Abstract
An efficient and facile green synthesis of spirooxindole derivatives bearing pyrano[2,3-c]pyrazole moiety has been achieved via a \(\mathrm{CeO}_{2}\)-NPs catalyzed four-component reaction in water. The protocol offers an environmentally benign and effective approach to highly functionalized and biologically interesting spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole] derivatives. The synthesized compounds exhibit potent antioxidant and antibacterial activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The spirooxindole framework is an important structural motif found in many natural products and bioactive compounds [1–5]. These spirooxindole-based molecules have shown to possess varieties of important biological activities, such as antimicrobial [6–8], anti-inflammatory [9], antimalarial [10], antimycobacterial [11], antitubercular [12], antitumor and anticancer [13, 14], and MDM2 inhibitor activity [15–17]. In addition, they are widely used as building blocks for the synthesis of bioactive natural products [18–20].
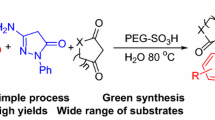
Due to their prominent biological and pharmacological activities, we became interested in the synthesis of a variety of spirooxindole derivatives using multicomponent reactions. Recently, we have developed simple and facile synthetic methods for the preparation of spirooxindole derivatives bearing hexahydroquinolines [21], dihydroquinazolinones [22], and 4-chromenes [23]. As a part of ongoing study on other spirooxindole derivatives, herein we examined four-component reactions of \(\beta \)-ketoesters, isatins, phenylhydrazines, and malononitrile to afford spirooxindole derivatives bearing pyrano[2,3-c]pyrazoles. It should be noted that a number of synthetic approaches using the three-component reaction of \(\beta \)-ketoesters with 3-methyl-2-pyrazolin-5-ones and malononitrile has been reported using \(\mathrm{InCl}_{3}\) [24], ZnS [25], \([\hbox {Ch}\hbox {-}\hbox {OSO}_{3}\hbox {H}]_{3}\hbox {W}_{12}\mathrm{PO}_{40}\) [26], CAN/sonication [13], NaCl/sonication [27], \(\hbox {I}_{2}\) [28], L-proline [29–35], 4-DMAP [36], and electrolysis [37] (Scheme 1). Also, several synthetic approaches to spirooxindole derivatives bearing pyrano[2,3-c]pyrazoles based on four-component reactions of \(\beta \)-ketoesters, isatins, phenylhydrazines, and malononitrile have been reported using \(\beta \)-cyclodextrin [38], chitosan/ionic liquid [39], piperidine [40, 41], L-proline [42], and \(\mathrm{ZrO}_{2}\) [43] conditions. Still, there is a demand for more efficient and environmentally benign synthetic approaches to spirooxindole derivatives bearing pyrano[2,3-c]pyrazoles.
Green and sustainable chemical processes with reduction or even elimination of the use and production of hazardous materials are in high demand. Consequently, the use of non-toxic catalysts and pollution abatement solvents has become a prime choice for the researchers in both academia and industry. Recently, cerium oxide nanoparticles have emerged as environmentally benign and economical heterogeneous catalysts [44–49]. They have exhibited various advantages, such as sustainability in water, low corrosiveness and toxicity, high catalytic reactivity, recoverability and reusability, and ease of handling [50, 51]. Because of these advantages, \(\mathrm{CeO}_{2}\)-NPs, including core-metal/shell \(\mathrm{CeO}_{2}\) nanoparticles, have been extensively used as efficient and useful catalysts in various organic transformations [52–57]. Moreover, commercially available \(\mathrm{CeO}_{2}\)-NPs are also being used in fluorescent applications [58], fuel cells [59], sunscreens [60], as antioxidants in cell model culture [61] and as gas sensors [62]. To the best of our knowledge, \(\mathrm{CeO}_{2}\) nanoparticle-catalyzed reactions of \(\beta \)-ketoesters with phenylhydrazines, malononitrile, and isatins for the construction of spirooxindoles have not been reported so far.
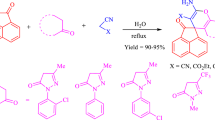
Herein, we describe a one-pot synthesis of biologically interesting spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole] derivatives using \(\mathrm{CeO}_{2}\) nanoparticle-catalyzed four-component reaction of \(\beta \)-ketoesters, phenylhydrazines, malononitrile, and isatins in water (Scheme 2). In addition, we report on the antibacterial and antioxidant activities of the synthesized spirooxindole derivatives.
Results and discussion
The four-component reaction of methyl acetoacetate (1a), phenylhydrazine (2a), malononitrile (3), and isatin (4a) was first examined in the presence of several catalysts and solvents (Table 1). Initially, reaction in the absence of catalyst in water at \(90\,^{\circ }\mathrm{C}\) afforded the product 5a in only 19 % yield (entry 1). Adding 30 mol% of ceric ammonium nitrate (CAN) and \(\mathrm{CeCl}_{3}\) at \(90\,^{\circ }\mathrm{C}\) allowed to increase the yield of 5a to 47 and 35 %, respectively (entries 2 and 3). Further reactions were attempted with \(\mathrm{CeO}_{2}\)-NPs in several solvents. The best yield (93 %) was obtained in the presence of 30 mol% of \(\mathrm{CeO}_{2}\)-NPs in water at \(90\,^{\circ }\mathrm{C}\) (entry 5). Moreover, in polar solvents, such as ethanol and acetonitrile, 5a was produced in a 65 and 81 % yield, respectively (entries 6 and 7) and in a non-polar solvent toluene, 5a was obtained only in trace amounts (entry 8). The decrease or increase in loading of the catalyst (\(\mathrm{CeO}_{2}\)-NPs) did not improve the yield of 5a (entries 9, 10, and 11). Using 20 mol % of Lewis acids such as \(\hbox {FeCl}_{3}\), \(\hbox {In}(\hbox {OTf})_{3}\) and \(\hbox {Cu}(\hbox {OTf})_{2}\) also gave the desired product in diminished yields (entries 12, 13, and 14). The identity of 5a was confirmed by analysis of its spectroscopic data in comparison to reported values [25]. The \({}^{1}\mathrm{H}\) NMR of 5a shows a methyl peak (\(\delta = 1.54\hbox { ppm}\), singlet) and an amide proton (\(\delta = 10.74\hbox { ppm}\), singlet). The \({}^{13}\hbox {C}\) NMR exhibits a characteristic quaternary carbon peak at 47.7 ppm and an amide carbon peak at 177.4 ppm.

Under the optimized reaction condition, the generality of this multicomponent reaction was further explored by employing various \(\beta \)-ketoesters 1a–1d, phenylhydrazines 2a–2e, and isatins 4a–4k (Table 2). Reactions of methyl 3-oxobutanoate (1a) with 2a, 3, and isatin 4b or 4c bearing electron-donating groups provided products 5b and 5c in an 84 and 86 % yield, respectively, whereas those with isatin 4d, 4e, or 4f bearing electron-withdrawing groups afforded the desired products 5d–5f, in a 91, 90, and an 87 % yield, respectively. Reactions of methyl and acetyl substituted isatins 4g and 4h provided products 5g–5h in an 80 and 89 % yield, respectively. With other \(\beta \)-ketoesters of methyl 3-oxohexanoate (1b) or methyl 4-methyl-3-oxopentanoate (1c), the desired products 5i–5q were produced in 86–94 % yields. Next, treatment of methyl 3-oxo-3-phenylpropanoate (1d) with phenylhydrazine (2a), malononitrile (3), and isatins 4a, 4g, or 4h afforded the desired products 5r–5t in 75–84 % yields.
In addition, further reactions of substituted phenylhydrazines 2b–2e bearing electron-donating or -withdrawing substituents on various positions of benzene ring were successful. For example, treatment of 1a with 4-methyl phenylhydrazine (2b), 3, and 4a provided the desired product 5u in an 84 % yield, whereas that of 1c with 2-ethyl phenylhydrazine (2c), 3, and 4h afforded the product 5v in a 79 % yield. Moreover, treatment of 1c or 1a with 4-chloro phenylhydrazine (2d) or 2-chloro phenylhydrazine (2e), 3, and 4a gave products 5w and 5x in 73 and 74 % yields. However, when malononitrile was replaced by cyanoesters like methyl cyanoacetate or ethyl cyanoacetate, no desired products were obtained, instead intractable mixtures were produced. Our procedure provides a rapid synthetic route to a variety of highly functionalized spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole] derivatives in good yield. Moreover, most of the synthesized compounds of 5f, 5i, 5k–5q, and 5t–5w are novel and reported for the first time.
The formation of 5a can be explained by the mechanism as shown in Scheme 3 [40, 41]. In the presence of \(\mathrm{CeO}_{2}\)-NPs, the intermediate 7 is first formed by condensation of methyl acetoacetate (1a) with phenylhydrazine (2a). The Knoevenagel condensation of 3a, derived from 3 and 4a, provides the intermediate 8, which is reacted with 7a to furnish 9 through Michael reaction. Tautomerism of 9 followed by intramolecular cyclization gives intermediate 10, which undergoes further isomerization to furnish the final product 5a.
In vitro antioxidant activity
Furthermore, the synthesized spirooxindoles were screened for their antioxidant activity by using ferric reducing/antioxidant tests [63]. The FRAP assay measures the ability of a compound to reduce the ferric 2,4,6-tripyridyl-s-triazine complex to the colored ferrous complex with development of intense blue color at the maximum wavelength of 593 nm. FRAP values were obtained by comparing the absorbance change in test reaction mixtures with those containing ferrous ions of known concentration. The results of the antioxidant test are expressed as Trolox equivalent antioxidant capacity (TEAC) values as shown in Fig. 1. A higher value of TEAC suggests a higher antioxidant capacity. The majority of the tested compounds in the series revealed moderate interactions with the FRAP reagent. Compounds 5f, 5h, 5k, and 5t exhibited superior activity as compared to other synthesized compounds.
The antioxidant activity of organic compounds seems to be related to the presence of hydroxyl groups, double bond conjugation, and resonance effects [64]. The synthesized compounds containing both electron-withdrawing and -donating groups showed moderate activity. Compounds containing electron-withdrawing or -donating group on ortho or para positions to the spirooxindolic NH or acetyl group on spirooxindolic nitrogen atom showed higher activity than other compounds. In FRAP tests, the antioxidant activities of the compounds are well correlated with their \(\mathrm{EC}_{50}\) (half maximal effective concentration) values, as shown in Fig. 2.
Antibacterial activity
The antibacterial activity of the synthesized spirooxindoles was tested against two gram-negative Escherichia coli (KCTC-1924) and Pseudomonas aeruginosa (KCTC-2004) and two gram-positive Staphylococcus aureus (KCTC-1916) and Bacillus cereus (KCTC-1012) bacteria, respectively, by using a modified Kirby-Bauer disk diffusion method [65]. The inhibition zone against the growth of the verified bacteria for the compounds is reported in Table 3. Aliquots of bacterial suspension (\(100\,\upmu \hbox {L}\)) were spread on \(\hbox {Difco}^{\mathrm{TM}}\) nutrient broth containing the test microorganism with an optical density of 0.7 at 595 nm. From the results, synthesized compounds showed antibacterial activity toward the investigated bacterial strains. In particular, compounds 5m and 5t exhibited excellent activity toward the gram-negative bacteria compared to standard ciprofloxacin. Compounds 5m, 5t, and 5v displayed moderate levels of antimicrobial activity toward the gram- positive bacteria. Compounds containing electron-withdrawing group on spirooxindolic NH or its para position showed higher activity than other compounds.
Conclusions
A green and efficient protocol for the construction of spirooxindole derivatives bearing pyrano[2,3-c]pyrazole] by a multicomponent coupling of \(\beta \)-ketoesters, phenylhydrazines, malononitrile, and isatins was developed. This method offers several advantages such as mild reaction conditions, ease of handling, high yields, and the use of an effective green catalyst. The synthesized spirooxindole derivatives show potent antioxidant and antibacterial activities.
Experimental
All experiments were conducted under a nitrogen atmosphere. Merck precoated silica gel plates (Art. 5554) with a fluorescent indicator were used for analytical TLC. Flash column chromatography was performed using silica gel 9385 (Merck). The melting points were determined using micro-cover glasses on a Fisher-Johns apparatus and were uncorrected. The \({}^{1}\hbox {H}\) NMR spectra were recorded on a Varian-VNS (300 MHz), DPX (300 MHz), and VNS (600 MHz) spectrometer in DMSO-\(d_{6}\) setting the solvent chemical shift at 2.50 ppm. The \({}^{13}\hbox {C}\) NMR spectra were recorded on a Varian-VNS (75 MHz), DPX (75 MHz), and VNS (150 MHz) spectrometer in DMSO-\(d_{6}\) setting the solvent chemical shift at 39.5 ppm. Chemical shifts (\(\delta \)) are expressed in units of ppm and J values are given in Hz. Multiplicities are abbreviated as follows: \(\hbox {s} = \hbox {singlet}\), \(\hbox {d} = \hbox {doublet}\), \(\hbox {t} = \hbox {triplet}\), \(\hbox {q} = \hbox {quartet}\), \(\hbox {br s} = \hbox {broad}\) singlet, \(\hbox {dd} = \hbox {doublet}\) of doublets, \(\hbox {tt} = \hbox {triplet}\) of triplet, and \(\hbox {m} = \hbox {multiplet}\). The IR spectra were recorded on PerkinElmer FT-IR spectrometer Spectrum \(\hbox {Two}^{\mathrm{TM}}\). High-resolution mass spectrometry (HRMS) was obtained with a JEOL JMS-700 spectrometer (EI) at the Korea Basic Science Institute.
General procedure for the synthesis of spirooxindole derivatives (5a–5x)
A mixture of \(\beta \)-ketoester 1 (1 mmol), phenylhydrazine 2 (1 mmol), malononitrile 3 (1 mmol), isatin 4 (1 mmol), and \(\mathrm{CeO}_{2}\)-NPs (30 mol%) in water (5 mL) was stirred at \(90\,^{\circ }\mathrm{C}\) for the time mentioned, until the completion of reaction as indicated by TLC. After completion of the reaction, the reaction mixture was cooled to room temperature. The precipitated product was then filtered and dissolved in EtOAc. The solution was then dried over \(\mathrm{MgSO}_{4}\) and filtered. After evaporating solvent, the residue was recrystallized from EtOAc to provide pure product.
6\(^\prime \)-Amino-3\(^\prime \)-methyl-2-oxo-1\(^\prime \)-phenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5a)
Compound 5a (342 mg, 93 %) was obtained as a white solid: mp 237–\(239\,^{\circ }\mathrm{C}\); \({}^{1}\hbox {H}\) NMR (300 MHz, DMSO-\(d_{6}\)) \(\delta \) 10.74 (1H, s), 7.78 (2H, d, \(J= 7.8\hbox { Hz}\)), 7.56–7.49 (4H, m), 7.37–7.26 (2H, m), 7.17 (1H, d, \(J = 7.2\hbox { Hz}\)), 7.02 (1H, t, \(J = 7.5\hbox { Hz}\)), 6.95 (1H, d, \(J = 7.5\hbox { Hz}\)), 1.54 (3H, s); \({}^{13}\hbox {C}\) NMR (75 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.4, 161.0, 144.9, 143.8, 141.6, 137.2, 132.1, 129.4, 129.2, 126.5, 124.8, 122.7, 120.1, 117.8, 109.8, 96.3, 56.2, 47.7, 11.6; IR (ATR) 3451, 3254, 3080, 2247, 1891, 1739, 1562, 1428, 1348, 1236, 1099, 988, \(931\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\hbox {C}_{21}\hbox {H}_{15}\hbox {N}_{5}\hbox {O}_{2}\): 369.1226. Found: 369.1229.
6\(^\prime \)-Amino-3\(^\prime \),5-dimethyl-2-oxo-1\(^\prime \)-phenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5b)
Compound 5b (320 mg, 84 %) was obtained as a white solid: mp 288–\(289\,^{\circ }\mathrm{C}\); \({}^{1}\hbox {H}\) NMR (300 MHz, DMSO-\(d_{6}\)) \(\delta \) 10.63 (1H, s), 7.79 (2H, d, \(J = 8.1\hbox { Hz}\)), 7.52 (4H, t, \(J= 8.1\hbox { Hz}\)), 7.35 (1H, t, \(J= 7.5\hbox { Hz}\)), 7.08 (1H, d, \(J= 7.5\hbox { Hz}\)), 7.0 (1H, s), 6.83 (1H, d, \(J= 7.8\hbox { Hz}\)), 2.23 (3H, s), 1.56 (3H, s); \({}^{13}\hbox {C}\) NMR (75 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.4, 161.0, 144.9, 144.0, 139.1, 137.3, 132.3, 131.6, 129.5, 129.4, 126.5, 125.3, 120.1, 118.0, 109.6, 96.5, 56.3, 47.8, 20.6, 11.7; IR (ATR) 3420, 3254, 3089, 2246, 1875, 1732, 1673, 1554, 1427, 1347, 1237, 1158, \(989\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\hbox {C}_{22}\hbox {H}_{17}\hbox {N}_{5}\hbox {O}_{2}\): 383.1382. Found: 383.1384.
6\(^\prime \)-Amino-5-methoxy-3\(^\prime \)-methyl-2-oxo-1\(^\prime \)-phenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5c)
Compound 5c (342 mg, 86 %) was obtained as a white solid: mp 213–\(215\,^{\circ }\mathrm{C}\); \({}^{1}\hbox {H}\) NMR (300 MHz, DMSO-\(d_{6}\)) \(\delta \) 10.54 (1H, s), 7.79 (2H, d, \(J= 8.1\hbox { Hz}\)), 7.54–7.49 (4H, m), 7.34 (1H, t, \(J = 7.8\hbox { Hz}\)), 6.8 (3H, d, \(J= 8.1\hbox { Hz}\)), 3.68 (3H, s), 1.56 (3H, s); \({}^{13}\hbox {C}\) NMR (75 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.5, 161.0, 155.7, 145.0, 144.0, 137.3, 134.8, 133.4, 129.5, 126.5, 120.1, 118.0, 114.4, 111.3, 110.4, 96.4, 56.3, 55.5, 48.3, 11.7; IR (ATR) 3318, 3184, 3062, 2203, 1696, 1486, 1392, 1293, 1199, 1029, \(962\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\hbox {C}_{22}\hbox {H}_{17}\hbox {N}_{5}\hbox {O}_{3}\): 399.1331. Found: 399.1328.
6\(^\prime \)-Amino-4-bromo-3\(^\prime \)-methyl-2-oxo-1\(^\prime \)-phenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5d)
Compound 5d (405 mg, 91 %) was obtained as a white solid: mp 268–\(270\,^{\circ }\mathrm{C}\); \({}^{1}\hbox {H}\) NMR (600 MHz, DMSO-\(d_{6}\)) \(\delta \) 11.02 (1H, s), 7.78 (2H, d, \(J = 9.0\hbox { Hz}\)), 7.65 (2H, s), 7.51 (2H, t, \(J= 7.8\hbox { Hz}\)), 7.35 (1H, t, \(J= 7.2\hbox { Hz}\)), 7.25 (1H, t, \(J= 7.8\hbox { Hz}\)), 7.18 (1H, d, \(J= 7.8\hbox { Hz}\)), 6.98 (1H, d, \(J= 7.8\hbox { Hz}\)), 1.60 (3H, s); \({}^{13}\hbox {C}\) NMR (150 MHz, DMSO-\(d_{6}\)) \(\delta \) 176.7, 161.7, 145.5, 143.7, 143.6, 137.2, 131.3, 129.5, 128.6, 126.7, 126.1, 120.1, 119.5, 117.7, 109.5, 94.1, 53.9, 49.4, \(11.7\hbox { cm}^{-1}\); IR (ATR) 3320, 3193, 2198, 1723, 1655, 1583, 1527, 1447, 1394, 1221, 1127, \(1037\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\hbox {C}_{21}\mathrm{H}_{14}\hbox {BrN}_{5}\hbox {O}_{2}\): 447.0331. Found: 447.0328.
6\(^\prime \)-Amino-5-chloro-3\(^\prime \)-methyl-2-oxo-1\(^\prime \)-phenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5e)
Compound 5e (362 mg, 90 %) was obtained as a white solid: mp 229–\(231\,^{\circ }\mathrm{C}\); \({}^{1}\hbox {H}\) NMR (300 MHz, DMSO-\(d_{6}\)) \(\delta \) 10.89 (1H, s), 7.79 (2H, d, \(J= 7.2\hbox { Hz}\)), 7.63 (2H, s), 7.53–7.49 (2H, m), 7.35–7.32 (3H, m), 6.97 (1H, d, \(J= 7.5\hbox { Hz}\)), 1.59 (3H, s); \({}^{13}\hbox {C}\) NMR (75 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.3, 161.1, 145.0, 143.8, 140.4, 137.2, 134.3, 129.4, 129.2, 126.7, 126.6, 125.2, 120.3, 117.9, 111.3, 95.7, 55.6, 48.0, 11.7; IR (ATR) 3305, 3181, 3015, 2194, 1842, 1725, 1646, 1451, 1382, 1216, 1123, \(1068\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\hbox {C}_{21}\mathrm{H}_{14}\mathrm{ClN}_{5}\mathrm{O}_{2}\): 403.0836. Found: 403.0835.
6\(^\prime \)-Amino-7-chloro-3\(^\prime \)-methyl-2-oxo-1\(^\prime \)-phenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5f)
Compound 5f (350 mg, 87 %) was obtained as a white solid: mp 235–\(237\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (300 MHz, DMSO-\(d_{6}\)) \(\delta \) 11.19 (1H, s), 7.78 (2H, d, \(J = 7.5\hbox { Hz}\)), 7.63 (2H, s), 7.52 (2H, t, \(J = 7.5\hbox { Hz}\)), 7.35 (2H, t, \(J = 7.5\hbox { Hz}\)), 7.18 (1H, d, \(J= 7.2\hbox { Hz}\)), 7.06 (1H, t, \(J= 7.8\hbox { Hz}\)), 1.57 (3H, s); \({}^{13}\hbox {C}\) NMR (75 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.5, 161.0, 144.9, 143.7, 139.3, 137.1, 133.9, 129.4, 129.2, 126.6, 123.9, 123.6, 120.2, 117.7, 114.1, 95.8, 55.7, 48.6, 11.7; IR (ATR) 3439, 3253, 3093, 2248, 1969, 1737, 1600, 1430, 1352, 1236, 1160, \(992\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\hbox {C}_{21}\mathrm{H}_{14}\mathrm{ClN}_{5}\mathrm{O}_{2}\): 403.0836. Found: 403.0837.
6\(^\prime \)-Amino-1,3\(^\prime \)-dimethyl-2-oxo-1\(^\prime \)-phenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5g)
Compound 5g (306 mg, 80 %) was obtained as a white solid: mp 212–\(214\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (600 MHz, DMSO-\(d_{6}\)) \(\delta \) 7.78 (2H, d, \(J= 8.4\hbox { Hz}\)), 7.60 (2H, s), 7.51 (2H, t, \(J= 8.4\hbox { Hz}\)), 7.39 (1H, t, \(J = 7.2\hbox { Hz}\)), 7.35 (1H, t, \(J= 7.2\hbox { Hz}\)), 7.23 (1H, d, \(J = 7.8\hbox { Hz}\)), 7.15 (1H, d, \(J= 7.8\hbox { Hz}\)), 7.11 (1H, t, \(J= 7.2\hbox { Hz}\)), 3.24 (3H, s), 1.45 (3H, s); \({}^{13}\hbox {C}\) NMR (150 MHz, DMSO-\(d_{6}\)) \(\delta \) 175.8, 161.2, 144.9, 143.8, 143.0, 137.2, 131.4, 129.5, 126.6, 124.6, 123.4, 120.2, 117.8, 108.9, 96.2, 55.8, 47.4, 26.5, 11.7; IR (ATR) 3445, 3236, 3095, 2229, 1780, 1541, 1419, 1331, 1214, \(952\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\hbox {C}_{22}\mathrm{H}_{17}\hbox {N}_{5}\mathrm{O}_{2}\): 383.1382. Found: 383.1381.
1-Acetyl-6\(^\prime \)-amino-3\(^\prime \)-methyl-2-oxo-1\(^\prime \)-phenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5h)
Compound 5h (364 mg, 89 %) was obtained as a white solid: mp 224–\(226\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (300 MHz, DMSO-\(d_{6}\)) \(\delta \) 8.20 (1H, d, \(J = 8.1\hbox { Hz}\)), 7.8 (4H, d, \(J= 8.1\hbox { Hz}\)), 7.55–7.43 (3H, m), 7.39–7.29 (3H, m), 2.64 (3H, s), 1.51 (3H, s); \({}^{13}\hbox {C}\) NMR (75 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.2, 170.6, 161.2, 144.9, 144.0, 139.3, 137.1, 130.4, 129.7, 129.5, 126.8, 126.2, 125.1, 120.4, 117.6, 115.8, 95.9, 56.0, 48.5, 26.3, 11.9; IR (ATR) 3379, 3319, 3192, 2924, 2202, 1723, 1651, 1517, 1393, 1257, 1162, 1031, \(908\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\hbox {C}_{23}\mathrm{H}_{17}\hbox {N}_{5}\mathrm{O}_{3}\): 411.1331. Found: 411.1335.
1-Acetyl-6\(^\prime \)-amino-2-oxo-1\(^\prime \)-phenyl-3\(^\prime \)-propyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5i)
Compound 5i (394 mg, 90 %) was obtained as a white solid: mp 224–\(226\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (300 MHz, DMSO-\(d_{6}\)) \(\delta \) 8.20 (1H, d, \(J= 8.1\hbox { Hz}\)), 7.83–7.81 (4H, m), 7.56–7.44 (3H, m), 7.41–7.29 (3H, m), 2.64 (3H, s), 1.80 (2H, t, \(J= 7.5\hbox { Hz}\)), 1.21–1.02 (2H, m), 0.58 (3H, t, \(J= 7.5\hbox { Hz}\)); \({}^{13}\hbox {C}\) NMR (75 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.6, 170.4, 161.0, 147.8, 144.8, 139.1, 137.1, 130.8, 129.7, 129.4, 126.8, 126.2, 125.2, 120.4, 117.6, 115.8, 95.5, 56.2, 48.6, 28.5, 26.2, 20.8, 13.6; IR (ATR) 3313, 3198, 2947, 2204, 1757, 1648, 1458, 1394, 1262, 1153, 1071, \(907\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\hbox {C}_{25}\mathrm{H}_{21}\hbox {N}_{5}\mathrm{O}_{3}\): 439.1644. Found: 439.1641.
6\(^\prime \)-Amino-3\(^\prime \)-isopropyl-2-oxo-1\(^\prime \)-phenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5j)
Compound 5j (372 mg, 94 %) was obtained as a white solid: mp 217–\(219\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (600 MHz, DMSO-\(d_{6}\)) \(\delta \) 10.75 (1H, s), 7.80 (2H, d, \(J= 7.8\hbox { Hz}\)), 7.53–7.51 (4H, m), 7.35 (1H, t, \(J= 6.6\hbox { Hz}\)), 7.27 (1H, t, \(J= 7.2\hbox { Hz}\)), 7.19 (1H, d, \(J= 7.2\hbox { Hz}\)), 7.02 (1H, t, \(J = 7.2\hbox { Hz}\)), 6.94 (1H, d, \(J= 7.8\hbox { Hz}\)), 2.10-2.05 (1H, m), 1.01 (3H, d, \(J = 6.6\hbox { Hz}\)), 0.69 (3H, d, \(J= 6.6\hbox { Hz}\)); \({}^{13}\hbox {C}\) NMR (150 MHz, DMSO-\(d_{6}\)) \(\delta \) 178.0, 160.7, 153.1, 144.7, 141.5, 137.3, 132.8, 129.4, 129.3, 126.5, 125.0, 122.6, 120.2, 117.9, 109.9, 95.0, 56.7, 47.8, 26.4, 21.4, 21.0; IR (ATR) 3412, 3254, 3091, 2248, 1891, 1675, 1619, 1425, 1345, 1437, 1160, 1096, \(989\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\mathrm{C}_{23}\mathrm{H}_{19}\mathrm{N}_{5}\mathrm{O}_{2}\): 397.1539. Found: 397.1540.
6\(^\prime \)-Amino-3\(^\prime \)-isopropyl-5-methoxy-2-oxo-1\(^\prime \)-phenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5k)
Compound 5k (388 mg, 91 %) was obtained as a white solid: mp 221–\(223\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (300 MHz, DMSO-\(d_{6}\)) \(\delta \) 10.57 (1H, s), 7.81 (2H, d, \(J= 8.1\hbox { Hz}\)), 7.54–7.52 (4H, m), 7.34 (1H, t, \(J= 7.2\hbox { Hz}\)), 6.85 (3H, s), 3.67 (3H, s), 2.16–2.07 (1H, m), 1.01 (3H, d, \(J= 6.9\)), 0.74 (3H, d, \(J= 6.6\)); \({}^{13}\hbox {C}\) NMR (75 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.9, 160.7, 155.7, 153.2, 144.7, 137.4, 134.7, 134.1, 129.4, 126.5, 120.2, 118.0, 114.5, 111.4, 110.4, 95.1, 56.8, 55.6, 48.4, 26.4, 21.5, 21.1; IR (ATR) 3313, 3188, 2964, 2193, 1839, 1704, 1645, 1475, 1390, 1199, 1030, \(760\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\mathrm{C}_{24}\mathrm{H}_{21}\mathrm{N}_{5}\mathrm{O}_{3}\): 427.1644. Found: 427.1642.
6\(^\prime \)-Amino-5-bromo-3\(^\prime \)-isopropyl-2-oxo-1\(^\prime \)-phenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5l)
Compound 5l (436 mg, 92 %) was obtained as a white solid: mp 200–\(202\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (300 MHz, DMSO-\(d_{6}\)) \(\delta \) 10.90 (1H, s), 7.80 (2H, d, \(J= 7.5\hbox { Hz}\)), 7.57–7.45 (6H, m), 7.36 (1H, t, \(J= 7.5\hbox { Hz}\)), 6.90 (1H, d, \(J= 8.1\hbox { Hz}\)), 2.13–2.06 (1H, m), 1.02 (3H, d, \(J= 6.9\hbox { Hz}\)), 0.74 (3H, d, \(J= 6.9\hbox { Hz}\)); \({}^{13}\hbox {C}\) NMR (75 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.6, 160.8, 152.9, 144.8, 140.7, 137.3, 135.4, 132.1, 129.4, 128.0, 126.6, 120.4, 117.8, 114.3, 111.9, 94.4, 56.0, 48.0, 26.5, 21.4, 21.1; IR (ATR) 3313, 3289, 2972, 2193, 1707, 1640, 1452, 1393, 1215, \(1085\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\mathrm{C}_{23}\mathrm{H}_{18}\hbox {BrN}_{5}\mathrm{O}_{2}\): 475.0644. Found: 475.0641.
6\(^\prime \)-Amino-5-chloro-3\(^\prime \)-isopropyl-2-oxo-1\(^\prime \)-phenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5m)
Compound 5m (396 mg, 92 %) was obtained as a white solid: mp 203–\(205\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (300 MHz, DMSO-\(d_{6}\)) \(\delta \) 10.90 (1H, s), 7.80 (2H, d, \(J= 8.1\hbox { Hz}\)), 7.58–7.50 (4H, m), 7.38–7.32 (3H, m), 6.96 (1H, d, \(J= 8.1\hbox { Hz}\)), 2.14–2.05 (1H, m), 1.02 (3H, d, \(J= 6.9\hbox { Hz}\)), 0.74 (3H, d, \(J= 6.6\hbox { Hz}\)); \({}^{13}\hbox {C}\) NMR (75 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.8, 160.8, 152.9, 144.8, 140.3, 137.3, 135.1, 129.4, 129.3, 126.7, 126.6, 125.3, 120.5, 117.9, 111.4, 94.4, 55.9, 48.1, 26.5, 21.5, 21.1; IR (ATR) 3345, 3225, 3016, 2248, 1751, 1680, 1561, 1423, 1351, 1220, 1095, \(920\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\mathrm{C}_{23}\mathrm{H}_{18}\mathrm{ClN}_{5}\mathrm{O}_{2}\): 431.1149. Found: 431.1153.
6\(^\prime \)-Amino-5-fluoro-3\(^\prime \)-isopropyl-2-oxo-1\(^\prime \)-phenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5n)
Compound 5n (385 mg, 93 %) was obtained as a white solid: mp 226–\(228\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (300 MHz, DMSO-\(d_{6}\)) \(\delta \) 10.79 (1H, s), 7.80 (2H, d, \(J= 7.8\hbox { Hz}\)), 7.57–7.49 (4H, m), 7.35 (1H, t, \(J= 7.2\hbox { Hz}\)), 7.22–7.19 (1H, m), 7.14–7.07 (1H, m), 6.96–6.91 (1H, m), 2.14–2.07 (1H, m), 1.02 (3H, d, \(J= 6.9\hbox { Hz}\)), 0.74 (3H, d, \(J= 6.9\hbox { Hz}\)); \({}^{13}\hbox {C}\) NMR (75 MHz, DMSO-\(d_{6}\)) \(\delta \) 178.0, 160.8, 158.7 (d, \(J= 237.9\hbox { Hz}\)), 153.0, 144.7, 137.5 (d, \(J= 46.1\hbox { Hz}\)), 134.7 (d, \(J= 15.5\hbox { Hz}\)), 129.4, 126.6, 120.3, 117.8, 115.7 (d, \(J= 46.1\hbox { Hz}\)), 112.8 (d, \(J= 48.5\hbox { Hz}\)), 110.9, 94.5, 56.2, 48.4, 26.4, 21.5, 21.0; IR (ATR) 3318, 3191, 2972, 2192, 1762, 1645, 1471, 1391, 1179, 1082, \(757\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\mathrm{C}_{23}\mathrm{H}_{18}\hbox {FN}_{5}\mathrm{O}_{2}\): 415.1445. Found: 415.1443.
6\(^\prime \)-Amino-3\(^\prime \)-isopropyl-1-methyl-2-oxo-1\(^\prime \)-phenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5o)
Compound 5o (353 mg, 86 %) was obtained as a white solid: mp 221–\(223\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (600 MHz, DMSO-\(d_{6}\)) \(\delta \) 7.80 (2H, d, \(J= 7.8\hbox { Hz}\)), 7.56 (2H, s), 7.52 (2H, t, \(J= 7.8\hbox { Hz}\)), 7.40–7.34 (2H, m), 7.26 (1H, d, \(J= 7.8\hbox { Hz}\)), 7.16 (1H, d, \(J= 8.4\hbox { Hz}\)), 7.11 (1H, t, \(J= 7.8\hbox { Hz}\)), 3.33 (3H, s), 1.92–1.87 (1H, m), 0.91 (3H, d, \(J= 7.2\hbox { Hz}\)), 0.69 (3H, d, \(J= 6.6\hbox { Hz}\)); \({}^{13}\hbox {C}\) NMR (150 MHz, DMSO-\(d_{6}\)) \(\delta \) 176.2, 160.8, 152.9, 144.7, 142.9, 137.3, 132.0, 129.4, 124.7, 123.3, 120.4, 120.2, 117.7, 108.9, 108.8, 94.8, 56.2, 47.5, 26.4, 26.4, 21.4, 20.7; IR (ATR) 3306, 3181, 2965, 2201, 1703, 1654, 1457, 1397, 1083, \(937\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\mathrm{C}_{24}\mathrm{H}_{21}\mathrm{N}_{5}\mathrm{O}_{2}\): 411.1695. Found: 411.1697.
1-Acetyl-6\(^\prime \)-amino-3\(^\prime \)-isopropyl-2-oxo-1\(^\prime \)-phenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5p)
Compound 5p (394 mg, 90 %) was obtained as a white solid: mp 221–\(223\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (300 MHz, DMSO-\(d_{6}\)) \(\delta \) 7.80 (2H, d, \(J= 7.8\hbox { Hz}\)), 7.56 (2H, s), 7.52 (2H, t, \(J= 7.8\hbox { Hz}\)), 7.40–7.34 (2H, m), 7.26 (1H, d, \(J= 7.8\hbox { Hz}\)), 7.16 (1H, d, \(J= 8.4\hbox { Hz}\)), 7.11 (1H, t, \(J= 7.8\hbox { Hz}\)), 3.33 (3H, s), 1.92–1.87 (1H, m), 0.91 (3H, d, \(J= 7.2\hbox { Hz}\)), 0.69 (3H, d, \(J= 6.6\hbox { Hz}\)); \({}^{13}\hbox {C}\) NMR (150 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.7, 170.3, 160.7, 152.9, 144.7, 139.0, 137.2, 131.1, 129.7, 129.4, 126.7, 126.1, 125.2, 120.5, 117.4, 115.8, 94.5, 56.5, 48.6, 26.5, 26.1, 21.5, 20.7; IR (ATR) 3422, 3254, 3093, 2247, 1875, 1675, 1610, 1425, 1343, 1243, 1160, 1099, \(989\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\mathrm{C}_{25}\mathrm{H}_{21}\mathrm{N}_{5}\mathrm{O}_{3}\): 439.1644. Found: 439.1641.
6\(^\prime \)-Amino-3\(^\prime \)-isopropyl-2-oxo-1,1\(^\prime \)-diphenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5q)
Compound 5q (435 mg, 92 %) was obtained as a white solid: mp 238–\(240\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (300 MHz, DMSO-\(d_{6}\)) \(\delta \) 7.82 (2H, d, \(J= 7.5\hbox { Hz}\)), 7.66–7.62 (4H, m), 7.55–7.53 (3H, m), 7.40–7.31 (5H, m), 7.18–7.14 (1H, m), 6.8 (1H, d, \(J = 7.8\hbox { Hz}\)), 2.19-2.12 (1H, m), 1.02 (3H, d, \(J= 6.6\hbox { Hz}\)), 0.78 (3H, d, \(J= 6.6\hbox { Hz}\)); \({}^{13}\hbox {C}\) NMR (150 MHz, DMSO-\(d_{6}\)) \(\delta \) 175.8, 160.6, 153.0, 144.8, 142.5, 137.3, 134.0, 131.8, 130.0, 129.5, 129.3, 128.5, 126.6, 126.3, 125.2, 124.0, 120.3, 117.6, 109.2, 94.5, 56.6, 47.7, 26.6, 21.6, 20.7; IR (ATR) 3456, 3278, 3160, 2962, 2191, 1704, 1651, 1595, 1489, 1384, 1211, 1084, \(933\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\mathrm{C}_{29}\mathrm{H}_{23}\mathrm{N}_{5}\mathrm{O}_{2}\): 473.1852. Found: 473.1850.
6\(^\prime \)-Amino-2-oxo-1\(^\prime \),3\(^\prime \)-diphenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5r)
Compound 5r (357 mg, 83 %) was obtained as a white solid: mp 208–\(210\,^{\circ }\mathrm{C}{}^{ 1}; \mathrm{H}\) NMR (600 MHz, DMSO-\(d_{6}\)) \(\delta \) 10.60 (1H, s), 7.90 (2H, d, \(J = 7.8\hbox { Hz}\)), 7.59–7.54 (4H, m), 7.42 (1H, t, \(J= 7.2\hbox { Hz}\)), 7.23–7.19 (3H, m), 7.13 (2H, t, \(J= 7.2\hbox { Hz}\)), 6.97–6.92 (3H, m), 6.79 (1H, d, \(J= 7.8\hbox { Hz}\)); \({}^{13}\hbox {C}\) NMR (75 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.6, 160.3, 147.4, 145.7, 141.7, 137.1, 133.5, 132.0, 129.5, 129.2, 128.3, 127.9, 127.3, 124.8, 122.5, 121.0, 120.8, 117.6, 109.8, 95.6, 57.5, 48.1; IR (ATR) 3408, 3254, 3095, 2246, 1891, 1816, 1730, 1671, 1549, 1422, 1345, 1158, 1099, \(989\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\mathrm{C}_{26}\mathrm{H}_{17}\mathrm{N}_{5}\mathrm{O}_{2}\): 431.1382 . Found: 431.1379.
6\(^\prime \)-Amino-1-methyl-2-oxo-1\(^\prime \),3\(^\prime \)-diphenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5s)
Compound 5s (333 mg, 75 %) was obtained as a white solid: mp 198–\(200\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (600 MHz, DMSO-\(d_{6}\)) \(\delta \) 7.89 (2H, d, \(J= 7.8\hbox { Hz}\)), 7.61 (2H, s), 7.57 (2H, t, \(J = 7.8\hbox { Hz}\)), 7.43–7.41 (1H, m), 7.28 (1H, t, \(J= 7.8\hbox { Hz}\)), 7.23 (2H, t, \(J= 7.8\hbox { Hz}\)), 7.14 (2H, t, \(J= 7.8\hbox { Hz}\)), 7.02 (1H, t, \(J= 7.8\hbox { Hz}\)), 6.93 (1H, d, \(J= 7.8\hbox { Hz}\)), 6.80 (2H, d, \(J = 7.2\hbox { Hz}\)), 2.98 (3H, s); \({}^{13}\)C NMR (150 MHz, DMSO-\(d_{6}\)) \(\delta \) 175.9, 160.5, 147.4, 145.4, 142.8, 137.1, 132.6, 131.8, 129.5, 129.3, 128.3, 127.9, 127.3, 127.2, 124.4, 123.2, 120.9, 117.6, 108.7, 95.8, 56.7, 47.7, 26.2; IR (ATR) 3364, 3185, 3068, 2199, 1863, 1660, 1515, 1382, 1249, \(1079\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\mathrm{C}_{27}\mathrm{H}_{19}\mathrm{N}_{5}\mathrm{O}_{2}\): 445.1539. Found: 445.1542.
1-Acetyl-6\(^\prime \)-amino-2-oxo-1\(^\prime \),3\(^\prime \)-diphenyl-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5t)
Compound 5t (397 mg, 84 %) was obtained as a white solid: mp 204–\(206\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (300 MHz, DMSO-\(d_{6}\)) \(\delta \) 7.97–7.90 (3H, m), 7.81 (2H, s), 7.58 (2H, t, \(J= 7.8\hbox { Hz}\)), 7.46–7.34 (3H, m), 7.27-7.21 (2H, m), 7.11 (2H, t, \(J= 7.8\hbox { Hz}\)), 6.79 (2H, d, \(J= 7.5\hbox { Hz}\)), 2.39 (3H, s); \({}^{13}\hbox {C}\) NMR (75 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.4, 169.9, 160.6, 147.5, 145.3, 139.0, 137.0, 131.8, 131.5, 129.5, 128.6, 128.0, 127.4, 127.3, 126.0, 125.0, 121.0, 117.4, 115.6, 96.0, 56.7, 48.8, 25.7; IR (ATR) 3317, 3197, 2925, 2200, 1717, 1645, 1454, 1385, 1682, 1153, 1027, \(913\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\mathrm{C}_{28}\mathrm{H}_{19}\mathrm{N}_{5}\mathrm{O}_{3}\): 473.1488. Found: 473.1487.
6\(^\prime \)-Amino-3\(^\prime \)-methyl-2-oxo-1\(^\prime \)-(p-tolyl)-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5u)
Compound 5u (321 mg, 84 %) was obtained as a white solid: mp 242–\(244\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (600 MHz, DMSO-\(d_{6}\)) \(\delta \) 10.72 (1H, s), 7.65 (2H, d, \(J = 8.4\hbox { Hz}\)), 7.54 (2H, s), 7.31–7.26 (3H, m), 7.16 (1H, d, \(J= 7.2\hbox { Hz}\)), 7.02 (1H, t, \(J= 7.2\hbox { Hz}\)), 6.94 (1H, d, \(J= 7.8\hbox { Hz}\)), 2.35 (3H, s), 1.53 (3H, s); \({}^{13}\hbox {C}\) NMR (150 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.5, 161.0, 144.7, 143.6, 141.6, 136.0, 134.9, 132.2, 129.9, 129.7, 129.2, 124.8, 122.6, 120.2, 120.1, 118.0, 109.8, 96.1, 56.2, 47.8, 20.5, 11.6; IR (ATR) 3443, 3326, 3155, 2189, 1707, 1471, 1394, 1328, 1224, 1068, 1044, 933, 751, \(615\hbox { cm}^{-1}\); HRMS m / z (\(M^{+}\)) calcd for \(\mathrm{C}_{22}\mathrm{H}_{17}\mathrm{N}_{5}\mathrm{O}_{2}\): 383.1382. Found: 383.1385.
1-Acetyl-6\(^\prime \)-amino-1\(^\prime \)-(2-ethylphenyl)-3\(^\prime \)-isopropyl-2-oxo-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5v)
Compound 5v (368 mg, 79 %) was obtained as a white solid: mp 211–\(213\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (600 MHz, DMSO-\(d_{6}\)) \(\delta \) 8.18 (1H, d, \(J= 8.4\hbox { Hz}\)), 7.59 (2H, s), 7.50–7.44 (4H, m), 7.41–7.38 (1H, m), 7.36–7.34 (2H, m), 2.63 (3H, s), 2.45–2.41 (2H, m), 1.97–1.92 (1H, m), 1.04 (3H, t, \(J= 7.2\hbox { Hz}\)), 0.91 (3H, d, \(J= 6.6\hbox { Hz}\)), 0.65 (3H, d, \(J = 6.6\hbox { Hz}\)); \({}^{13}\hbox {C}\) NMR (150 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.8, 170.4, 160.8, 152.4, 145.5, 141.3, 139.0, 134.8, 131.3, 129.8, 129.6, 127.6, 126.8, 126.2, 124.9, 117.5, 115.8, 92.7, 56.6, 48.8, 26.5, 26.1, 23.9, 21.6, 20.8, 14.4; IR (ATR) 3395, 3322, 2965, 2204, 1766, 1648, 1526, 1393, 1331, 1268, 1155, 1090, \(1040\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\mathrm{C}_{27}\mathrm{H}_{25}\mathrm{N}_{5}\mathrm{O}_{3}\): 467.1957. Found: 467.1954.
1-Acetyl-6\(^\prime \)-amino-1\(^\prime \)-(4-chlorophenyl)-3\(^\prime \)-isopropyl-2-oxo-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5w)
Compound 5w (357 mg, 83 %) was obtained as a white solid: mp 250–\(252\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (300 MHz, DMSO-\(d_{6}\)) \(\delta \) 10.75 (1H, s), 7.84 (2H, d, \(J= 8.7\hbox { Hz}\)), 7.58–7.54 (4H, m), 7.27 (1H, t, \(J= 7.5\hbox { Hz}\)), 7.19 (1H, d, \(J = 7.2\hbox { Hz}\)), 7.01 (1H, t, \(J= 7.5\hbox { Hz}\)), 6.90 (1H, d, \(J= 7.8\hbox { Hz}\)), 2.11–2.02 (1H, s), 0.99 (3H, d, \(J= 6.9\hbox { Hz}\)), 0.68 (3H, d, \(J = 6.9\hbox { Hz}\)); \({}^{13}\hbox {C}\) NMR (150 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.8, 160.6, 153.5, 144.7, 141.4, 136.2, 132.6, 130.6, 129.3, 125.0, 122.6, 121.6, 117.7, 109.9, 95.3, 56.7, 47.8, 26.3, 21.3, 20.9; IR (ATR) 3460, 3334, 3186, 2190, 1852, 1709, 1644, 1508, 1391, 1329, 1221, 1092, 1044, \(931\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\mathrm{C}_{23}\mathrm{H}_{18}\mathrm{ClN}_{5}\mathrm{O}_{2}\): 431.1149. Found: 431.1146.
6\(^\prime \)-Amino-1\(^\prime \)-(2-chlorophenyl)-3\(^\prime \)-methyl-2-oxo-1\(^\prime \)H-spiro[indoline-3,4\(^\prime \)-pyrano[2,3-c]pyrazole]-5\(^\prime \)-carbonitrile (5x)
Compound 5x (298 mg, 74 %) was obtained as a white solid: mp 250–\(252\,^{\circ }\mathrm{C}\); \({}^{1}\mathrm{H}\) NMR (600 MHz, DMSO-\(d_{6}\)) \(\delta \) 10.72 (1H, s), 7.72 (1H, d, \(J= 7.8\hbox { Hz}\)), 7.64 (1H, d, \(J= 6.6\hbox { Hz}\)), 7.59–7.53 (2H, m), 7.40 (2H, s), 7.29 (1H, t, \(J= 7.8\hbox { Hz}\)), 7.11 (1H, d, \(J= 7.2\hbox { Hz}\)), 7.04 (1H, t, \(J= 7.2\hbox { Hz}\)), 6.94 (1H, d, \(J = 7.2\hbox { Hz}\)), 1.53 (3H, s); \({}^{13}\hbox {C}\) NMR (150 MHz, DMSO-\(d_{6}\)) \(\delta \) 177.4, 161.0, 146.1, 144.2, 141.6, 133.9, 132.2, 131.3, 130.8, 130.4, 129.9, 129.3, 128.3, 124.7, 122.6, 118.0, 109.8, 95.0, 56.2, 48.0, 11.7; IR (ATR) 3315, 3160, 2922, 2198, 1711, 1531, 1473, 1381, 1128, 1042, 932, 750, \(623\hbox { cm}^{-1}\); HRMS m / z (\(\hbox {M}^{+}\)) calcd for \(\hbox {C}_{21}\mathrm{H}_{14}\hbox {ClN}_{5}\mathrm{O}_{2}\): 403.0836. Found: 403.0833.
Ferric reducing antioxidant power (FRAP) assay
The FRAP assay was carried out according to the procedure described by Benzie and Strain [63]. FRAP reagent was prepared freshly by adding 10 vol. of 300 mM acetate buffer, pH 3.6 (3.1 g sodium acetate and 16 mL glacial acetic acid) and 1 vol. of 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) prepared in 40 mM HCl and 1 vol. of 20 mM \(\hbox {FeCl}_{3}\). For each assay, 3 mL of FRAP reagent was mixed with 0.1 mL diluted synthesized compound. The mixture was shaken and incubated at \(37\,^{\circ }\mathrm{C}\) for 30 mins. Absorbance of the reaction mixture was measured at 593 nm. The standard calibration curve was carried out using Trolox and values expressed in terms of TEAC (\(\upmu \hbox {M}\)). For calculating \(\mathrm{EC}_{50}\) values, aliquots of the different concentrations of the synthesized compounds were prepared and incubated as described above.
Antibacterial activity
The antibacterial activities of synthesized compounds were determined using a modified Kirby-Bauer disk diffusion method [65]. Briefly, the test bacteria were grown in 10 mL of fresh \(\hbox {Difco}^{\mathrm{TM}}\) nutrient broth for 24 h. Optical density of test bacteria was measured using an Optizer 3220 (Double beam) UV–Vis spectrophotometer and found to be 0.7 at 595 nm. Aliquots of above bacterial suspension (\(100\,\upmu \hbox {L}\)) were then spread on \(\hbox {Difco}^{\mathrm{TM}}\) nutrient broth agar, which corresponded to the broth in which they were maintained. Two gram-negative bacteria Escherichia coli (KCTC-1924) and Pseudomonas aeruginosa (KCTC-2004) and two gram-positive bacteria Staphylococcus aureus (KCTC-1916) and Bacillus cereus (KCTC-1012) were obtained from the Korean Collection for Type Cultures (KCTC). The bacteria were incubated at \(37\,^{\circ }\mathrm{C}\) for 20–36 h, and then the diameters of the inhibition zones were measured in millimeters. Two mg of each test compound was dissolved in DMSO and further diluted to \(100\,\upmu \hbox {g/mL}\). Standard disks of ciprofloxacin served as positive controls and filter disks impregnated with DMSO as negative controls. Further, the depth of the agar in the plate is a factor to be considered in the disk diffusion method. Blank paper disks of diameter of 8.0 mm were impregnated with \(10\,\upmu \hbox {L}\) of above diluted sample solutions. When a filter disk impregnated with a tested chemical is placed on agar, the chemical diffuses from the disk into the agar. The solubility of the chemical and its molecular size determine the size of the area of infiltration. When an organism is placed on the agar, it will not grow in the area around the disk, if the chemical is active. This area of no growth around the disk is referred to as the zone of inhibition.
References
Galliford CV, Scheidt KA (2007) Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew Chem Int Ed 46:8748–8758. doi:10.1002/anie.200701342
Cheng D, Ishihara Y, Tan B, Barbas CF III (2014) Organocatalytic asymmetric assembly reactions: synthesis of spirooxindoles via organocascade strategies. ACS Catal 4:743–762. doi:10.1021/cs401172r
Xu J, Shao LD, Li D, Deng X, Liu YC, Zhao QS, Xia C (2014) Construction of tetracyclic 3-spirooxindole through cross dehydrogenation of pyridinium: applications in facile synthesis of (\(\pm )\)-Corynoxine and (\(\pm )\)-Corynoxine B. J Am Chem Soc 136:17962–17965. doi:10.1021/ja5121343
Santos MMM (2014) Recent advances in the synthesis of biologically active spirooxindoles. Tetrahedron 70:9735–9757. doi:10.1016/j.tet.2014.08.005
Marti C, Carreira EM (2003) Construction of spiro[pyrrolidine-3,3-oxindoles] recent applications to the synthesis of oxindole alkaloids. Eur J Org Chem 2003:2209–2219. doi:10.1002/ejoc.200300050
Bhaskar G, Arun Y, Balachandran C, Saikumar C, Perumal PT (2012) Synthesis of novel spirooxindole derivatives by one pot multicomponent reaction and their antimicrobial activity. Eur J Med Chem 51:79–91. doi:10.1016/j.ejmech.2012.02.024
Wu G, Ouyang L, Liu J, Zeng S, Huang W, Han B, Wu F, He G, Xiang M (2013) Synthesis of novel spirooxindolo-pyrrolidines, pyrrolizidines, and pyrrolothiazoles via a regioselective three-component \([3+2]\) cycloaddition and their preliminary antimicrobial evaluation. Mol Divers 17:271–283. doi:10.1007/s11030-013-9432-3
Thangamani A (2010) Regiospecific synthesis and biological evaluation of spirooxindolopyrrolizidines via \([3+2]\) cycloaddition of azomethine ylide. Eur J Med Chem 45:6120–6126. doi:10.1016/j.ejmech.2010.09.051
Sun Y, Liu J, Sun T, Zhang X, Yao J, Kai M, Jiang X, Wang R (2014) Anti-cancer small molecule JP-8g exhibits potent in vivo anti-inflammatory activity. Sci Rep 4:4372/1–4372/5. doi:10.1038/srep04372
Rottmann M, McNamara C, Yeung BKS, Lee MCS, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, González-Páez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT (2010) Spiroindolones, a potent compound class for the treatment of malaria. Science 329:1175–1180. doi:10.1126/science.1193225
Rajesh SM, Permual S, Menendez JC, Yogeeswari P, Sriram D (2011) Antimycobacterial activity of spirooxindolo-pyrrolidine, pyrrolizine and pyrrolothiazole hybrids obtained by a three-component regio- and stereoselective 1,3-dipolar cycloaddition. Med Chem Commun 2:626–630. doi:10.1039/c0md00239a
Haddad S, Boudriga S, Akhaja TN, Raval JP, Porzio F, Soldera A, Askri M, Knorr M, Rousselin Y, Kubicki MM, Rajani D (2015) A strategic approach to the synthesis of functionalized spirooxindole pyrrolidine derivatives: in vitro antibacterial, antifungal, antimalarial and antitubercular studies. New J Chem 39:520–528. doi:10.1039/c4nj01008f
Zhao Y, Yu S, Sun W, Liu L, Lu J, McEachern D, Shargary S, Bernard D, Li X, Zhao T, Zou P, Sun D, Wang S (2013) A potent small-molecule inhibitor of the MDM2-p53 interaction (MI-888) achieved complete and durable tumor regression in mice. J Med Chem 56:5553–5561. doi:10.1021/jm4005708
Zhou R, Wu Q, Guo M, Huang W, He X, Yang L, Peng F, He G, Han B (2015) Organocatalytic cascade reaction for the asymmetric synthesis of novel chroman-fused spirooxindoles that potently inhibit cancer cell proliferation. Chem Commun 51:13113–13116. doi:10.1039/c5cc04968g
Zhao Y, Liu L, Sun W, Lu J, McEachern D, Li X, Yu S, Bernard D, Ochsenbein P, Ferey V, Carry JC, Deschamps JR, Sun D, Wang S (2013) Diastereomeric spirooxindoles as highly potent and efficacious MDM2 inhibitors. J Am Chem Soc 135:7223–7234. doi:10.1021/ja3125417
Yu S, Qin D, Shangary S, Chen J, Wang G, Ding K, McEachern D, Qiu S, Nikolovska-Coleska Z, Miller R, Kang S, Yang D, Wang S (2009) Potent and orally active small-molecule inhibitors of the MDM2-p53 interaction. J Med Chem 52:7970–7973. doi:10.1021/jm901400z
Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S, Nikolovska-Coleska Z, Ding K, Wang G, Chen J, Bernard D, Zhang J, Lu Y, Gu Q, Shah RB, Pienta KJ, Ling X, Kang S, Guo M, Sun Y, Yang D, Wang S (2008) Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci 105:3933–3938. doi:10.1073/pnas.0708917105
Singh GS, Desta ZY (2012) Isatins as privileged molecules in design and synthesis of spiro fused cyclic frameworks. Chem Rev 112:6104–6155. doi:10.1021/cr300135y
Hong L, Wang R (2013) Recent advances in asymmetric organocatalytic construction of 3,3\(^\prime \)-spirocyclic oxindoles. Adv Synth Catal 355:1023–1052. doi:10.1002/adsc.201200808
Dalpozzo R, Bartoli G, Bencivenni G (2012) Recent advances in organocatalytic methods for the synthesis of disubstituted 2-and 3-indolinones. Chem Soc Rev 41:7247–7290. doi:10.1039/C2CS35100E
Kang SR, Lee YR (2013) Efficient one-pot synthesis of spirooxindole derivatives bearing hexahydroquinolines using multicomponent reactions catalyzed by ethylenediamine diacetate. Synthesis 45:2593–2599. doi:10.1055/s-0033-1338506
Narasimhulu M, Lee YR (2011) Ethylenediamine diacetate-catalyzed three-component reaction for the synthesis of 2,3-dihydroquinazolin-4(1\(H)\)-ones and their spirooxindole derivatives. Tetrahedron 67:9627–9634. doi:10.1016/j.tet.2011.08.018
Park JH, Lee YR, Kim SH (2013) A novel synthesis of diverse 2-amino-5-hydroxy-4\(H\)-chromene derivatives with a spirooxindole nucleus by \(Ca(OH)_{2}\)-mediated three-component reactions of substituted resorcinols with isatins and malononitrile. Tetrahedron 69:9682–9689. doi:10.1016/j.tet.2013.09.021
Shanthi G, Subbulakshmi G, Perumal PT (2007) A new \(\text{ InCl }_{3}\)-catalyzed, facile and efficient method for the synthesis of spirooxindoles under conventional and solvent-free microwave conditions. Tetrahedron 63:2057–2063. doi:10.1016/j.tet.2006.12.042
Dandia A, Parewa V, Jain AK, Rathore KS (2011) Step-economic, efficient, ZnS nanoparticle-catalyzed synthesis of spirooxindole derivatives in aqueous medium via Knoevenagel condensation followed by Michael addition. Green Chem 13:2135–2145. doi:10.1039/c1gc15244k
Satasia SP, Kalaria PN, Avalani JR, Raval DK (2014) An efficient approach for the synthesis of spirooxindole derivatives catalyzed by novel sulfated choline based heteropolyanion at room temperature. Tetrahedron 70:5763–5767. doi:10.1016/j.tet.2014.06.050
Dandia A, Jain AK, Bhati DS (2011) NaCl as a novel and green catalyst for the synthesis of biodynamic spiro heterocycles in water under sonication. Synth commun 41:2905–2919. doi:10.1080/00397911.2010.515365
Kidwai M, Jain A, Nemaysh V, Kumar R, Luthra PM (2013) Efficient entry to diversely functionalized spirooxindoles from isatin and their biological activity. Med Chem Res 22:2717–2723. doi:10.1007/s00044-012-0249-x
Tanaka K, Toda F (2000) Solvent-free organic synthesis. Chem Rev 100:1025–1074. doi:10.1021/cr940089p
Rothenberg G, Downie AP, Raston CL, Scott JL (2001) Understanding solid/solid organic reactions. J Am Chem Soc 123:8701–8708. doi:10.1021/ja0034388
Cave GWV, Raston CL, Scott JL (2001) Recent advances in solventless organic reactions: towards benign synthesis with remarkable versatility. Chem Commun 21:2159–2169. doi:10.1039/b106677n
Kaupp G (2003) Solid-state molecular syntheses: complete reactions without auxiliaries based on the new solid-state mechanism. CrystEngComm 5:117–133. doi:10.1039/b303432a
Schneider F, Szuppa T, Stolle A, Ondruschka B, Hopf H (2009) Energetic assessment of the Suzuki-Miyaura reaction: a curtate life cycle assessment as an easily understandable and applicable tool for reaction optimization. Green Chem 11:1894–1899. doi:10.1039/b915744c
Choudhary G, Peddinti RK (2011) An expeditious, highly efficient, catalyst-free and solvent-free synthesis of nitroamines and nitrosulfides by Michael addition. Green Chem 13:276–282. doi:10.1039/c0gc00830c
Cheng C, Jiang B, Tu SJ, Li G (2011) \([4+2+1]\) Domino cyclization in water for chemo- and regioselective synthesis of spiro-substituted benzo[\(b\)]furo[3,4-\(e\)][1,4]diazepine derivatives. Green Chem 13:2107–2115. doi:10.1039/c1gc15183e
Feng J, Ablajan K, Sali A (2014) 4-Dimethylaminopyridine-catalyzed multi-component one-pot reactions for the convenient synthesis of spiro[indoline-3,4’-pyrano[2,3-\(c\)]pyrazole] derivatives. Tetrahedron 70:484–489. doi:10.1016/j.tet.2013.11.019
Elinson MN, Dorofeev AS, Miloserdov FM, Nikishin GI (2009) Electrocatalytic multicomponent assembling of isatins, 3-methyl-2-pyrazolin-5-ones and malononitrile: facile and convenient way to functionalized spirocyclic [indole-3,4-pyrano[2,3-\(c\)]pyrazole] system. Mol Divers 13:47–52. doi:10.1007/s11030-008-9100-1
Tayade YA, Padvi SA, Wagh YB, Dalal DS (2015) \(\beta \)-Cyclodextrin as a supramolecular catalyst for the synthesis of dihydropyrano[2,3-\(c\)] pyrazole and spiro[indoline-3,40-pyrano [2,3-\(c\)]pyrazole] in aqueous medium. Tetrahedron Lett 56:2441–2447. doi:10.1016/j.tetlet.2015.03.084
Rai P, Srivastava M, Singh J, Singh J (2014) Chitosan/ionic liquid forms a renewable and reusable catalyst system used for the synthesis of highly functionalized spiro derivatives. New J Chem 38:3181–3186. doi:10.1039/c3nj01545a
Zou Y, Hu Y, Liu H, Shi D (2012) Rapid and efficient ultrasound-assisted method for the combinatorial synthesis of spiro[indoline-3,4\(\prime \)-pyrano[2,3-\(c\)]pyrazole] derivatives. ACS Comb Sci 14:38–43. doi:10.1021/co200128k
Liu X, Xu X, Wang X, Yang W, Qian Q, Zhang M, Song L, Deng H, Shao M (2013) A facile and convenient way to functionalized trifluoromethylated spirocyclic[indole-3,4-pyrano[2,3-\(c\)]pyrazole] derivatives. Tetrahedron Lett 54:4451–4455. doi:10.1016/j.tetlet.2013.06.038
Yu J, Zhou Y, Shen T, Mao W, Chen K, Song Q (2013) Novel and efficient one-pot synthesis of spiro[indoline-3,4\(^\prime \)-pyrano [2,3-c]pyrazole]derivatives catalysed by L-proline in aqueous medium. J Chem Res 37:365–368. doi:10.3184/174751913X13687116634925
Bodhak C, Kundu A, Pramanik A (2015) \(\text{ ZrO }_{2}\) nanoparticles as a reusable solid dual acid-base catalyst for facile one-pot synthesis of multi-functionalized spirooxindole derivatives under solvent free condition. RSC Adv 5:85202–85213. doi:10.1039/C5RA16259A
Tamura M, Tomishige K (2015) Redox properties of \(\text{ CeO }_{2}\) at low temperature: the direct synthesis of imines from alcohol and amine. Angew Chem Int Ed 54:864–867. doi:10.1002/anie.201409601
Honda M, Tamura M, Nakagawa Y, Nakao K, Suzuki K, Tomishige K (2014) Organic carbonate synthesis from \(\text{ CO }_{2}\) and alcohol over \(\text{ CeO }_{2}\) with 2-cyanopyridine: scope and mechanistic studies. J Catal 318:95–107. doi:10.1016/j.jcat.2014.07.022
Tamura M, Noro K, Honda M, Nakagawa Y, Tomishige K (2013) Highly efficient synthesis of cyclic ureas from \(\text{ CO }_{2}\) and diamines by a pure \(\text{ CeO }_{2}\) catalyst using a 2-propanol solvent. Green Chem 15:1567–1577. doi:10.1039/c3gc40495a
Edayadulla N, Lee YR (2014) Cerium oxide nanoparticle-catalyzed three component protocol for the synthesis of highly substituted novel quinoxalin-2-amine derivatives and 3,4-dihydroquinoxalin-2-amines in water. RSC Adv 4:11459–11468. doi:10.1039/C4RA00717D
Shelkar R, Sarode S, Nagarkar J (2013) Nano ceria catalyzed synthesis of substituted benzimidazole, benzothiazole, and benzoxazole in aqueous media. Tetrahedron Lett 54:6986–6990. doi:10.1016/j.tetlet.2013.09.092
Akhlaghinia B, Ebrahimabadi H, Goharshadi EK, Samiee S, Rezazadeh SJ (2012) Ceria nanoparticles as an efficient catalyst for oxidation of benzylic CH bonds. Mol Catal A: Chem 357:67–72. doi:10.1016/j.molcata.2012.01.020
Kim M, DiMaggio C, Yan S, Salley SO, Ng KYS (2011) The effect of support material on the transesterification activity of CaO-\(\text{ La }_{2}\text{ O }_{3}\) and CaO-\(\text{ CeO }_{2}\) supported catalysts. Green Chem 13:334–3390. doi:10.1039/c0gc00828a
Honda M, Sonehara S, Yasuda H, Nakagawa Y, Tomishige K (2011) Heterogeneous \(\text{ CeO }_{2}\) catalyst for the one-pot synthesis of organic carbamates from amines, CO\(_{2}\) and alcohols. Green Chem 13:3406–3413. doi:10.1039/c1gc15646b
Mitsudome T, Yamamoto M, Maeno Z, Mizugaki T, Jitsukawa K, Kaneda K (2015) One-step synthesis of core-gold/shell-ceria nanomaterial and its catalysis for highly selective semihydrogenation of alkynes. J Am Chem Soc 137:13452–13455. doi:10.1021/jacs.5b07521
Li HQ, Liu X, Zhang Q, Li SS, Liu YM, He HY, Cao Y (2015) Deoxygenative coupling of nitroarenes for the synthesis of aromatic azo compounds with CO using supported gold catalysts. Chem Commun 51:11217–11220. doi:10.1039/c5cc03134f
Tamura M, Ito K, Nakagawa Y, Tomishige K (2015) \(\text{ CeO }_{2}\)-catalyzed direct synthesis of dialkylureas from \(\text{ CO }_{2}\) and amines. J Catal. doi:10.1016/j.jcat.2015.11.015
Wang S, Zhao L, Wang W, Zhao Y, Zhang G, Ma X, Gong J (2013) Morphology control of ceria nanocrystals for catalytic conversion of \(\text{ CO }_{2}\) with methanol. Nanoscale 5:5582–5588. doi:10.1039/c3nr00831
Tamura M, Honda M, Noro K, Nakagawa Y, Tomishige K (2013) Heterogeneous \(\text{ CeO }_{2}\)-catalyzed selective synthesis of cyclic carbamates from \(\text{ CO }_{2}\) and amino alcohols in acetonitrile solvent. J Catal 305:191–203. doi:10.1016/j.jcat.2013.05.013
Tamura M, Tonomura T, Shimizu K, Satsuma A (2012) \(\text{ CeO }_{2}\)-catalyzed one-pot selective synthesis of N-alkyl amides from nitriles, amines and water. Appl Catal A 417–418:6–12. doi:10.1016/j.apcata.2011.12.004
Woan K, Tsai YY, Sigmund W (2010) Synthesis and characterization of luminescent cerium oxide nanoparticles. Nanomedicine 5:233–242. doi:10.2217/nnm.09.106
Minh NQ, Takahashi T (1995) Science and technology of ceramic fuel cells. Elsevier, New York
Faure B, Salazar-Alvarez G, Ahniyaz A, Villaluenga I, Berriozabal G, De Miguel YR, Bergström L (2013) Dispersion and surface functionalization of oxide nanoparticles for transparent photocatalytic and UV-protecting coatings and sunscreens. Sci Technol Adv Mater 14:023001. doi:10.1088/1468-6996/14/2/023001
Tarnuzzer RW, Colon J, Patil S, Seal S (2005) Vacancy engineered eeria nanostructures for protection from radiation-induced cellular damage. Nano Lett 5:2573–2577. doi:10.1021/nl052024f
Shehata N, Meehan K, Leber D (2013) Study of fluorescence quenching in aluminum-doped ceria nanoparticles: potential molecular probe for dissolved oxygen. J Fluoresc 23:527–532. doi:10.1007/s10895-013-1186-x
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 23:70–76. doi:10.1006/abio.1996.0292
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956. doi:10.1016/0891-5849(95)02227-9
Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496
Acknowledgments
This work was supported by the 2014 Yeungnam University Research Grant (215A555002).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shrestha, R., Sharma, K., Lee, Y.R. et al. Cerium oxide-catalyzed multicomponent condensation approach to spirooxindoles in water. Mol Divers 20, 847–858 (2016). https://doi.org/10.1007/s11030-016-9670-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-016-9670-2