Abstract
Three different production systems are used for perch ongrowing: (1) traditional extensive polyculture system, (2) semi-intensive culture farming and (3) intensive perch farming under RAS (Recirculating Aquaculture System(s)). Extensive and semi-intensive culture systems have many production limitations. Therefore, intensive perch farming has been developed in Europe for more continuous and predictive marketable perch production.
Marketable perch production under RAS is affected by several main factors of production system. Optimal value and condition of each factor for stable and maximal perch production under RAS are described and recommended in details in this chapter.
Overall, white, grey and black tank walls with light regime 12L:12D or 18 L:8D and light intensity 200–1,100 lx create optimal light conditions for intensive ongrowing perch culture. Freshwater or water with salinity under 4 ‰ with temperature 22–24 °C, oxygen saturation around 60–72 % and very low ammonia (below 0.3 mg N-NH3 · L−1) and nitrite (below 0.5 mg NO2 − · L−1) concentrations are optimal conditions for intensive perch production. Disturbance (cleaning of tanks, fish size-sorting etc.) must be reduced at minimum level for providing of maximal production which is the highest under optimal fish biomass from 10 to 20 kg · m−3 for 10 g perch to 60–70 kg · m−3 for 150 g perch under RAS.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Nowadays, three different production systems for culture of Eurasian perch (Perca fluviatilis) during ongrowing phase (body weight ranging from 1–2 to 100–300 g) can be recognized (Kestemont et al. 2008). Ongrowing of perch has traditionally taken place in production ponds and reservoirs under extensive polyculture systems (Tamazouzt et al. 1993). Semi-intensive culture using cage farming in lakes or sea bays or the combination of pond and RAS (Recirculating Aquaculture System(s)) culture in ongrowing perch has been used in different countries such as: Switzerland (Janssens 2013, personal communication), Sweden (Ȍberg 2008), Germany (Schmidt and Wedekind 2008) and Czech Republic (Policar et al. 2009). However, conditions for more intensive aquaculture of perch have been investigated over last 20 years (Overton and Paulsen 2005). Therefore, intensive perch farming under RAS has been developed and used for more predictive marketable fish production mainly in Switzerland, Ireland and France (Wernicke von Siebenthal 2013; Toner 2012; Martin and Vandevorede 2008).
2 Extensive Perch Farming Under Pond Conditions
Perch farming in extensive polyculture system accounts for a substantial portion of marketable perch production, especially in central and eastern part of Europe (Kestemont et al. 2008) including following countries: Russia, Ukraine, Czech Republic, Romania, Latvia and Bulgaria (FAO 2013). French perch production from polyculture ponds occurs in three specific areas (Dombes, Lorraine and Brenne) of north-east France (Tamazouzt 2008).
The perch production cycle takes 3–4 years under extensive pond culture to produce a 250–400 g market size (Policar et al. 2009). Marketable perch are harvested maximum twice per year, once during autumn and once during spring harvest season when ponds are harvested (Kratochvíl 2012).
Young perch (0+; final TL around 70 mm) are produced in monoculture system with or without the presence of prey fish, when ponds are stocked at density 120,000 fish per hectare. Prey fish (e.g. roach, Rutilus rutilus, topmouth gudgeon, Pseudorasbora parva, or other small cyprinids species) up to mentioned perch size (TL = 70 mm) have not positive effect on perch growth and survival rate compared to perch culture without prey fish. Macrophytes have a positive effect on macroinvertebrate (phytomacrofauna) community that are the main food for reared perch in ponds. Their abundance seems to be more effective to increase perch production than using of prey (Bláha et al. 2013). Survival rate from larvae up to TL = 70 mm perch ranged from 12 % to 36 % with final perch density of 14–43 thousands fish per hectare. A SGR of 1.3 % · day−1 is recorded during the rearing period from the end of April till the end of September (Bláha et al. 2013).
Ongrowing perch (1+ to 3+) are cultured in ponds of several hectares in polyculture (Policar et al. 2009). In this rearing system, the production of marketable perch represents 0.25–1 % only from the total final biomass of fish stock, which is dominated by cyprinids species such as common carp (Cyprinus carpio) 80–85 %, Chinese carps (5–10 %) such as grass carp (Ctenopharyngodon idella) and bighead carp (Hypophthalmichthys molitrix) (Adámek et al. 2010; Kratochvíl 2012). Perch as a predatory species plays an important role in the control and regulation of the overpopulated and less valued small cyprinids such as: roach, bleak (Alburnus alburnus), bream (Abramis brama), topmouth gudgeon and ruffe (Gymnocephalus cernua) in production ponds (Musil and Adámek 2003; Adámek et al. 2012). Thus, perch is an interesting supplementary fish to regulate carp production in ponds (Adámek et al. 2010).
3 Semi-intensive Perch Farming Using Cages and Ponds
Commercial culture of perch in cages was used by Perlac company, Switzerland under ambient water temperature in lake Neuchâtel near to Chez–le–Bart during 1999–2005 (Janssens 2013, personal communication). The Swedish company, Stannafisk AB, followed the same procedure using cages and tarpaulin tanks for perch production in the sea bay near to Ȍstergötland between 2006 and 2011 (Ȍberg 2008, 2012). Very low fish growth and survival mainly during winter period, poor efficiency of culture system (Ȍberg 2012), fatal bacterial infection caused by Aeromonas sobria, as well as social and political problems with operating fish farms in Swiss lake (Janssens 2013, personal communication) resulted in the failure of this production system for marketable perch production in the mentioned countries.
The greatest drawback of this perch farming system within Europe is suboptimal temperature for growth during whole year. It requires two successive growing seasons (May–October, Fig. 16.1) to obtain marketable-size fish (80–100 g minimum) when water temperature ranges between 14 and 20 °C (Tamazouzt et al. 1993). Temperature during ongrowing season highly affected perch production ranging from 15 to 160 g · m−3 · day−1 when fish were reared under relatively high final density (30 kg · m−3) (Kestemont and Mélard 2000).
Growth curve of perch in cage under natural fluctuated temperature (2–23 °C) or at stable temperature (23 °C) in RAS (Mélard 2008)
Fontaine et al. (1996) recommended using the pond – cage combination system for perch farming. In this system, 1-year old perch of 10–20 g body weight were harvested from ponds during spring and subsequently cultured in cages for two summer seasons. However, the perch growth recorded in this cage culture system was very low and a few fish reached a marketable size (80–100 g). When Tamazouzt et al. (1996) reared perch with initial body weight 25 g in floating cages from July to September survival rate was between 70 % and 79 % and body weight perch, ranging from 48 to 49 g, far less than minimal market size. Perch cultured in cages had a higher protein and a lower lipid and energy content compared to perch reared in recirculating aquaculture system(s) (RAS) under 22 °C (Tamazouzt et al. 1996). Nowadays, this farming system is not widely used for commercial perch production in Europe because it does not provide stable, high-quality and profitable production of marketable fish (Kestemont and Mélard 2000).
4 Semi-intensive Perch Farming Using the Combination of Pond and RAS Culture
Initial larval and juvenile culture under pond conditions up to perch of 1–3.2 g body weight has been combined with intensive ongrowing of fish to a commercial size in RAS using artificial food (Schmidt and Wedekind 2008; Stejskal et al. 2009a, 2010). This production system using advantages of both pond and RAS culture systems is very efficient.
Survival rate during habituation of the pond-reared fingerlings was 95 % after one week adaptation and weaning period when perch were fed frozen Chironomus or Chaoborus larvae with artificial food. The advantages of the pond culture system are: natural food, rapid fish growth, high-quality of produced juveniles, low production cost, elimination of abnormal fish, environmentally friendly and sustainable fish production without any fish deformity. RAS provides controlled and stable production conditions throughout year resulting in rapid growth rate and thus shorter production cycle of marketable fish (Stejskal et al. 2010; Policar et al. 2013). Currently, the greatest limitation of this system for perch rearing is only one batch production per year, when initial pond culture of this system is possible to carry out during later spring or summer period. The second limitation is the risk to introduce diseases in the RAS when stocking with juveniles fish coming from ponds (Schmidt and Wedekind 2008; Stejskal et al. 2010). This system is successfully used mainly in countries of Central Europe (Czech Republic, Germany, Hungary etc.) where large pond area is available (Policar et al. 2011).
5 Intensive Perch Farming Under RAS
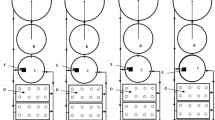
Intensive perch culture in recirculating aquaculture system(s) provides optimal culture conditions for rapid fish growth, high survival rate, shorter production cycle, year round and predictable production, reduction of fish stress and cannibalism. In order to ensure a high productivity and reduced production costs, several rearing conditions have to be optimized (Fig. 16.1, Kestemont and Mélard 2000): colour of rearing tank walls (Staffan 2004; Mairesse et al. 2005; Strand et al. 2007a), light regime and intensity (Jourdan et al. 2000; Strand et al. 2007a; Stejskal et al. 2009a, 2010; Jacquemond 2004), water temperature (Karas and Thoresson 1992; Kestemont and Mélard 2000; Mélard et al. 1995, 1996a; Overton and Paulsen 2005; Strand et al. 2007b; Mélard 2008), water quality including mainly oxygen (Zakes and Demska-Zakes 2005; Mélard 2008; Stejskal 2009b, 2012), salinity (Overton et al. 2008), ammonia and nitrite levels (Mélard 2008; Vandecan et al. 2008; Kroupová et al. 2013), disturbance during tank cleaning, fish size-sorting (Mélard et al. 1995, 1996a; Kestemont and Baras 2001; Strand et al. 2007b; Mélard 2008) and fish density and biomass (Mélard et al. 1996a, b; Mélard 2008).
6 Factors Affecting Growth, Survival Rates and Productivity of Perch Under Intensive Culture
6.1 Colour of Tank Walls, Light Intensity and Regime
The interaction between colour of tank walls and light intensity creates specific light conditions within rearing tanks which significantly affects feed detection and feeding success of farmed perch under intensive conditions, thus influencing perch growth (Strand et al. 2007a).
In general, larvae of percid species are strongly phototactic, but older individuals may be more sensitive to high light intensities (Craig 2000; Kestemont and Mélard 2000; Kestemont et al. 2003) such as 2,200 lx (Staffan 2004). However, Strand et al. (2007a) showed that different light intensities (at least when 200 and 1,100 lx were used) did not affect feed intake, growth rate and growth efficiency. Instead, the mentioned study showed that food intake and consequently growth rate were significantly higher in white or grey tanks compared to black ones under low light intensity (200 lx). The reason was reported to be the increased feed visibility, probably due to the feed’s higher contrast against the background colour of the tank walls. When the authors used higher light intensity (1,100 lx) for different wall colour tanks they didn’t find any significant differences in feed intake and growth rates between different colours of tank wall. The explanation of this observation is that higher light intensity increased the ability of fish to detect feed in dark tanks (Strand et al. 2007a) and the effect of colour of tank walls on the feed intake has been reduced. Careful consideration of tank colour therefore was reported to be of greater importance at low light intensities than at high light intensities (Strand et al. 2007a). Any effect of tank colour and light intensity on the energy expenditure was not evident during mentioned experiment. Probably, stress was not induced by any tested environmental factors or their combination for the cultured perch. Staffan (2004) tested three different light intensities (16; 200 and 2,200 lx) during light regime 12L:12D and found that perch were more active during daytime in the highest light intensity compared to two lower intensities. These results can indicate increased stress at higher light intensity that is not recommended for perch aquaculture. Staffan (2004) also studied preference of perch for three different colours of tank walls (white, grey and black), when perch could move freely among tank colours. No general preferences were found for any specific colour. As growth did not differ among the three different tank colours, the study indicated that white, grey and black tank wall colours are equally suitable for farming of perch. A clear difference in body colour was interestingly noted for perch kept in black and white tanks. Almost, all perch coming from the black tanks were dark and perch coming from the white tanks were light grey (Mairesse et al. 2005; Strand et al. 2007a). This phenomenon indicated that the capacity of perch to change body colour in accordance with its background could reduce conspicuousness and thus reduce this potential source of stress in cultured fish (Strand et al. 2007a).
Light regime 12L:12D with an intensity of 105–250 lx at water surface (Stejskal 2009a, 2010; Jacquemond 2004) or 16L:8hD (Strand et al. 2007a) were used during perch ongrowing phase under controlled and intensive conditions. When Jourdan et al. (2000) increased light regime from 12L:12D to 18L:6D and even 24L:0D, specific growth rates of perch significantly increased but without any differences between both light regimes 18L:6D and 24L:0D.
6.2 Water Temperature
Eurasian perch is a thermophilic species and optimum temperature for rapid growth ranges from 22 to 24 °C (Mélard et al. 1996a). Intensive ongrowing of perch under this range of temperature gives the highest productivity level (Kestemont and Mélard 2000). Temperatures of 22–24 °C maintained during the whole ongrowing phase results in market size perch (130–150 g) obtained in about 14 months including larval rearing period (Mélard et al. 1996a). When perch juveniles (0.5 g initial body weight) were cultured under RAS at 23 °C the minimal commercial marketable size (100 g) could be obtained after 9 months (Fig. 16.2, Mélard 2008). Maximum daily growth rates (0.06–1.80 g · fish−1) for 3–300 g fish were also observed at 23 °C. This constant elevated temperature inhibited the sexual maturation in female. This phenomenon supported female higher investment in somatic growth rate. However, males presented a normal gonadal development in the same temperature conditions (Mélard et al. 1996a; Overton and Paulsen 2005).
Effect of water temperature on growth of mixed sex perch in intensive rearing conditions (Mélard 2008)
Rearing at higher (27 °C) or lower temperatures (11–20 °C) reduces growth rate, i.e. the growth of 15 g fish at 27 °C was 12 % lower than at 23 °C. Similarly, 100 g perch reared under 20 °C had a 20 % lower growth compared to 23 °C (Mélard et al. 1996a). A relative low growth rate was observed at 11 °C which is 29 % of that found at 23 °C. This low temperature is not suitable for intensive perch culture and it has been used within extensive (pond) or semi-intensive (cage) perch culture during spring and autumn (Kestemont and Mélard 2000). Nevertheless, Mélard et al. (1995) found higher SGR (1.86 % · day−1) in 1.9 g perch cultured at 26.5 °C compared to 22.9 °C (1.76 % · day−1). These results were not significantly different because both temperatures were within optimal range of temperature for perch growth. Strand et al. (2007b) indicated the lack of temperature effect on energy expenditures of Eurasian perch and concluded that this fish is equally well adapted for growth at both 17 and 23 °C. Also they found that perch does not seem to be energetically more sensitive to disturbance at 23 than at 17 °C. However, the overall effect of temperature was significant for feed intake and growth rate, with higher feed intake and growth rate at 23 than at 17 °C. Karas and Thoresson (1992) showed a maximum food consumption of perch between 23 and 28 °C.
Despite this, Overton and Paulsen (2005) recommended temperature around 20 °C for perch rearing because this temperature is better for easier control of infections and problems occurred by Saprolegnia spp. Mélard et al. (1995) found the higher infection level of cultured ongrowing perch by Heteropolaria spp. at 26.5 °C. The survival of ongrowing perch (83.2 %) was negatively affected by this infection at 26.5 °C compared to lower temperature 22.9 °C without any infection and with survival rate 91.5 % (Mélard et al. 1995). Effect of bacteria (Aeromonas sp., A. veronii, A. hydrophila, Streptococcus sp., Staphylococcus sp., Vibrio fluviatilis and Enterobacter agglomerans) and protozoa (Ichtyobodo necator, Trichodina sp., Ambiphyra sp. and Heteropolaria sp.) species on the perch health inducing mass mortality of fish under stable and optimal temperature in intensive perch culture was well reviewed by Grignard et al. (1996).
6.3 Water Quality Including Oxygen, Salinity and Ammonia Level
Generally, water quality directly affects physiological condition of cultured perch and indirectly their feed intake and growth rate (Wedemeyer 1996). The oxygen level should be maintained above 5 mg O2 · L−1 (60 % oxygen saturation) and higher oxygen concentrations do not significantly increase the growth rate of perch under intensive ongrowing conditions at 23 °C (Mélard 2008). However, Kestemont et al. (2008) recommended optimal oxygen level up to 6 mg O2 · L−1, i.e. 68–72 % oxygen saturation for intensive perch juvenile culture under 20–23 °C. Stejskal et al. (2009b) used 6.8 ± 1.2 mg O2 · L−1 oxygen level, i.e. 80 % oxygen saturation, for ongrowing perch under intensive culture. Effect of three different oxygen levels (hypoxia: 50–60 % oxygen saturation; normoxia: 90–100 % and hyperoxia: 140–150 %) on the feed intake and conversion and growth rate of intensively cultured pikeperch (Sander lucioperca) was examined by Stejskal et al. (2012). These authors found that higher oxygen saturation provides higher feed intake, better feed utilization (lower FCR) and growth rate. Perch has very similar environmental requirements as pikeperch (Craig 2000), therefore found results could be also generalized to intensive perch culture. Optimization of oxygen level during intensive perch culture needs additional studies mainly for obtaining more precise data regarding to the growth, feed conversion under high oxygen level and the impact on production cost in intensive perch farms. Biological oxygen consumption (OC in mg O2 · kg−1 · h−1) in six size groups (18.4–82.3 g) of intensively farmed perch was determined at 23 °C by Zakes and Demska-Zakes (2005). OC decreased from 336.2 to 185.0 mg O2 · kg−1 · h−1 in all tested size groups. An increase of body weight by 1 g led to average decrease in OC by a mean of 2.53 O2 · kg−1 · h−1. Stejskal et al. (2009b) observed diurnal course of OC in two groups (fed and feed-deprived) of intensively cultured perch. Fed perch with body weight from 44.8 to 279.4 g had average OC 288.3–180.6 mg O2 · kg−1 · h−1 with significant peak observed 6 h after the onset of feeding and relatively stable values of OC up to the end of feeding during day. Significantly lower average OC values (181.1–110.5 mg O2 · kg−1 · h−1) were found in feed-deprived perch with body weight 57.9–336.2 g without any significant peak of OC during day.
Oxygen levels under 5 mg · L−1 may induce significant stress leading to pathology and mortality (Stejskal et al. 2009b; Mélard 2008). Mortality of perch mainly appears when oxygen deficit is combined with intensive feeding resulting in additional oxygen consumption (Stejskal et al. 2009b).
Overton et al. (2008) found that growth rate and condition factor of perch from freshwater population (1.6 g) can be negatively affected by salinity exceeding 10 ‰ during 126 days rearing at 20 °C. These authors showed significantly higher growth rate in freshwater and at salinity 4 ‰, compared to salinity 10 ‰. Moreover, salinity 10 ‰ caused higher FCR (2.48) compared to freshwater or salinity 4–8 ‰ (1.80). Condition factor was reduced at salinity 10 ‰ compared to perch reared in freshwater with salinity 0 ‰. These results show that a freshwater strain of perch presents the best growth performance in fresh water or low saline conditions (4 ‰). Regarding to the fact that some perch strains are found in brackish waters, Overton et al. (2008) recommended choosing salinity adapted strains for brackish water production as found in the lower Baltic region.
Overton et al. (2008) found negative effect of higher salinity 13 ‰ and 18 ‰ on perch survival which was highly affected by water temperature (12, 15, 20 and 25 °C). No mortality of perch was found when fish were moved into brackish water of 13 ‰ at 12 and 15 °C. However, a dramatic increase in mortality rate was seen when perch were transferred into brackish water of 13 ‰ at 20 or 25 °C. A total of 50 % mortality was reached at 109 and 62 h for the two mentioned temperatures. Perch transfer to brackish water at 18 ‰ resulted in high mortality. Again, the mortality rate was positively related to temperature and 50 % mortality was reached at 178, 119, 69 and 39 h at 12, 15, 20 and 25 °C, respectively (Overton et al. 2008).
In intensive perch culture under RAS, high concentrations of ammonia and nitrite in water may take place when biological filters do not work properly. Ammonia (0.3 mg N-NH3 · L−1) and nitrite (0.5–0.7 mg NO2 − · L−1) concentrations induce physiological changes in perch such as reduced excretion of ammonia, conversion of haemoglobin to methaemoglobin resulting in limited oxygen transport (Jensen 2003; Svobodová 2005; Mélard 2008; Kroupová et al. 2013). These physiological changes negatively affect growth performances then induce mortality (Svobodová et al. 2005; Mélard 2008; Vandecan et al. 2008). A chronic exposure of 140–150 g perch at concentrations of 0.3–0.4 mg N-NH3 · L−1 induces a 50 % decrease of growth rate compared to fish in control groups under temperature 23.1 °C, oxygen saturation 100 % and pH 8.1 (Fig. 16.3) (Vandecan et al. 2008; Mélard 2008). Higher ammonia concentration (0.8 mg N-NH3 · L−1) has lethal effect for 50 % of the population after 96 h exposure at 23 °C (96hLC50) (Vandecan et al. 2008; Mélard 2008).
Effect of long time exposure to ammonia on growth of 150 g perch at 23 °C (Mélard 2008)
Acute toxicity of nitrite for 10.8 g perch was found by Kroupová et al. (2013). These authors established lethal concentration of nitrite (48hLC50) at 11 mgNO2 − · L−1. Concentration of 3.8 mg NO2 − · L−1 was found safe after 48 h exposure. However, these results are obtained from laboratory experiments and according to our field experience, lethal concentration of nitrite is even significantly lower (1–3 mg NO2 − · L−1) during intensive perch culture under RAS.
6.4 Disturbance
Disturbance from cultivation procedure like cleaning of tanks, service disturbance, fish size-sorting and other stressful events, reduce feed intake and increase energy expenditure (Strand et al. 2007b) and indirectly decrease growth in perch (Jentoft et al. 2005; Strand et al. 2007b). All mentioned operation must be carefully done for maintenance of good zoohygienic conditions, sorting of perch, elimination of growth heterogeneity and cannibalism with minimal negative effect on production (Mélard et al. 1996a; Acerete et al. 2004; Jentoft et al. 2005; Strand et al. 2007b). Proper culture management may reduce the response of fish to disturbance (Strand et al. 2007b).
Higher frequency of disturbance, included cleaning of tanks and other service disturbance, decreased feed intake and growth rate under lower (17 °C) and optimal temperature (23 °C). Severely disturbed perch at 17 °C had higher energy expenditures than undisturbed fish. The energetic growth efficiencies for disturbed fish were reduced by 19–38 % compared to undisturbed fish causing a reduction in weight increase of 24–56 %. No significant effect of higher frequency of disturbance on energy expenditures was observed at 23 °C (Strand et al. 2007b). The timing of this disturbance in relation to time of feeding is also very important factor affected feed intake in perch. Kestemont and Baras (2001) indicated that feed intake for perch disturbed by human activity prior to their first meal was reduced by 60 %, but if the fish were disturbed later in the day, after being fed, the daily feed intake was little affected. In contrast, Strand et al. (2007b) executed disturbance after the morning meal found 7 and 28 % reduction of feed intake at 17 °C, and 31 and 22 % at 23 °C for moderately and severely disturbed perch.
6.5 Growth Heterogeneity
Fish size-sorting is a very important operation within intensive perch culture for the reduction of growth heterogeneity and cannibalism with minimal negative effect on production. However this operation is stressful for fish and, paradoxically, may induce new social interactions within perch population after a grading. Generally, frequency and process of fish size-sorting must be optimized according to current situation in perch farming (Mélard et al. 1995, 1996a, b; Kestemont and Mélard 2000; Mélard 2008). Fish size-sorting to reduce growth heterogeneity results in the emergence of fast growing perch in each sorted group and strongly reduces cannibalism. However, this technique induces a new increase of heterogeneity in future culture phase (Mélard et al. 1996b; Mélard 2008; Kestemont et al. 2000). Paradoxically, Kestemont et al. (2000) found that the higher initial heterogeneity caused the lower final heterogeneity (Table 16.1). However, high initial heterogeneity promoted cannibalism exerted upon small fish and induced a decrease of heterogeneity and survival rate under its initial value.
Heterogeneity in perch growth is high. Body weight can range from 7 to 89 g for 7-month-old perch averaging 25.9 g (Mélard et al. 1996a) and from 21 to 452 g for 1-year-old perch (Fig. 16.4). In general, size heterogeneity (coefficient of variation of body weight) tends to stabilize around 40–45 % (Kestemont et al. 2000). The origin of the huge heterogeneity in perch is not only due to sexual dimorphism, when female grows around 20–30 % faster than males (Fontaine et al. 1997; Rougeot and Mélard 2008), but also to genetic traits (Mandiki et al. 2004) and social behaviour (Mélard et al. 1995). Therefore, the sorting process does not guarantee the improvement of the global productivity of perch culture. For instance, non-sorted populations had a growth rate 5–6 % higher than in size-graded populations of same origin and body weight (Mélard et al. 1995, 1996a; Mélard 2008). Generally, intensive perch farming needs to reduce frequency of fish size-sorting. Perch intensive culture with lower needs for fish size-sorting can be provide by the production of monosex female populations with higher growth rate, shortening production cycle and lowering size heterogeneity (Mélard et al. 1996a; Rougeot and Mélard 2008).
Generally, mortality resulting from direct and indirect effect of handling is only a small part of overall mortality, which is mainly due to parasites and bacteria (Mélard et al. 1996a).
6.6 Body Weight
Increasing body weight of intensively cultured perch under 23 °C decreased SGR and production capacity. Perch of 5 g body weight reared at an optimal biomass of 35 kg · m−3 gave production around 600 g · m−3 · day−1. At an optimal biomass of 75 kg · m−3, the production of 150 g body weight fish was 320 g · m−3 · day−1, only (Fig. 16.6, Mélard 2008). Overton et al. (2008) obtained the same results related to growth rate for perch reared during 4 months. Decreasing specific growth rate was found from 6 % · day−1 for perch reared 1–14 days after the start of the experiment up to growth rate 1 % for older perch reared 85–126 days of the experiment.
Continuous mortality may occur throughout intensive perch culture within the 1–200 g body weight range and result in an overall 50 % survival rate after 14 months (Mélard et al. 1996a). In RAS, where rearing conditions are optimal, Mélard (2008) reported that mortality takes place during first months of intensive culture of ongrowing perch from 0.3 to 200 g body weight and overall survival rate is fluctuating between 60 % and 70 % (Fig. 16.5).
Evolution of perch survival in RAS starting from 0.3 g weaned juveniles to 1 year 200 g fish (Mélard 2008)
6.7 Fish Density and Biomass
In general, higher fish density significantly increases growth rate and decreases growth heterogeneity in initial phase of perch intensive ongrowing (0.5–15 g) (Mélard et al. 1996a). Increased stocking density from 400 to 10,000 fish · m−3 resulted in a 67 % increased growth rate of 1 g perch juveniles cultured under 23 °C during 74 days. Growth rate was 0.12 and 0.20 g · fish−1 · day−1 at densities of 400 and 10,000 fish · m−3, respectively (Mélard et al. 1996a). Mélard et al. (1996b) examined the effect of stocking density on 45-days perch juveniles and showed that higher densities (1,430 and 2,380 fish · m−2) significantly increased specific growth rate of perch in comparison to low density (95 fish · m−2). After 74-days rearing, juveniles with initial body weight 0.86 g reached a final body weight of 9.75 g (SGR = 3.44 % · day−1) and 15.83 g (SGR = 3.87 % · day−1) under low (95 fish · m−2) and high fish density (2,380 fish · m−2), respectively.
However, this positive relationship between perch biomass and growth rate was valid until fish reached 10–16 g body weight (Mélard et al. 1996a). Growth rate of ongrowing perch with initial body weight up to 16 g decreased with increasing stocking biomass up to 20–60 kg · m−3 (Mélard 2008).
For 5–10 g perch increasing density induces a decreasing of growth heterogeneity and consequently of cannibalism (Mélard et al. 1996b). Coefficient of variation of fish body weight was decreased from 98.4 % to 57.9 % with increasing stocking density from 400 to 10,000 fish · m−3 (Mélard et al. 1996a). Negative effect of higher density on cannibalism rate was found by Mélard et al. (1996b). Density up to 1,430 fish · m−2 resulted in lower numbers of cannibals. Emergence of cannibalism depending on perch density was modelled as a second order polynomial equation (R2 = 0.995, df = 9, P < 0.01): NCa = 0.157 + 6.394 × 10−2 ISD – 1.779 × 10−5 (ISD)2, where NCa is numbers of cannibalism (fish · m−2) and ISD is initial stocking perch density (fish · m−2) (Mélard et al. 1996b). Cannibalism caused overall mortality rates of 3.0, 4.5, 6.7, 11.4 and 7.1 % at densities 95, 240, 480, 1,430 and 2,380 fish · m−2, respectively. However, these authors found that cannibalism is not the main reason of overall mortality for ongrowing perch, when survival rate varied between 75.4 % and 92.2 % at perch density 1,430 and 95 fish · m−3, respectively. Excluding cannibalism, the main reason of perch mortality during ongrowing phase is parasites, bacteria and stress that are indirectly affected by different perch densities and biomass (Mélard et al. 1996a, b).
6.8 Relationship Between Body Weight, Optimal Biomass and Production
In intensive farming, the optimal biomass of perch giving the maximal production depends of body weight. The higher is perch body weight, the higher optimal biomass is. Optimal biomass ranges from 35 kg · m−3 for 5 g perch to 80 kg · m−3 for 150 g fish. Relationship between optimal biomass (y) and body weight (x) is expressed as follow: y = 24 × 0.23 (Fig. 16.6, Mélard 2008). Production (y) is decreasing when body weight increases (x) as shown by the following relationship: y = 824 × 0.18 (Fig. 16.6, Mélard 2008). Maximal daily production in intensive culture ranges from 0.6 kg m−3 for 5 g fish to 0.35 kg m−3 for 150 g perch. The combination of the two models gives the maximal potential of production of a fish farm in relation to fish body weight and the total volume of rearing tanks available in the facility. Thus, the maximal fish farm productivity is obtained when perch are reared around optimal biomass.
Relationships between body weight, biomass and production level of perch reared at 23 °C (Mélard 2008)
7 Conclusion
Nowadays, three different production systems for perch culture during ongrowing phase are described: (1) traditional extensive polyculture system in earthen ponds, (2) semi-intensive culture farming using cage or tarpaulin tanks in lakes or sea bays or the combination of pond and RAS (Recirculating Aquaculture System(s)) culture and (3) intensive perch farming under RAS. Extensive and semi-intensive culture systems have many production limitations such as: suboptimal water temperature, acute risks of diseases, long production cycle, unpredictable production, etc. Therefore, intensive perch farming has been developed in Europe (mainly in Switzerland, Ireland and France) for more continuous and predictive marketable perch production.
Intensive ongrowing perch culture has to provide stable and optimal culture conditions for rapid fish growth, shorter production cycle, year round and predictable production, fish stress and cannibalism reduction, resulting in higher survival rate. Currently perch ongrowing under intensive culture cannot be considered as a bottleneck for stable and high-quality perch production when rearing facilities and all husbandry conditions are carefully optimized in order to ensure significant production profitability. Several factors such as: colour of tank walls, light regime and intensity, water temperature, water quality (including mainly oxygen, salinity, ammonia and nitrite levels), disturbance during tank cleaning, fish size-sorting, density and biomass affect marketable perch production under intensive conditions.
Different tank wall colours with the combination of 12L:12D or 18L:6D light regime and light intensity between 200 and 1,100 lx are equally suitable for farming of ongrowing perch. Optimum temperature for rapid growth ranges from 22 to 24 °C. Significantly higher growth and survival rates of freshwater perch strains are recorded under freshwater or water with salinity under 4 ‰. Optimum oxygen level and saturation for perch ongrowing should be up to 5–6 mg O2 · L−1 and 60–72 % oxygen saturation. Very low ammonia (0.3 mg N-NH3 · L−1) and nitrite (0.5–0.7 mg NO2 − · L−1) concentrations may induce physiological changes and decrease growth in perch. Disturbance resulting from cultivation procedure like cleaning of tanks, service disturbance, fish size-sorting and other stressful events cause directly reduction of feed intake and an increase of energy expenditure and indirectly decreasing growth rate. The frequency of grading must be optimized by proper culture management or using of monosex female population to reduce growth heterogeneity. Mortality of perch resulting from direct and indirect effect of handling is only a very small part of overall mortality, which is mainly caused by parasite and bacteria disease. Low and high stocking densities result in slower growth rates. Optimal stocking biomass for production ranges from 10–20 kg · m−3 for 10 g perch to 60–70 kg · m−3 for 150 g fish.
References
Acerete L, Balasch JC, Espinnosa E, Josa A, Tort L (2004) Physical responses in Eurasian perch (Perca fluviatilis, L.) subjected to stress by transport and handling. Aquaculture 237:167–178
Adámek Z, Helešic J, Maršálek B, Rulík M (2010) Applied hydrobiology. Second edition FFPW USB, 350 p, in Czech only
Adámek Z, Linhart O, Kratochvíl M, Flajšhans M, Randák T, Policar T, Masojídek J, Kozák P (2012) Aquaculture the Czech Republic in 2012: modern European prosperous sector based on thousand-year history of pond culture. Aquac Eur 37:5–14
Bláha M, Šetlíková I, Musil J, Policar T (2013) No reason for keeping 0+ perch (Perca fluviatilis L.ú with prey fish. Aquac Int 2:883–896
Craig JF (2000) Age, growth and mortality In: Craig JF (ed) Percids fishes systematics, ecology and exploitation. Fish and aquatic resources series, vol 3. Blackwell Sciences, Oxford, UK, pp 39–54
FAO (2013) Fisheries and aquaculture information and statistics service – 12/09/2013. http://www.fao.org/fishery/statistics/global-aquaculture-production/query/en
Fontaine P, Tamazouzt L, Capdeville B (1996) Growth of the Eurasian perch (Perca fluviatilis) reared in floating cages and in water recirculated system: first results. J Appl Ichthyol 12:181–184
Fontaine P, Gardeur JN, Kestemont P, Georges A (1997) Influence of feeding level on growth, intraspecific weight variability and sexual growth dimorphism of Eurasian perch Perca fluviatilis L. reared in a recirculation system. Aquaculture 157:1–9
Grignard JC, Mélard C, Kestemont P (1996) A preliminary study of parasites and diseases of perch in an intensive culture system. J Appl Ichthyol 12:195–199
Jacquemond F (2004) Separated breeding of perch fingerlings (Perca fluviatilis L.) with and without initial inflated swim bladder: comparison of swim bladder development, skeleton conformation and growth performance. Aquaculture 239:261–273
Jensen FB (2003) Nitrite disrupts multiple physiological functions in aquatic animals. Comp Biochem Physiol A Mol Integr Physiol 135:9–24
Jentoft S, Aastveit AH, Torjesen PA, Andersen Ø (2005) Effects of stress on growth, cortisol and glucose levels in non-domesticated Eurasian perch (Perca fluviatilis) and domesticated rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol A Mol Integr Physiol 141:353–358
Jourdan S, Fontaine P, Boujard T, Vandeloise E, Gardeur JA, Anthouard M, Kestemont P (2000) Influence of daylength on growth, heterogeneity, gonad development, sexual steroid and thyroid levels, and N and P budgets in Perca fluviatilis. Aquaculture 186:253–265
Karas P, Thoresson G (1992) An application of a bioenergetics model to Eurasian perch (Perca fluviatilis L.). J Fish Biol 41:217–230
Kestemont P, Baras E (2001) Environmental factors and feed intake: mechanisms and interactions. In: Houlihan D, Boujard T, Jobling M (eds) Food intake in fish. MPG Books, Cornwall, pp 88–156
Kestemont P, Mélard Ch (2000) Aquaculture. In: Craig JF (ed) Percids fishes systematics, ecology and exploitation. Fish and aquatic resources series, vol 3. Blackwell Sciences, Oxford, UK, pp 191–224
Kestemont P, Cuvier A, Houbart M, Mélard Ch, Baras E, Fontaine P, Jourdan S, Gardeur JN, Anthouard M, Boujard T, Kentouri M, Paspatis M, Ducarme C (2000) An integrated study of interindividual competition and its relationships with feeding physiology and behaviour in culture predatory fish. FAIR CT96-1572, final report, Facultes Universitaires Notre-Dame de la Paix, Namur, Belgium, p 118
Kestemont P, Jourdan S, Houbart M, Mélard C, Paspatis M, Fontaine P, Cuvier A, Kentouri M, Baras E (2003) Size heterogeneity, cannibalism and competition in cultured predatory fish larvae: biotic and abiotic influences. Aquaculture 227:333–356
Kestemont P, Rougeot C, Musil J, Toner D (2008) Larval and juvenile production. In: Rougeot C, Torner D (eds) Farming of Eurasian Perch, special publication BIM no 24, Bord Iascaigh Mhara, Dublin, Ireland, pp 30–41
Kratochvíl M (2012) Harvesting of marketable fish in members of Czech fish farmers association in 2011 and using of fish production in the Czech Republic during 1990–2011. Report of Czech Fish Farmers Association, 31 p, in Czech only
Kroupová H, Stejskal V, Kouřil J, Máchová J, Piačková V, Zusková E (2013) A wide difference in susceptibility to nitrite between Eurasian perch (Perca fluviatilis L.) and largemouth bass (Micropterus salmoides Lac.). Aquac Int 21:961–967
Mairesse G, Thomas M, Gardeur JN, Brun-Bellut J (2005) Appearance and technological characteristics in wild and reared Eurasian perch, Perca fluviatilis (L.). Aquaculture 246:295–311
Mandiki SNM, Blanchard G, Mélard C, Koskela J, Kuchaczyk D, Fontaine P, Kestemont P (2004) Effect of geographic origin on growth and food intake in Eurasian perch (Perca fluviatilis L.) juveniles under intensive culture conditions. Aquaculture 229:117–128
Martin B, Vandevorede D (2008) Lucas Perches: production of Eurasian perch in recirculating system. In: Fontaine P, Kestemont P, Teletchea F, Wang N (eds) Proceeding of Percid Fish Culture From Research to Production, Presses Universitaires de Namur, p 66
Mélard Ch (2008) Growth and husbandry effects in percids. In: Fontaine P, Kestemont P, Teletchea F, Wang N (eds) Proceeding of Percid Fish Culture From Research to Production, Presses Universitaires de Namur, pp 53–58
Mélard C, Baras E, Kestemont P (1995) Preliminary results of European perch (Perca fluviatilis) intensive rearing trials: effect of temperature and size grading on growth. Bull Fr Pȇch Pisc 336:19–27
Mélard C, Kestemont P, Grignard JC (1996a) Intensive culture of juvenile and adult Eurasian perch (P. fluuiatilis): effect of major biotic and abiotic factors on growth. J Appl Ichthyol 12:175–180
Mélard C, Baras E, Mary L, Kestemont P (1996b) Relationships between stocking density, growth, cannibalism and survival rate in intensively cultured larvae and juveniles of perch (Perca fluviatilis). Ann Zool Fenn 33:643–651
Musil J, Adámek Z (2003) Predation pressure of European perch (Perca fluviatilis) towards topmouth gudgeon (Pseudorasbora parva) under the model pond condition. Bull VÚRH Vodňany 39:75–81
Ȍberg O (2008) Perch farming, Swedish experience. In: Fontaine P, Kestemont P, Teletchea F, Wang N (eds) Proceeding of Percid Fish Culture From Research to Production, Press Universitaires de Namur, pp 71–74
Ȍberg O (2012) ABBORÖS Slutrapport. KTH Architecture and the built environment, Trita-LWR report 3038: 89 p, in Swedish only
Overton JL, Paulsen H (2005) Ongrowing of perch (Perca fluviatilis) juveniles: (Videreopdraet af aborreyngel). Danmarks Fiskeriundersøgelser, DFU-rapport, no 151-05, p 36
Overton JL, Bayley M, Paulsen H, Wang T (2008) Salinity tolerance of cultured Eurasian perch, Perca fluviatilis L: effect on growth and on survival as a function of temperature. Aquaculture 277:282–286
Policar T, Stejskal V, Bláha M, Alavi SMH, Kouřil J (2009) Technology of intensive Eurasian perch (Perca fluviatilis L.) farming. Edition of practical handbooks, USB FFPW 89: 51 p, in Czech only
Policar T, Bláha M, Křišťan J, Stejskal V (2011). High quality and stable production of advanced juveniles in pikeperch (Sander lucioperca) under ponds. Edition of practical handbook, USB FFWP 110: 33 p, in Czech only
Policar T, Stejskal V, Křišťan J, Podhorec P, Švinger V, Bláha M (2013) The effect of fish size and density on the weaning success in pond-cultured pikeperch (Sander lucioperca L.) juveniles. Aquac Int 21:869–882
Rougeot C, Mélard Ch (2008) Genetic improvement of growth. In: Rougeot C, Torner D (eds) Farming of Eurasian perch, special publication BIM no 24, Bord Iascaigh Mhara, Dublin, Ireland, pp 42–51
Schmidt G, Wedekind H (2008) Dry feed adaptation and rearing of one-year old Eurasian perch (Perca fluviatilis) fed with dry feed with varying oil supplementation. In: Fontaine P, Kestemont P, Teletchea F, Wang N (eds) Proceeding of percid fish culture from research to production, Presses Universitaires de Namur, pp 130–131
Staffan F (2004) Food competition and its relation to aquaculture in juveniles Perca fluviatilis. PhD thesis, Acta Universitatis Agriculturae Sueciae, Silvestria 329: 24 p
Stejskal V, Kouřil J, Musil J, Hamackova J, Policar T (2009a) The growth pattern of all-female perch (Perca fluviatilis L.) juveniles – is monosex perch stock beneficial? J Appl Ichthyol 25:432–437
Stejskal V, Kouřil J, Valentová O, Hamáčková J, Policar T (2009b) Size-related oxygen consumption and ammonia excretion of Eurasian perch (Perca fluviatilis L.) cultured in a recirculating system. Aquac Res 41:135–142
Stejskal V, Policar T, Bláha M, Křišťan J (2010) Marketable Eurasian perch (Perca fluviatilis) production with the combination of pond and RAS. Edition of practical handbooks, USB FFPW 105: 34 p
Stejskal V, Matoušek J, Drozd B, Bláha M, Policar T, Kouřil J (2012) The effect of long-term hyperoxia and hypoxia on growth in pikeperch (Sander lucioperca). In: Abstract book from AQUA 2012 conference, USB of Abstracts, Prague, p 1058
Strand Å, Alanärä A, Staffan F, Magnhagen C (2007a) Effects of tank colour and light intensity on feed intake, growth rate and energy expenditure of juvenile Eurasian perch, Perca fluviatilis L. Aquaculture 272:312–318
Strand Å, Magnhagen C, Alanärä A (2007b) Effects of repeated disturbances on feed intake, growth rates and energy expenditures of juvenile perch, Perca fluviatilis. Aquaculture 265:163–168
Svobodová Z, Máchová J, Poleszczuk G, Huda J, Hamáčková J, Kroupová H (2005) Nitrite poisoning of fish in aquaculture facilities with water-recirculating systems. Acta Vet Brno 74:129–137
Tamazouzt L (2008) The French restocking market for percids. In: Fontaine P, Kestemont P, Teletchea F, Wang N (eds) Proceeding of percid fish culture from research to production, Presses Universitaires de Namur, pp 17–18
Tamazouzt L, Dubois JP, Fontaine P (1993) Enquȇte sur la production et les marches européens de la perche. La Pisculture Française 114:4–8
Tamazouzt L, Dubois JP, Fontaine P, Capdeville B, Terver D (1996) Zootechnical performance and body composition of Perca fluviatilis pelleted diet in a floating cage: effect of daily ration. Ann Zool Fenn 33:635–641
Toner D (2012) Culture of European perch Perca fluviatilis in Ireland. Recent advances and developments towards a sustainable production model. In: Abstract book from AQUA 2012 conference, USB of abstracts, Prague, p 1100
Vandecan M, Gbamou P, Mélard Ch (2008) Ammonia toxicity in percids. In: Fontaine P, Kestemont P, Teletchea F, Wang N (eds) Proceeding of percid fish culture from research to production, Presses Universitaires de Namur, pp 138–139
Wedemeyer GA (1996) Physiology of fish in intensive culture systems. Chapman and Hall, New York, 232 p
Wernicke von Siebenthal E (2013) How to protect a new RAS from disease introduction on an existing site with several health issues. In: Abstract book from Aquaculture Europe 2013, USB of abstracts, Trondheim, pp 459–460
Zakes Z, Demska-Zakes K (2005) Oxygen consumption and ammonia excretion by Eurasian perch (Perca fluviatilis L.) reared under optimal thermal conditions. Archiwum Rybactwa Polskiego 13:251–258
Acknowledgments
This chapter was prepared thanks to following projects: the Ministry of Education, Youth and Sports of the Czech Republic – CENAKVA (CZ.1.05/2.1.00/01.0024) and CENAKVA II (LO1205 under the NPU I program), NAZV (QI 101C033) and POSTDOC JU (CZ.1.07/2.3.00/30.0006).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Policar, T., Samarin, A.M., Mélard, C. (2015). Culture Methods of Eurasian Perch During Ongrowing. In: Kestemont, P., Dabrowski, K., Summerfelt, R. (eds) Biology and Culture of Percid Fishes. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7227-3_16
Download citation
DOI: https://doi.org/10.1007/978-94-017-7227-3_16
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7226-6
Online ISBN: 978-94-017-7227-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)