Abstract
Lipids and essential fatty acids are required for normal brain development and continued photoreceptor membrane biogenesis for the maintenance of vision. The blood-brain barrier and blood-eye barriers prohibit the free diffusion of solutes into the brain and eye so that transporter-mediated uptake predominates at these barriers. The major facilitator superfamily of transporters constitutes one of the largest families of facilitative transporters across all domains of life. A unique family member, major facilitator superfamily domain containing 2a (Mfsd2a) is a lysophosphatidylcholine (LPC) transporter expressed at the blood-brain and blood-retinal barriers and demonstrated to be the major pathway for brain and eye accretion of docosahexaenoic acid (DHA) as an LPC. In addition to LPC-DHA, Mfsd2a can transport other LPCs containing mono- and polyunsaturated fatty acids. Mfsd2a deficiency in mouse and humans results in severe microcephaly, underscoring the importance of LPC transport in brain development. Beyond its role in brain development, LPC-DHA uptake in the brain and eye negatively regulates de novo lipogenesis. This review focuses on the current understanding of the physiological roles of Mfsd2a in the brain and eye and the proposed transport mechanism of Mfsd2a.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Major facilitator superfamily (MFS)
- Major facilitator superfamily domain containing 2a (Mfsd2a)
- Lipid transfer activity
- Lysolipids
- Brain
- Eye
14.1 Major Facilitator Superfamily
Lipids are organic compounds that are essential in living cells. Mammalian cell membranes are largely made up of glycerolipids, phospholipids, and cholesterol, organized into a lipid bilayer. The transport of molecules across this hydrophobic membrane is vital for cell growth, metabolism, and signal transduction, and are facilitated by transport proteins such as channels and transporters. Primary active transporters like ATP-binding cassette transporters are fueled by energy released from ATP hydrolysis. Conversely, secondary facilitative transporters do not utilize ATP hydrolysis for transport and can be generally categorized as facilitative or active facilitate. The former transports solutes down their concentration gradients across membranes, while active facilitative transporters transport solutes against their concentration gradients either as symporters or antiporters. Facilitative active transporters derive their energy for transport through coupling solute transport with the transport of ions such as sodium or protons down their concentration gradients across membranes [45, 60, 75].
The major facilitator superfamily (MFS) is one of the largest families of secondary transporters. The vast majority of characterized MFS transporters transport minimally polar hydrophilic substrates such as mono- and disaccharides, amino acids, and nucleosides [45, 53]. However, there are three MFS transporters that are exceptions to this general feature of the MFS family, namely, Spns2, Mfsd2a, and Mfsd2b [39, 52, 73]. Spns2 and Mfsd2b are sphingosine-1-phosphate transporters [39, 73], while Mfsd2a, the subject of this review article, is a lysophosphatidylcholine transporter.
MFS proteins have a highly conserved fold that is composed of 12 transmembrane alpha helices separated into two 6-transmembrane units that exhibit a pseudo twofold symmetry about an axis perpendicular to the membrane plane [45, 53]. Within each of these 6-transmembrane domains, the two 3-transmembrane units are organized as inverted repeats [45, 60, 75].
The majority of crystal structures of MFS proteins are bacterial proteins, with some exceptions being human glucose transporters [26, 27, 75], where the first MFS structures to be elucidated are that of Escherichia coli Lactose:H+ symporter (LacY) [1] and Escherichia coli Glycerol-3-phosphate:Pi antiporter (GlpT) [36]. A common transport mechanism that has been proposed as a result of these structural and biochemical studies is that substrates are transported in a rocker-switch, alternating access mechanism [75]. The N- and C- terminal domains rotate about the central substrate binding site and open exclusively to either the cytoplasm or extracellular space at any one time, rocking between an inward open or outward open state [1, 36, 45]. The four important transmembrane helices that surround the central pocket and essential for transport activity are domains 1, 4, 7, and 10. Transmembrane domains consisting of 2, 5, 8, and 11 or 3, 6, 9, and 12 are positioned just outside the core helices and mediate the interference between the N- and C- domains and support the structural integrity of the transporter, respectively [75].
14.2 Major Facilitator Superfamily Containing 2a
Major facilitator superfamily domain containing 2a (MFSD2A) was first identified by Angers et al. as an orphan transporter to be significantly induced in brown adipose tissue (BAT) of mice lacking both nuclear receptors retinoid-related orphan receptor alpha and gamma (RORα and RORγ) [5]. The Mfsd2a gene is approximately 14.3kb long, with 14 exons and 13 introns. Analysis of the amino acid sequence indicates it is most closely related to the bacterial-sodium melibiose symporter MelB at a 43–37% similarity [29]. Importantly, amino acid sequence of both mouse and human MFSD2A proteins is approximately 85% identical and is highly conserved from fish to human [11].
Mfsd2a is expressed in the brain, spinal cord, BAT, liver, kidney, lung, placenta, testes [11], and eye [74]. mRNA expression of Mfsd2a is greatly induced in murine liver and BAT during fasting and follows an oscillatory expression profile consistent with a circadian rhythm, with peak expression at circadian time 12 [5]. Additionally, Mfsd2a mRNA was also significantly upregulated exclusively in BATs by cold exposure and β-adrenergic receptor signaling pathway [5]. Berger et al. identified Mfsd2a to be induced by fasting and regulated by both peroxisome proliferator-activated receptor alpha (PPARα) and glucagon signaling in the liver, which turns over rapidly in liver upon refeeding [11]. While mRNA can be detected in BAT, Mfsd2a protein level is extremely low [11]. The function of Mfsd2a in BAT has not been determined.
14.3 Lipids and Essential Fatty Acids Are Important for Brain Growth
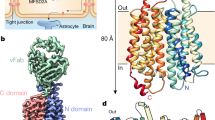
The brain is made up of glycerophospholipids, cholesterol, and sphingolipids, making it one of the most lipid-rich organs in the body [41]. Prenatal brain development is a complex developmental process that begins with the development of the neural tube, which ultimately differentiates into the brain and spinal cord. This is also the time where hundreds of specialized cell types come together, organizing a network of synaptic connectivity and a functioning blood-brain barrier (BBB) (Fig. 14.1) [6, 24, 61]. The BBB separates the brain from blood and serves to maintain a tightly controlled environment where toxins and pathogens are prevented from freely entering or leaving the brain by diffusion. The BBB is governed by tight junctions of endothelial cells of blood vessels, supported by astrocytes and pericytes [6, 24]. This is followed by postnatal brain growth, which is accompanied by the proliferation of astrocytes and oligodendrocytes [14, 28, 42] and myelination of axons and synaptogenesis [8, 28, 51]. Massive amounts of membrane phospholipids are therefore required for brain growth, where it has been postulated that lipids are derived exclusively from de novo biosynthesis within cells of the brain.
De novo lipogenic gene expression is controlled by sterol regulatory element-binding proteins (Srebp-1 and Srebp-2). In support of the vital role of de novo lipogenesis in brain development, the genetic deficiency of Scap, an essential chaperone protein for Srebp, in neurons in the developing central nervous system resulted in microcephaly and early postnatal lethality [65]. In addition, deficiency of Scap in mature astrocytes and oligodendrocytes have profound effects on myelination [71].
14.4 LPC-DHA Transport into the Brain
Docosahexaenoic acid (DHA) is an omega-3 fatty acid composed of 22 carbons and 6 double bonds. DHA can be synthesized by the liver through chain extension and desaturation of the essential fatty acid linolenic acid. DHA is highly enriched in brain phospholipids, particularly in the phosphatidylethanolamine (PE), phosphatidylserine (PS), and to a lesser extent, phosphatidylcholine (PC) pools within membranes, and comprises up to 15% or more of the total fatty acid composition of the prefrontal cortex [13, 50]. In humans, DHA is rapidly taken up as early as the end of the second trimester, coinciding with the development of the BBB where considerable amounts of membrane phospholipids are required for the growing brain [17, 38, 61]. DHA is continuously acquired from early postnatal days until approximately 2 years of age [23, 47, 66]. While DHA supplementation studies in term infants or pregnant and lactating women have been inconclusive for enhancing cognitive development [25, 31, 55], DHA supplementation in preterm infants has shown some benefit to cognitive development, presumably because preterm infants might have lower brain DHA levels [7, 18]. Likewise, decreased levels of DHA in the developing brain have been associated with negative effects on cognitive function [33, 49] and neurodevelopmental disorders [19, 35, 48]. Importantly, DHA itself cannot be de novo synthesized and must be transported across the BBB into brain.
The form by which DHA gets taken up into brain, either as unesterified DHA or DHA esterified as lysophosphatidylcholine-DHA (LPC-DHA), has been a point of debate. LPCs circulate in blood bound to albumin [20, 56, 67] where it was first shown by Illingworth and Portman to be taken up and reacylated readily in brains of squirrel monkeys [37]. As early as 1965, it was hypothesized by Switzer and Eder that plasma LPCs serve as precursors for the renewal of cellular membranes [67]. Importantly, Thiès et al. reported a preference for unsaturated fatty acids esterified as 2-acyl-LPC in young rat brains where LPC-DHA was transported 12-fold more than unesterified DHA, suggesting that LPCs might be an efficient delivery of polyunsaturated fatty acids (PUFAs) into the developing brain [69, 70]. Moreover, Lagarde et al. was the first to propose that LPC is the preferred carrier of PUFAs like DHA or arachidonic acid (AA) to the brain [43]. As will be further discussed below, Mfsd2a is the LPC transporter that explains the LPC transport activity first described by Lagarde and co-workers. More recently, it was demonstrated that supplementing adult mice with dietary LPC-DHA, but not unesterified DHA, were able to increase brain DHA levels twofold [64]. Collectively, these findings support the conclusion that LPC-DHA, and not unesterified DHA, is the primary carrier of DHA delivery to the brain. However, Mfsd2a KO mice have residual phospholipid containing DHA in the brain and eye, indicating the possibility of either compensatory de novo biosynthesis, other transport mechanisms, or acquisition of DHA during embryogenesis in the brain and eye prior to blood-barrier formation. It is important to note that single cell sequencing projects and bulk RNA-seq of the blood-brain barrier in mice [68, 72, 79] have shown that mRNA expression for proteins proposed to be involved in the uptake of unesterified DHA by the BBB endothelium, such as LPL, and its essential chaperone GPIHBP1 [78], CD36, and FATP1-6 (Slc27a1-6), and ACSL6 are not expressed by the endothelium of the BBB.
14.5 Mfsd2a Deficiency in the Brain
Importantly, Nguyen et al. and Ben-Zvi et al. discovered Mfsd2a to be highly expressed at the endothelium of the BBB [10, 52]. Through targeted lipidomic analysis, Mfsd2a was found to be the major pathway for brain DHA accretion, where a significant 60–70% reduction in steady-state levels of total percentage DHA-containing phospholipids was observed in brains of 2aKO mice relative to wild-type controls [15, 52]. Conversely, brains of 2aKO mice had a modest 35% increase in steady-state levels of total percentage AA-containing phospholipids [52], a phenomenon commonly observed in rodent models of DHA deficiency [62].
More recently, using endothelial-specific and inducible endothelial-specific Mfsd2a deletion mouse models, Chan et al. showed that Mfsd2a deficiency results in a unique form of postnatal microcephaly, with DHA deficiency preceding the onset of microcephaly [15]. Only adult 2aKO mice exhibit a minor loss of Purkinje cells in the cerebellum and a decrease in neuronal cell density in the CA1 and CA3 regions of the hippocampus [52]. Because the brains of 2aKO embryos are deficient in DHA but are not microcephalic until postnatal life, these cell loss phenotypes are secondary events. These findings also indicate that DHA deficiency is an unlikely cause underlying microcephaly, but rather the absence of bulk LPC transport, where LPCs are phospholipid membrane building blocks.
Recently, transcriptomic and lipidomic analysis in Mfsd2a deficiency mouse models was used as a tool to understand how the brain adapts to DHA deficiency, thus revealing functions of DHA in the brain [15]. It was discovered that Mfsd2a deficiency resulted in a de-repression of the Srebp1 and Srebp2 pathways leading to an increase in de novo synthesis of unsaturated fatty acids in phospholipids. It was shown that Mfsd2a is expressed in neural stem cells (NSCs) isolated from early postnatal mice and that NSCs treated with LPC-DHA and other LPC-PUFAs can acutely downregulate Srebp1 and Srebp2 target gene expression in an Mfsd2a-dependent fashion and that the mechanism is in part through inhibition of Srebp-1 receptor processing [15]. Moreover, Mfsd2a itself is regulated by Srebp, forming a negative feedback loop on Srebp processing that can balance de novo lipogenesis with exogenous uptake of LPC-DHA. The regulation of brain Srebp function by LPC-DHA transported by Mfsd2a might serve the purpose of fine-tuning membrane phospholipid saturation and hence biophysical properties during brain development [15].
Another reported feature of Mfsd2a deficiency in the brain and eye is that Mfsd2a knockout mice have increased transcytosis resulting in increased BBB permeability [4, 10]. It has been suggested that the microcephaly and DHA deficiency in 2aKO mice could be due to a leaky BBB, but it is unclear how a leaky BBB would result in less DHA uptake and not more relative to wild-type (WT) mice. Nonetheless, this issue has been resolved in that BBB permeability, but not microcephaly and DHA deficiency, can be completely rescued in Mfsd2a-deficient mice by genetic deficiency of Cav1 [4]. Andreone et al. generated a transporter-dead Mfsd2a knockin mouse model bearing a D96A aspartate to alanine point mutation, a conserved residue with D97 in the human Mfsd2a constituting the sodium binding site, and showed that consistent with the lack of transport activity, Mfsd2aD96A/D96A mice exhibited microcephaly and DHA deficiency in the brain [4]. These findings indicate that microcephaly and DHA deficiency are primary phenotypes of Mfsd2a deficiency, and not a result of a leaky BBB [4], and that LPC transport via Mfsd2a is essential for DHA accretion and postnatal brain growth. Of note, the increased transcytosis phenotype in the BBB or blood-retina barrier of 2aKO mice reported by the Gu lab [4, 10, 16] has not been observed in other studies [46, 74].
14.6 Mfsd2a Deficiency in the Eye
The retina is a highly organized structure, with photoreceptors (PR), extensive retinal glial network, and retinal pigment epithelium (RPE) organized into distinct layers. Rods and cones are the two types of PR found in mammalian eyes, which make up 70% majority of cells in the retina. DHA, localized with rhodopsin [30], is found primarily in phospholipids of membrane discs that make up rod PR outer segments (OS), making the retina a tissue with the highest concentration of DHA per unit area in the body [58]. With daily daylight exposure, OS discs which are photosensitive, accumulate photo-damaged proteins and lipids [9] and must be synthesized continuously throughout one’s lifetime for the maintenance of healthy vision [63, 77]. The villi-containing apical membrane of the RPE is particularly important for this renewal process, where through its interaction with the distal ends of the OS (Fig. 14.2) facilitates the daily phagocytosis of OS discs that make up one-tenth of the OS length. This process of phagocytosis is balanced with an equal rate of disc regeneration, so that the OS length is maintained [76], thus highlighting the importance of lipids and essential fatty acids for membrane biogenesis and turnover.
Blood-retinal barrier. The BRB is made up of the inner BRB, formed by tight junctions (TJ) of the endothelium of retinal capillaries (EC), supported by pericytes (P), astrocytes (A) and Müller cells (MC). The outer BRB is governed by TJ of the retinal pigment epithelium (RPE). DHA is found primarily in phospholipids of the outer segment (OS) discs of rod and cones and interact closely with the apical membrane villi of the RPE. As photo-damaged discs need to undergo a constant renewal process for the maintenance of vision, inner segments that contain metabolic machinery synthesize new membrane discs that move along the length of the OS where they are eventually phagocytosed by the RPE. CB cell body, IS inner segments, OS outer segments, AM apical membrane, RPE retinal pigment epithelium, BI basal infoldings, Ch choroid
Similar to the BBB of the brain, the eye contains cellular barriers that prevent the diffusion of blood-borne material or lipids from entering the retina freely. The eye contains two blood-eye barriers (Fig. 14.2), the inner blood-retina barrier (inner BRB) that is established by tight junctions between retinal endothelial cells, supported by the pericytes, astrocytes, and Müller cells [22, 59] and the outer BRB that is governed by tight junctions of the RPE [21].
Mfsd2a is expressed at the endothelium of the BRB and RPE. The RPE is the major site of Mfsd2a expression and is quantitatively important for DHA accretion into the retina via LPC-DHA transport [74] (Fig. 14.3). Whole-body Mfsd2a-deficient (2aKO) mice displayed a unique form of a slow, progressive retina degeneration [46, 74]. However, a 40% deficiency in phospholipids containing DHA in eyes of 2aKO mice did not result in the expected severe and rapid retinal degeneration nor significant visual dysfunction [46, 74]. Like the brain, upregulation of de novo lipogenesis pathways was observed in eyes of 2aKO mice which might serve, as a compensatory mechanism to synthesize new OS discs in the absence of Mfsd2a [46, 74]. In addition, the BRB was found to be intact in 2aKO mice [46, 74], which is inconsistent with a report that Mfsd2a is required to suppresses transcytosis for the development and maintenance of a functional BRB [16]. This discrepancy is not due to strain-specific differences as the strain used in the Lobanova study was the same as reported by Chow et al. [16]. The most remarkable finding from studying 2aKO retinas is that phototransduction tested by electroretinography [74] or light evoked potential recordings of single rods [46] indicated that phototransduction in 2aKO and WT was indistinguishable. These findings might suggest that the compensatory changes in lipid composition in 2aKO retinas of increased monounsaturated fatty acids and arachidonic acid in phospholipid pools might compensate for the severe reduction in DHA.
MFSD2A transports LPC-DHA across the BBB and BRB. DHA can come preformed from the diet or its precursor linolenic acid, conjugated to LPC in the liver, and transported in blood plasma bound to albumin. At the BBB, Mfsd2a translocates LPC-DHA across the endothelial plasma membrane into the brain. Mfsd2a is expressed at both the inner and outer BRB, but Mfsd2a at the RPE is the major route by which LPC-DHA gets into the eye. IS inner segments, OS outer segments, RPE retinal pigment epithelium, Ch choroid
14.7 Inactivating Mutations of MFSD2A in Humans
To date, four unrelated consanguineous families with homozygous non-synonymous inactivating mutations in MFSD2A have been identified that presented with severe microcephaly and intellectual impairments [2, 32, 34]. The first two families, one from Libya and the other from Egypt, harbored a p.Thr159Met or p.Ser166Leu protein change [32]. Mutant Mfsd2a proteins were stably expressed and localized to the plasma membrane when expressed in HEK 293 cells, comparable to WT Mfsd2a, but had complete inactivation of transport activity [32]. The third family from Pakistan was a large pedigree, with ten affected family members harboring a p.Ser339Leu protein change that presented with severe non-lethal microcephaly [2]. Again, mutant Mfsd2a proteins were stably expressed and had proper membrane localization when expressed in HEK 293 cells but exhibited a partial inactivation of transport activity relative to WT protein [2]. A fourth family was identified in Israel that harbored a p.Pro402His protein change, with complete inactivation of transport activity, and presented with severe non-lethal microcephaly [34]. Consistent with reduced or complete inactivation of transport activity that would be expected to reduce brain and eye LPC uptake, increased plasma LPC levels have been observed in all affected family members [2, 32, 34]. In further support of this explanation for increased plasma LPC in patients with inactivating mutations in Mfsd2a, plasma LPC levels were also found to be increased by 40% in 2aKO mice, consistent with 85–90% reduction in LPC transport in the brain and eye using tracer studies [32, 52, 74].
Both p.Thr159Met and p.Ser166Leu mutations were found on transmembrane domain 4 of Mfsd2a, p.Ser339Leu was found on transmembrane domain 8, while p.Pro402His was found on the extracellular loop between transmembrane 10 and 11. A molecular explanation for the loss-of-function caused by Ser166Leu and Pro402His is not known. However, Thr159met is homologous to Thr121 in MelB, which is essential for establishing a hydrogen bond with conserved aspartate residues at the sodium binding pocket. Therefore, it can be predicted that Thr159Met inactivity is a consequence of absence of sodium binding [32].
14.8 Proposed Transport Mechanism of Mfsd2a
Mfsd2a does not transport unesterified PUFAs, but PUFAs esterified as a LPC [52]. It was determined through structure-activity relationship studies that lysophospholipid with a minimal acyl chain length of 14 carbons and a zwitterionic headgroup (e.g., PC, PE, and PS) is essential for transport by Mfsd2a [52]. More recently, Quek et al. showed that the acyl-carnitines can also be transported by Mfsd2a, again underscoring the importance of a zwitterionic headgroup and not strictly a phosphorylcholine headgroup as a necessary feature for lysolipid transport [57]. Notably, Mfsd2a has a higher transport capacity for LPCs having unsaturated fatty acids like DHA relative to LPCs with saturated fatty acids like palmitate [52]. This latter finding is important, because it indicates that LPC transport capacity is inversely correlated with the physiological levels of LPCs in human plasma, where LPC-palmitate is the most abundant [2, 32, 56]. Presumably, this preference for LPC-PUFA by Mfsd2a would allow the brain to obtain the lower abundant essential fatty acids diluted in a larger milieu of LPCs containing non-essential fatty acids.
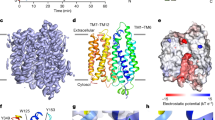
Using homology modeling based upon crystal structures of MelB and LacY, and further refinement by site-directed mutagenesis and biochemical transport analysis, Quek et al. identified the following four important structural features of human Mfsd2a: a sodium binding site, a hydrophobic cleft, a lipid phosphate headgroup binding residue (Lys436), and ionic locks [57]. The hydrophobic cleft is likely involved in LPC acyl chain binding, while the Lys436 is involved in coordinating the LPC phosphate headgroup interaction. The ionic locks are presumably involved in stabilizing the outward open conformation during the transport cycle as previously proposed for similar ionic locks identified on MelB [29]. This proposed model of transport co-opts the standard rocker-switch model, with the exception that LPCs bound to albumin would first bind to the outer leaflet of the plasma membrane and diffuse laterally into Mfsd2a facing the outward open conformation until hydrophobic forces position the acyl chain of the LPC into the hydrophobic cleft and headgroup binding to Lys436 (Fig. 14.4). Sodium binding to its binding site comprising residues Asp93, Asp97, and Thr159 would drive a conformational change to an inward-open conformation that would push the LPC-DHA down along the hydrophobic cleft and flip over to the inner lipid leaflet, where it exits the transporter by diffusing laterally along the inner membrane [57]. This “flipping” activity would in theory allow LPCs to bypass the tight junctions of the BBB endothelium [12]. Once LPCs reach other cells at the BBB such as astrocytes, it could be converted to PC-DHA through activity of the LPCATs [44].
Proposed mechanism of LPC transport. (a) Mfsd2a in the outward-open conformation facing the extracellular surface. (b) Sodium binds to sodium binding site, while LPC diffuses laterally into the central cavity and binds to Lys436. (c) In the presence of sodium, Mfsd2a undergoes a conformational change, and LPC “flips” along hydrophobic cleft. (d) LPC diffuses laterally into the inner leaflet and converts to PC-DHA
14.9 Concluding Remarks
Mfsd2a is a sodium-dependent lysophosphatidylcholine co-transporter highly expressed at the blood-brain barrier and blood-eye barriers that is essential for normal human brain development. Mfsd2a shows high specificity for the transport of LPCs with long chain and unsaturated fatty acyl chains. LPC-DHA in particular negatively regulates Srebp activity during brain development, and this function is likely important to maintain proper membrane phospholipid saturation. An important question that remains to be answered is a determination of the transport mechanism of LPCs by Mfsd2a. This determination awaits the development of new biochemical assays to reconstitute transport on purified Mfsd2a and the determination of atomic resolution structures. Interestingly, Mfsd2a is expressed by other cell types and tissues such as liver and a determination of the function of Mfsd2a outside of the brain and eye will likely reveal new biology into the function of LPCs. For example, Piccirillo and others have shown that Mfsd2a is required for the maintenance of memory T cells [54], perhaps in part through TOX, which might regulate Mfsd2a [3, 40]. Lastly, a word of caution, many recent papers have been published using non-validated Mfsd2a antibodies that are likely leading to erroneous conclusions on the regulation of, site of, expression of, and involvement of Mfsd2a in particular biological and pathophysiological processes. It is critical that Mfsd2a antibodies be validated using both cell-based overexpression and Mfsd2a deficiency cell or mouse models.
References
Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S (2003) Structure and mechanism of the lactose permease of Escherichia coli. Science 301:610–615
Alakbarzade V, Hameed A, Quek DQ, Chioza BA, Baple EL, Cazenave-Gassiot A, Nguyen LN, Wenk MR, Ahmad AQ, Sreekantan-Nair A et al (2015) A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet 47:814–817
Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim HE, Roelli P, Utzschneider DT, von Hoesslin M, Cullen JG, Fan Y et al (2019) TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 571:265–269
Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C (2017) Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron 94:581–594. e585
Angers M, Uldry M, Kong D, Gimble JM, Jetten AM (2008) Mfsd2a encodes a novel major facilitator superfamily domain-containing protein highly induced in brown adipose tissue during fasting and adaptive thermogenesis. Biochem J 416:347–355
Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K et al (2010) Pericytes regulate the blood-brain barrier. Nature 468:557–561
Baack ML, Puumala SE, Messier SE, Pritchett DK, Harris WS (2016) Daily enteral DHA supplementation alleviates deficiency in premature infants. Lipids 51:423–433
Baumann N, Pham-Dinh D (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871–927
Bazan NG (2006) Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci 29:263–271
Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C (2014) Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509:507–511
Berger JH, Charron MJ, Silver DL (2012) Major facilitator superfamily domain-containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism. PLoS One 7:e50629
Betsholtz C (2015) Lipid transport and human brain development. Nat Genet 47:699–701
Carver JD, Benford VJ, Han B, Cantor AB (2001) The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Res Bull 56:79–85
Catalani A, Sabbatini M, Consoli C, Cinque C, Tomassoni D, Azmitia E, Angelucci L, Amenta F (2002) Glial fibrillary acidic protein immunoreactive astrocytes in developing rat hippocampus. Mech Ageing Dev 123:481–490
Chan JP, Wong BH, Chin CF, Galam DLA, Foo JC, Wong LC, Ghosh S, Wenk MR, Cazenave-Gassiot A, Silver DL (2018) The lysolipid transporter Mfsd2a regulates lipogenesis in the developing brain. PLoS Biol 16:e2006443
Chow BW, Gu C (2017) Gradual suppression of transcytosis governs functional blood-retinal barrier formation. Neuron 93:1325–1333. e1323
Clandinin MT, Chappell JE, Heim T, Swyer PR, Chance GW (1981) Fatty acid utilization in perinatal de novo synthesis of tissues. Early Hum Dev 5:355–366
Collins CT, Sullivan TR, McPhee AJ, Stark MJ, Makrides M, Gibson RA (2015) A dose response randomised controlled trial of docosahexaenoic acid (DHA) in preterm infants. Prostaglandins Leukot Essent Fat Acids 99:1–6
Colombo J, Kannass KN, Shaddy DJ, Kundurthi S, Maikranz JM, Anderson CJ, Blaga OM, Carlson SE (2004) Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev 75:1254–1267
Croset M, Brossard N, Polette A, Lagarde M (2000) Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem J 345(Pt 1):61–67
Cunha-Vaz J (1979) The blood-ocular barriers. Surv Ophthalmol 23:279–296
Cunha-Vaz JG, Shakib M, Ashton N (1966) Studies on the permeability of the blood-retinal barrier. I. On the existence, development, and site of a blood-retinal barrier. Br J Ophthalmol 50:441–453
Cunnane SC, Francescutti V, Brenna JT, Crawford MA (2000) Breast-fed infants achieve a higher rate of brain and whole body docosahexaenoate accumulation than formula-fed infants not consuming dietary docosahexaenoate. Lipids 35:105–111
Daneman R, Zhou L, Kebede AA, Barres BA (2010) Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468:562–566
Delgado-Noguera MF, Calvache JA, Bonfill Cosp X (2010) Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development Cochrane Database Syst Rev:CD007901
Deng D, Sun P, Yan C, Ke M, Jiang X, Xiong L, Ren W, Hirata K, Yamamoto M, Fan S et al (2015) Molecular basis of ligand recognition and transport by glucose transporters. Nature 526:391–396
Deng D, Xu C, Sun P, Wu J, Yan C, Hu M, Yan N (2014) Crystal structure of the human glucose transporter GLUT1. Nature 510:121–125
Dobbing J, Sands J (1979) Comparative aspects of the brain growth spurt. Early Hum Dev 3:79–83
Ethayathulla AS, Yousef MS, Amin A, Leblanc G, Kaback HR, Guan L (2014) Structure-based mechanism for Na(+)/melibiose symport by MelB. Nat Commun 5:3009
Fliesler SJ, Anderson RE (1983) Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res 22:79–131
Gould JF, Smithers LG, Makrides M (2013) The effect of maternal omega-3 (n-3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 97:531–544
Guemez-Gamboa A, Nguyen LN, Yang H, Zaki MS, Kara M, Ben-Omran T, Akizu N, Rosti RO, Rosti B, Scott E et al (2015) Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet 47:809–813
Guesnet P, Alessandri JM (2011) Docosahexaenoic acid (DHA) and the developing central nervous system (CNS) – implications for dietary recommendations. Biochimie 93:7–12
Harel T, Quek DQY, Wong BH, Cazenave-Gassiot A, Wenk MR, Fan H, Berger I, Shmueli D, Shaag A, Silver DL et al (2018) Homozygous mutation in MFSD2A, encoding a lysolipid transporter for docosahexanoic acid, is associated with microcephaly and hypomyelination. Neurogenetics 19:227–235
Heird WC, Lapillonne A (2005) The role of essential fatty acids in development. Annu Rev Nutr 25:549–571
Huang Y, Lemieux MJ, Song J, Auer M, Wang DN (2003) Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science 301:616–620
Illingworth DR, Portman OW (1972) The uptake and metabolism of plasma lysophosphatidylcholine in vivo by the brain of squirrel monkeys. Biochem J 130:557–567
Innis SM (2005) Essential fatty acid transfer and fetal development. Placenta 26(Suppl A):S70–S75
Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N (2009) The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 323:524–527
Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, Werner MT, Huang AC, Alexander KA, Wu JE et al (2019) TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature 571:211–218
Korade Z, Kenworthy AK (2008) Lipid rafts, cholesterol, and the brain. Neuropharmacology 55:1265–1273
Kriegstein A, Alvarez-Buylla A (2009) The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 32:149–184
Lagarde M, Bernoud N, Brossard N, Lemaitre-Delaunay D, Thies F, Croset M, Lecerf J (2001) Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J Mol Neurosci 16:201–204; discussion 215-221
Lands WE (1960) Metabolism of glycerolipids. 2. The enzymatic acylation of lysolecithin. J Biol Chem 235:2233–2237
Law CJ, Maloney PC, Wang DN (2008) Ins and outs of major facilitator superfamily antiporters. Annu Rev Microbiol 62:289–305
Lobanova ES, Schuhmann K, Finkelstein S, Lewis TR, Cady MA, Hao Y, Keuthan C, Ash JD, Burns ME, Shevchenko A et al (2019) Disrupted blood-retina lysophosphatidylcholine transport impairs photoreceptor health but not visual signal transduction. J Neurosci 39:9689–9701
Martinez M (1992) Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr 120:S129–S138
Martinez M (1996) Docosahexaenoic acid therapy in docosahexaenoic acid-deficient patients with disorders of peroxisomal biogenesis. Lipids 31(Suppl):S145–S152
McNamara RK (2010) DHA deficiency and prefrontal cortex neuropathology in recurrent affective disorders. J Nutr 140:864–868
McNamara RK, Liu Y, Jandacek R, Rider T, Tso P (2008) The aging human orbitofrontal cortex: decreasing polyunsaturated fatty acid composition and associated increases in lipogenic gene expression and stearoyl-CoA desaturase activity. Prostaglandins Leukot Essent Fat Acids 78:293–304
Micheva KD, Beaulieu C (1996) Quantitative aspects of synaptogenesis in the rat barrel field cortex with special reference to GABA circuitry. J Comp Neurol 373:340–354
Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, Silver DL (2014) Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509:503–506
Pao SS, Paulsen IT, Saier MH Jr (1998) Major facilitator superfamily. Microbiol Mol Biol Rev 62:1–34
Piccirillo AR, Hyzny EJ, Beppu LY, Menk AV, Wallace CT, Hawse WF, Buechel HM, Wong BH, Foo JC, Cazenave-Gassiot A et al (2019) The lysophosphatidylcholine transporter MFSD2A is essential for CD8(+) memory T cell maintenance and secondary response to infection. J Immunol 203:117–126
Qawasmi A, Landeros-Weisenberger A, Leckman JF, Bloch MH (2012) Meta-analysis of long-chain polyunsaturated fatty acid supplementation of formula and infant cognition. Pediatrics 129:1141–1149
Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL et al (2010) Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res 51:3299–3305
Quek DQ, Nguyen LN, Fan H, Silver DL (2016) Structural insights into the transport mechanism of the human sodium-dependent lysophosphatidylcholine transporter Mfsd2a. J Biol Chem 291:9383–9394
SanGiovanni JP, Chew EY (2005) The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res 24:87–138
Shakib M, Cunha-Vaz JG (1966) Studies on the permeability of the blood-retinal barrier. IV. Junctional complexes of the retinal vessels and their role in the permeability of the blood-retinal barrier. Exp Eye Res 5:229–234
Shi Y (2013) Common folds and transport mechanisms of secondary active transporters. Annu Rev Biophys 42:51–72
Silbereis JC, Pochareddy S, Zhu Y, Li M, Sestan N (2016) The cellular and molecular landscapes of the developing human central nervous system. Neuron 89:248–268
Simopoulos AP (2008) The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 233:674–688
Steinberg RH (1985) Interactions between the retinal pigment epithelium and the neural retina. Doc Ophthalmol 60:327–346
Sugasini D, Thomas R, Yalagala PCR, Tai LM, Subbaiah PV (2017) Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci Rep 7:11263
Suzuki R, Ferris HA, Chee MJ, Maratos-Flier E, Kahn CR (2013) Reduction of the cholesterol sensor SCAP in the brains of mice causes impaired synaptic transmission and altered cognitive function. PLoS Biol 11:e1001532
Svennerholm L (1968) Distribution and fatty acid composition of phosphoglycerides in normal human brain. J Lipid Res 9:570–579
Switzer S, Eder HA (1965) Transport of lysolecithin by albumin in human and rat plasma. J Lipid Res 6:506–511
Tabula Muris C, Overall c, Logistical c, Organ c, processing, Library, p., sequencing, Computational data a, Cell type a, Writing g et al (2018) Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562:367–372
Thies F, Delachambre MC, Bentejac M, Lagarde M, Lecerf J (1992) Unsaturated fatty acids esterified in 2-acyl-l-lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. J Neurochem 59:1110–1116
Thies F, Pillon C, Moliere P, Lagarde M, Lecerf J (1994) Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Am J Phys 267:R1273–R1279
van Deijk AF, Camargo N, Timmerman J, Heistek T, Brouwers JF, Mogavero F, Mansvelder HD, Smit AB, Verheijen MH (2017) Astrocyte lipid metabolism is critical for synapse development and function in vivo. Glia 65:670–682
Vanlandewijck M, He L, Mae MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Lavina B, Gouveia L et al (2018) A molecular atlas of cell types and zonation in the brain vasculature. Nature 560:475–480
Vu TM, Ishizu AN, Foo JC, Toh XR, Zhang F, Whee DM, Torta F, Cazenave-Gassiot A, Matsumura T, Kim S et al (2017) Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets. Nature 550:524–528
Wong BH, Chan JP, Cazenave-Gassiot A, Poh RW, Foo JC, Galam DL, Ghosh S, Nguyen LN, Barathi VA, Yeo SW et al (2016) Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid (DHA) in eye and is important for photoreceptor cell development. J Biol Chem 291:10501–10514
Yan N (2015) Structural biology of the major facilitator superfamily transporters. Annu Rev Biophys 44:257–283
Young RW (1967) The renewal of photoreceptor cell outer segments. J Cell Biol 33:61–72
Young RW (1976) Visual cells and the concept of renewal. Invest Ophthalmol Vis Sci 15:700–725
Young SG, Davies BS, Voss CV, Gin P, Weinstein MM, Tontonoz P, Reue K, Bensadoun A, Fong LG, Beigneux AP (2011) GPIHBP1, an endothelial cell transporter for lipoprotein lipase. J Lipid Res 52:1869–1884
Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N et al (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34:11929–11947
Acknowledgments
This work was supported by grants from the National Research Foundation, Singapore (NRF-NRFI2017-05 to D.L.S.), and the Ministry of Health (MOH-000217-00).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Wong, B.H., Silver, D.L. (2020). Mfsd2a: A Physiologically Important Lysolipid Transporter in the Brain and Eye. In: Jiang, XC. (eds) Lipid Transfer in Lipoprotein Metabolism and Cardiovascular Disease. Advances in Experimental Medicine and Biology, vol 1276. Springer, Singapore. https://doi.org/10.1007/978-981-15-6082-8_14
Download citation
DOI: https://doi.org/10.1007/978-981-15-6082-8_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-6081-1
Online ISBN: 978-981-15-6082-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)