Abstract
In this study, alginate and alginate:chitosan semi interpenetrating polymer network (IPN) scaffolds were prepared by freeze-drying process. Alginate scaffolds were crosslinked with different concentrations of CaCl2, i.e. 0.5, 1 or 3% (w/v), in 96% (v/v) ethanol solutions for two different periods, i.e. 4 and 24 h, after freeze-drying. Scanning electron microscope (SEM)/ Energy Dispersive Analysis by X-ray (EDAX) analysis and swelling studies indicated that crosslinking of scaffolds with 3% (w/v) CaCl2 for 24 h was effectively created suitable alginate scaffolds in terms of optimum porosity and mechanical stability. This is why, alginate:chitosan semi IPN scaffolds were prepared at the crosslinking condition mentioned above in 70:30, 60:40 and 50:50% (v/v) alginate:chitosan ratios. Besides the attachment and proliferation abilities of ATDC5 murine chondrogenic cells on alginate, 70:30% (v/v) alginate:chitosan and 50:50% (v/v) alginate:chitosan scaffolds, their cellular responses were assessed for chondrogenic potential. These structural and cellular outcomes demonstrate potential utility of chitosan semi IPNs in alginate scaffolds. Comparative results found in relation to alginate scaffolds, support the necessity for alginate:chitosan scaffolds for improved cartilage tissue engineering.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tissue engineering is recognized as a promising strategy for creating biological body parts as alternatives for transplanting harvested tissues and organs [1]. For cartilage treatment in tissue engineering, a scaffolding material plays a critical role in guiding chondrocyte cell attachment and proliferation for new tissue formation in 3D [2, 3]. There are some potent biopolymers that can be used as starting materials to prepare stable scaffolds for cartilage tissue engineering including poly(l-lactide)/poly(ε-caprolactone) [4], polyglycolide [5] and poly(lactide-co-glycolide) [6]. Nowadays, using naturally occurring biopolymers as scaffolds has become a new trend for cartilage tissue engineering which may be due to their biodegradability, low toxicity and low disposal costs. For example, cellulose provides the structure of higher plants, chitin is the main component of exoskeleton of several molluscs, keratin causes thermoinsulation in hair and collagen supports connective tissues mechanically [7]. However, selecting an appropriate biomaterial for the target tissue is a key factor since the ideal cell-carrier substance should be one that closely mimics the natural environment in the extracellular matrix (ECM).
Alginates, are naturally occurring polysaccharides composed of (1-4)-linked β-d-mannuronic acid (M units) and α-l-guluronic acid (G units) monomers which vary in amount and sequential distribution along the polymer chain depending on the source of the alginate, have been extensively used as synthetic ECMs [8, 9]. Sodium alginate forms relatively stable hydrogels through ionotropic gelation. Divalent cations like Ca2+ cooperatively bind between the G blocks of adjacent alginate chains, creating ionic interchain bridges which cause gelling of aqueous alginate solutions. Not only several therapeutic agents, including antibiotics, enzymes, growth factors and DNA, have already been successfully incorporated in alginate gels [10], but also alginate hydrogels have been widely studied for bone and cartilage regeneration applications as scaffolds [11, 12]. Chitosan is a partially deacetylated derivative of chitin which is biodegradable, biocompatible, nonantigenic, nontoxic, and biofunctional [13]. Moreover, it represents some articular cartilage components like glycosaminoglycans (GAGs) and hyaluronic acid [14] and has been used as a scaffold for cartilage repair [13–15]. Since chitosan is regarded as a cationic polysaccharide, shows excellent cell adhesive properties, it is reported that the alginate-chitosan hybrid polymers have potential as a scaffold material for cartilage and bone tissue engineering [16, 17]. The alginate-based chitosan hybrid polymer fibers improved adhesion capacity of fibroblasts compared with alginate polymer fiber in ligament and tendon tissue engineering [18]. Moreover, alginate blends with chitosan were studied as an improved alternative to chitosan for cartilage repair and regeneration [19]. However, the results obtained were inconsistent since anchorage dependent mammalian cells were unable to interact with alginate polysaccharides or their hydrogels due to minimal protein adsorption arising from their highly hydrophilic nature [20, 21]. It has been previously reported that alginate microspheres were able to discourage cell adhesion which is improved by incorporation of calcium phosphate [22]. Rowley et al. demonstrated that alginate surfaces could not support cell adhesion and RGD modified alginate hydrogels were reported as another approach to promote cell adhesion [23].
In the present study, we aimed to investigate potential effects of chitosan semi-interpenetrating networks (IPNs) on alginate scaffolds and to determine the biological aspects of these novel scaffolds which are beneficial for cartilage tissue engineering. Previous studies investigated alginate-chitosan blends in terms of a compact alginate:chitosan hybrid scaffold relating to cartilage repair [16, 18, 19]. It has been previously reported that alginate-chitosan hydrogel blends forming semi-IPNs were used for several drug delivery applications [24, 25]. However, alginate scaffolds containing chitosan semi-IPNs have not been investigated in terms of cartilage repair. In this paper, we generated alginate scaffolds containing different amounts of chitosan semi-IPNs for cartilage tissue engineering. Our first goal is to determine the feasibility of alginate scaffolds containing chitosan semi-IPNs over alginate scaffolds, then to investigate the compositional amount of chitosan in constructs regarding to material and chondrocytic cell interactions. We first outlined the preparation and structural characteristics of alginate and alginate:chitosan semi-IPNs. Then ATDC5 chondrogenic cell line was used to demonstrate cell responses related to chondrogenesis, evaluated by scanning electron microscopy (SEM) and GAG and DNA quantities.
2 Materials and methods
2.1 Materials
Chitosan derived from crab shell with a deacetylation degree (DD) of minimum 85% was purchased from Aldrich (Cat. No: 417963, Germany). Sodium alginate was obtained from Fluka AG (Cat. No: 71240, Germany). Fluorescein 5(6)-isothiocyanate (FITC) were obtained from Sigma (Germany). Acetic acid (HPLC grade), ethanol (96% v/v) and acetone were from Merck (Germany). For cell culture studies papain from papaya latex, cysteine–HCl, hexamethyldisilazane and glutaraldehyde were purchased from Sigma Corporation (Germany). ITS premix (insulin, transferrin and selenium), 1,9-dimethyl-methylene blue and Hoechst 33258 were purchased from Sigma–Aldrich. All solvents used in this study are analytical grade.
2.2 Preparation of alginate scaffolds
Porous alginate scaffold samples were prepared by freeze-drying method which was described in our previous publication with regard to preparation of chitosan scaffolds [26]. In brief, sodium alginate solutions with concentration of 2% (w/v) were prepared by dissolving sodium alginate in ultra-pure water and were filtered using a 0.45 μm filter (Millipore) in order to eliminate the impurities. These solutions were poured into 24-well tissue-culture polystyrene dishes (TCPS, TPP Switzerland), having a diameter of 15 mm, to a depth of approximately 5 mm and frozen at −20°C for 24 h. Then they were transferred into a freeze-drier (Christ, Germany) and lyophilized at −80°C for 4 days to ensure that they were completely dried. Freshly lyophilized alginate scaffolds were crosslinked by different concentrations of CaCl2 (0.5, 1 or 3% (w/v)) in 96% (v/v) ethanol solutions for 4 or 24 h periods. Crosslinked alginate scaffolds were washed with distilled water and freeze-dried for 1 day.
2.3 Preparation of alginate scaffolds containing chitosan semi-IPNs
Chitosan was dissolved in 0.2 M acetic acid to prepare the solutions of 2% (w/v) concentration, then these solutions were filtered using a 0.22 μm filter to eliminate the impurities. Chitosan solutions mixed with previously prepared 2% (w/v) alginate solutions in proportions of (alginate:chitosan) (50:50)% (v/v), (60:40)% (v/v) and (70:30)% (v/v). The mixtures were poured into 24-well TCPS dishes and frozen at −20°C for 24 h. Then they were transferred into a freeze-drier and lyophilized at −80°C for 4 days. Freshly lyophilized scaffolds were first immersed in 96% (v/v) ethanol for overnight in order to stabilize chitosan in alginate:chitosan scaffolds. Then porous scaffolds were incubated in CaCl2 (3% w/v in ethanol) solution for crosslinking of alginate for 24 h. Crosslinked alginate-chitosan scaffolds were washed with distilled water and freeze-dried for 1 day.
2.4 Microstructural characterization of alginate scaffolds
2.4.1 SEM and EDAX analysis
Surface and cross-section morphologies of lyophilized alginate and alginate:chitosan semi IPN scaffolds were examined by using a JEOL (JSM-840A, Japan) SEM after coating with a gold-palladium layer. The elements present in alginate scaffolds were shown by EDAX (Oxford, UK) in order to indicate Na/Ca content in structure.
2.4.2 Swelling studies
Stabilized alginate and alginate:chitosan scaffolds were fully rehydrated in Dulbecco’s phosphate buffered saline (DPBS; pH 7.4) at 37°C in order to investigate the swelling characteristics. Samples were taken out of the buffer solution and the excess of buffer was removed by blotting with filter paper. Swelling ratios were determined gravimetrically in dry basis by using the following equation:
where W 0 is dry weight and W is wet weight of alginate scaffold. All the swelling experiments were repeated at least three times and results were reported as average values.
2.4.3 Porosity
The porosity of the alginate:chitosan scaffolds was measured by liquid displacement [27]. Hexane was used as the displacement liquid as it permeates through scaffolds without swelling or shrinking the matrix. Scaffolds (dry weight, W) were immersed in a known volume (V1) of hexane in a graduated cylinder for 5 min. The total volume of hexane and the hexane-impregnated scaffold was recorded as V2. The hexane-impregnated scaffold was then removed from the cylinder and the residual hexane volume was recorded as V3. The total volume of the scaffold was
V2 − V1 is the volume of the polymer scaffold and V1 − V3 is the volume of hexane within the scaffold. The porosity of the scaffold (ε) was obtained by
2.4.4 Visualization of chitosan semi IPNs in alginate:chitosan scaffolds
To visualize chitosan in scaffolds, a fluorescent dye, fluorescein 5(6)-isothiocyanate (FITC, Sigma, Germany), was used to label primary amine groups on chitosan structure by dialysis tubing cellulose membrane technique [28]. Briefly, for each ml of chitosan solution 50 μl FITC solution (1 mg/ml) was added and incubated in dark for 8 h at 4°C. Finally, prepared solution was dialysed by cellulose membrane (Sigma, Germany) for two days. Then FITC labeled chitosan was used for preparation of alginate:chitosan scaffold as described above. Visualization of prepared scaffolds was performed by fluorescence microscope (Olympus, USA).
2.5 Cell culture studies
Cell culture studies were carried out with ATDC5 cell line, a murine chondrogenic cell line, which was purchased from Riken Cell Bank (Japan). The cells were subcultured in flasks using Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F12 (Sigma Co., Germany) supplemented with 5% (v/v) fetal bovine serum (FBS, Sigma Co.) and 1% penicillin–streptomycin (Biological Ind., Israel). The cells, maintained at 37°C in a humidified CO2 (5%) atmosphere (Heraus Instruments, Germany), were dissociated with 0.25% trypsin-EDTA (Sigma, Germany), centrifuged and resuspended in medium prior to cell seeding. Cells at passage number 3 were used in this study.
2.5.1 Cell culture and seeding
Cell cultures were conducted in sterile 24-well TCPS dishes in stationary conditions. Prior to cell culture experiments, 24-well TCPS dishes were precoated with parafilm and were soaked in 96% ethanol and placed under UV light for 30 min for sterilization. Alginate and alginate:chitosan semi IPN scaffolds (50:50)% (v/v), 70/30% (v/v) (alginate:chitosan), having 1.4 mm diameter and 2 mm thickness (average dry weight is 0.01 g), were sterilized with 70% ethanol for 1 h and equilibrated in sterile Dulbecco’s PBS (pH 7.4, 24 h). Then scaffolds were immersed in conditioning medium for 1.5 h prior to cell seeding. Finally, 1 ml of culture medium supplemented with 1× ITS premix (insulin, 10 μg/ml; transferrin, 5.5 μg/ml; and selenium, 5 ng/ml) was added in order to maintain 4 × 104 cells/ml inoculation density for each scaffold. The medium was replenished every two days.
2.5.2 Cell attachment and viability
Cell attachment and viability of cells in scaffolds were quantitatively assessed by mitochondrial activity assay with 3-[4,5-dimethylthiazol-2-yl]-diphenyltetrazolium bromide (MTT) formazan. In brief, culture medium was aspirated and washed with 600 μl prewarmed PBS (pH 7.4). 600 μl prewarmed culture medium supplemented with 60 μl MTT solution (2.5 mg/ml MTT dissolved in PBS) was added to each sample which were incubated at 37°C for 3 h. After incubation period, the medium was removed from each well and scaffolds were transferred to another 24-well Petri dish. 400 μl of 0.04 M HCl in isopropanol solution was added to each well to dissolve formazan crystals. The resulting solution of crystal violet color was removed and centrifuged at 13,000 rpm for 2 min. 200 μl supernatant was used for measuring optical density spectrophotometrically at 570 nm with reference to 690 nm using a microplate reader (Asys UVM 340, Austria). MTT assay was also applied to the scaffolds without cells as control and the data was subtracted from measured values. In terms of cell attachment, the growth index was determined from MTT assay results at the end of first day since only attached cells remain viable in scaffolds. Viability of cells was checked by MTT assay results after 1 week of incubation period.
2.5.3 Biochemical analysis
Cell-scaffold constructs (n = 3 per group) were frozen, lyophilized, and papain digested [29], using 1 ml enzyme solution per 4–40 mg dry weight of the sample. The number of cells per construct was assessed from the DNA content using a spectrofluorometer (Cary Eclipse, Australia) and conversion factor of 7.7 pg DNA per chondrocyte [30]. GAG contents were measured spectrophotometrically using dimethylmethylene blue [31] and bovine chondroitin sulfate as a standard. GAG assay was also applied to the scaffolds without cells as control and the data was subtracted from measured values.
2.5.4 Microscopic imaging of cells within scaffolds
The morphology of the cells in the scaffolds was observed by a SEM (JSM-840A, Japan) at the end of 1 and 4 weeks of incubation. The scaffolds were gently washed with PBS and cells were fixed with 2.5% (v/v) glutaraldehyde in 0.1 M PBS (pH 7.4) for 1 h at 4°C. Then the scaffolds were dehydrated in ethanol series (30, 50, 70, 90 and 100% (v/v)) and rinsed with hexamethyldisilazane.
2.6 Statistical analysis
All data are expressed as means ± standard deviations of a representative of three similar experiments carried out in triplicate. Statistical analysis was performed by one-way analysis of variance (ANOVA) in conjunction with Tukey’s post hoc test for multiple comparisons using SPSS version 9.0 software.
3 Results and discussion
3.1 Fabrication of alginate scaffolds
Porous alginate scaffolds were prepared by freeze-drying method. In this method, alginate solutions were frozen to maintain ice crystals and freezing alginate solutions were lyophilized in order to maintain porous and interconnected structure by removal of ice crystals from the frozen solutions. Alginate scaffolds were crosslinked with different concentrations of CaCl2, i.e. 0.5, 1 or 3% (w/v) in 96% (v/v) ethanol solutions for two different periods, i.e. 4 and 24 h. The sample became insoluble due to the physical crosslinking through the Ca2+ bridges. Since crosslinking is performed by exchange of sodium and calcium ions [32], Na/Ca ratios of fabricated alginate scaffolds, indicating success of crosslinking, are calculated by determining Na and Ca amounts (wt.%) by EDAX analysis. Results showed that with increasing crosslinking concentration and time period, Na/Ca ratio is decreased as expected (Table 1).
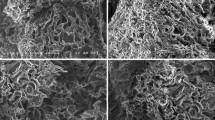
The morphological structure of crosslinked alginate scaffolds are shown in Fig. 1a–c. Cross-section SEM images of alginate scaffolds with three different crosslinking concentrations and 4 h periods showed not only a complete porous morphology but also an interconnected structure was observed for all types of scaffolds. However, it was observed that alginate scaffolds with high crosslinking concentration showed a more uniform structure and pores are seemed to be close to each other (Fig. 1c). Moreover, it was observed that not only changing crosslinking concentration from 0.5 to 3.0% (w/v) but also changing crosslinking time period from 4 to 24 h affected the morphological structure of scaffolds. It was observed that alginate scaffolds fabricated with 24 h crosslinking time periods showed the same morphological changes of alginate scaffolds for high crosslinking concentrations. According to the SEM images, the pore sizes of alginate scaffolds prepared with 0.5 or 1% (w/v) crosslinking density for 4 h crosslinking period were found to be in the range of 150–200 μm (Fig. 1a), while the pore sizes of alginate scaffold prepared with 3% crosslinking density for 4 h crosslinking period was found to be ~100 μm (Fig. 1c). On the other hand, the pore sizes of alginate scaffolds prepared with 0.5 or 1% (w/v) crosslinking density for 24 h crosslinking period were found to be in the range of 100–150 μm, while the pore sizes of alginate scaffold prepared with 3% crosslinking density for 24 h crosslinking period was found to be in the range of 80–100 μm. It must be noted that all alginate scaffolds were found to be having favorable pore sizes for cell proliferation and function [33].
3.2 Swelling and porosity of alginate scaffolds
Swelling studies were performed by rehydrating samples in PBS (pH 7.4) at 37°C and equilibrium swelling ratios of fabricated alginate scaffolds were calculated. The results shown in Table 2, indicating that there is little variation between equilibrium swelling ratios of alginate scaffolds crosslinked with 0.5 or 1% (w/v) CaCl2 concentration. Their swelling ratios were about 50. However, equilibrium swelling ratios of alginate scaffolds crosslinked with 3% (w/v) CaCl2 concentration were calculated as in the range of ~25. Porosity of alginate scaffolds were calculated from Eq. 3 and results were shown in Table 2. As evident from Table 2, porosities of alginate scaffolds with different crosslinking densities and crosslinking time periods was found in the range of 70% with very little variation. Since porosities did not vary significantly, results obtained from swelling ratios suggested structural stability and integrity of alginate scaffolds crosslinked with 3% (w/v) CaCl2. The affects of high crosslinking ratio and period on alginate were studied by several investigators and concluded as enhancement of structural stability and integrity [34, 35], which is consistent with our results. Thus, both SEM/EDAX analysis and swelling studies pointed out that the most effective fabrication condition of alginate scaffolds are 3% (w/v) CaCl2 crosslinking concentration for 24 h time period.
3.3 Alginate scaffolds containing chitosan semi-IPNs
Cross-section SEM images of alginate scaffolds containing different proportions of chitosan ((alginate:chitosan), (50:50)% (v/v), (60:40)% (v/v) and (70:30)% (v/v)) are shown in Fig. 2. Results demonstrated that chitosan was successfully incorporated into alginate scaffolds. Moreover, incorporation of chitosan into alginate scaffold was seemed to be creating bigger pores in scaffold (~200 μm) and pores were highly interconnected. As it is seen from Fig. 2, porous chitosan structure was collapsed in alginate. When a mixture of two or more cross-linked networks that are dispersed or mixed at a molecular segmental level, the system is termed as a semi-IPN if only one polymer of IPN is cross-linked leaving the other in linear form [36]. The existence of semi-IPN structure of chitosan in alginate scaffolds were visualized by fluorescence microscope (Fig. 3). As evident from Fig. 3, free chitosan chains were collapsed in alginate network and distributed in alginate scaffold homogeneously which indicates the formation of chitosan semi-IPNs clearly. Results are in good correlation with SEM images with regard to chitosan semi-IPN structure in alginate scaffolds.
Fluorescence microscope images of alginate scaffolds containing chitosan semi-IPNs showing distribution of FITC labeled chitosan throughout the structure (×10). a (70:30)% (v/v) (alginate:chitosan) scaffolds, b (60:40)% (v/v) (alginate:chitosan) scaffolds, c (50:50)% (v/v) (alginate:chitosan) scaffolds. Shining areas correspond to FITC labeled chitosan. Bar shows 200 μm
Li et al. reported alginate:chitosan scaffolds which is technically created by the same method of our work, freeze-drying [19]. However, they maintained a compact alginate:chitosan hybrid scaffold which is due to the exact differences of preparation methods of scaffolds compared to this study. Basically, Li et al. reported that chitosan and alginate blends were heated at 70°C and resulted pH of the solution was adjusted to 7–7.4 in order to decrease solubility of chitosan and to form an ionic complex of chitosan:alginate [17]. Moreover, they demonstrated SEM figures of maintained alginate:chitosan scaffolds, seemed a compact alginate:chitosan scaffold, which is definitely different compared to our alginate scaffolds containing semi-IPNs [19].
The results from swelling studies showed that alginate scaffold having the lowest amount of chitosan (30% (v/v)), was found to be having highest equilibrium swelling ratio. However, it was recorded that there is not a significant difference between equilibrium swelling ratios of (60:40)% (v/v) and (50:50)% (v/v) alginate:chitosan scaffolds (Table 2). Recorded porosities for alginate:chitosan scaffolds were in the range of 84.7–87.4% without significant differences. Thus, cell culture studies were conducted with alginate, (70:30)% (v/v) and (50:50)% (v/v) alginate:chitosan scaffolds. It should be noted that cell culture studies were not conducted with chitosan percentage higher than 50% or pure chitosan due to the ability of evaluating alginate scaffolds with chitosan improvements.
3.4 Cell culture studies
3.4.1 Cell attachment, viability and proliferation
Chondrogenic cell attachment was assessed with MTT assay by calculating growth index values at the end of 1 day since only attached cells remain viable in scaffolds (Table 3). As seen from Table 3, growth index values were increased with increasing chitosan percentage in alginate scaffolds which may be due to the chitosan’s cationic structure. Moreover, statistically significant high growth index values were obtained from (50:50)% (v/v) alginate:chitosan scaffolds (P < 0.05) indicating the role of chitosan for cellular attachment.
Cell viability is checked by MTT assay results after 1 week of incubation period. Results indicated that (50:50)% (v/v) alginate:chitosan scaffolds has statistically significant high metabolic activity (P < 0.001) which leads to comparatively increasing proliferation. In contrary to the some previous studies of other researchers [37, 38] we suggested that alginate suppressed cell-material interactions in terms of cellular proliferation. Our results are consistent with some studies previously demonstrated by other authors [22, 23]. Barrias et al. have investigated the adhesion of osteoblastic-like cells to calcium phosphate-alginate microspheres of different compositions [22]. They observed that cells could not adhere to the surface of control alginate scaffolds properly and ceramic-to-polymer ratio strongly influenced the ability of cellular adhesion and spreading on the surface of microspheres. Majima et al. showed that alginate-based chitosan hybrid polymer fibers could provide superior support for rabbit tendon fibroblast adhesion compared to that provided by alginate polymer fiber [18]. Seo et al. investigated the hepatocyte adhesion onto alginate and alginate/chitosan films [39] and they obtained higher cell adhesion on alginate/chitosan films than that of alginate films.
3.4.2 Microscopic images of cells within scaffolds
Cell morphologies on alginate, 70:30% (v/v) alginate:chitosan and 50:50% (v/v) alginate:chitosan semi IPN scaffolds for the first and fourth weeks of culture are given in Fig. 4. SEM images demonstrated that the existence of chitosan and the amount of chitosan incorporated in alginate scaffolds strongly affected initial cell proliferation and cell morphology (Figs. 4a, c, e). At day 7, most of the chondrocytes on both alginate:chitosan semi IPN scaffolds (50:50 and 70:30) attached to the matrix and migrated through the matrix. It is concluded that increasing the amount of chitosan in alginate scaffolds resulted in enhancement of chondrogenic cell attachment. This accelerated cell attachment on alginate:chitosan scaffolds, resulted comparable increase of cell proliferation between alginate and alginate:chitosan scaffolds, attributed to chitosan semi-IPNs since the affinity between cells and materials should be improved with incorporation of chitosan. After 4 weeks of culture period cell morphologies on alginate and alginate:chitosan scaffolds were observed and compared with alginate scaffolds (Fig. 4b, d, f). At day 28, most of the cells exhibited spherical morphology and formed clusters since maintaining the spherical shape of the cells by reduced cell-matrix interaction is another important condition for cartilage regeneration [40]. As seen in Fig. 4, cells could survive and created their ECMs mainly on 50:50% (v/v) alginate:chitosan scaffolds.
SEM images of ATDC5 cells cultured on alginate and alginate:chitosan scaffolds. (50:50)% (v/v) (alginate:chitosan) scaffolds a incubated for 7 days (×5.01 K), b incubated for 28 days (×5.48 K); (70:30)% (v/v) (alginate:chitosan) scaffolds c incubated for 7 days (×2.58 K), d incubated for 28 days (×5.04 K); alginate scaffolds e incubated for 7 days (×1.74 K), f incubated for 28 days (×2.18 K)
3.4.3 Chondrogenic activities of alginate and alginate:chitosan scaffolds
In this study, ATDC5 chondrogenic cells were cultivated in a chondrogenic media (DMEM/Ham’s F12 supplemented with 1× ITS premix), the composition of which has been previously described [41, 42]. Since chondrogenic differentiation of ATDC5 cells in chondrogenic media has previously been proven [41, 42], these cell lines were considered suitable to determine the alginate-chitosan scaffold’s potential for cartilage repair. Indeed, ATDC5 cell line is a well-defined chondrogenic cell line which has previously been reported as a suitable cell source for cell-material interaction studies in the field of cartilage tissue engineering [43]. Moreover, the authors considered that the use of this cell line could bring advantages over primary cells for in vitro studies with regard to concept of this study. In particular, cell lines can serve to provide important information concerning seeding densities, time frames, biocompatibility, nutritional status, and judicious use of growth factors and differentiating agents, thereby significantly reducing the time and cost associated with culture of primary human cells [43]. Therefore, easily obtainable ATDC5 chondrogenic cell line was found to be preferable over articular chondrocytes which are susceptible to de-differentiation in ex vivo cultures [44].
GAG contents of chitosan scaffolds were measured by dimethylmethylene blue assay in order to evaluate initial tissue-material interactions in alginate and alginate:chitosan scaffolds. Table 4 demonstrates GAG contents of in vitro samples grown for 14, 21 and 28 days. As seen from this table after 4 weeks of incubation period, 50:50% (v/v) alginate:chitosan scaffolds significantly generated GAG contents under control group of alginate scaffolds (P < 0.001). Furthermore, GAG contents of alginate, 70:30% (v/v) alginate:chitosan and 50:50% (v/v) alginate:chitosan scaffolds was quantified as 1.64 mg GAG/g dry scaffold, 1.88 mg GAG/g dry scaffold and 3.63 mg GAG/g dry scaffold, respectively. Results clearly indicated that ECM formation was accelerated by incorporation of high amount of chitosan to alginate scaffolds. Cell densities of alginate scaffolds were quantified by their DNA contents. As seen from Table 4, 50:50% (v/v) alginate:chitosan scaffolds were found to be having significantly higher DNA contents after 28 days of culture period under control group of alginate scaffolds (P < 0.001). ATDC5 chondrocytes cultured on alginate, 70:30% (v/v) alginate:chitosan and 50:50% (v/v) alginate:chitosan scaffolds for 4 weeks in vitro reached a number of 1.04 × 107, 1.49 × 107 and 1.69 × 107 cells per scaffold, respectively (Table 4). So, it is concluded that amount of chitosan incorporated in alginate strongly affected both proliferation of ATDC5 cells and initial material-tissue interactions. It has been reported [45] that chitosan can promote attachment of cells since chitosan is a cationic polymer carrying primary amine groups at neutral pH. Cells attach to scaffolds by nonspecific electrostatic interactions occurring directly between protonated amine groups and surface of cell membrane [26]. Initial cell attachment occurring in the first step of cell/material interactions suggesting that the increased cell–polymer interactions play a crucial role in stimulating cell proliferation, as reported earlier by many research groups [46–48]. Since 50:50% (v/v) alginate:chitosan scaffolds were found to be having highest DNA and GAG contents, selection of alginate scaffolds containing high amounts of chitosan semi-IPNs seems to be a logical for the improvement of alginate scaffolds by means of chondrogenic activity for cartilage regeneration.
4 Conclusion
Here we studied the suitability of a novel type of scaffold prepared by the combination of alginate and chitosan in different ratios. Unlike other alginate-chitosan hybrid materials, this novel scaffold was prepared by introducing chitosan into the alginate matrix in semi IPN form. The preliminary results of this study indicated that alginate:chitosan semi IPN scaffolds can promote chondrocyte proliferation and retain cell functionality and phenotype significantly compared to alginate scaffolds. Both structural and cellular analysis concluded that 50:50% (v/v) alginate:chitosan scaffolds was promising regarding in vitro cartilage tissue engineering. Our ongoing studies are doing under development of cell proliferation and phenotypic characterization on alginate:chitosan semi IPN scaffolds with selected growth factors.
References
R. Langer, J. Vacanti, Science 260, 920 (1993). doi:10.1126/science.8493529
B.S. Kim, D.J. Mooney, Trends Biotechnol. 16, 224 (1998). doi:10.1016/S0167-7799(98)01191-3
W.W. Minuth, M. Sittinger, S. Kloth, Cell Tissue Res. 291, 1 (1998). doi:10.1007/s004410050974
J. Zhao, X. Yuan, Y. Cui, Q. Ge, K. Yao, J. Appl. Polym. Sci. 91, 1676 (2003). doi:10.1002/app.13323
L.E. Freed, J.C. Marquis, A. Nohria, J. Emmanual, A.G. Mikos, R. Langer, J. Biomed. Mater. Res. 27, 11 (1993). doi:10.1002/jbm.820270104
M. Sittinger, D. Reitzel, M. Dauner, H. Hierlemann, C. Hammer, E. Kastenbauer, H. Planck, G.R. Burmester, J. Bujia, J. Biomed. Mater. Res. 33, 57 (1996). doi:10.1002/(SICI)1097-4636(199622)33:2<57::AID-JBM1>3.0.CO;2-K
M.A. Barbosa, P.L. Granja, C.C. Barrias, I.F. Amaral, ITBM-RBM 26, 212 (2005). doi:10.1016/j.rbmret.2005.04.006
A. Martinsen, G. Skjåk-Bræk, O. Smidsrød, Biotechnol. Bioeng. 33, 79 (1989). doi:10.1002/bit.260330111
W.R. Gombotz, S.F. Wee, Adv. Drug Deliv. Rev. 31, 267 (1998). doi:10.1016/S0169-409X(97)00124-5
O. Smidsrod, K.I. Draget, Carbohydr. Eur. 14, 6 (1996)
V. Alsberg, K. Anderson, A. Albeiruti, R.T. Franceshi, D.J. Mooney, J. Dent. Res. 80, 2025 (2001)
K. McConell, M. Jarman-Smith, K. Stewart, J.B. Chaudhuri, Food and Bioproduct Processing C2 Special Issue. Tissue Eng. 82, 126 (2004)
J.K. Suh, H.W. Matthew, Biomaterials 21, 2589 (2000). doi:10.1016/S0142-9612(00)00126-5
V.F. Sechriest, Y.J. Miano, C. Niyibizi, A. Westerhausen-Larson, H.W. Matthew, C.H. Evans, F.H. Fu, J.K. Suh, J. Biomed. Mater. Res. 49, 534 (2000). doi:10.1002/(SICI)1097-4636(20000315)49:4<534::AID-JBM12>3.0.CO;2-#
A. Lahiji, A. Sohrabi, D.S. Hungerford, C.G. Frondoza, J. Biomed. Mater. Res. 51, 586 (2000). doi:10.1002/1097-4636(20000915)51:4<586::AID-JBM6>3.0.CO;2-S
N. Iwasaki, S.T. Yamane, T. Majima, Y. Kasahara, A. Minami, K. Harada, S. Nonaka, N. Maekawa, H. Tamura, S. Tokura, M. Shiono, K. Monde, S.I. Nishimura, Biomacromolecules 5, 828 (2004). doi:10.1021/bm0400067
Z. Li, H.R. Ramay, K.D. Hauch, D. Xiao, M. Zhang, Biomaterials 26, 3919 (2005). doi:10.1016/j.biomaterials.2004.09.062
T. Majima, T. Funakosi, N. Iwasaki, S.T. Yamane, K. Harada, S. Monaka, A. Minami, S.I. Nishimuna, J. Orthop. Sci. 10, 302 (2005). doi:10.1007/s00776-005-0891-y
Z. Li, M. Zhang, J. Biomed. Mater. Res. Part A 75A2, 485 (2005)
K. Smentana, Biomaterials 14, 1046 (1993). doi:10.1016/0142-9612(93)90203-E
N.G. Genes, J.A. Rowley, D.J. Mooney, L.J. Bonassar, Arch. Biochem. Biophys. 422, 161 (2004). doi:10.1016/j.abb.2003.11.023
C.C. Barrias, C.C. Ribeiro, M.C. Sá Miranda, M.A. Barbosa, Key Eng. Mater. 284, 689 (2005)
J.A. Rowley, G. Madlambayan, D.J. Mooney, Biomaterials 20, 45 (1999). doi:10.1016/S0142-9612(98)00107-0
Y.H. Lin, H.F. Liang, C.K. Chung, M.C. Chen, H.W. Sung, Biomaterials 26, 2105 (2005). doi:10.1016/j.biomaterials.2004.06.011
S.C. Chena, Y.C. Wua, F.L. Mib, Y.H. Lina, L.C. Yua, H.W. Sunga, J. Control. Release 96, 285 (2004). doi:10.1016/j.jconrel.2004.02.002
R.S. Tığlı, A. Karakeçili, M. Gümüşderelioğlu, J. Mater. Sci.: Mater. Med. 18, 1665 (2007). doi:10.1007/s10856-007-3066-x
U.-J. Kim, J. Park, H.J. Kim, M. Wada, D.L. Kaplan, Biomaterials 26, 2775 (2005). doi:10.1016/j.biomaterials.2004.07.044
T.H. The, T.E.W. Feltkamp, Immunology 18(6), 865 (1970)
R.L.Y. Sah, I.J. Kim, J.Y.H. Doong, A.J. Godzinsky, A.H.K. Plaas, J.D. Sandy, J. Orthop. Res. 7, 619 (1989). doi:10.1002/jor.1100070502
Y.J. Kim, R.L. Sah, J.Y. Doong, A.J. Grodzinsky, Anal. Biochem. 174(1), 168 (1988). doi:10.1016/0003-2697(88)90532-5
R.L. Goldberg, L.M. Kolibas, Connect. Tissue Res. 24, 265 (1990). doi:10.3109/03008209009152154
G.T. Grant, E.R. Morris, D.A. Rees, P.J.C. Smith, D. Thom, FEBS Lett. 32, 195 (1973). doi:10.1016/0014-5793(73)80770-7
S. Yang, K.F. Leong, Z. Du, C.K. Chua, Tissue Eng. 7, 679 (2001). doi:10.1089/107632701753337645
C.K. Kuo, P.X. Ma, Biomaterials 22, 511 (2001). doi:10.1016/S0142-9612(00)00201-5
S. Sakai, H. Masuhara, Y. Yamada, T. Ono, H. Ijima, K. Kawakami, J. Biosci. Bioeng. 100, 127 (2005). doi:10.1263/jbb.100.127
X. Hou, K.S. Siow, Polymer (Guildf) 42, 4181 (2001). doi:10.1016/S0032-3861(00)00818-1
J.S. Lee, B. Kim, M.S. Kim, S.J. Lee, S.W. Kim, D.W. Cho, J.S. Kim, G. Lim, Key Eng. Mater. 326, 883 (2006)
C.N. Yen, Y.R. Lin, M.D.T. Chang, C.W. Tien, Y.C. Wu, C.J. Liao, Y.C. Hu, Biotechnol. Prog. 24, 452 (2008). doi:10.1021/bp0702828
S.J. Seo, I.Y. Kim, Y.J. Cho, T. Akaike, C.S. Cho, Biomaterials 27, 1487 (2006). doi:10.1016/j.biomaterials.2005.09.018
J. Bonaventure, N. Kadhom, L. Cohen-Solal, K.H. Ng, J. Bourguignon, C. Lasselin, P. Freisinger, Exp. Cell Res. 212, 97 (1994). doi:10.1006/excr.1994.1123
C. Shukunami, K. Ishizeki, T. Atsumi, Y. Ohta, F. Suzuki, Y. Hiraki, J. Bone Miner. Res. 12, 1174 (1997). doi:10.1359/jbmr.1997.12.8.1174
C. Shukunami, C. Shigeno, T. Atsumi, K. Ishizeki, F. Suzuki, Y. Hiraki, J. Cell Biol. 133, 457 (1996). doi:10.1083/jcb.133.2.457
R.S. Tare, D. Howard, J.C. Pound, H.I. Roach, O.C. Oreffo, BBRC 333, 609 (2005)
M.R. Homicz, B.L. Schumacher, R.L. Sah, D. Watson, Otolaryngol. Head Neck Surg. 127, 398 (2002). doi:10.1067/mhn.2002.129730
G.I. Howling, P.W. Dettmar, P.A. Goddard, F.C. Hampson, M. Dornish, E.J. Wood, Biomaterials 22, 2959 (2001). doi:10.1016/S0142-9612(01)00042-4
A.G. Karakeçili, T.T. Demirtaş, C. Satriano, M. Gümüşderelioğlu, G. Marletta, J. Biosci. Bioeng. 104(1), 111 (2007). doi:10.1263/jbb.104.69
U. Hersel, C. Dahmen, H. Kessler, Biomaterials 24, 4385 (2003). doi:10.1016/S0142-9612(03)00343-0
R.S. Tığlı, M. Gümüşderelioğlu, Int. J. Biol. Macromol. 43, 121 (2008). doi:10.1016/j.ijbiomac.2008.04.003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tığlı, R.S., Gümüşderelioğlu, M. Evaluation of alginate-chitosan semi IPNs as cartilage scaffolds. J Mater Sci: Mater Med 20, 699–709 (2009). https://doi.org/10.1007/s10856-008-3624-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-008-3624-x