Abstract
Myocardial ischemia and reperfusion cause injury to the heart in myocardial ischemic disease. Both processes increase autophagy. In this chapter, we will provide an overview of the autophagic mechanism caused by myocardial ischemia/reperfusion injury and the role of autophagy in myocardial ischemia/reperfusion injury.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Coronary artery disease arises from luminal stenosis or occlusion caused by atherosclerosis lesions in the coronary artery, resulting in myocardial ischemia, hypoxia, or necrosis. Based on severity, this condition is divided into five clinical types: asymptomatic myocardial ischemia (occult coronary artery disease), angina pectoris, myocardial infarction, ischemic heart failure (ischemic heart disease), and sudden death. The total number of patients with coronary artery diseases in China is approximately 130,000,000, and approximately 1,000,000 people die of various coronary heart diseases every year. Therefore, coronary artery disease is the most common disease severely threatening people’s health. Most patients die of acute myocardial infarction or sudden death. The main reasons are myocardial ischemic necrosis and malignant arrhythmia or cardiogenic shock after complete obstruction of the coronary artery caused by coronary vasospasm or intravascular atherosclerotic plaque rupture due to tiredness, stress, and smoking. The main symptom of myocardial infarction is typical chest pain in the retrosternal or precordial region, accompanied by pyrexia, diaphoresis, panic, nausea, and vomiting. Approximately one-third of patients show sudden death in the first onset. The current main treatment is to alleviate stenocardia symptoms, quickly recover the blocked blood flow, and reduce the myocardial infarction range and long-term mortality rate.
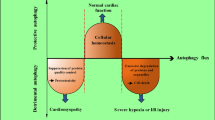
At the time of myocardial infarction, the coronary artery participating in supplying nutrients and oxygen to the myocardium is occluded; hence, necessary molecules for cell survival are lacking. When the artery is reopened, the surviving cells become injured by the sudden increase in oxygen. The whole process is called ischemia/reperfusion (IR), which induces a series of expression changes in proteins and genes associated with death promotion and survival inhibition. Because the cells are injured, the contraction capability of the heart is reduced, accompanied by post-injury remodeling. Early manifestations of cardiac remodeling include ventricular dilatation, cardiomyocyte hypertrophy, increased cytokine levels, and autorhythmicity changes in cardiomyocytes, thus maintaining cardiac function. Manifestations at late remodeling include fibrosis, collagen deposition, calcium flow disorder, thinner ventricular walls, and persistently increased cell death rate, finally leading to heart failure. The death of cardiomyocytes under ischemia is an initiating factor for cardiac remodeling; thus, exploration of protective mechanisms for avoiding cardiomyocyte death is necessary.
In theory, under ischemia and hypoxia, cardiomyocytes die via apoptosis or necrosis. In-depth studies have revealed that autophagy can regulate the survival of cardiomyocytes under ischemia and hypoxia.
1 Reasons for Autophagy Induced by IR
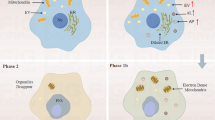
Recently, multiple studies have demonstrated that the number of autophagosomes formed in the myocardial ischemic stage or reperfusion stage is significantly increased. Sybers et al. found that autophagosomes were generated in the mouse embryonic heart under reoxygenation conditions after hypoxia and glucose deprivation (Sybers et al. 1976). Decker and Gurusamy showed that ischemia for 20 min alone could not induce autophagy in the Langendorff heart IR model, but ischemia for 40 min induced the formation of autophagosomes. After reperfusion, the number of autophagosomes was further increased, and the expression of autophagy-related genes (LC3-II and Beclin 1) was significantly increased (Decker and Wildenthal 1980; Gurusamy and Das 2009). Furthermore, autophagy was found to be increased after chronic ischemia in human and pig heart tissues, and the formation of autophagosomes and increased lysosomal activity was observed in rat and mouse hearts after acute IR. In the myocardial infarction model induced by left coronary artery ligation, autophagosome markers (LC3 and p62) were increased, and many autophagosomes were generated in the infarction margin.
The mechanisms causing autophagy during IR include:
-
(1)
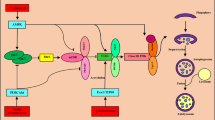
Change of ATP level and activation of the AMP-activated protein kinase (AMPK) signaling pathway in the myocardial ischemic stage: during in vitro culture, deprivation of glucose can reduce the intracellular ATP level, which is consistent with upregulation of autophagy. In vivo, the intracellular ATP content is insufficient, and the AMP and ATP ratios increase to activate AMPK at the time of myocardial ischemia. Activated AMPK induces autophagy by indirectly or directly modifying Unc-51-like autophagy activating kinase 1 (ULK1). Matsui et al. found that glucose deprivation in cardiomyocytes cultured in vitro led to autophagosome formation by activating AMPK and negatively regulating the mTOR signaling pathway (inhibitor of autophagy) (Matsui et al. 2007). AMPK inhibits mTORC1 by phosphorylating TSC2 and Raptor and then indirectly activates ULK1 (Alers et al. 2012). Recently, AMPK was shown to directly phosphorylate and activate ULK1, thus inducing autophagy. When the activity of AMPK is inhibited, the autophagy induced by myocardial ischemia is reduced.
-
(2)
SIRT1–FOXO-dependent mechanism: Sebastiano et al. found that under starvation, SIRT1 mediates the deacetylation of FOXO, increases the expression of the GTP-binding protein Rab7, mediates autophagosome-lysosome fusion and enhances autophagy. When FOXO1 is downregulated or deacetylation is inhibited, autophagy is attenuated. The abovementioned studies indicate that myocardial ischemia may regulate autophagy via a SIRT1–FOXO-dependent mechanism.
-
(3)
The BNIP3 pathway (a proapoptotic protein Bc122 family member): Zhang et al. revealed that during myocardial hypoxia, BNIP3 mediated the upregulation of hypoxia-inducible factor 1 and activated autophagy. After siRNA silencing of BNIP3, hypoxia-induced autophagy was attenuated, indicating that BNIP3 also participates in the regulation of autophagy at the time of myocardial hypoxia (Azad et al. 2008).
-
(4)
Calcium overload in mitochondria: the Ca2+ concentration in ischemic cardiomyocytes significantly increases with the opening of sodium–calcium exchanger calcium channels and becomes an inducing factor of autophagy. Ca2+ mobilization agents, such as vitamin D, ionomycin, ATP, and carotene, can inhibit the mTOR signaling pathway, leading to the accumulation of Beclin 1- and ATG7-dependent autophagosomes.
-
(5)
ROS: IR increases the intracellular ROS level. However, an in vitro study indicated that LPS promotes the generation of ROS in cardiomyocytes of suckling mice and induces autophagy formation. ROS scavengers could reduce this formation (Hickson-Bick et al. 2008). Autophagosome formation is inhibited by oxidation of the serine subunit of ATG4 by hydrogen peroxide, which is generated during starvation, thereby deactivating autophagy.
-
(6)
Endoplasmic reticulum (ER) stress and the unfolded protein response: ER stress and the unfolded protein response in cardiomyocytes of IR and myocardial infarction model in mice are activated. Tunicamycin and carotene (two activators of ER stress) can induce autophagosome formation. As an effective molecule of ER stress, IRE1 is necessary for autophagosome formation. All these investigations suggest that ER stress is a regulatory mechanism formed by autophagy in ischemic cardiomyocytes.
-
(7)
Opening of mitochondrial permeability transition pores (MPTPs): MPTPs on the mitochondrial membrane are closed under physiological conditions and open when cells are stimulated. MPTP opening during IR promotes cell autophagy. Cyclosporine or sanglifehrin-A can inhibit cardiomyocyte autophagy in the reperfusion stage.
2 Protective Effects of Autophagy in IR Injury
2.1 Maintenance of ATP Balance
The basic function of autophagy is degradation. In autolysosomes, proteins and cell membranes are degraded, and free fatty acids and amino acids are released as raw materials to synthesize ATP in the tricarboxylic acid cycle. Matsui et al. showed that glucose deprivation reduces the intracellular ATP level (Matsui et al. 2007), which is associated with the upregulation of autophagy. The autophagy inhibitor 3-methyladenine (3-MA) further reduces the ATP concentration and increases the mortality rate of cells. This finding suggests that autophagy activated during the ischemic process can promote cell survival by maintaining the ATP concentration.
2.2 Alleviation of Mitochondrial Autophagy in Mitochondrial Injury
Mitochondria in ischemic cardiomyocytes are damaged because of energy deficiency. The damaged mitochondria can generate excessive amounts of ROS and further consume ATP. Moreover, the damaged mitochondria release cytochrome C, apoptosis-inducing factor (AIF) and other proapoptotic factors, thus damaging more mitochondria. Scavenging the damaged mitochondria is a protective effect of cells. Autophagy is the only way to degrade organelles. Scanning electron microscopy has shown that the autophagosomes formed during IR contain mitochondria. The numbers of mitochondria and sarcomeres were increased in cardiomyocytes of mice with Atg5 gene deletion. Mitochondrial lysis is an essential condition for mitophagy. In the myocardium after IR, chondriokinesis occurs earlier than autophagosome formation. Furthermore, chondriokinesis inhibition can reduce autophagosome formation in ischemic myocardium.
2.3 Protein Scavenging
During ischemic hypoxic injury in cardiomyocytes, high levels of unfolded proteins are observed in the cells. Scavenging of these proteins requires autophagy and the ubiquitin protease system. Rapamycin-activated autophagy was shown to promote scavenging of intracellular aggregated proteins and reduce protein aggregation. While proteasome activity is inhibited, autophagy inhibition will increase the aggregation of intracellular proteins. Therefore, autophagy scavenges proteins and alleviates ER stress caused by these proteins; thus, it has protective effects on ischemic myocardium.
3 Possible Mechanism of Autophagy Promotion in Cardiac IR Injury
In contrast, several studies have indicated that activated autophagy induced in the reperfusion stage promotes cell death. Zhai et al. found that during IR, excessive autophagy accelerated the degradation of organelles and proteins that inhibit cardiomyocyte death, leading to more death and aggravation of cardiac injury caused by myocardial infarction. During IR in Beclin 1+/− mice, autophagy was reduced, as well as the myocardial infarction area; thus, cardiac function was improved. The results of this study indicated that during IR, myocardial injury induced by excessive autophagy can be attenuated by low temperatures and inhibition of oxidative stress. The uncertainty regarding autophagy during reperfusion could be interpreted as follows.
In the cardiac reperfusion stage, although the energy crisis (such as ATP) has been eased, other proautophagy mechanisms, such as oxidative stress, mitochondrial injury, ER stress, and the inflammatory response, can activate autophagy. Autophagy is a nonspecific degradation mechanism. Degradation of some key hydrolases leads to enhanced oxidative stress and promotes apoptosis. For example, during reperfusion, the generated ROS induce autophagy to degrade catalase, generating hydrogen peroxide. The elevated hydrogen peroxide level aggravates autophagy. Positive feedback between autophagy and oxidative stress leads to autophagic cell death. ROS can directly stimulate autophagosome formation by oxidative modification. For example, hydrogen peroxide oxidizes the cysteine residues of Atg4, inhibits the activity of cysteine proteases and stimulates starvation-induced autophagy. ROS can induce permeability changes in mitochondrial membranes and activate autophagy by mitochondrial injury. These findings indicate that autophagy protects cardiomyocytes, whereas other studies indicate that autophagy promotes cardiomyocyte apoptosis.
There are many linkages between autophagy and the apoptotic pathway: on one hand, some autophagy-related proteins simultaneously participate in the regulation of autophagy and apoptosis. For example, ATG5 can transfer into mitochondria, interact with Bcl-xl (an antiapoptotic molecule), and induce cytochrome C release and thus apoptosis. On the other hand, some antiapoptotic proteins simultaneously participate in the regulation of autophagy and apoptosis. For example, Bcl-2 affects autophagy. The proautophagy effect of Beclin 1 is often negatively regulated by Bcl-2, which interacts with it. During reperfusion, the increase in Bcl-2/Bcl-xl-binding proteins (such as BNIP3) and the decrease in Bcl-2 aggravate Beclin 1-induced cell apoptosis and autophagy. Elevated BNIP3 can destroy the integrity of mitochondria and increase the levels of hyperoxides and proapoptotic factors. Furthermore, there is a mutual effect between autophagy and apoptosis. For example, Beclin 1 possesses one BH3 structural domain that can bind to and inhibit the proapoptotic Bcl-xl, suggesting that Beclin 1 can directly activate apoptosis. When Beclin 1 is hydrolyzed by Calpain and the domain is exposed, Beclin 1 can be transferred to a proapoptotic protein beyond the protective capability of autophagy. ATG5 is upregulated during heart IR. ATG5 has been demonstrated to directly interact with the Fas-related protein death domain and stimulate autophagic cell death. The above studies indicate that autophagy has dual effects on heart IR. The determining factor is still not clear, but it may be associated with the autophagy level and duration.
Autophagy induced by reperfusion injury may be directly related to the preceding ischemic degree. If it is mild, the enhancement of induced autophagy at the reperfusion stage is beneficial. In contrast, overactivation of autophagy will result in scavenging necessary proteins or organelles in the cells, leading to cell dysfunction and autophagic death. Furthermore, autophagy is associated with inducing factors, such as hypoxia and lack of glucose, which may lead to the opposite effects.
With continuous investigations of autophagy, selective regulation of autophagy may be a new therapeutic target for the prevention and treatment of cardiac IR. Because autophagy may have dual functions at the myocardial IR stage, it is very important to use autophagy as a therapeutic target to differentiate between its protective and aggravation effects on tissue injury. More in-depth investigations are needed to explore the autophagy-activated transduction pathway, activation degree, and correlation between time and cell survival via gene or drug intervention under different conditions.
References
Alers S, Loffler AS, Wesselborg S et al (2012) Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol 32:2–11
Azad MB, Chen Y, Henson ES et al (2008) Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy 4:195–204
Decker RS, Wildenthal K (1980) Lysosomal alterations in hypoxic and reoxygenated hearts. I. Ultrastructural and cytochemical changes. Am J Pathol 98:425–444
Gurusamy N, Das DK (2009) Is autophagy a double-edged sword for the heart? Acta Physiol Hung 96:267–276
Hickson-Bick DL, Jones C, Buja LM (2008) Stimulation of mitochondrial biogenesis and autophagy by lipopolysaccharide in the neonatal rat cardiomyocyte protects against programmed cell death. J Mol Cell Cardiol 44:411–418
Matsui Y, Takagi H, Qu X et al (2007) Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100:914–922
Sybers HD, Ingwall J, Deluca M (1976) Autophagy in cardiac myocytes. Recent Adv Stud Cardiac Struct Metab 12:453–463
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Science Press and Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Du, J., Li, Y., Zhao, W. (2020). Autophagy and Myocardial Ischemia. In: Le, W. (eds) Autophagy: Biology and Diseases. Advances in Experimental Medicine and Biology, vol 1207. Springer, Singapore. https://doi.org/10.1007/978-981-15-4272-5_15
Download citation
DOI: https://doi.org/10.1007/978-981-15-4272-5_15
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-4271-8
Online ISBN: 978-981-15-4272-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)