Abstract
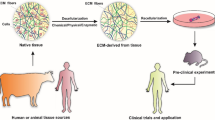
Tissue Engineering consists of cells, a scaffold and cytokines. Decellularization represents the removal of cells from tissues or organs. Recently, decellularized tissue has been investigated as a scaffold for tissue engineering, termed decellularized tissue engineering. Importantly, the decellularized organ retains its original structure, which is then used as a template for organ construction. The decellularized organ also retains the tissue-specific extracellular matrix. Therefore, decellularized tissue can be used as a matrix to provide a suitable microenvironment for inoculated cells. Based on these concepts, the reconstruction of tissues/organs with decellularized tissue/organ has been attempted using decellularized tissue engineering. In this chapter, we introduce the typical methods used, history and attainment level for the reconstruction of specific tissues/organs. First, the different decellularized techniques and characteristics are introduced. Then, the commonly used analysis methods and cautionary points during decellularization and reconstruction with decellularized tissues/organs are explained. Next, the specific methods and characteristics of decellularized tissue engineering for specific tissues/organs are introduced. In these sections, the current conditions, problems and future work are explained. Finally, we conclude with a summary of this chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Tissue engineering
- Decellularization
- Decellularized organ
- Extracellular matrix
- Blood vessel
- Heart
- Cartilage
- Liver
- Lung
- Adipose
- Skin
- Kidney
- Tendon
- Nerve
- Pancreas
- Solubilized decellularized tissue
1 Introduction
Tissue engineering was propounded by Langer and Vacanti (1993) about two decades ago. The concept was to construct tissues by combining cells, scaffolds, and cytokines. Recently, the use of decellularized (DC) tissues as a scaffold for culturing cells or as a template for organs has been the focus of research. DC-tissues are obtained by removing cells from tissues that consist of an organ-specific extracellular matrix (ECM). Therefore, DC-tissues are expected to be an effective scaffold that has suitable components for the construction of tissues. In addition, DC-organs are also expected to be effective templates for the construction of organs because they retain their original three-dimensional structure. The seeding of cells to DC-tissues or DC-organs is termed “recellularization”. Currently, the reconstruction of various tissues or organs by the recellularization of DC-tissues or DC-organs is being investigated.
Decellularization techniques can be divided into two categories: chemical agents and physical treatments. Table 1 shows the major agents/treatments of each decellularization mechanism and their characteristics (derived from Crapo et al. (2011) and summary of this chapter). Decellularization of tissues or organs is performed by using one agent/treatment or combining agents/treatments. In addition, the procedures for using chemical agents follow a pattern, for example, perfusion via blood vessels, or soaking on orbital shaker. The procedure depends on the structure or characteristics of each tissue/organ (Shirakigawa et al. 2012). The important characteristics and behaviors that should remain after decellularization depend on the target tissue/organ. Therefore, different methods of decellularization are used for different tissues/organs.
To date, DC-blood vessels, heart (containing heart valves), cartilage, liver, lung, adipose, dermal, kidney, tendon, nerve, and pancreas have been reported. The search results with “PubMed” are shown in Fig. 1. “Decellularized” and each organ/tissue name (such as “decellularized vascular/vessel” or “decellularized heart”) were used as keywords for the search. Studies reporting decellularization are shown in this distribution. Although decellularization can be performed in any tissue/organ, the tissues/organs usually require reconstruction as a scaffold.
Each organ has a unique structure that performs a specific function, and this is important when evaluating which methods should be used for each tissue/organ. However, some basic methods of decellularization are common. First, this review will describe the common analysis methods and important points of decellularization and recellularization. Then, we focus on each tissue and organ in order and important points regarding each tissue/organ will be explained.
2 Common Analysis Methods and Cautionary Points During Decellularization and Recellularization
The method of decellularization depends on the target tissue/organ characteristics. However, some basic methods are common to all tissue/organ decellularization. These methods can be divided into three categories.
-
Perfect cell removal
-
Remaining tissue/organ-specific three-dimensional structure
-
Remaining tissue/organ-specific ECM components.
Here, we introduce the widely used methods. First, histological evaluation, especially hematoxylin and eosin staining, is usually performed in most studies of DC-tissue/organs. Hematoxylin and eosin stains cell nuclei and other cell components purple and pink, respectively. Therefore, the above three points can be evaluated qualitatively by comparing the staining tissue/organ before and after decellularization.
Second, DNA content as an index of cell removal is widely used for quantitative analysis. The ideal value of DNA content of DC tissue was reported to be <50 ng dsDNA per mg ECM dry weight (Crapo et al. 2011), although, evidence for this was not shown. However, another study reported that DNA content should be evaluated based on wet weight (Mazza et al. 2015). If the dry weight is used, the value will be affected by the weight of cells because the tissue/organ weight is changed by cell removal during decellularization. We have measured the wet and dry weight of rat native/DC-liver right lobe and evaluated the water content ratios from these values. The water content of native liver was about 70%. However, the water content of DC-liver was greater than 99%. These results suggested that the dry cell weight was about 30% of the wet weight and the dry ECM weight was less than 1% of the wet weight. Therefore, the weight of cells can affect the dry weight of the target tissue/organ. Therefore, the wet weight should be used for quantitative analysis. By contrast, Lee et al. (2014) reported the ratio of remaining DNA in DC-liver versus that of native liver. They suggested that the remaining 3–4% DNA in the DC-tissue/organ was not a problem. Although the standard for analysis of DNA content is yet to be determined, the quantitative analysis of decellularization is important.
Once cells have been removed by decellularization, it is important that the ECM should remain. The ECM mainly consists of collagen fibers and proteoglycan complexes. Therefore, collagen and glycosaminoglycan are often evaluated as typical components of collagen fibers and proteoglycan complexes, respectively. These are sometimes evaluated quantitatively using an evaluation kit (Methe et al. 2014), or using qualitative immunostaining methods (Uygan et al. 2010). The ideal values of collagen and glycosaminoglycan have not been reported. Even if the value decreases by decellularization, if it will not affect the organ function or construction; therefore, the values are not critical. Furthermore, the specific components of each tissue/organ are evaluated. For example, collagen IV and laminin in the kidney (Peloso et al. 2015). In addition, each organ-specific structure is evaluated, such as the blood vessel network in the liver and alveolar size in the lung.
Recellularization of DC-tissues/organs will be performed if necessary. The cell adhesion and histological analysis of recellularized tissue are commonly evaluated. Then, further evaluations of functions are performed depending on the specific organ function such as the pumping movement in the heart and gas exchange in the lung. In summary, following the common analyses of DC-tissues/organs, further analyses are required for each tissue/organ. Next, we discuss some cautionary points during DC and recellularization.
First is the time from harvest of the tissue/organ to the finish of decellularization and sterilization. When reconstruction of the tissue/organ is performed using DC-tissue/organ, the length of time from harvest of the tissue/organ to the finish of decellularization and sterilization directly affects the time until the constructed tissue/organ can be administered to the patient. Long-term decellularization can denature or reduce the resolution of the ECM of the DC-tissue/organ. Furthermore, if the time is prolonged, a risk of contamination is increased and the cost for construction of the tissue/organ is increased.
Second, the washing process is important because the chemical agents used for decellularization are cytotoxic. The DC-tissue/organ should be washed before use as a scaffold for cell culture or transplantation. The washing process is usually performed for a few hours or days, which is the same or longer than that of incubation with the decellularization agent.
Third, the circulation system is important. During decellularization and recellularization, some solutions should be used sequentially. The circulation system is built by combining pumps and tubes, and is usually developed by each researcher based on their specific requirements and therefore, they are not standardized. In some organs, air bubbles in the flow solution can disrupt organ structures such as the vascular network in liver. Therefore, the flow solution should be continuous, and air vents should be contained in the circulation system when sensitive organs are used.
Fourth, stability of the flow solution speed or flow pressure in the tissue/organ is very important to maintain the structure of the DC-tissue/organ. Circulation of a solution at a high speed may cause the collapse of internal structures of the DC-tissue/organ. In addition, control of the speed of circulating culture medium is important especially during cell seeding because it affects cell adhesion. Furthermore, the seeding process and conditions should be optimized, e.g. the type and number of seeded cells, the seeding method such as via the artery, and the period until restart of the culture medium circulation after cell seeding.
Finally, the sterilization of DC-tissues/organs is important for cell culture or construction of the tissue/organ for transplantation. Bacterial growth in DC-tissues/organs should be avoided during decellularization and recellularization. Therefore, antibacterial agents are often added to the circulating solution such as washing solution. In addition, the sterilization procedure is sometimes performed before transplantation or cell seeding. However, sterilization can damage the ECM of DC-tissues/organs. For example, gamma irradiation and peracetic acid can denature proteins. Therefore, the sterilization procedure should be selected depending on the organ characteristics. Based on the above points, the decellularization and recellularization procedure should be performed under mild conditions. In the Sect. 3 (3.1–3.12), we focus on specific tissues/organs and explain their background, structure, decellularization, and recellularization processes. In addition, some recent studies are introduced. The strain, decellularization method, tissue/organ specific evaluation except common analysis and future works are shown and summarized in each table.
3 Decellularization of Various Organs
3.1 Blood Vessels
Background
Injury of blood vessels due to accident or illness requires reparative surgery. Autografts are sometimes used, but these processes also cause the injury to the normal tissues and the limited with the range of uses. Thus, it is not considered a suitable treatment approach. Injured blood vessels are sometimes reinforced by stents. However, stents treatment has a limited range of application. Therefore, artificial blood vessels are required for clinical treatment. Each year, 1.4 million patients in the USA need arterial prostheses (Hasan et al. 2014).
Artificial blood vessels are the oldest artificial organ. Large artificial blood vessels (inner diameter (ID) ≥ 6 mm) were developed using synthetic polymers such as Dacron and polytetrafluoroethylene (PTFE). These Artificial blood vessels are good and have long-term patency for clinical treatment (Conte 1998). However, small (narrow) artificial blood vessels (internal diameter; ID < 6 mm) with suitable function have not been developed. Retaining patency in these vessels is difficult. Furthermore, artificial blood vessels consisting of synthetic polymers cannot replace native tissue. The ID of blood vessels in children should increase with growth, but this does not occur with synthetic artificial blood vessels. Conte (1998) reported “The ‘ideal’ vascular graft would be characterized both by its mechanical attributes and post implantation healing responses”. Given the economic considerations, low cost and long-term durability are also important issues. Therefore, the development of tissue-engineered artificial blood vessels to replace native tissues is required. Currently, materials such as cell-seeded synthetic materials, biodegradable polymers, cell sheets, and biopolymer are being studied. An example of biopolymer based artificial blood vessels that is close to clinical application is discussed. Sugiura et al. (2016) reported that 50:50 Poly (1-lactide-co-Ɛ-caprolactone) is allowed to flow into a glass tube and lyophilized. Then, the tube was coated with polylactic acid by electrospinning. The obtained tube was 3 mm in length and transplanted into mice for 8 weeks by infrarenal aortic interposition with microsurgery. Endothelialization and cell invasion were observed, but how to replace the native tissue in long term is being evaluated. However, products with sufficient function have not been developed. Another issue is that mechanical strength decreases with time by biodegradation. Therefore, the balance between biodegradation and maturation of blood vessels is a problem. However, when a DC-blood vessel is used, biodegradation is not necessary because it is an original basic structure.
Structure of blood vessels
The structure of blood vessels is shown in Fig. 2. The internal surface is covered with endothelial cells that provide antithrombogenicity. The medium layer consists of smooth muscle cells and controls the expansion and shrinkage of blood vessels. The ECM fixes the layers between the internal surface and medium layer. Mechanical strength is an important characteristic of blood vessels. It is determined in two directions, circumferential and longitudinal tensile strength. However, the values are dependent between studies.
Decellularized blood vessels
It is important to develop small artificial blood vessels. When scaled up from animals to humans, the size of vessels should be carefully considered because although the diameter is similar between species the length is different in humans. There have been many reports about DC-blood vessels (Moroni and Mirabella 2014; Mahara et al. 2015; Umashanakar et al. 2016). The mechanical strength of DC-blood vessels is important when used as a scaffold for the construction of blood vessels. However, the structure is a simple straight tube. Therefore, the decellularization methods used are common and include the use of flowing detergent and physical treatments such as high hydrostatic pressure. After decellularization, recellularization is usually performed using DC tissue engineering. Since, cells flow in the blood, so flowing cells can attach to the surface of DC-blood vessels indicating recellularization may not be necessary. The products made from synthetic polymers such as Dacron and PTFE are not recellularized. Therefore, recellularization might not be necessary for small artificial blood vessels. A trend of recent studies is non-seeding, which provides anti-thrombogenicity and improvement of cell adhesion. Table 2 summarizes the findings of three studies.
These studies used interesting techniques. Mahara et al. (2015) reported the decellularization of ostrich carotid artery using a non-detergent method. This material is very interesting because the ostrich carotid artery is narrow, long and straight without branching. Blood vessels usually have many branches. If the branches are tied to form a straight scaffold, the knot will prevent the smooth flow of blood and coagulation may occur. Therefore, the use of a straight blood vessel without unexpected branches is important for obtaining an ideal scaffold. They showed the long-term patency of the transplanted DC-artery using a porcine experiment. The advancement of this research for clinical use is expected. Umashanakar et al. (2016) proposed the use of DC-blood vessels as a patch for treatment of a blood vessel hole. This method is expected to be used for a wide variety of areas including accidents during surgery. The development of this patch is expected. Gong et al. (2016) suggested that mechanical strength was decreased during decellularization treatment. Therefore, they coated DC-blood vessels with a nanofibrous material using electrospinning to improve its strength. Using suitable material for specific role in association with other materials is a good idea. In these studies, recellularization was not performed.
Future work
Tissue-engineered artificial blood vessels should have anti-blood clotting property and mechanical strength. To achieve these objectives, artificial blood vessel should be sufficiently endothelialized and have an elastic force similar to the native tissue. The perfect replacement of ECM of DC-vessels by native cells and tissue will hopefully achieve these objectives. Collagen is the main constituent of ECM and is a trigger for coagulation. Therefore, the use of pre-endothelialization or anti-blood clotting coating for complete endothelialization is necessary for clinical use. Furthermore, the long-term stability of endothelialization is needed. The mechanical strength of DC-blood vessels is expected to be close to the strength of the original, so the hope for its use as a scaffold for the construction of blood vessels is high.
3.2 Heart Valves
Background
In Germany, 29,672 heart valve surgeries were performed in 2013, and the patient number is increasing globally (Theodoridis et al. 2016). Currently there are two basic types of artificial heart valve products: mechanical heart valves and bioprosthetic heart valves. Both have advantages and disadvantages. Mechanical heart valves are made of artificial biomaterials (mainly pyrolytic carbon) that allow them to be used for a lifetime, but patients are treated daily with Warfarin, an anticoagulation medicine. In addition, the level of the medicine is checked more than once per month. Bioprosthetic heart valves are made of porcine aortic valves or bovine pericardial xenografts. These are treated with glutaraldehyde (GA). The GA treatment devitalizes and sterilizes tissues and also reduces tissue immunogenicity (Roosens et al. 2016). Patients receiving transplanted bioprosthetic heart valves have to take Warfarin for the first few months, but the valves suffer from calcification and have to be transplanted again 10–20 years later. Therefore, the development of a novel heart valve that can be used for a lifetime without Warfarin is desired.
Structure of heart valves
The structure and characteristics of heart valves are shown in Fig. 3. As shown in Fig. 3b, the surface of the heart valve consists of endothelial cells. The internal middle layer consists of interstitial cells. The heart valves perform repetitive opening and closing movements many times during lifetime (Fig. 3a). Therefore, durability is important.
Decellularization of heart valves
Mechanical grafts require mechanical strength and biocompatibility when used for heart valves. However, the endothelialization of the surface is needed for anti-coagulation. Recent studies are shown in Table 3.
The commonly used detergents are not powerful for decellularization. Although the decellularization of heart valves is thought to be difficult because of its internal vascular network, but reports suggest all cells can be removed. However, bioprosthetic heart valves, which retain some cells, are widely used. Therefore, the complete removal of cells may not be important. The disadvantages of mechanical valves are anti-coagulation and for bioprosthetic valves it is prolonging the effective application period by anti-calcification. DC-heart valves are expected to resolve these problems. Therefore, characteristic analysis of DC-heart valves should include anti-coagulation and anti-calcification. Some studies have reported the clinical use of DC-heart valves. In these studies, recellularization was not performed. The endothelialization of DC-heart valves is thought to occur after transplantation in vivo. Mechanical valves and bioprosthetic valves were not endothelialized but have been used in clinical treatment. Therefore, endothelialization before transplantation may not be important.
Future work
The structure of heart valves is simple, therefore, the clinical application of artificial heart valves is hoped for in the near future. To improve the current treatment, the long-term (more than 20 years) stability of endothelialization and mechanical strength should be evaluated. To achieve this, both the endothelialization of the surface but the replacement of native tissues in the middle layer of heart valves may be necessary to construct a complete heart valve. If this can be achieved, the heart valves will grow in conjunction with the growth of a child to adulthood.
3.3 Heart
Background
Heart disease is the leading cause of death in the USA and throughout many advanced countries (Sánchez et al. 2015). Although the gold standard treatment for end-stage heart failure is still heart transplantation through surgery, the shortage of donor organs is a severe problem. Even if a matching donor is found, there are risks of postsurgical complications (Zia et al. 2016). As with any other organ transplantation, patients are treated with long-term immune-suppressants that cause a variety of side effects including immunodeficiency, hypertension, diabetes and renal insufficiency. Therefore, 24% patients die within 5 years after transplantation. To solve these problems, the development of tissue-engineered hearts is required.
Structure of the heart
The heart mainly consists of muscle and blood vessels as shown in Fig. 4. Its function is to pump blood throughout the whole body and its internal surface is composed of endothelial cells that allow blood cells to adhere. The internal middle layer consists of high-density muscle except in the heart valves.
Decellularization of the heart
To construct an artificial heart, DC-tissues should retain the original three-dimensional structure as a scaffold for cell adhesion. Endothelial cells should attach and cover the internal surface. Muscle cells should invade into the middle layer and grow. To perform its functions, the muscle cells have to beat strongly and steadily. A summary of recent studies is shown in Table 4.
SDS and Triton X-100 are usually used for decellularization of the heart. These solutions enter the heart via the ascending aorta. The heart muscle contains a very narrow vascular network; therefore, detergents cannot be used with this network because detergents attack the middle layer cells of the heart wall by diffusion from an internal surface. Thus, a powerful detergent for decellularization is required. Furthermore, Triton X-100 is used to remove remaining SDS or cell debris inside the DC-matrix.
Recellularization of DC-heart
The heartbeat is maintained by heart muscles. Therefore, recellularization with cardiomyocytes is necessary for reconstruction of the heart. To develop sufficient muscle strength, a high cell density and culture of muscle inside the DC-heart are important. Furthermore, electrical activation is required during recellularized DC-heart culture to improve the strength of seeded cardiomyocytes. Although Ott et al. (2007) have performed electrical activation of heart cells during a medium circulation culture. Kitahara et al. (2016) suggested that differentiation of mesenchymal stem cells (MSCs) to heart muscle or endothelial cells was not sufficient without electrical activation. Although circulation culture systems containing an electrical activation system are complicated, this is required for the construction of functional muscle. Furthermore, the placement of each suitable cell to a suitable place is difficult. Kitahara et al. (2016) also suggested that the transmission of electric signals will not be successful if certain cells such as immature cells (e.g. myoblasts) are seeded and differentiated inside the DC-heart. Recent studies reported the use of heterotopic transplantation. However, coagulation occurred after the transplantation because complete endothelialization was not performed, and long-term transplantation with blood flow was not achieved.
Future work
The improvement of muscle strength of recellularized DC-heart is necessary to construct an alternative organ for the heart. To date, only a few of the adult heart functions gave been reported (Ott et al. 2008). To improve the muscle strength of recellularized DC-heart, high-density placement of muscle cells is important. In addition, electric activation is also important for training the constructed muscle. A pacemaker might be required to maintain a steady heartbeat. During the recellularization of DC-heart, uniform recellularization is required in each heart atrium and cardiac chamber. Furthermore, the complete endothelialization of internal surfaces is important for anti-coagulation in the heart. These studies can be performed with animal models, but when the scale-up will be performed for humans, much higher cell numbers will be needed. The adult human heart contains 2 × 109 cardiomyocytes (Smit and Dohmen 2014). The growth and maturation of seeded cells inside the DC-heart during organ culture will be important. However, the construction of a bioreactor system to train muscle to achieve the required blood pressure and flow is important for recellularized DC-heart cultures. In addition, a reinforcement product such as a heart cell sheet or reinforcement patch with DC-heart powder may be useful alternatives to whole heart products.
3.4 Liver
Background
There are about 27,000 deaths annually in the USA due to liver disease (Yagi et al. 2013). End stage liver diseases including cirrhosis, chronic viral hepatitis, hepatocellular carcinoma, injuries from alcohol abuse, or even inborn metabolic disorders, often lead to demands for organ transplantation (Sabetkish et al. 2015). However, 20% of patients die on the waiting list due to a shortage of organ donors (Mazza et al. 2015). The liver is a regenerative organ and even if 70% of the liver is harvested, the donor can survive (Shirakigawa et al. 2013). Often, a living liver transplantation is performed in the clinic because of a cadaveric donor shortage. However, this treatment has a potential risk of death for the donor, and therefore, alternative treatments should be developed such as tissue-engineered liver construction.
Structure of liver
The structure of the liver is shown in Fig. 5. The liver consists of millions hepatic lobules, which are the minimum functional unit of the liver. Hepatocytes are the main cell type involved in liver functions. The liver has a fine blood vessel network, and hepatocytes require oxygen for their survival and function.
Decellularization of liver
Liver, composed of hepatocytes, has many functions and is the central organ of metabolism. Because hepatocytes require oxygen, recellularization of hepatocytes and construction of blood vessel networks are important for the construction of a functional liver. A summary of recent studies is shown in Table 5.
To construct a fine vascular network, the original three-dimensional structure of the liver should be used as a scaffold. Therefore, chemical agents are used for the decellularization of liver via the portal vein, the major blood vessel in the liver. The vascular network structure was evaluated by forming a template of it with resin. Previous reports observed an intact vascular-tree network. However, the quality of the remaining structure is different between studies. At the macroscopic levels, they show similarities to the original structure. However, the liver requires oxygen, so the thickness of the hepatocytes aggregate that can survive is a maximum of 50 µm. The remaining vascular network structure should contain small diameter vessels for use as a template in DC-liver. Thus, the ideal distance between blood vessels is less than 100 µm. Even if the cell growth (regeneration of liver) occurs during the culturing of recellularized DC-liver or transplantation in vivo, the length between blood vessel structures in the template (i.e. DC-liver) should be less than 1 mm (Shirakigawa et al. 2013).
Recellularization is necessary for functional liver construction because hepatocytes perform the main functions of the liver. For recellularization, various liver cells were used (Table 5). After recellularization of DC-liver with hepatocytes, liver specific functions were determined as follows: albumin synthesis as an index of protein synthesis ability, cytochrome P450 (CYP) activity as an index of drug metabolism ability and urea secretion. Albumin synthesis or urea secretion was reported in recellularized DC-liver cultures. Therefore, drug metabolism functions such as CYP activity should also be analyzed in recellularized DC-liver. Currently, the function of recellularized DC-liver is reduced compared with native liver. An improvement in seeded cell density is expected. It is assumed that the minimum liver weight required to support a patient with acute liver failure is approximately 5–10% of total liver weight. Therefore, about 10 billion human cells, or 50–100 million rat cells must survive and function in the reconstructed liver (Caralt et al. 2014).
Heterotopic transplantations of porcine and rat DC-liver were reported. However, coagulation occurred inside the transplanted graft in the liver. In addition, long-term transplantation with blood flow was not achieved. Therefore, the perfect endothelialization is needed.
Future work
Hepatocytes require an oxygen supply for survival and function indicating a functional vascular network is necessary. Therefore, endothelialization is required in DC-liver. The inoculation of a high cell density should improve liver specific functions of DC-liver. However, to achieve a high cell density similar to native numbers in vivo is difficult. Cells need to be seeded at a low density of about 1/10 that in vivo. Then, reconstruction to the in vivo cell density should be occur following cell growth and neo-vascularization during recellularized DC-liver culture or transplantation in vivo. Additionally, it would be useful to have bile secretion in DC-liver. In the future, it is hoped reconstructed liver grafts are used for orthotopic transplantation. The ECM containing ratio of the liver is very low compared with other organs and the mechanical strength of DC-liver is weak; thus, it might be difficult to maintain abdominal pressure. Reinforcement of the constructed graft may be needed.
3.5 Lung
Background
In the USA, nearly 30 million patients currently suffer from end-stage lung disease, with 12.1 million adults affected by chronic obstructive pulmonary disease, the fourth leading cause of death (Song et al. 2011). Lung transplantation remains the only definitive treatment, but donor shortage is a severe problem. Therefore, the construction of an alternative donor lung is required.
Structure of lung
The lung function is to exchange gas. Oxygen is supplied from the atmosphere to blood, and carbon dioxide is released from the blood to the atmosphere. To achieve this, the lung has fine vascular networks and many alveoli as shown in Fig. 6. The surface of an alveolus consists of epidermal cells and the lung can shrink and expand with the movement of the diaphragm.
Decellularization of lung and recellularization
The lung is a special organ because it has three phases: gas, liquid, and solids that exchange gas between the blood and atmosphere. Cells are usually cultured in liquid culture medium, but epidermal cells on the surface of alveolus have to be exposed to the atmosphere. The three-dimensional structure of the airways should be retained before and after decellularization of the lung. Therefore, the solution circulation system for decellularization has to contain a channel for retaining the airway structure including fine structures such as alveoli and the vascular network. Therefore, the detergent method is usually used for decellularization. The solutions usually enter the lung via the lung artery, but the airways can also be used. A summary of four recent reports are shown in Table 6.
As mentioned above, because oxygen should be supplied from the atmosphere to blood in the lung, the construction of blood vessels and alveolar surfaces is important. Therefore, the seeding and culture of epidermal cells and endothelial cells is necessary. After recellularization of DC-lung, specific analyses are performed: comparison of alveolar size and number before and after decellularization, and evaluation of gas exchange of oxygen and carbon dioxide. In addition, the fetal cells or cell lines were seeded on airways instead of primary lung cells. The growth ability of primary lung cells might be poor. Orthotopic transplantation for a few days was reported, but the size of the transplanted recellularized DC-lung was reduced by macroscopic observation (Song et al. 2011). The engraftment of recellularized DC-lung is still difficult.
Future work
Functional lungs should perform gas exchange with breathing. To achieve this, there are three important points: (1) no leakage of air from airways by epidermal cellularization of the airways; (2) the constructed organ should have sufficient mechanical strength for breath; and (3) blood can flow in the vascular network without clotting. These points should be achieved before its clinical use. In addition, the long-term stability and the homogeneity of constructed lung should be determined.
3.6 Kidney
Background
Chronic kidney disease (CKD) is a global public health issue with an estimated prevalence of 8–16% worldwide. End-stage renal disease (ESRD) eventually develops in 0.15–2% of patients with overt CKD annually, and renal replacement therapy with dialysis or transplantation is required (Figliuzzi et al. 2014). Nearly 1 million patients in the USA live with ESRD, with over 100,000 new diagnoses every year. Although hemodialysis has increased the survival of patients with ESRD, transplantation remains the only available curative treatment. However, donors are lacking. In addition, even patients that receive a transplanted kidney from a donor, 20% of recipients experience an episode of acute rejection within 5 years of transplantation, and approximately 40% of recipients lose graft function within 10 years after transplantation (Song et al. 2013). The development of tissue-engineered kidney is required as a solution to these problems.
Structure of the kidney
The kidney filters the blood and creates urine to control fluid balance, and regulate the balance of electrolytes. All the blood in our bodies passes through the kidneys several times a day. The structure of the kidney is shown in Fig. 7.
Decellularization of kidney and recellularization
Kidney filters the blood plasma to form urine. Then, reabsorption is performed via renal tubules. The kidney consists of two systems: blood vessels and urinary ducts. A summary of recent studies is shown in Table 7.
Decellularization of the kidney is usually performed by passing the detergent solution through the artery because the kidney structure is important for reconstruction using the kidney as a scaffold. As a detergent, SDS is widely used for kidney decellularization indicating removing cells in the kidney is difficult. Reconstruction of the kidney requires the construction of a blood vessel system and urinary duct system. To achieve this, endothelial cells were seeded via the artery and kidney cells were seeded via the urethra (Song et al. 2013). Another study used stem cells seeded via the artery to allow the cells to differentiate in DC-kidney (Guan et al. 2015). Currently, the construction of a blood vessel system and urinary duct system is difficult. During orthotopic transplantation, coagulation occurred in DC-kidney when the endothelial cells were seeded suggesting that the complete construction of a vascular network system is required for the construction of a kidney. When the recellularization of DC-kidney was performed with kidney cells, creatinine or urea syntheses as kidney functions were evaluated. However, these functions should be improved as an alternative to hemodialysis or kidney donors.
Future work
The complete construction of a vascular network is necessary because of the high blood flow through kidneys. Kidney cells should be inoculated at a suitable area and the construction of the urethra is required. The performance of kidney function should be improved; however, functions such as filtration by the glomerulus or reabsorption via the renal tubules will be difficult to achieve.
3.7 Adipose
Background
In plastic surgery, the construction of adipose tissue is valuable for reconstruction and cosmetics. After resection for breast cancer, breast reconstruction requires adipose construction of cm in size. Reconstruction can be achieved by allografts; however, the allograft has to move with blood vessels. Therefore, the dermis or muscle must be cut open. High biocompatibility synthetic materials such as clinical silicon have been developed. However, atrophy occurs in 10% of transplanted patients and the shape of the transplanted material can change. In addition, although the adipose tissue loses elasticity with age, artificial materials remain unchanged. Therefore, the balance of the body changes with time. Recently, studies have reported the injection of autograft adipose, obtained from the abdomen or thigh by absorption of adipose. However, survival and shape control of injected adipose is difficult. Thus, the development of tissue-engineered adipose is required.
Structure of adipose
Adipose consists of adipose cells, ECM, and a fine vascular network, which is simple in structure. Adipose cells contain a fat reservoir, so the size of the cell depends on the amount of fat.
Decellularization of adipose
A summary of recent reports of decellularization of adipose is shown in Table 8. Adipose does not contain large blood vessels. Therefore, it cannot be perfused with chemical agents via blood vessels. Thus, DC-adipose was obtained by repeated freeze-thaw cycles and soaking in decellularized agent solution. The differentiated ratio of adipose was increased when adipose-derived stem cells were seeded onto decellularized adipose. The mixture of cells and DC-adipose is usually transplanted subcutaneously, and the engraftment of the transplanted tissue is examined for functional analysis. In addition, the density of the vascular network required for engraftment of transplanted tissue is often performed. Oil red O staining of the adipose is used for analysis of transplanted tissues.
Future work
Adipose tissue injection is performed in plastic surgery, but the survivability of transplanted adipose obtained from the abdomen or thigh by absorption of adipose is less than 50%. The survivability can be improved to 85–90% by injection of a mixture consisting of adipose tissue and adipose-derived stem cells selected by centrifugation. However, the reconstruction of a sufficient volume adipose is difficult using these methods alone. These problems might be resolved by combining a scaffold such DC-adipose tissue with adipose-derived stem cells. As a scaffold, it should be effective for adipose construction because it will promote adipose-derived stem cell differentiation. In addition, the formation of a vascular network using DC-adipose as a scaffold will allow the survival of transplanted adipose. The improvement of survival rate of the transplanted tissue or the acceleration of adipose tissue formation should be examined in the future. However, the control of its three-dimensional shape may be difficult. Combining other materials such as synthetic biodegradable polymers that become a gel in situ might allow its flexible formation.
3.8 Dermis
Background
The skin is our first line of defense against the outside world and provides a barrier against physical and biological attack, moisture retention, thermoregulation or excretion of waste products by sweating, as well as transmitting touch sensations. The skin heals itself by natural reconstruction when lightly injured. However, larger wounds may result in prolonged healing time complicated by infection or which might not heal (Nyame et al. 2015). When the skin is destroyed over a large area, it might be life threatening. Autografts remain the gold standard for the management of large wounds. However, injuries such as large area burns cannot be treated with autografting. In addition, when autograft treatment is performed, the surrounding skin is injured forming a new wound. To resolve these problems, many bioproducts have been developed to promote the healing of skin functions.
Structure of the skin
The skin consists of a subcutaneous bilayer formed by the epidermis and dermis as shown in Fig. 8. The epidermis is 0.1–0.3 mm thick and mostly consists of keratinocytes (about 95%). The dermis is tightly connected to the epidermis by a basement membrane. The dermis consists of ECM and fibroblasts, and has a fine vascular network connected to the subcutaneous tissue. The dermis contains nerves, hair roots, sebaceous glands, and sweat glands.
Bioproducts and decellularized skin
In the tissue engineering field, Rheinwold and Green (1975) reported a method of expanding keratinocytes in vitro and keratinocyte cell sheets are sold as a tissue-engineered bioproducts (Epicel®, Genzyme Biosurgery, Corp., Cambridge, MA, and Jace®, Japan Tissue Engineering Co. Ltd., Aichi, Japan). The protocol uses a skin sample larger than 1 cm2 isolated from the patient. Then, the keratinocytes are isolated and cultured. After 2–3 weeks, a sheet of cultured epidermis measuring 1000 cm2 is produced. However, this product only consists of the epidermis. In a deep wound, the dermis is also injured and because the keratinocyte cell sheet preparation requires the long-term culture of a patient’s cells with high cost, this treatment is not suitable for acute injury.
Allograft is another treatment after autograft. Allografts need to be sterilized to avoid disease transmission. During sterilization, graft proteins are denatured killing the graft cells. Therefore, this product consists of denatured human ECM and another person’s dead cells. It can be used as a temporary alternative skin that functions as a barrier. After transplantation, the allograft is slowly replaced by the regenerating host tissue. Although it does not have a special function, low cost production may be achieved because it is produced by the sterilization of human skin. Another product, GammaGraft® (Promethean LifeSciences, Inc., Pittsburgh, PA) is a ready-to-use, gamma-irradiated allograft.
Decellularized allograft products are also available. Allograft products contain dead cells, which have no healing effect. Therefore, its function is similar to the allograft product. Decellularization allows a space for the patient’s cells to invade, and it can perform as a scaffold for the reconstruction of skin. AlloDerm® (LifeCell Corporation, Branchburg, NJ) or Graftjacket® (Wright Medical Technology, Inc., Memphis, TN) are already available. Recently, MatrACELL® (LifeNet Health, Inc., Virginia Beach, VA) is a human DC-dermis product that uses a non-denaturing anionic detergent (N-lauroyl sarcosinate) (Moore et al. 2015). Gentler decellularization procedures should be developed. In addition, allogenic skin has been developed, consisting of human cells and biomaterials such as collagen. For example, Apligraf® (Organogenesis, Canton, MA) has a bilayer structure. The upper epidermal layer is formed by promoting human keratinocytes (epidermal cells). The lower dermal layer combines bovine collagen I and human fibroblasts (dermal cells), which produce additional matrix proteins. However, these products do not contain a vascular network, hair roots or sweat glands, and therefore can be improved.
Other products use xenografts or DC-xenografts. The most important benefit of these products is that the material can be obtained in large quantities and with a large size. Porcine xenografts are the most commonly used xenograft (Nyame et al. 2015). Xenografts act as a temporary barrier and decrease the healing time. DC-xenografts might act as a template for the reconstruction of skin and may have a function similar to DC-allografts if a suitable treatment is developed and performed.
Future work
Although many bioproducts have been developed for skin treatment, and clinical studies have been reported for these products, the optimal procedure for each product or treatment method has not been developed, and should be investigated in the future. However, the high cost of these techniques is a disadvantage. Because these products will be used to treat large areas, the cost should be reduced. New products of DC-skin should follow one of two paths: (i) perfect skin that is identical to native skin that functions immediately after transplantation, and does not require replacement by the patient’s own skin; and (ii) a low cost skin with wide versatility that can be stored at room temperature, and used as a template for the reconstruction of skin. This would be invaded by the patient’s cells and help skin cell growth and neo-vascularization.
Currently, a recellularized DC-dermis is not available. The recellularization of DC-dermis has advantages and disadvantages. An advantage is that seeded cells may promote replacement with the patient’s cells or produce ECM if human fibroblast cells are also seeded. If a patient’s cells are seeded, the time to replacement may be decreased. A disadvantage is the high cost of cell seeding, culture processes for recellularization, and transportation or storage. After recellularization and culture, the products should be used within a few days and transported at 37 °C, or otherwise stored in a freezer to keep the seeded cells alive. However, it may become a perfect skin immediately. Furthermore, the cell source is a problem. For cells that are ready-to-use, identification of the patient immune type or a highly efficient method for cell growth will be needed. To develop low cost skin, DC-skin using xenografts may be useful compared with allografts. To improve the function of DC-skin as a scaffold, some additional functions are required such as promoting cell growth or neo-vascularization, and antibacterial activity to decrease the risk of infections.
Finally, no products or studies have reported the reconstruction of nerves, hair roots, sebaceous glands, and sweat glands, and this should be addressed in future studies. In addition, the visual appearance of the reconstructed skin should be as natural as possible to enhance the quality of life of the patients. Even if this raises the costs, some patients will hope for a natural visual reconstruction.
3.9 Cartilage
Background
Aging population has increased the number of patients suffering from cartilage disorders such as osteoarthritis, the most common joint disease in the USA, affecting an estimated 27 million Americans (Bautista et al. 2016). Additionally, if the cartilage is injured, self-repair is limited because it consists of dense ECM without a vascular network. When the injury is small, treatments such as bone drilling or autograft transplantation can be used. Bone drilling describes the drilling of the subchondral bone to induce blood flow. The blood coagulates at the injured cartilage area and becomes cartilage. However, the reconstructed cartilage is fiber cartilage not hyaline cartilage. Autograft transplantation describes the harvesting of a small rod of cartilage from an area that is not subject to strain from body weight and that is then transplanted to the injured area. Using this method, the transplanted cartilage is similar to the original cartilage, but its usable range is limited. However, these methods cannot be performed in patients with a large injury. When the healing of cartilage injury is difficult, an artificial joint might be transplanted. However, the durability of artificial joints is limited to 10–20 years. Therefore, this treatment is not suitable for young patients.
In the tissue-engineering field, tissue-engineered cartilage is available as Jack™ (Japan Tissue Engineering Co.). This method harvest a small cartilage sample from the patient, then the chondrocytes are isolated and cultured in Atelocollagen gel for four weeks. Furthermore, the proliferating chondrocytes with gel are transplanted to the injured site. This method is a novel treatment. However, the healing time is long, about 6–12 months is needed until the patient can walk. Thus, the development of a new treatment method for the reconstruction of hyaline cartilage in a short-term is required.
Structure
Cartilage overlaid on the subchondral bone consists of dense ECM (Fig. 9). The superficial zone consists of collagen II fibers aligned in parallel to the articular surface to resist shear stress, and the deep zone consists of the same fibers aligned perpendicularly to the bone interface to absorb compressive loads. There is no vascular network in cartilage.
Decellularized cartilage
A summary of recent studies about DC-cartilage are shown in Table 9.
Because there is no vascular network in cartilage, decellularization of cartilage is usually performed by freeze-thawing or diffusion of detergents from the surface. Some studies have used cartilage directly without chopping. Decellularization of cartilage is often performed after chopping. The reason is that cartilage consists of dense ECM and the diffusion of a detergent may be difficult. In addition, when it is transplanted to the injured site, its shape may be controlled better when added as a chopped sample. For recellularization, MSCs are widely used. The necessity of recellularization is unclear, but the cartilage cells are needed to maintain the cartilage for a long time. However, the seeding density and method must be optimized.
Future work
When DC-cartilage is used without chopping, it might be used as a template for the reconstruction of cartilage. If the original ECM exists, then reconstruction may be achieved in a short time. However, the control of its shape to match the injured site and the method of seeding cells inside the dense ECM will be difficult. The decellularized chopped cartilage or solubilized DC-cartilage may be better for shape control, but the construction of a three-dimensional structure will also be difficult. Evidence for the promotion of reconstructed cartilage using DC-cartilage is needed.
3.10 Tendon
Background
Tendons connect bone and muscle. The most commonly affected tendons are the finger and hand flexors and extensors, the rotator cuff, and the Achilles tendon. In particular, acute Achilles tendon ruptures have an increasing incidence of 18 per 100,000 (Lovati et al. 2016). When injured seriously, movement of the connected part is difficult, and self-repair is difficult. Large tendon damage needs to be repaired using any tissue substitutes. Allograft transplantation is usually performed, but there are disadvantages, such as slow incorporation into host tissues, potential disease transmission, danger of infection, tunnel widening caused by immune responses, delayed tendon-bone healing, and lower mechanical character (Dong et al. 2015). In addition, both synthetic and biological scaffolds have been used in studies, but a viable tendon substitute is not yet widely available for clinical applications. Therefore, a tissue-engineered tendon should be developed.
Structure
Tendons consist of a low cell density (5%) of collagen fibers containing collagen I (more than 90%) (Lovati et al. 2016).
Decellularized tendon
There are many reports about DC-tendons (Lovati et al. 2016). A summary of recent reports is shown in Table 10.
The diffusion of detergent for the decellularization of tendons is difficult because of the presence of dense collagen fibers. Therefore, freeze/thaw cycles are widely used for the decellularization of tendons, and sliced tendons are sometimes used. The sliced tendon is used to form a rod by “rolling up” the tissue. However, slicing can change its mechanical strength. A rabbit model is often used for the analysis of tendon repair (Lovati et al. 2016). After transplantation, histological analyses and biomechanical tests are performed (Pan et al. 2015). Results suggest that the DC-tendon will be useful for tendon repair. However, it is not currently ready for clinical use. Previous analyses were performed in animal models. For human clinical use, the mechanical strength will be different. Therefore, an index of its strength for clinical use should be developed. In addition, long-term stability analysis is required and the role of DC-tendons should be determined during healing.
Future work
The promotion of tendon reconstruction of tendon is expected by the use of DC-tendon as a template. However, the necessity of recellularization of DC-tendon is still unclear. In addition, its mechanical strength and durability should be studied further. Although tendon injury is not life threatening, it can reduce the quality of life for patients. Its structure is very simple, so the quick development of reconstruction techniques is desired.
3.11 Pancreas
Background
According to the World Health Organization, at least 285 million people worldwide suffer from diabetes. While pharmaceutical interventions and insulin supplementation are the most common treatment of diabetes, these do not represent a cure and can potentially lead to long term complications (Goh et al. 2013). β-cell replacement through islet or pancreas transplantation is the only therapy that can reliably re-establish a stable euglycemic state (Peloso et al. 2016). However, transplantations have a disadvantage of a severe donor shortage. To solve this problem, studies have attempted to develop artificial pancreas using a machine to control blood glucose levels in an ideal range by the injection of insulin based on monitoring of the blood glucose level. Recently, the downsizing of machines and the stabile control of blood glucose levels have been reported (Haidar et al. 2015). However, long-term stability is still a problem. Therefore, a tissue-engineered pancreas might resolve these problems.
Structure
The pancreas contains endocrine and exocrine tissues. Endocrine islets contain α-cells and β-cells, which produce glucagon and insulin, respectively that control the blood glucose level. Although the pancreas consists of exocrine islets (more than 90%), it is abnormalities of the endocrine islets that affect the patient’s life. Therefore, reconstruction of the endocrine system should be developed.
Decellularization and recellularization
DC-pancreas might be used as a scaffold for the transplantation of islets that can survive in vivo. A summary of recent studies of DC-pancreas are shown in Table 11.
The pancreas is a soft organ with a vascular network. Therefore, decellularization of the pancreas is usually performed by detergents without physical treatment. After decellularization, recellularization is required for full functionality. Either pancreas cells or endothelial cells are seeded and cultured and then the vascular density or insulin secretion is evaluated. However, the seeding of each cell type at a suitable location will be difficult.
Future work
Previous studies have not reported an improvement of the mass transfer between islets and blood because of the difficulty of constructing a vascular network. In addition, the stabilization of transplanted islets in vivo is also a problem that needs to be solved. Therefore, for transplanted islets to survive, the construction of a vascular network is needed. DC-pancreas may be a suitable ECM for islets, but it is very soft. A previous study reported that the kidney was used as a scaffold to construct pancreas (Willenberg et al. 2015). Furthermore, the reconstructed pancreas should be strengthened to endure abdominal pressure. DC-pancreas might be achieved if a fine vascular network can be achieved in the scaffold. Finally, by seeding each cell type at each suitable location, the DC-pancreas function might be improved.
3.12 Others
Tissues and organs consist of cells and ECM. Therefore, the decellularization of any tissue and organ can be performed in theory. However, the difficulty of decellularization depends on the specific structure of each tissue and organ. A summary of studies on the decellularization of other tissues and organs is shown in Table 12.
As shown in Table 12, decellularization has been applied to bone and nerves. In these tissues, the promotion of reconstruction might be achieved by decellularized tissues containing the original ECM components and structure for use as a template. If the efficiency of DC-tissues for reconstruction is shown, then other DC-tissues may be studied further.
4 Solubilized Decellularized Tissues/Organs
Background
The survival, original function, and growth of isolated cells or transplanted cells from tissues or organs can be difficult. The construction of an in vivo-like microenvironment is required for such cells. The solubilization of DC-tissues/organs will allow the collection of material for tissue culture or construction. However, it is still unclear what factors affect cell differentiation, for example, physical specifications such as hydrophobic character, or ECM content.
Efficient cell differentiation is a problem in the tissue engineering field. It was reported that differentiation can be promoted by culturing cells on specific ECM (Nakamura and Ijima 2013). Solubilized DC-tissues/organs might be used as a potential scaffold for differentiation. In addition, for solubilized DC-tissues/organs, the shape of the material may be controlled by coating or gelation of the three-dimensional structure, which might be useful for cell culture material and construction of the tissue/organ. A summary of recent studies of solubilized decellularized tissues/organs is shown in Table 13.
For the solubilization of DC-tissues/organs, there are two methods: (i) the tissue/organ is chopped before decellularization; and (ii) the tissue/organ is chopped after decellularization. When a tree-like vascular network is present, the perfusion of a chemical agent via the network will achieve efficient decellularization. Therefore, after decellularization, DC-tissues/organs might be easier to dissociate. However, if the chopping is performed before decellularization, a greater decrease in ECM might occur during decellularization because of the increased surface area. When there are many fine vascular structures, the severed ends of the vascular structures will be present at the chopped surface allowing the detergent to diffuse more efficiently. When few vascular structures are present, it is better to perform decellularization after chopping because the diffusion of the detergent depends on the surface area. Retention of the three-dimensional native structure is not required because the DC-tissue/organ will be solubilized. Physical treatments such as freeze/thaw cycles are often combined. For the solubilization of DC-tissues/organs, 0.1 N HCl and pepsin are often used. Collagen, which is the main component of ECM, is usually derived with HCl; however, pepsin is also used because HCl alone cannot solubilize DC-tissue/organ efficiently. However, acid or enzymes can damage the ECM. Therefore, the amount used or length of incubation should be reduced.
Future works
Solubilized DC-tissue/organ can be used as an ECM matrix for culture dish coating or as a three-dimensional culture material by gelation. The concentration of the matrix or gel strength should be optimized. Furthermore, the construction of an organ might require a combination of three-dimensional printing technology or decellularized organs. In addition, its application by injection-like spray, as a patch of tissue or to reinforce tissues will be useful.
5 Conclusions
Dermis and heart valve bioproducts from bovine or porcine have already been developed and decellularized human dermis is already available. These tissues are easy to use clinically because their function is simple or it is used at a denuded area. However, the knowledge gained using these materials can be applied to other tissues/organs. The use of other tissues/organs with xenografts is also expected to be developed in the near future. Of course, DC-tissue/organ allografts can be used, but these treatments can be expanded by using xenografts. Organs such as the heart or liver from cadaveric donors are not usually used for transplantation. However, DC-organs can be obtained from cadaveric donors. We have discussed the various studies on the reconstruction of tissues/organs based on DC-tissue/organ in Sect. 3. However, the most common problem for the reconstruction of a functional organ is the endothelialization or construction of a vascular network in the DC-organ. By developing efficient vascularization methods, these studies will be improved. Another common problem is the seeding method used to inoculate cells to a suitable location. Mature cells could be used or immature cells could be seeded first and mature cells seeded second. The latter method is expected to be more successful, but there is a risk of cancer from the immature cells. Therefore, differentiation methods should be improved. The DC-tissue/organ can be used to construct a suitable microenvironment for cells. The reconstruction of various tissues/organs is expected based on the use of decellularized tissues/organs.
Abbreviations
- CKD:
-
Chronic kidney disease
- CYP:
-
Cytochrome P450
- DC:
-
Decellularized
- DNA:
-
Deoxyribonucleic acid
- ECM:
-
Extracellular matrix
- ESRD:
-
End-stage renal disease
- GA:
-
Glutaraldehyde
- HCl:
-
Hydrochloric acid
- MSCs:
-
Mesenchymal stem cells
- PTFE:
-
Polytetrafluoroethylene
- SDS:
-
Sodium dodecyl sulfate
References
Bautista CA, Park HJ, Mazur CM et al (2016) Effects of chondroitinase ABC-mediated proteoglycan digestion on decellularization and recellularization of articular cartilage. PLoS ONE 11:e0158976
Bhumiratana S, Bernhard JC, Alfi DM et al (2016) Tissue-engineered autologous grafts for facial bone reconstruction. Sci Transl Med 8:343ra83
Caralt M, Velasco E, Lanas A et al (2014) Liver bioengineering: from the stage of liver decellularized matrix to the multiple cellular actors and bioreactor special effects. Organogenesis 10:250–259
Cebotari S, Tudorache I, Ciubutaru A et al (2011) Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults early report. Circulation 124(Suppl 1):S115–S123
Conte MS (1998) The ideal small arterial substitute: a search for the Holy Grail? FESEB J 12:43–45
Crapo PM, Gilbert TW, Badylak SF (2011) An overview of tissue and whole organ decellularization processes. Biomaterial 32:3233–3243
Crapo PM, Medberry CJ, Reing JE et al (2012) Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials 33:3539–3547
DeQuach JA, Yuan SH, Goldstein LS et al (2011) Decellularized porcine brain matrix for cell culture and tissue engineering scaffolds. Tissue Eng Part A 17:2583–2592
Dong S, Huangfu X, Xie G et al (2015) Decellularized versus fresh-frozen allografts in anterior cruciate ligament reconstruction: an in vitro study in a rabbit model. Am J Sports Med 43:1924–1934
Farnebo S, Woon CY, Bronstein JA et al (2014) Decellularized tendon-bone composite grafts for extremity reconstruction: an experimental study. Plast Reconstr Surg 133:79–89
Figliuzzi M, Remuzzi G, Remuzzi A (2014) Renal bioengineering with scaffolds generated from rat and pig kidneys. Nephron Exp Nephrol 126:113–118
Goh SK, Bertera S, Olsen P et al (2013) Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 34:6760–6772
Gong W, Lei D, Li S et al (2016) Hybrid small-diameter vascular grafts: anti-expansion effect of electrospun poly ε-caprolactone on heparin-coated decellularized matrices. Biomaterials 76:359–370
Graham ME, Gratzer PF, Bezuhly M et al (2016) Development and characterization of decellularized human nasoseptal cartilage matrix for use in tissue engineering. Laryngoscope. doi:10.1002/lary.25884
Guan Y, Liu S, Sun C et al (2015) The effective bioengineering method of implantation decellularized renal extracellular matrix scaffolds. Oncotarget 6:36126–36138
Haidar A, Legault L, Messier V et al (2015) Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol 3:17–26
Han TT, Toutounji S, Amsden BG et al (2015) Adipose-derived stromal cells mediate in vivo adipogenesis, angiogenesis and inflammation in decellularized adipose tissue bioscaffolds. Biomaterials 72:125–137
Hasan A, Memic A, Annabi N et al (2014) Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater 10:11–25
Kitahara H, Yagi H, Tajima K et al (2016) Heterotopic transplantation of a decellularized and recellularized whole porcine heart. Interact CardioVasc Thorac Surg 22:571–579
Ko IK, Peng L, Peloso A et al (2015) Bioengineered transplantable porcine livers with re-endothelialized vasculature. Biomaterials 40:72–79
Langer R, Vacanti JP (1993) Tissue engineering. Science 260:920–926
Lee JS, Shin J, Park HM et al (2014) Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules 15:206–218
Lovati AB, Bottagisio M, Moretti M (2016) Decellularized and engineered tendons as biological substitutes: a critical review. Stem Cells Int 2016:7276150
Lu Q, Li M, Zou Y et al (2014) Delivery of basic fibroblast growth factors from heparinized decellularized adipose tissue stimulates potent de novo adipogenesis. J Control Release 174:43–50
Mahara A, Somekawa S, Kobayashi N et al (2015) Tissue-engineered acellular small diameter long-bypass grafts with neointima-inducing activity. Biomaterials 58:54–62
Mazza G, Rombouts K, Hall AR et al (2015) Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci Rep 5:13079
Mei J, Yu Y, Li M et al (2016) The angiogenesis in decellularized scaffolt-mediated the renal regeneration. Oncotarget 7:27085–27093
Methe K, Bächdahl H, Johansson BR et al (2014) An alternative approach to decellularize whole porcine heart. BioRes Open Access 3:327–338
Moore MA, Samsell B, Wallis G et al (2015) Decellularization of human dermis using non-denaturing anionic detergent and endonuclease: a review. Cell Tissue Bank 16:249–259
Moroni F, Mirabella T (2014) Decellularized matrices for cardiovascular tissue engineering. Am J Stem Cells 3:1–20
Nakamura S, Ijima H (2013) Solubilized matrix derived from decellularized liver as a growth factor-immobilizable scaffold for hepatocyte culture. J Biosci Bioeng 116:746–753
Nichols JE, Francesca SL, Vega SP et al (2016) Giving new life to old lungs: methods to produce and assess whole human paediatric bioengineered lungs. J Tissue Eng Regen Med. doi:10.1002/term.2113
Nyame TT, Chiang HA, Leavitt T et al (2015) Tissue-engineered skin substitutes. Plast Reconstr Surg 136:1379–1388
Ota T, Taketani S, Iwas S et al (2007) Novel method of decellularization of porcine valves using polyethylene glycol and gamma irradiation. Ann Thorac Surg 83:1501–1507
Ott HC, Matthiesen TS, Goh SK et al (2008) Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med 14:213–221
Ott HC, Clippinger B, Conrad C et al (2010) Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 16:927–933
Pan J, Liu GM, Ning LJ et al (2015) Rotator cuff repair using a decellularized tendon slices graft: an in vivo study in a rabbit model. Knee Surg Sports Traumatol Arthrosc 23:1524–1535
Peloso A, Ferrario J, Maiga B et al (2015) Creation and implantation of acellular rat renal ECM-based scaffolds. Organogenesis 11:58–74
Peloso A, Urbani L, Cravedi P et al (2016) The human pancreas as a source of protolerogenic extracellular matrix scaffold for a new-generation bioartificial endocrine pancreas. Ann Surg 264:169–179
Ren X, Moser PT, Gilpin SE et al (2016) Engineering pulmonary vasculature in decellularized rat and human lungs. Nat Biotechnol 33:1097–1102
Rheinwald JG, Green H (1975) Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6:331–343
Roosens A, Somers P, Somer FD et al (2016) Impact of detergent-based decellulairization method on porcine tissues for heart balve engieering. Ann Biomed Eng 44:2827–2839
Rowland CR, Colucci LA, Guilak F (2016) Fabrication of anatomically-shaped cartilage constructs using decellularized cartilage-derived matrix scaffolds. Biomaterials 91:57–72
Sabetkish S, Kajbafzadeh AM, Sabetkish N et al (2015) Whole-organ tissue engineering: decellullarization and recellularization of three-demensional matrix liver scaholds. J Biomed Mater Res A 103A:1498–1508
Sánchez PL, Fernández-Santos ME, Costanza S et al (2015) Acellular human matrix: a critical step toward whole heart grafts. Biomaterials 61:279–289
Sánchez PL, Fernández-Santos ME, Espinosa MA et al (2016) Data from acellular human heart matrix. Data Brief 8:211–219
Sarikouch S, Horke A, Tudorache I et al (2016) Decellularized fresh homografts for pulomonary valve replacement: a decade of clinical experience. Eur J Cardiothorac Surg 50:281–290
Schmitt T, Fox PM, Woon CY et al (2013) Human flexor tendon tissue engineering: in vivo effects of stem cell reseeding. Plast Reconstr Surg 132:567e–576e
Seif-Naraghi SB, Salvatore MA, Schup-Magoffin PJ et al (2010) Design and characterization of an injectable pericardial matrix gel: a potentially autologous scaffold for cardiac tissue engineering. Tissue Eng Part A 16:2017–2027
Shirakigawa N, Ijima H, Takei T (2012) Decellularized liver as a practical scaffold with a vascular network template for liver tissue engineering. J Biosci Bioeng 114:546–551
Shirakigawa N, Takei T, Ijima H (2013) Base structure consisting of an endothelialized vascular-tree network and hepatocytes for whole liver engineerig. J Biosci Bioeng 116:740–745
Smit FE, Dohmen PM (2014) Bio-artificial heart as ultimate treatment of end-stage heart failure. Med Sci Monit Basic Res 20:161–163
Song JJ, Kim SS, Liu Z et al (2011) Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg 92:998–1006
Song JJ, Guyette JP, Gilpin SE et al (2013) Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 19:646–651
Sugiura T, Tara S, Nakayama H et al (2016) Novel bioresorbable vascular graft with sponge-type scaffold as a small-diameter arterial graft. Ann Thorac Surg (in press)
Theodoridis K, Müller J, Ramm R et al (2016) Effects of combined cryopreservation and decellularization on the biomechanical, structural and biochemical properties of porcine pulmonary heart valves. Acta Biomater (in press)
Umashankar PR, Sabareeswaran A, Sachin JS (2016) Long-term healing of mildly cross-linked decellularized bovine pericardial aortic patch. J Biomed Mater Res B (in press)
Uygan BE, Sato-Gutierrez A, Yagi H et al (2010) Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 16:814–820
Wakimura Y, Wang W, Itoh S et al (2015) An experimental study to bridge a nerve gap with a decellularized allogeneic nerve. Plast Reconstr Surg 136:319e–327e
Wang L, Johnson JA, Zhang Q et al (2013) Combining decellularized human adipose tissue extracellular matrix and adipose-derived stem cells for adipose tissue engineering. Acta Biomater 9:8921–8931
Willenberg BJ, Oca-Cossio J, Cai Y et al (2015) Repurposed biological scaffolds: kidney to pancreas. Organogenesis 11:47–57
Yagi H, Fukumitsu K, Fukuda K et al (2013) Human-scale whole-organ bioengineering for liver transplantation: a regenerative medicine approach. Cell Transplant 22:231–242
Yasui H, Lee JK, Yoshida A et al (2014) Excitation propagation in three-dimensional engineered hearts using decellularized extracellular matrix. Biomaterials 35:7839–7850
Yin Z, Chen X, Zhu T et al (2013) The effect of decellularized matrices on human tendon stem/progenitor cell differentiation and tendon repair. Acta Biomater 9:9317–9329
Zhan JS, Wang ZB, Lin KZ et al (2015) In vivo regeneration of renal vessels post whole decellularized kidneys transplantation. Oncotarget 6:40433–40442
Zhang Q, Johnson JA, Dunne LW et al (2016a) Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps. Acta Biomater 35:166–184
Zhang S, Lu Q, Cao T et al (2016b) Adipose tissue and extracellular matrix development by injectable decellularized adipose matrix loaded with basic fibroblast growth factor. Plast Reconstr Surg 137:1171–1180
Zhou J, Ding J, Nie B et al (2015) Promotion of adhesion and proliferation of endothelial progenitor cells on decellularized valves by covalent incorporation of RGD peptide and VEGF. J Mster Sci: Mater Med 27:142
Zia S, Mozafari M, Natasha G et al (2016) Hearts beating through decellularized scaffolds: whole-organ engineering for cardiac regeneration and transplantation. Crit Rev Biotechnol 36:705–715
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Shirakigawa, N., Ijima, H. (2017). Decellularized Tissue Engineering. In: Tripathi, A., Melo, J. (eds) Advances in Biomaterials for Biomedical Applications. Advanced Structured Materials, vol 66. Springer, Singapore. https://doi.org/10.1007/978-981-10-3328-5_5
Download citation
DOI: https://doi.org/10.1007/978-981-10-3328-5_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3327-8
Online ISBN: 978-981-10-3328-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)