Abstract
Hydrogen production by biological methods, particularly dark fermentation, has recently gained in interest for the scientific as well as the socio-economic communities. This theme has been intensively investigated over the past decade since biohydrogen can be produced under a large range of conditions. This chapter aims to present the basic concepts of biohydrogen production by dark fermentation , including microbial metabolism and related microorganisms . The wide range of substrate possibilities, as well as the most important operational parameters, are also reviewed. Integration of the dark fermentation bioprocesses into the concept of environmental biorefinery is further discussed by proposing alternatives to overcome the limitations prior to their application at industrial scale. Finally, the current situation of hydrogen as main energetic vector for the future, as well as the role of dark fermentation in the environmentally friendly hydrogen production are discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In the recent years, a growing awareness of the environmental damage caused by the use of fossil fuels has arisen. It is well admitted that fossil fuels contribute to climate change and that their production and consumption are associated with the generation of large amounts of non-biodegradable wastes. These issues have led to a growing interest of the scientific community to seek alternative renewable energy sources. In this context, hydrogen is considered to be one of the most promising alternative fuels for the future. Other than generating only water during its combustion , its high energetic value of 120 MJ kg−1 (more than twice that of common natural gas or gasoline) makes this gas a cleaner and competitive alternative to common fossil fuels [1]. When compared to other energy sources and in particular those producing electricity, hydrogen presents the main advantages of being storable and generated from various renewable sources i.e., by using the surplus of electricity of wind turbines or solar panels [2]. Different scenarios of hydrogen-based energy systems have been proposed so far and, in all cases, hydrogen will supply energy for diverse applications, such as industrial, commercial, residential or transportation activities [3]. In particular, H2-based electro-mobility is expected to gradually replace the use of fossil fuels and special efforts will be made on renewable H2, so-called green hydrogen.
Currently about 96% of all the hydrogen produced worldwide is based on chemical processes that use fossil materials as raw materials [4]. To stand as an environmental-friendly and renewable alternative, hydrogen must be produced using sustainable processes, such as physico-chemical techniques (e.g. water electrolysis , biomass gasification or solar thermo-chemical processes) or biological processes. These latter are based on the biological capability of some microorganisms to produce hydrogen gas by the degradation of organic matter, as found in Nature. In addition to the production of clean hydrogen, these processes can be used to treat organic wastes , converting them into more valuable products. This is the case of dark fermentation (DF), a fermentation process in which microorganisms degrade complex organic matter to simpler molecules and simultaneously generate hydrogen. The added-value co-products are mainly composed of volatile fatty acids (e.g. acetate and butyrate), other organic acids (e.g. lactate) and organic solvents (e.g. ethanol). All of these are valuable chemicals that are also used in the chemical industry. Therefore, DF appears as a promising technology that can be included in the concepts of environmental biorefinery and circular economy, where organic residues are not anymore considered as a waste but as a resource. Moreover, more than 220 billion tons of agricultural organic waste accumulate per year because of intensive agricultural production that constitute one of the most abundant renewable sources for producing H2 by DF [5].
This chapter aims to describe the main aspects of the production of hydrogen by DF, including the bases of the microbial metabolism involved, the main operational parameters affecting the process and the different substrates that DF can accommodate. The integration of DF within the concept of environmental biorefinery will also be discussed. Finally, the current situation of hydrogen as fuel and its potential implications for the future energy systems are also assessed.
2 Dark Fermentation Microbiology and Metabolisms
Production of dark fermentative hydrogen is a ubiquitous phenomenon that occurs in most of anaerobic natural environments. It consists in an obligate cascade of reduction-oxidation (redox) reactions that must be kept in balance. Although these reactions are mostly thermodynamically favorable and spontaneous, they are also constrained by biological regulations within microorganisms and by interspecies interactions in microbial communities [6].
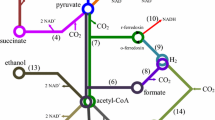
Dark fermentation can involve any type of organic molecules, being glucose the most common substrate investigated in literature. Many biological pathways have been proposed using glucose as model substrate (Fig. 1). The hydrogen production is a natural response of the cellular need for releasing the excess of electrons and is always coupled with volatile fatty acids and/or alcohols production. The most common co-products in the glucose fermentation are acetate, butyrate, and formate. The hydrogen–acetate couple produces more ATP per mol of substrate than alcohols such as ethanol and butanol and is therefore the energetically ‘‘preferred’’ bacterial fermentation product from sugars [7]. The stoichiometric yields are 4 mol of hydrogen for each mole of glucose when acetic acid is the co-product [8] and 2 mol of hydrogen if butyric acid is produced [9,10,11]. In practice, the hydrogen yields are within the range of 10–20% of COD [12], which is equivalent to 1.17–2.34 mol H2 mol glucose−1 [13,14,15]. Indeed, each molecule of glucose can potentially produce 4 mol of hydrogen if no biomass production is considered. However, the fermentation process naturally implies maximizing the cell growth and not the hydrogen production and thus the maximum hydrogen yield is rarely achieved in practice, especially with mixed microbial cultures [10, 14]. In any case, glucose is first converted into pyruvate, producing adenosine triphosphate (ATP) from adenosine diphosphate (ADP) and the reduced form of nicotinamide adenine dinucleotide (NADH) via the glycolytic pathway. Pyruvate is then converted to acetyl coenzyme A (acetyl-CoA) and carbon dioxide by pyruvate–ferredoxin oxidoreductase (PFOR) (Fig. 1b), when strict anaerobes break down glucose . In contrast, facultative anaerobes convert pyruvate to acetyl-CoA and formate by pyruvate formate lyase (PFL) (Fig. 1a). In both cases acetyl-CoA is finally converted into acetate, butyrate, or ethanol, depending on the involved microorganisms and the environmental conditions [11, 16, 17].
Pathways for hydrogen production by dark fermentation from glucose under anaerobic conditions using mixed cultures; a Pyruvate formate lyase (PFL) is the common pathway in facultative anaerobes; b Pyruvate-ferredoxin oxidoreductase (PFOR) is the common pathway in strict anaerobes; c Additional hydrogen-production by hydrogenases at low hydrogen partial pressure (<60 Pa).
As key parameter, the microbial inoculum used to start the DF process can substantially impact the hydrogen yields. This is because the fermentation end products are directly influenced by the type of bacterial metabolism [16]. A wide variety of obligate and/or facultative bacteria have been used for hydrogen production by DF. This includes mixed cultures and pure hydrogen-producing cultures [17, 18].
In strict anaerobes, the oxidation of pyruvate into acetyl-CoA requires the reduction of ferredoxin (Fd) by PFOR, which is then oxidized by a hydrogenase that regenerates oxidized Fd and hydrogen [18]. Additional hydrogen can be produced from the NADH excess that is generated during glycolysis (Fig. 1c). The NADH is oxidized by NADH-[FeFe] hydrogenase, but only at very low partial pressures of hydrogen (<60 Pa) [11, 16]. Some strict anaerobes are particularly efficient in producing hydrogen by DF such as C. acetobutylicum, C. beijerinckii or C. butyricum, among others [11, 19]. C. butyricum is a widely studied clostridia species, responsible for the production of butyric acid as the major product of fermentation together with acetate and hydrogen. Mostly, clostridia are identified as dominant HPB in DF operated with mesophilic mixed cultures [20].
Facultative anaerobes can grow under anaerobic and aerobic conditions . In anaerobic conditions, formate is produced to get rid of extra reducing equivalents that would have been lost through the reduction of NAD+ under aerobic conditions. Subsequently, formate can be degraded into hydrogen and carbon dioxide under acid conditions to maintain the pH of the system and lower the formate concentration in the cell [11]. Some facultative anaerobes capable of producing hydrogen by DF include E. coli, E. Cloacae, and E. aerogenes, among others [11, 19].
Working with pure cultures allows detecting easily the metabolic shifts due to the low diversity of the microbial biomass . Studies employing pure cultures can reveal important information regarding the operating conditions to be applied for increasing the hydrogen yields [19]. Although relatively high hydrogen yields have been obtained with pure cultures of hydrogen-producing bacteria (HPB), their use is not always feasible when dealing with the transformation of complex substrates , providing indigenous bacterial contamination. Moreover, during the DF process, a wide consortium of microorganisms is required for hydrolyzing the complex substrates prior to fermentation of the released sugars into hydrogen. Besides, it has been argued that it is more practical and economically feasible to use mixed cultures on larger scale rather than pure cultures [21,22,23]. Nonetheless, the use of mixed cultures has a major constraint. Besides containing HPB, mixed microflora also consist of a wide variety of microorganisms such as hydrogen-consuming bacteria and other microorganisms that compete with HPB for organic substrates. This may eventually decrease the net hydrogen yield. These non-hydrogen producers include hydrogenotrophic methanogens , homoacetogenic bacteria (HAB), sulphate-reducing bacteria (SRB), nitrate-reducing bacteria (NRB), propionate producers, iron-reducing bacteria and lactic acid bacteria (LAB) [10, 24, 25]. Consequently, the use of mixed microflora may result in direct hydrogen consumption, lower hydrogen yields, increased formation of end products and further process inhibition [26]. The main strategy to eliminate the hydrogen-consuming microorganisms is the pretreatment of the microbial inoculum prior to DF. The different kinds of pretreatment will be discussed in the coming Sect. (3.1.1).
3 Main Operating Conditions Affecting Dark Fermentation
Several bioprocess parameters can influence the hydrogen production by DF, impacting the hydrogen yields and/or the hydrogen production rates. The main operational parameters affecting the DF process are: the inoculum source and pre-treatment, the organic substrate used, the reactor operation/type, the temperature , the pH and the hydraulic retention time (HRT) [19, 22, 27]. These parameters have been separated into two different sections: (i) parameters for reactor start-up and (ii) parameters to be monitored during DF.
3.1 Parameters for Reactor Start–up
3.1.1 Inoculum and Pre-treatments
As aforementioned, hydrogen production by DF can be performed using pure cultures (such as Clostridium sp.) or mixed cultures (such as anaerobic sludge) and both have their own benefits and disadvantages. However, the use of mixed cultures is more practical in terms of control, operation and may be able to degrade a broader range of feedstock, being more attractive for industrial use [28, 29], especially since sterile conditions are not necessary [30, 31]. Despite these benefits, the hydrogen yields using mixed cultures are relatively low due to the presence of hydrogen-consuming microorganisms such as methanogens (archaea) [26, 32]. Commonly, these microorganisms are inhibited and/or eliminated by both inoculum pre-treatment and adapted operating conditions. The most common pretreatment techniques reported in the literature include: heat-shock, pH shock, loading-shock, chemical pretreatment, swinging the oxidation-reduction potential (ORP) (e.g. by aeration) and combination of different methods [26, 30].
The heat shock treatment allows removing the non-spore forming microorganisms, like archaea (methanogens ), thereby enriching the culture media with spore-forming bacteria, such as Clostridium sp., which is a very well-known hydrogen producer [33]. The control conditions for heat-shock pretreatment usually listed on the literature range from 90 to 100 °C, with exposure time between 15 to 60 min [28, 29, 33,34,35,36,37,38].
A pH shock consists in removing the hydrogen- consuming bacteria and the methanogens while protecting the spore-forming microorganisms [33]. Acid (lower than 6.3) or basic treatment (higher than 7.8) are efficient options for inhibiting the growth of methanogens [39]. The acid treatment is the most widely used option, using HCl 1 or 2 M at an adjusted pH of 3 maintained during 24 h [40, 41]. The base treatment is usually undertaken by adjusting the pH of the inoculum at 10 using NaOH 1 M maintained for 24 h at 25 °C [38, 41].
Chemical treatment in mixed cultures eliminates the hydrogen consuming methanogens by chemical inhibition, using molecules such as 2-bromoethanesulfonic acid (BES), iodopropane or chloroform, which are toxic to these archaea [28, 42]. BESA is a structural molecule analog to the co-enzyme M reductase complex found in methanogens and blocks this reaction. Iodopropane is a corrinoid antagonist who prevents functioning of B12 enzymes as a methyl group carrier, therefore inhibiting cell growth and hydrogen consumption for methane production [43].
However, inoculum treatment can also affect the production of hydrogen if it is not properly managed. In a batch study, Luo et al. [37] applied various pretreatment methods on mixed inocula, reaching the highest hydrogen yield without pretreatment (65.3 mL H2 g VS−1) and the lowest one after a base and heat shock (51.3 ± 1.8 and 51.4 ± 1.8 mL H2 g VS−1 respectively).
3.1.2 Micro- and Macro-nutrients Requirements for Efficient DF Nutritional Requirements
When talking about substrate , this refers to the carbon and energy source (generally sugars). However, microorganisms need other elements for their growth, such as nitrogen, phosphorous and other important micronutrients. That is why, the nutritional requirements and the composition of the culture medium are important variables that directly affect the microbial metabolism during DF and therefore are critical for hydrogen production [44,45,46].
Concerning nitrogen, it is an important component in proteins , including enzymes, and nucleic acids, whose synthesis is crucial for the growth of bacteria. However, there are still disagreements with respect to the optimum concentration. It is known that a nitrogen excess can affect the intracellular pH and eventually inhibit the activity of nitrogenases , inhibiting also bacterial growth. High nitrogen concentrations can induce ammonification, which is not favorable for the hydrogen production [22, 44]. It has also been shown that appropriate C/N and C/P ratios are fundamental for fermentative hydrogen production. However, it exists a certain disagreement on the optimal values, because all the studies have utilized different substrates , inoculums and C/N- C/P ranges [22, 46].
Within the micronutrients, metal ions are also suspected to play an important role because they assist cell growth and both enzyme and co-enzyme activation [26]. Nonetheless, high concentrations of metal ions might lead to inhibition of the hydrogen production. Metal ions can be classified into light metal ions (Mg2+, Na+ and Ca2+) or heavy metal ions (Fe2+ and Ni2+) [22, 26]. Among the later, Iron is the most studied, since it is required for bacterial growth and for biosynthesis of enzymes and proteins, such as hydrogenases and ferredoxins, which are critical for hydrogen production by DF [14, 26, 47].
3.1.3 Bioreactor Configuration and Operational Mode
For hydrogen production by DF, the reactors can be operated in batch or continuous mode, batch tests being more reported in the literature because of their simplicity and flexibility [19, 27]. Continuous processes are more recommended when considering industrial applications, mainly because of their economic feasibility and their practical engineering design when treating large amounts of substrates [16, 19, 25].
Different kinds of bioreactor configurations have been used for continuous hydrogen production by DF. Nowadays, the suspended-cell completely stirred tank reactor (CSTR) is the most commonly applied option. However, up-flow anaerobic sludge blanket (UASB) reactors, anaerobic membrane bioreactors and immobilized (e.g. fluidized bed) bioreactors are becoming popular due to their improved hydrogen producing potentials [30, 48]. The use of CSTRs is generally associated with relatively short start-up phase when compared to other configurations due to better mass transfer , but it also needs rigorous supervision due to the disposition of cells to be washed out at inadequate operating bioreactor regimen (e.g. HRT). This risk of wash out can be avoided by retained-biomass systems such as the membrane reactors or immobilized systems [30, 48].
3.2 Parameters to be Monitored During DF
3.2.1 pH
pH is one of the most important parameters in DF. It affects the hydrolysis of substrates (when complex), the activity of important enzymes for hydrogen production (such as hydrogenase ), the predominant microbial population and their main metabolic pathways [27, 49]. The range of operational pH for hydrogen production has been reported between 4.5 and 8.0 [49, 50]. Such wide range of optimal pH can be explained by the variability of inocula and substrates [20]. Indeed, for simple substrates such as glucose, the highest hydrogen yields were reported at pH of 6.0 (1.83 mol H2 mol−1) in batch experiments [51]. When fermenting a complex substrate (food waste), maximum hydrogen yields were reported at pH 8.0 (1.92 mol H2 mol hexose−1) [52]. However, there is an agreement of the negative effect of pH values below 4.5–5.5, generally caused by the accumulation of volatile fatty acids , which can reduce the hydrogen production due to shifts in the metabolite production pathways towards solventogenesis (acetone, butanol, ethanol) [50].
3.2.2 Temperature
Temperature plays an important role in reducing the activity of hydrogen consumers [20]. The range of operational temperature is mesophilic (35 °C), thermophilic (55 °C) and extreme thermophilic (>65 °C). Varying the temperature affects greatly the structure of the bacterial community. Lazaro et al. [53] explained that significant differences between the microbial communities at 37 °C and 55 °C exist. A shift from Clostridium at mesophilic conditions to Thermoanaerobacterium when thermophilic conditions were applied was shown. However, the hydrogen yield was not impacted by the temperature regime (2.31 and 2.23 mmol H2 g−1CODinfluent at mesophilic and thermophilic respectively) [54]. As reported by Ghimire et al. [20], the temperature also affects the metabolic pathways , thus modifying the by-products produced during DF. Consistently, the study of Valdez et al. [23] showed a significant difference on the average distribution of metabolites between thermophilic and mesophilic conditions. The predominant metabolite produced under mesophilic temperatures was butyrate, while in thermophilic conditions acetate was the main metabolite.
3.2.3 Hydraulic Retention Time
The hydraulic retention time (HRT), as defined in Eq. 1, is one of the major critical parameters affecting the continuous production of hydrogen. In suspended-cell reactors, such as CSTRs, the HRT corresponds to the inverse of the dilution rate (D). In these systems, D (and thus the HRT) will determine which microorganisms will be dominant in the reactor. Basically, if D is equal to the microbial growth rate (μ), the system reaches equilibrium, also called steady-state. If D is higher than μmax, (maximum growth rate), the slow-growing microorganisms are washed out from the reactor and if D is lower than μmax, slow-growers will also survive, although they could be washed out by lack of nutrients (competitive exclusion between microorganisms).
Therefore, to favor the emergence of certain hydrogen-producing microbial populations, it is important to know the μmax of the microorganisms to further establish an adequate HRT and avoid the wash-out of the biomass from the reactor, maximizing at the same time the microbial growth and the production of desired metabolites. Indeed, unlike pure cultures, mixed cultures have a greater microbial richness and contain different microorganisms with different μmax. In this context, the HRT is a key parameter that allows the selection of the desired populations (i.e., washing-out slow-growing microorganisms from the reactors). Focusing on hydrogen producing reactors by DF, this is a good way to eliminate methanogenic microorganisms, which grow slower (HRT ≥ 1 d) than HPB (HRT ≤ 24 h). However, it is important to consider the type of substrate and the inoculum sources. As an illustration, several studies reported that in order to decrease the methanogenic activity during DF, it is sufficient to work at short HRTs (<few h) and low pH (5–5.5), in what has been called a “biokinetic control” [16, 20, 55].
4 Subtrates for Dark Fermentation: Solid Wastes and Wastewaters
Fermenting bacteria can utilize several types of substrates, mostly the ones rich in carbohydrates , such as first generation fuel crops (i.e., sugar cane, wheat, corn, and sugar beets), second generation biomass like agricultural residues as well as industrial waste and wastewaters [20]. Since DF allows coupling organic waste treatment with the production of renewable energy , the utilization of waste as substrates is particularly attractive from an environmental and economic point of view [31]. Therefore, nowadays this alternative is being widely researched, aiming to reduce the costs of organic waste treatment, while generating added-value end-products. Thus, the Table 1 shows the different main wastes and wastewaters that have been used for hydrogen production by DF.
The choice of the type of substrate is a key decision that affects greatly the hydrogen yields, the hydrogen production rates and the overall process economy. These variables are largely dependent on the carbohydrate content of the substrate (with higher hydrogen yields at higher contents of soluble carbohydrates), its bioavailability and its biodegradation rate [25, 56,57,58]. Substrates rich in carbohydrates have been widely used in studies focused on DF, particularly pure glucose and mixtures of sucrose and starch [20]. However, using this type of substrates at an industrial level is not economically profitable. In this context, wastewaters and solid wastes appear as perfect possibilities to generate ‘green’ hydrogen from renewable sources.
Recent studies have dealt with the dark fermentation of complex substrates, such as the organic fraction of municipal solid waste (OFMSW) , agricultural residues (e.g. rice straw, wheat straw and corn stalks), agro-industrial wastes (e.g. olive mill wastewater or cheese whey), effluents from livestock farms or aquatic plants. Moreover, if DF is integrated within the concept of environmental biorefinery (i.e., multi-substrates to multi end-products), the co-products generated during biofuel production such as crude glycerol, de-oiled algal cake or cotton seed cake, could be further used as substrates for DF.
To achieve satisfactory hydrogen yields using complex organic wastes as substrates for DF, pretreatments are frequently required to facilitate the hydrolysis step, especially with substrates containing significant lignocellulosic fractions. These pretreatments increase the soluble fraction of carbohydrates, improving the hydrogen yields [20]. Among all the possible substrate pretreatments, the most relevant are: physical methods (e.g. mechanical comminution, irradiation with gamma-rays, electro-beam or microwaves, hydrothermal treatment, high pressure steaming and pyrolysis) , chemicals methods (e.g. ozonolysis, acid or alkaline hydrolysis, solvent extraction and explosion with steam ammonia fiber or carbon dioxide) and even biological methods, using fungi [20]. It has been reported that it is possible to increase from 2 to 50 times the hydrogen yields by pretreating the substrates [25, 59,60,61]. However, economic and energetic assessments are required before application of a pretreatment.
Concerning livestock wastes, they are also suitable as DF substrates and can be categorized in: urine waste, solid manure and wastewaters from process water collection (e.g. feedlot runoff, silage juices, bedding, disinfectants and liquid manure) [62]. The proper disposal and treatment of these wastes is crucial because they can contaminate the air and natural water courses. Nutrient leaching and pathogen contamination can also cause important health problems [25]. When using this type of substrate for DF, it is necessary to include thermal pretreatment not only to eliminate the indigenous methanogenic activity but also to hygienize the wastewater, which are inherent to this waste due to the presence of native archaea and enteric pathogens [25].
Due to its high biodegradability and energy content, food industry waste has been regarded as ideal for microbial growth. In addition, this waste is commonly disposed in landfills, causing environmental problems, such as of odors, methane emissions and groundwater contamination. Therefore, its treatment and valorization by DF is clearly beneficial. Kitchen refuse [63], organic fraction of municipal waste [64], food industry co-products (such as oil mill) [65, 66], cheese whey [67] and starch-manufacturing waste [68] are representative waste of this category that have been efficiently applied for hydrogen production by DF.
In general, all the wastes aforementioned have shown a great potential as substrates for producing hydrogen by DF, with various yields mainly depending on their content in readily accessible carbohydrates . Nonetheless, hydrogen yields will not only depend on the composition of the waste, but also on the correct choice of the key operational parameters and the microbial consortium, which must be optimized for each particular DF feed since it contains its own indigenous microbial communities.
5 Dark Fermentation as Core Process in Future Environmental Biorefineries
The concept of environmental biorefinery lies on the idea of integrating different bioprocess to convert biomass into several added-value products [69]. The main aim of this approach is to obtain a global process which is self-sufficient, environmentally sustainable and economically beneficial. The integration of DF with other processes will reduce the amount of organic residues produced (and the associated disposal costs), increasing at the same time the total revenues by synthetizing added-value chemicals and improving the global energy yields [70]. In addition, the development of a comprehensive biorefinery would help to overcome two of the main bottlenecks for commercial hydrogen production from DF: the low yields of the process and the incomplete biomass conversion/stabilization.
A main advantage of DF, when compared to other processes for organic waste treatment and energy production, is the wide variety of substrates that it can accommodate. Thus, DF can be integrated within existing or novel biomass valorization (bio)-processes treating several substrates , such as residues from agricultural activities, forestry activities, macro- and micro-algae activities, food industry, municipal waste and bio-industrial waste [70]. In a DF-based biorefinery, these wastes could be transformed into several added-value products, such as hydrogen, methane, liquid fuels, lipids , bioplastics, electricity, fine chemicals or proteins , among others.
Several biorefinery models including DF have been proposed. These models are flexible and can be adapted to local specific conditions (geographical location, seasonal variability in substrate production, among others). Figure 2 shows a comprehensive (but not exhaustive) schematic representation of DF biorefinery frameworks. The most common one (Fig. 2, process 3) is the so-called “acidogenic model” or two-stage anaerobic digestion (AD) [71]. In this process, DF represents the first stage, producing hydrogen and different metabolites, such as alcohols and volatile fatty acids . DF metabolites, being value-added products, can be (all or some of them) extracted and purified, while the remaining organic matter in DF effluent enters the second stage, which consists in an anaerobic reactor for methane production and waste stabilization. It has been stated that this process integration could have a tremendous positive impact in the economic viability of AD processes by maximizing the substrate conversion [72]. The integration of these two stages increases the sustainability of the process, achieving at the same time a complete waste treatment. Combined DF and AD has been proved to be economically and technically feasible using a wide variety of substrates, with high yields of both hydrogen and methane [20]. Therefore, AD can clearly be applied to improve the economic performance of commercial hydrogen production by DF.
Different options for coupling dark fermentation with other bio-processes in a biorefinery framework for organic waste valorization. The numbers stand for: (1) microbial electrolysis, (2) photo-fermentation, (3) anaerobic digestion , (4) microalgae cultivation and (5) direct application/recovery (Adapted from [20, 57, 73, 75, 76, 95, 96])
In addition to this approach, several other options exist for coupling DF with other processes which can use DF by-products. Among them, some of the most promising alternatives (Fig. 2) that have been proposed are: DF and direct recovery of value-added compounds in the effluent [73], DF and photofermentation for hydrogen production [74], DF and microbial electrolysis for hydrogen production [75] and DF and microalgae growth in the effluents for biofuel production [76]. Eventually, a final AD stage could always be included to further valorize and stabilize the residual biomass [20].
Among other biotechnologies that could utilize the metabolic by-products generated by DF processes, bio-electrochemical systems have been proposed as a technology that can be coupled with fermentative hydrogen production [77]. More specifically, microbial electrolysis cells (MECs) , a recent emerging technology related to microbial fuel cells (MFCs) , is a promising candidate for the improvement of classical, single-stage DF to generate hydrogen gas with a better efficiency [78]. Microbial electrolysis is accomplished in an electrochemical reactor, in which bacteria referred as exoelectrogens [79] oxidize a substrate and release electrons to the anode providing an electric current that is then used at the cathode to electrochemically produce hydrogen from water. However, this process requires a small external power supply in order to make the hydrogen production thermodynamically favorable [79]. Hydrogen from MECs is considered a very promising route with near term commercialization potential [80] and it has been recently demonstrated that coupling DF and MEC for organic waste/wastewater treatment and/or by-products transformation highly increases the hydrogen yield compared to DF alone and thus constitutes not only a suitable but also a highly promising route for producing bio-hydrogen within the scheme of an environmental biorefinery [75, 81,82,83].
To produce further hydrogen from DF effluents, another option that has received a lot of attention in the recent years is the coupling of DF with photo-fermentation (Fig. 2, process 2). In this process, the effluents from DF are consumed by purple non sulfur photosynthetic bacteria in a secondary anaerobic reactor. These microorganisms use light as energy source and the organic matter from DF as electron donor , converting VFA to hydrogen and carbon dioxide. A great advantage of this process is that purple non sulfur bacteria are able to use a wide range of organic acids as substrate , making photofermentation a suitable post-treatment of DF effluents. This further hydrogen production has served to increase the productions yields significantly. As reported in Ghimire et al. [20], combined hydrogen yields up to 10.25 mol H2 mol sucrose−1 were achieved. In addition, from a total yield of 5.48 mol H2 mol glucose−1, 4.16 mol H2 mol glucose−1 were produced in the photo-fermentation stage, indicating its importance to improve the global hydrogen yields of this process [84]. Eventually, residual organic matter from the photobioreactor can be send to an AD reactor (Fig. 2, process 3) for completing the biomass final stabilization.
Indeed, as aforementioned, AD is the most widely applied process and it can be considered as the final step of most of DF biorefinery pathways (including DF, MEC, photofermentation and algae cultivation) to further stabilize the end products (Fig. 2).
Instead of producing further hydrogen, an interesting alternative is the production of biodiesel by using the effluents from DF as substrate for cultivation of microalgae . This is an attractive options because it allows the production of both gaseous (hydrogen by DF) and liquid (i.e., biodiesel from algal lipids ) biofuels (Fig. 2, process 4). This alternative relies on the heterotrophic growth of microalgae , which can uptake the organic matter present in the DF effluents (preferably acetate) for their growth [76]. Afterwards, the lipids produced by the algal biomass could be converted into biodiesel by transesterification and the remaining biomass could be used for methane production by AD. Still on its infancy, this is clearly a process worthy to be pursued in the future.
Moreover, as mentioned before, the direct utilization of the DF effluent or the direct recovery of the most value-added compounds already present in this stream have are options that have also been considered (Fig. 2, process 5). Indeed, as listed by Ghimire et al. [20], the effluent from DF has been directly used as carbon sources for biological nutrient removal from wastewater, for sulfur and sulfide reduction and for producing phosphate solubilizing biofertilizer. In addition, depending on the DF working conditions, high concentrations of value-added co-products, such as ethanol, butyric acid, caproic acid or 1,3-propanediol in the effluent can be achieved [70, 73]. Although the direct recovery/purification of these compounds from DF effluents remains unexplored, the high prices associated with these co-products make this alternative a simple approach to improve the economic viability of DF.
Finally, some authors have pointed out the feasibility of generating other value-added products from DF effluents, such as polyhydroxyalkanoates and microbial lipids [20, 70].
In order to consolidate DF as a main technology for the future, the aforementioned biorefineries should have high energy efficiencies, generating value-added products while applying almost zero-waste production processes [71]. Holistic studies are needed to evaluate the environmental impacts and the economic feasibility of these systems and more research must be carried out to increase the yields of products.
6 Outlook of Bio-hydrogen as Energy Carrier
6.1 Hydrogen as Energetic Vector for Future Transportation
Fossil fuels are finite. No wonder that today there is a constant search for alternative sources of clean energy worldwide. And it is this quest that will determine the next “champions” of the world race for energy security in several sectors of the energy system such as the power sector, the industry, the building sector and transportation .
The hydrogen marketFootnote 1 is growing incredibly fast due to the flexibility of its production, which can be from any prevalent primary energy source (i.e., biomass, natural gas and coal). Furthermore, hydrogen can be stored in large quantities over long periods although it requires high pressurization (700 bars) and adapted materials to avoid leakages. H2 can then be distributed in both centralized and decentralized systems as energy carrier for diverse end-use applications.
According to the International Energy Agency [85] hydrogen can be re-transformed into (i) electricity for powering buildings and industries (power-to-power); it can be mixed into (ii) the natural gas grid or converted to synthetic methane (power-to-gas); or even sold as (iii) fuel for fuel cell electric vehicle (FCEV) to the transport sector (power-to-fuel).
To date, the status of hydrogen-based technologies for the aforementioned alternatives are presented as follows: (i) Power-to-power storage systems still must to achieve the leveled cost of electricity (LCOEFootnote 2) of USD 90 per MWh, as in the breakthrough scenario, the cost of investment attributable to both the electrolyzer (i.e., to achieve the electrolysisFootnote 3) and the fuel cell would need to drop to around USD 400 per MWh, and efficiencies would need to increase to up to 90% for electrolyzers and 60% for fuel cells higher heating value (HHV) [85]. (ii) A low blend share of 5% hydrogen mixed with natural gas are close to the benchmark [85]; (iii) around 550 FCEV (passenger cars and buses) are running in several demonstration projects across the world. Toyota launched its Mirai (“Future”) model in Japan in 2014, Hyundai is planning to begin the sale of FCEVs in the near future (the Hyundai Tucson FCEV has been available for lease since summer 2014), and Honda announced plans to launch its next generation FCEV in 2016 [85, 86].
Only focusing on improving the technology is not sufficient, new and more integrated approaches need to be applied to create viable business cases. The association of i–iii point towards a link between the different energy sectors and networks, increasing the operational flexibility of future low-carbon energy systems as illustrate in Fig. 3.
Transformation of today’s energy system with hydrogen as renewable energy linking different energy sectors (collected from International Energy Agency, 2015, [85])
According to Kapdan and Kargi [57], it is expected that hydrogen will account for 8–10% of the total energy market in the United States of America by 2025 with hydrogen power and transport systems available in all regions of the country by 2040. A similar trend can be observed in Germany with a remarkable concentration of activity on hydrogen-based large-scale energy storage and Japan ranking first for delivered systems due to the successful upscaling of the Ene-Farm micro co-generation power system [85].
6.2 Potential Role of Dark Fermentation in ‘Green’ Hydrogen Production
Undeniable progress on hydrogen and fuel cell technologies have been achieved since the first FCEV developed in the 1960s [87]. However, the adoption of renewable hydrogen is still in the early stages of commercialization and currently struggle to compete with alternative technologies (fossil-derived hydrogen with or without carbon capture and storage—CCS), including other low-carbon options, due to high costs [85].
Highlighting renewable pathways of hydrogen generation, more specifically electrolysis versus DF process, both have been supporting the hydrogen market progress and unlocking public and private funds for research, development and demonstration though electrolysis represents the only process modelled explicitlyFootnote 4 and counting with 8 GW of capacity installed worldwide [85]. However, even under optimistic assumptions, in relation to the electrolyze techno-economic parameters, electrolytic hydrogen remains considerably more expensive than hydrogen from natural gas reforming, unless very low cost renewable electricity is available and carbon or natural gas prices are high [85]. Based on that, the question of how to produce the required hydrogen remains a main issue to be addressed.
The future of DF as a core technology for hydrogen generation lies within the concept of the biorefinery . In such scenario, DF process has the advantage over other pathways for ensuring the biological hydrogen generation associated with the production of value-added compounds (organic acids, solvents, etc.) or alternatively, to the treatment of residual liquid stream, when a methanogenic reactor is coupled to the fermentative system.
However, biohydrogen generation by DF is still a technological challenge for being a very sensitive process, requiring careful balancing of pH [88], temperature [89], organic loading rate [90] and specific organic loading rate [91, 92]. Moreover, the hydrogen yields in fermentative systems are mostly between 1.2–2.3 mol H2 mol hexose−1 [20], representing only 30–50% of the theoretical maximum hydrogen yield (4 mol H2 mol glucose−1).
As an illustration, in Ferraz Júnior et al. [90, 93] the theoretical calculation of energy conversion for one liter of sugarcane vinasse in a two-stage system (acidogenic/methanogenic) was 45.5 W, with only 1.5 W corresponding to the hydrogen generation in the first stage. Corroborating these findings, it is expected that DF will account for no more than the 10–12% of the total hydrogen produced by 2050 [85].
Recent studies have speculated that the construction of industrial DF processes would be economically feasible. The economic viability of a DF system in a solid wastes plant depends mainly on the evolution of the biohydrogen price in the near future [94] and the cost optimization of the operational conditions (i.e., improved metabolic pathway of hydrogen at low energy costs). Economic evaluation should consider the energy costs of the process, as assessed on a thermophilic hydrogen production system with a working volume of 1947.8 m3 and fed with sugarcane vinasses to support the investments made in system implementation within 2 years [88].
Optimistically, new alternatives are coming up to valorize biohydrogen, such as the biohythane (i.e., a fuel that blends until 20% hydrogen with 80% natural gas) [70]. Those alternatives could be interesting options to increase the calorific value in the natural gas grid, to stabilize the energy supply in rural areas where the access to the grid might be limited, and to act as backup system when other energy sources are insufficient to supply the required demand. Therefore, a strong policy, regulatory framework and finance (hydrogen-based) associated to improving the efficiency of DF systems (optimization of reactors design and operation; and most important, hydrogen productivities and yields) will guarantee the economic feasibility of waste valorization by DF.
Notes
- 1.
In this section, it is important to take into consideration that hydrogen is referred to as energy carrier and not as an energy source: although hydrogen as a molecular component is abundant in nature, energy needs to be used to generate pure hydrogen which incurs a cost and suffers from thermodynamic losses.
- 2.
LCOE is a measure of a power source which attempts to compare different methods of electricity generation on a consistent basis. It is an economic assessment of the average total cost to build and operate a power-generating asset over its lifetime divided by the total energy output of the asset over that lifetime. The LCOE can also be regarded as the minimum cost at which electricity must be sold in order to break-even over the lifetime of the project.
- 3.
Electrolysis is a process of splitting water into hydrogen and oxygen by applying a direct current, converting electricity into chemical energy.
- 4.
Explicit methods calculate the state of a system at a later time from the state of the system at the current time.
References
Dutta S (2014) A review on production, storage of hydrogen and its utilization as an energy resource. J Ind Eng Chem 20(4):1148–1156
ADEME (2016) L’hydrogène dans la transition énergétique p 1–7
Marbán G, Valdés-Solís T (2007) Towards the hydrogen economy? Int J Hydrog Energy 32(12):1625–1637
Lin CY, Lay CH, Sen B, Chu CY, Kumar G, Chen CC, Chang JS (2012) Fermentative hydrogen production from wastewaters: a review and prognosis. Int J Hydrog Energy 37(20):15632–15642
Ren N, Wang A, Cao G, Xu J, Gao L (2009) Bioconversion of lignocellulosic biomass to hydrogen: potential and challenges. Biotechnol Adv 27(6):1051–1060
Moscoviz R, Toledo-Alarcón J, Trably E, Bernet N (2016) Electro-fermentation: How to drive fermentation using electrochemical systems. Trends Biotechnol 34(11):856–865
Logan BE, Oh SE, Kim IS, Van Ginkel S (2002) Biological hydrogen production measured in batch anaerobic respirometers. Environ Sci Technol 36(11):2530–2535
Nandi R, Sengupta S (1998) Microbial production of hydrogen: an overview. Critic Rev Microbiol 24(1):61–84
Hwang MH, Jang NJ, Hyun SH, Kim IS (2004) Anaerobic bio-hydrogen production from ethanol fermentation: the role of pH. J Biotechnol 111(3):297–309
Saady NMC (2013) Homoacetogenesis during hydrogen production by mixed cultures dark fermentation: unresolved challenge. Int J Hydrog Energy 38(30):13172–13191
Mathews J, Wang G (2009) Metabolic pathway engineering for enhanced biohydrogen production. Int J Hydrog Energy 34(17):7404–7416
Kalia VC, Purohit HJ (2008) Microbial diversity and genomics in aid of bioenergy. J Ind Microbiol Biotechnol 35(5):403–419
Angenent LT, Karim K, Al-Dahhan MH, Wrenn BA, Domíguez-Espinosa R (2004) Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol 22(9):477–485
Hallenbeck PC, Benemann JR (2002) Biological hydrogen production; fundamentals and limiting processes. Int J Hydrog Energy 27(11–12):1185–1193
Logan BE (2004) Extracting hydrogen and electricity from renewable resources. Environ Sci Technol 38(9):160–167
Ramírez-Morales JE, Tapia-Venegas E, Toledo-Alarcón J, Ruiz-Filippi G (2015) Simultaneous production and separation of biohydrogen in mixed culture systems by continuous dark fermentation. Water Sci Technol 71(9):1271–1285
Tapia-Venegas E, Ramirez-Morales JE, Silva-Illanes F, Toledo-Alarcón J, Paillet F, Escudie R, Ruiz-Filippi G (2015) Biohydrogen production by dark fermentation: scaling-up and technologies integration for a sustainable system. Rev Environ Sci Bio 14(4):761–785
Nath K, Das D (2004) Improvement of fermentative hydrogen production: various approaches. Appl Microbiol Biotechnol 65(5):520–529
Elsharnouby O, Hafez H, Nakhla G, El MH (2013) A critical literature review on biohydrogen production by pure cultures. Int J Hydrog Energy 38(12):4945–4966
Ghimire A, Frunzo L, Pirozzi F, Trably E, Escudie R, Lens PNL, Esposito G (2015) A review on dark fermentative biohydrogen production from organic biomass: process parameters and use of by-products. Appl Energ 144:73–95
Li C, Fang HHP (2007) Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Environ Sci Technol 37(1):1–39
Wang JL, Wan W (2009) Factors influencing fermentative hydrogen production: a review. Int J Hydrog Energy 34(2):799–811
Valdez-Vazquez I, Ríos-Leal E, Esparza-García F, Cecchi F, Poggi-Varaldo HM (2005) Semi-continuous solid substrate anaerobic reactors for H2 production from organic waste: Mesophilic versus thermophilic regime. Int J Hydrog Energy 30(13–14):1383–1391
Valdez-Vazquez I, Poggi-Varaldo HM (2009) Hydrogen production by fermentative consortia. Renew Sustain Energy Rev 13(5):1000–1013
Guo XM, Trably E, Latrille E, Carrère H, Steyer JP (2010) Hydrogen production from agricultural waste by dark fermentation: a review. Int J Hydrog Energy 35(19):10660–10673
Bundhoo MAZ, Mohee R (2016) Inhibition of dark fermentative bio-hydrogen production: a review. Int J Hydrog Energy 41:6713–6733
Azwar MY, Ma Hussain, Abdul-Wahab aK (2014) Development of biohydrogen production by photobiological, fermentation and electrochemical processes: a review. Renew Sust Energy Rev 31:158–173
Wang JL, Wan W (2008) Comparison of different pretreatment methods for enriching hydrogen-producing bacteria from digested sludge. Int J Hydrog Energy 33(12):2934–2941
Kannaiah Goud R, Sarkar O, Venkata Mohan S (2014) Regulation of biohydrogen production by heat-shock pretreatment facilitates selective enrichment of Clostridium sp. Int J Hydrog Energy 39(14):7572–7586
Bakonyi P, Nemestóthy N, Simon V, Bélafi-Bakó K (2014) Review on the start-up experiences of continuous fermentative hydrogen producing bioreactors. Renew Sustain Energy Rev 40:806–813
Boboescu IZ, Ilie M, Gherman VD, Mirel I, Pap B, Negrea A, Maróti G (2014) Revealing the factors influencing a fermentative biohydrogen production process using industrial wastewater as fermentation substrate. Biotechnol Biofuels 7(1):139–149
Ben-yi X, Jun-xin L (2006) Effects of thermally pretreated temperature on bio-hydrogen production from sewage sludge. J Environ Sci 18(1):6–12
Mohan SV (2008) Fermentative hydrogen production with simultaneous wastewater treatment: Influence of pretreatment and system operating conditions. J Sci Ind Res India 67(11):950–961
Penteado ED, Lazaro CZ, Sakamoto IK, Zaiat M (2013) Influence of seed sludge and pretreatment method on hydrogen production in packed-bed anaerobic reactors. Int J Hydrog Energy 38(14):6137–6145
Cisneros-Pérez C, Carrillo-Reyes J, Celis LB, Alatriste-Mondragón F, Etchebehere C, Razo-Flores E (2015) Inoculum pretreatment promotes differences in hydrogen production performance in EGSB reactors. Int J Hydrog Energy 40(19):6329–6339
El-Bery H, Tawfik A, Kumari S, Bux F (2013) Effect of thermal pre-treatment on inoculum sludge to enhance bio-hydrogen production from alkali hydrolysed rice straw in a mesophilic anaerobic baffled reactor. Environ Technol 34(13):1965–1972
Luo G, Xie L, Zou Z, Wang W, Zhou Q (2010) Evaluation of pretreatment methods on mixed inoculum for both batch and continuous thermophilic biohydrogen production from cassava stillage. Bioresour Technol 101(3):959–964
Kan E (2013) Effects of pretreatments of anaerobic sludge and culture conditions on hydrogen productivity in dark anaerobic fermentation. Renew Energy 49:227–231
Chen CC, Lin CY, Lin MC (2002) Acid-base enrichment enhances anaerobic hydrogen production process. Appl Microbiol Biotechnol 58(2):224–228
Chaganti SR, Kim DH, Ja Lalman (2012) Dark fermentative hydrogen production by mixed anaerobic cultures: Effect of inoculum treatment methods on hydrogen yield. Renew Energ 48:117–121
Yin Y, Hu J, Wang J (2014) Enriching hydrogen-producing bacteria from digested sludge by different pretreatment methods. Int J Hydrog Energy 39(25):13550–13556
Zhu H, Beland M (2006) Evaluation of alternative methods of preparing hydrogen producing seeds from digested wastewater sludge. Int J Hydrog Energy 31(14):1980–1988
Kenealy W, Zeikus JG (1981) Influence of corrinoid antagonists on methanogen metabolism. J Bacteriol 146(1):133–140
Chandrasekhar K, Lee Y, Lee D (2015) Biohydrogen production: Strategies to Improve Process Efficiency through Microbial Routes. Int J Mol Sci 16(4):8266–8293
Lin CY, Lay CH (2005) A nutrient formulation for fermentative hydrogen production using anaerobic sewage sludge microflora. Int J Hydrog Energy 30(3):285–292
Lin CY, Lay CH (2004) Carbon/nitrogen-ratio effect on fermentative hydrogen production by mixed microflora. Int J Hydrog Energy 29(1):41–45
Das D, Veziroglu T (2008) Advances in biological hydrogen production processes. Int J Hydrog Energy 33(21):6046–6057
Hallenbeck PC, Ghosh D (2009) Advances in fermentative biohydrogen production: the way forward? Trends Biotechnol 27(5):287–297
Kim DH, Kim SH, Jung KW, Kim MS, Shin HS (2011) Effect of initial pH independent of operational pH on hydrogen fermentation of food waste. Bioresour Technol 102(18):8646–8652
Xie GJ, Feng LB, Ren N, Ding J, Liu C, Xing DF, Ren HY (2010) Control strategies for hydrogen production through co-culture of Ethanoligenens harbinense B49 and immobilized Rhodopseudomonas faecalis RLD-53. Int J Hydrog Energy 35(5):1929–1935
Li Z, Wang H, Tang Z, Wang X, Bai J (2008) Effects of pH value and substrate concentration on hydrogen production from the anaerobic fermentation of glucose. Int J Hydrogen Energ 33(24):7413–7418
De Gioannis G, Friargiu M, Massi E, Muntoni A, Polettini A, Pomi R, Spiga D (2014) Biohydrogen production from dark fermentation of cheese whey: Influence of pH. Int J Hydrog Energy 39(36):20930–20941
Karadag D, Puhakka JA (2010) Effect of changing temperature on anaerobic hydrogen production and microbial community composition in an open-mixed culture bioreactor. Int J Hydrog Energy 35(20):10954–10959
Lazaro CZ, Perna V, Etchebehere C, Varesche MBA (2014) Sugarcane vinasse as substrate for fermentative hydrogen production: The effects of temperature and substrate concentration. Int J Hydrog Energy 39(12):6407–6418
Si B, Li J, Li B, Zhu Z, Shen R, Zhang Y, Liu Z (2015) The role of hydraulic retention time on controlling methanogenesis and homoacetogenesis in biohydrogen production using upflow anaerobic sludge blanket (UASB) reactor and packed bed reactor (PBR). Int J Hydrog Energy 40(35):11414–11421
Ren N, Guo W, Liu B, Cao G, Ding J (2011) Biological hydrogen production by dark fermentation: challenges and prospects towards scaled-up production. Curr Opin Biotech 22(3):365–370
Kapdan IK, Kargi F (2006) Bio-hydrogen production from waste materials. Enzyme Microb Tech 38(5):569–582
Chong ML, Sabaratnam V, Shirai Y, Hassan MA (2009) Biohydrogen production from biomass and industrial wastes by dark fermentation. Int J Hydrog Energy 34(8):3277–3287
Li D, Chen H (2007) Biological hydrogen production from steam-exploded straw by simultaneous saccharification and fermentation. Int J Hydrog Energy 32(12):1742–1748
Zhang ML, Fan YT, Xing Y, Pan CM, Zhang GS, Lay JJ (2007) Enhanced biohydrogen production from cornstalk wastes with acidification pretreatment by mixed anaerobic cultures. Biomass Bioenerg 31(4):250–254
Ivanova G, Rakhely G, Kovacs KL (2009) Thermophilic biohydrogen production from energy plants by Caldicellulosiruptor saccharolyticus and comparison with related studies. Int J Hydrog Energy 34(9):3659–3670
Burton CH, Turner C (2003) Manure management: treatment strategies for sustainable agriculture. Silsoe Research Institute, London
Jayalakshmi S, Joseph K, Sukumaran V (2009) Biohydrogen generation from kitchen waste in an inclined plug flow reactor. Int J Hydrog Energy 34(21):8854–8858
Liu D, Liu D, Zeng RJ, Angelidaki I (2006) Hydrogen and methane production from household solid waste in the two-stage fermentation process. Water Res 40(11):2230–2236
Eroglu E, Erolu I, Gunduz U, Yucel M (2009) Treatment of olive mill wastewater by different physicochemical methods and utilization of their liquid effluents for biological hydrogen production. Biomass Bioenerg 33(4):701–705
Chookaew T, O-Thong S, Prasertsan P (2012) Fermentative production of hydrogen and soluble metabolites from crude glycerol of biodiesel plant by the newly isolated thermotolerant Klebsiella pneumoniae TR17. Int J Hydrog Energy 37(18):13314–13322
Venetsaneas N, Antonopoulou G, Stamatelatou K, Kornaros M, Lyberatos G (2009) Using cheese whey for hydrogen and methane generation in a two-stage continuous process with alternative pH controlling approaches. Bioresour Technol 100(15):3713–3717
Yokoi H, Maki R, Hirose J, Hayashi S (2002) Microbial production of hydrogen from starch-manufacturing wastes. Biomass Bioenerg 22(5):389–395
Rama Mohan S (2016) Strategy and design of innovation policy roadmapping for a waste biorefinery. Bioresour Technol 215:76–83
Bastidas-Oyanedel JR, Bonk F, Thomsen MH, Schmidt JE (2015) Dark fermentation biorefinery in the present and future (bio)chemical industry. Rev Environ Sci Bio 14(3):473–498
Venkata SM, Nikhil GN, Chiranjeevi P, Nagendranatha Reddy C, Rohit MV, Naresh Kumar A, Sarkar O (2016) Waste biorefinery models towards sustainable bioeconomy: critical review and future perspectives. Bioresour Technol 2015:2–12
Sawatdeenarunat C, Nguyen D, Surendra KC, Shrestha S, Rajendran K, Oechsner H, Khanal SK (2016) Anaerobic biorefinery: current status, challenges, and opportunities. Bioresour Technol 215:304–313
Sarma SJ, Pachapur V, Brar SK, Le Bihan Y, Buelna G (2015) Hydrogen biorefinery: potential utilization of the liquid waste from fermentative hydrogen production. Renew Sustain Energy Rev 50:942–951
Zong W, Yu R, Zhang P, Fan M, Zhou Z (2009) Efficient hydrogen gas production from cassava and food waste by a two-step process of dark fermentation and photo-fermentation. Biomass Bioenerg 33(10):1458–1463
Marone A, Ayala-Campos OR, Trably E, Carmona-Martinez AA, Moscoviz R, Latrille E, Bernet N (2016) Coupling dark fermentation and microbial electrolysis to enhance bio-hydrogen production from agro-industrial wastewaters and by-products in a bio-refinery framework. Int J Hydrog Energy 42(3):1609–1621
Turon V, Trably E, Fouilland E, Steyer JP (2016) Potentialities of dark fermentation effluents as substrates for microalgae growth: a review. Proc Biochem 51(11):1843–1854
Rozendal RA, Hamelers HVM, Euverink GJW, Metz SJ, Buisman CJN (2006) Principle and perspectives of hydrogen production through biocatalyzed electrolysis. Int J Hydrog Energ 31(12):1632–1640
Kumar G, Bakonyi P, Kobayashi T, Xu K, Sivagurunathan P, Kim S, Béla K (2016) Enhancement of biofuel production via microbial augmentation: The case of dark fermentative hydrogen. Renew Sustain Energ Rev 57:879–891
Logan B, Regan J (2006) Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol 14:512–518
Lee HS, Vermaas WFJ, Rittmann BE (2010) Biological hydrogen production: prospects and challenges. Trends Biotechnol 28(5):62–271
Moreno R, Escapa A, Cara J, Carracedo B, Gómez X (2015) A two-stage process for hydrogen production from cheese whey: Integration of dark fermentation and biocatalyzed electrolysis. Int J Hydrog Energ 40(1):168–175
Dhar BR, Elbeshbishy E, Hafez H, Lee HS (2015) Hydrogen production from sugar beet juice using an integrated biohydrogen process of dark fermentation and microbial electrolysis cell. Bioresour Technol 198:223–230
Li XHH, Liang DWW, Bai YXX, Fan YTT, Hou HWW (2014) Enhanced H2 production from corn stalk by integrating dark fermentation and single chamber microbial electrolysis cells with double anode arrangement. Int J Hydrog Energy 39(17):8977–8982
Su H, Cheng J, Zhou J, Song W, Cen K (2009) Combination of dark- and photo-fermentation to enhance hydrogen production and energy conversion efficiency. Int J Hydrog Energ 34(21):8846–8853
International Energy Agency (2015) Technology roadmap: hydrogen and fuel cells
The Linde Group (2017) Hydrogen energy applications
Chan CC (2015) Overview of electric, hybrid, and fuel cell vehicles. Encyc Autom Eng
Koyama MH, Araújo Júnior MM, Zaiat M, Ferraz Júnior ADN (2016) Kinetics of thermophilic acidogenesis of typical Brazilian sugarcane vinasse. Energy 116(1):1097–1103
Ferraz Junior ADN, Etchebehere C, Zaiat M (2015) High organic loading rate on thermophilic hydrogen production and metagenomic study at an anaerobic packed-bed reactor treating a residual liquid stream of a Brazilian biorefinery. Bioresour Technol 186:81–88
Ferraz Junior ADN, Wenzel J, Etchebehere C, Zaiat M (2014) Effect of organic loading rate on hydrogen production from sugarcane vinasse in thermophilic acidogenic packed bed reactors. Int J Hydrog Energy 39(30):16852–16862
Ferraz Junior ADN, Etchebehere C, Zaiat M (2015) Mesophilic hydrogen production in acidogenic packed-bed reactors (APBR) using raw sugarcane vinasse as substrate: influence of support materials. Anaerobe 34:94–105
MdelP Anzola-Rojas, da Fonseca SG, da Silva CC, de Oliveira VM, Zaiat M (2015) The use of the carbon/nitrogen ratio and specific organic loading rate as tools for improving biohydrogen production in fixed-bed reactors. Biotechnol Rep 5:46–54
Ferraz Júnior ADN, Koyama MH, Araújo Júnior MM, Zaiat M (2016) Thermophilic anaerobic digestion of raw sugarcane vinasse. Renew Energy 89:245–252
Han W, Fang J, Liu Z, Tang J (2016) Techno-economic evaluation of a combined bioprocess for fermentative hydrogen production from food waste. Bioresour Technol 202:107–112
Capson-Tojo G, Rouez M, Crest M, Steyer JP, Delgenès JP, Escudié R (2016) Food waste valorization via anaerobic processes: a review. Rev Environ Sci Bio 15(3):499–547
Redwood MD, Orozco RL, Majewski AJ, Macaskie LE (2012) An integrated biohydrogen refinery: synergy of photofermentation, extractive fermentation and hydrothermal hydrolysis of food wastes. Bioresour Technol 119:384–392
Acknowledgements
Javiera Toledo-Alarcon is grateful to the Chilean National Commission for Scientific Research and Technology (CONICYT) for the award of her PhD scholarship. Gabriel CAPSON-TOJO is thankful to Suez, which has financed his research under the CIFRE convention 434 N° 2014/1146. A. Marone postdoctoral program was funded by the Marie Curie Intra-European Fellowship WASTE2BIOHY (FP7-MCIEF-326974) under the 7th Framework Programme of the European Community. F. Paillet Ph.D. work was supported by the TRIFYL center, administrative department syndicate for treatment and valorization of municipal solid waste , the French Environment and Energy Management Agency (ADEME) and the French Institute for Agricultural and Food Research (INRA). Antônio Djalma N. FERRAZ JÚNIOR gratefully acknowledges the financial support from FAPESP (Project 2013/15665-8 and 2015/21650-9).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Toledo-Alarcón, J. et al. (2018). Basics of Bio-hydrogen Production by Dark Fermentation. In: Liao, Q., Chang, Js., Herrmann, C., Xia, A. (eds) Bioreactors for Microbial Biomass and Energy Conversion. Green Energy and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-10-7677-0_6
Download citation
DOI: https://doi.org/10.1007/978-981-10-7677-0_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7676-3
Online ISBN: 978-981-10-7677-0
eBook Packages: EnergyEnergy (R0)