Abstract
Postoperative pancreatic fistula (PF) is a common surgical complication following the Whipple procedure (pancreaticoduodenectomy). Several interventions have been advocated to diminish the rate of clinically significant fistulae, which can approach 30% in high-risk patients. The method of pancreatic anastomosis has been the subject of considerable study, and herein we describe the through-and-through transpancreatic duct-to-mucosa pancreaticojejunostomy, also known as the Blumgart anastomosis. Clinical evidence, including retrospective institutional series, comparison studies, and single-institution randomized trials all demonstrate a decreased incidence of PF, as well as improved secondary endpoints such as length of stay and overall surgical complications. Given these findings, we describe here the key considerations in this surgical technique and review the body of clinical evidence surrounding its utilization in the clinical setting.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
11.1 Introduction (Literature Review)
Pancreatic fistula (PF) remains a common and significant source of postoperative morbidity in patients undergoing pancreaticoduodenectomy. While mortality following the Whipple procedure has improved substantially to rates close to 1% in several large Western centers [1, 2], morbidity remains high with rates of 30–40% [3]. Whereas for most operative interventions surgical complications typically include anastomotic leak, hemorrhage, and wound infection, it is the pancreatic leak or fistula that is the most common source of perioperative morbidity. Classified by the International Study Group on Pancreatic Fistula (ISGPF) criteria, the clinically relevant Grade B and C fistulae historically required reoperation and now are commonly addressed by percutaneous or endoscopic techniques [4]. These technical advances notwithstanding, pancreatic fistulae remain an intractable source of morbidity following pancreaticoduodenectomy.
The risk factors for pancreatic fistula have been well studied. These include pancreas-specific features [type of pathology, soft texture to the pancreatic parenchyma, or small duct size (often occurring when there is a lack of antecedent pancreatic ductal obstruction)] and patient-specific features [intraoperative blood loss, poor blood supply, diabetes, obesity] that either make the technical reconstruction or wound healing, respectively, considerably more challenging [5]. In patients with both pancreas- and patient-specific risk factors, the incidence of clinically significant postoperative pancreatic fistula approaches 30% [6]. However, even in those patients with low fistula risk scores, the risk of PF remains upward of 6% [7].
Several approaches have been undertaken to prevent pancreatic fistulae. The pharmacologic approach has sought to minimize pancreatic secretions, typically through the use of somatostatin analogues such as octreotide. The extensive data on octreotide are mixed, with a recent Cochrane review suggesting a decreased rate of pancreatic fistula (RR 0.66; 95% CI 0.55–0.79; n = 2206) and postoperative complications (RR 0.70; 95% CI 0.61–0.80; n = 1903) with the use of octreotide. These findings, however, were not significant in those studies using ISGPF criteria to determine clinical relevance (RR 0.69; 95% CI 0.38–1.28; n = 292) [8]. Pasireotide, a somatostatin analog with a longer half-life than octreotide, was shown in a randomized controlled trial performed at our institution to decrease the incidence of clinically significant pancreatic fistulae following pancreaticoduodenectomy as compared to placebo (10% vs. 21%; p = 0.04) [9]. Several subsequent studies have since suggested that this intervention is cost-effective [10,11,12].
Other factors that have been evaluated have been the type of suture material, the use of fibrin sealants (to better secure the pancreaticojejunal anastomosis), and the use of external and internal pancreatic stents. One retrospective comparison study has evaluated the outcomes following the use of absorbable and nonabsorbable sutures, finding no difference between suture types [13]. Similarly, fibrin sealants appear to offer no benefit in terms of perioperative morbidity and mortality, with an incidence of pancreatic fistula following pancreatic resection across several randomized studies of 29.6% when fibrin sealants were used and 31.0% in control groups (RR 0.93; 95% CI 0.71–1.21; p = 0.58) [14]. With respect to stent placement, internal stenting across the pancreaticojejunal anastomosis does not reduce the rate of PF formation [15], but there does appear to be a risk reduction with externalized stents (6% stent vs. 22% no stent; p = 0.04) [16]. However, systematic review does not support the use of either external or internal stenting [17].
Perhaps the most abundantly researched in the effort to lower the incidence of pancreatic fistulae has been the specific technique of pancreatic anastomosis. Among the many established approaches are the typical duct-to-mucosa pancreaticojejunostomy (with or without parenchymal sutures), invagination into the jejunum, and pancreaticogastrostomy [18]. Despite extensive study, the literature fails to offer clarity on which approach is associated with improved outcomes. Recent randomized controlled trials have suggested a superiority of pancreaticogastrostomy over pancreaticojejunostomy when subjected to meta-analysis with rates of PF reduced from 18.7% to 11.2% [19]. Another contemporaneous meta-analysis showed similar findings across seven randomized controlled trials, with a reduction in PF among pancreaticogastrostomy patients (10.6% vs. 18.5%; p = 0.0002). Subgroup analysis restricted to those studies in which ISGPF criteria were used also showed a significant difference (8.3% vs. 20.5%; p < 0.00001) [20]. Additional meta-analyses have also suggested a decreased incidence of PF with pancreaticogastrostomy and a modest impact on overall morbidity [21,22,23]. However, a more recent multicenter German randomized controlled trial (RECOPANC) included 320 patients in an intent-to-treat analysis, finding no difference in the incidence of Grade B/C pancreatic fistulae between pancreaticogastrostomy and pancreaticojejunostomy (20% vs. 22%; p = 0.617) [24], and a greater number of postoperative hemorrhage in the PG group. In the end, while the preponderance of data suggests that pancreaticogastrostomy should be preferred over pancreaticojejunostomy, it is crucial to note that the pancreaticojejunostomy techniques employed in the aforementioned studies were invariably heterogeneous.
Indeed, the outcomes following alternative methods of pancreaticojejunostomy can be quite varied. In an early randomized trial comparing invagination to a duct-to-mucosa anastomosis, the former was associated with a lower rate of pancreatic fistula formation (12% vs. 24%; p < 0.05) [25]. This contrasts with the prior randomized trial from Italy showing no difference in outcomes between the two techniques, including a similar incidence of pancreatic fistula (13% for duct-to-mucosa anastomosis and 15% for invagination; p = ns) [26]. Additional trials showing conflicting data regarding these methods have since followed [27], and a recent meta-analysis showed no significant difference (OR = 1.23 for duct-to-mucosa vs. invagination; p = 0.38) across the published studies [28].
At our institution, we utilize an altogether different reconstructive method—the Blumgart pancreaticojejunostomy—that combines a duct-to-mucosa anastomosis with transpancreatic sutures. Because the jejunum is imbricated over the entire transected pancreatic parenchymal surface, this technique essentially couples a duct-to-mucosa anastomosis to the proposed advantages of invagination. Performed over the last 25 years at our institution and by hundreds of trainees across the world, this technique has become our preferred means of pancreaticojejunal anastomosis. Here we discuss the critical aspects of the surgical technique and review the literature regarding its use in the reconstruction following pancreaticoduodenectomy.
11.2 Surgical Technique
Resection is performed in the standard fashion, with removal of the head of the pancreas and the duodenum. Pylorus preservation can be performed as dictated by the indication for operative intervention. During pancreatic transection, four stay sutures—typically consisting of a 3-0 monofilament absorbable suture (such as PDS)—are placed at the superior and inferior aspect of the pancreas on both sides of the intended line of transection. Two of these remain on the pancreatic remnant following extirpation of the specimen. At this point, the remnant is prepared by dissecting it free of the splenic artery and vein for a length of 1–2 cm. The jejunal limb is then brought through a defect in the transverse mesocolon that is created to the right of the middle colic vessels, such that the proximal jejunal end sits to the right of the inferior aspect of the pancreatic remnant. Absorbable stay sutures are placed in the jejunum approximately 10–15 cm apart—representing the full extent of the jejunum incorporated into the future anastomosis—in order to splay out the small intestine during the creation of the retrocolic pancreaticojejunostomy.
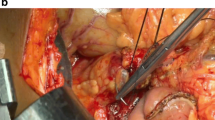
The critical component of the through-and-through (Blumgart) approach is the careful placement of several transpancreatic U-sutures. At our institution, we use 3-0 braided absorbable sutures (such as VICRYL) that are placed in interrupted fashion beginning at the superior aspect of the pancreatic remnant. The first suture is placed adjacent to the superior 3-0 PDS stay suture, taking care to avoid the pancreaticoduodenal vessels. As with all the transpancreatic sutures, this stitch is first placed in the anterior surface of the pancreas and brought out the posterior aspect. A horizontal seromuscular stitch in the jejunum—approximately 1 cm of travel along the longitudinal axis of the bowel and well-posterior to the anticipated duct-to-mucosa anastomosis (i.e., closer to the posterior mesentery)—is then placed, with the stitch then taken back through the pancreas posterior to anterior, exiting about 0.5 cm from the initial entry of the stitch on the anterior surface. Six of these ‘U’ horizontal mattress stitches are placed—three cranial and three caudal to the pancreatic duct—taking extreme care on the interior two stitches to avoid placing the stitch through the duct itself. (A plastic stent can be inserted into the duct to avoid this issue.) Each stitch travels along the length of the bowel, incorporating a total of 8–10 cm of jejunum into the anastomosis. The stitches are not tied, and the needles are left on all six stitches; importantly, the jejunum is not approximated to the pancreas at this time. It is critical in the placement of these sutures to maintain organization for later completion (Fig. 11.1).
The duct-to-mucosa anastomosis is then performed. A small enterotomy using the needlepoint on the electrocautery is made in the jejunum, between the third and fourth ‘U’ stitches and exactly on the antimesenteric side of the jejunum. The full anastomosis will usually consist of 6–8 interrupted monofilament absorbable sutures (usually 4-0 or 5-0 PDS) for larger ducts and 4–6 sutures for smaller ducts. Invariably, the first stitch is placed in the 6 o’clock position on the inside of the pancreatic duct, exiting through the posterior aspect of the remnant and then full thickness through the jejunum, from the serosa to the lumen (Fig. 11.2). Adjacent sutures are then placed in the 3 and 9 o’clock positions (for small ducts) or in the 4 and 8 o’clock positions (for normal or larger ducts). As with the initial duct-to-mucosa suture, the needle is first passed from the lumen of the pancreatic duct out the posterior parenchyma and then from serosa to mucosa on the jejunal side.
At this point, the jejunum is parachuted down to the pancreatic remnant, and the transpancreatic U-sutures are tied (with the needles left on). This approximates the jejunum to the posterior face of the pancreatic remnant and decreases tension on the stitches of the duct-to-mucosa anastomosis. The 6 o’clock duct-to-mucosa suture is then tied, followed by the additional posterior stitches (4 and 8 o’clock or 3 and 6 o’clock, as dictated by duct size) (Fig. 11.3). To complete the duct-to-mucosa anastomosis, the anterior set of stitches are then placed in similar fashion —except the 12 o’clock stitch, for which the needle is first placed on the anterior pancreatic parenchymal surface, brought through the duct and then from mucosa to serosa on the jejunal side. These sutures are tied as they are placed.
With the duct-to-mucosa inner layer complete, attention is turned to the completion of the transpancreatic sutures. The needle of the most cranial transpancreatic suture is taken, and two seromuscular bites are taken through the jejunum. The first of these is transversely from the posterior mesenteric side (incorporated in the first part of the anastomosis) to the anterior mesenteric side. The second is parallel to the longitudinal axis of the bowel. This represents the corner transpancreatic U-suture, and the stitch is tied so that the jejunum forms a wrap over the anterior surface of the pancreas at its most superior aspect. The next four transpancreatic sutures are completed by taking 1 cm travel seromuscular stitches in the jejunum (again along the longitudinal axis of the bowel), close to the anterior mesentery so that there is adequate distance from the duct-to-mucosa anastomosis. Each of these is tied so that the jejunum is brought over the anterior surface of the pancreatic parenchyma (Fig. 11.4). The last transpancreatic suture is also a corner stitch with two bites taken—the first is longitudinally on the bowel and the second transversely such that the final needle exit on the jejunum is 0.5 cm from the suture taken through the posterior surface of the jejunum.
The final result of the anastomosis is depicted in Fig. 11.5, showing the entire transected surface of the pancreas completely covered by jejunal serosa. While the Cattell-Warren pancreaticojejunostomy and most other technical approaches do also cover the transected surface, the Blumgart anastomosis differs in that the pancreatic remnant should appear imbricated into the jejunum.
11.3 Results
As with the multitude of measures described in the foregoing, the through-and-through duct-to-mucosa (Blumgart) pancreaticojejunostomy has been the subject of extensive clinical investigation. In a multi-institution report of 187 consecutively treated patients, the Blumgart pancreaticojejunostomy described herein was associated with a 6.9% incidence of Grade B and C pancreatic fistula (by ISGPF criteria). Perioperative mortality was low at 1.6%, and the incidence of reoperation was 5.3%; notably, neither mortality nor reoperation occurred in a patient with a postoperative pancreatic fistula [29]. There was a 13.4% incidence of Grade A pancreatic fistula not altering clinical management. Compared with other retrospective studies employing the ISGPF criteria, this report compares favorably with the results of other technical approaches. Similarly, a group in India applied this technique in 98 consecutive patients, finding an incidence of Grade B and C pancreatic fistula of 7.14%, with only one patient requiring re-exploration due to leak [30]. As various contemporaneous retrospective studies examining the outcomes of other reconstruction techniques have shown an incidence of Grade B and C fistulae of 10–15%, the outcomes following Blumgart pancreaticojejunostomy in these two studies represent a significant improvement [31, 32].
Several studies have provided higher quality evidence by comparing the Blumgart technique directly with other technical methods. One such study employed a before-after retrospective design from a single institution in Germany examining the outcomes following the Blumgart pancreaticojejunostomy versus a Cattell-Warren anastomosis. The Blumgart anastomosis was performed in the fashion described herein, while the latter consisted of interrupted anterior and posterior rows of sutures between the seromuscular jejunum and the anterior and posterior pancreatic capsule, respectively (in conjunction with a duct-to-mucosa anastomosis). The authors of this study observed a statistically significant decrease in operative time and a trend toward decreased blood loss in the Blumgart anastomosis group. Importantly, the rate of surgical complications and the rate of Grade B and C pancreatic fistula were statistically significantly lower (4% vs. 13%; p = 0.032). Finally, in a multivariate analysis, the type of anastomosis (Blumgart vs. Cattell-Warren) was a significant predictor of both major local complications and systemic complications [33]. In another line of evidence, a second German group published similar findings in a randomized study comparing a transpancreatic mattress suture anastomosis to the conventional Cattell-Warren pancreaticojejunostomy. Their technique, representing a variation on the Blumgart anastomosis, employed transpancreatic U-sutures, not in conjunction with a duct-to-mucosa anastomosis, but with the invagination of the entire cut surface of the pancreas into a large jejunal opening; in their hands, there was a trend toward fewer complications in the group using transpancreatic mattress sutures with invagination compared to a group in whom Cattell-Warren anastomoses were performed [34]. In both studies, the authors theorize that the shear forces on the pancreatic parenchyma are lessened by the transpancreatic sutures, as knot-tying compresses the full-thickness parenchyma rather than generates perpendicular force that in the soft pancreas tears through the tissue.
More recently, further evidence to support the use of the Blumgart anastomosis has been offered by additional comparison studies. In one, a Japanese group compared the Kakita method (interrupted full-thickness pancreatic sutures + a duct-to-mucosa anastomosis) to the transpancreatic mattress method described here. Of note, externalized pancreatic stents were employed in all patients with a pancreatic duct of less than 3mm in diameter. In this single-institution matched historical control study with 120 patients in each arm, the Blumgart technique was associated with a significantly lower rate of Grade B and C pancreatic fistula (2.5% vs. 36%; p < 0.001), shorter duration of drain placement, and shorter postoperative stay [35]. Another Japanese group also examined in a retrospective fashion the outcomes of the Kakita method with the Blumgart anastomosis, finding a lower rate of Grade B and C fistula in the through-and-through mattress method (20.5% vs. 37.2%; p = 0.033) [36]. It should be noted that the rate of clinically significant fistulae was quite high in this report and does well exceed those described in Western centers for most methods of reconstruction.
An important piece of evidence has also been recently offered from a Taiwanese group comparing the outcomes of the Blumgart anastomosis with that of pancreaticogastrostomy. Given that several studies have compared various methods of pancreaticojejunostomy with pancreaticogastrostomy and that there is suggestion that the latter may be superior, this comparison is an important one. In a retrospective analysis of a prospectively maintained database, the Taiwanese study examines 206 matched patients undergoing pancreaticoduodenectomy and either Blumgart pancreaticojejunostomy or pancreaticogastrostomy. In this series, the former was associated with a decreased incidence of clinically relevant pancreatic fistulae (7% vs. 20%; p = 0.007), shorter hospital stay, and decreased perioperative mortality (0% vs. 4.9%; p = 0.03) [37]. Taken together, the largely retrospective series throughout the literature do concur that there are fewer complications, and specifically a decreased rate of clinically significant pancreatic fistulae, when the method of reconstruction is that described here.
Despite the foregoing, there remains a paucity of high-level clinical evidence to delineate the optimal method of pancreaticojejunostomy. To address this issue, the PANasta trial has been announced. A multicenter, double-blinded, randomized controlled trial, PANasta, aims to evaluate if the Blumgart pancreaticojejunostomy is superior to the Cattell-Warren anastomosis. The primary endpoint of the study will be the rate of pancreatic fistula, and secondary endpoints include mortality, surgical complications, nonsurgical complications, hospital stay, and completion of adjuvant chemotherapy. Aiming to enroll 253 patients per study arm, the study is powered to detect a 10% absolute risk reduction in the rate of pancreatic fistula and is expected to be completed following an enrollment period of 3 years and a 1-year follow-up [38]. However, in the absence of level I clinical evidence, the literature supports the use of the transpancreatic Blumgart anastomosis over alternative methods of pancreaticojejunostomy with respect to clinical outcomes.
Final considerations in support of the Blumgart anastomosis are the technical advantages for the operating surgeon. The transpancreatic mattress method provides a suitable window for creation of the duct-to-mucosa portion of the anastomosis, as the jejunum is not apposed to the cut surface of the pancreas until the latter is completed. In the Cattell-Warren anastomosis, the jejunum is in apposition with the pancreas when the posterior row of duct-to-mucosa sutures is placed, rendering visualization more difficult. Finally, the transpancreatic method has in our institutional experience proven to be facile to teach to trainees. That many MSKCC-trained surgeons continue to employ this method of reconstruction described herein supports the notion that the anastomosis is reproducible in the hands of surgeons both nationally and internationally.
Conclusions
As surgical complications and specifically pancreatic fistula following pancreaticoduodenectomy are a considerable source of perioperative morbidity, a large body of clinical research has centered on the various pharmacologic and technical measures that can be employed to decrease the incidence of postoperative PF. While the role for several interventions is limited by a paucity of clinical evidence, there are several reports supporting the use of a through-and-through transpancreatic duct-to-mucosa pancreaticojejunostomy, also known as the Blumgart anastomosis. Technically facile, this technique is associated with lower rates of pancreatic fistula across several institutions and in comparison to alternative methods. In the absence of high-level clinical evidence, there remains of preponderance of data to support widespread use of the Blumgart anastomosis as the reconstruction method of choice in patients undergoing pancreaticoduodenectomy.
References
Grant F, Brennan MF, Allen PJ, DeMatteo RP, Kingham TP, D’Angelica M, Fischer ME, Gonen M, Zhang H, Jarnagin WR. Prospective randomized controlled trial of liberal vs restricted perioperative fluid management in patients undergoing Pancreatectomy. Ann Surg. 2016;264:591–8.
Zureikat AH, Postlewait LM, Liu Y, Gillespie TW, Weber SM, Abbott DE, Ahmad SA, Maithel SK, Hogg ME, Zenati M, Cho CS, Salem A, Xia B, Steve J, Nguyen TK, Keshava HB, Chalikonda S, Walsh RM, Talamonti MS, Stocker SJ, Bentrem DJ, Lumpkin S, Kim HJ, Zeh HJ 3rd, Kooby DA. A multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy. Ann Surg. 2016;264:640–9.
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M, International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13.
Tilara A, Gerdes H, Allen P, Jarnagin W, Kingham P, Fong Y, DeMatteo R, D’Angelica M, Schattner M. Endoscopic ultrasound-guided transmural drainage of postoperative pancreatic collections. J Am Coll Surg. 2014;218:33–40.
House MG, Fong Y, Arnaoutakis DJ, Sharma R, Winston CB, Protic M, Gonen M, Olson SH, Kurtz RC, Brennan MF, Allen PJ. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg. 2008;12:270–8.
Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1–14.
Miller BC, Christein JD, Behrman SW, Drebin JA, Pratt WB, Callery MP, Vollmer CM Jr. A multi-institutional external validation of the fistula risk score for pancreatoduodenectomy. J Gastrointest Surg. 2014;18:172–9; discussion 179-80.
Gurusamy KS, Koti R, Fusai G, Davidson BR. Somatostatin analogues for pancreatic surgery. Cochrane Database Syst Rev. 2013:CD008370.
Allen PJ, Gönen M, Brennan MF, Bucknor AA, Robinson LM, Pappas MM, Carlucci KE, D’Angelica MI, DeMatteo RP, Kingham TP, Fong Y, Jarnagin WR. Pasireotide for postoperative pancreatic fistula. N Engl J Med. 2014;370:2014–22.
Abbott DE, Sutton JM, Jernigan PL, Chang A, Frye P, Shah SA, Schauer DP, Eckman MH, Ahmad SA, Sussman JJ. Prophylactic pasireotide administration following pancreatic resection reduces cost while improving outcomes. J Surg Oncol. 2016;113:784–8.
Goyert N, Eeson G, Kagedan DJ, Behman R, Lemke M, Hallet J, Mittmann N, Law C, Karanicolas PJ, Coburn NG. Pasireotide for the prevention of pancreatic fistula following pancreaticoduodenectomy: a cost-effectiveness analysis. Ann Surg. 2017;265:2–10.
Ma LW, Dominguez-Rosado I, Gennarelli RL, Bach PB, Gonen M, D’Angelica MI, DeMatteo RP, Kingham TP, Brennan MF, Jarnagin WR, Allen PJ. The cost of postoperative pancreatic fistula versus the cost of pasireotide: results from a prospective randomized trial. Ann Surg. 2017;265:11–6.
Andrianello S, Pea A, Pulvirenti A, Allegrini V, Marchegiani G, Malleo G, Butturini G, Salvia R, Bassi C. Pancreaticojejunostomy after pancreaticoduodenectomy: Suture material and incidence of post-operative pancreatic fistula. Pancreatology. 2016;16:138–41.
Cheng Y, Ye M, Xiong X, Peng S, Wu HM, Cheng N, Gong J. Fibrin sealants for the prevention of postoperative pancreatic fistula following pancreatic surgery. Cochrane Database Syst Rev. 2016;2:CD009621.
Winter JM, Cameron JL, Campbell KA, Chang DC, Riall TS, Schulick RD, Choti MA, Coleman J, Hodgin MB, Sauter PK, Sonnenday CJ, Wolfgang CL, Marohn MR, Yeo CJ. Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2006;10:1280–90; discussion 1290
Motoi F, Egawa S, Rikiyama T, Katayose Y, Unno M. Randomized clinical trial of external stent drainage of the pancreatic duct to reduce postoperative pancreatic fistula after pancreaticojejunostomy. Br J Surg. 2012;99:524–31.
Dong Z, Xu J, Wang Z, Petrov MS. Stents for the prevention of pancreatic fistula following pancreaticoduodenectomy. Cochrane Database Syst Rev. 2016:CD008914.
Yeo CJ, Cameron JL, Maher MM, Sauter PK, Zahurak ML, Talamini MA, Lillemoe KD, Pitt HA. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–8; discussion 588-92.
Menahem B, Guittet L, Mulliri A, Alves A, Lubrano J. Pancreaticogastrostomy is superior to pancreaticojejunostomy for prevention of pancreatic fistula after pancreaticoduodenectomy: an updated meta-analysis of randomized controlled trials. Ann Surg. 2015;261:882–7.
Liu FB, Chen JM, Geng W, Xie SX, Zhao YJ, Yu LQ, Geng XP. Pancreaticogastrostomy is associated with significantly less pancreatic fistula than pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: a meta-analysis of seven randomized controlled trials. HPB (Oxford). 2015;17:123–30.
Clerveus M, Morandeira-Rivas A, Picazo-Yeste J, Moreno-Sanz C. Pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy: a systematic review and meta-analysis of randomized controlled trials. J Gastrointest Surg. 2014;18:1693–704.
Hallet J, Zih FS, Deobald RG, Scheer AS, Law CH, Coburn NG, Karanicolas PJ. The impact of pancreaticojejunostomy versus pancreaticogastrostomy reconstruction on pancreatic fistula after pancreaticoduodenectomy: meta-analysis of randomized controlled trials. HPB (Oxford). 2015;17:113–22.
Xiong JJ, Tan CL, Szatmary P, Huang W, Ke NW, Hu WM, Nunes QM, Sutton R, Liu XB. Meta-analysis of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Br J Surg. 2014;101:1196–208.
Keck T, Wellner UF, Bahra M, Klein F, Sick O, Niedergethmann M, Wilhelm TJ, Farkas SA, Börner T, Bruns C, Kleespies A, Kleeff J, Mihaljevic AL, Uhl W, Chromik A, Fendrich V, Heeger K, Padberg W, Hecker A, Neumann UP, Junge K, Kalff JC, Glowka TR, Werner J, Knebel P, Piso P, Mayr M, Izbicki J, Vashist Y, Bronsert P, Bruckner T, Limprecht R, Diener MK, Rossion I, Wegener I, Hopt UT. Pancreatogastrostomy Versus Pancreatojejunostomy for RECOnstruction After PANCreatoduodenectomy (RECOPANC, DRKS 00000767): Perioperative and Long-term Results of a Multicenter Randomized Controlled Trial. Ann Surg. 2016;263:440–9.
Berger AC, Howard TJ, Kennedy EP, Sauter PK, Bower-Cherry M, Dutkevitch S, Hyslop T, Schmidt CM, Rosato EL, Lavu H, Nakeeb A, Pitt HA, Lillemoe KD, Yeo CJ. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized, prospective, dual-institution trial. J Am Coll Surg. 2009;208:738–47; discussion 747-9.
Bassi C, Falconi M, Molinari E, Mantovani W, Butturini G, Gumbs AA, Salvia R, Pederzoli P. Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery. 2003;134:766–71.
El Nakeeb A, El Hemaly M, Askr W, Abd Ellatif M, Hamed H, Elghawalby A, Attia M, Abdallah T, Abd EWM. Comparative study between duct to mucosa and invagination pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized study. Int J Surg. 2015;16:1–6.
Hua J, He Z, Qian D, Meng H, Zhou B, Song Z. Duct-to-mucosa versus invagination pancreaticojejunostomy following pancreaticoduodenectomy: a systematic review and meta-analysis. J Gastrointest Surg. 2015;19:1900–9.
Grobmyer SR, Kooby D, Blumgart LH, Hochwald SN. Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J Am Coll Surg. 2010;210:54–9.
Mishra PK, Saluja SS, Gupta M, Rajalingam R, Pattnaik P. Blumgart’s technique of pancreaticojejunostomy: an appraisal. Dig Surg. 2011;28:281–7.
Pratt WB, Callery MP, Vollmer CM Jr. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg. 2008;32:419–28.
Reid-Lombardo KM, Farnell MB, Crippa S, Barnett M, Maupin G, Bassi C, Traverso LW, Pancreatic Anastomotic Leak Study Group. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1,507 patients: a report from the Pancreatic Anastomotic Leak Study Group. J Gastrointest Surg. 2007;11:1451–8; discussion 1459.
Kleespies A, Rentsch M, Seeliger H, Albertsmeier M, Jauch KW, Bruns CJ. Blumgart anastomosis for pancreaticojejunostomy minimizes severe complications after pancreatic head resection. Br J Surg. 2009;96:741–50.
Langrehr JM, Bahra M, Jacob D, Glanemann M, Neuhaus P. Prospective randomized comparison between a new mattress technique and Cattell (duct-to-mucosa) pancreaticojejunostomy for pancreatic resection. World J Surg. 2005;29:1111–9; discussion 1120-1.
Fujii T, Sugimoto H, Yamada S, Kanda M, Suenaga M, Takami H, Hattori M, Inokawa Y, Nomoto S, Fujiwara M, Kodera Y. Modified Blumgart anastomosis for pancreaticojejunostomy: technical improvement in matched historical control study. J Gastrointest Surg. 2014;18:1108–15.
Oda T, Hashimoto S, Miyamoto R, Shimomura O, Fukunaga K, Kohno K, Ohshiro Y, Akashi Y, Enomoto T, Ohkohchi N. The tight adaptation at pancreatic anastomosis without parenchymal laceration: an institutional experience in introducing and modifying the new procedure. World J Surg. 2015;39:2014–22.
Wang SE, Chen SC, Shyr BU, Shyr YM. Comparison of Modified Blumgart pancreaticojejunostomy and pancreaticogastrostomy after pancreaticoduodenectomy. HPB (Oxford). 2016;18:229–35.
Halloran CM, Platt K, Gerard A, Polydoros F, O’Reilly DA, Gomez D, Smith A, Neoptolemos JP, Soonwalla Z, Taylor M, Blazeby JM, Ghaneh P. PANasta Trial; Cattell Warren versus Blumgart techniques of pancreatico-jejunostomy following pancreato-duodenectomy: Study protocol for a randomized controlled trial. Trials. 2016;17:30.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Chandwani, R., Jarnagin, W.R. (2018). Through-and-Through Transpancreatic Duct-to-Mucosa (Blumgart) Pancreaticojejunostomy. In: Tewari, M. (eds) Surgery for Pancreatic and Periampullary Cancer. Springer, Singapore. https://doi.org/10.1007/978-981-10-7464-6_11
Download citation
DOI: https://doi.org/10.1007/978-981-10-7464-6_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-7463-9
Online ISBN: 978-981-10-7464-6
eBook Packages: MedicineMedicine (R0)