Abstract
The alkaline technology has been applied to phosphate (Pi) recovery from sewage sludge ash (SSA) at wastewater treatment plants (WWTP). Pi is extracted from mono-incinerated sewage sludge with NaOH and recovered from the leachate using chemical precipitation with Ca(OH)2. Approximately 30–40% of Pi could be recovered from SSA as calcium Pi while minimizing the leaching of toxic heavy metals at high pH. The recovered P product can be recycled as a fertilizing material for agriculture.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Phosphorus (P) needs to be removed from sewage to control eutrophication in natural bodies of water. The P removed from sewage ultimately ends up in sewage sludge at wastewater treatment plants (WWTP). Dewatered sewage sludge is often mono-incinerated to reduce its volume and to recover energy at WWTP. This allows phosphate (Pi) to be concentrated in the sewage sludge ash (SSA). Since SSA has a high P content, typically ranging from 20 to 30 wt% P2O5, it is becoming increasingly a priority target for P recovery in the wastewater treatment sector.
In Japan, about 300 incinerators are operating at WWTP. Together, they have a capacity of incinerating c. 25,000 t/year (tons per year) of dewatered sludge, generating c. 33,000 t/year of SSA. Approximately 70% of the SSA has been accepted by the recycling sector, mainly the cement industry in Japan. However, since Pi can cause a detrimental effect on the cement quality, it is becoming a nuisance issue for the cement industry to accept Pi-rich SSA from the wastewater treatment sector. Hence, it is critical to develop an alternative technology option that enables the valorization of ever-increasing Pi-rich SSA. In this chapter, the alkaline P leaching technology to recycle P from SSA to farmland will be described.
2 Alkaline Pi Leaching Technology
Figure 8.1 shows a schematic diagram of Pi recovery from SSA using the alkaline leaching technology (Yanase 2009). In the alkaline leaching process, ash is mixed with 1.0 M NaOH solution to extract Pi (PO4 3−). The Pi-rich leachate is separated from the rest (de-phosphorus ash) by mechanical dewatering. Pi is recovered as calcium phosphate (Ca3(PO4)2) by the addition of Ca(OH)2 to the leachate in the precipitation process. The phenomenological reaction for Pi leaching and precipitation may be given by:
After the precipitation process, the slurry is subjected to mechanical dewatering to recover Pi. The high-pH rejected liquor is returned to the Pi extraction process. This is effective in saving the chemical costs for Pi recovery from SSA.
Figure 8.2 shows the full-scale process for Pi recovery from SSA using the alkaline leaching technology (Moriya 2009). Ash, which is collected by a dust collector of the incineration system, is fed to the Pi leaching tank through a buffer ash hopper. SSA is then mixed with 1.0 M NaOH solution at temperature of 50–70 °C for about 30 min of gentle mixing. NaOH is dissolved in hot water, which is prepared using waste heat energy of the exhaust gas from the mono-incinerator. Although the temperature required for Pi leaching is about 50–70 °C, the heat energy can be supplied by the waste heat from the incinerator, thereby saving the energy costs.
In the Pi leaching tank, the concentration of slurry is kept around 8–10%. In case that the Pi content in SSA is high, the Pi leaching step can be repeated to increase the amount of Pi leached. After leaching Pi, the mixture is subjected to mechanical solid-liquid separation. Then the Pi-rich liquor is sent to Pi precipitation tank where Ca(OH)2 is fed at Ca/P molar ratio of 1–1.5. The mixture is slowly stirred for about 6–18 h to gradually dissolve Ca(OH)2 and to generate calcium Pi as precipitates.
Calcium Pi precipitates are recovered by mechanical dewatering, washed with water, dried, and stored for shipping (Fig. 8.3a). To reduce the chemical cost, high-pH rejected water is returned to the NaOH tank for NaOH recycling. The solid material remaining after Pi leaching is washed two times with water and finally with dilute H2SO4 at pH of 4.5–5.5 to remove toxic heavy metals. The resulting solid (de-phosphorus ash, Fig. 8.3b), which meets the environmental quality standard for soil in Japan, can be used for cement, asphalt filler, and roadbed.
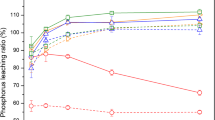
The alkaline leaching technology can recover approximately 30–60% of Pi from SSA in the form of calcium Pi. Importantly, the rate of Pi leaching from SSA is strongly dependent on the CaO content of SSA. Figure 8.4 shows the relationship between the CaO content in SSA and the rate of Pi leaching in the alkaline extraction step (Moriya 2009). There is a clear relationship between them. The alkaline Pi leaching becomes difficult when the CaO content of SSA exceeds 20%. Hence, if lime is used for sludge dewatering, the alkaline leaching technology is not a suitable option for Pi recovery from SSA.
3 Recovered Products
The weight of recovered P product is about 30–50% of SSA on a dry weight basis. The typical composition of recovered P product is shown in Table 8.1 (Yanase 2009). The total P content of recovered product is about 30% P2O5, most of which is citric acid-soluble P. The levels of toxic substances such as As, Cd, and Se are much lower than their regulation levels for by-product Pi fertilizer in Japan. Since the main component of recovered product is calcium Pi, it can also be used as a substitute for Pi rock in a variety of technical applications.
The alkaline Pi leaching process generates about 0.75 tons of de-phosphorus ash from each ton of SSA treated. De-phosphorus ash is a microporous material whose average particle size is typically about 50% smaller than that of SSA. Therefore, the specific surface area of de-phosphorus ash is about one order of magnitude larger than that of SSA. As mentioned before, de-phosphorus ash meets the environmental quality standard for soil in Japan so that it can be used for cement, asphalt filler, and roadbed. The stable sale of recovered P product and de-phosphorus ash is critical to bring economic benefits to WWTP. However, fertilizer companies do not accept recovered P product unless it brings some economic benefits to their business. The expanded use of this technology requires further reduction of the cost of chemicals required for Pi leaching and recovery.
4 Conclusions
The alkaline leaching technology can be applied to Pi recovery from sewage sludge ash at wastewater treatment plants. Approximately 30–40% of Pi could be recovered from sewage sludge ash as calcium phosphate while preventing toxic heavy metals from leaching at high pH. The rate of alkaline Pi leaching from SSA is strongly dependent on the CaO content of SSA. The alkaline Pi leaching technology is suited for Pi recovery from SSA having a low CaO content. Recovered P product can be used as a fertilizing material for agriculture, while the rest is usable for cement, asphalt filler, and roadbed after being washed with a dilute acid solution.
References
Moriya Y (2009) Phosphorus recovery technology from sewage sludge ash. J Resour Environ 45(6):35–39. (in Japanese)
Yanase T (2009) Phosphorus recovery and detoxification system from sewage sludge ash. J Earth Environ 40(4):72–73. (in Japanese)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sonoda, Ki. (2019). Alkaline Leaching of Phosphate from Sewage Sludge Ash. In: Ohtake, H., Tsuneda, S. (eds) Phosphorus Recovery and Recycling . Springer, Singapore. https://doi.org/10.1007/978-981-10-8031-9_8
Download citation
DOI: https://doi.org/10.1007/978-981-10-8031-9_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-8030-2
Online ISBN: 978-981-10-8031-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)