Abstract
An efficient, convenient and environmentally benign procedure for the construction of various bioactive spirooxindoles has been developed by condensation reactions of isatins, malononitrile and α-methylene carbonyl compounds/enols in the presence of starch solution as expedient, eco-friendly and biodegradable catalyst at 60 °C. The prominent features of the above protocol are short reaction time, high atom economy, simple work-up, cost-effectiveness, avoidance of toxic chemicals.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
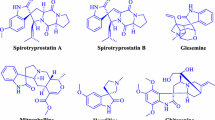
Spirooxindole based compounds possess wide array of activities such as antitumuor [1], antimicrobial [2, 3], antitubercular [4], antimycobacterial [5], antiproliferative properties [6]. The unique structure of spirooxindoles and their highly pronounced biological activity has attracted the interest among various researchers [7,8,9].
Moreover design and development of methods to access biologically relevant complex molecules has become increasingly popular at the forefront of contemporary organic synthesis. Multicomponent reactions (MCRs), provide one of the most dominant platforms for the sustainable synthesis of polyfunctionalized heterocyclic compounds owing to their atom economy, operational simplicity, environmental friendliness and green chemistry characteristics [10,11,12,13].
As a part of our research interest toward the development of environmentally benevolent, efficient and economically viable protocol for the synthesis of heterocycles [14,15,16,17,18,19,20], we envisaged on the synthesis of spiropyran annulated heterocycles through one pot condensation of isatins with malononitrile and α-methylene carbonyl compounds/enols. Starch solution which is easily available, inexpensive, biodegradable as well as non-toxic in nature was used as catalyst for the synthesis of aforementioned heterocycles.
2 Results
In the present work, a novel, proficient and green protocol for the synthesis of spirooxindoles has been described via one pot condensation reactions of isatins, malononitrile and α-methylene carbonyl compounds/enols namely 5,5-dimethylcyclohexane-1,3-dione (dimedone), cyclohexane-1,3-dione, 4-hydroxycoumarin, 2,4-dihydro-5-methyl-pyrazol-3-one in presence of starch solution at 60 °C.
In order to optimise the reaction conditions isatin, malononitrile and cyclohexane-1,3-dione were selected as model substrates. Various reactions of isatin (1.0 mmol) with malononitrile (1.5 mmol) and cyclohexane-1,3-dione (1.0 mmol) were attempted in presence of varying amount of starch solution which serve as dual role of catalyst as well as reaction media at different temperature. The impact of different amounts of catalyst load as well as reaction temperature on the yield of desired product i.e. 2-amino-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carbonitrile (Ia) and reaction time is shown in Table 1. The best result was obtained using 5.0 mL starch solution at 60 °C. The reaction was complete in 10 min. and gave 93% of Ia.
Using these optimised reaction conditions, reactions of isatin, malononitrile with 3,3-dimethylcyclohexane-1,3-dione/4-hydroxycoumarin/2,4-dihydro-5-methyl-pyrazol-3-one were also performed. All the reactions underwent completion in 10–15 min and afforded the corresponding spirooxindoles (Ib-d).
The scope of the above condensation reaction was also examined by using 5-bromoisatin for reactions with malononitrile and 1,3-dicarbonyl compounds (dimedone, cyclohexane-1,3-dione) under otherwise identical conditions and 2-amino-5′-bromo-7,7-dimethyl-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carbonitrile (Ie), 2-amino-5′-bromo-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carbonitrile (If) were obtained respectively. All these results have been shown in Table 2 and represented in Eq. 1.

3 Discussion
The proposed mechanism for the synthesis of spirooxindoles is shown in Fig. 1. The starch is expected to form micelle-like structure, which is capable of holding the molecules and thereby catalysing the reaction. Initially, Knoevenagel condensation of isatin with malononitrile takes place to give an intermediate. The intermediate so formed underwent Michael addition with α-methylene carbonyl compounds/enol to furnish an intermediate. The hydroxyl group of this intermediate underwent cycloaddition to cyano group to yield I.
4 Materials and Methods
All the melting points were measured using Buchi melting point 545 apparatus and are uncorrected. IR spectra were recorded on a Perkin Elmer FTIR spectrophotometer using KBr pellets. The 1H NMR spectra were recorded on Jeol JNM ECX-400P (at 400 MHz) with DMSO-d6 as solvent and using TMS as internal reference. Thin Layer Chromatography (TLC) was performed on precoated silica gel plates (Merck).
General procedure for preparation of starch solution: The starch solution employed for carrying out synthesis of spirooxindoles was prepared by stirring a mixture of solid starch (1.5 g) in water (15.0 mL) at 25 °C for 30 min. After 30 min. the solution was filtered and the filtrate was used for synthesis of I.
Procedure for synthesis of spirooxindoles: To a 50 mL round-bottomed flask isatins (1.0 mmol), malononitrile (1.5 mmol), α-methylene carbonyl compounds/enols (1.0 mmol) and starch solution (5.0 mL) were added. The contents were stirred vigorously at 60 °C for the appropriate times as mentioned in Table 2. After completion of reaction (monitored by TLC), the content was cooled to room temperature. The precipitate so obtained was filtered, washed with water and subsequently with ethanol. All the synthesised products were known compounds and were characterized by FT-IR, 1H NMR and comparison of their melting points with known compounds [21,22,23,24].
-
Spectral Data of Representative Spirooxindoles:
2-Amino-7,7-dimethyl-2’,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carbonitrile (Ib): White solid; Yield = 94%; M.pt.: 300 °C IR (KBr) υmax-/cm−1 = 3377, 3314, 3145, 2960, 2192, 1722, 1682, 1656, 1471, 1348, 1327, 1223; 1H NMR (400 MHz, DMSO-d6) δ: 10.32 (1H, s, NH), 7.15 (2H, s, NH2), 7.09-7.04 (1H, m, Ar-H), 6.91-6.80 (2H, m, Ar-H), 6.73-6.71 (1H, m, Ar-H), 2.44-2.43 (2H, m, CH2), 2.10 and 2.08 (AB system, 2H, J = 16 Hz, CHaHbC(CH3)2), 0.96 (3H, s, CCH3), 0.93 (3H, s, CCH3).
2′-Amino-2,5′-dioxo-5′H-spiro[indoline-3,4′-pyrano[3,2-c]chromene]-3′-carbonitrile (Ic): White solid; Yield = 95%; M.pt.: 294–296 °C; IR (KBr) υmax-/cm−1 = 3350, 3297, 3196, 2955, 2206, 1736, 1673, 1604, 1541, 1471, 1359, 1219, 1169; 1H NMR (400 MHz, DMSO-d6) δ: 10.69 (1H, s, NH), 7.94 (1H, d, Ar-H, J = 7.8 Hz), 7.77 (1H, t, Ar-H, J = 7.7 Hz), 7.66 (2H, s, NH2), 7.56 (1H, t, Ar- H, J = 7.6 Hz), 7.49 (1H, d, Ar-H, J = 8.4 Hz), 7.22 (2H, t, Ar-H, J = 7.6 Hz), 6.93 (1H, t, Ar-H, J = 7.6 Hz), 6.86 (1H, d, Ar-H, J = 7.9 Hz).
5 Conclusion
In this work, we report a rapid and green synthesis of spirooxindoles via multicomponent approach in the presence of starch solution. The benefits of this novel environmentally benign protocol are excellent yields, short reaction time, ease of product isolation and purification, use of environment-friendly catalyst.
References
Girgis AS (2009) Regioselective synthesis of dispiro[1H-indene-2,3′-pyrrolidine-2′,3″-[3H]indole]-1,2″(1″H)-diones of potential anti-tumor properties. Eur J Med Chem 44:91–100. https://doi.org/10.1016/j.ejmech.2008.03.013
Thangamani A (2010) Regiospecific synthesis and biological evaluation of spirooxindolopyrrolizidines via [3 + 2] cycloaddition of azomethine ylide. Eur J Med Chem 45:6120–6126. https://doi.org/10.1016/j.ejmech.2010.09.051
Bhaskar G, Arun Y, Balachandran C, Saikumar C, Perumal PT (2012) Synthesis of novel spirooxindole derivatives by one pot multicomponent reaction and their antimicrobial activity. Eur J Med Chem 51:79–91. https://doi.org/10.1016/j.ejmech.2012.02.024
Rouatbi F, Askri M, Nana F, Kirsch G, Sriram D, Yogeeswari P (2016) Synthesis of new spirooxindole derivatives through 1,3-dipolar cycloaddition of azomethine ylides and their antitubercular activity. Tetrahedron Lett 57:163–167. https://doi.org/10.1016/j.tetlet.2015.11.056
Maheswari SU, Balamurugan K, Perumal S, Yogeeswari P, Sriram D (2010) A facile 1,3-dipolar cycloaddition of azomethine ylides to 2-arylidene-1,3-indanediones: synthesis of dispiro-oxindolylpyrrolothiazoles and their antimycobacterial evaluation. Bioorg Med Chem Lett 20:7278–7282. https://doi.org/10.1016/j.bmcl.2010.10.080
Yu B, Sun X-N, Shi X-J, Qi P-P, Zheng Y-C, Yu D-Q, Liu HM (2015) Efficient synthesis of novel antiproliferative steroidal spirooxindoles via the [3 + 2] cycloaddition reactions of azomethine ylides. Steroids 102:92–100. https://doi.org/10.1016/j.steroids.2015.08.003
Santos MMM (2014) Recent advances in the synthesis of biologically active spirooxindoles. Tetrahedron 70:9735–9757. https://doi.org/10.1016/j.tet.2014.08.005
Xia M, Ma R-Z (2014) Recent progress on routes to spirooxindole systems derived from isatin. J Hetereocyclc Chem 51:539–554. https://doi.org/10.1002/jhet.1114
Ziarani GM, Moradi R, Lashgari N (2016) Synthesis of spiro-fused heterocyclic scaffolds through multicomponent reactions involving isatin. ARKIVOC (i):1–81. https://doi.org/10.3998/ark.5550190.p009.385
Ugi I (2001) Recent progress in the chemistry of multicomponent reactions. Pure Appl Chem 73:187–191. https://doi.org/10.1351/pac200173010187s
Tietze LF, Bsasche C, Gericke KM (2006) Domino reactions in organic synthesis. Wiley, New York (2006)
Tietze LF (1996) Domino reactions in organic synthesis. Chem Rev 96:115–136. https://doi.org/10.1021/cr950027e
Ramon DJ, Yus M (2005) Asymmetric multicomponent reactions (AMCRs): the new frontier. Angew Chem Int Ed 44:1602–1634. https://doi.org/10.1002/anie.200460548
Khurana JM, Chaudhary A, Lumb A, Nand B (2012) An expedient four-component domino protocol for the synthesis of novel benzo[a]phenazine annulated heterocycles and their photophysical studies. Green Chem 14:2321. https://doi.org/10.1039/C2GC35644A
Khurana JM, Chaudhary A, Nand B, Lumb A (2012) Aqua mediated indium(III) chloride catalyzed synthesis of fused pyrimidines and pyrazoles. Tetrahedron Lett 53:3018–3022. https://doi.org/10.1016/j.tetlet.2012.04.001
Saluja P, Chaudhary A, Khurana JM (2014) Synthesis of novel fluorescent benzo[a]pyrano[2,3-c]phenazine and benzo[a]chromeno[2,3-c]phenazine derivatives via facile four-component domino protocol. Tetrahedron Lett 55:3431–3335. https://doi.org/10.1016/j.tetlet.2014.04.072
Khanna G, Chaudhary A, Khurana JM (2014) An efficient catalyst-free synthesis of novel benzo[a][1, 3]oxazino[6,5-c]phenazine derivatives via one pot four-component domino protocol in water. Tetrahedron Lett 55:6652–6654. https://doi.org/10.1016/j.tetlet.2014.10.067
Khurana JM, Lumb A, Chaudhary A, Nand B (2013) Synthesis and in vitro evaluation of antioxidant activity of diverse naphthopyranopyrimidines, diazaanthra[2,3-d][1, 3]dioxole-7,9-dione and tetrahydrobenzo[a]xanthen-11-ones. RSC Adv 3:1844–1854. https://doi.org/10.1039/C2RA22406B
Rajeshwari M, Khanna G, Chaudhary A, Khurana JM (2015) Multicomponent Domino Process for the Synthesis of Some Novel Benzo[a]chromenophenazine Fused Ring Systems Using H2SO4, Phosphotungstic Acid, and [NMP]H2PO4. Synth Commun 45:1426–1432. https://doi.org/10.1080/00397911.2015.1024324
Chaudhary A, Gaba R, Sharma K, Upadhyay A, Mathur P, Sharma N, Mishra A, Kapoor K (2016) Green synthesis of spirooxindoles via multicomponent approach. In: Proceedings of national conference in chemistry (NCC 2016): “environmental and harmonious development”, New Delhi, India, pp 190–192. ISBN: 9789385824012
Khurana JM, Yadav S (2012) Highly monodispersed PEG-stabilized Ni Nanoparticles: proficient catalyst for the synthesis of biologically important spiropyrans. Aust J Chem 65:314–319. https://doi.org/10.1071/CH11444
Dabiri M, Bahramnejad M, Baghbanzadeh M (2009) Ammonium salt catalyzed multicomponent transformation: simple route to functionalized spirochromenes and spiroacridines. Tetrahedron 65:9443–9447. https://doi.org/10.1016/j.tet.2009.08.070
Li Y, Chen H, Shi C, Shi D, Ji S (2010) Efficient one-pot synthesis of spirooxindole derivatives catalyzed by l-proline in aqueous medium. J Comb Chem 12:231–237. https://doi.org/10.1021/cc9001185
Guo RY, An ZM, Mo LP, Yang ST, Liu HX, Wang SX, Zhang ZH (2013) Meglumine promoted one-pot, four-component synthesis of pyranopyrazole derivatives. Tetrahedron 69:9931–9938. https://doi.org/10.1016/j.tet.2013.09.082
Acknowledgements
P.S. and G.K. are thankful to UGC and, CSIR, New Delhi respectively, for the grant of junior and senior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Chaudhary, A., Saluja, P., Khanna, G. (2018). Expedient Synthesis of Diverse Spirooxindoles via Multicomponent Approach in Presence of Green Catalyst. In: Parmar, V., Malhotra, P., Mathur, D. (eds) Green Chemistry in Environmental Sustainability and Chemical Education. Springer, Singapore. https://doi.org/10.1007/978-981-10-8390-7_2
Download citation
DOI: https://doi.org/10.1007/978-981-10-8390-7_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-8389-1
Online ISBN: 978-981-10-8390-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)