Abstract
Ca2+ is essential for proper structure and function of skeletal muscle. It not only activates contraction and force development but also participates in multiple signaling pathways. Low levels of Ca2+ restrain muscle regeneration by limiting the fusion of satellite cells. Ironically, sustained elevations of Ca2+ also result in muscle degeneration as this ion promotes high rates of protein breakdown. Moreover, transforming growth factors (TGFs) which are well known for controlling muscle growth also regulate Ca2+ channels. Thus, therapies focused on changing levels of Ca2+ and TGFs are promising for treating muscle-wasting disorders. Three principal systems govern the homeostasis of Ca2+, namely, excitation-contraction (EC) coupling, excitation-coupled Ca2+ entry (ECCE), and store-operated Ca2+ entry (SOCE). Accordingly, alterations in these systems can lead to weakness and atrophy in many hereditary diseases, such as Brody disease, central core disease (CCD), tubular aggregate myopathy (TAM), myotonic dystrophy type 1 (MD1), oculopharyngeal muscular dystrophy (OPMD), and Duchenne muscular dystrophy (DMD). Here, the interrelationship between all these molecules and processes is reviewed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Numerous biological processes depend on the levels of intracellular Ca2+. The neuromuscular transmission (NMT) is an emblematic example. It begins with the arrival of an action potential (AP) to the nerve terminal, with the ensued release and accumulation of acetylcholine (ACh) into the synaptic cleft. Subsequently, precise coordination of the gating of many types of ion channels (and transporters) results in a transitory increase in the levels of free myoplasmic Ca2+ ([Ca2+]i). More specifically, the influx of Na+ through skeletal muscle ACh receptors depolarizes the membrane and thereby activates voltage-gated Na+ channels, an AP is fired, and a process known as excitation-contraction (EC) coupling begins. During EC coupling, the voltage sensors of a voltage-gated Ca2+ channel (CaV1.1) activate the opening of ryanodine receptors (RyR1s, located in the sarcoplasmic reticulum or SR), which allows a massive release of Ca2+ to the cytosol. The resulting rise of [Ca2+]i activates, in turn, not only the contractile machinery but also the SR Ca2+ ATPase (SERCA) that pumps Ca2+ back into the SR (reviewed recently in [1]).
Many human diseases course with skeletal muscle weakness, which (not surprisingly) can be explained by alterations in either NMT or EC coupling. Nevertheless, such modifications can also elicit a chronic loss of muscle mass. For example, by inhibiting the activity of the Ca2+-calmodulin-dependent protein kinase (CamK). This kinase is important to not only stimulate the differentiation of precursor cells (myoblasts) [2] but also to induce transactivation of genes involved in hypertrophy. Apparently, CamK stimulates hypertrophy by inactivating a protein named glycogen synthase kinase 3 beta (GSK3β) [3], whose function is to limit the synthesis of proteins. Thus, by downregulating CamK, low levels of Ca2+ are well suited to generate atrophy. Paradoxically, a sustained rise of [Ca2+]i also results in muscle wasting. This is because the amount of muscle mass depends on a balance between protein synthesis and degradation, and the elevated levels of Ca2+ can activate proteases and thereby promote the breakdown of proteins (Fig. 14.1) [4]. Accordingly, both agonists of the CamK signaling pathway and inhibitors of Ca2+-dependent proteases represent intriguing candidates for treating the pathological loss of skeletal muscle (reviewed in [4, 5]). Herein, the interrelationship between all these physiological and pathological processes is reviewed. An emphasis is put on the role of Ca2+ as a critical node that manages the transition, from a healthy muscular structure to weakness and atrophy.
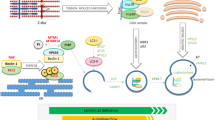
The scheme depicts how pathological alterations of [Ca2+]i can lead to atrophy. Changes in the levels of Ca2+, in the up-and-down direction, activate two different signaling pathways that converge in promoting a significant loss of muscle mass. High: Sustained elevations of Ca2+ can activate a Ca2+-dependent protease (calpain) and thereby result in the breakdown of proteins and atrophy. Low: On the other hand, a decrease in resting Ca2+ levels leads to an impaired formation of myotubes, preventing the proper regeneration of muscle and thus promoting the development of atrophy. See the text for further details
2 Dynamic Changes in Myoplasmic Ca2+
The following three major physiological processes contribute to regulating the homeostasis of Ca2+. They reflect the expression and activity of both Ca2+ channels and the SERCA pump.
2.1 Excitation-Contraction (EC) Coupling
EC coupling is the process by which an AP induces contraction and force development. A transitory increase in [Ca2+]i (Ca2+ transient) is responsible for activating the contractile machinery, whose relaxation occurs as the Ca2+ levels return to normal values, thanks to the activity of SERCA. The source of Ca2+ for EC coupling is the SR, and it has been firmly established that extracellular Ca2+ is irrelevant for this process. For example, in the absence of extracellular Ca2+, the skeletal muscle fiber contracts vigorously, for several minutes [6]. Additionally, the maximum levels of both [Ca2+]i and contractile force can be elicited at membrane potentials where the influx of Ca2+ is practically null [7, 8]. Moreover, in 1973 Schneider and Chandler published what is known as the hypothesis of the physical link for EC coupling. It states that mobile particles embedded in the sarcolemma (voltage sensors) sense APs and mechanically activate the release of Ca2+ from the SR [9]. The molecular identity of voltage sensors was subsequently defined. They form part of a voltage-gated Ca2+ channel, also known as the dihydropyridine receptor (DHPR), or CaV1.1 [10, 11]. The junctional gap between transverse tubes of the sarcolemma (T-tubes) and terminal cisterns of the SR contains electron dense structures, termed “feet.” They reflect the presence of the SR Ca2+ release channel, also known as RyR1 [12]. Indeed, mice knockout for the RyR1 gene lack feet [13]. Thus, CaV1.1 and RyR1 are both essential for EC coupling. Accordingly, they are also critical for survival [14,15,16].
2.2 Excitation-Coupled Ca2+ Entry (ECCE)
The Ca2+-conducting activity of CaV1.1 is irrelevant for EC coupling [17]. This fact indirectly reinforces the concept that the SR is the only source of Ca2+ for this process (see Sect. 14.2.1). Nevertheless, it has been proposed that the entry of Ca2+ through CaV1.1 might participate in replenishing the SR during sustained depolarizations. A process known as excitation-coupled Ca2+ entry (ECCE, [18]) provides indirect support for this speculation. ECCE is a slow increase in the entry of Ca2+ in response to either sustained or repetitive depolarization (for review see [19]). A large amount of data suggests that in both, developing myotubes and adult muscle fibers, an entry of Ca2+ via CaV1.1 represents the underlying mechanism for ECCE [20,21,22].
The following direct evidence supports the notion that ECCE effectively contributes to SR Ca2+ loading. Robin and Allard (2015) reported that the SR Ca2+ loading is potentiated in response to an increase in the magnitude of Ca2+ current associated to ECCE. Moreover, they also found that Mn2+ is not only able to permeate during ECCE but also produces quenching of the fluo-5 N trapped in the SR [22]. Although these findings could be interpreted to suggest that ECCE is physiologically relevant, neither the development nor performance of skeletal muscle is altered in response to the elimination of Ca2+ influx via CaV1.1 [23]. Thus, the possibility that a reduced magnitude of ECCE be of pathophysiological relevance is practically null. Nevertheless, future work may lead to the exciting discovery that, conversely, an increase in ECCE leads to pathological symptoms.
2.3 Store-Operated Ca2+ Entry (SOCE)
SOCE is the process in which a decrease in the load of SR Ca2+ induces a protein of the SR to oligomerize and directly activate a Ca2+ channel of the sarcolemma: STIM is the SR protein, whereas Orai is the Ca2+ channel. Three isoforms of Orai have been identified in human, namely, Orai1, Orai2, and Orai3. They conform the well-known calcium release-activated Ca2+ channels (CRAC) [24]. STIM, on the other hand, consists of two isoforms, which have been detected in vertebrates (STIM1 and STIM2). The principal isoforms that underlie SOCE in skeletal muscle are STIM1 and Orai1 [25]. The C-terminal portion of STIM1 is cytosolic and presents domains critical for binding to—and activating—Orai1. On the other hand, the NH2-terminal segment of STIM1 is located in the lumen of the SR. It contains two regions that are critical for sensing the levels of luminal Ca2+. More specifically, the following domains, EF-hand and sterile alpha-motif (SAM), are thought to constitute the sensor of Ca2+ (EF-SAM). Under normal levels of SR Ca2+ loading, the binding of Ca2+ to EF-SAM keeps STIM1 in its monomeric form. However, the EF-SAM conforms dimers and oligomers in response to depletion and thus promotes both binding of STIM1 to Orai1 and the subsequent entry of Ca2+ [21, 24, 26, 27].
It has been proposed that SOCE participates in refilling the SR of Ca2+, but this idea is controversial. Evidently, an SR depletion is required for activating SOCE, but this condition is difficult to reach, not only physiologically but also experimentally [28]. The following evidence supports the view that SOCE, in effect, contributes to refilling the SR of Ca2+. Mice knockout for myostatin (Sect. 14.3.3) develop a severe reduction in expression levels of STIM1 and Orai1, which correlates with an inhibition of SOCE and a faster SR depletion (induced by repetitive release of Ca2+) [29]. Indeed, this tendency to readily exhaust the SR might explain why those mice deficient in myostatin also exhibit a significant muscle weakness (low specific force), in the face of an excessive muscle mass [30].
3 Myogenesis
3.1 Myogenesis Is Critical for Muscle Growth and Force Development
This is a brief explanation of how precursor cells contribute to the genesis and regeneration of skeletal muscle. The reader is encouraged to consult more extensive reviews on this topic [31,32,33]. During the embryonic development, precursor cells (termed myoblasts) fuse and form multinucleated cells, known as myotubes. The myoblasts withdraw from the cell cycle, adopt a spindle shape, and align with each other—forming a braid—and the fusion occurs. Subsequently, the myotubes are transformed into muscle fibers, through a maturation process that involves (among other things) the formation of T-tubes. The fusion of myoblasts is also known as “terminal differentiation” because it implies that DNA from the fused myoblasts will no longer replicate, and thereby the cell proliferation is arrested. In the adults, myotubes continually form. The corresponding precursor cells are known as satellite cells (SCs). Although not fully differentiated, proliferating myoblasts and SCs are committed to the myogenic lineage (i.e., they already express transcription factors of the MyoD family). Depending on specific conditions, precursor cells can be either mitotically quiescent or induced to proliferate. For example, injury stimulates SCs to proliferate, and the resulting colony provides for generating both a stock of quiescent cells and a significant number of fusion-competent myoblasts. The latter eventually will either form a new fiber or fuse into injured fibers contributing to healing [31,32,33].
In vitro, the fusion of myoblasts is often quantified as the “fusion index”: that is, the number of nuclei per myotube, divided by the total number of nuclei per field of observation. The fusion index is crucial for in vivo conditions because the myofiber size and thereby the contractile strength depend on the number of nuclei in the fiber. Accordingly, it is well known that the number of nuclei in the myofiber declines during atrophy. Conversely, the restoration of muscle mass requires myonuclear accretion [34]. Remarkably, SCs also contribute to a robust neuromuscular junction (NMJ) [35, 36]. Indeed, the deterioration of NMJs, in aging, is more closely related to deficiencies in SCs and myogenesis rather than to denervation [36].
3.2 Role of Ca2+ in Skeletal Muscle Development
Myogenesis involves a dramatic change in phenotype which in turn depends on a coordinated activation of skeletal muscle-specific genes [37,38,39]. Apart from the expression of myogenic factors (e.g., MyoD, Myf5, Myf6, and myogenin), this process requires Ca2+. More precisely, a Ca2+-dependent signaling pathway that involves calmodulin and the family of transcription factors known as NFAT leads to the fusion of myoblasts (for review see [5, 39, 40]). The recent discovery of a feedback mechanism by which SOCE and NFATc3 control the fusion of myoblasts highlights the relevance of this Ca2+-dependent pathway [41].
Because myogenesis requires Ca2+, a reduced entry of this ion tends to inhibit the proper regeneration of muscle. Ironically, however, sustained elevations of [Ca2+]i also contribute to the degeneration of skeletal muscle (Fig. 14.1). This is because Ca2+-dependent proteases lead to protein degradation (i.e., calpains, which contain Ca2+-binding domains) [4]. Indeed, an increase in intracellular Ca2+ is frequently observed in both congenital myopathies and muscular dystrophies (see Sect. 14.4). Additionally, high rates of protein breakdown have been reported in many muscle-wasting diseases [42].
During myogenesis, the expression of several proteins involved in the homeostasis of Ca2+ is induced. An intricate relationship exists because Ca2+, in turn, regulates the expression of at least two of these proteins (i.e., SERCA and CaV1.1) [43,44,45]. Therefore, dissecting the role of a specific protein in myogenesis is complicated. Nevertheless, the use of knockout animals has provided irrefutable proofs pointing to a leading role for CaV1.1 and RyR1. For example, it has been reported that dyspedic and dysgenic mice (i.e., RyR1 and CaV1.1 knockout) die both at birth. More interestingly, these two strains of mice also develop malformations, consisting in delayed development of skeletal muscle [14,15,16, 46]. Thus, RyR1 and CaV1.1 are both of paramount relevance for not only EC coupling (Sect. 14.2.1) but also myogenesis. On the other hand, a recent work elegantly showed that the Ca2+-conducting activity of CaV1.1 is irrelevant for skeletal muscle development and function [23]. Thus, most likely this protein exerts its regulatory actions via mechanical control of RyR1 (as opposed to regulating the entry of Ca2+, see Sect. 14.2).
In mice, the voltage-gated Ca2+ channel isoform CaV3.2 is expressed during embryonic development and then gradually disappears, after birth [47, 48]. In 2000, Biglenga et al. proposed that the entry of Ca2+ through this channel stimulates myogenesis [49]. More recently, this idea was tested and discarded because the fusion of myoblasts was unaltered by nickel (a CaV3.2 blocker) [50]. In addition to CaV3.2, both Orai1 (see Sect. 14.2.3) and a transient receptor potential channel (TRCP1) have also been proposed as necessary for myogenesis [51, 52].
3.3 Transforming Growth Factors Regulate Both Myogenesis and Ca2+ Channels
Several extracellular signaling factors participate in controlling distinct phases of myogenesis. For example, the hepatocyte growth factor (HGF) and fibroblast growth factor (FGF) are both considered of critical relevance for SCs activation [53]. Myostatin (growth differentiation factor 8, GDF-8) is a member of the transforming growth factor-β (TGF-β) superfamily, and it has also proven essential to regulate myogenesis [54, 55]. The TGF-β superfamily includes many other types of growth factors, which, similarly to myostatin, also inhibits the development of skeletal muscle. Specifically, in less than 24 h, the bone morphogenetic protein type 2 (BMP-2) and transforming growth factor β1 (TGF-β1) decrease both the expression of MyoD and myogenin. The effect on these transcription factors precedes a drastic inhibition of myotube formation (Fig. 14.2) [56], which saturates at nanomolar concentrations [57].
TGF-β1 and BMP-2 inhibit myotube formation. Light-field images of myoblasts that were obtained from newborn mice and then kept 6 days under control differentiation conditions (upper panel) and the presence of either BMP-2 (5 nM, middle panel) or TGF-β1 (40 pM, lower panel). The scale bar represents 50 μm
Because myogenesis requires Ca2+ (Sect. 14.3.2), it is possible that BMP-2 and TGF-ß1 arrest this process by interfering with the activity of Ca2+ channels. In support of this view, both growth factors also inhibit the functional expression of CaV3 channels (in semi-differentiated myotubes, see Fig. 14.2). Moreover, TGF-β1, but not BMP-2, also downregulates the activity of CaV1.1 [56]. Although these data suggest that CaV1.1 and CaV3 channels participate in myogenesis, a role for only CaV1.1 has been firmly established (see Sect. 14.3.2).
4 Role of Ca2+ in Diseases That Course with Skeletal Muscle Atrophy
The calcium ions are of paramount relevance in the context of muscle atrophy (Sect. 14.3.2). Thus, not surprisingly, the list of diseases in which alterations in the homeostasis of Ca2+ and skeletal muscle atrophy concur is vast. This section discusses examples where dysregulation of Ca2+ channels and SERCA has been observed. It also explains how such dysregulation contributes to understanding the corresponding loss of muscle mass. It is highly recommended to consult the following excellent reviews on these topics [58, 59].
4.1 Congenital Myopathies
4.1.1 Brody Disease
Brody disease is a congenital myopathy characterized by muscle cramping that usually manifests after exercise (especially in the cold) and is accompanied by impairment of muscle relaxation. Muscles from the legs, arms, and eyelids are principally affected, and they slowly return to relaxation if maintained at rest (reviewed in [60]). This disease is linked to mutations in the gene encoding the skeletal muscle SERCA (i.e., SERCA1) [61]. A related myopathy has also been observed but in the absence of SERCA mutations (termed Brody syndrome). Thus, in more general terms, these disorders are just referred to as “Brody myopathy.” It has been reported that patients with advanced phases of this myopathy also show skeletal muscle weakness and atrophy (of both type I and type II fibers) [60, 62, 63].
A reduced SERCA activity is observed in muscle samples of Brody myopathy patients, and this alteration explains an increase in time needed for myoplasmic Ca2+ extrusion after repetitive stimulation. Although this mechanism underlies the damaged muscle relaxation, stiffness, and cramping [64, 65], the primary functional defect responsible for the loss of SERCA activity remains unknown [60]. Likewise, the molecular basis underlying loss of muscle mass has yet to be elucidated. Because an increase in time needed for myoplasmic Ca2+ extrusion is ostensibly involved in this myopathy, it seems reasonable to speculate that an elevated level of [Ca2+]i recruits Ca2+-dependent proteases and thereby induces protein degradation (Fig. 14.1; see also Sect. 14.3.2). Dantrolene and verapamil, two inhibitors of EC coupling, are promising therapeutic agents for Brody myopathy. They limit the amount of Ca2+ released, and thereby the low Ca2+ pumping capacity readily restores the normal resting [Ca2+]i levels, preventing Ca2+ overload ([65], discussed in [60]). Thus, in the near future, it will be interesting to investigate if these compounds also prevent the development of atrophy.
4.1.2 Central Core Disease
The following congenital myopathies have been related to mutations in the gene encoding RyR1: central core disease (CCD), multiminicore disease (MmD), core myopathies with rods, centronuclear myopathy (CNM), and congenital fiber-type disproportion (CFTD). They conform the also known as “RyR1-related congenital myopathies” (RyR1-RCM) [66, 67]. CCD was the first one being linked to RyR1, and thus the corresponding mutations have been more thoroughly investigated.
CCD is of early onset and courses with proximal weakness, wasting, and skeletal deformities. These symptoms can range from very mild to extremely severe. The diagnosis is based on the identification of areas located within the center of the myofiber, depleted of mitochondria and with poor oxidative enzymatic activity (for recent reviews, see [68, 69]).
Several CCD RyR1 mutant proteins exhibit an overactive or “leaky” behavior that depletes the SR of Ca2+ and thereby decreases the magnitude of the Ca2+ transient [43, 45, 70]. Another set of mutations, located nearby the pore leaning segment of RyR1 (i.e., exon 102, within the C-terminus region), results in mutant proteins with poor Ca2+ permeability. Thus, rather than being leaky, these “pore mutations” result in a functional uncoupling of SR Ca2+ release from the electrical stimulus (termed “EC uncoupling”) [71,72,73]. A third mechanism indicates that certain CCD mutations induce a reduced expression level of RyR1 and thus also promote a lower magnitude of Ca2+ transients [74,75,76,77]. These three primary defects (i.e., leaky, Ca2+-impermeable, and decreased expression) are not mutually exclusive. For example, it has been reported that the Y4864H mutation results in mutant RyR1 proteins that exhibit both, low expression level and altered functional properties (leaky behavior). Remarkably, this mutation also elicits a reduced magnitude of Ca2+ transients, and this defect is attributed to a modified gating of the channel (as opposed to a reduced number of Ca2+ release units) [77].
Although mutations located in many regions of the RyR1 result in leaky behavior, evidence exists suggesting that this alteration ultimately depends on a structural modification of the protein portion facing the lumen of the SR. In particular, it has been reported that the leak depends on a reduced threshold for store overload-induced Ca2+ release (SOICR) [78].
As reviewed above (Sect. 14.3.2), mice knockout for RyR1 exhibit several malformations, including a delayed development of skeletal muscle. Conceivably, these alterations could simply arise from the physical absence of RyR1. Nevertheless, the following evidence indicates that they are due to the inevitable loss of SR Ca2+ release. A point RyR1 mutation that renders Ca2+ impermeable channels (equivalent to I4897T in humans) also inhibits the fusion of C2C12 myoblasts [45]. Moreover, mice knock-in for the same mutation also exhibit a delayed development, which includes a reduced and amorphous skeletal muscle, and very small myotubes [72]. Thus, a reduced level of SR Ca2+ release is sufficient for disrupting myogenesis and thereby also contributes to explaining the atrophy seen in the corresponding CCD patients (Fig. 14.1).
On the contrary, in patients expressing leaky CCD mutations, the atrophy is likely due to a sustained increase in the levels of [Ca2+]i [43, 45, 70]. More specifically, Ca2+-dependent proteolysis [4] may result in increased rate of protein degradation [42] and thereby promote the corresponding loss of muscle mass (Fig. 14.1).
In a mouse model of CCD, the I4897T mutation (see above) was found to induce the development of endoplasmic reticulum stress, unfolded protein response, mitochondrial reactive oxygen species (ROS) production, muscle weakness, and atrophy. Currently, it is unclear how this Ca2+-impermeable mutant protein results in all these alterations. Nevertheless, it is important to note that they were reverted by treatment with the chemical chaperone 4-phenylbutyrate (4-PBA) [79]. Similarly to 4-PBA, agonists of the Gs subgroup of G-protein-coupled receptors have also been reported to be of therapeutic potential in CCD [45, 80]. These findings are encouraging since no effective treatment exists for CCD.
4.1.3 Tubular Aggregate Myopathy
Tubular aggregate myopathy (TAM) is a condition characterized by the presence of “tubular aggregates,” cramps, weakness, and myalgia. Such aggregates contain proteins of the SR and thereby are thought to represent structural alterations of this organelle. A genetic cause of the disease was recently found. Specifically, in 2013 Böhm and collaborators discovered a form of TAM that is inherited with an autosomal dominant pattern and is associated with mutations in the gene encoding STIM1 [81]. This finding was confirmed more recently [82,83,84]. Most of the naturally occurring mutations in STIM1 are punctual substitutions, and they are positioned within the NH2-terminal sequence, just where the EF-hand is located (Sect. 14.2.3). Accordingly, these mutations result in mutant proteins that exhibit an altered capability to bind luminal Ca2+ and thereby also present constitutive oligomerization [81, 83, 85]. The principal role of STIM1 is to activate the entry of Ca2+ via Orai1 channels (during SOCE, Sect. 14.2.3). Thus, prominent levels of SOCE may represent an important functional defect of this myopathy. Indeed, TAM has also been linked to mutations in Orai1, and the corresponding mutant proteins allow an exacerbated influx of Ca2+ [86,87,88].
A TAM STIM1 mutation that consists of an extension of amino acids (I484RfsX21) was reported recently. Remarkably, it resides in the cytosolic part of the protein (C-terminal portion) and, in contrast to mutations of the lumen, it inhibits the entry of Ca2+ [84]. In addition, TAM has been linked to three different mutations in the gene encoding calsequestrin (CASQ1, which is responsible for Ca2+ storage in the SR). Interestingly, while all CASQ1 mutant proteins show a reduced ability to store Ca2+, only two appear to stimulate SOCE [89]. These findings suggest that TAM, and the corresponding atrophy, can both arise from other pathophysiological mechanisms, in addition to elevated levels of SOCE.
4.2 Muscular Dystrophies
4.2.1 Myotonic Dystrophy Type 1 (MD1)
This disease is caused by the expansion of a CTG repeat in the gene encoding a protein kinase termed MDPK. Increased excitability, delayed relaxation, atrophy, and weakness represent the most common symptoms. The CTG-repeat expansion results in both lower MDPK protein levels and trapping of the corresponding mRNA into nuclear foci. Interestingly, muscle degeneration has been related to increased rates of myofibrillar protein breakdown [42], which in turn could be explained by an exacerbated activity of Ca2+-dependent proteases [4]. Indeed, elevated levels of [Ca2+]i have been observed in myotubes derived from both MD1 patients and DMPK knockout mice [90,91,92]. Nevertheless, it is important to note that a deficiency in DMPK has functional effects in neither cardiac nor skeletal muscle. Thus, the MD1 symptoms likely arise from toxic effects of the trapped transcripts, rather than to decreased levels of the protein [93]. Transcripts of at least both, transcription factors and alternative splicing factors can be trapped, which explains why in this myopathy the expression of multiple genes is altered. Remarkably, the trapping of mRNAs modifies not only the function but also the structure of the nuclei [94].
MD1 has also been associated with misregulated alternative splicing; for example, MD1 patients show repressed alternative splicing of exon 29 in CaV1.1. Of note, the degree of exon skipping correlates with the severity of muscle weakness, suggesting that the corresponding functional alteration in CaV1.1 contributes to exacerbating symptoms [95]. Additionally, the alternative splicing of both RyR1 and SERCA (1 and 2) is misregulated. Thus, aberrant splicing of the corresponding transcripts most likely also contribute (by affecting Ca2+-dependent pathways) [92, 96].
4.2.2 OPMD
Oculopharyngeal muscular dystrophy, or OPMD, is a late-onset autosomal dominant congenital myopathy. The first symptoms begin between the fifth and sixth decades of life. They consist of progressive drooping of eyelids (ptosis), swallowing difficulty (dysphagia), muscle atrophy, and proximal upper and lower weakness. OPMD is linked to mutations in the gene encoding poly(A)-binding protein nuclear 1 (PABPN1). The OPMD mutations consist of an expansion of a tract that contains 10 alanines (to 12–17). The pathological hallmark is that the nuclei of skeletal muscle fibers develop aggregates or inclusions (termed intranuclear inclusions, INI), which contain a misfolded PABPN1 and sequester poly(A) RNA [97, 98]. This disease is also frequently accompanied by other severe symptoms, such as weakness and atrophy of the tongue, dysphonia, limitation of upward gaze, and facial muscle weakness [99].
Although the precise underlying mechanism is not yet clear, it has been proposed that the INIs generate toxic effects, likely by interfering with the cellular traffic of poly(A) RNA, and thus affecting gene expression [97, 98]. The expression of at least 202 genes is misregulated, as shown by microarray assays performed in muscle fibers from a mouse model of OPMD [100]. A recent study reported that an OPMD mutant protein (PABPN1-17A) promotes structural alterations of the nucleus, which contributes to explaining the wide range of genes whose expression is misregulated [101].
Interestingly, PABPN1 stimulates the fusion of myoblasts, and this property is missing in the PABPN1-17A mutant protein [101]. Thus, an altered capacity to regenerate muscle may explain the corresponding muscle atrophy and weakness in OPMD. In C2C12 myotubes, PABPN1-17A also elicits many alterations in the homeostasis of Ca2+ [101]. For example, it promotes a ~50% reduction of the magnitude of Ca2+ transients. This effect can be explained by parallel changes in the expression of RyR1 and SR Ca2+ content. In fibers from adult mice, however, this mutant protein is unable to modify the magnitude of Ca2+ transients [101]. This finding indirectly supports the notion that atrophy, due to inability to stimulate myogenesis (Fig. 14.1), likely represents the most significant pathophysiological consequence of PABPN1 mutant proteins [101,102,103,104].
4.2.3 Duchenne Muscular Dystrophy
The absence of dystrophin, a cytosolic protein that is critical for proper structure of the muscle, results in a genetic disorder known as Duchenne muscular dystrophy (DMD). This disease is characterized by shorter lifespan, cardiac involvement, and skeletal muscle degeneration and weakness. An increased structural fragility of muscle fibers and altered homeostasis of Ca2+ represent two relevant pathophysiological mechanisms. Indeed, an increased entry of Ca2+ (which promotes protein degradation and higher levels of ROS) has been proposed to explain the corresponding atrophy [42, 105]. Accordingly, myotubes of mdx mice (a commonly used model of DMD) exhibit a higher activity of Ca2+ channels at resting membrane potentials, compared with controls. This hyperactivity is due to the presence of a mechano-transducing Ca2+ channel, which likely contributes to the high influx of Ca2+ [106, 107]. Although the identity of the corresponding stretch-activated Ca2+ channel(s) (SACs) has yet to be firmly established, members of the transient receptor potential channel (TRPC) family may be involved. TRPCs participate in muscle differentiation, and thus changes in their function/expression might also contribute to generating the corresponding loss of muscle mass. For a recent and comprehensive review, see [108].
An exacerbated SOCE has also been linked to DMD. For example, muscle fibers from mdx mice show not only increased levels of SOCE but also higher expression level of both Orai1 and STIM1 [109, 110]. Accordingly, it has been reported that the severity of this disease can be reduced by expressing a dominant negative Orai1, in two mouse models of DMD [111].
Like in many human myopathies, no effective treatment exists for DMD (other than palliatives focused on easing the symptoms). Thus, the search for a more effective treatment continues. With regard to “fixing” alterations in the homeostasis of Ca2+, pharmacological approaches have been investigated. More precisely, the efforts have focused on using blockers of Ca2+ channels, as well as on regulating the activity and expression of SERCA (reviewed in [112, 113]). Knocking down the expression and activity of myostatin (see Sect. 14.3.2) also represents a promising therapy. This intervention is particularly beneficial to counteract muscle weakness and wasting, in not only DMD [114, 115] but also many other disorders [116].
5 Conclusions
In skeletal muscle fibers, much work has evolved in acquiring a deep knowledge of the mechanisms that control the homeostasis of Ca2+, under both physiological and pathological conditions. Meanwhile, significant efforts have firmly established a pivotal role for Ca2+ in determining the amount of muscle mass. Accordingly, it is now generally accepted that this ion controls not only muscle mechanical properties but also the corresponding development, regeneration, atrophy, and hypertrophy. Therefore, treating wasting disorders with therapies based on a precise tune-up of the activity/expression of Ca2+ channels and transporters could eventually become a daily clinical practice.
References
Franzini-Armstrong C (2018) The relationship between form and function throughout the history of excitation-contraction coupling. J Gen Physiol 150:189–210
Konig S, Béguet A, Bader CR, Bernheim L (2006) The calcineurin pathway links hyperpolarization (Kir2.1)-induced Ca2+ signals to human myoblast differentiation and fusion. Development 133:3107–3114
Sacchetto R, Bovo E, Salviati L, Damiani E, Margreth A (2007) Glycogen synthase binds to sarcoplasmic reticulum and is phosphorylated by CaMKII in fast-twitch skeletal muscle. Arch Biochem Biophys 459:115–121
Costelli P, Reffo P, Penna F, Autelli R, Bonelli G, Baccino FM (2005) Ca(2+)-dependent proteolysis in muscle wasting. Int J Biochem Cell Biol 37:2134–2146
Al-Shanti N, Stewart CE (2009) Ca2+/calmodulin-dependent transcriptional pathways: potential mediators of skeletal muscle growth and development. Biol Rev Camb Philos Soc 84:637–652
Armstrong CM, Bezanilla FM, Horowicz P (1972) Twitches in the presence of ethylene glycol bis( −aminoethyl ether)-N,N’-tetracetic acid. Biochim Biophys Acta 267:605–608
Miledi R, Parker I, Schalow G (1977) Measurement of calcium transients in frog muscle by the use of arsenazo III. Proceedings of the Royal Society of London. Series B Biol Sci 198:201–210
Caputo C, Bezanilla F, Horowicz P (1984) Depolarization-contraction coupling in short frog muscle fibers. A voltage clamp study. J Gen Physiol 84:133–154
Schneider MF, Chandler WK (1973) Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature 242:244–246
Rios E, Brum G (1987) Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature 325:717–720
Tanabe T, Beam KG, Powell JA, Numa S (1988) Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature 336:134–139
Inui M, Saito A, Fleischer S (1987) Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem 262:1740–1747
Takekura H, Nishi M, Noda T, Takeshima H, Franzini-Armstrong C (1995) Abnormal junctions between surface membrane and sarcoplasmic reticulum in skeletal muscle with a mutation targeted to the ryanodine receptor. Proc Natl Acad Sci U S A 92:3381–3385
Beam KG, Knudson CM, Powell JA (1986) A lethal mutation in mice eliminates the slow calcium current in skeletal muscle cells. Nature 320:168–170
Takeshima H, Iino M, Takekura H, Nishi M, Kuno J, Minowa O, Takano H, Noda T (1994) Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature 369:556–559
Buck ED, Nguyen HT, Pessah IN, Allen PD (1997) Dyspedic mouse skeletal muscle expresses major elements of the triadic junction but lacks detectable ryanodine receptor protein and function. J Biol Chem 272:7360–7367
Dirksen RT, Beam KG (1999) Role of calcium permeation in dihydropyridine receptor function. Insights into channel gating and excitation-contraction coupling. J Gen Physiol 114:393–403
Cherednichenko G, Hurne AM, Fessenden JD, Lee EH, Allen PD, Beam KG, Pessah IN (2004) Conformational activation of Ca2+ entry by depolarization of skeletal myotubes. Proc Natl Acad Sci U S A 101:15793–15798
Bannister RA, Beam KG (2013) Ca(V)1.1: the atypical prototypical voltage-gated Ca2+ channel. Biochim Biophys Acta 1828:1587–1597
Bannister RA, Pessah IN, Beam KG (2009) The skeletal L-type Ca(2+) current is a major contributor to excitation-coupled Ca(2+) entry. J Gen Physiol 133:79–91
Dirksen RT (2009) Checking your SOCCs and feet: the molecular mechanisms of Ca2+ entry in skeletal muscle. J Physiol Lond 587:3139–3147
Robin G, Allard B (2015) Voltage-gated Ca(2+) influx through L-type channels contributes to sarcoplasmic reticulum Ca(2+) loading in skeletal muscle. J Physiol Lond 593:4781–4797
Dayal A, Schrötter K, Pan Y, Föhr K, Melzer W, Grabner M (2017) The Ca2+influx through the mammalian skeletal muscle dihydropyridine receptor is irrelevant for muscle performance. Nat Commun 8:475
Stathopulos PB, Ikura M (2013) Structural aspects of calcium-release activated calcium channel function. Channels 7:344–353
Lyfenko AD, Dirksen RT (2008) Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J Physiol Lond 586:4815–4824
Stathopulos PB, Li G-Y, Plevin MJ, Ames JB, Ikura M (2006) Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J Biol Chem 281:35855–35862
Launikonis BS, Murphy RM, Edwards JN (2010) Toward the roles of store-operated Ca2+ entry in skeletal muscle. Pflugers Archiv 460:813–823
Kurebayashi N, Ogawa Y (2001) Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol Lond 533:185–199
Sztretye M, Geyer N, Vincze J, Al-Gaadi D, Oláh T, Szentesi P, Kis G, Antal M, Balatoni I, Csernoch L, Dienes B (2017) SOCE is important for maintaining sarcoplasmic calcium content and release in skeletal muscle fibers. Biophys J 113:2496–2507
Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, Voit T, Muntoni F, Vrbóva G, Partridge T, Zammit P, Bunger L, Patel K (2007) Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci U S A 104:1835–1840
Brack AS, Rando TA (2012) Tissue-specific stem cells: lessons from the skeletal muscle satellite cell. Cell Stem Cell 10:504–514
Randolph ME, Pavlath GK (2015) A muscle stem cell for every muscle: variability of satellite cell biology among different muscle groups. Front Aging Neurosci 7:190
Crist C (2017) Emerging new tools to study and treat muscle pathologies: genetics and molecular mechanisms underlying skeletal muscle development, regeneration, and disease. J Pathol 241:264–272
Mitchell PO, Pavlath GK (2001) A muscle precursor cell-dependent pathway contributes to muscle growth after atrophy. Am J Physiol Cell Physiol 281:C1706–C1715
Liu W, Wei-LaPierre L, Klose A, Dirksen RT, Chakkalakal JV (2015) Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions. elife 4. https://doi.org/10.7554/eLife.09221
Liu W, Klose A, Forman S, Paris ND, Wei-LaPierre L, Cortés-Lopéz M, Tan A, Flaherty M, Miura P, Dirksen RT, Chakkalakal JV (2017) Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. elife 6. https://doi.org/10.7554/eLife.26464
Chen JCJ, Goldhamer DJ (2003) Skeletal muscle stem cells. Reprod Biol Endocrinol 1:101
Parker MH, Seale P, Rudnicki MA (2003) Looking back to the embryo: defining transcriptional networks in adult myogenesis. Nat Rev Genet 4:497–507
Horsley V, Pavlath GK (2004) Forming a multinucleated cell: molecules that regulate myoblast fusion. Cells Tissues Organs 176:67–78
Benavides Damm T, Egli M (2014) Calcium’s role in mechanotransduction during muscle development. Cell Physiol Biochem 33:249–272
Phuong TTT, Yun Y-H, Kim SJ, Kang TM (2013) Positive feedback control between STIM1 and NFATc3 is required for C2C12 myoblast differentiation. Biochem Biophys Res Commun 430:722–728
Warnes DM, Tomas FM, Ballard FJ (1981) Increased rates of myofibrillar protein breakdown in muscle-wasting diseases. Muscle Nerve 4:62–66
Tong J, McCarthy TV, MacLennan DH (1999) Measurement of resting cytosolic Ca2+ concentrations and Ca2+ store size in HEK-293 cells transfected with malignant hyperthermia or central core disease mutant Ca2+ release channels. J Biol Chem 274:693–702
Avila G, O’Connell KM, Groom LA, Dirksen RT (2001) Ca2+ release through ryanodine receptors regulates skeletal muscle L-type Ca2+ channel expression. J Biol Chem 276:17732–17738
Vega AV, Ramos-Mondragón R, Calderón-Rivera A, Zarain-Herzberg A, Avila G (2011) Calcitonin gene-related peptide restores disrupted excitation-contraction coupling in myotubes expressing central core disease mutations in RyR1. J Physiol Lond 589:4649–4669
Filipova D, Henry M, Rotshteyn T, Brunn A, Carstov M, Deckert M, Hescheler J, Sachinidis A, Pfitzer G, Papadopoulos S (2018) Distinct transcriptomic changes in E14.5 mouse skeletal muscle lacking RYR1 or Cav1.1 converge at E18.5. PLoS One 13:e0194428
Beam KG, Knudson CM (1988) Effect of postnatal development on calcium currents and slow charge movement in mammalian skeletal muscle. J Gen Physiol 91:799–815
Berthier C, Monteil A, Lory P, Strube C (2002) Alpha(1H) mRNA in single skeletal muscle fibres accounts for T-type calcium current transient expression during fetal development in mice. J Physiol Lond 539:681–691
Bijlenga P, Liu JH, Espinos E, Haenggeli CA, Fischer-Lougheed J, Bader CR, Bernheim L (2000) T-type alpha 1H Ca2+ channels are involved in Ca2+ signaling during terminal differentiation (fusion) of human myoblasts. Proc Natl Acad Sci U S A 97:7627–7632
Bidaud I, Monteil A, Nargeot J, Lory P (2006) Properties and role of voltage-dependent calcium channels during mouse skeletal muscle differentiation. J Muscle Res Cell Motil 27:75–81
Louis M, Zanou N, Van Schoor M, Gailly P (2008) TRPC1 regulates skeletal myoblast migration and differentiation. J Cell Sci 121:3951–3959
Darbellay B, Arnaudeau S, König S, Jousset H, Bader C, Demaurex N, Bernheim L (2009) STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J Biol Chem 284:5370–5380
Shefer G, Yablonka-Reuveni Z, Schiaffino S, Partridge T (2008) The ins and outs of satellite cell Myogenesis: the role of the ruling growth factors. In: Skeletal muscle repair and regeneration. Springer, Dordrecht, pp 107–144
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90
Tsuchida K (2008) Targeting myostatin for therapies against muscle-wasting disorders. Curr Opin Drug Discov Devel 11:487–494
Mejia-Luna L, Avila G (2004) Ca2+ channel regulation by transforming growth factor-beta 1 and bone morphogenetic protein-2 in developing mice myotubes. J Physiol Lond 559:41–54
Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T (1994) Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 127:1755–1766
Rossi AE, Dirksen RT (2006) Sarcoplasmic reticulum: the dynamic calcium governor of muscle. Muscle Nerve 33:715–731
Dowling JJ, Lawlor MW, Dirksen RT (2014) Triadopathies: an emerging class of skeletal muscle diseases. Neurotherapeutics 11:773–785
Voermans NC, Laan AE, Oosterhof A, van Kuppevelt TH, Drost G, Lammens M, Kamsteeg EJ, Scotton C, Gualandi F, Guglielmi V, van den Heuvel L, Vattemi G, van Engelen BG (2012) Brody syndrome: a clinically heterogeneous entity distinct from Brody disease: a review of literature and a cross-sectional clinical study in 17 patients. Neuromuscul Disord 22:944–954
Odermatt A, Taschner PE, Khanna VK, Busch HF, Karpati G, Jablecki CK, Breuning MH, MacLennan DH (1996) Mutations in the gene-encoding SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+ ATPase, are associated with Brody disease. Nat Genet 14:191–194
MacLennan DH, Loke JC (2002) Brody disease associated with defects in a calcium pump. In: Karpati G (ed) Structural and molecular basis of skeletal muscle disease. ISN Neuropath, Basel, pp 103–105
Guglielmi V, Vattemi G, Gualandi F, Voermans NC, Marini M, Scotton C, Pegoraro E, Oosterhof A, Kósa M, Zádor E, Valente EM, De Grandis D, Neri M, Codemo V, Novelli A, van Kuppevelt TH, Dallapiccola B, van Engelen BG, Ferlini A, Tomelleri G (2013) SERCA1 protein expression in muscle of patients with Brody disease and Brody syndrome and in cultured human muscle fibers. Mol Genet Metab 110:162–169
Karpati G, Charuk J, Carpenter S, Jablecki C, Holland P (1986) Myopathy caused by a deficiency of Ca2+−adenosine triphosphatase in sarcoplasmic reticulum (Brody’s disease). Ann Neurol 20:38–49
Benders AA, Veerkamp JH, Oosterhof A, Jongen PJ, Bindels RJ, Smit LM, Busch HF, Wevers RA (1994) Ca2+ homeostasis in Brody’s disease. A study in skeletal muscle and cultured muscle cells and the effects of dantrolene an verapamil. J Clin Investig 94:741–748
Fauré J, Lunardi J, Monnier N, Marty I (2014) Ryanodine receptor 1 and associated pathologies. In: Pathologies of calcium channels. Springer, Berlin/Heidelberg, pp 167–187
Marty I, Fauré J (2016) Excitation-contraction coupling alterations in myopathies. J Neuromuscul Dis 3:443–453
Jungbluth H (2007) Central core disease. Orphanet J Rare Dis 2:25
Guerrero-Hernández A, Avila G, Rueda A (2014) Ryanodine receptors as leak channels. Eur J Pharmacol 739C:26–38
Avila G, Dirksen RT (2001) Functional effects of central core disease mutations in the cytoplasmic region of the skeletal muscle ryanodine receptor. J Gen Physiol 118:277–290
Avila G, O’Brien JJ, Dirksen RT (2001) Excitation--contraction uncoupling by a human central core disease mutation in the ryanodine receptor. Proc Natl Acad Sci U S A 98:4215–4220
Zvaritch E, Depreux F, Kraeva N, Loy RE, Goonasekera SA, Boncompagni S, Kraev A, Gramolini AO, Dirksen RT, Franzini-Armstrong C, Seidman CE, Seidman JG, Maclennan DH (2007) An Ryr1I4895T mutation abolishes Ca2+ release channel function and delays development in homozygous offspring of a mutant mouse line. Proc Natl Acad Sci U S A 104:18537–18542
Loy RE, Orynbayev M, Xu L, Andronache Z, Apostol S, Zvaritch E, MacLennan DH, Meissner G, Melzer W, Dirksen RT (2011) Muscle weakness in Ryr1I4895T/WT knock-in mice as a result of reduced ryanodine receptor Ca2+ ion permeation and release from the sarcoplasmic reticulum. J Gen Physiol 137:43–57
Monnier N, Ferreiro A, Marty I, Labarre-Vila A, Mezin P, Lunardi J (2003) A homozygous splicing mutation causing a depletion of skeletal muscle RYR1 is associated with multi-minicore disease congenital myopathy with ophthalmoplegia. Hum Mol Genet 12:1171–1178
Zhou H, Brockington M, Jungbluth H, Monk D, Stanier P, Sewry CA, Moore GE, Muntoni F (2006) Epigenetic allele silencing unveils recessive RYR1 mutations in core myopathies. Am J Hum Genet 79:859–868
Zhou H, Lillis S, Loy RE, Ghassemi F, Rose MR, Norwood F, Mills K, Al-Sarraj S, Lane RJ, Feng L, Matthews E, Sewry CA, Abbs S, Buk S, Hanna M, Treves S, Dirksen RT, Meissner G, Muntoni F, Jungbluth H (2010) Multi-minicore disease and atypical periodic paralysis associated with novel mutations in the skeletal muscle ryanodine receptor (RYR1) gene. Neuromuscul Disord 20:166–173
Cacheux M, Blum A, Sébastien M, Wozny AS, Brocard J, Mamchaoui K, Mouly V, Roux-Buisson N, Rendu J, Monnier N, Krivosic R, Allen P, Lacour A, Lunardi J, Fauré J, Marty I (2015) Functional characterization of a central Core disease RyR1 mutation (p.Y4864H) associated with quantitative defect in RyR1 protein. J Neuromuscul Dis 2:421–432
Chen W, Koop A, Liu Y, Guo W, Wei J, Wang R, MacLennan DH, Dirksen RT, Chen SRW (2017) Reduced threshold for store overload-induced Ca2+release is a common defect of RyR1 mutations associated with malignant hyperthermia and central core disease. Biochem J 474:2749–2761
Lee CS, Hanna AD, Wang H, Dagnino-Acosta A, Joshi AD, Knoblauch M, Xia Y, Georgiou DK, Xu J, Long C, Amano H, Reynolds C, Dong K, Martin JC, Lagor WR, Rodney GG, Sahin E, Sewry C, Hamilton SL (2017) A chemical chaperone improves muscle function in mice with a RyR1 mutation. Nat Commun 8:14659
Messina S, Hartley L, Main M, Kinali M, Jungbluth H, Muntoni F, Mercuri E (2004) Pilot trial of salbutamol in central core and multi-minicore diseases. Neuropediatrics 35:262–266
Böhm J, Chevessier F, Maues De Paula A, Koch C, Attarian S, Feger C, Hantaï D, Laforêt P, Ghorab K, Vallat JM, Fardeau M, Figarella-Branger D, Pouget J, Romero NB, Koch M, Ebel C, Levy N, Krahn M, Eymard B, Bartoli M, Laporte J (2013) Constitutive activation of the calcium sensor STIM1 causes tubular-aggregate myopathy. Am J Hum Genet 92:271–278
Böhm J, Chevessier F, Koch C, Peche GA, Mora M, Morandi L, Pasanisi B, Moroni I, Tasca G, Fattori F, Ricci E, Pénisson-Besnier I, Nadaj-Pakleza A, Fardeau M, Joshi PR, Deschauer M, Romero NB, Eymard B, Laporte J (2014) Clinical, histological and genetic characterisation of patients with tubular aggregate myopathy caused by mutations in STIM1. J Med Genet 51:824–833
Hedberg C, Niceta M, Fattori F, Lindvall B, Ciolfi A, D’Amico A, Tasca G, Petrini S, Tulinius M, Tartaglia M, Oldfors A, Bertini E (2014) Childhood onset tubular aggregate myopathy associated with de novo STIM1 mutations. J Neurol 261:870–876
Okuma H, Saito F, Mitsui J, Hara Y, Hatanaka Y, Ikeda M, Shimizu T, Matsumura K, Shimizu J, Tsuji S, Sonoo M (2016) Tubular aggregate myopathy caused by a novel mutation in the cytoplasmic domain of STIM1. Neurol Genet 2:e50
Walter MC, Rossius M, Zitzelsberger M, Vorgerd M, Müller-Felber W, Ertl-Wagner B, Zhang Y, Brinkmeier H, Senderek J, Schoser B (2015) 50 years to diagnosis: autosomal dominant tubular aggregate myopathy caused by a novel STIM1 mutation. Neuromuscul Disord 25:577–584
Nesin V, Wiley G, Kousi M, Ong EC, Lehmann T, Nicholl DJ, Suri M, Shahrizaila N, Katsanis N, Gaffney PM, Wierenga KJ, Tsiokas L (2014) Activating mutations in STIM1 and ORAI1 cause overlapping syndromes of tubular myopathy and congenital miosis. Proc Natl Acad Sci U S A 111:4197–4202
Endo Y, Noguchi S, Hara Y, Hayashi YK, Motomura K, Miyatake S, Murakami N, Tanaka S, Yamashita S, Kizu R, Bamba M, Goto Y, Matsumoto N, Nonaka I, Nishino I (2015) Dominant mutations in ORAI1 cause tubular aggregate myopathy with hypocalcemia via constitutive activation of store-operated Ca2+ channels. Hum Mol Genet 24:637–648
Garibaldi M, Fattori F, Riva B, Labasse C, Brochier G, Ottaviani P, Sacconi S, Vizzaccaro E, Laschena F, Romero NB, Genazzani A, Bertini E, Antonini G (2017) A novel gain-of-function mutation in ORAI1 causes late-onset tubular aggregate myopathy and congenital miosis. Clin Genet 91:780–786
Barone V, Del Re V, Gamberucci A, Polverino V, Galli L, Rossi D, Costanzi E, Toniolo L, Berti G, Malandrini A, Ricci G, Siciliano G, Vattemi G, Tomelleri G, Pierantozzi E, Spinozzi S, Volpi N, Fulceri R, Battistutta R, Reggiani C, Sorrentino V (2017) Identification and characterization of three novel mutations in the CASQ1 gene in four patients with tubular aggregate myopathy. Hum Mutat 38:1761–1773
Jacobs AE, Benders AA, Oosterhof A, Veerkamp JH, van Mier P, Wevers RA, Joosten EM (1990) The calcium homeostasis and the membrane potential of cultured muscle cells from patients with myotonic dystrophy. Biochim Biophys Acta 1096:14–19
Benders AA, Groenen PJ, Oerlemans FT, Veerkamp JH, Wieringa B (1997) Myotonic dystrophy protein kinase is involved in the modulation of the Ca2+ homeostasis in skeletal muscle cells. J Clin Investig 100:1440–1447
Santoro M, Piacentini R, Masciullo M, Bianchi MLE, Modoni A, Podda MV, Ricci E, Silvestri G, Grassi C (2014) Alternative splicing alterations of Ca2+ handling genes are associated with Ca2+ signal dysregulation in myotonic dystrophy type 1 (DM1) and type 2 (DM2) myotubes. Neuropathol Appl Neurobiol 40:464–476
Carrell ST, Carrell EM, Auerbach D, Pandey SK, Bennett CF, Dirksen RT, Thornton CA (2016) Dmpk gene deletion or antisense knockdown does not compromise cardiac or skeletal muscle function in mice. Hum Mol Genet 25:4328–4338
Rodríguez R, Hernández-Hernández O, Magaña JJ, González-Ramírez R, García-López ES, Cisneros B (2015) Altered nuclear structure in myotonic dystrophy type 1-derived fibroblasts. Mol Biol Rep 42:479–488
Tang ZZ, Yarotskyy V, Wei L, Sobczak K, Nakamori M, Eichinger K, Moxley RT, Dirksen RT, Thornton CA (2012) Muscle weakness in myotonic dystrophy associated with misregulated splicing and altered gating of ca(V)1.1 calcium channel. Hum Mol Genet 21:1312–1324
Kimura T, Nakamori M, Lueck JD, Pouliquin P, Aoike F, Fujimura H, Dirksen RT, Takahashi MP, Dulhunty AF, Sakoda S (2005) Altered mRNA splicing of the skeletal muscle ryanodine receptor and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase in myotonic dystrophy type 1. Hum Mol Genet 14:2189–2200
Calado A, Tomé FM, Brais B, Rouleau GA, Kühn U, Wahle E, Carmo-Fonseca M (2000) Nuclear inclusions in oculopharyngeal muscular dystrophy consist of poly(A) binding protein 2 aggregates which sequester poly(A) RNA. Hum Mol Genet 9:2321–2328
Abu-Baker A, Rouleau GA (2007) Oculopharyngeal muscular dystrophy: recent advances in the understanding of the molecular pathogenic mechanisms and treatment strategies. Biochim Biophys Acta 1772:173–185
Trollet C, Gidaro T, Klein P, Périé S, Butler-Browne G, Lacau St Guily J (1993) Oculopharyngeal muscular dystrophy. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, LJH B, Stephens K, Amemiya A (eds) GeneReviews® [internet]. University of Washington, Seattle
Corbeil-Girard L-P, Klein AF, Sasseville AM-J, Lavoie H, Dicaire MJ, Saint-Denis A, Pagé M, Duranceau A, Codère F, Bouchard JP, Karpati G, Rouleau GA, Massie B, Langelier Y, Brais B (2005) PABPN1 overexpression leads to upregulation of genes encoding nuclear proteins that are sequestered in oculopharyngeal muscular dystrophy nuclear inclusions. Neurobiol Dis 18:551–567
García-Castañeda M, Vega AV, Rodríguez R, Montiel-Jaen MG, Cisneros B, Zarain-Herzberg A, Avila G (2017) Functional impact of an oculopharyngeal muscular dystrophy mutation in PABPN1. J Physiol Lond 595:4167–4187
Périé S, Mamchaoui K, Mouly V, Blot S, Bouazza B, Thornell L-E, St Guily JL, Butler-Browne G (2006) Premature proliferative arrest of cricopharyngeal myoblasts in oculo-pharyngeal muscular dystrophy: therapeutic perspectives of autologous myoblast transplantation. Neuromuscul Disord 16:770–781
Wang Q, Bag J (2006) Ectopic expression of a polyalanine expansion mutant of poly(A)-binding protein N1 in muscle cells in culture inhibits myogenesis. Biochem Biophys Res Commun 340:815–822
Apponi LH, Corbett AH, Pavlath GK (2013) Control of mRNA stability contributes to low levels of nuclear poly(A) binding protein 1 (PABPN1) in skeletal muscle. Skelet Muscle 3:23
Turner PR, Westwood T, Regen CM, Steinhardt RA (1988) Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature 335:735–738
Franco A, Lansman JB (1990) Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature 344:670–673
Franco-Obregón A, Lansman JB (1994) Mechanosensitive ion channels in skeletal muscle from normal and dystrophic mice. J Physiol Lond 481(Pt 2):299–309
Saüc S, Frieden M (2017) Neurological and motor disorders: TRPC in the skeletal muscle. Adv Exp Med Biol 993:557–575
Edwards JN, Friedrich O, Cully TR, von Wegner F, Murphy RM, Launikonis BS (2010) Upregulation of store-operated Ca2+ entry in dystrophic mdx mouse muscle. Am J Phys Cell Phys 299:C42–C50
Zhao X, Moloughney JG, Zhang S, Komazaki S, Weisleder N (2012) Orai1 mediates exacerbated ca(2+) entry in dystrophic skeletal muscle. PLoS One 7:e49862
Goonasekera SA, Davis J, Kwong JQ, Accornero F, Wei-LaPierre L, Sargent MA, Dirksen RT, Molkentin JD (2014) Enhanced Ca2+ influx from STIM1-Orai1 induces muscle pathology in mouse models of muscular dystrophy. Hum Mol Genet 23:3706–3715
Burr AR, Molkentin JD (2015) Genetic evidence in the mouse solidifies the calcium hypothesis of myofiber death in muscular dystrophy. Cell Death Differ 22:1402–1412
Spinazzola JM, Kunkel LM (2016) Pharmacological therapeutics targeting the secondary defects and downstream pathology of Duchenne muscular dystrophy. Expert Opin Orphan Drugs 4:1179–1194
Malerba A, Kang JK, McClorey G, Saleh AF, Popplewell L, Gait MJ, Wood MJ, Dickson G (2012) Dual Myostatin and dystrophin exon skipping by Morpholino nucleic acid oligomers conjugated to a cell-penetrating peptide is a promising therapeutic strategy for the treatment of Duchenne muscular dystrophy. Mol Ther-Nucleic Acids 1:e62
St Andre M, Johnson M, Bansal PN, Wellen J, Robertson A, Opsahl A, Burch PM, Bialek P, Morris C, Owens J (2017) A mouse anti-myostatin antibody increases muscle mass and improves muscle strength and contractility in the mdx mouse model of Duchenne muscular dystrophy and its humanized equivalent, domagrozumab (PF-06252616), increases muscle volume in cynomolgus monkeys. Skelet Muscle 7:25
Smith RC, Lin BK (2013) Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr Opin Support Palliat Care 7:352–360
Acknowledgments
The lab has been supported by CONACyT. I thank Lizbeth Mejía-Luna for help in preparing Fig. 14.2.
Competing Financial Interests
The author declares no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Avila, G. (2018). Disturbed Ca2+ Homeostasis in Muscle-Wasting Disorders. In: Xiao, J. (eds) Muscle Atrophy. Advances in Experimental Medicine and Biology, vol 1088. Springer, Singapore. https://doi.org/10.1007/978-981-13-1435-3_14
Download citation
DOI: https://doi.org/10.1007/978-981-13-1435-3_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1434-6
Online ISBN: 978-981-13-1435-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)