Abstract
The fabrication of fully functional active components via additive manufacturing, also known as 3D printing, has progressed beyond mere prototype. With composites, metals, ceramics, concrete, and polymers, it is a flexible production technique. This article focuses on the evolution of additive products in the biomedical and sports industries. Additionally, a number of instances of additive manufacturing techniques used in the creation of unique goods are provided. The use of additive manufacturing as a collaborative tool with the idea of innovative problem-solving techniques in the creation of new items has also been given a conceptual framework.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
A novel approach to traditional manufacturing techniques is additive manufacturing (AM). In traditional manufacturing methods, parts are created by eliminating undesired components from raw materials before converting them into finished products. The amount of material wasted with this process is substantial, and fabricating complex pieces is challenging or time-consuming. AM is frequently referred to as 3D printing, a process that overhauled the manufacturing sector. In this method, which is known as additive manufacturing, parts are created by putting a layer on top of another [1]. Rapid prototyping, on-demand manufacturing, digital fabrication, desktop manufacturing, layer manufacturing, direct manufacturing technology, and 3D printing are other names for additive manufacturing [2]. Stereolithography (SLA), digital light processing (DLP), selective laser sintering (SLS), electron beam melting (EBM), fusion deposition modelling (FDM), multijet/polyjet 3D printing (M/P 3D printing), selective laser melting (SLM), and laminated object manufacturing are a few examples of additive manufacturing processes (LOM). These procedures involve the utilisation of a wide variety of materials, including ceramics, plastic, metal, liquid, powder, and even live cells [3].
It has drawn attention because of its simplicity in fabrication, limitless design potential, low level of complexity, decreased part count, weight reduction, and increased system effectiveness. With such a response, AM revenue was previously anticipated to be $2.7 billion; nevertheless, it will increase to almost $100 billion over the next twenty years [4]. It has demonstrated promising results in the biological sciences, industrial applications, aeronautical technology, and academic research. This technique is applied to the aerospace industry to boost system efficiency overall, reduce part weight by reducing the number of parts, and increase fuel efficiency, which in turn affects cost [5].
Additive manufacturing, which enables the printing of customised body parts with intrinsic geometry and offers each patient a unique set of treatments, is quickly revolutionising the medical sectors. AM has developed over the last few decades into a flexible and useful technique for creating geometrically challenging structures in the medical sector. Dental implants, heart valves, joint replacements, cranial plates, spinal fusion cages, valve, stent, and knee implant pieces have all been created using additive manufacturing technology. However, in the medical field, 2D radiographic pictures from CT scans, MRIs, and X-rays can be transformed to 3D digital print files, enabling the construction of specialised anatomical, intricate, and clinical structures [6].
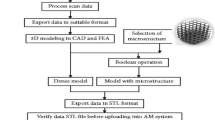
To create a computer-aided design (CAD) model, software is used. The model is then transferred via slicing software in the subsequent step to produce a format for its layered representation known as Standard Tessellation Language (STL) [7]. To begin printing, the STL file is loaded onto a 3D printer. Immediately following printing, the part is sent for post-processing, where it is essential to clean it, remove it from the build plate using electric discharge machining (EDM) [8], and then heat the substrate in a furnace to relieve internal stresses. The numerous manufacturing processes stages are shown in Fig. 1.
Schematic of processing steps in AM [9]
Objects made from a variety of metals, polymers, ceramics, photopolymer resins, and wax grades have been created via 3D printing. Because raw materials are handled substantially differently than they would be in a regular production method, materials are crucial to the additive manufacturing process. As a result, it is now easy to transition from traditional to additive manufacturing. The seven primary approaches used in the AM process were categorised by the American Society for Testing and Materials (ASTM F42). Fused deposition modelling, often known as material extrusion (ME), is a method that employs plastic and polymer-based wire filament as its raw material (FDM). The form of the spool-wrapped wire serves as the primary raw material for this technique. Fused layer manufacturing (FLM) and fused filament fabrication are other names for the FDM process (FFF) [9]. Binder Jetting (BJ), also known as 3D inkjet technique, adheres one layer of a particle on top of another using liquid binders. To create different grade objects, multi jetting (MJ) sprays a liquid photo-reactive chemical over a construction platform [10]. In order to make a polymer product (DLP), stereolithography (SLA) and digital light processing (DLP) both used a vat of liquid photopolymer [11]. Sheet lamination is the mechanical bending of sheets or foils to join or laminate them (SL) [12]. A technique called direct energy deposition (DED) uses a laser beam and powder to construct structures at the same time. Typically, wire-feed and powder-fed systems are the foundation of the DED process [13]. Powder bed fusion (PBF), the fourth frequently employed technique, involves inserting material in the form of powder into a platform that has been built using a powder recoater system. The energy source used to scan the geometry for the melting of the powder is a laser beam.
Healthcare is expected to alter as a result of additive manufacturing, which has recently shown tremendous potential in the medical sector. Some of the most frequently used applications of additive manufacturing include the creation of tissue and organs, anatomical models, the development of specialised prosthetics and implants, advancements in the pharmaceutical industry, particularly with regard to drug dosage forms, discovery and delivery, and other biomedical applications [14]. Due to its many benefits, including the ability to customise and personalise medical products, biocompatibility, cost effectiveness, increased productivity, accessibility, quick production times, simple assembly, collaboration, and democratisation, additive manufacturing is widely used in the medical industry [15]. However, additive manufacturing is still not widely used due to its slow uptake in industrial production [5].
But in the medical industry, where highly precise and tailored products are needed in smaller quantities, this limitation is seen as a benefit. This is because each patient has different needs for medication and individualised treatment. As a result, clinical and biomedical applications are where additive manufacturing is best suited and most frequently used [16]. Researchers are now exploring for bio-comparable materials to produce vascularized people after several have attempted to map human organs and transform them into virtual three-dimensional creations. There are still certain regulatory and scientific challenges to be solved, nevertheless [15]. Figure 2 depicts the distinct stages needed to build the 3D medical models. Images are acquired, or the structural target area is chosen, three-dimensional geometry is evolved through processing of the medical images, materials and three-dimensional printing equipment are chosen for implants, post-processing is applied as needed, testing is done, and finally the implant body is implemented. A better grasp of diseases, their costs, the surgical procedure, the use of surgical instruments, and the design of implanted devices that are patient-specific are just a few examples of how medical models can improve a surgeon's knowledge and skill [17]. Custom fit masks, the development of novel organs, and surgical practise, among other things, are best served by medical additive manufacturing [18].
AM used for orthopaedic implants [19]
The market for sporting goods is now utilising additive manufacturing. Due to the market's strong emphasis on customer perception, the advantages of unique items and customisation made available by additive manufacturing may be crucial. Because every human's anatomy is programmed differently, each person has different preferences for fit and form. The equipment that players often use in sports, such as a racket with greater grip, a suit with better aerodynamics, or a cleat with higher traction, has a significant effect on their performance. In addition, the right equipment can prevent accidents, whereas the wrong equipment can easily result in injuries. Companies are continuously challenged to develop new goods that surpass those of the competition and offer a good fit and performance in the fast-moving sports market. However, many goods in the sports equipment sector are now mature and hardly have room for improvement.
AM provides the chance to create products that are fully tailored to match individual structure and performance needs, potentially enhancing comfort, reducing injuries (especially overuse problems), and enhancing performance. Notably, the sparse amount of sports AM research that is now available rarely provides goods with thorough case studies or statistics, despite the fact that [20] claim that the acceptance of sports business is just in the “initiating phase.” The 2019 research by Meier et al. on AM awareness in the sports industry Despite signs that additive manufacturing is being more widely used in other sectors, the aforementioned restrictions—such as a scarcity of usable materials, a lack of expertise in designing products for additive manufacturing, and expensive equipment costs—remain obstacles. The study also found that, although rapid prototyping, a term used to describe the application of AM technologies for producing prototypes rather than finished products, helps to drive innovation in product design, it is not being significantly used to enhance the production of new products. This is because the sports industry lacks a general understanding of additive manufacturing (AM) technology.
The sporting uses of 3D printing that have received a lot of attention recently include shoes by Nike, Adidas (such as Future craft 4D14) [20], and New Balance (such as Zante Generate), as well as shin pads, bicycle helmets, Olympic speed skating gloves, prosthetics used in the Paralympics [21] and countless other examples. Carbon (Redwood City, CA, USA), the inventor of Continuous Liquid Interface Production (CLIP) technology, has created three famous sports products that are either already on the market or will be in the near future [22]. These products include the S-Works cycling seat, the Adidas Future Craft 4D footwear, and a football helmet liner that can be customised (Des Plaines, IL, USA). The Power Saddle for Specialized has a lattice construction with more than 14,000 struts (Specialized Bicycle Components, Morgan Hill, CA, USA) [23]. Although it's possible that these instances of AM acceptance in the sports sector are the exception rather than the rule, their popularity online and rising consumer awareness do point to a growing market readiness to accept new performance-driven features.
Due to the rapid rate of AM invention, information regarding new shoes and other AM-produced products is frequently only accessible through company media releases and websites for mainstream news outlets rather than more established academic sources. This issue has been highlighted, especially within formal AM education, where project-based learning and flipped classroom formats encourage the use of numerous digital resources to support learning that is up-to-date and industry-aligned [24]. Given these disparate depictions of AM in sports, this study looked for any accessible scientific proof that AM results in better sporting goods. The main objectives were to describe the various AM-related digital technologies, materials, software, and equipment used, to document the scope of research investigations, to summarise the prospects and constraints of AM from the literature, and to list the different sports and products that use AM.
The objective of review paper is to compile work of numerous research’s in the field of additive manufacturing, with a particular emphasis on applications in the medical and sporting industries that make use of diverse AM technologies and materials. This review article might end up carrying the research in this field into the future.
2 Manufacturing Methods for Biomedical and Sports Industry Using Additive Manufacturing
2.1 Biomedical Applications Using Additive Manufacturing
The primary materials used in the production of contemporary medical implants are ferrous alloy steel, cobalt-chromium-based materials, and titanium-based materials. Additive manufacturing (metal-AM) is one of the modern fabrication processes used to provide bespoke characteristics that satisfy structural requirements. Figure 3 illustrates the usage of biomaterials manufactured by additive manufacturing technology used in different parts of human body.
The schematic depiction of the biomedical application of additive manufacturing of biometals includes the cranial prosthesis, dental implants, acetabular cup, interbody fusion cage, hip prosthesis, and knee prosthesis [25]
Currently, Metallic biomaterials are currently used in the production of various biomedical devices. This section discusses the use of various AM techniques and the impact of different processing parameters.
2.1.1 Titanium Alloys
AM of individual materials is popular in the titanium market titanium metal matrix composites and titanium-based structures for use in biomedical applications. The titanium metal matrix composites are given special attention in a distinct subsection because to their significance. Ti-based materials have shown to be very biocompatible. They also exhibit outstanding resilience to corrosion and fatigue stress. Additionally, Ti-based alloys are lighter than other metallic materials due to an inherent high strength to weight ratio.
Furthermore, there are a number of drawbacks to materials made of titanium. Tribology-wise, Ti-based materials perform poorly. The limitations of Ti-based materials’ mechanical properties are the cause of this issue. Ti-based alloys are frequently utilised for implants that must support weight, including total hip or total knee replacements. A strong bond between the new implant and the human tissue is crucial in these conditions. It has been shown that a layer of hard tissue slowly develops on the Ti implant. In light of this, there are still problems that need to be resolved even though Ti-based materials are a well-established materials system for use in biomedical implants. Utilizing additive manufacturing techniques can aid in resolving some of these problems. These options include creating porous or structurally graded components to promote bone regeneration as well as altering the surface of Ti-based materials to enhance their wear performance.
Kelly et al. used the Laser Additive Manufacturing (LAM) system of AeroMet Corporation to explore the micro-structural and thermo modelling of laser-produced Ti64 alloy [26]. Similar to earlier finds, researchers found -Ti laths with -Ti grains around them. There were many of equiaxed -Ti grain colonies in each of these bands. The LAM-deposited Ti64 alloy tested by Vicker as a microhardness sample has a hardness of roughly 350 HV. Porous Ti structures were shown using LENSTM by Balla et al. and Xue et al. [27]. The key to producing porous Ti components in this work was partial feedstock powder melting, in contrast to other attempts at processing Ti-based materials, which employed whole feedstock particle melting and solidification.
According to this theory, the central cores of the powder remained unaltered and only the exterior surfaces were melted, creating the porous structures depicted in Fig. 4 [28]. Particles bonded to one another as a result of these melted surfaces, and any remaining porosity was also created. Additionally, finished components displayed a Young’s Modulus that was comparable to that of genuine bone [29]. The porous Ti produced by LENSTM has better biological characteristics than bare Ti plate, as illustrated in Fig. 4, according to in vitro analyses [27].
a The Ti-6Al-4V alloy’s general microstructure, produced by laser additive manufacturing [29]. b Porous Ti structures that have undergone LENSTM processing; c-a the impact of density on 0.2% proof strength; and c-b a study of the porous Ti samples’ effective moduli as calculated empirically and theoretically [27]. d Ti plate and porous Ti were seen using confocal microscopy on days 10 and 21 as well as during MTT assays on days 3, 10, and 21
Direct Electron Beam Melting was used by Heinl et al. to fabricate cellular Ti structures [30]. Ti64 powder was used to create the structures, which were then vacuum-fabricated at a pressure between 10–4 and 10–5 mbar to eliminate any potential oxygen or nitrogen contamination. Samples with three distinct porosities—25%, 38%, and 60% by volume—were effective in each of the three attempts to generate connected porous structures.
Harrysson et al. demonstrated how to employ EBM to produce Ti64 structures with densities ranging from 4 to 40%, and then used the 3P bend technique to assess their flexural and compression strength. It was shown that the compressive strength dropped when cell size or structural porosity increased. A few examples of these EBM-processed structures are shown in Fig. 5 [31]. Structures with a cell size of 3 mm and a relative density of 0.41 had a compressive strength of 85.72 MPa, whereas structures with a cell size of 12 mm and a relative density of 0.04 had a compressive strength of 0.84 MPa.
Shows various Ti-6Al-4V assemblies created using the E-grin melting method [31] in (a) and mesh Ti64 alloy assemblies and a model of a human vertebrae created using the SLS/DLF approach (b)
Ti-based materials that are dense and porous have been processed using selective laser sintering (SLS) or selective laser melting (SLM) for use in biomedical applications. Hot isostatic pressing (HIP) and SLS were used by Das et al. to manufacture net forms of Ti64 alloy [32]. To create a 92% dense component, Ti64 powder was first selectively sintered with a laser source. The part’s density was greatly raised during HIP post-processing. Since the pieces made in this way were almost net-shaped, more machining was required. From the exterior to the interior, the micrographs of SLS/HIP treated products apparently change. While the bulk microstructure was made up of coarser grains, the skin or outer walls displayed a fine Widmanstatten structure with a high aspect ratio of the lamellar phase. A temperature gradient created by the surface melting of powders was thought to be the cause of the disparity in microstructure. A sub-scaled replica of an AIM-9 Sidewinder missile housing component was produced to show the viability of the SLS/HIP process. This component's microstructure was completely dense.
Fisher et al. also demonstrated the sintering of Ti powder. Ti64 components were produced by Hollander et al. using SLS or DLF (Direct Laser Forming) technology, and they were then tested on vitro with consideration of human primary osteoblast cells. Figure 5 [33] shows the procedure the authors used to verify the feasibility of SLS/DLF component manufacturing. The DLF pieces’ unpolished surfaces had a granulated appearance, necessitating finishing work. Nominal pore sizes for porous components ranged from 500 to 700 m, and most pores were cylindrical in character. The SLS/DLF items’ mechanical characteristics considering the strength of 1200 MPa tensile & an 6.5% deflection. Elongation at failure doubled to 13.0% following post-process annealing heat treatment, which also decreased tensile strength to 1042 MPa.
Results from in vitro tests showed that the materials were not harmful. The authors cultured cells in vitro on porous materials with 500 m, 700 m, and 1000 m average pore sizes. After 14 days, samples with 500 m hole diameters demonstrated the best interactions between cells and materials. Kruth et al. worked on the comparative wear characteristics for several materials created using the SLS/SLM process. Several materials tested on fretting wear tests with weights of 2 N and 6 N, and damages on the wear was calculated as mm3 of material lost over 10 × 103 cycles [34]. At 2.1 N load, Ti64 alloy has a wear volume of 7.362 × 103 mm3, while at 6 N load, it has a wear volume of 9.337 × 103 mm3. For 2 N and 6 N stresses, the damages for the CoCrMo alloy were 1.474 × 103 mm3 and 2.252 × 103 mm3.
Three-dimensional (3D) fibre deposition, which was initially intended for 3D manufacture of polymeric components, is another technique for treating porous Ti-based materials. In the scaffolds created by 3D fibre deposition, Li et al. revealed the aspect of bonematerial interaction [35]. A slurry was pumped via a syringe in a Bioplotter device using Ti64 alloy powder and 0.6% aqueous methylcellulose solution. A complete 3D scaffold is created through such layer-by-layer deposition and is afterwards sintered for two hours at 1200 °C. These samples ranged in porosity from 39 to 68%, while the diameters of the square and rectangular pores ranged from 200 to 800 m. After that, samples were placed inside the lumbar goat spines for in vivo testing. After a year, animals were sacrificed.
No sign of toxicity or inflammation were present. Even when the implant’s porosity and pore size grew, the amount of new bone formation increased. Additionally, Ryan et al. used wax models manufactured on a Thermojet 3D printer to create porous Ti scaffolds [36]. The wax mould was filled with a Ti powder and ethylene glycol slurry, which was then dried. The Ti structure was sintered between 1100 and 1300 °C in a vacuum after the wax had been removed. These sintered Ti scaffolds were used to cultivate SAOS-2 pre-osteoblast cells for more than 3 weeks without any issues of toxicity. These have a average pore size of 465 170 m and a porosity of 45%. Additionally, scaffolds with pore diameters of 200, 300, and 400 m with a porosity of up to 66.9% were created.
LENSTM was used to treat Ti-TiO2 compositionally graded structures [37]. TiO2 content was raised from 50 to 90%. The inclusion of 50% TiO2 in Ti led to a hardness of 1100 HV. LENSTM has also been used to strengthen SiC in Ti [38, 39]. With a hardness of up to 1000 HV, the Ti substrate’s tribological performance was increased by more than an order of magnitude. Through nitrogen injection in the oxygen free-argon rich chamber with LENSTM operation, Zhang et al. demonstrated the formation of nitrides of Ti and Si on Ti substrates. This technique is a relatively new way to create an in-the-moment ceramic phase [40]. The surface of Ti was then coated after being fed a combination of Ti and Si powders. The resultant structures had an alpha Ti matrix and were composed of dendrites of TiN and Si3N4. These in-situ produced ceramic coatings ranged in hardness from 1300 to 2100 HV and were 200 times more resistant to wear damage than a Ti substrate.
2.1.2 Cobalt Alloys
Understanding the viability of these materials in bulk form is crucial from the perspective of biomedical applications. Medical grade CoCrMo alloy was demonstrated by Janaki Ram et al. using LENSTM-based additive manufacturing [41]. LENSTM 285 W laser power was used to treat samples that were 6 mm thick. Each layer's microstructure was made up of very small equiaxed dendrites and slightly coarser columnar dendritic phase. These changes in the solidification circumstances led to these microstructural variances. It was also noted that the equiaxed and columnar dendritic sections contained carbide phase. The carbide layer was either solitary particles or a resilience network in the interdendritic zones. The CoCrMo alloy utilised in LENSTM technology has a hardness of 40 HRC, which is comparable to the hardness of 41 HRC of the wrought base. The abrasive wear resistance was found to be below that of the substrate. The peculiar morphology of the carbide phase, which was predominantly present as thin and long connected particles rather than being noticeably more regular, spherical, and equally dispersed across the treated substrate, is credited by the researchers as the cause of this.
E-beam melting has also been used to create dense, porous co-based alloys [42]. Figure 6 displays a number of test specimens and joint replacement components made utilising the E-beam technique. CoCrMo alloys having a density of 8.4 g/cm3 were treated to produce completely dense components. Additionally, components for open cellular mesh and femoral knee implants were produced utilising an electron beam-based additive manufacturing technique. The usual ASTM F75 CoCr alloy treatment and annealing heat treatment were also applied to the components of the femoral knee implant as they were being manufactured. The microstructure of fully dense components revealed columnar grains, arrays of carbides, and zigzag carbide phases.
E-beam treated porous femoral knee replacement component and dense CoCrMo alloy [42]
The average hardness in the horizontal plane was 4.4 GPa for a rectangular shape of fully dense CoCrMo alloy treated in an E-beam and 4.7 GPa for a cylinder specimen. These specimens were made with CoCrMo powder, which is around 30% harder than these hardness ratings. The prototype femoral knee component’s microstructure was made up of columnar carbides and arrays of carbides that ranged in size from 2 to 3 m. As demonstrated in Fig. 7, the microstructure of polished and annealed femoral components revealed an intergranular fcc structure with observable annealing twins and finer carbide phases. The CoCrMo alloy’s struts in the reticulated mesh structure resembled totally dense block and cylinder samples in terms of their microstructure. The hardness of the struts was 6.8 GPa in the horizontal plane and 5.6 GPa in the vertical plane, which was about 25% greater than that of the dense structures.
Microstructure following E-beam processing and annealing [42] depicts annealing twins in CoCrMo
2.1.3 Shape Memory Alloys
Recently, there has been an increasing interest in using these smart materials in the biomedical sectors of tissue engineering, drug delivery, endovascular surgery, orthodontics, and orthopaedics. Biomedical polymers must be produced from highly biocompatible and nontoxic components in order to function without inducing immune-inflammatory responses. Their biodegradability/biostability, mechanical characteristics, and activation processes are additional factors to be taken into account while constructing biomedical SMPs. The high-temperature SMPs, which have transition temperatures close to that of the body (37 C), are the activation methods that have generated the greatest study on polymers for biological uses [43]. Medical implants won’t perform as well if biomedical SMPs are used at varying temperatures above or below body temperature. In order to circumvent this issue, the SME may also be activated remotely utilising apparatus such as infrared (IR), laser, ultrasound, etc. [44]. Other strategies exist, such as adding magnetic nanoparticles and functional fillers to the polymer matrix, which enables the implanted device to be activated inside the human body at the right temperature and position with the help of an external magnetic field.
According to their adaptable physical and mechanical qualities, aliphatic polyester-based SMPs are frequently utilised for a variety of biomedical applications, including resorbable implants, sutures, and wound closures [45]. SMPs are used in biodegradable smart sutures, which are used to close wounds (Fig. 8a) [46]. The right amount of stress must be applied to the wound margins for optimal primary wound closure, which has long been a major challenge in endoscopic suturing [47]. Applying larger strains will cause the knot of the suture to be secured by tissue necrosis, whilst employing weaker forces would cause the formation of scar tissue and possibly hernias. Lendlein and Langer [48] were the first to propose a disposable smart thermo-plastic suture based on oligo(e-caprolactone) diol as a viable remedy for attaining optimal wound healing in endoscopy treatment. This suture can be tied inside the wound, then extended outward with controlled force before being shrunk and tightened inside the body (Fig. 8a).
Self-fitting tissue scaffolds might be developed to treat abnormally shaped bones because of the special properties of SMPs. A PCL diacrylate-based SMP scaffold that can accommodate a craniomaxillofacial bone abnormality was created by Zhang et al. [49] (Fig. 9). When heated over Trans, the scaffold initially became pliable and mushy (55 C). Next, a model defect was hand jammed into the scaffold. The structure was enlarged to accommodate the crooked shape once the force had been released. After cooling, the scaffold became firm once more, and it was eventually sealed inside a typical flaw.
Uses of medical devices based on SMP include: a tightening the biodegradable suture at 40C; b the malleability of self-fitting foam at its Ttrans; c the fitting of the foam into the irregular defect model; d the removal of the foam from the defect area after cooling; and e SMP foam before and after exposure to air [50]
3 Applications of Additive Manufacturing in Sports Industry
The first company to apply AM technologies to sports was Nike. Nike was able to decrease the sampling process from weeks to days by prototyping 30 alternative plate variations for its Zoom Superfly Fly knit. By utilising AM to create a running shoe midsole, Adidas was able to reduce weight and increase durability without compromising stability. Adidas intends to make custom shoes quickly and in-store using digital foot measurements and foot scanning technologies to deliver the most personalised shopping experience possible. In order to create a 3D model of a running shoe, New Balance works with athletes to gather mechanical data [51]. AM is used to make a biathlon rifle support. In order to create a rifle support that would fit the athlete's body and allow for a steady firing action, a 3D scan of the athlete's body was performed. Then, in order to reduce component weight while also providing structural protection and stiffness, topology optimization was paired with a FE review. It was discovered that the printed rifle support could sustain a force of 13.92 KN, or 14,294 times its weight (99.4 g). This outcome shows a 40% improvement over the original design, proving the viability of the suggested topology optimization plan. The printable rifle support can be utilised in biathlon because it weighs less than 100 g [52].
Using the fused filament fabrication (FFF) process, Aferdita et al. created a novel, precise approach for incorporating shock sensors in the interior of sports helmets [53]. Figure 10 demonstrates FFF printed PLA and TPU blocks with silica-coated fibres implanted in them. The use of FFF to incorporate Fiber Bragg grating sensors (FBGs) inside intricate polymer constructions, such as padding components for American football helmets, was reported and validated. Additionally, the possibility for embedding FBGs with FFF was validated, and a novel technique was presented employing stereolithography equipment (SLA). SLA produces prints of higher quality than FFF, however it cannot be used to embed polyimide fibres because of how they react chemically with the SLA resin. Depending on the application use-case, the higher quality prints produced by SLA must be weighed against SLA's greater cost and longer print time.
4 Customizing Surfboard and Stand-Up Paddle Board Fins Using a Parametric Method for Additive Manufacturing
Fins for stand-up paddle (SUP) and surfboards are made using 3D printing [54]. Figure 11 depicts the 3D printing of two fin designs, one of which was created using SLS and the other using a desktop FFF machine (Wanhao Duplicator i3, Acrylonitrile Butadiene Styrene material). The fins were affixed on a 2016 G-Whiz 9′4″ SUP board from Slingshot Sports. Ocean paddling on flat water was successful with both fins.
Comparison of the 2016 G-Whiz Slingshot SUP's original centre fin with versions made similarly using desktop FFF (second), and selective laser sintering (third) (3rd). One of the fins installed on the SUP board is shown in the right image [54]
In order to build the cam systems for a compound bow, Serena Graziosi and colleagues considered the functional, manufacturing, and assembly limitations as well as the techniques used to assure the object’s printability. The manufacturing method used the selective laser melting technology. The absence of integrated design techniques and the sizeable number of factors that need to be taken into account when making design decisions were recognised as the challenges faced by practitioners while designing for additive manufacturing. Figure 12a shows the redesigned geometry with the supports highlighted in red, and Fig. 12b displays the printed result as it was designed to be. Supports were needed to disperse heat, enabling a straightforward separation of the item from the platform, and support overhanging surfaces that were below the 45° limit. About half of the initial value will need to be sustained in terms of surfaces (Fig. 12a). Last but not least, Fig. 12c displays the sandblasted and support-free printed item [55].
a A summary of the supports required to ensure the object may be printed (in red); b the printed cam; and c the new cam following support removal and sandblasting [55]
Particularly in the field of athletics, orofacial injuries are a frequent cause of impairment. The main contributing factors to this injury occurrence are physical contact and a lack of protection. The hard and soft tissues of the mouth are protected by polymeric mouth protectors, commonly referred to as mouthguards, which are devices designed to be worn in the athlete's mouth while engaging in physical activity. A range of steps are involved in this paper manufacturing technique for creating personalised mouthguards, including preparing the mouth prototype, manipulating the chosen thermoplastic sheet, and the final forming process as show in Fig. 13. A PEEK-based mouthguard might be designed using digital software, according to a study by Li and colleagues [56]. The finished produced devices and other guards made using conventional techniques showed no discernible differences in retention (vacuum-pressure forming). The dental design of 3-D printed mouthguards has to be improved in order to maximise impact attenuation. According to a separate study that used digital technology to build a high-elastic silicone rubber mouthguard, this strategy can help to enhance fit accuracy [57].
Pressure equipment and moulding circumstances (condition MP) [58]
5 Challenges in Production for Biomedical and Sports Industry Using Additive Manufacturing
-
Given the lack of longitudinal evaluations and small sample numbers in the majority of research, the long-term durability of AM materials for sporting applications is unknown.
-
Compared to conventional production methods, additive manufacturing is often slower.
-
Postprocessing might take a lot of time and effort.
-
Many AM-produced pieces still need to work with traditional parts to complete products, which restricts geometry.
-
Manual involvement and an understanding of design for are still needed when using optimization software.
-
New items created with AM might not adhere to athletic laws.
-
The results of laboratory testing might not apply to real-world situations.
6 Conclusion
Particularly in the medical industry, additive manufacturing is a powerful, innovative, ground breaking, and quickly expanding technology. Patient-specific surgical models, surgical equipment, and personalised prostheses are the four main fundamental applications of additive manufacturing (AM) in the medical industry that are linked to recent advances. AM has recently offered a variety of applications in medicine, including prosthetic dentistry, the creation of various surgical cutting and drilling instruments, and the use of craniofacial implants for hip, knee, and spinal implants.
Due to its outstanding manufacturing methodology, additive manufacturing (AM) has significantly increased growth in the biomedical and sports industries. It has completely changed the tested testing procedures and methods. The biomedical industry can now approach implants in a different way because some places were beyond the capabilities of conventional production techniques. However, with additive manufacturing, more complicated implant designs like cranial plates, stem implants, and knee joint implants may be quickly created. Since these implants are individually-specific and cannot be produced similarly for every patient, patient specification is strongly advised. Similar to other industries, the sport industry has profited from additive manufacturing in terms of lightweight, part customisation, and sophisticated design solutions. For a spacecraft or communication satellite, reducing weight is frequently a top concern because it increases system effectiveness and uses less fuel.
References
De Krijger J, Rans C, Van Hooreweder B, Lietaert K, Pouran B, Zadpoor AA (2017) Effects of applied stress ratio on the fatigue behavior of additively manufactured porous biomaterials under compressive loading. J Mech Behav Biomed Mater 70:7–16
Afshar M, Anaraki AP, Montazerian H, Kadkhodapour J (2016) Additive manufacturing and mechanical characterization of graded porosity scaffolds designed based on triply periodic minimal surface architectures. J Mech Behav Biomed Mater 62:481–494. https://doi.org/10.1016/j.jmbbm.2016.05.027
Pecci R, Baiguera S, Ioppolo P, Bedini R, del Gaudio C (2020) 3D printed scaffolds with random microarchitecture for bone tissue engineering applications: Manufacturing and characterization. J Mech Behav Biomed Mater 103:103583. https://doi.org/10.1016/j.jmbbm.2019.103583
Liu YJ, Li SJ, Hou WT, Wang SG, Hao YL, Yang R et al (2016) Electron beam melted beta-type Ti–24Nb–4Zr–8Sn porous structures with high strength-to-modulus ratio. J Mater Sci Techno 32(6):505–508
Zhang LC, Attar H, Calin M, Eckert J (2016) Review on manufacture by selective laser melting and properties of titanium based materials for biomedical applications. Mater Technol Adv Perform Mater 31(2):66–76
Zhang LC, Xu J, Ma E (2002) Mechanically alloyed amorphous Ti50(Cu0.45Ni0.55) 44–xAl xSi4B2 alloys with supercooled liquid region. J Mater Res 17(07):1743–1749
Zhang LC, Shen Z, Xu J (2003) Glass formation in a (Ti, Zr, Hf)–(Cu, Ni, Ag)–Al high-order alloy system by mechanical alloying. J Mater Res 18(9):2141–2149
Rayegani F, Onwubolu GC (2014) Fused deposition modelling (FDM) process parameter prediction and optimization using group method for data handling (GMDH) and differential evolution (DE). Int J Adv Manuf Technol 73(1–4):509–519
Pant M, Singari RM, Arora PK, Moona G, Kumar H (2020) Mater Res Express 7:11
Melchels FP, Feijen J, Grijpma DW (2010) Biomaterials 31:6121
Gibson I, Rosen D, Stucker B, Khorasani M (2014) Additive manufacturing technologies, 3rd ed. Springer, New York, p 675. ISBN: 978-3-030-56126-0
Lee H, Lim CHJ, Low MJ, Tham N, Murukeshan VM, Kim YJ (2017) Int J Pr Eng Man-Gt 4:307
Tofail SA, Koumoulos EP, Bandyopadhyay A, Bose S, Donoghue L, Charitidis C (2018) Mater Today 21:22
Ronga M, Fagetti A, Canton G, Paiusco E, Surace MF, Cherubino P (2013) Clinical applications of growth factors in bone injuries: experience with BMPs. Injury 44:S34–S39
Zhang LC, Attar H (2016) Selective laser melting of titanium alloys and titanium matrix composites for biomedical applications: a review. Adv Eng Mater 18(4):463–475
Lu H, Poh C, Zhang LC, Guo Z, Yu X, Liu H (2009) Dehydrogenation characteristics of Ti-and Ni/Ti-catalyzed Mg hydrides. J Alloys Compd 481(1):152–155
Ehtemam-Haghighi S, Prashanth KG, Attar H, Chaubey AK, Cao GH, Zhang LC (2016) Evaluation of mechanical and wear properties of Ti–xNb–7Fe alloys designed for biomedical applications. Mater Des 111:592–599
Liu X, Shen Y, Yang R, Zou S, Ji X, Shi L et al (2012) Inkjet printing assisted synthesis of multicomponent mesoporous metal oxides for ultrafast catalyst exploration. Nano Lett 12(11):5733–5739
Ehtemam-Haghighi S, Liu Y, Cao G, Zhang LC (2016) Phase transition, microstructural evolution and mechanical properties of Ti–Nb–Fe alloys induced by Fe addition. Mater Des 97:279–286
Wohlers T, Campbell I, Huff R et al (2019) Wohlers report 2019: 3D printing and additive manufacturing state of the industry. Wohlers Associates, Fort Collins, CO
Gibson I, Rosen D, Stucker B (2015) Additive manufacturing technologies: 3D printing, rapid prototyping, and direct digital manufacturing, 2nd edn. Springer, New York
Murray CJ (2015) 3D Systems going mainstream in heathcare: 3D printing is becoming a go-to for customized healthcare solutions. Des News 70:27–28
Quinlan HE, Hasan T, Jaddou J et al (2017) Industrial and consumer uses of additive manufacturing: a discussion of capabilities, trajectories, and challenges. J Ind Ecol 21:S15–S20
Sandström CG (2016) The non-disruptive emergence of an ecosystem for 3D printing—insights from the hearing aid industry’s transition 1989–2008. Technol Forecast Soc Change 102:160–168
Jang TS, Kim D, Han G et al (2020) Powder based additive manufacturing for biomedical application of titanium and its alloys: a review. Biomed Eng Lett 10:505–516. https://doi.org/10.1007/s13534-020-00177-2
Kelly SM, Kampe SL (2004) Microstructural evolution in laser-deposited multilayer Ti-M-4V builds: Part I. Microstructural characterization. Metall Mater Trans-Phys Metall Mater Sci 35A:1861–1867
Xue W, Krishna BV, Bandyopadhyay A, Bose S (2007) Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater 3:1007–1018
Krishna BV, Bose S, Bandyopadhyay A (2007) Low stiffness porous Ti structures for load-bearing implants. Acta Biomater 3:997–1006
Kobryn PA, Semiatin SL (2001) The laser additive manufacture of Ti-6Al-4V. JOM 53:40–42
Heinl P, Rottmair A, Körner C, Singer RF (2007) Cellular titanium by selective electron beam melting. Adv Eng Mater 9:360–364
Harrysson OLA, CansiZoglu O, Marcellin-Little DJ, Cormier DR, West HA (2008) Direct metal fabrication of titanium implants with tailored materials and mechanical properties using electron beam melting technology. Mater Sci Eng C-Biomim Supramol Syst 28:366–373
Das S, Wohlert M, Beaman JJ, Bourell DL (1999) Processing of titanium net shapes by SLS/HIP. Mater Des 20:115–121
Hollander DA, Von Walter M, Wirtz T, Sellei R, Schmidt-Rohlfing B, Paar O et al (2006) Structural, mechanical and in vitro characterization of individually structured Ti–6Al–4V produced by direct laser forming. Biomaterials 27:955–963
Kumar S, Kruth J-P (2008) Wear performance of SLS/SLM materials. Adv Eng Mater 10:750–753
Li JP, Habibovic P, van Den Doel M, Wilson CE, de Wijn JR, van Blitterswijk CA et al (2007) Bone ingrowth in porous titanium implants produced by 3D fiber deposition. Biomaterials 28:2810–2820
Ryan GE, Pandit AS, Apatsidis DP (2008) Porous titanium scaffolds fabricated using a rapid prototyping and powder metallurgy technique. Biomaterials 29:3625–3635
Balla VK, Devasconcellos PD, Xue W, Bose S, Bandyopadhyay A (2009) Fabrication of compositionally and structurally graded Ti–TiO2 structures using laser engineered net shaping (LENS). Acta Biomater 5:1831–1837
Das M, Balla VK, Basu D, Bose S, Bandyopadhyay A (2010) Laser processing of SiC-particle-reinforced coating on titanium. Scr Mater 63:438–441
Das M, Bysakh S, Basu D, Sampath Kumar TS, Balla VK, Bose S et al (2011) Microstructure, mechanical and wear properties of laser processed SiC particle reinforced coatings on titanium. Surf Coat Technol 205:4366–4373
Zhang Y, Sahasrabudhe H, Bandyopadhyay A (2015) Additive manufacturing of Ti-Si-N ceramic coatings on titanium. Appl Surf Sci 346:428–437
Janaki Ram GD, Esplin CK, Stucker BE (2008) Microstructure and wear properties of LENS deposited medical grade CoCrMo. J Mater Sci Mater Med 19:2105–2111
Gaytan SM, Murr LE, Martinez E, Martinez JL, Machado BI, Ramirez DA et al (2010) Comparison of microstructures and mechanical properties for solid and mesh cobalt-base alloy prototypes fabricated by electron beam melting. Metall Mater Trans A 41:3216–3227
Chan BQY, Low ZWK, Heng SJW, Chan SY, Owh C, Loh XJ, Appl ACS (2016) ACS Appl Mater Interfaces 8:10070
Small W IV, Wilson TS, Benett WJ, Loge JM, Maitland DJ (2005) Opt Express 13:8204
Peterson GI, Dobrynin AV, Becker ML (2017) Adv Healthc Mater 6
Fernandes DJ, Peres RV, Mendes AM, Elias CN (2011) ISRN Dent 2011:1
Petrini L, Migliavacca F (2011) J Metall 2011
Lendlein A, Langer R (2002) Science (80–):296, 1673
Zhang D, George OJ, Petersen KM, Jimenez-Vergara AC, Hahn MS, Grunlan MA (2014) Acta Biomater 10:4597
Jung YC, Cho JW (2010) J Mater Sci Mater Med 21:2881
Paolini A, Kollmannsberger S, Rank E (2019) Additive manufacturing in construction: a review on processes, applications, and digital planning methods. Addit Manuf 30(July):100894. https://doi.org/10.1016/j.addma.2019.100894
Yuan L (2019) Solidification defects in additive manufactured materials. Jom 71(9):3221–3222. https://doi.org/10.1007/s11837-019-03662-x
Xhameni A, Cheng R, Farrow T (2022) A precision method for integrating shock sensors in the lining of sports helmets by additive manufacturing. IEEE Sens Lett 6(10):1–4. Article number 5000704. https://doi.org/10.1109/LSENS.2022.3205249
Novak JI (2020) A parametric method to customize surfboard and stand up paddle board fins for additive manufacturing. Comput Aided Des Appl 18(2):297–308. https://doi.org/10.14733/cadaps.2021.297-308
Graziosi S, Rosa F, Casati R, Solarino P, Vedani M, Bordegoni M (2017) Designing for metal additive manufacturing: a case study in the professional sports equipment field. Procedia Manuf 11:1544–1551. https://doi.org/10.1016/j.promfg.2017.07.288
Li Z, Wang S, Ye H, Lv L, Zhao X, Liu Y, Zhou Y (2020) Preliminary clinical application of complete workflow of digitally designed and manufactured sports mouthguards. Int J Prosthodont 33:99–104
Yanagi T, Kakura K, Tsuzuki T, Isshi K, Taniguchi Y, Hirofuji T, Kido H, Yoneda M (2019) Fabrication of mouthguard using digital technology. Dentistry 9:1000531
Takahashi M, Koide K, Mizuhashi F (2014) Optimal heating conditions for forming a mouthguard using a circle tray: effect of different conditions on the thickness and fit of formed mouthguards. J Prosthodont Res 58:171–176
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sollapur, S.B., Sharath, P.C., Waghmare, P. (2024). Applications of Additive Manufacturing in Biomedical and Sports Industry. In: Rajendrachari, S. (eds) Practical Implementations of Additive Manufacturing Technologies. Materials Horizons: From Nature to Nanomaterials. Springer, Singapore. https://doi.org/10.1007/978-981-99-5949-5_13
Download citation
DOI: https://doi.org/10.1007/978-981-99-5949-5_13
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-5948-8
Online ISBN: 978-981-99-5949-5
eBook Packages: EngineeringEngineering (R0)