Abstract
With advances in modern medicine, there has been a constant need to develop a single material that caters to all demands such as high tensile strength, biocompatibility and biodegradability. The development of an interpenetrating polymer network (IPN) is both an outstanding innovation and contribution that has led to massive technological advances across a wide spectrum of applications in medicine. IPNs comprising natural and synthetic polymers are typically endowed with improved properties compared to monolithic materials and offer superior properties. Most importantly, synergism of properties has also been observed in most of the systems. This chapter discusses the potential of alginate-based IPN carriers for biomedical applications.
Alginate is a naturally occurring anionic polysaccharide widely employed in a broad spectrum of biomedical applications. The ability to assemble alginate with a diversity of polymers and to fabricate IPNs makes it a promising choice for various applications in biomedicine. This chapter discusses at length the various inherent properties of alginate that make it suitable as a biomaterial. The state-of-art applications of alginate IPNs in drug delivery, wound healing and tissue engineering have also been elaborated. The prospective of alginate in delivery of small molecule drugs as well as protein drugs has been presented. This chapter further focuses on the potential of alginate IPNs in wound dressings and regenerative medicine.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Utilization of naturally occurring polysaccharides towards the design of biomaterials for biomedical applications has blossomed into a promising approach owing to their biocompatibility and biodegradability. The diversity in the properties of these natural polysaccharides could be well ascribed to the presence of a large number of reactive groups, wide range of molecular weight and varying chemical composition (Prabaharan 2011). Due to the presence of various functional groups in their backbone, these polysaccharides are greatly suitable for chemical and biochemical modifications resulting in a wide range of derivatives (Prabaharan and Jayakumar 2009). In particular, their abundant resources in nature and low processing cost have fuelled up their utilities in pharmaceutical industries. In view of these magnificent attributes, alginate, a naturally occurring anionic polysaccharide, has carved a niche for itself for myriad biomedical applications. Alginate has been extensively investigated in biomedical utilities due to its natural abundance, relative low cost, biocompatibility and ease of fabrication of formulations (Gombotz and Wee 1998).
4.2 Chemistry of Alginate
4.2.1 Sources and Extraction
Alginate is a naturally occurring anionic polysaccharide primarily extracted from the species of brown seaweed viz. Laminaria hyperborea, Laminaria digitata, Laminaria japonica, Ascophyllum nodosum and Macrocystis pyrifera (Smidsrod and Skjak-Braek 1990). In all these algae, alginate constitutes almost 40% of dry weight (Sutherland 1991). Alginate is extracted from the dried algae after treatment with dilute mineral acid (mostly HCl) (Rinaudo 2008). Free alginic acid, thus obtained, is then converted to a salt, mostly the sodium salt, i.e. sodium alginate, which finds prominence in biomedical applications (Tonnesen and Karlsen 2002). Alginate also exists as mixed salts of various cations found in seawater such as Mg2+, Ca2+, Ba2+ and Na+. To extract the alginate, the cations are exchanged for H+. The alginate is then converted to its soluble sodium salt by addition of sodium carbonate while maintaining the pH below 10 (Sutherland 1991).
Bacterial alginates have also been isolated from Azotobacter vinelandii and several species of Pseudomonas (Smidsrod and Skjak-Braek 1990). The pathway for alginate biosynthesis mostly comprises of four decisive steps: (i) synthesis of precursor substrate, (ii) polymerization and cytoplasmic membrane transfer, (iii) periplasmic transfer and modification and, lastly, (iv) export through the outer membrane (Lee and Mooney 2012; Remminghorst and Rehm 2006). Further regulation and tailored development features in bacterial alginate biosynthesis could provide a new direction towards alginate production and its wide applications in biomedicine.
4.2.2 Chemical Structure
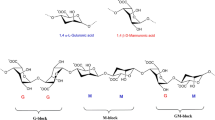
Alginates are a family of linear block copolymers comprising of 1,4’-linked β-D-mannuronic and α-L-guluronic acid residues (George and Abraham 2006). The composition and properties of alginates are strongly dependent on the ratio of guluronate (G) to mannuronate (M) residues. Depending on the source of extraction, the contents of M and G vary and to date, more than 200 different alginates have been manufactured (Thakur et al. 2018). The blocks might be arranged with consecutive G residues, M residues and alternating G and M residues. Because of the difference in the mode of linkage between the G residues and M residues, the geometries of the G-block regions, M-block regions and the alternating regions are significantly different (Fig. 4.1a) (from Lee and Mooney 2012). The G-blocks are considered to be buckled shaped whereas M-blocks are often referred to as extended ribbons. The specific arrangement of G residues results in diamond-shaped holes of suitable dimensions that are ideal for accommodating divalent cations such as Ca+2 to form stacked egg-box-like structures of the G residues resulting in gelation (Fig. 4.1b) (from Lee and Mooney 2012) (George and Abraham 2006). Thus, the composition (M/G ratio), the sequence and the G-block length are crucial factors that determine the physical properties of the alginate and its resultant formulations (George and Abraham 2006).
Chemical structures of G-block, M-block and alternating block in alginate. (Lee and Mooney 2012)
Alginate hydrogels prepared by ionic crosslinking (egg-box model). (Lee and Mooney 2012)
4.2.3 Molecular Weight and Viscosity
The molecular weights (MW) of commercially available Na-alginates mostly range between 32,000 and 400,000 g/mol (Rinaudo 1992). The intrinsic viscosity ([η]) of alginate solution depends on its MW. According to Mark-Houwink relationship ([η] = KM v a) the parameters are K = 2 × 10−3 and a = 0.97 for Na-alginate in 0.1 M NaCl and at 25 °C, where M v is the viscosity average molecular weight (Rinaudo 1992). The viscosity of alginate solutions is also sensitive to the pH and it increases as the pH is lowered. A maximum viscosity is observed around pH 3–3.5 wherein the carboxylate groups in the polysaccharide become protonated and take part in hydrogen bonding (Berth 1992). It has also been demonstrated that alginate solutions with high MW become greatly viscous which is undesirable for further processing (LeRoux et al. 1999). Thus, judicious manipulation of MW can tailor the solution viscosity and render alginates with desired properties. Ideally, by employing a combination of high and low MW polymers, the viscosity of the resulting alginate gels could be adjusted (Kong et al. 2002).
4.3 Properties of Alginate Making It Suitable for Biomedical Applications
4.3.1 Gelation
The gel formation capacity or gelation property of alginate is of great importance in widespread applications of this polysaccharide in food and pharmaceutical industries. The ability of alginate to form two types of gels (i) pH dependent (acid gel) and (ii) ionotropic gel confers unique advantages over neutral polysaccharides. Hydration of alginic acid, especially the high MW variants, leads to formation of high viscosity ‘acid gels’ due to strong intermolecular hydrogen bonding. These gels can entrap large amount of labile water molecules and are of great importance in many applications such as cell immobilization and encapsulation (Nahar et al. 2017). Alginate can form ionotropic gels with a variety of metal ions under mild conditions. Monovalent cations generally form soluble salt with alginate whereas divalent and multivalent cations often result in gelation. Ionic crosslinking mostly involves divalent cations such as Ca2+, Ba2+ and Sr2+. The ionotropic gelation of alginate is mostly due to the ion-induced association of G-block regions in the polymer. Various functional properties such as porosity, swelling behaviour, stability, mechanical strength, biodegradability, immunological characteristics and biocompatibility of these gels are found to be greatly influenced by the chemical structure, MW, gel formation kinetics of the polysaccharide and the cation. Alginate gels can be prepared in various shapes and sizes among which alginate beads are largely explored.
Alginate beads have been prepared by extruding a solution of sodium alginate containing the desired protein or drug, as droplets, into divalent crosslinking solutions of Ca2+, Ba2+ and Sr2+ (Sutherland 1991). The viscosity of alginate solution and the diameter of the extruder are paramount in influencing the shapes and sizes of the resulting beads. Recent studies point to alginate gelation by covalent crosslinking (mostly esterification, carbodiimide, methacrylation, click chemistry) or by photocrosslinking (Bidarra et al. 2014).
4.3.2 pH Sensitivity
Alginate is a well-known pH-responsive polymer due to its ionic nature. At low pH, (gastric environment) it shrinks into a porous insoluble matrix usually referred to as alginic acid skin but as soon as it experiences higher pH in the intestinal tract, the alginic acid skin converts to a soluble layer (Chen et al. 2004). The strong pH-dependent responsivity of alginate could be accredited to the acidic β-D-mannuronic and α-L-guluronic acid structural units on its backbone that get protonated in acidic environment (pH 1.2 of gastric fluid). In alkaline media (pH 7.4 of intestinal fluid) the repulsion between the deprotonated COO− groups renders the alginate matrix a greater swellability (Kajjari et al. 2012). This pH-sensitive behaviour has been categorically exploited to customize drug release kinetics from alginate matrices. The various biomedical applications pertaining to pH sensitivity of alginate have been discussed in the later sections.
4.3.3 Mechanical Properties
Materials intended as extracellular matrices (ECMs) often require specific and reproducible elastic moduli that can be modulated in a controlled manner (Schneider et al. 2006). The mechanical properties of a polymer such as stiffness can mostly be manipulated by physical factors like crosslinker type, crosslinking density, MWs and also by chemical modification of the polymer (Peppas 2004). Generally, in the case of high MW alginate, an increase in stiffness is observed upon increasing the polymer concentration. This, however, leads to a greatly viscous polymer solution that makes further processing difficult (Kong et al. 2003; Augst et al. 2006). To circumvent this hindrance, specifically formulated combination of high and low MW alginates is usually employed. In these formulations, the low MW chains reduce the physical interactions in solution while the high MW chains maintain the long-range interactions in the gel (Augst et al. 2006). Thus, the synergistic effects of these two factors tend to minimize the alginate gel brittleness.
Reinforcement of cationic polymers such polyethylene imine into alginate is also an effectual strategy to improve its mechanical strength (Kong and Mooney 2003). Gelling condition such as gelation time and type of crosslinker are pivotal that dramatically influence the swelling and mechanical properties of the alginate gel.
4.3.4 Bioadhesiveness
Studies have revealed that polymers possessing a charge density serve as good mucoadhesive agents (Chickering and Mathiowitz 1995; Chang et al. 1985; George and Abraham 2006). More so, anionic polymers are more efficient bioadhesive candidates than their polycationic and nonionic counterparts. Literature reports have illustrated alginate with excellent bioadhesion characteristics (El-Kamel et al. 2002; Pinkas et al. 2017; Singh et al. 2017; Shtenberg et al. 2017; Hong et al. 2018;). Moreover, due to adherence of alginate to mucosal tissues, protein transit time is delayed and thus the drug is localized to the absorptive surfaces, thereby improving the drug bioavailability and efficacy (Szekalska et al. 2016). In a recent development, an oral mucoadhesive drug delivery system constituting alginate and liposomes were fabricated by Shtenberg and co-workers that displayed retention of around 80% of the biomaterial in the tongue tissues (Shtenberg et al. 2018).
4.3.5 Biodegradation
Degradation of a biomaterial is often a key issue in tissue replacement and drug release. Ionically crosslinked alginates mostly dissolve in neutral pH by losing their divalent crosslinking cation (Augst et al. 2006). Gamma irradiation-assisted alginate degradation has also been reported at the optimum dose 100 kGy (Lee et al. 2003). Enzymic degradation of alginates is a well-known phenomenon (Gacesa 1992). Schaumann and Weide (1990) have explicitly detailed on alginase-mediated structural and molecular degradation of Na-alginate by marine fungi. The review article by Wong and co-authors (2000) particularly emphasizes on the enzyme characteristics of alginate lyase to engineer and fine-tune the degradation of alginate-based biomaterials. Recently, controlled degradation of alginate has also been achieved by crosslinked enzyme aggregates of alginate lyase (Kunjukunju et al. 2018). The study revealed that controlled degradation of alginate was obtained over a period of 28 days. These findings altogether suggest that it is possible to tailor the degradation kinetics of alginate and its derivatives with proper use of conditions.
4.3.6 Biocompatibility
The biocompatibility of alginate has been studied extensively by various authors in vitro as well as in vivo. However there still remains some debate about the varying levels of impurity particularly for commercially available alginates. Since crude alginates often contain contaminants such as proteins or endotoxins, it is desirable to properly purify the alginates to minimize the risk of an immune response. Tam et al. (2011) have studied the factors influencing the alginate gel biocompatibility and inferred that the mannuronate/guluronate content is the key factor. Orive and co-authors (2005) have presented a battery of in vitro techniques to access the biocompatibility of alginates with different compositions and purities. Their findings established that the differences in protein and polyphenol content amount to purified and non-purified alginates.
4.4 Limitations of Pure Alginate
The foremost hindrance associated with pure alginate is its poor mechanical strength which usually warrants reinforcement (Li et al. 2005; Zineh et al. 2018). The application of alginate is further limited by its high moisture sensitivity (Jost and Reinelt 2018). Composite materials usually exhibit better tensile strength in comparison to monolithic materials and thus, alginate has been assimilated with a diversity of polymers to engineer composites of biomedical interest.
4.5 Modifications of Alginate
In recent years multicomponent drug delivery systems have been developed for potential therapeutic and diagnostic applications and among these, semi-interpenetrating polymeric networks (semi-IPNs) and interpenetrating polymeric networks (IPNs) have emerged as innovative biomaterials for myriad biomedical applications (Dragan 2014). The network properties can be tailored by the type of polymer and its concentration, by the applied crosslinking method as well as by the overall procedure used for their preparation.
IPNs are ‘alloys’ of crosslinked polymers, at least one of them being synthesized and/or crosslinked within the immediate presence of the other, without any covalent bonds between them, which cannot be separated unless chemical bonds are broken (Dragan 2014). IPNs can be basically classified as simultaneous IPN and sequential IPN based on the preparation method. When the precursors of both networks are mixed and the two networks are synthesized at the same time, simultaneous IPN results. A sequential IPN is typically performed by swelling of a single-network hydrogel into a solution containing the mixture of monomer, initiator and activator, with or without a crosslinker. In the presence of a crosslinker, a full IPN results while the absence of a crosslinker forms a semi-IPN (Matricardi et al. 2013; Dragan 2014; Lohani et al. 2014).
The high rates of success of IPNs and/or semi-IPNs used for biomedical and pharmaceutical applications could be attributed to the combination of favourable properties of each constituent polymer. Formation of IPNs leads to new systems with improved properties, which quite often are substantially different from those of the individual polymers. Most importantly, synergism of properties has also been observed in most of the IPNs (Matricardi et al. 2013). The combination and synergism of properties can be judiciously exploited to modify the characteristics of the resulting biomaterial to cater to specific needs, particularly in the biomedical and pharmaceutical fields. Development of IPNs has led to an assortment of architectures such as hydrogels, microspheres, microgels, nanoparticles, tablets, capsules and scaffolds, among others, to accomplish the biomedical objective (Lohani et al. 2014).
4.6 IPNs of Alginate
Alginates have been assembled with a diversity of polymers for the fabrications of IPNs and/or semi-IPNs intended for biomedical applications in the form of films, microspheres, microgels, nanoparticles or depot matrices. They have also been designed as scaffolds in tissue engineering for bone, cartilage and soft tissue generation. Because of the high versatility and tailorable properties, IPNs of alginate are promising candidates in pharmacy. Alginates have often been taken in combination with synthetic polymers or with naturally occurring biopolymers for the generation of IPNs. Depending on the nature and the intrinsic properties of components, the resulting IPNs can be fine-tuned. Moreover, the range of reachable properties can be broadened substantially.
4.6.1 IPNs with Synthetic Polymers
A combination of synthetic polymers with alginate further widens the window of pharmaceutical and biomedical utilities. Synthetic polymers such as polyvinyl alcohol (PVA), polyethylene glycol (PEG), polyacrylic acid (PAA), polyacrylamide (PAAm), poly(N-isopropylacrylamide) (PNIPAAm), polycaprolactone (PCL), polylactic acid (PLA), polyvinyl pyrrolidone (PVP), etc., have been taken in conjugation with alginate to form IPNs.
Table 4.1 reviews the IPNs of alginate with various synthetic polymers and their potential applications in biomedicine.
4.6.2 IPN with Biopolymers
Biopolymers such as chitosan, guar gum, cellulose, pectin, soya protein and others have been integrated with alginate and explored for manifold biomedical applications. Table 4.2 enlists the recent advances in the IPNs of alginates with various biopolymers and their diverse pharmaceutical applications.
4.7 Biomedical Applications of IPN of Alginate
Owing to its biocompatible, non-immunogenic, non-toxic, mucoadhesive and biodegradable features, applications of alginate are manifold in biomedical field. Alginate biocompatibility has been affirmed in vivo after ocular (Lin et al. 2004), nasal (Sarei et al. 2013), topical (Coskun et al. 2014), vascular (Rottensteiner et al. 2014; Amirian et al. 2017) and oral (Nunamaker et al. 2007; Thomas et al. 2018; Yang et al. 2018a, b) administration. Alginate has been greatly studied for pharmaceutical applications like targeted drug delivery, protein delivery, tissue engineering and wound healing purposes. The following sections explore the biomedical wonders of alginates in greater details.
4.7.1 Pharmaceutical Applications
Oral formulations are currently the most frequent and preferred mode for pharmaceutical applications (Nahar et al. 2017). The design of oral dosage forms usually follows any one of these two principles: (i) the entire drug dose is in the same physical unit or (ii) the dose is encapsulated in an assembly of small subunits (Nahar et al. 2017). In the latter case, the subunits are further compressed into tablets which, thereby, experience a ‘barrier’ that is pivotal to provide a controlled release profile. In particular, hydrocolloids like alginates undergo an immediate hydration to create a high viscosity layer around themselves which act as diffusion barrier, thereby decreasing the migration of small chemical drugs, thus sustaining the delivery. Additionally, the intrinsic pH responsivity of alginates makes them wonderful agents for colon-specific drug delivery. Alginates are widely used as delivery vehicles due to their ability to encapsulate and release a wide range of drugs in a gentle and biocompatible manner.
4.7.1.1 Delivery of Small Chemical Drugs
Alginate hydrogels may be broadly useful for the sustained and localized delivery of traditional small chemical drugs. Conventional approaches to drug delivery (oral mostly) usually lead to burst release of drugs and poor targeting to the site of interest causing side effects and inefficacy. The release kinetics of low MW drugs from alginate gels can be controlled by regulating drug-alginate interactions. When there are no chemical interactions between the drug and the polymer, the release depends largely on the charge polarization of the molecule; i.e. hydrophilic molecules may diffuse very quickly while hydrophobic drugs diffuse slowly through the gel pores (Augst et al. 2006).
pH-Sensitive Alginate Hydrogels in Drug Delivery
A plethora of alginate-based IPN formulations have been developed towards the controlled delivery of drug molecules to the target. pH-sensitive composite hydrogel beads of Ca-alginate and agar have been developed towards controlled drug delivery (Yin et al. 2018). Biodegradable and biocompatible semi-IPN hydrogels of Na-alginate and poly(methacrylic acid) have been utilized for controlled delivery of theophylline to colon (Ganguly et al. 2018). Upadhay and co-workers (2018) have optimized capecitabine-loaded IPN beads of locust bean gum and Na-alginate by ionotropic gelation method and investigated their controlled drug delivery features. In vitro drug release studies indicated sustained delivery for 12 h. More so, cytotoxicity assay against HT-29 cells revealed significant reduction in cell growth, validating their worth towards the treatment of colon cancer. IPN hybrid hydrogels comprising Ca-alginate and peptide Fmoc-tyrosine have been constructed with an aim to achieve a controlled release drug profile for small molecules (Chen et al. 2018b). The IPN hydrogels exhibited enhanced storage moduli and fracture energy in comparison to Ca-alginate and revealed minimal drug release at acidic pH and sustained release at intestinal pH. Basu et al. (2017) have developed silver nanocomposite semi-IPN hydrogels of Na-alginate and acrylamide and explored their drug delivery features. The results demonstrated sustained release of ciprofloxacin for colonic delivery. Anwar and co-authors (2017) constructed Na-alginate and PVA hydrogels by free radical polymerization using 2-ccylamido-2-methylpropane-sulfonic acid. The hydrogels exhibited prolonged delivery of tramadol HCl with improved entrapment efficiency.
Samanta and Ray (2014) synthesized various grades of Na-alginate-acrylamide IPN hydrogels using N,N’-methylenebisacrylamide as crosslinker and studied the release of acetaminophen. It was observed that the IPN with 6 wt% Na-alginate content was the best of the lot in terms of optimum swelling, drug entrapment and controlled release drug profile at physiological pH. Na-alginate has also been assimilated with carrageenan to formulate pH-responsive IPN hydrogel beads (Mohamadnia et al. 2007). The beads displayed sustained release profile for betamethasone acetate at pH 7.4. Literature reports pertaining to carrageenan and alginate-based IPN suggest their utility as colon-targeted controlled drug delivery systems (Mohamadnia et al. 2008; Kulkarni et al. 2011; Kulkarni et al. 2012; Mahdavinia et al. 2014).
In situ forming IPN hydrogels of Ca-alginate and hydroxyethyl-methacrylate-derivatized dextran have been prepared by Pescosolido and co-workers (2011) and bovine serum albumin (BSA) was loaded as a model drug to evaluate the drug delivery efficacy of the hydrogels. Surprisingly the drug release spanned approximately for 15 days. Treenate and Monvisade (2017) investigated the release profile of paracetamol from hydroxyethylacryl chitosan and Na-alginate IPN hydrogels crosslinked by ionic crosslinkers such as Ca2+, Zn2+ and Cu2+. The comprehensive results of the study demonstrated their potential in the applications of site-specific oral drug delivery to the intestine and colon. Na-alginate-carboxymethyl cellulose hydrogel beads have been prepared in ferric chloride solution that showed excellent pH responsivity and controlled delivery features for metformin in pH 7.4 (Swamy and Yun 2015). IPN hydrogel membranes of Na-alginate and PVA prepared by Kulkarni et al. (2010) by solvent casting method were explored for prolonged transdermal delivery of antihypertensive drug prazosin hydrochloride.
Temperature-Sensitive Alginate Hydrogels in Drug Delivery
Thermo-sensitive smart polymers are one of the most common classes of smart polymers studied in drug delivery research. Two major classifications of thermo-responsive IPNs can be categorized as:
-
1.
IPNs that exhibit an upper critical solution temperature (UCST) that show a transition from gel to sol state above this temperature
-
2.
IPNs that exhibit a lower critical solution temperature (LCST) that show a transition from gel to sol state below this temperature
Temperature-sensitive smart IPN hydrogels of alginate have often been prepared by taking in combination with one of the most commonly used thermo-responsive polymers, poly(N-isopropylacrylamide) (PNIPAAm) that has its phase transition near body temperature (∼33 °C). The use of PNIPAAm in interpenetrating architectures has been particularly investigated for tailoring the release of drugs. Integrating alginates with PNIPAAm result in a special class of dual stimuli-sensitive composites with responsivity to both pH and temperature.
Doxorubicin-loaded alginate-g-PNIPAAm IPN hydrogels demonstrated a sustained release profile of drug in physiological pH and were found to be effective in cancer therapy (Liu et al. 2017). Alginate-g-PNIPAAm injectable temperature-sensitive hydrogels with potential for localized and sustained delivery of stem cells and bioactive molecules have been successful and are ready to serve their purpose (Pentlavalli et al. 2017). Temperature-sensitive alginate beads synthesized via microwave-assisted graft copolymerization were found to prolong the delivery rate of indomethacin (Isiklan and Kucukbalci 2016). Muniz and co-workers studied the effect of temperature on the mechanical properties and permeability of PNIPAAm-Ca-alginate semi-IPNs and IPNs (Guilherme et al. 2002; de Moura et al. 2005; Guilherme et al. 2005). The authors evaluated the IPN hydrogel strength and observed a synergism of the mechanical performances of the polymers both below and above the LCST. The effect was more pronounced above LCST where the uniaxial compressive modulus of the hydrogel was much higher than individual components. More so, sustained release of drug was observed above LCST, which is attributed to the tighter structure of the hydrogel matrices with smaller pore sizes.
Ju and co-workers (2002) prepared semi-IPN hydrogels of alginate and amine-terminated PNIPAAm using calcium chloride as crosslinker. Also, they reported that semi-IPN hydrogel exhibited a remarkable sensitivity to temperature, pH and ionic strength of swelling. Li et al. (2018) employed Ca-alginate-PNIPAAm IPN onto cotton fibre surfaces using 1,2,3,4-butanetetracarboxylic acid crosslinking. The cotton fibre-supported hydrogels exhibited stiffer mechanical properties and controlled drug release characteristics at 37 °C. The in vitro drug release of anticancer drug 5-fluorouracil from semi-IPN networks of Na- alginate-PNIPAAm microspheres was reported by Reddy and co-workers (2008). The drug release at 25 °C and 37 °C confirmed about the thermo-responsive nature of the microspheres.
pH/Temperature Dual-Sensitive Alginate Hydrogels in Drug Delivery
Several studies have also focused on the development of pH and temperature dual stimuli-sensitive smart alginate hydrogels. The properties of alginate-PNIPAAm semi-IPNs or IPNs were found to be strongly affected not only by pH but also by temperature. Shi et al. (2006) studied the effect of pH and temperature on the release of indomethacin from semi-IPN beads of Ca-alginate and PNIPAAm. At pH 2.1 and 37 °C, the drug release was slow and less than 10% of drug was released in 7 h while the release completed in 3 h at pH 7.4. Temperature-dependant drug release was also observed from the same gel, leading to a faster release at 37 °C than 25 °C. An overview of the properties and performances of alginate-based smart IPNs for drug delivery applications has been elucidated in the review article by Matricardi and co-authors (2013).
Alginate Microparticles in Drug Delivery
Alginate-based microparticles have been fabricated by various groups and are found to be quite efficacious towards oral targeted drug delivery. The microparticles have been formulated mostly by the conventional shredding method in a commercial food processor or by a water/oil (w/o) emulsion and external gelation method. Chitosan-reinforced alginate microparticles for sustained release of antineoplastic drugs have been successfully synthesized and drug delivery potential has been explored (Yu et al. 2008). The same authors have also developed composite microparticle drug delivery systems composed of alginate, chitosan and pectin for site-specific delivery via oral route (Yu et al. 2009). Moebus et al. (2009) illustrated the efficacy of alginate-poloxamer microparticles for controlled drug delivery to mucosal tissues. The recently published review article by Aguero and co-authors (2017) summarizes the utility of alginate microparticles as oral colonic drug delivery devices with greater precision.
Alginate Nanoparticles in Drug Delivery
The development of nanoparticles has proven to be a boon to the pharmaceutical sector. The idea of IPN nanoparticles as drug delivery agents may be utilized to modify or control the drug distribution at the tissue, cellular or subcellular levels (Lohani et al. 2014). Chitosan-alginate nanoparticles as delivery systems for ε-polylysine were designed and a sustained release profile was achieved (Liu et al. 2018a, b, c). In another report of the above nanoparticulate systems by Motwani and co-workers (2008), prolonged ocular delivery of the ophthalmic antibiotic gatifloxacin was revealed for a period of 24 h. Hybrid nanoparticles of alginate and stearic acid-polyethylene glycol were loaded with an antiviral drug, zidovudine (Joshy et al. 2017). The optimized formulations were stable up to a period of six months and represented potential carrier for the drug by enhancing its efficiency.
Alginate Microgels in Drug Delivery
Microgels offer unique advantages for polymeric drug delivery systems in terms of high degree of control over properties such as stability for prolonged circulation in blood stream, control over particle size and biodegradability for sustained release of drugs. The potential of alginate microgels as sustained drug delivery agents is well-established. Chen and co-authors (2018a) have demonstrated the controlled release of an antacid (magnesium hydroxide) from alginate-pectin microgels. Microgels of varying dimensions were formed using either a handheld syringe or a vibrating nozzle encapsulation device with different nozzle sizes. The slowest antacid releasing features was observed from the microgel containing 80% alginate and 20% pectin. The authors inferred that the role of alginate was crucial for the design of microgels for the release of antacids in stomach. In another study by Wang and Newby (2018), coating of poly(allylamine) and poly(styrene sulfonate) was deposited layer by layer on alginate microgels and evaluated for sustained release of two hydrophilic drugs, sodium benzoate and zosteric acid. The results revealed that a prolonged release of the drugs could be achieved from the microgels spanning from hours to at least three days. Na-alginate microgel spheres encapsulated with tea polyphenol were designed for the treatment of bone infection (Chen et al. 2018d). The drug loading efficiency was reported to be 92.96% and the polyphenol release was therapeutic. Apart from exhibiting excellent antibacterial activity against S. aureus, the microgels promoted proliferation and differentiation of osteoblasts. Microgel suspensions of alginate have been developed for controlled release of vascular endothelial growth factor (VEGF)-encoding lentivectors (Madrigal et al. 2018). Babu and co-workers (2005) have reported IPN microgels of Na-alginate-acrylic acid prepared by w/o emulsion technique for the controlled release of ibuprofen. The developed systems showed pH responsivity and prolonged the drug delivery at physiological pH.
4.7.1.2 Protein Delivery
The various inimitable features of alginate have enabled it to be used as a matrix for encapsulation and delivery for a variety of protein drugs. These features include (Gombotz and Wee 2006):
-
1.
An almost inert aqueous environment within the matrix
-
2.
An encapsulation process devoid of any organic solvents that proceeds at room temperature
-
3.
A high gel porosity which allows for high diffusion rates for macromolecules
-
4.
Ease of controlling the porosity with simple procedures
-
5.
Biodegradation of the system under normal physiological conditions
Proteins encapsulated in alginate matrices are essentially released by two mechanisms: (i) diffusion of the protein through the pores of the polymer network and (ii) degradation of the polymeric network (George and Abraham 2006). In order to achieve a sustained release profile for protein drugs, the matrix degradation may not be the suitable method since it usually results in the rapid release of the protein. Therefore, for protein delivery, it is apt that the matrix remains intact and the protein diffuses out through the pores. Numerous reports have been published on the encapsulation and release of proteins from alginate matrices.
In a recently published article by Lima et al. (2018), pH-responsive alginate hydrogels have been evaluated for oral delivery of bovine serum albumin (BSA) in acidic and alkaline environments. The hydrogels were found to be compatible with living cells and higher BSA release was observed at pH 7.4, thereby validating them to be ideal for oral protein delivery. In another study utilizing Na-alginate-glycerol dressings for the delivery of therapeutic proteins to wounds, the authors found that the protein release was sustained for more than 72 h (Momoh et al. 2015). The films also showed ideal moisture content that is required for protein conformation and exhibited a good balance of flexibility and toughness.
Rahmani and Sheardown (2018) have explored the potential of protein-alginate complexes as pH-/ion-sensitive carriers for protein delivery. The authors used cytochrome C, lysozyme, myoglobin, chymotrypsin and BSA as model proteins for preparing the complexes. They observed that the proteins could be complexed with alginate in the absence of a cation and the complexes displayed decreased release rates. Furthermore, the protein release was facilitated by environmental triggers such as pH and ionic strength. Reports pertaining to protein encapsulation in alginate hydrogel beads also point to the utility of alginate for controlled protein delivery. Whey protein was encapsulated in Ca-alginate beads using an extrusion device (Zhang et al. 2016). The results suggested that hydrogel beads were suitable for encapsulation and the pH-triggered release of proteins was monitored. L-arginine-g-alginate beads were synthesized by Eldin et al. (2015) to be utilized as carriers for BSA. The grafting of alginate improved its release profile. The preliminary results clearly suggested that the Arg-g-alginate hydrogel may be a potential candidate for polymeric carrier for oral delivery of protein. IPN hydrogel beads composed of Na-alginate-carboxymethyl chitosan have shown great potential towards the oral delivery of BSA (Hu et al. 2016).
Injectable pH-/thermo-responsive hydrogels of poly(ethylene glycol) methacrylate, N-isopropylacrylamide and methacrylated alginate were prepared by Zhao and co-workers (2014). BSA as a model protein drug was encapsulated in situ in the hydrogel. BSA release results indicated that these hydrogels, as carriers, have great potential for long-term localized protein release.
A study by Pescosolido et al. (2011) demonstrated the potential of in situ forming IPN hydrogels based on a physical network of Ca-alginate, interpenetrated with a chemical one based on hydroxyethyl-methacrylate-derivatized dextran (dex-HEMA). BSA was gradually released from the IPNs over approximately 15 days. In situ semi-IPN hydrogels of Ca-alginate and dextran methacrylate (Dex-MA) were obtained by a dispersion of Dex-MA chains into a Ca-alginate hydrogel (Matricardi et al. 2008). The release of protein from these hydrogels validated their efficacy as sustained protein delivery systems.
4.7.2 Wound Dressing
An ideal dressing should always protect the wound from bacterial infection as well as promote healing. Hydrogels have received widespread applications as wound healing agents because they provide a moist environment, accelerate wound healing capacity, allow gaseous exchange and protect against bacterial infection. Moreover, they are non-toxic and have suitable mechanical and water vapour retention capabilities that can facilitate the healing process. Alginate-based formulations have been used in an array of wound healing applications. Bakhshayesh et al. (2018) reviewed that alginate-based wound dressing formulations such as sponges, hydrogels and electrospun mats are promising candidates for wound healing and have numerous advantages in terms of haemostatic capability and gel-forming ability upon absorption of wound exudates.
The antibacterial and wound healing properties of Na-alginate-PAAm hydrogels have been reported by Zhou et al. (2018). The influence of different divalent ion crosslinking (Cu, Zn, Sr and Ca) on the efficacy of the hydrogels has been explored. In vitro and in vivo study results showed that Zn-crosslinked hydrogel has a spectrum of advantages such as antibacterial activities, cell viability, better mechanical strength and higher ability of wound closure. Studies comprising chitosan-alginate-collagen have also proven them to be quite effective wound dressing materials (Xie et al. 2018). In another study, ZnO nanoparticle-loaded Na-alginate-gum acacia hydrogels displayed excellent wound healing effect on fibroblast cells (Raguvaran et al. 2017).
Summa et al. (2018) studied the in vitro biocompatibility and the efficiency of Na-alginate and povidone iodine (PVPI) polymeric composite in a mouse model for wound healing features. The hydrogel exhibited outstanding wound healing properties of alginates with the bactericidal and fungicidal properties of PVPI, providing a controlled release of antiseptic; thus, it is a good candidate for reduction in inflammatory response both in human foreskin fibroblasts and in rodents after wound induction. The synthesis of ciprofloxacin-loaded electrospun hydrophobic poly(lactic-co-glycolic acid) (PLGA) fibrous mats modified by Na-alginate microparticles was reported by Liu et al. (2018b). The results revealed that alginate improved the wettability and water absorption capacity and improved the release rate of ciprofloxacin from the PLGA fibrous mats and reduced the stiffness of the mats for better protection of the injured area. Furthermore, the burst release of ciprofloxacin which resulted from the addition of alginate could offer an improved antibacterial effect to the PLGA mats.
Alginate crosslinked by calcium gluconate crystals deposited in poly(ε-caprolactone)-b-poly(ethylene glycol)-b-poly(ε-caprolactone) porous microspheres was developed by Liao et al. (2018) for skin wound healing. The porous structure of the microspheres offered additional anchor points for fibroblast attachment and growth, enhancing the cell growth in the hybrid hydrogel.
N-Carboxymethyl chitosan and alginate-based hydrogels were prepared by electrostatic interaction and divalent chelation with epidermal growth factor (EGF) by Hu et al. (2018b) for cell proliferation and wound healing activities. Their investigation suggested that the loading of EGF did not depreciate the mechanical properties of hydrogels. Moreover, the porous 3D structure of the hydrogels permitted sufficient loading and release of EGF and improved cell proliferation indicating that the hydrogel is an excellent candidate for wound healing applications. Carboxymethyl chitosan (CMCS)-alginate and CMCS-alginate-chitosan oligosaccharide (COS) hydrogels were synthesized by Lv et al. (2018) for in situ wound healing treatment. CMCS-alginate and CMCS-alginate-COS hydrogels demonstrated faster wound contraction, while the healing speed of CMCS-alginate-COS hydrogels was faster as compared with CMCS-alginate. At 11 days of treatment, both the hydrogels showed remarkable increase in thickness and integrity of epidermal tissue and increased construction of collagen fibres (Fig. 4.2.) (Wound healing and cytotoxicity from Lv et al. 2018).
Photographs at each time point of 10 mm diameter wounds (a) and wound closure curves (b) demonstrating the accelerated healing for wounds treated with CMCS/alginate hydrogel and CMCS/alginate hydrogel with 0.5% COS. (Lv et al. 2018)
Akbar and co-authors (2018) evaluated the in vivo anti-diabetic and wound healing potential of curcumin-loaded chitosan-alginate-maltodextrin-pluronic-based mixed polymeric micelles. They reported that after 2 weeks of treatment of wound with different formulations, effective wound healing responses in rats were observed. Shahzad et al. (2018) studied the wound healing activity of cefazolin nanoparticles of chitosan loaded into Na-alginate/pectin films crosslinked by calcium chloride. Their finding suggested that after 7 days the films were fairly protective against the growth of the secondary bacterial infections at the wound area. Kamoun et al. (2018) have demonstrated Na-alginate/PVA hydrogels containing sodium ampicillin as an ideal candidate in wound care. It has also been reported that alginate- and gelatin-based biocomposite wafers containing silver sulfadiazine have a potential wound healing application (Boateng et al. 2015).
The main problem associated with chronic non-healing wounds is drug-resistant infection as the skin barrier functionality reduced. Also, biofilm formation due to the existence of aerobic and anaerobic bacteria produces a drug-resistant infection that escapes the host immune response. Tarusha and co-workers (2018) fabricated alginate hydrogels loaded with hyaluronic acid-lactose-modified chitosan and silver nanoparticles to stimulate wound healing and to regulate bacterial contamination of non-healing wounds. In vitro investigation revealed that hyaluronic acid released by the membrane is able to stimulate the wound healing whereas the silver nanoparticle exploits an effectual antibacterial activity against both planktonic bacteria and biofilms.
Amniotic fluid (AF) is enriched with a varied range of growth factors such as fibroblast growth factor (FGF), epidermal growth factor (EGF) and transforming growth factor beta 1 (TGF- β1) that are ideal to promote cellular response and wound healing. AF has specified functions and applications like cell proliferation, migration and differentiation which have enormous effect on improvement of the wound healing process. Moreover, AF has revealed to enhance healing in bone, regeneration in tendon tissue and prevention of scar formation in nerve cells. Ghalei and co-workers (2018) fabricated alginate hydrogel-electrospun silk fibroin fibres to deliver AF to the wound site. Fibroblast culturing on the fabricated dressings demonstrated that cellular proliferation, spreading and secretion of collagen enhanced with increasing AF. Taken together, the results provided a novel bioactive dressing with great potentials for speeding up the healing process in severe wounds.
4.7.3 Tissue Engineering
Tissue engineering has emerged as a viable approach to treat disease or repair damage in tissues and organs. The ideal paradigm in tissue engineering is introduction of tissue grafts native to the wounded areas to facilitate the regenerative process. Tissue engineering mostly involves the in vitro seeding and proliferation of cells in a scaffold-based or injectable supports. To ensure that adequate cells reach the target tissue, it is important to have effective cell transplantation process that is capable of sustaining the survival of implanted cells while maintaining their function and improving their adhesion with the host (Sun and Tan 2013). The delivery of cells with the help of a biocompatible material has emerged as an efficient strategy. In this regard, alginate is a classic example because of its versatility and tunability. The potential of alginate as an artificial three-dimensional cellular matrix in a diversity of applications in regenerative medicine has been elucidated beautifully by Giri et al. (2012) and Sun and Tan (2013).
4.7.3.1 Bone Tissue Regeneration
Bone is a complex tissue that comprises of hydroxyapatite and collagen. Bone defects or fractures occur under various medical conditions such as osteoporosis, arthritis, neoplasm, congenital defects, etc. The current standard treatment is based on the use of autograft, allograft and xenograft. Despite the introduction of several augmentation techniques and bone graft materials, bone regeneration still remains a subject of clinical challenge. Autografts and allografts are inevitably associated with certain shortcomings such as donor site morbidity, risk of immune reaction and post-operative pain and infection (Venkatesan et al. 2015). Therefore, there has been a constant need for development of biomaterials that apart from restoring/repairing the damaged bone should also be structurally, functionally and mechanically equivalent to a healthy bone and favour cell adhesion, proliferation and differentiation for bone tissue regeneration. In this regard, alginate has been appraised as a promising biomaterial because of its biocompatibility, non-immunogenicity and biodegradability. A broad overview of alginate composites, their preparation and subsequent applications in bone tissue engineering has been vividly provided by Venkatesan et al. (2015) in their review article.
Over the years, natural polymer-ceramic composites have evolved to be a promising bone graft substitute. While the ceramic provide strength and osteoconductivity, the polymer imparts flexibility and resorbability. Recently, R. A. Popescu et al. (2018) have combined bioactive glass-ceramics with alginate-pullulan hydrogel for the synthesis of new biocompatible hydrogels for in vivo bone tissue regeneration. The proliferation rates for the fibroblast and osteoblast cell viability assays were excellent for the composites. Additionally, the histopathological results displayed good biocompatibility, thus validating their worth in bone regeneration applications. In another report by Tohamy et al. (2018), alginate-hydroxyethyl cellulose-hydroxyapatite composite scaffolds were potentially explored for enhanced in vitro bone regeneration. The authors witnessed higher protein adsorption, cell proliferation and cell viability for human mesenchymal cells. X. Zhang and co-workers (2018) prepared composite hydrogels of Na-alginate-akermanite-glutamate to promote irregular bone regeneration through stem cell recruitment. Their findings revealed no cytotoxicity and higher gene expressions in human bone marrow stromal cells after culturing with hydrogel extracts. The implanted hydrogel also assisted bone mesenchymal stem cell migration to the injured area via CXCR4 (C-X-C chemokine receptor type 4) elevation and stimulated osteogenic differentiation of these cells through the MAPK pathway. This study validated the hydrogels as competent materials for the regeneration of irregular bone cavities.
Clinically used supraphysiological dose of bone morphogenetic protein-2 (BMP-2) usually carries the risk of adverse effects. Thus, an injectable hydrogel composed of BMP-2-loaded recombinant collagen-based microspheres and alginate were developed by Mumcuoglu et al. (2018). BMP-2 doses of 10 μg, 3 μg and 1 μg per implant (50 μg/mL, 15 μg/mL and 5 μg/mL, respectively) were effectively injected subcutaneously in rats in a time- and dose-dependent manner for both ectopic and calvarial rat defect models. Lin et al. (2018) reported about the implantation of alginate fibre with diclofenac and bone cells coated with chitosan for bone regeneration during inflammation. The outcome revealed that on days 7 and 10, when diclofenac was consumed and the concentrations of inflammatory compounds surged, the coating efficiently blocked the harmful compounds and protected the bone cells within the fibres. In another study by Chen and co-authors (2018c) the effect of an IPN of sodium hyaluronate-Na-alginate scaffold combined with berberine on osteochondral repair was investigated in vivo. The authors explored the mechanism of the osteochondral repair and found that the IPN could simultaneously regenerate not only the cartilage but also the subchondral bone and hence is a promising material for osteochondral defect regeneration. Hydrogels comprising of chitosan-alginate-hydroxyapatite have also been successfully designed and efficaciously utilized for bone tissue engineering therapies with amazing biocompatibility and proliferation with osteoblast cells (Kim et al. 2015; Sharma et al. 2016).
4.7.3.2 Cartilage Regeneration
Despite intensive research, regeneration of articular cartilage largely remains an unresolved medical concern due to their very limited reparative capacity. For cartilage treatment in tissue engineering, the implantation of autologous chondrocytes and stem cells have been explored in the pursuit of cartilage repair (Bidarra et al. 2014). There have been many recent examples where alginate has been employed for cartilage tissue regeneration.
Ruvinov and co-authors (2018) assessed the feasibility and long-term efficiency of a bilayered injectable acellular affinity-binding alginate hydrogel in a mini pig model of osteochondral defects and the outcomes were evaluated after 6 months. Macroscopical and histological evaluation of the defects treated with the hydrogel revealed about the effective reconstruction of articular cartilage layer, glossy surface and cellular organization hyaline tissue associated with noticeable deposition of proteoglycans and type II collagen. The authors concluded that the model showed promising potential of an injectable acellular growth factor-loaded affinity-binding candidate for effective repair and regeneration of articular hyaline cartilage (Fig. 4.3) (Chen et al. 2018c).
Preparation and characterization of sodium hyaluronate and sodium alginate IPN scaffold. (a) Microscopic structure of the scaffold. (b) Cell viability in the presence of the scaffold. (c) Live-dead cell staining. Scale bars = 200 μm. (d) Cell proliferation assay. (e) SEM micrographs of BMSCs on the scaffold. (f) Standard curve of BER. (g) Controlled release profile of BER. (Chen et al. 2018c)
Three-dimensional cell printing is a unique technique that enables free-form fabrication of cell-laden hydrogel scaffolds with controllable features and interconnected pores for tissue engineering applications. Thus, bioink materials that are able to offer good printability and favourable cellular interaction are highly desirable. Recent literature has pointed towards the surge of 3D bioprinted alginate scaffolds for cartilage engineering. Yang et al. (2018a, b) constructed printed cartilage tissue using collagen type I or agarose mixed with Na-alginate to serve as 3D bioprinting bioinks by incorporating chondrocytes. The Na-alginate/collagen scaffold could noticeably facilitate cell adhesion and proliferation and improved the expression of cartilage-specific genes such as Acan, Col2al and Sox9 than Na-alginate/agarose. The authors further observed that Na-alginate/collagen scaffold effectively suppressed the differentiation of chondrocytes and preserved the phenotype and hence is a promising biomaterial in cartilage tissue engineering. In a study by Markstedt and co-workers (2015), 3D bioprinted human chondrocytes with nanocellulose/alginate bioink for cartilage tissue engineering were developed. The bioinks enabled the printing of both 2D grid-like structures and 3D constructs. Additionally, the human chondrocytes bioprinted in the bioink exhibited a cell viability of 86% even after seven days of culture. This study established the efficacy of nanocellulose/alginate bioinks for 3D bioprinting of living tissues. Kundu et al. (2015) have developed 3D cell-printed scaffolds using layer-by-layer deposition of polycaprolactone and chondrocyte cell-encapsulated alginate hydrogel. The 3D cell-printed scaffolds were then implanted in the dorsal subcutaneous spaces of female nude mice. After 4 weeks, the retrieved implants revealed much enhanced cartilage tissue formation in the scaffold.
Several studies have also demonstrated the use of injectable hydrogels for cartilage regeneration purposes because of the ease of handling and complete filling of defect area through minimally invasive surgical instruments. In a study by Liao et al. (2017) an injectable 3D alginate hydrogel crosslinked by calcium gluconate-loaded porous microspheres was assessed for cartilage regeneration features. The authors reported that the defects could be completely healed by 18 weeks and the repaired chondrocytes regained a normal tissue structure. A series of injectable in situ self-crosslinking poly(L-glutamic acid)-alginate hydrogels were fabricated and rabbit chondrocytes were encapsulated in them (Yan et al. 2014). The results affirmed the injectability and rapid in vivo gel formation along with mechanical stability, cell growth and ectopic cartilage formation.
4.7.3.3 Cardiac Tissue Regeneration
Heart failure is one of the most common causes of death globally. Majority cases of heart failure have been due to myocardial infarction (MI) associated with the left ventricle. Since the adult heart lacks regenerative capacity, loss of myocardium is irreversible and ultimately leads to failure. Existing heart failure therapies aim to compensate for the insufficient and low intrinsic regenerative ability of the adult heart. In order to repopulate the areas of cell loss in the damaged hearts, cell regeneration has gained thrust (Bidarra et al. 2014). Alginate has been identified as one building block to accomplish the therapeutic regeneration of cardiomyocytes.
Liberski et al. (2016) have reviewed the current applications of alginate in cardiac regeneration and valve replacement techniques. In another review article by Ruvinov and Cohen (2016), the authors have summarized the versatile applications of alginate as a supporting cardiac implant after acute MI to its employment as delivery vehicles for stem cells and other bioactive molecules and/or regenerative factors to the heart. The preclinical and first-in-man clinical trials using alginate hydrogels leading to myocardial repair and tissue regeneration have been discussed in greater details.
Recently, Sondermeijer et al. (2018) reported the development of a porous, biocompatible 3D alginate scaffold covalently modified with RGDfK (Arg-Gly-Asp-D-Phe-Lys) peptide. Following implantation in the abdominal rectus muscles in rats, the authors observed that the scaffolds seeded with human mesenchymal precursor cells and patched to the epicardial surface of infarcted myocardium induced myocardial neoangiogenesis and significantly improved cardiac function. The authors established this biomaterial as a potential strategy to deliver cells to myocardial infarct areas to improve neovascularization and cardiac function.
Rosellini and co-workers (2018) have lately fabricated a new class of porous scaffolds by integrating a protein (collagen or gelatin) with alginate for mimicking native ECM for cardiac tissue engineering applications. The gelatin-alginate scaffolds better mimicked the native tissues and exhibited superior mechanical properties. A high viability of the resulting cardiac constructs was observed from the scaffolds after culturing with neonatal rat cardiomyocytes. The authors strongly proposed the protein-alginate scaffolds as viable substitutes for application in cardiac tissue regeneration.
Cardiac stem cells (CSC) are a heterogenic group of cells concentrated in particular areas of heart such as the atria or pericardium. CSCs represent a logical cell type to be explored as a regenerative treatment option for tissues damaged due to MI. Since the isolation of CSCs is time consuming and expensive, O’Neill and co-authors (2018) proposed the incorporation of growth factor-eluting alginate microparticles into collagen-based scaffolds to promote the recruitment and expansion of CSCs. The alginate microparticles were encapsulated with two types of proteins, hepatocyte growth factor and insulin-like growth factor-1, and subsequently incorporated into the collagen matrix. The in vitro assays with isolated CSCs demonstrated that the sustained protein release (which extended up to 15 days) from the scaffolds resulted in motogenic and proliferative effect.
4.7.3.4 Liver Tissue Regeneration
Liver tissue engineering basically deals with the possibility of reproducing in total or in part the functions of the liver in order to treat acute or chronic liver disorders and, ultimately, create a fully functional organ to be transplanted or used as an extracorporeal device. The technological strategies in this direction are based on allocating hepatocytes/hepatocyte-like cells within a 3D structure to ensure their survival and maintain their functional phenotype. The recently published review article by Mazza et al. (2018) elucidates liver tissue engineering with precision in terms of implantable liver tissues to whole organ engineering.
A recent study carried out by Liu and co-authors (2018c) employed Ca-alginate gel sheets, embedded with liver cells (RLC-18) with the intention to imitate liver lobule tissue. The Ca-alginate sheets having hepatic lobule-shaped patterns were deposited onto a microelectrode device using electrodeposition and the viability of embedded cells exceeded 80%. The cell sheets were removed from the electrode substrate and stacked onto a 3D multilayered structure to mimic the morphology of liver lobule tissue. The authors concluded that the developed method could provide a new bottom-up paradigm to build 3D macroscopic liver tissue similar to that in vivo.
Developing 3D cell culture systems is necessary for investigating the mechanism of hepatocellular carcinoma (HCC) metastasis and screen therapeutic drugs. Sun et al. (2018) constructed decellularized liver matrix-alginate (DLM-ALG) hybrid gel beads for the 3D culture of HCC cells. DLM-ALG beads exhibited better activity of matrix metalloproteinases (MMPs) of HCCLM3 cells, including MMP2 and MMP9 and urokinase plasminogen activator system. The findings of this study established DLM-ALG beads as potential in HCC research and subsequently in liver tissue engineering.
Injectable hydrogels synthesized from glycyrrhizin, alginate and calcium for 3D cell culture in liver tissue engineering has been reported by Tong et al. (2018). The hydrogels showed good biocompatibility and could maintain the viability, proliferation and liver function for longer periods of time. Moreover, the hydrogels enhanced the mRNA expression of cytochrome P450, which were key enzymes to the metabolization of hepatocytes and could be a potential 3D cell culture system for liver tissue engineering.
4.7.3.5 Nerve Tissue Regeneration
Neural regeneration research is designed to develop strategies for therapy for nerve damage incurred by disease or injury. In order to fabricate fully functional and biomimetic nerve substitutes, various kinds of polymeric-based scaffolds have been proposed. Artificial nerve grafts need to mimic the native extracellular matrix both structurally and mechanically in order to provide the appropriate environment for the neotissues. Furthermore, artificial nerve grafts need to be electrically conductive to support the electrical conduction of injured nerve during regeneration and enhance regeneration. The biomaterial further needs to induce appropriate chemical and physical signalling cues, which transduce into intracellular biochemical responses to moderate the cellular function. Alginate being a negatively charged polysaccharide has been widely applied to develop artificial constructs for peripheral nerve tissues (Prang et al. 2006).
Bioprinting Schwann cell-encapsulated scaffolds using alginate, fibrin, hyaluronic acid and/or RGD peptide were synthesized by Ning et al. (2018) for nerve tissue engineering. The printed scaffolds enhanced the alignment of Schwann cells inside scaffolds and provided haptotactic cues to direct the extension of dorsal root ganglion neurites, which has potential applications in nerve tissue engineering. A composite hydrogel of PAAm-graphene oxide-gelatin-Na-alginate for accelerating peripheral nerve regeneration was fabricated through in situ free radical polymerization (Zhao et al. 2018). The hydrogel displayed remarkable adhesion and proliferation of cultured Schwann cells. The results of this study validated the hydrogel for neural tissue engineering applications by promoting Schwann cell growth. In another study, Golafshan and co-workers (2017) developed graphene-Na-alginate-PVA scaffolds for engineering neural constructs. The scaffolds displayed superior electrical and mechanical properties with enhanced PC12 cell interaction. Overall, the developed scaffolds were promising devices for peripheral nerve regeneration. Wu et al. (2017) studied about the neural tissue engineering through chitosan-polylactide-alginate fibres. Nerve growth factor (NGF)-induced neurite extension of PC12 cells confirmed about the bioactivity of NGF released from fibres was well retained. Bu and co-authors (2018) have synthesized a conductive polymer of Na-alginate-carboxymethyl chitosan crosslinked with Ca2+ ions. The conductivity of the hydrogel was implemented by doping with polypyrrole. The conductive hydrogel exhibited excellent biocompatibility and repair features as a bioactive biomaterial and is potent for neural tissue engineering applications.
In a novel methodology, Buyukoz et al. (2018) utilized a combined strategy of thermally induced phase separation and porogen leaching to create interconnected macropores and nanofibrous structures. Gelatin scaffolds integrated with nerve growth factor-loaded alginate microspheres were prepared by the aforementioned strategy. The scaffolds had good topologic and mechanical properties similar to brain tissue and pore structure suitable for cell growth and differentiation and can have potential applications in brain tissue engineering.
4.8 Conclusion and Future Perspectives
To summarize, alginate has been extensively utilized in drug/protein delivery or as building blocks for tissue repair and regeneration. Owing to their versatility, alginate IPNs have been tailored with the desired structures, properties and functions. Alginate-based biomaterials are promising substrates for tissue engineering applications with the advantage that both drugs and cells can be readily integrated into the scaffolding matrix. The surge in alginate bioinks in 3D bioprinting points to the greater utility and success of the biopolymer in tissue engineering. Successful exploitation of alginate-based biomaterials in different tissues and organs such as bone, cartilage, cardiac, liver and nerve suggests their promising future for repair and regeneration applications. The applications of alginate IPNs as wound healing dressings are manifold. Alginate has rendered itself as a hugely potent wound dressing agent.
Engineering of more alginate-based biomaterials endowed with precisely designed physical, chemical and biological properties should be carried out to mimic the environment of natural tissues. The design of such alginate IPNs could further revolutionize the applicability of alginate IPNs in the world of biomedicine.
References
Aguero L, Zaldivar-Silva D, Pena L, Dias ML (2017) Alginate microparticles as oral colon drug delivery device: a review. Carbohydr Polym 168:32–43
Akbar MU, Zia KM, Akash MSH, Nazir A, Zuber M, Ibrahim M (2018) In-vivo anti-diabetic and wound healing potential of chitosan/alginate/maltodextrin/pluronic-based mixed polymeric micelles: curcumin therapeutic potential. Int J Biol Macromol 120:2418–2430
Ali M, Husain Q (2018) Guar gum blended alginate/agarose hydrogel as a promising support for the entrapment of peroxidase: stability and reusability studies for the treatment of textile effluent. Int J Biol Macromol 116:463–471
Aljohani W, Ullah MW, Li W, Shi L, Zhang X, Yang G (2018) Three-dimensional printing of alginate-gelatin-agar scaffolds using free-form motor assisted microsyringe extrusion system. J Polym Res 25:62
Amirian J, Van TTT, Bae S-H, Jung H-I, Choi H-J, Cho H-D, Lee B-T (2017) Examination of in vitro and in vivo biocompatibility of alginate-hyaluronic acid microbeads as a promising method in cell delivery for kidney regeneration. Int J Biol Macromol 105:143–153
Anwar H, Ahmad M, Minhas MU, Rehmani S (2017) Alginate-polyvinyl alcohol based interpenetrating polymer network for prolonged drug therapy, Optimization and In-vitro characterization. Carbohydr Polym 166:183–194
Arjmandi M, Ramezani M, Nand A, Neitzert T (2018a) Tribological characterization of polyacrylamide-alginate hybrid hydrogels as a potential candidate for cartilage replacement. Key Eng Mater 775:109–114
Arjmandi M, Ramezani M, Nand A, Neitzert T (2018b) Experimental study on friction and wear properties of interpenetrating polymer network alginate-polyacrylamide hydrogels for use in minimally-invasive joint implants. Wear 406:194–204
Augst AD, Kong HJ, Mooney DJ (2006) Alginate hydrogels as biomaterials. Macromol Biosci 6:623–633
Babu VR, Rao KSVK, Sairam M, Naidu BVK, Hosamani KM, Aminabhavi TM (2006) pH sensitive interpenetrating network microgels of sodium alginate-acrylic acid for the controlled release of ibuprofen. J Appl Polym Sci 99:2671–2678
Bajpai SK, Saxena SK, Sharma S (2006) Swelling behavior of barium ions-crosslinked bipolymeric sodium alginate–carboxymethyl guar gum blend beads. React Funct Polym 66:659–666
Bakhshayesh ARD, Annabi N, Khalilov R, Akbarzadeh A, Samiei M, Alizadeh E, Alizadeh-Ghodsi M, Davaran S, Montaseri A (2018) Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif Cells Nanomed Biotechnol 46:691–705
Basu S, Samanta HS, Ganguly J (2017) Green synthesis and swelling behavior of Ag-nanocomposite semi-IPN hydrogels and their drug delivery using Dolichos biflorus Linn. Soft Materials 16:7–19
Baysal K, Aroguz AY, Adiguzel Z, Baysal BM (2013) Chitosan/alginate crosslinked hydrogels: Preparation, characterization and application for cell growth purposes. Int J Biol Macromol 59:342–348
Belhadji L, Hadj-Sadok A, Moulai-Mostefa N (2018) Design and characterization of calcium-free in-situ gel formulation based on sodium alginate and chitosan. Drug Develop Ind Pharm 44:662–669
Benfattoum K, Haddadine N, Bouslah N, Benaboura A, Maincent P, Barille R, Sapin-Minet A, El-Shall MS (2018) Formulation characterization and in vitro evaluation of acacia gum–calcium alginate beads for oral drug delivery systems. Polym Adv Technol 29:884–895
Bernela M, Kaur P, Chopra M, Thakur R (2014) Synthesis, characterization of nisin loaded alginate–chitosan–pluronic composite nanoparticles and evaluation against microbes. LWT-Food Sci Technol 59:1093–1099
Berth G (1992) Methodical aspects of characterization of alginate and pectate by light scattering and viscometry coupled with GCP. Carbohydr Polym 19:1–9
Bhutani U, Laha A, Mitra K, Majumdar S (2016) Sodium alginate and gelatin hydrogels: Viscosity effect on hydrophobic drug release. Mater Lett 164:76–79
Bidarra SJ, Barrias CC, Granja PL (2014) Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater 10:1646–1662
Boateng J, Burgos-Amador R, Okeke O, Pawar H (2015) Composite alginate and gelatin based bio-polymeric wafers containing silver sulfadiazine for wound healing. Int J Biol Macromol 79:63–71
Boppana R, Mohan GK, Nayak U, Mutali S, Sa B, Kulkarni RV (2015) Novel pH-sensitive IPNs of polyacrylamide-g-gum ghatti and sodium alginate for gastro-protective drug delivery. Int J Biol Macromol 75:133–143
Bu Y, Xu H-X, Li X, Xu W-J, Yin Y-X, Dai H-L, Wang X-B, Huang Z-J, Xu P-H (2018) A conductive sodium alginate and carboxymethyl chitosan hydrogel doped with polypyrrole for peripheral nerve regeneration. RSC Adv 8:10806–10817
Buyukoz M, Erdal E, Altinkaya SA (2018) Nanofibrous gelatin scaffolds integrated with NGF-loaded alginate microspheres for brain tissue engineering. J Tissue Eng Regen Med 12:e707–e719
Chai F, Sun L, He X, Li J, Liu Y, Xiong F, Ge L, Webster TJ, Zheng C (2017) Doxorubicin-loaded poly (lactic-co-glycolic acid) nanoparticles coated with chitosan/alginate by layer by layer technology for antitumor applications. Int J Nanomedicine 12:1791–1802
Chandy T, Mooradian DL, Rao GHR (1998) Chitosan/polyethylene glycol–alginate microcapsules for oral delivery of hirudin. J Appl Polym Sci 70:2143–2153
Chang A (2015) pH-sensitive starch-g-poly(acrylic acid)/sodium alginate hydrogels for controlled release of diclofenac sodium. Iran Polym J 24:161–169
Chang H, Park H, Kelly P, Robinson J (1985) Bioadhesive polymers as platforms for oral controlled drug delivery. Synthesis and evaluation of some swelling, water-insoluble bioadhesive polymers. J Pharm Sci 74:399–405
Chen SC, Wu YC, Mi FL, Lin YH, Yu LC, Sung HW (2004) A novel pH-sensitive hydrogel composed of N, O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J Controlled Release 96:285–300
Chen F, Zhang Z, Deng Z, Zhang R, Fan G, Ma D, McClements DJ (2018a) Controlled-release of antacids from biopolymer microgels under simulated gastric conditions: impact of bead dimensions, pore size, and alginate/pectin ratio. Food Res Int 106:745–751
Chen J, Tao N, Fang S, Chen Z, Liang L, Sun X, Li J, Liu Y-N (2018b) Incorporation of Fmoc-Y nanofibers into Ca-alginate hydrogels for improving their mechanical properties and the controlled release of small molecules. New J Chem 42:9651–9657
Chen P, Xia C, Mo J, Mei S, Lin X, Fan S (2018c) Interpenetrating polymer network scaffold of sodium hyaluronate and sodium alginate combined with berberine for osteochondral defect regeneration. Mater Sci Eng C 91:190–200
Chen Z, Lv X, Zhao M, Zhang P, Ren X, Mei X (2018d) Encapsulation of green tea polyphenol by pH responsive, antibacterial, alginate microgels used for minimally invasive treatment of bone infection. Colloid Surf B: Biointerface 170:648–655
Cheow WS, Kiew TY, Hadinoto K (2014) Controlled release of Lactobacillus rhamnosus biofilm probiotics from alginate-locust bean gum microcapsules. Carbohydr Polym 103:587–595
Chickering DE, Mathiowitz E (1995) Bioadhesive microspheres: I. A novel electrobalance-based method to study adhesive interactions between individual microspheres and intestinal mucosa. J Controlled Release 34:251–261
Coskun G, Karaca E, Ozyurtlu M, Ozbek S, Yermezler A, Cavusoglu I (2014) Histological evaluation of wound healing performance of electrospun poly (vinyl alcohol)/sodium alginate as wound dressing in vivo. Biomed Mater Eng 24:1527–1536
Darnell MC, Sun J-Y, Mehta M, Johnson C, Arany PR, Suo Z, Mooney DJ (2013) Performance and biocompatibility of extremely tough alginate/polyacrylamide hydrogels. Biomaterials 34:8042–8048
de Moura MR, Guilherme MR, Campese GM, Radovanovic E, Rubira AF, Muniz EC (2005) Porous alginate-Ca2+ hydrogels interpenetrated with PNIPAAm networks: interrelationship between compressive stress and pore morphology. Eur Polym J 41:2845–2852
Desai NP, Sojomihardjo A, Yao Z, Ron N, Soon-Shiong P (2000) Interpenetrating polymer networks of alginate and polyethylene glycol for encapsulation of islets of Langerhans. J Microencapsul 17(6):677–690
Dragan ES (2014) Design and applications of interpenetrating polymer network hydrogels. A review. Chem Eng J 243:572–590
Eldin MSM, Kamoun EA, Sofan MA, Elbayomi SM (2015) l-Arginine grafted alginate hydrogel beads: A novel pH-sensitive system for specific protein delivery. Arab J Chem 8:355–365
El-Kamel A, Sokar M, Naggar V, Gamal SA (2002) Chitosan and sodium alginate- based bioadhesive vaginal tablets. AAPS PharmSci 4:224–230
Fareez IM, Lim SM, Lim FT, Mishra RK, Ramasamy K (2017) Microencapsulation of lactobacillus sp. using chitosan-alginate-xanthan gum-β-cyclodextrin and characterization of its cholesterol reducing potential and resistance against pH, temperature and storage. J Food Process Eng 40:e12458
Fattahpour M, Shamanian M, Tavakoli N, Fathi M, Sheykhi SR, Fattahpour S (2015) Design and optimization of alginate−chitosan−pluronic nanoparticles as a novel meloxicam drug delivery system. J Appl Polym Sci 132:42241
Gacesa P (1992) Enzymic degradation of alginates. Int J Biochem 24:545–552
Ganguly S, Maity PP, Mondal S, Das P, Bhawal P, Dhara S, Das NC (2018) Polysaccharide and poly(methacrylic acid) based biodegradable elastomeric biocompatible semi-IPN hydrogel for controlled drug delivery. Mater Sci Eng C 92:34–51
George M, Abraham TE (2006) Polyionic hydrocolloids for the intestinal delivery of protein drugs: Alginate and chitosan — a review. J Controlled Release 114:1–14
George M, Abraham TE (2007) pH sensitive alginate–guar gum hydrogel for the controlled delivery of protein drugs. Int J Pharm 335:123–129
Giri T, Thakur D, Alexander A, Ajazuddin, Badwaik H, Tripathi D (2012) Alginate based hydrogel as a potential biopolymeric carrier for drug delivery and cell delivery systems: present status and applications. Curr Drug Deliv 9:539–555
Golafshan N, Kharaziha M, Fathi M (2017) Tough and conductive hybrid graphene-PVA: Alginate fibrous scaffolds for engineering neural construct. Carbon 111:752–763
Gombotz WR, Wee SF (1998) Protein release from alginate matrices. Adv Drug Delivery Rev 31:267–285
Goncalves VSS, Gurikov P, Poejo J, Matias AA, Heinrich S, Duarte CMM, Smirnova I (2016) Alginate-based hybrid aerogel microparticles for mucosal drug delivery. Eur J Pharm Biopharm 107:160–170
Graulus G-J, Mignon A, Vlierberghe SV, Declercq H, Feher K, Cornelissen M, Martins JC, Dubruel P (2015) cross-linkable alginate-graft-gelatin copolymers for tissue engineering applications. Eur Polym J 72:494–506
Guilherme MR, Toledo EA, Rubira AF, Muniz EC (2002) Water affinity and permeability in membranes of alginate-Ca2+ containing poly(N-isopropylacrylamide). J Membr Sci 210:129–136
Guilherme MR, de Moura MR, Radovanovic E, Geuskens G, Rubira AF, Muniz EC (2005) Novel thermo-responsive membranes composed of interpenetrated polymer networks of alginate-Ca2+ and poly(N-isopropylacrylamide). Polymer 46:2668–2674
Guo J, Giusti MM, Kaletunc G (2018) Encapsulation of purple corn and blueberry extracts in alginate-pectin hydrogel particles: impact of processing and storage parameters on encapsulation efficiency. Food Res Int 107:414–422
Hanif M, Abbas G (2018) pH-responsive alginate–pectin polymeric rafts and their characterization. Adv Polym Technol 37:1496–1506
Hardy A, Seguin C, Brion A, Lavalle P, Schaaf P, Fournel S, Bourel-Bonnet L, Frisch B, De Giorgi M (2018) Cyclodextrin-functionalized chitosan/alginate compact polyelectrolyte complexes (CoPECs) as functional biomaterials with anti-inflammatory properties. ACS Appl Mater. Interface 10:29347–29356
Hong SH, Kim S, Park JP, Shin M, Kim K, Ryu JH, Lee H (2018) Dynamic bonds between boronic acid and alginate: hydrogels with stretchable, self-healing, stimuli-responsive, remoldable, and adhesive properties. Biomacromolecules 19:2053–2061
Hu W-W, Ting J-C (2019) Gene immobilization on alginate/ polycaprolactone fibers through electrophoretic deposition to promote in situ transfection efficiency and biocompatibility. Int J Biol Macromol 119:1337–1345
Hu Y, Peng J, Ke L, Zhao D, Zhao H, Xiao X (2016) Alginate/carboxymethyl chitosan composite gel beads for oral drug delivery. J Polym Res 23:129
Hu W-W, Wu Y-C, Hu Z-C (2018a) The development of an alginate/ polycaprolactone composite scaffold for in situ transfection application. Carbohydr Polym 183:29–36
Hu Y, Zhang Z, Li Y, Ding X, Li D, Shen C, Xu F-J (2018b) Dual-crosslinked amorphous polysaccharide hydrogels based on chitosan/alginate for wound healing applications. Macromol Rapid Commun 39:e1800069
Isiklan N, Kucukbalci G (2016) Synthesis and characterization of pH- and temperature-sensitive materials based on alginate and poly(N-isopropylacrylamide/acrylic acid) for drug delivery. Polym Bull 73:1321–1342
Jalababu R, Veni SS, Reddy KVNS (2018) Synthesis and characterization of dual responsive sodium alginate-g-acryloyl phenylalanine-poly N-isopropyl acrylamide smart hydrogels for the controlled release of anticancer drug. J Drug Deliv Sci Technol 44:190–204
Jalil A, Khan S, Naeem F, Haider MS, Sarwar S, Raiz A, Ranjha NM (2016) The structural, morphological and thermal properties of grafted pH-sensitive interpenetrating highly porous polymeric composites of sodium alginate/acrylic acid copolymers for controlled delivery of diclofenac potassium. Des Monomers Polym 20:308–324
Jana S, Gandhi A, Sheet S, Sen KK (2015) Metal ion-induced alginate–locust bean gum IPN microspheres for sustained oral delivery of aceclofenac. Int J Biol Macromol 72:47–53
Joshy KS, George A, Jose J, Kalarikkal N, Pothen LA, Thomas S (2017) Novel dendritic structure of alginate hybrid nanoparticles for effective anti-viral drug delivery. Int J Biol Macromol 103:1265–1275
Jost V, Reinelt M (2018) Effect of Ca2+ induced crosslinking on the mechanical and barrier properties of alginate cast films. J Appl Polym Sci 135:45754
Ju HK, Kim SY, Kim SJ, Lee YM (2002) pH/Temperature-responsive remi-IPN hydrogels composed of alginate and poly(N-isopropylacrylamide). J Appl Polym Sci 83:1128–1139
Kajjari PB, Manjeshwar LS, Aminabhavi TM (2012) Novel pH- and temperature responsive blend hydrogel microspheres of sodium alginate and PNIPAAm-g-GG for controlled release of isoniazid. AAPS PharmSciTech 13:1147–1157
Kamoun EA, Kenawy ES, Tamer TM, El-Meligy MA, Eldin MSM (2018) Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arab J Chem 8:38–47
Khalid I, Ahmad M, Minhas MU, Barkat K (2018) Preparation and characterization of alginate-PVA-based semi-IPN: controlled release pH-responsive composites. Polym Bull 75(3):1075–1099
Kim MS, Kim GH (2014) Three-dimensional electrospun polycaprolactone (PCL)/ alginate hybrid composite scaffolds. Carbohydr Polym 114:213–221
Kim H-L, Jung G-Y, Yoon J-H, Han J-S, Park Y-J, Kim D-G, Zhang M, Kim D-J (2015) Preparation and characterization of nano-sized hydroxyapatite/alginate/chitosan composite scaffolds for bone tissue engineering. Mater Sci Eng C 54:20–25
Kolanthai E, Sindu PA, Khajuria DK, Veerla SC, Kuppuswamy D, Catalani LH, Mahapatra DR (2018) Graphene oxide–a tool for preparation of chemically crosslinking free alginate-chitosan-collagen scaffold for bone tissue engineering. ACS Appl Mater Interface 10:12441–12452
Kong HJ, Mooney DJ (2003) The effects of poly(ethyleneimine) (PEI) molecular weight on reinforcement of alginate hydrogels. Cell Transplant 12:779–785
Kong HJ, Lee KY, Mooney DJ (2002) Decoupling the dependence of rheological/ mechanical properties of hydrogels from solids concentration. Polymer 43:6239–6246
Kong HJ, Smith MK, Mooney DJ (2003) Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 24:4023–4029
Kulkarni RV, Sreedhar V, Mutalik S, Setty CM, Sa B (2010) Interpenetrating network hydrogel membranes of sodium alginate and poly(vinyl alcohol) for controlled release of prazosin hydrochloride through skin. Int J Biol Macromol 47:520–527
Kulkarni RV, Baraskar VV, Setty CM, Sa B (2011) Interpenetrating polymer network matrices of sodium alginate and carrageenan for controlled drug delivery application. Fiber Polym 12:352–358
Kulkarni RV, Boppana R, Mohan GK, Mutalik S, Kalyane NV (2012) pH-responsive interpenetrating network hydrogel beads of poly(acrylamide)-g-carrageenan and sodium alginate for intestinal targeted drug delivery: Synthesis, in vitro and in vivo evaluation. J Colloid Interface Sci 367:509–517
Kumar A, Rao KM, Han SS (2017) Development of sodium alginate-xanthan gum-based nanocomposite scaffolds reinforced with cellulose nanocrystals and halloysite nanotubes. Polym Test 63:214–225
Kundu J, Shim J-H, Jang J, Kim S-W, Cho D-W (2015) An additive manufacturing-based PCL–alginate–chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med 9(11):1286–1297
Kunjukunju S, Roy A, Shekhar S, Kumta PN (2018) Cross-linked enzyme aggregates of alginate lyase: A systematic engineered approach to controlled degradation of alginate hydrogel. Int J Biol Macromol 115:176–184
Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci 37:106–126
Lee DW, Choi WS, Byun MW, Park HJ, Yu YM, Lee CM (2003) Effect of gamma-irradiation on degradation of alginate. J Agric Food Chem 51:4819–4823
LeRoux MA, Guilak F, Setton LA (1999) Compressive and shear properties of alginate gel: effects of sodium ions and alginate concentration. J Biomed Mater Res 47:46–53
Li Z, Zhang M (2005) Chitosan–alginate as scaffolding material for cartilage tissue engineering. J Biomed Mater Res 75A:485–493
Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M (2005) Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials 26:3919–3928
Li B, Li D, Yang Y, Zhang L, Xu K, Wang J (2018) Study of thermal-sensitive alginate-Ca+2/poly(N-isopropylacrylamide) hydrogels supported by cotton fabric for wound dressing applications. Textile Res J. https://doi.org/10.1177/0040517518755790
Liao JF, Wang BY, Huang YX, Qu Y, Peng JR, Qian ZY (2017) Injectable alginate hydrogel cross-linked by calcium gluconate-loaded porous microspheres for cartilage tissue engineering. ACS Omega 2:443–454
Liao JF, Jia YP, Wang BY, Shi K, Qian ZY (2018) Injectable hybrid poly(ε caprolactone)-b-poly(ethyleneglycol)-b-poly(ε-caprolactone) porous microspheres/ alginate hydrogel cross-linked by calcium gluconate crystals deposited in the pores of microspheres improved skin wound healing. ACS Biomater Sci Eng 4:1029–1036
Liberski A, Latif N, Raynaud C, Bollensdorf C, Yacoub M (2016) Alginate for cardiac regeneration: from seaweed to clinical trials. Glob Cardiol Sci Pract 1:e201604
Lim H-P, Ooi C-W, Tey B-T, Chan E-S (2017) Controlled delivery of oral insulin aspart using pH-responsive alginate/κ-carrageenan composite hydrogel beads. React Funct Polym 120:20–29
Lima DS, Tenorio-Neto ET, Lima-Tenorio MK, Guilherme MR, Scariot DB, Nakamura CV, Muniz EC, Rubira AF (2018) pH-responsive alginate-based hydrogels for protein delivery. J Mol Liq 262:29–36
Lin H-R, Sung KC, Vong W-J (2004) In situ gelling of alginate/ pluronic solutions for ophthalmic delivery of pilocarpine. Biomacromolecules 5:2358–2365
Lin Y-S, Liang H-F, Chung C-K, Chen M-C, Sung H-W (2005) Physically crosslinked alginate/N,O-carboxymethyl chitosan hydrogels with calcium for oral delivery of protein drugs. J Controlled Release 26:2105–2113
Lin H-Y, Tsang-Wen C, Peng T-K (2018) 3D plotted alginate fibers embedded with diclofenac and bone cells coated with chitosan for bone regeneration during inflammation. J Biomed Mater Res A 106(6):1511–1521
Lin F, Zheng R, Chen J, Su W, Dong B, Lin C, Huang B, Lu B (2019) Microfibrillated cellulose enhancement to mechanical and conductive properties of biocompatible hydrogels. Carbohydr Polym 205:244–254
Liu M, Song X, Wen Y, Zhu Z-L, Li J (2017) Injectable thermoresponsive hydrogel formed by alginate-g-poly(N-isopropylacrylamide) that releases doxorubicin-encapsulated micelles as a smart drug delivery system. ACS Appl Mater Interfaces 9:35673–35682
Liu J, Xiao J, Li F, Shi Y, Li D, Huang Q (2018a) Chitosan-sodium alginate nanoparticle as a delivery system for ε-polylysine: preparation, characterization and antimicrobial activity. Int J Biol Macromol 91:302–310
Liu X, Nielsen LH, Klodzinska SN, Nielsen HM, Qu H, Christensen LP, Rantanen J, Yang M (2018b) Ciprofloxacin-loaded sodium alginate/poly (lactic-co-glycolic acid) electrospun fibrous mats for wound healing. Eur J Pharm Biopharm 123:42–49
Liu Z, Lu M, Takeuchi M, Yue T, Hasegawa Y, Huang Q, Fukuda T (2018c) In vitro mimicking the morphology of hepatic lobule tissue based on Ca-alginate cell sheets. Biomed Mater 13:035004
Livnat M, Beyar R, Seliktar D (2005) Endoluminal hydrogel films made of alginate and polyethylene glycol: physical characteristics and drug-eluting properties. J Biomed Mater Res Part A 75A:710–722
Lohani A, Singh G, Bhattacharya SS, Verma A (2014) Interpenetrating polymer networks as innovative drug delivery systems. J Drug Deliv 2014:1–11
Lv X, Liu Y, Song S, Tong C, Shi X, Zhao Y, Zhang J, Hou M (2018) Influence of chitosan oligosaccharide on the gelling and wound healing properties of injectable hydrogels based on carboxymethyl chitosan/alginate polyelectrolyte complexes. Carbohydr Polym 198:86–93
Madrigal JL, Sharma SN, Campbell KT, Stilhano RS, Gijsbers R, Silva EA (2018) Microgels produced using microfluidic on-chip polymer blending for controlled released of VEGF encoding lentivectors. Acta Biomater 69:265–276
Mahdavinia GR, Rahmani Z, Karami S, Pourjavadi A (2014) Magnetic/pH-sensitive κ-carrageenan/sodium alginate hydrogel nanocomposite beads: preparation, swelling behavior, and drug delivery. J Biomater Sci Polym Ed 25:1891–1906
Markstedt K, Mantas A, Tournier I, Avila HM, Hagg D, Gatenholm P (2015) 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules 16:1489–1496
Matricardi P, Pontoriero M, Coviello T, Casadei MA, Alhaique F (2008) In situ cross-linkable novel alginate-dextran methacrylate IPN hydrogels for biomedical applications: Mechanical characterization and drug delivery properties. Biomacromolecules 9:2014–2020
Matricardi P, Di Meo C, Coviello T, Hennink WE, Alhaique F (2013) Interpenetrating Polymer Networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv Drug Deliv Rev 65:1172–1187
Mazza G, Al-Akkad W, Rombouts K, Pinzani M (2018) liver tissue engineering: from implantable tissue to whole organ engineering. Hepatol Commun 2:131–141
Moebus K, Siepmann J, Bodmeier R (2009) Alginate–poloxamer microparticles for controlled drug delivery to mucosal tissue. Eur J Pharm Biopharm 72:42–53
Mohamadnia Z, Zohuriaan-Mehr MJ, Kabiri K, Jamshidi A, Mobedi H (2007) pH-sensitive IPN hydrogel beads of carrageenan-alginate for controlled drug delivery. J Bioactiv Compat Polym 22:342–356
Mohamadnia Z, Zohuriaan-Mehr MJ, Kabiri K, Jamshidi A, Mobedi H (2008) Ionically cross-linked carrageenan-alginate hydrogel beads. J Biomater Sci Polym Ed 19:47–59
Momoh FU, Boateng JS, Richardson SCW, Chowdhury BZ, Mitchell JC (2015) Development and functional characterization of alginate dressing as potential protein delivery system for wound healing. Int J Bio Macromol 81:137–150
Motwani SK, Chopra S, Talegaonkar S, Kohli K, Ahmad FJ, Khar RK (2008) Chitosan–sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: formulation optimisation and in vitro characterisation. Eur J Pharm Biopharm 68:513–525
Mumcuoglu D, Fahmy-Garcia S, Ridwan Y, Nickel J, Farrell E, Kluijtmans SG, van Osch GJ (2018) Injectable bmp-2 delivery system based on collagen derived microspheres and alginate induced bone formation in a time-and dose-dependent manner. Eur Cell Mater 35:242–254
Naghizadeh Z, Karkhaneh A, Khojasteh A (2018) Self-crosslinking effect of chitosan and gelatin on alginate based hydrogels: injectable in situ forming scaffolds. Mater Sci Eng C 89:256–264
Nahar K, Hossain MK, Khan TA (2017) Alginate and its versatile applications in drug delivery. J Pharm Sci Res 9:606–617
Narayanan LK, Huebner P, Fisher MB, Spang JT, Starly B, Shirwaiker RA (2016) 3D-bioprinting of polylactic acid (PLA) nanofiber–alginate hydrogel bioink containing human adipose-derived stem cells. ACS Biomater Sci Eng 2:1732–1742
Ning L, Sun H, Lelong T, Guilloteau R, Zhu N, Schreyer DJ, Chen X (2018) 3D bioprinting of scaffolds with living Schwann cells for potential nerve tissue engineering applications. Biofabrication 10:035014
Nooeaid P, Chuysinuan P, Techasakul S, Lirdprapamongkol K, Svasti J (2017) Physico-chemical and in vitro cytotoxic properties of alginate/soy protein isolated scaffolds for tissue engineering. Key Eng Mater 757:46–51
Nunamaker EA, Purcell EK, Kipke DR (2007) In vivo stability and biocompatibility of implanted calcium alginate disks. J Biomed Mater Res 83A:1128–1137
O’Neill HS, O’Sullivan J, Porteous N, Ruiz-Hernandez E, Kelly HM, O’Brien FJ, Duffy GP (2018) A collagen cardiac patch incorporating alginate microparticles permits the controlled release of hepatocyte growth factor and insulin-like growth factor-1 to enhance cardiac stem cell migration and proliferation. J Tissue Eng Regen Med 12:e384–e394
Orive G, Carcaboso AM, Hernandez RM, Gascon AR, Pedraz JL (2005) Biocompatibility evaluation of different alginates and alginate-based microcapsules. Biomacromolecules 6:927–931
Park D, Hong Z, Kim J-C (2017) Gold nanoparticles-loaded cinnamoyl pluronic F-127/ cinnamoyl alginate microparticles prepared by a spray drying method. Int J Polym Mater Polym Biomater 66:753–761
Pentlavalli S, Chambers P, Sathy BN, O’Doherty M, Chalanqui M, Kelly DJ, Haut-Donahue T, McCarthy HO, Dunne NJ (2017) Simple radical polymerization of poly(alginate-graft-N-isopropylacrylamide) injectable thermoresponsive hydrogel with the potential for localized and sustained delivery of stem cells and bioactive molecules. Macromol Biosci 17:1700118
Peppas NA (2004) Hydrogels. In: Rattner BD, Hoffmann AS, Schoen FJ, Lemons JE (eds) Biomaterials science, 2nd edn. Elsevier, San Diego, CA, pp 100–106
Pescosolido L, Vermonden T, Malda J, Censi R, Dhert WJA, Alhaique F, Hennink WE, Matricardi P (2011) In situ forming IPN hydrogels of calcium alginate and dextran-HEMA for biomedical applications. Acta Biomater 7:1627–1633
Pinkas O, Goder D, Noyvirt R, Peleg S, Kahlon M, Zilberman M (2017) Structuring of composite hydrogel bioadhesives and its effect on properties and bonding mechanism. Acta Biomater 51:125–137
Popescu RA, Magyari K, Taulescu M, Vulpoi A, Berce C, Bogdan S, Lelescu C, Dreanca A, Tudoran O, Papuc I, Baia L (2018) New alginate-pullulan-bioactive glasses composites with copper oxide for bone tissue regeneration trials. J Tissue Eng Regen Med 12:2112–2121
Prabaharan M (2011) Prospective of guar gum and its derivatives as controlled drug delivery systems. Int J Biol Macromol 49:117–124
Prabaharan M, Jayakumar R (2009) Chitosan-graft-beta-cyclodextrin scaffolds with controlled drug release capability for tissue engineering applications. Int J Biol Macromol 44:320–325
Prang P, Muller R, Eljaouhari A, Heckmann K, Kunz W, Weber T, Faber C, Vroemen M, Bogdahn U, Weidner N (2006) The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials 27:3560–3569
Raguvaran R, Manuja BK, Chopra M, Thakur R, Anand T, Kalia A, Manuja A (2017) Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int J Biol Macromol 96:185–191
Rahmani V, Sheardown H (2018) Protein-alginate complexes as pH-/ion-sensitive carriers of proteins. Int J Pharm 535:452–461
Rassu G, Salis A, Porcu EP, Giunchedi P, Roldo M, Gavini E (2016) Composite chitosan/alginate hydrogel for controlled release of deferoxamine: A system to potentially treat iron dysregulation diseases. Carbohydr Polym 136:1338–1347
Reddy KM, Babu VR, Rao KSVK, Subha MCS, Rao KC, Sairam M, Aminabhavi TM (2008) Temperature sensitive semi-IPN microspheres from sodium alginate and N- Isopropylacrylamide for controlled release of 5-fluorouacil. J Appl Polym Sci 107:2820–2829
Remminghorst U, Rehm BHA (2006) Bacterial alginates: from biosynthesis to applications. Biotechnol Lett 28:1701–1712
Riaz S, Malik S, Hussain T, Ashraf M, Iftikhar F, Younus A, Abid S, Zahir A (2018) Development of antibacterial fibers and study on effect of guar-gum addition on properties of carboxymethylcellulose (CMC)/alginate fibers. IOP Conf Ser: Mater Sci Eng 414:012020
Rinaudo M (1992) On the abnormal exponents αη and αD in Mark–Houwink type equations for wormlike chain polysaccharides. Polym Bull 27:585–589
Rinaudo M (2008) Main properties and current applications of some polysaccharides as biomaterials. Polym Int 57:397–430
Rosellini E, Zhang YS, Migliori B, Barbani N, Lazzeri L, Shin SR, Dokmeci MR, Cascone MG (2018) Protein/polysaccharide-based scaffolds mimicking native extracellular matrix for cardiac tissue engineering applications. J Biomed Mater Res Part A 106:769–781
Rottensteiner U, Sarker B, Heusinger D, Dafinova D, Rath SN, Beier JP, Kneser U, Horch RE, Detsh R, Boccaccini AR, Arkudas A (2014) In vitro and in vivo biocompatibility of alginate dialdehyde/gelatin hydrogels with and without nanoscaled bioactive glass for bone tissue engineering applications. Materials 7:1957–1974
Ruvinov E, Cohen S (2016) Alginate biomaterial for the treatment of myocardial infarction: Progress, translational strategies, and clinical outlook: from ocean algae to patient bedside. Adv Drug Deliv Rev 96:54–76
Ruvinov E, Re’em TT, Witte F, Cohen S (2018) Articular cartilage regeneration using acellular bioactive affinity-binding alginate hydrogel: a 6-month study in a mini-pig model of osteochondral defects. J Orthop Trans. https://doi.org/10.1016/j.jot.2018.08.003
Samanta HS, Ray SK (2014) Synthesis, characterization, swelling and drug release behavior of semi-interpenetrating network hydrogels of sodium alginate and polyacrylamide. Carbohydr Polym 99:666–678
Sarei F, Dounighi NM, Zolfagharian H, Khaki P, Bidhendi SM (2013) Alginate nanoparticles as a promising adjuvant and vaccine delivery system. Ind J Pharm Sci 75:442–449
Sarika PR, James NR, Anil Kumar PR, Raj DK (2016) Preparation, characterization and biological evaluation of curcumin loaded alginate aldehyde–gelatin nanogels. Mater Sci Eng C 68:251–257
Schaumann K, Weide G (1990) Enzymatic degradation of alginate by marine fungi. Hydrobiologia 204/205:589–596
Schneider A, Francius G, Obeid R, Schwinte P, Hemmerle J, Frisch B, Schaaf P, Voegel JC, Senger B, Picart C (2006) Polyelectrolyte multilayers with a tunable young’s modulus: influence of film stiffness on cell adhesion. Langmuir 22:1193–1200
Seeli DS, Dhivya S, Selvamurugan S, Prabaharan M (2016) Guar gum succinate-sodium alginate beads as a pH-sensitive carrier for colon-specific drug delivery. Int J Biol Macromol 91:45–50
Shahzad A, Khan A, Afzal Z, Umer MF, Khan J, Khan GM (2018) Formulation development and characterization of cefazolin nanoparticles-loaded cross-linked films of sodium alginate and pectin as wound dressings. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2018.11.090
Shahzad A, Khan A, Afzal Z, Umer MF, Khan J, Khan GM (2019) Formulation development and characterization of cefazolin nanoparticles-loaded cross-linked films of sodium alginate and pectin as wound dressings. Int J Biol Macromol 124:255–269
Shankar S, Rhim J-W (2018) Antimicrobial wrapping paper coated with a ternary blend of carbohydrates (alginate, carboxymethyl cellulose, carrageenan) and grapefruit seed extract. Carbohydr Polym 196:92–101
Shao X, Hunter CJ (2007) Developing an alginate/chitosan hybrid fiber scaffold for annulus fibrosus cells. J Biomed Mater Res 82A:701–710
Sharma C, Dinda AK, Potdar PD, Chou C-F, Mishra NC (2016) Fabrication and characterization of novel nano-biocomposite scaffold of chitosan–gelatin–alginate–hydroxyapatite for bone tissue engineering. Mater Sci Eng C 64:416–427
Shi J, Alves NM, Mano JF (2006) Drug release of pH/temperature-responsive calcium alginate/poly(N-isopropylacrylamide) semi-IPN beads. Macromol Biosci 6:358–363
Shi J, Alves NM, Mano JF (2008) Chitosan coated alginate beads containing poly(N-isopropylacrylamide) for dual-stimuli-responsive drug release. J Biomed Mater Res 84B:595–603
Shi X, Zheng Y, Wang G, Lin Q, Fan J (2014) pH- and electro-responsive characteristics of bacterial cellulose nanofiber/ sodium alginate hybrid hydrogels for the dual controlled drug delivery. RSC Adv 4:47056–47065
Shtenberg Y, Goldfeder M, Schroeder A, Bianco-Peled H (2017) Alginate modified with maleimide-terminated PEG as drug carriers with enhanced mucoadhesion. Carbohydr Polym 175:337–346
Shtenberg Y, Goldfeder M, Prinz H, Shainsky J, Ghantous Y, El-Naaj IA, Schroeder A, Bianco-Peled H (2018) Mucoadhesive alginate pastes with embedded liposomes for local oral drug delivery. Int J Biol Macromol 111:62–69
Singh B, Varshney L, Francis S, Rajneesh (2017) Designing sterile biocompatible moxifloxacin loaded trgacanth-PVA-alginate wound dressing by radiation crosslinking method. Wound Med 17:11–17
Smidsrod O, Skjak-Braek G (1990) Alginate as immobilization matrix for cells. Trends Biotechnol 8:71–78
Sondermeijer HP, Witkowski P, Seki T, van der Laarse A, Itescu S, Hardy MA (2018) RGDfK-peptide modified alginate scaffold for cell transplantation and cardiac neovascularization. Tissue Eng Part A 24:740–751
Su X, Chen B (2018) Tough, resilient and pH-sensitive interpenetrating polyacrylamide/alginate/montmorillonite nanocomposite hydrogels. Carbohydr Polym 197:497–507
Summa M, Russo D, Penna I, Margaroli N, Bayer IS, Bandiera T, Athanassiou A, Bertorelli R (2018) A biocompatible sodium alginate/povidone iodine film enhances wound healing. Eur J Pharm Biopharm 122:17–24
Sun J, Tan H (2013) Alginate-based biomaterials for regenerative medicine applications. Materials 6:1285–1309
Sun D, Liu Y, Wang H, Deng F, Zhang Y, Zhao S, Ma X, Wu H, Sun G (2018) Novel decellularized liver matrix-alginate hybrid gel beads for the 3D culture of hepatocellular carcinoma cells. Int J Biol Macromol 109:1154–1163
Sutherland IW (1991) Alginates. In: Byrom D (ed) Biomaterials: novel materials from biological sources. Stockton Press, New York, pp 309–331
Swamy BY, Yun Y-S (2015) In vitro release of metformin from iron (III) cross-linked alginate–carboxymethyl cellulose hydrogel beads. Int J Biol Macromol 77:114–119
Szekalska M, Pucilowska A, Szymanska E, Ciosek P, Winnicka K (2016) Alginate: current use and future perspectives in pharmaceutical and biomedical applications. Int J Polym Sci 2016:1–17
Tam SK, Dusseault J, Bilodeau S, Langlois G, Halle J-P, Yahia L (2011) Factors influencing alginate gel biocompatibility. J Biomed Mater Res Part A 98A:40–52
Tansaz S, Durmann A-K, Detsch R, Boccaccini AR (2017) Hydrogel films and microcapsules based on soy protein isolate combined with alginate. J Appl Polym Sci 134:44358
Tareq AZ (2016) Preparation and characterization of PVA-NaAlg interpenetrating polymer network (IPN) hydrogel for controlled delivery of carbidopa. Am Chem Sci J 17:1–10
Tarusha L, Paoletti S, Travan A, Marsich E (2018) Alginate membranes loaded with hyaluronic acid and silver nanoparticles to foster tissue healing and to control bacterial contamination of non-healing wounds. J Mater Sci Mater Med 29:22
Thakur S, Sharma B, Verma A, Chaudhary J, Tamulevicius S, Thakur VK (2018) Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J Clean Prod 198:143–159
Thankam FG, Muthu J, Sankar V, Gopal RK (2013) Growth and survival of cells in biosynthetic poly vinyl alcohol–alginate IPN hydrogels for cardiac applications. Colloid Surf B: Biointerface 107:137–145
Thomas D, Latha MS, Thomas KK (2018) Synthesis and in vitro evaluation of alginate-cellulose nanocrystal hybrid nanoparticles for the controlled oral delivery of rifampicin. J Drug Deliv Sci Technol 46:392–399
Tigli RS, Gumusderelioglu M (2009) Evaluation of alginate-chitosan semi IPNs as cartilage scaffolds. J Mater Sci Mater Med 20:699–709
Tohamy KM, Mabrouk M, Soliman I, Beherei HH, Aboelnasr MA (2018) Novel alginate/hydroxyethyl cellulose/hydroxyapatite composite scaffold for bone regeneration: In vitro cell viability and proliferation of human mesenchymal stem cells. Int J Biol Macromol 112:448–460
Tong X-F, Zhao F-Q, Ren Y-Z, Zhang Y, Cui Y-L, Wang Q-S (2018) Injectable hydrogels based on glycyrrhizin, alginate, and calcium for three-dimensional cell culture in liver tissue engineering. J Biomed Mater Res A 106:3292–3302
Tonnesen HH, Karlsen J (2002) Alginate in drug delivery systems. Drug Develop Ind Pharm 28:621–630
Treenate P, Monvisade P (2017) In vitro drug release profiles of pH-sensitive hydroxyethylacryl chitosan/sodium alginate hydrogels using paracetamol as a soluble model drug. Int J Biol Macromol 99:71–78
Upadhyay M, Adena SKR, Vardhan H, Yadav SK, Mishra B (2018) Development of biopolymers based interpenetrating polymeric network of capecitabine: a drug delivery vehicle to extend the release of the model drug. Int J Biol Macromol 115:907–919
Vega-Sagardia M, Rocha J, Saez K, Smith CT, Gutierrez-Zamorano C, Garcia-Cancino A (2018) Encapsulation, with and without oil, of biofilm forming Lactobacillus fermentum UCO-979C strain in alginate-xanthan gum and its anti-Helicobacter pylori effect. J Funct Food 46:504–513
Venkatesan J, Bhatnagar I, Manivasagan P, Kang KH, Kim SK (2015) Alginate composites for bone tissue engineering: a review. Int J Biol Macromol 72:269–281
Vignesh S, Gopalakrishnan A, Poorna MR, Nair SV, Jayakumar R, Mony U (2018) Fabrication of micropatterned alginate-gelatin and k-carrageenan hydrogels of defined shapes using simple wax mould method as a platform for stem cell/induced Pluripotent Stem Cells (iPSC) culture. Int J Biol Macromol 112:737–744
Volic M et al (2018) Alginate/soy protein system for essential oil encapsulation with intestinal delivery. Carbohydr Polym 200:15–24
Wang Q, Newby BZ (2018) Layer-by-layer polyelectrolyte coating of alginate microgels for sustained release of sodium benzoate and zosteric acid. J Drug Deliv Sci Technol 46:46–54
Wang Q, Zhang N, Hu X, Yang J, Du Y (2007) Alginate/polyethylene glycol blend fibers and their properties for controlled drug release. J Biomed Res 82A:122–128
Wang Q, Zhang J, Wang A (2009) Preparation and characterization of a novel pH-sensitive chitosan-g-poly (acrylic acid)/attapulgite/sodium alginate composite hydrogel bead for controlled release of diclofenac sodium. Carbohydr Polym 78:731–737
Wong TY, Preston LA, Schiller NL (2000) Alginate Lyase: review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu Rev Microbiol 54:289–340
Wu H, Liu J, Fang Q, Xiao B, Wan Y (2017) Establishment of nerve growth factor gradients on aligned chitosan-polylactide/alginate fibers for neural tissue engineering applications. Colloid Surf B Biointerface 160:598–609
Xie H, Chen X, Shen X, He Y, Chen W, Luo Q, Ge W, Yuan W, Tang X, Hou D, Jiang D, Wang Q, Liu Y, Liu Q, Li K (2018) Preparation of chitosan-collagen-alginate composite dressing and its promoting effects on wound healing. Int J Biol Macromol 107:93–104
Yan S, Wang T, Feng L, Zhu J, Zhang K, Chen X, Cui L, Yin J (2014) Injectable in situ self-cross-linking hydrogels based on poly(l-glutamic acid) and alginate for cartilage tissue engineering. Biomacromolecules 15:4495–4508
Yang D, Wu W, Wang S (2018a) Biocompatibility and degradability of alginate-poly-L-arginine microcapsules prepared by high-voltage electrostatic process. Int J Polym Mater Polym Biomater 67:1087–1095
Yang X, Lu Z, Wu H, Li W, Zheng L, Zhao J (2018b) Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater Sci Eng C 83:195–201
Yigitoglu M, Aydin G, Isiklan N (2014) Microwave-assisted synthesis of alginate-g-polyvinylpyrrolidone copolymer and its application in controlled drug release. Polym Bull 71:385–414
Yin Z-C, Wang Y-L, Wang K (2018) A pH-responsive composite hydrogel beads based on agar and alginate for oral drug delivery. J Drug Deliv Sci Technol 43:12–18
Yu C-Y, Zhang X-C, Zhou F-Z, Zhang X-Z, Cheng S-X, Zhuo R-X (2008) Sustained release of antineoplastic drugs from chitosan-reinforced alginate microparticle drug delivery systems. Int J Pharm 357:15–21
Yu C-Y, Yin B-C, Zhang W, Cheng S-X, Zhang X-Z, Zhuo R-X (2009) Composite microparticle drug delivery systems based on chitosan, alginate and pectin with improved pH-sensitive drug release property. Colloid Surf B: Biointerfaces 68:245–249
Zhang Z, Zhang R, Zou L, McClements DJ (2016) Protein encapsulation in alginate hydrogel beads: Effect of pH on microgel stability, protein retention and protein release. Food Hydrocolloid 58:308–315
Zhang X, Zhu Y, Cao L, Wang X, Zheng A, Chang J, Wu J, Wen J, Jiang X, Li H, Zhang Z (2018) Alginate-aker injectable composite hydrogels promoted irregular bone regeneration through stem cell recruitment and osteogenic differentiation. J Mater Chem B 6:1951–1964
Zhao S, Cao M, Li H, Li L, Xu W (2010) Synthesis and characterization of thermo-sensitive semi-IPN hydrogels based on poly(ethylene glycol)-co-poly(ɛ-caprolactone) macromer, N-isopropylacrylamide, and sodium alginate. Carbohydr Res 345:425–431
Zhao J, Zhao X, Guo B, Ma PX (2014) Multifunctional interpenetrating polymer network hydrogels based on methacrylated alginate for the delivery of small molecule drugs and sustained release of protein. Biomacromolecules 15:3246–3252
Zhao Y, Wang Y, Niu C, Zhang L, Li G, Yang Y (2018) Construction of polyacrylamide/graphene oxide/gelatin/sodium alginate composite hydrogel with bioactivity for promoting Schwann cells growth. J Biomed Mater Res A 106:1951–1964
Zhou Q, Kang H, Bielec M, Wu X, Cheng Q, Wei W, Dai H (2018) Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohydr Polym 197:292–304
Zineh BR, Shabgard MR, Roshangar L (2018) Mechanical and biological performance of printed alginate/ methylcellulose/halloysite nanotube/polyvinylidene fluoride bio-scaffolds. Mater Sci Eng C 92:779–789
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Das, S., Subuddhi, U. (2020). Alginate-Based Interpenetrating Network Carriers for Biomedical Applications. In: Jana, S., Jana, S. (eds) Interpenetrating Polymer Network: Biomedical Applications. Springer, Singapore. https://doi.org/10.1007/978-981-15-0283-5_4
Download citation
DOI: https://doi.org/10.1007/978-981-15-0283-5_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0282-8
Online ISBN: 978-981-15-0283-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)