiology (ESC) and the european cardiac arrhythmia society (ECAS); and in collaboration with the american college of cardiology (ACC), american heart association (AHA), the asia pacific heart rhythm society (APHRS), and the society of thoracic surgeons (STS). Endorsed by the governing bodies of the American college of cardiology foundation, the American heart association, the European cardiac arrhythmia society, the European heart rhythm association, the society of thoracic surgeons, the Asia pacific heart rhythm society, and the heart rhythm society. Heart Rhythm 9:632–696.e21
Schreiber D, Rostock T, Fröhlich M, Sultan A, Servatius H, Hoffmann BA, Lüker J, Berner I, Schäffer B, Wegscheider K, Lezius S, Willems S, Steven D (2015) Five-year follow up after catheter ablation of persistent atrial fibrillation using the “stepwise approach” and prognostic factors for success. Circ Arrhythm Electrophysiol 8:308–317
Scherr D, Khairy P, Miyazaki S, Aurillac-Lavignolle V, Pascale P, Wilton SB, Ramoul K, Komatsu Y, Roten L, Jadidi A, Linton N, Pedersen M, Daly M, O’Neill M, Knecht S, Weerasooriya R, Rostock T, Manninger M, Cochet H, Shah AJ, Yeim S, Denis A, Derval N, Hocini M, Sacher F, Haïssaguerre M, Jaïs P (2014) Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol 8:18–24
Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P, Sanders P, Investigators STARAFII (2015) Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 372:1812–1822
Verma A, Mantovan R, Macle L, De Martino G, Chen J, Morillo CA, Novak P, Calzolari V, Guerra PG, Nair G, Torrecilla EG, Khaykin Y (2010) Substrate and trigger ablation for reduction of atrial fibrillation (STAR AF): a randomized, multicentre, international trial. Eur Heart J 31:1344–1356
Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T (2004) A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 43:2044–2053
Haissaguerre M, Sanders P, Hocini M, Takahashi Y, Rotter M, Sacher F, Rostock T, Hsu LF, Bordachar P, Reuter S, Roudaut R, Clémenty J, Jaïs P (2005) Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol 16:1125–1137
Rostock T, Salukhe TV, Steven D, Drewitz I, Hoffmann BA, Bock K, Servatius H, Müllerleile K, Sultan A, Gosau N, Meinertz T, Wegscheider K, Willems S (2011) Long-term single- and multiple-procedure outcome and predictors of success after catheter ablation for persistent atrial fibrillation. Heart Rhythm 8:1391–1397
Tilz RR, Rillig A, Thum AM, Arya A, Wohlmuth P, Metzner A, Mathew S, Yoshiga Y, Wissner E, Kuck KH, Ouyang F (2012) Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the hamburg sequential ablation strategy. J Am Coll Cardiol 60:1921–1929
Fiala M, Bulková V, Škňouřil L, Nevřalová R, Toman O, Januška J, Špinar J, Wichterle D (2015) Sinus rhythm restoration and arrhythmia noninducibility are major predictors of arrhythmia-free outcome after ablation for long-standing persistent atrial fibrillation: a prospective study. Heart Rhythm 12:687–698
Nademanee K, Lockwood E, Oketani N, Gidney B (2010) Catheter ablation of atrial fibrillation guided by complex fractionated atrial electrogram mapping of atrial fibrillation substrate. J Cardiol 55:1–12
Miwa Y, Minamiguchi H, Bhandari AK, Cannom DS, Ho IC (2014) Amiodarone reduces the amount of ablation during catheter ablation for persistent atrial fibrillation. Europace 16:1007–1014
Mohanty S, Di Biase L, Mohanty P, Trivedi C, Santangeli P, Bai R, Burkhardt JD, Gallinghouse JG, Horton R, Sanchez JE, Hranitzky PM, Zagrodzky J, Al-Ahmad A, Pelargonio G, Lakkireddy D, Reddy M, Forleo G, Rossillo A, Themistoklakis S, Hongo R, Beheiry S, Casella M, Dello Russo A, Tondo C, Natale A (2014) Effect of periprocedural amiodarone on procedure outcome in longstanding persistent atrial fibrillation undergoing extended pulmonary vein antrum isolation: results from a randomized study (SPECULATE). Heart Rhythm 12:477–483
Oral H, Chugh A, Ozaydin M, Good E, Fortino J, Sankaran S, Reich S, Igic P, Elmouchi D, Tschopp D, Wimmer A, Dey S, Crawford T, Pelosi F, Jongnarangsin K, Bogun F, Morady F (2006) Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 114:759–765
Ammar S, Hessling G, Reents T, Paulik M, Fichtner S, Schön P, Dillier R, Kathan S, Jilek C, Kolb C, Haller B, Deisenhofer I (2013) Importance of sinus rhythm as endpoint of persistent atrial fibrillation ablation. J Cardiovasc Electrophysiol 24:388–395
Scherr D, Khairy P, Miyazaki S, Aurillac-Lavignolle V, Pascale P, Wilton SB, Ramoul K, Komatsu Y, Roten L, Jadidi A, Linton N, Pedersen M, Daly M, O’Neill M, Knecht S, Weerasooriya R, Rostock T, Manninger M, Cochet H, Shah AJ, Yeim S, Denis A, Derval N, Hocini M, Sacher F, Haïssaguerre M, Jaïs P (2014) Five-Year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol 8:18–24
Pecha S, Aydin MA, Ahmadzade T, Hartel F, Hoffmann B, Steven D, Willems S, Reichenspurner H, Wagner FM (2015) Implantable loop recorder monitoring after concomitant surgical ablation for atrial fibrillation (AF): insights from more than 200 continuously monitored patients. Heart Vessels. doi:10.1007/s00380-015-0735-4
Goy JJ, Kaufmann U, Kappenberger L, Sigwart U (1988) Restoration of sinus rhythm with flecainide in patients with atrial fibrillation. Am J Cardiol 62(6):38D–40D
Wang Z, Feng J, Nattel S (1995) Idiopathic atrial fibrillation in dogs: electrophysiologic determinants and mechanisms of antiarrhythmic action of flecainide. J Am Coll Cardiol 26:277–286
Shinagawa K, Shiroshita-Takeshita A, Schram G, Nattel S (2003) Effects of antiarrhythmic drugs on fibrillation in the remodeled atrium: insights into the mechanism of the superior efficacy of amiodarone. Circulation 107:1440–1446
Donovan KD, Power BM, Hockings BE, Dobb GJ, Lee KY (1995) Intravenous flecainide versus amiodarone for recent-onset atrial fibrillation. Am J Cardiol 75:693–697
Hoshiyama T, Yamabe H, Koyama J, Kanazawa H, Ogawa H (2015) Left atrial electrophysiologic feature specific for the genesis of complex fractionated atrial electrogram during atrial fibrillation. Heart Vessels. doi:10.1007/s00380-015-0672-2
Singh SM, Davila A, Kim SJ, Houghtaling C, Dukkipati SR, Reddy VY (2010) Intraprocedural use of ibutilide to organize and guide ablation of complex fractionated atrial electrograms: preliminary assessment of a modified step-wise approach to ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol 21:608–616
Shan Z, Van Der Voort PH, Blaauw Y, Duytschaever M, Allessie MA (2004) Fractionation of electrograms and linking of activation during pharmacologic cardioversion of persistent atrial fibrillation in the goat. J Cardiovasc Electrophysiol 15:572–580
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflicts of interest to report.
Rights and permissions
About this article
Cite this article
Lüker, J., Sultan, A., Sehner, S. et al. Use of antiarrhythmic drugs during ablation of persistent atrial fibrillation: observations from a large single-centre cohort. Heart Vessels 31, 1669–1675 (2016). https://doi.org/10.1007/s00380-015-0771-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-015-0771-0