Abstract
Recently, many reports have shown that the outcomes of Laparoscopic Kasai Portoenterostomy (Lap Kasai PE) are comparable to those of open Kasai portoenterostomy. We have established surgical procedures for Lap Kasai PE. We performed an umbilical Mercedes incision for multiple purposes. We prefer using the AirSeal® Intelligent Flow System as a pneumoperitoneum and a 3 mm bipolar cautery. Laparoscopic surgery is not very different from open surgery. The dissection of the porta hepatis is similar to that in laparotomy. The fibrous biliary remnants were not entirely resected.
Lap Kasai PE provides excellent visibility for the resection of the fibrous biliary remnants in the hilar plate, which can ensure good results. The results are expected to be equivalent to those of open surgery.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Laparoscopic Kasai portoenterostomy (Lap Kasai PE) was introduced by Esteves in 2002 [1]. With rapid advances in minimally invasive surgery, the replacement of open Kasai portoenterostomy with Lap Kasai PE is currently being considered [2, 3]. Recently, many reports have shown that the outcomes of Lap Kasai PE are comparable to those of open Kasai portoenterostomy [4,5,6].
We perform laparoscopic surgery for almost all cases of biliary atresia. We have established surgical procedures for Lap Kasai PE. The advantage of laparoscopic surgery is that it is minimally invasive; this means it is less painful, causes smaller wounds, and enables a faster recovery than open surgery. Laparoscopic surgery is beneficial not only for the patients, but also for the surgeons. The surgeons can visualize the hepatic hilum in a magnified view. They can share the surgical field using one monitor. All surgical procedures can be recorded and verified later. The recorded operative procedures can be easily performed throughout the body. The operative procedure we used for our patient is described below.
22.1 Preoperative Preparation
A decrease in bile excretion into the intestinal bilirubin can lead to vitamin K malabsorption; therefore, intravenous vitamin K should be administered before surgery to maintain coagulation ability. No other special pretreatment is performed for laparoscopic surgery.
22.2 Surgical Procedure
22.2.1 Position and Port Insertion
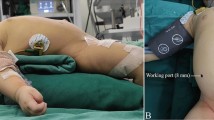
The patient was positioned at the foot of the operating table in a supine frog position under general anesthesia. The surgeon stood at the patient’s foot; an assistant with a camera was positioned at the patient’s left, and another assistant at the patient’s right. A multichannel port with two 5-mm ports was inserted from an umbilical Mercedes incision, which can provide a wide enough fascial opening with minimal esthetic impact (Fig. 22.1a). One was a camera port, and the other was an assistant port for suction or securing the field of view. We prefer using the AirSeal® Intelligent Flow System as a pneumoperitoneum to secure the working space and operative vision. This system ensures that the pneumoperitoneum pressure remains constant despite the opening and closing of the port and suction. Without this pneumoperitoneum device, it is difficult to deal with accidental bleeding. Two 3-mm ports were inserted into the right abdomen and upper left abdomen as the working ports. The upper left abdominal port was inserted considerably into the upper abdomen, assuming a hilar jejunal anastomosis. The other port inserted into the upper right abdomen was the assistant port (Fig. 22.1b).
22.2.2 Cholangiography
A laparoscope was inserted, and the liver surface was observed. If the Makibishi sign (Fig. 22.2a) is visible, it is a strong indicator of biliary atresia. In order to make a definitive diagnosis of biliary atresia and a rapid intraoperative pathological diagnosis, a liver biopsy must first be performed, followed by cholangiography. The gallbladder was punctured using a 20-G needle, and a contrast study was performed (Fig. 22.2b). The findings confirmed the diagnosis of biliary atresia.
22.2.3 Securing the Surgical Field
The teres hepatis ligament of the liver was pulled up toward the epigastric region and the gallbladder was pulled to the upper right abdomen to view the hepatic hilum (Figs. 22.2c and 22.2d).
22.2.4 Identification of Common Bile Duct Cord-like Tissue and Approaching the Hilar Region
The operative procedure was performed using 3-mm forceps. The fibrous remnants of the common bile duct were identified on the cranial side of the duodenum near the surface of the hepatoduodenal ligament and dissected with ligation. This cord-like tissue is easily found in most cases (Fig. 22.3a). If the cord-like tissue is firmly lined up in the hilum of the liver, the cranial margin must be searched, but if the cord-like tissue is considerably thin, this step is not necessary (Fig. 22.3b). The hepatic hilum was directly approached and detached. The cranial margin of the portal vein was found (Fig. 22.3c). If there are many fibrous remnants in the hepatic hilum, it takes some time to identify the portal vein (Fig. 22.3d).
22.2.5 Dissection of the Hepatic Hilum
After locating the cranial edge of the portal vein, the branches of the caudate lobe were cut off by bipolar cautery (Figs. 22.4a and 22.4b). The median branch of the hepatic artery was dissected since it crossed the hilum of the liver (Figs. 22.4c and 22.4d). First, the fibrous biliary remnants in the hepatic hilum were separated from the center of the porta hepatis toward the right porta hepatis (Fig. 22.5a). The fibrous biliary remnants were not entirely resected. A few remnants were preserved at the surface of the porta hepatis (Figs. 22.5b and 22.5c). The hepatic capsule must not be broken. The right porta hepatis extended to the point where the artery of the right anterior segment branch of the hepatic artery and portal vein entered the liver (Fig. 22.5d).
After the fibrous remnants in the right hepatic hilum were resected, the remaining fibrous remnants were separated from the center to the left porta hepatis (Fig. 22.6a). The depth of resection was the same on the right and left sides (Figs. 22.6b and 22.6c). Often, the fibrous remnants in the left are thinner than those on the right side. The left porta hepatis was dissected up to the point where the left branch of the portal vein entered the liver (Fig. 22.6d). Then, total resection of the fibrous remnants was performed at the porta hepatis (Fig. 22.6e).
Resection of fibrous remnants at left porta hepatis. The fibrous biliary remnants at the porta hepatis are not completely resected. (a, b, c, d) The fibrous biliary remnant cone is dissected from the center of the porta hepatis toward the left edge of the porta hepatis. (e) Total resection of fibrous remnants at the porta hepatic
22.2.6 Establishing the Roux-En-Y Limb with Exteriorization Via the Umbilical Mercedes Incision
We laparoscopically confirmed the presence of the ligament of Treitz and measured 15 cm of the jejunum. At 45 cm distal to the efferent cut end, the afferent limb was anastomosed. The roux limb was transferred in the retrocolic route, and an incision was made in the anterior wall. Then, the completed Roux-en-Y limb was returned to the abdominal cavity. After confirming that there were no twists in the intestinal tract, we closed the Petersen defect (Fig. 22.7a).
Portoenterostomy at the dorsal site of the porta hepatis. (a) Closure of Petersen’s defect. (b) First stitch: liver parenchyma at the right side of the porta hepatis. (c) Third stitch: liver parenchyma at the dorsal site of portal vein. (d) Third stitch: from liver parenchyma at the dorsal site of portal vein to Roux limb. (e) Fifth stitch: from liver parenchyma to the left edge of the dissection
22.2.7 Portoenterostomy
Portoenterostomy was performed between the liver parenchyma and the roux limb using 5–0 monofilament sutures. The anastomosis was started in the 9 o’clock position. The first suture was made from the right edge of the fibrous remnants to the hepatic parenchyma (Fig. 22.7b). On the dorsal side, the hepatic parenchyma behind the portal vein was anastomosed to the jejunum (Figs. 22.7c and 22.7d). The fifth suture was made from the liver at 2 o’clock position in the hilum to the left edge of the dissection (Fig. 22.7e).
The anterior wall was sutured from the liver toward the boundary between the hilar dissection and hepatic parenchyma (Fig. 22.8a). Three or four stitches were made. The portoenterostomy was performed, covering all the transecting fibrous biliary remnants (Fig. 22.8b).
Portoenterostomy at the abdominal site and postoperative wounds. (a) The anterior wall is sutured from the liver toward the boundary between the hilar dissection area and the liver parenchyma. (b) The portoenterostomy is covering all the transecting fibrous biliary remnants. (c) Glazing with anti-adhesive agents. (d) 2 months after the operation
22.2.8 Drain Insertion and Abdominal Closure
After cleaning the abdominal cavity, the muscular layer at the port insertion site was sutured. A 10-Fr break drain was inserted into the dorsal site of the portoenterostomy from the port skin incision on the right abdomen since the muscular layer in the peritoneal insertion site was different from that in the port insertion site. By doing so, it is possible to prevent the omentum from escaping after the drain is removed. Finally, the portoenterostomy site was glazed with anti-adhesive agents (Fig. 22.8c). The operation was thus completed. Postoperative wounds, including the shape of the navel, showed good healing (Fig. 22.8d).
22.3 Postoperative Management
Postoperative management was the same as for laparotomy. Antibiotics were discontinued 48 hours after the operation, and oral drinking was started 3 days after the operation. Oral prednisolone was started 5 days after the operation. The dose was reduced every 5 days in the following sequence: 4, 2, 1, to 0.5 mg/kg. Ursodeoxycholic acid was administered intravenously from the day after surgery, and oral Urso was (20 mg/kg) was prescribed when oral administration was started.
Laparoscopic surgery is not very different from open surgery. The dissection of the porta hepatis is similar to that in laparotomy. The fibrous biliary remnants were not entirely resected. A few remnants were preserved at the surface of the porta hepatis to ensure the hepatic capsule is not broken. However, if bleeding from the portal vein occurs, it should be stopped with the AirSeal® system. When bleeding occurs, it is necessary to use various techniques under laparoscopy, including the Pringle maneuver.
Lap Kasai PE provides excellent visibility for the resection of the fibrous biliary remnants in the hilar plate, which can ensure good results. The results are expected to be equivalent to those of open surgery.
References
Esteves E, et al. Laparoscopic Kasai portoenterostomy for biliary atresia. Pediatr Surg Int. 2002;18:737–40.
Lishuang M, et al. Laparoscopic portoenterostomy versus open portoenterostomy for the treatment of biliary atresia: a systematic review and meta-analysis of comparative studies. Pediatr Surg Int. 2015;31:261–9.
Hussain MH, et al. Outcomes of laparoscopic Kasai portoenterostomy for biliary atresia: a systematic review. J Pediatr Surg. 2017;52:264–7.
Murase N, et al. A new era of laparoscopic revision of Kasai Portoenterostomy for the treatment of biliary atresia. Biomed Res Int. 2015;2015:173014.
Nakamura H, et al. Comprehensive assessment of prognosis after laparoscopic portoenterostomy for biliary atresia. Pediatr Surg Int. 2016;32:109–12.
Shirota C, et al. Laparoscopic Kasai portoenterostomy is advantageous over open Kasai portoenterostomy in subsequent liver transplantation. Surg Endosc. 2020;34:3375–81.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Uchida, H., Shirota, C., Tainaka, T. (2021). Operative Procedures: Laparoscopic Kasai Procedure. In: Nio, M. (eds) Introduction to Biliary Atresia. Springer, Singapore. https://doi.org/10.1007/978-981-16-2160-4_22
Download citation
DOI: https://doi.org/10.1007/978-981-16-2160-4_22
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-2159-8
Online ISBN: 978-981-16-2160-4
eBook Packages: MedicineMedicine (R0)