Abstract
Leaves from beets have been used since ancient times for nutrition and the swollen roots were one of the first sweeteners in the Middle Ages that could be stored through the winter. Breeding of beets to increase sugar content began only in the 18th century, after it was uncovered that the nature of the sweet taste of sugar cane and that of beet roots relies on the same sugar molecule. The major breakthrough in breeding sugar beet was the selection of beet progenies that, unlike their wild ancestors, did not flower in the first year of growth, correlating to a high root and thus sugar yield. This was the birth of the sugar beet that became a major crop in Europe and later on worldwide. Genetics has shown that the switch from annual to biennial beets relies mainly on one gene: the ‘bolting gene’ B. However, research from model plants has shown that the regulation of flowering is complex and involves many regulatory pathways, which perceive, transduce and integrate both endogenous and environmental cues for the fine tuning of flowering. Therefore, broad approaches to study flowering time in beets have been initiated, including both forward and reverse genetic studies to elucidate the molecular nature of B as well as other components of what is likely an intricate regulatory network also in beet. This chapter will give a short history of beet use and breeding as well as strategies and results from recent and current efforts to understand the regulation of flowering time in sugar beet.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 The Sugar Beet Crop and Its Cultivated and Wild Relatives
Sugar beet (B. vulgaris L. ssp. vulgaris Sugar Beet Group) is the only sucrose-storing species of moderate climates. It belongs to the genus Beta that is now grouped in the Amaranthaceae (formerly Chenopodiaceae) subfamily Chenopodiaceae (The Angiosperm Phylogeny Group 2009).
Species of the genus Beta show great morphological variation of leaves and roots, including colors that vary from red to yellow due to the production of two betalains, betacyanine (red-violett) or betaxanthine (yellow). Domesticated forms of B. vulgaris have been used since antiquity. Leaf beets (syn. mangold, swiss chard) (B. vulgaris L. ssp. vulgaris Leaf Beet Group) form fleshy leaves and have a long tradition as a vegetable. Red table beets (beetroot) (B. vulgaris L. ssp. vulgaris Garden Beet Group) form a thickened root and hypocotyl with an intense dark red color. They have a high content of free folic acid (vitamin B12), and are used as a vegetable and for production of natural colors for food additives. Fodder beets (B. vulgaris L. ssp. vulgaris Fodder Beet Group) form a thickened root and hypocotyl and are traditionally used as animal feed, mainly for dairy cattle.
Sugar beets form a thickened root, which like other cultivated forms store substantial amounts of sucrose. While sucrose concentration in fodder beets ranges between 4 and 10 %, sugar beet roots may contain more than 20 % sucrose. Under central European growing conditions the sucrose concentration typically is 17–18 % (Biancardi et al. 2005).
The development of sugar beet as a cultivated species began in 1747 when the German chemist A. S. Marggraf detected cane sugar within the roots of garden beets, frequently grown as a vegetable at that time. Cultivation started on a very small scale at the end of the 18th century in Germany when F. C. Achard grew ‘sugar beet’ near Berlin. The first beet root processing sugar factory was constructed in 1801 in Silesia, a Prussian province at that time. That year is regarded as the beginning of sugar beet cultivation.
The sucrose content of beet roots at the beginning of beet cultivation was estimated to be around 4 %. By mass selection, sucrose content was raised to 16 % by the end of the 19th century. At that time, the market share of sugar beet sucrose was 62 %, the rest coming from sugar cane. After World War I, it dropped dramatically to 23 %. In 2010, the world beet harvest reached 227 Mt fresh weight giving rise to 32.3 Mt of sugar that is 19 % of the total world production (166.8 Mt of sugar (http://www.zuckerverbaende.de/zuckermarkt/zahlen-und-fakten/weltzuckermarkt/erzeugung-verbrauch.html). Sugar beets are grown in many countries of the Northern hemisphere. The total sugar beet area harvested was 4.3 Mha (2009). Major producers were the Russian Federation (770,000 ha), the United States of America (465,000 ha), Germany (384,000 ha), and France (374,000 ha) (http://faostat3.fao.org/faostat-gateway/go/to/download/Q/QC/E).
The species of the genus Beta are divided into two sections (Kadereit et al. 2006) (Fig. 1.1). All cultivated forms belong to the same subspecies B. vulgaris ssp. vulgaris. Together with their wild progenitor B. vulgaris ssp. maritima (L.) Arcang, they belong to section I (Beta). B. vulgaris ssp. maritima and the related wild species ssp. adanensis(Pamuk) Ford-Lloyd & Will., B. patulaAit.and B. macrocarpaGuss. grow around the Mediterranean and the coasts of northwest Europe up to Scandinavia and between the Capverdian Islands and Bangladesh. There are only wild species in the section II (Corollinae). Apart from diploid species, tetraploids, pentaploids and hexaploids also exist within this section. Those species grow on the hilly and mountainous regions in Turkey and adjacent countries (Fig. 1.1).
The species of the genus Beta (as revised by Kadereit et al. 2006)
The systematics within this genus have been disputed for a long time until a new taxonomy was proposed in 2006 (Kadereit et al. 2006) which became official in June 2009. The species B. nana, which formerly belonged to section III, was moved to section II and the former section IV (Procumbentes) (Lange et al. 1999) became the new genus Patellifolia(Kadereit et al. 2006) (Fig. 1.1).
2 Sugar Beet Breeding and Genetics
For 200 years, sugar beet has been cultivated for sucrose production. The main breeding aims were high sucrose yield in combination with quality traits such as low Na/K content and low α-amino acid content to reduce the amount of molasses. Mass selection for sucrose content was extremely successful in the early period of beet breeding because the dry matter, whose content was easily measurable, mainly consists of sucrose. Today sucrose is measured directly by refractrometry and α-amino acid and Na/K content are determined separately. Mass selection has been replaced by single plant selection in the 1930s and today hybrids are exclusively used in the major beet-growing areas. But also a number of other traits have been substantially improved during the past 50 years. The introduction of monogermic seeds laid the foundation for beet production at an industrial scale. The successful breeding for rhizomania resistance was a breakthrough for the cultivation of sugar beet in many growing areas of the Northern hemisphere where soils are often contaminated with the beet necrotic yellow vein virus (BNYVV).
Recently, sugar beet became an interesting alternative as a renewable energy resource in central Europe. Biomass production in this part of the world heavily relies on maize and alternatives are urgently needed. Sugar beet has the highest dry matter production capacity under central European growth conditions. The beet with its high sucrose content is also suitable for loading fermenters to produce methane. Thus, breeding biomass beets, which do not necessarily have to exhibit the quality traits of ‘sucrose beets’, has become an interesting option. One means to increase biomass yields is to grow winter beets. Those beets are sown before winter (preferentially in August). They overwinter in the field and develop their shoot mass early in spring. Harvest time is expected to be earlier than for conventionally grown ‘spring’ beets. The yielding potential of winter beet has been estimated to be ~ 20 % higher than that of conventional beet (Hoffmann and Kluge-Severin 2011), but requires a strict bolting control (see Sect. 1.9).
The haploid chromosome number of Beta species is 9 (x = 9). Sugar beet is a diploid species with 2n = 2x = 18 chromosomes and a haploid genome size of 758 Mb (Arumuganathan and Earle 1991). Triploids and tetraploids exist, which have been frequently used in beet breeding. Thus, the sugar beet crop is a rare example of a seed-propagated triploid crop species. Several molecular marker-based genetic maps have been published (Barzen et al. 1992; Pillen et al. 1992; Schondelmaier and Jung 1997; Schumacher et al. 1997; Grimmer et al. 2007; McGrath et al. 2007; Schneider et al. 2007) and used for mapping major genes and polygenes of agronomic importance. Unfortunately, routine procedures for doubled haploid production such as microspore or anther culture are so far lacking. Doubled haploids can only be produced by costly and time-consuming gynogenesis. Therefore, F2 or advanced inbred populations have been used for mapping. Apart from bolting time genes, which will be discussed in Sects. 1.6–1.8, major genes for nematode resistance (Cai et al. 1997; Kleine et al. 1998), rhizomania resistance (Barzen et al. 1992; Barzen et al. 1997; Lein et al. 2007a) and monocarpic seeds (Barzen et al. 1992) have been mapped. Also, a number of QTLs have been placed on the beet chromosomes such as Cercospora leaf spot resistance (Nilsson et al. 1999; Schäfer-Pregl et al. 1999; Setiawan et al. 2000), Rhizoctonia root rot resistance (Lein et al. 2007b), fertility restorer genes (Hjerdin-Panagopoulos et al. 2002) and quality traits (Schneider et al. 2002). The efficiency of association mapping in sugar beet was recently demonstrated by mapping a number of quantitative traits (e.g. sucrose content) in a panel of 460 elite sugar beet lines (Würschum et al. 2011). Other resources for studying the beet genome have been established in the past years and will be discussed in Sect. 1.4.
3 Phenology of Beta Species
The development of sugar beet after sowing in spring is characterized by secondary root growth and the formation of a large leaf rosette in the first year. During the vegetative phase, sugar beets develop a large harvestable organ, which is mainly formed by the root and contains only small portions of epicotyl and hypocotyl. The beet root results from secondary thickening with up to 12 successive concentric rings of cambia (Bell et al. 1996). Each cambium forms a cylindrical ring of xylem and phloem tissue and parenchyma cells in between two rings (Bhambie et al. 2000). The number of rings is much smaller in fodder beet and red beet (3–5 rings).
Sugar beets enter the generative phase only after exposure to cold temperatures typical for winter periods under central European conditions. The first visible event is the elongation of the shoot, referred to as ‘bolting’, usually followed by flower formation (Fig. 1.2a). A plant can have more than one flowering shoot, which are panicles and carry numerous hermaphrodite flowers (up to 10,000) that are formed in the axils of bracts. In wild beets, 2–4 flowers are merged and develop a multigerm seed ball. B. vulgaris is an allogamous species due to a gametophytic self-incompatibility system controlled by two series of sterility alleles (S1-Sn, Z1-Zn). Thus, self-pollination is avoided leading to highly heterozygous and heterogeneous wild populations. However, a self-fertility locus with a self-fertility allele SF exists, which is frequently used for selfing sugar beets to produce inbred lines (Biancardi et al. 2005).
Wild beets from the Mediterranean area are annuals, flower early without vernalization and finish their life cycle within the first year (Fig. 1.2b). By contrast, wild beets growing in the northern regions are biennials with a marked requirement for cold temperatures for flowering (Fig. 1.2a). Furthermore, long-lived, iteroparous perennials exist in the subspecies maritima, which produce offspring in successive cycles (Hautekeete et al. 2002) (Fig. 1.2c). However, all Beta species are strict long-day (LD) plants.
The onset of bolting is of greatest importance for the cultivation of sugar beet as well as of root and leaf beet. High root yield is only guaranteed if beets do not flower (Fig. 1.2d). The storage root of bolting and flowering beets is much smaller, thus sucrose yield is drastically reduced after bolting (Bürcky 1986, Fig. 1.3a) and bolting during beet production must be completely avoided. Consequently, breeders have selected against early bolting since the beginning of beet breeding. This has been quite successful because early bolters can be easily identified and eradicated during mass selection. However, when seed production was moved to southern Europe, where annual wild beets are abundant there was an increased risk of cross pollination between wild beets and male sterile sugar beet seed parents in seed production fields. Since early bolting is controlled by a single dominant allele (see Sect. 1.5) heterozygous beets resulting from cross pollination would bolt early, creating a need for rigorous elimination of wild beets and strict isolation of seed production fields. Today, molecular markers (Gaafar et al. 2005, see Sect. 1.5) are employed for testing seed lots for the presence of early bolters. However, even biennial beets can have a tendency towards early bolting under certain environmental conditions such as exposure to cold temperatures in spring (Fig. 1.3b). Therefore, sowing time is delayed in some areas with a risk of cold temperatures late in spring (Milford and Burks 2010). On the other hand, for seed production beets must bolt and flower readily after winter (Fig. 1.3c). Therefore, breeders have selected for early flowering after winter, and completely bolting-resistant beets (that will never bolt) are not found among cultivated beets.
4 Genomic Resources for Beet
Genetic mapping has been used in model and crop plants to map and clone many flowering time genes in recent years (Turck et al. 2008). In contrast to model species, no collection of defined flowering time mutants is available for sugar beet. However, phenotypic variation for flowering time is easily observable among natural accessions and in structured populations derived from crosses between annual wild beets, beet cultivars and/or breeding lines. QTL analyses have been performed and linkage maps are available, but efforts to construct high density molecular marker maps thus far are rare (Lange et al. 2010). Therefore, cloning of flowering time genes from beet by mapping procedures is still challenging and time consuming (McGrath et al. 2007).
Other resources for studying the beet genome have been established in the past years. Large insert libraries exist for several beet genotypes which representatively cover the whole beet genome (Hohmann et al. 2003; Hagihara et al. 2005; Schulte et al. 2006; Lange et al. 2008). A sugar beet EST database can be found at Michigan State University (http://genomics.msu.edu/sugarbeet/index.html) and approximately 30,000 B. vulgaris ESTs are listed in GenBank (http://www.ncbi.nlm.nih.gov/sites/entrez?db=nucest&cmd=DetailsSearch&term=(beta+vulgaris)+AND+%22Beta+vulgaris%22%5Bporgn%5D&save_search=true). From The GenBank EST database, 315 ESTs have been placed on a sugar beet map (Schneider et al. 2007) and 2,752 were used to produce macroarrays for expression analyses (Pestsova et al. 2008). An Agilent 15 K oligonucleotide microarray has been established, which was used for mapping 392 BAC-end derived sequences and 119 ESTs (Lange et al. 2010). A beet genome mapping and sequencing consortium has started working in 2004 with the aim to physically map (GABI—The German Plant Genome Research Program Progress Report 2004–2007;http://www.gabi.de/client/media/3/gabi_progrep_ii_web.pdf ) and sequence the whole beet genome using second generation sequencing technology (http://www.gabi.de/projekte-alle-projekte-neue-seite-144.php). Approximately 67,000 genomic survey sequence fragments including BAC end sequences have been deposited in GenBank (http://www.ncbi.nlm.nih.gov/nucgss/?term=%22Beta%20vulgaris%22) and the preliminary annotation yielded approximately 28,000 gene models (Weisshaar et al. 2011). To characterize sugar beet seed vigor, a proteomic analysis was performed and 759 proteins with specific root, cotyledon and perisperm expression profiles were identified (Catusse et al. 2008). Finally, at least two transcriptomics projects are underway to generate genome-wide expression profiles and/or transcript sequences for specific developmental processes (vernalization) or tissues (shoot apex) that are relevant for the study of flowering time control in beet (http://www.gabi.de/projekte-alle-projekte-neue-seite-171.php;http://www.ncbi.nlm.nih.gov/bioproject/73561).
5 Genetics of Bolting Time in Beet
While beets in natural environments require long days for bolting to occur, there is considerable intraspecific variation in vernalization requirement, which follows a latitudinal cline. Wild beets from the southern part of the species distribution area (the Mediterranean) bolt in the first year without experiencing prolonged periods of cold temperatures and generally behave as annuals, but may live and flower for up to three consecutive years (Van Dijk 2007). Beets from northern latitudes including the Atlantic and North Sea coasts have a longer lifespan of approximately 4–17 years or more (Hautekeete et al. 2002; Van Dijk 2007) and commonly require vernalization, but there is quantitative variation among natural populations of different geographic origins in the extent of cold exposure required (Van Dijk et al. 1997; Boudry et al. 2002). Owen et al. (1940) coined the term ‘photothermal induction’ to describe the inductive effects of low temperatures and long photoperiods on bolting in B. vulgaris and showed that, although not required in their natural habitats, exposure to cold also promotes and accelerates bolting in annuals. As a result of strict selection during the breeding process against the annual character, which is associated with poor root yield and interferes with harvest operations, sugar beet and other cultivated beet forms require vernalization to bolt and, for seed production, are grown as biennials.
Genetically, the annual growth habit is under the control of a major dominant gene that has long been referred to as the ‘bolting gene’ or ‘B’ (Abegg 1936). Plants which are derived from crosses between homozygous annual (BB) and biennial beets (bb) and are heterozygous at the B locus (Bb) behave as annuals under favorable conditions but may bolt several days later than homozygous annuals (Munerati 1931; Abegg 1936; Mutasa-Göttgens et al. 2010). Heterozygotes may also fail to bolt in the first year under suboptimal photothermal conditions as they are present e.g. in late spring, summer or autumn sowings (Owen 1954; Boudry et al. 1994; Abe et al. 1997). In addition to appropriate environmental conditions, the manifestation of the annual character is also influenced by additional, modifying genes (Abe et al. 1997; Büttner et al. 2010; Abou-Elwafa et al. 2012). Furthermore, Owen et al. (1940) defined a locus for easy-bolting tendency (Bʼ) in a biennial beet accession which does not bolt without prior vernalization under field conditions, but bolts easily and early without vernalization under relatively low temperatures and long photoperiods in the greenhouse. On the basis of linkage data between the B locus and the R locus for hypocotyl color, and between Bʼ and R, the authors concluded that Bʼ is allelic to B.
Following the original observation by Rimpau (1876, 1880) that the annual habit is dominantly inherited in beet, and later work by Munerati (1931), who on the basis of phenotypic data for large F2 populations segregating for annuality suggested further that this trait follows a monogenic mode of inheritance, Abegg (1936) was able to show that the B gene (or ‘factor’ in the language of his time) in Munerati’s annual accessions was linked to the hypocotyl color factor ‘R’. Abegg calculated a cross-over value of 15.5 % between B and R, and by considering the previously identified linkage relationship between R and Y, another locus affecting pigmentation, defined the first linkage group with three morphological markers in beet. The Y-R-B linkage group together with additional markers was later assigned to chromosome II according to the standard nomenclature for beet chromosomes suggested by Schondelmaier and Jung (1997). Using a backcross population derived from a biennial parent and a different annual accession than had been analyzed in Munerati’s and Abegg’s early studies, Boudry et al. (1994) confirmed linkage of a locus for annuality, which was presumed to be B, to R in their population, and were able to identify several restriction fragment length polymorphism (RFLP) markers more closely linked to B. The B locus was further fine-mapped by anonymous fragment length polymorphism (AFLP) mapping to a 0.37 cM interval of chromosome II using another unrelated mapping population (El-Mezawy et al. 2002). BAC library screening and bulked segregant analysis identified, respectively, several sequence-based markers which flank the B locus on either side (Hohmann et al. 2003; Gaafar et al. 2005) or completely co-segregate with B (Büttner et al. 2010).
Besides the major bolting locus B, two recent studies identified additional, previously unknown loci, which contribute to annual bolting in wild beets (Büttner et al. 2010; Abou-Elwafa et al. 2012). A screen for bolting mutants derived from ethyl methanesulfonate (EMS) mutagenesis of the same annual beet accession that was used for fine-mapping of B (El-Mezawy et al. 2002) identified five M3 families which bolted only after vernalization and thus behaved as biennials (Hohmann et al. 2005). Somewhat surprisingly at the time, in two out of several F2 mapping populations derived from crosses between these biennial genotypes and an annual wild beet accession, the annual bolting phenotype did not co-segregate with the B locus, but instead was mapped to a new locus on chromosome IX which was named B2 (Büttner et al. 2010). Because all plants in these populations carried the dominant allele for annuality at the B locus, but approximately one quarter of plants failed to bolt without vernalization, the authors concluded that B2 acts epistatically with B to co-regulate vernalization-independent bolting. The genetic and phenotypic data further indicated that B2, similar to B, harbors a major gene which is inherited in a dominant-recessive fashion. Co-segregation analyses of the remaining populations segregating for annuality revealed that the natural accession used as annual parent carries at least one additional locus which also promotes bolting, but in contrast to B2 appears to act independently of B. A QTL analysis of annual bolting in two populations showed that the B locus and the newly identified locus, termed B4, contributed equally to phenotypic variation in bolting behavior, and that B4 also exhibited a dominant gene action (Abou-Elwafa et al. 2012). The B4 locus is genetically linked to the B locus and was mapped to chromosome II at a genetic distance of 11 cM from B.
6 Flowering Time Genes and Their Regulation in Beet
6.1 Beet Homologs of the FLC Gene and Putative Regulators
Vernalization, a prolonged exposure of plants to cold temperatures over winter, is a prerequisite for many plants to flower in the following spring or summer. For Arabidopsis and other Brassicaceae, it has been shown that the MADS box gene FLC is the main regulator of the vernalization response. FLC acts as a flowering time repressor showing a characteristic expression pattern: before vernalization FLC mRNA accumulates to high levels, but during vernalization expression declines and remains low post-vernalization. In winter-annual Arabidopsis accessions, FLC is repressed by an epigenetic mechanism and is only de-repressed in the next generation. There are five paralogs of FLC (MAF1–MAF5) that are also reported to be regulated by vernalization in Arabidopsis, but these show only a mild response compared to FLC (Ratcliffe et al. 2003).
In cereals, wheat and barley winter varieties also exhibit a clear vernalization requirement for flowering. However, the vernalization response involves different major players, often without clear homologs in dicot species, indicating that the regulation of vernalization response evolved independently in dicots and monocots (Kim et al. 2009). One exception is VRN3, an FT ortholog from Arabidopsis, as both FT and VRN3 integrate signals from various regulatory pathways and promote flowering.
Recently, in beet a homolog of FLC that was named BvFL1 has been identified in EST libraries by using a phylogenetic approach (Reeves et al. 2007). The authors showed that four splice variants of BvFL1 RNA were present in beets, which they constitutively expressed in an Arabidopsis flc3 null mutant. All splice variants caused later flowering (Reeves et al. 2007) but to a much lesser extent than transgenic plants overexpressing the endogenous Arabidopsis FLC gene (Michaels and Amasino 1999). Nevertheless, BvFL1 also acts as a repressor for flowering in transgenic Arabidopsis and two of the four splice variants are also down-regulated in beet leaves in response to a vernalization treatment of 90 days. However, after vernalization, expression of these splice variants was not stably repressed and the expression recovered to pre-vernalization levels. In addition, BvFL1 is expressed at equal levels in annual and vernalization-requiring biennial beets, suggesting that the difference in vernalization requirement cannot be attributed to differences in the abundance of BvFL1 transcripts (Reeves et al. 2007). Therefore, it seems unlikely that BvFL1 is the primary target for the vernalization response in biennial sugar beets.
In Arabidopsis, FLC is also regulated by a number of genes assigned to the ‘autonomous pathway’ of flowering time control (Simpson 2004). Beet homologs of several pathway members, namely BvFLK, BvFVE1, BvLD and BvLDL1, have recently been identified by Abou-Elwafa et al. (2011). It was shown that BvFLK overexpression leads to earlier flowering and can complement an Arabidopsis flk mutant. BvFLK also repressed the endogenous FLC gene in transgenic Arabidopsis, suggesting that gene function is at least conserved to some extent between Arabidopsis and beets. However, the authors also found indications for evolutionary divergence of autonomous pathway gene homologs in Arabidopsis and beets. Overexpression of BvFVE1 in an Arabidopsis fve mutant did not rescue the late flowering mutant phenotype. Furthermore, in apparent contrast to its homolog in Arabidopsis, BvFVE1 is under circadian clock control. Since beet carries a second closely related FVE homolog (BvFVE2), it is conceivable that BvFVE1 and BvFVE2 underwent sub-functionalization and that BvFVE2 is a functional FVE ortholog (Abou-Elwafa et al. 2011).
6.2 Photoperiodic Pathway and CO Homologs
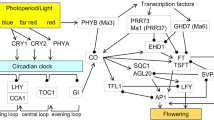
While the key regulators of vernalization requirement and response differ between distantly related species such as A. thaliana and cereals, a core component of the photoperiodic regulation of flowering appears to be largely conserved among angiosperms. The central regulator of the photoperiod pathway in Arabidopsis is the CONSTANS, CONSTANS-LIKE and TIMING OF CAB EXPRESSION 1 (CCT) domain transcription factor gene CONSTANS (CO), which promotes flowering in response to LD conditions (Suarez-Lopez et al. 2001). CO activity is diurnally regulated both at the transcriptional level and post-translationally, and is highest at the end of the light phase in long days when high levels of CO transcription and high CO protein stability coincide (Turck et al. 2008; Srikanth and Schmid 2011). The concurrent effects of both exogenous and endogenous factors on CO activity at critical times of the day, which involve circadian clock-regulation of transcriptional CO regulators and light-regulated stabilization of the protein, suggest that regulation of CO can account for much of the molecular basis of the ‘external’ and ‘internal coincidence’ models proposed by Bünning (1936), Pittendrigh and Minis (1964) and Pittendrigh (1972) for the induction of flowering (and other biological processes) by photoperiod (Turck et al. 2008; Srikanth and Schmid 2011). Once stably expressed under inductive LD conditions, CO transcriptionally activates FT in the leaf vasculature. Although the exact mode of this activation is not well understood, several co-regulatory proteins which interact with CO and contribute to the regulation of FT have been identified (Wenkel et al. 2006; Song et al. 2012).
CO homologs have been identified in numerous dicotyledonous and monocotyledonous species, including species which flower in response to different photoperiodic conditions such as short-day and day-neutral plants (reviewed in Turck et al. 2008). For several of these species, including both LD and short-day (SD) plants among monocots, a functional role of CO homologs in photoperiodic regulation of flowering has been demonstrated. However, the mode of action of CO genes differs to some extent between species and may be modified by various interactions with co-regulatory genes and/or light-induced changes of the protein, as has been suggested for rice (Turck et al. 2008; Ishikawa et al. 2011). Furthermore, there is also increasing evidence for CO-independent photoperiodic regulation in monocots including rice, where a regulatory mechanism involving the species-specific transcriptional regulators Grain number, plant height and heading date 7 (Ghd7) and Early heading date 1 (Ehd1) enables expression of the FT homolog Heading date 3a (Hd3a) under SD conditions irrespective of Heading date 1 (Hd1), the rice ortholog of CO (Doi et al. 2004; Itoh et al. 2010). In barley, the major determinant of LD response was identified as Photoperiod-H1 (Ppd-H1), which also carries a CCT domain but is otherwise unrelated to CO (Turner et al. 2005), whereas the function of CO homologs in barley is less understood and allelic variants have not been identified. A recent study of transgenic plants over-expressing HvCO1, the closest barley homolog of CO and the putative ortholog of Hd1 in rice (Griffiths et al. 2003), showed that HvCO1 indeed also promotes flowering in barley, in a process involving activation of the FT homolog HvFT1 (Campoli et al. 2011). Interestingly, natural variation at the Ppd-H1 locus affected flowering time irrespective of high transgenic expression of HvCO1, leading the authors to suggest that Ppd-H1 may ‘bypass’ the regulatory CO-FT interaction (Campoli et al. 2011) and raising the possibility that, while HvCO1 is a functional regulator of flowering time, the photoperiod response in barley may also involve an HvCO1-independent pathway.
Like in other species, a family of CO-like genes has also been identified in beet (Chia et al. 2008). However, none of the closest CO homologs in beet identified thus far (BvCOL1 and BvCOL2) appear to be true orthologs of CO, but instead are more closely related to CO-LIKE 1 (COL1) and COL2 in Arabidopsis. Consistently, the diurnal expression profile of BvCOL1 more closely resembled the profiles of COL1 and COL2, and showed that BvCOL1, in contrast to CO, was not or only very weakly expressed at the end of the light phase in LDs (Chia et al. 2008). Nevertheless, over-expression of BvCOL1 in Arabidopsis rescued the late-flowering phenotype of the loss-of-function co-2 mutant and activated FT expression, suggesting at least a certain degree of functional conservation of the BvCOL1 gene product (Chia et al. 2008). Perhaps noteworthy, the over-expression of HvCO1 failed to complement the same mutant, which was suggested to result from sequence variation at conserved positions in a B-Box-type zinc finger domain (B-Box2) (Campoli et al. 2011), whereas this domain is highly conserved between CO in Arabidopsis and BvCOL1. Like the B and B4 loci, BvCOL1 was mapped to chromosome II, but at large genetic distances of approximately 22–24 cM upstream of B and 35–38 cM upstream of B4 (Chia et al. 2008; Abou-Elwafa et al. 2012).
6.3 Two Copies of FT Homologs with Different Function in Beet
FT is a member of a protein family with structural similarities to mammalian phosphatidylethanolamine-binding protein (PEBP) domains (Kardailsky et al. 1999; Kobayashi et al. 1999) and a hitherto unknown biochemical function. In Arabidopsis, the PEBP protein family consists of three phylogenetically distinct groups represented by FT, TFL1 and MFT. FT and TSF are components of the long-sought florigen signals that promote flowering in Arabidopsis under LDs, but also integrate signals from other flowering time pathways, whereas TFL1 acts antagonistically to prevent flowering. It has been shown in other species (including both LD and SD plants) that expression of FT orthologs rises in response to inductive photoperiods, and that constitutive expression induces early flowering whereas mutations in FT orthologs delay flowering. As had been expected for the long elusive florigen, the FT protein moves from the leaves to the apex where it establishes flowering (Turck et al. 2008).
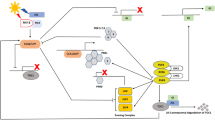
In sugar beet, Pin et al. (2010) identified two paralogous FT genes. Surprisingly, these genes, which were termed BvFT1 and BvFT2, have antagonistic functions. While BvFT1 acts as a repressor, BvFT2 promotes flowering. After vernalization, biennial sugar beets are competent to flower, but the vernalized plants still require long days for floral induction. Diurnal expression studies in annual, biennial and vernalized biennial plants in different photoperiods showed that under non-inductive SD conditions BvFT2 expression was hardly detectable, whereas BvFT1 showed a distinct morning expression peak. Without vernalization, only annual beets bolt in LDs which was found to be coincident with very low BvFT1 expression, whereas BvFT2 expression peaked after 12 hours of illumination in an 18 hour photoperiod. However, unvernalized biennial beets exhibited a very different LD expression profile. Here, BvFT2 was not expressed, whereas BvFT1 showed a peak of expression in the morning not dissimilar to that also observed in non-inductive SD. During vernalization, BvFT1 was down-regulated and BvFT2 was up-regulated, indicating that BvFT2 may be repressed by BvFT1. Moreover, when vernalized biennial plants were transferred to SD conditions, which lead to de-vernalization and suppression of bolting, BvFT1 expression was induced and BvFT2 was repressed. Finally, the observed correlation of BvFT2 expression with the initiation of flowering in both annual and biennial beet and a complementation analysis in Arabidopsis ft mutants suggested that BvFT2 is the functional FT ortholog in sugar beet.
In transgenic approaches overexpressing BvFT2 under the control of the 35S promoter or down-regulating BvFT2 expression by RNA interference (RNAi), it was demonstrated that BvFT2 is essential for flowering in sugar beet. 35S::BvFT2 biennial plants flowered prematurely in tissue culture without vernalization and annual BvFT2 RNAi plants failed to bolt and continued to produce leaves in LD for more than 400 days, which was correlated with strongly reduced BvFT2 transcript levels. Since in these transformants BvFT1 expression was not altered, the results indicate that the modulation of flowering time is directly regulated by BvFT2 and not indirectly via modulation of BvFT1 expression by BvFT2. Transgenic annual beets constitutively expressing BvFT1 also failed to bolt and showed a strong down-regulation of BvFT2, indicating that BvFT1 represses BvFT2 expression. Vernalized transgenic biennial beets over-expressing BvFT1 showed a non-bolting phenotype for more than 6 months under inductive LD and a strong down-regulation of BvFT2. Together, these results indicate that BvFT1 suppresses flowering under non-inductive conditions, which at least in part may be mediated by repression of BvFT2 expression. BvFT1 is down-regulated during vernalization, which eliminates the repressive effect of BvFT1 on BvFT2 and allows sugar beet plants to respond to inductive LD conditions (Pin et al. 2010). Interestingly, Chenopodium rubrum, a SD plant and close relative of B. vulgaris, also carries two close homologs of FT that show different expression profiles, suggesting different roles of the encoded proteins (Cháb et al. 2008). However, a functional study in Chenopodium has not been performed yet. It would be highly interesting to compare the functional roles of the FT genes in these closely related species and their possible contributions to the differentiation of these species into SD and LD plants.
Since BvFT1 and BvFT2 are paralogs and show a high degree of sequence similarity, it was interesting to evaluate the encoded proteins with respect to activating or repressing functions. From previous studies in Arabidopsis, it was known that a swap of the fourth exon from FT (i.e. a promoter of flowering) to TFL (i.e. a repressor) converted FT to TFL function and vice versa (Ahn et al. 2006). Strikingly, this could also be achieved by swapping just a single amino acid of an external loop of exon 4 (Hanzawa et al. 2005). The external loops encoded by exon 4 of BvFT1 and BvFT2 only differ by three amino acids out of 14. Exchanging exon 4 or only the external loop regions between BvFT1 and BvFT2 also resulted in opposite protein functions, indicating that the functional domains for the repression or promotion of flowering through PEBP proteins are well conserved in different eudicot clades (Pin et al. 2010).
6.4 GA Metabolism and Early Bolting
It has been shown that treatments with gibberellins (GAs) can induce bolting and flowering especially in LD plants that form rosettes under non-inductive conditions (Zeevaart 1983). In some LD plants, the amount of endogenous GAs is increased when these plants are transferred into inductive environments (Metzger 1995), but GA-deficient and GA-insensitive mutants of Arabidopsis have also provided strong evidence that GAs are required for floral induction in SD and play only a minor role in LD (Wilson et al. 1992). In Arabidopsis, the response to GA with regard to flowering time regulation is integrated by LFY and SOC1. SOC1 is activated by GAs and overexpression of SOC1 can rescue the non-flowering phenotype of ga1-3 mutants in SD, whereas soc1 mutants show a reduced sensitivity to GAs. The expression of LFY is directly regulated by the GA-activated GAMYB protein, while SOC1 also activates LFY by directly binding to its promoter. Therefore, GAs regulate LFY transcription by both SOC1-dependent and -independent pathways (Lee and Lee 2010).
Previous studies in sugar beet have shown that GAs play a role during reproductive growth (Lenton et al. 1975), and that GA contents after vernalization and during bolting increases significantly (Sorce et al. 2002). Exogenously applied GAs can stimulate cell growth in a genotype specific manner (Sadeghian et al. 1993b), but endogenous levels of GAs are not limiting for bolting and the application of exogenous GAs failed to induce bolting and flowering in non-vernalized sugar beets (Mutasa-Göttgens et al. 2009). Mutasa-Göttgens et al. (2010) studied the interaction of GAs, the bolting gene B and vernalization under controlled physiological conditions by analyzing the effect of GA4 addition in inductive LDs and non-inductive SDs in sugar beet populations segregating for the B allele and grown with or without vernalization. Under SD conditions, GA4 promoted bolt initiation independently of the B allele, but stem elongation did not occur without prior vernalization. Moreover, vernalization and addition of GA4 could not substitute for LD conditions, indicating that long days are absolutely required for flowering in sugar beets. Finally, the authors showed that exogenous application of GA4 had no significant effect on bolting and flowering in LD, suggesting that GAs are not a limiting factor for bolting and flowering under these conditions.
7 A Model for Bolting Time Regulation in Beet
While the nature and regulatory roles of the major bolting loci in beet that were detected genetically await identification and further characterization, the presence of FT genes in beet as well as of homologs of photoperiod, vernalization and autonomous pathway genes suggest that important components of flowering time regulation are also conserved in beet. However, the pioneering study by Pin et al. (2010) on the antagonistic action of the FT genes in beet and the finding that neither B nor the other two mapped bolting loci, B2 and B4, correspond to any of the closest beet homologs of the major flowering time regulators FT, CO and FLC, indicate that in beet different or additional components may have evolved as central regulators of floral transition. According to the current data, BvFT2 is a functional ortholog of FT in Arabidopsis and may also in beet act as a central ‘executive’ component of florigen which under favorable conditions transduces the promotive signals perceived by the plant in LD or under vernalizing conditions (Pin et al. 2010). However, upstream of BvFT2, the FT paralog BvFT1, which does not have a counterpart in Arabidopsis, acts as a repressor of BvFT2 in SD (in both annuals and biennials) and, in biennials, in the absence of vernalization regardless of day-length. Similar to FLC in Arabidopsis, BvFT1 expression in biennials is gradually down-regulated during vernalization, resulting in the release of BvFT2 repression and enabling its activation in the long days that follow winter. In contrast to FLC, however, BvFT1 is also down-regulated in LD, but only in annuals the LD signal suffices for stable repression of BvFT1 throughout the course of the day and activation of BvFT2 (Pin et al. 2010). Because annual beets, carrying the dominant B or B4 alleles, bolt readily in long days, whereas biennial beets require vernalization as an additional stimulus for bolting to occur, it is conceivable that the recessive alleles may be impaired in sensing the inductive LD signal, and that B and/or B4 are part of a (CO-dependent or independent) photoperiod pathway in beet which mediates the effect of day-length on the two FT genes. Because B2 acts epistatically to B, it may constitute another component of the same floral induction pathway. Large-scale mapping and sequencing projects are underway to identify the underlying genes (Müller, Jung and colleagues, unpublished) and are expected to help elucidating the molecular basis of the long-postulated interaction of photoperiod and vernalization response mechanisms in beet (Owen et al. 1940). However, the currently available data for beet suggest that not only monocots and dicots have evolved different molecular mechanisms to adapt the timing of reproduction to the climatic conditions of their natural habitats, but that different strategies have also evolved within the dicot clade (Jarillo and Piñeiro 2011).
8 Exploiting Natural Variation for Bolting Time
All cultivated beets are non-bolting under non-inductive environmental conditions, typically after sowing in (late) spring. Beet varieties grown as winter beets in some regions of southern Europe, where mild winters are predominant, have a high bolting resistance. On the other hand, plants with an extremely high bolting resistance would create problems in sugar beet seed production and have thus been avoided by breeders (Sadeghian and Johansson 1993).
Although undesirable, beets can have a tendency for early bolting under central European growing conditions and differences can be found among breeding lines (Sadeghian et al. 1993a). Longer periods of cold temperatures (5–8 °C) in spring can result in a significant increase of bolting beets in the field (Jolliffe and Arthur 1993) (Fig. 1.3b). Several studies have also measured the genetic effects underlying early bolting in biennial beets grown under flowering inductive conditions, e.g. by sowing early in spring (Jolliffe and Arthur 1993; Sadeghian and Johansson 1993). Under these conditions the frequency of bolters in July increased to 75 % (Jolliffe and Arthur 1993). Early bolting in biennial cultivated beets was found to be a quantitative character and high additive genetic effects were calculated (Sadeghian and Johansson 1993). In most studies bolting resistance was dominant over bolting susceptibility (Jolliffe and Arthur 1993).
Rich phenotypic variation for bolting time has been found in recent studies from our institute. In the course of a project to breed winter beets (see Sect. 1.9) a worldwide collection of cultivated and wild B. vulgaris (Fig. 1.3d) accessions was grown over winter at different locations across Europe. While annual flowering was abundant among wild beets, all cultivated types including chards and red table beets were biennial. Interestingly, all sugar beets bolted early after winter whereas wild beets showed a larger variation for bolting behavior even after winter (unpublished data). This demonstrates the potential to breed beets with altered bolting characters from the primary gene pool of B. vulgaris. With the sequences of major flowering time regulators at hand, it has also become possible to analyze phenotypic variation in bolting time for associations with sequence variation in candidate genes, and a corresponding project has been initiated. Finally, we are also using TILLING (Targeting Induced Local Lesion in Genomes; McCallum et al. 2000) to detect new sequence variation in flowering time regulators. In brief, candidate gene sequences are amplified by PCR and subjected to endonuclease digests to detect mismatches between a reference wild type sequence and variant alleles, which arose either as a result of natural variation or after EMS-induced mutagenesis. While the frequency of induced mutations was found to be very low in two independent EMS-mutagenized beet populations, a number of ‘natural’ sequence variants have been found which in some cases could be correlated with altered bolting behaviour (Frerichman et al., in preparation).
9 Strategies to Breed Winter Beets by Manipulating Major Bolting Time Regulators
Sugar beets are sown in spring under central European growth conditions (temperate climates). Winter beets, i.e. beets sown before winter, are only grown on a limited scale in some parts of southern Europe. There has been increasing evidence in the past years that winter beets cultivated under temperate climates could be superior in yield to traditionally grown beets (Hoffmann and Kluge-Severin 2010). Under these conditions beets would be sown in August and stay in the field over winter. Thus, winter hardiness is a prerequisite for winter beet cultivation, and experimental data indicate that there is sufficient genetic variation for winter hardiness within the primary gene pool of B. vulgaris. A total of 396 cultivated and wild B. vulgaris accessions were grown at five different locations across central Europe. As expected for a character which has never been targeted by breeding, survival rates varied widely across the test locations and ranged from 0.7 to 86.3 % (Kirchhoff et al. 2012). The sugar beet accessions showed the highest winter hardiness and had a survival rate of 39.7 % across all environments, suggesting that there is sufficient genetic variation for breeding winter beets.
Winter beet field trials have been performed on a limited scale in Europe. Due to the lack of bolting resistant beets traditional spring beet cultivars were used, which, when sown before winter, started bolting in spring and set flowers a few weeks later. Thus, their root yield was considerably reduced, and bolting beets have a lower sugar but a higher marc content which makes them unsuitable for sugar recovery (Hoffmann and Kluge-Severin 2011). Because root mass is highly correlated with the season and duration of radiation absorption, the yield potential of winter beets was estimated by the absorbed light and the radiation use efficiency. An increase of up to 26 % was estimated independently by two different research groups (Jaggard and Werker 1999; Hoffmann and Kluge-Severin 2010) which makes autumn sown beets an interesting alternative. Moreover, winter beets will reach harvest time earlier. It has been speculated that winter beets will reach the same yield as spring beets 6–8 weeks earlier (Hoffmann and Kluge-Severin 2010). This alone could be a strong advantage over spring beet cultivation because sugar factories would be able to make better use of their capacities if the beet harvest period will be extended. However, a substantial increase in beet yield requires a higher sink capacity (Hoffmann and Kluge-Severin 2010), and it remains to be seen whether this will be the case in winter beets. Furthermore, for the yield potential to be realized in the field, high disease pressure before winter has to be taken into account, which requires new plant protection management strategies.
The breeding of beets which are bolting-resistant after winter is a specific challenge for beet breeders. No genetic variation for this character is expected among the elite germplasm because breeders have selected for relatively early bolting and rapid flowering after winter, as otherwise seed production would not be economical. Field trials at our institute confirmed that most beet cultivars bolt readily after winter. However, we also found some variation in B. vulgaris for bolting behaviour, with some accessions bolting late or comprising high ratios of non-bolting plants after winter. Some accessions repeatedly did not flower until the end of summer, which is the desired phenotype of a winter beet (unpublished data). The genetic causes underlying this phenotype are presently being investigated. It remains to be seen whether the causative alleles can be useful in winter beet breeding because flowering is necessary for seed production.
Our increasing understanding of flowering time regulators in beet (see Sect. 1.6) offers a chance for genetic modification of bolting and flowering time. In principle, they rely on either repression of floral promoters or over-expression of floral repressors. While disruption of gene function can be obtained by mutagenesis (e.g. TILLING) the only realistic option currently available for gene over-expression is genetic modification by transformation. Thus, the resulting plants will be transgenic which causes some legal constraints. Examples of floral promoters as targets for mutagenesis or RNAi are BvFT2, as demonstrated by Pin et al. (2010) using the RNAi approach, or the bolting gene B, whereas the repressor gene BvFT1 or homologs of floral repressors in other species are potential candidates for gene over-expression. For BvFT1, Pin et al. (2010) demonstrated that constitutive expression from a strong promoter can indeed delay bolting after vernalization for at least 6 months.
Reverting the non-bolting phenotype to induce bolting and flowering for seed production is another challenge (Fig. 1.4a). Different strategies have been suggested (Jung and Müller 2009). The expression of a transgene can be controlled if it is under transcriptional regulation of an inducible promoter, e.g. ethanol- or acetaldehyde-inducible promoters (Fig. 1.4b). Alternatively, a floral repressor such as BvFT1 is brought under the transcriptional regulation of a constitutive or any other promoter only after cleavage of a spacer fragment separating both sequences (hybrid/recombinase approach). For site-directed cleavage, recombinase target sites flanking the spacer are introduced into a construct (parent 1). The second parent is transformed with a recombinase gene. Both parents would be expected to flower normally after vernalization, and hybrid seeds can be produced as usual. The hybrids, however, would not bolt after vernalization because the spacer fragment is removed as a result of recombinase activity, thus allowing the floral repressor gene to be transcribed from the promoter. Transgenic plants carrying both constructs have been obtained and are presently under investigation (Fig. 1.4c). A third alternative could be the use of two late flowering parents whose phenotypes are caused by different gene actions. Depending on the nature and genetic interactions of these genes, the hybrid may not flower, or at least flower later than the parents, due to additive effects of the parental alleles. If bolting can be delayed until the time of harvest, this approach could be an interesting alternative because it avoids, in principal, a need for genetic modification. Late bolting accessions which have been already identified from the primary gene pool of B. vulgaris (unpublished data) can be used as donors of late flowering alleles. A caveat of this approach is that breeding with late flowering lines is slow as a result of prolonged generation cycles. In general, hybrid approaches as described above seem a realistic option for winter beet breeding because all beet cultivars presently on the market are hybrids. Genetic modification of flowering time genes to breed non-bolting beets would be a rare (and to date unique) example of transgenic plants with improved yield potential.
Strategies to breed winter beets, which are bolting resistant after winter. a Selection of non-bolting beets after winter. b Bolting induction after transformation of non-bolting beets with an inducible flowering time control gene (after Jung and Müller 2009). c A transgenic hybrid approach to breed non-bolting F1 hybrids by activating a flowering time repressor. Parental lines containing either a FLP recombinase or different inactive repressors for flowering time control could be used. After a genetic cross the FLP recombinase activates the repressor in F1 hybrids by depleting a spacer between promoter and coding sequence
Note Added in Proof
Since the writing of this chapter the identification of the B locus by map-based cloning was described (Pin et al. 2012). In this report it was shown that the pseudo-response regulator gene BOLTING TIME CONTROL 1 (BvBTC1) at the B locus is required for bolting in the absence of vernalization. Surprisingly, the recessive Bvbtc1 allele in biennials facilitates bolting, too, but does so only after vernalization. Induction of bolting by BvBTC1 or Bvbtc1 is mediated through the regulation of BvFT1 and BvFT2 in both non-vernalized (annual) and vernalized (biennial) beets. According to the proposed model, expression of the dominant and fully functional BvBTC1 allele in annual beets is enhanced in the late afternoon hours of long days, which leads to repression of BvFT1 and activation of BvFT2, and in turn bolting and flowering. Biennial sugar beet cultivars carry a partial loss-of-function allele which is insufficiently active in response to long days without prior vernalization. The prolonged exposure to cold over winter enhances Bvbtc1 activity, and possibly that of co-regulatory genes, thus restoring the plant’s responsiveness to long days in the following spring. Under climate chamber conditions, down-regulation of Bvbtc1 by RNAi resulted in suppression of bolting after vernalization, suggesting that this gene can be part of strategies towards the development of winter beets. The central role of BvBTC1 and the BvFT1/BvFT2 module in the regulation of vernalization requirement and photoperiod response also distinguishes beet from the well-studied flowering time control systems in Arabidopsis and cereals and illustrates the evolution of diverse floral regulatory mechanisms among angiosperms.
References
Abe J, Guan GP, Shimamoto Y (1997) A gene complex for annual habit in sugar beet (Beta vulgaris L.). Euphytica 94:129–135
Abegg FA (1936) A genetic factor for the annual habit in beets and linkage relationship. J Agr Res 53:493–511
Abou-Elwafa SF, Büttner B, Chia T et al (2011) Conservation and divergence of autonomous pathway genes in the flowering regulatory network of Beta vulgaris. J Exp Bot 62:3359–3374
Abou-Elwafa SF, Büttner B, Kopisch-Obuch FJ et al (2012) Genetic identification of a novel bolting locus in Beta vulgaris which promotes annuality independently of the bolting gene B. Mol Breed 29:989–998
Ahn JH, Miller D, Winter VJ et al (2006) A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J 25:605–614
Arumuganathan K, Earle ED (1991) Nuclear DNA content of some important plant species. Plant Mol Biol Rep 9:208–218
Barzen E, Mechelke W, Ritter E et al (1992) RFLP markers for sugar beet breeding—chromosomal linkage maps and location of major genes for rhizomania resistance, monogermy and hypocotyl colour. Plant J 2:601–611
Barzen E, Stahl R, Fuchs E et al (1997) Development of coupling-repulsion phase SCAR markers diagnostic for the sugarbeet Rr1 allele conferring resistance to rhizomania. Mol Breed 3:231–238
Bell CI,Milford GFJ, Leigh RA (1996) Sugar beet. In: Zamski E, Schaffer AA (eds) Marcel, New York, pp 691–707
Bhambie S, Goyal R, Gupta S (2000) Ontogeny of cambium in two genera of Chenopodiaceae. Acta Bot Indica 18:252–255
Biancardi E, Campbell LG, Skaracis GN, De Biaggi M (2005) Genetics and breeding of sugar beet. Science, Enfield
Boudry P, McCombie H, Van Dijk H (2002) Vernalization requirement of wild beet Beta vulgaris ssp maritima: among population variation and its adaptive significance. J Ecol 90:693–703
Boudry P, Wieber R, Saumitou-Laprade P et al (1994) Identification of RFLP markers closely linked to the bolting gene B and their significance for the study of the annual habit in beets (Beta vulgaris L.). Theor Appl Genet 88:852–858
Bünning E (1936) Die endonome tagesrhythmik als grundlage der photoperiodischen reaktion. Ber Deut Bot Ges 54:590–607
Bürcky K (1986) Ertrag und qualität von zuckerrüben bei unterschiedlichem anteil an schossern im bestand. Zuckerindustrie 111:862–867
Büttner B, Abou-Elwafa SF, Zhang W et al (2010) A survey of EMS-induced biennial Beta vulgaris mutants reveals a novel bolting locus which is unlinked to the bolting gene B. Theor Appl Genet 121:1117–1131
Cai D, Kleine M, Kifle S et al (1997) Positional cloning of a gene for nematode resistance in sugar beet. Science 275:832–834
Campoli C, Drosse B, Searle I et al (2011) Functional characterisation of HvCO1, the barley (Hordeum vulgare) flowering time ortholog of CONSTANS. Plant J 69:868–880
Catusse J, Strub JM, Job C et al (2008) Proteome-wide characterization of sugarbeet seed vigor and its tissue specific expression. Proc Natl Acad Sci U S A 105:10262–10267
Cháb D, Kolář J, Olson M, Štorchová H (2008) Two FLOWERING LOCUS T (FT) homologs in Chenopodium rubrum differ in expression patterns. Planta 228:929–940
Chia T, Müller A, Jung C, Mutasa-Göttgens E (2008) Sugar beet contains a large CONSTANS-LIKE gene family including a putative CO homologue that is independent of the early-bolting (B) gene locus. J Exp Bot 59:2735–2748
Doi K, Izawa T, Fuse T et al (2004) Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev 18:926–936
El-Mezawy A, Dreyer F, Jacobs G, Jung C (2002) High resolution mapping of the bolting gene B of sugar beet. Theor Appl Genet 105:100–105
Gaafar RM, Hohmann U, Jung C (2005) BAC-derived molecular markers for early bolting in sugar beet. Theor Appl Genet 110:1027–1037
Griffiths S, Dunford RP, Coupland G, Laurie DA (2003) The evolution of CONSTANS-like gene families in barley, rice, and Arabidopsis. Plant Physiol 131:1855–1867
Grimmer MK, Trybush S, Hanley S et al (2007) An anchored linkage map for sugar beet based on AFLP, SNP anbd RAPD markers and QTL mapping of a new source of resistance to beet necrotic yellow vein virus. Theor Appl Genet 114:1151–1160
Hagihara E, Matsuhira H, Ueda M et al (2005) Sugar beet BAC library construction and assembly of a contig spanning Rf1, a restorer-of-fertility gene for Owen cytoplasmic male sterility. Mol Genet Genomics 274:316–323
Hanzawa Y, Money T, Bradley D (2005) A single amino acid converts a repressor to an activator of flowering. Proc Natl Acad Sci U S A 102:7748–7753
Hautekeete NC, Piquot Y, Van Dijk H (2002) Life span in Beta vulgaris ssp maritima: the effects of age at first reproduction and disturbance. J Ecol 90:508–516
Hjerdin-Panagopoulos A, Kraft T, Rading IM et al (2002) Three QTL regions for restoration of Owen CMS in sugar beet. Crop Sci 42:540–544
Hoffmann CM, Kluge-Severin S (2010) Light absorption and radiation use efficiency of autumn and spring sown sugar beets. Field Crops Res 119:238–244
Hoffmann CM, Kluge-Severin S (2011) Growth analysis of autumn and spring sown sugar beet. Eur J Agron 34:1–9
Hohmann U, Jacobs G, Jung C (2005) An EMS mutagenesis protocol for sugar beet and isolation of non-bolting mutants. Plant Breed 124:317–321
Hohmann U, Jacobs G, Telgmann A et al (2003) A bacterial artificial chromosome (BAC) library of sugar beet and a physical map comprising the bolting gene B. Mol Genet Genomics 269:126–136
Ishikawa R, Aoki M, Kurotani K et al (2011) Phytochrome B regulates Heading date 1 (Hd1)-mediated expression of rice florigen Hd3a and critical day length in rice. Mol Genet Genomics 285:461–470
Itoh H, Nonoue Y, Yano M, Izawa T (2010) A pair of floral regulators sets critical day length for Hd3a florigen expression in rice. Nat Genet 42:635–638
Jaggard KW, Werker AR (1999) An evaluation of the potential benefits and costs of autumn-sown sugarbeet in NW Europe. J Agri Sci 132:91–102
Jarillo JA, Piñeiro M (2011) Timing is everything in plant development. The central role of floral repressors. Plant Sci 181:364–378
Jolliffe TH, Arthur AE (1993) Diallel analysis of bolting in sugarbeet. J Agric Sci 121:327–332
Jung C, Müller A (2009) Flowering time control and applications in plant breeding. Trends Plant Sci 14:563–573
Kadereit G, Hohmann S, Kadereit JW (2006) A synopsis of Chenopodiaceae subfam. Betoidae and notes on the taxonomy of Beta. Willdenowia 36:9–19
Kardailsky I, Shukla VK, Ahn JH et al (1999) Activation tagging of the floral inducer FT. Science 286:1962–1965
Kim D-H, Doyle MR, Sung S, Amasino RM (2009) Vernalization: winter and the timing of flowering in plants. Annu Rev Cell Dev Biol 25:277–299
Kirchhoff M, Svirshchevskaya A, Hoffmann C et al (2012) High degree of genetic variation of winter hardiness in a panel of Beta vulgaris L. Crop Sci 52:179–188
Kleine M, Voss H, Cai D, Jung C (1998) Evaluation of nematode resistant sugar beet (Beta vulgaris L.) lines by molecular analysis. Theor Appl Genet 97:896–904
Kobayashi Y, Kaya H, Goto K et al (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286:1960–1962
Lange C, Holtgräwe D, Schulz B et al (2008) Construction and characterization of a sugar beet (Beta vulgaris) fosmid library. Genome 51:948–951
Lange C, Mittermayr L, Dohm J et al (2010) High-throughput identification of genetic markers using representational oligonucleotide microarray analysis. Theor Appl Genet 121:549–565
Lange W, Brandenburg WA, De Bock TSM (1999) Taxonomy and cultonomy of beet (Beta vulgaris L.). Bot J Linn Soc 130:81–96
Lee J, Lee I (2010) Regulation and function of SOC1, a flowering pathway integrator. J Exp Bot 61:2247–2254
Lein JC, Asbach K, Tian YY et al (2007a) Resistance gene analogues are clustered on chromosome 3 of sugar beet and co-segregate with QTL for rhizomania resistance. Genome 50:61–71
Lein JC, Sagstetter C, Schulte D et al (2007b) Mapping of rhizoctonia root rot resistance genes in sugar beet. Plant Breed 127:602–611
Lenton JR,Pocock TO, Radley ME (1975) Endogenous gibberellins and bolting of sugar beet.vol 1. UK: Rothamsted Experimental Station, 44–46
McCallum CM, Comai L, Greene EA, Henikoff S (2000) Targeting Induced Local Lesions IN Genomes (TILLING) for plant functional genomics. Plant Phys 123:439–442
McGrath JM, Trebbi D, Fenwick A et al (2007) An open-source first-generation molecular genetic map from a sugarbeet × table beet cross and its extension to physical mapping. Crop Sci 47:S27–S47
Metzger J (ed) (1995) Hormones and reproductive development. Kluwer, Dordrecht
Michaels SD, Amasino RM (1999) FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11:949–956
Milford G, Burks E (2010) Managing the risk of bolters. Brit Sugar Beet Rev 78:32–35
Munerati O (1931) L’eredita della tendenza alla annualita nella comune barbabietola coltivata. Ztschr Züchtung, Reihe A, Pflanzenzüchtung 17:84–89
Mutasa-Göttgens E, Qi A, Mathews A, Thomas S et al (2009) Modification of gibberellin signalling (metabolism & signal transduction) in sugar beet: analysis of potential targets for crop improvement. Transgenic Res 18:301–308
Mutasa-Göttgens ES, Qi A, Zhang W et al (2010) Bolting and flowering control in sugar beet: Relationships and effects of gibberellin, the bolting gene B and vernalization. AoB Plants. doi:10.1093/aobpla/plq012
Nilsson NO, Hansen M, Panagopoulos AH et al (1999) QTL analysis of Cercospora leaf spot resistance in sugar beet. Plant Breed 118:327–334
Owen FV (1954) The significance of single gene reactions in sugar beets. Proc Amer Soc Sugar Beet Technol 8:392–398
Owen FV, Carsner E, Stout M (1940) Photothermal induction of flowering in sugar beets. J Agric Sci 61:101–124
Pestsova E, Meinhard J, Menze A et al (2008) Transcript profiles uncover temporal and stress-induced changes of metabolic pathways in germinating sugar beet seeds. BMC Plant Biol 8:122
Pillen K, Steinrücken G, Wricke G et al (1992) A linkage map of sugar beet (Beta vulgaris L.). Theor Appl Genet 84:129–135
Pin PA, Benlloch R, Bonnet D et al (2010) An antagonistic pair of FT homologs mediates the control of flowering time in sugar beet. Science 330:1397–1400
Pin PA, Zhang W, Vogt SH et al (2012) The role of a pseudo-response regulator gene in life cycle adaptation and domestication of beet. Curr Biol 22:1095–1101
Pittendrigh CS (1972) Circadian surfaces and the diversity of possible roles of circadian organization in photoperiodic induction. Proc Natl Acad Sci U S A 69:2734–2737
Pittendrigh CS, Minis DH (1964) The entrainment of circadian oscillations by light and their role as photoperiodic clocks. Amer Natur 98:261–294
Ratcliffe OJ, Kumimoto RW, Wong BJ, Riechmann JL (2003) Analysis of the Arabidopsis MADS AFFECTING FLOWERING gene family: MAF2 prevents vernalization by short periods of cold. Plant Cell 15:1159–1169
Reeves PA, He Y, Schmitz RJ et al (2007) Evolutionary conservation of the FLOWERING LOCUS C-mediated vernalization response: evidence from the sugar beet (Beta vulgaris). Genetics 176:295–307
Rimpau W (1876) Das Aufschiessen der Runkelrüben. Landw Jahrb 5:31–45
Rimpau W (1880) Das Aufschiessen der Runkelrüben. Landw Jahrb 9:191–203
Sadeghian SY, Johansson E (1993) Genetic study of bolting and stem length in sugar beet (Beta vulgaris L.) using a factorial cross design. Euphytica 65:177–185
Sadeghian SY, Becker HC, Johansson E (1993a) Inheritance of bolting in three sugar beet crosses after different periods of vernalization. Plant Breed 110:328–333
Sadeghian SY, Johansson E, Lexander K (1993b) A genetic analysis of the numer of cells, length of cell, and gibberellic acid sensitivity in sugar beet and their relation to bolting mechanism. Euphytica 68:59–67
Schäfer-Pregl R, Borchardt D, Barzen E et al (1999) Localization of QTLs for tolerance to Cercospora beticola on sugar beet linkage groups. Theor Appl Genet 99:829–836
Schneider K, Kulosa D, Rosleff-Soerensen T et al (2007) Analysis of DNA polymorphisms in sugar beet (Beta vulgaris L.) and development of an SNP-based map of expressed genes. Theor Appl Genet 115:601–615
Schneider K, Schäfer-Pregl R, Borchardt DC, Salamini F (2002) Mapping QTLs for sucrose content, yield and quality in a sugar beet population fingerprinted by EST-related markers. Theor Appl Genet 104:1107–1113
Schondelmaier J, Jung C (1997) Chromosomal assignment of the nine linkage groups of sugar beet (Beta vulgaris L.) using primary trisomics. Theor Appl Genet 95:590–596
Schulte D, Cai D, Kleine M et al (2006) A complete physical map of a wild beet (Beta procumbens) translocation in sugar beet. Mol Genet Genomics 275:504–511
Schumacher K, Schondelmaier J, Barzen E et al (1997) Combining different linkage maps in sugar beet (Beta vulgaris L.) to make one map. Plant Breed 116:23–38
Setiawan A, Koch G, Barnes SR, Jung C (2000) Mapping quantitative trait loci (QTLs) for resistance to Cercospora leaf spot disease (Cercospora beticola Sacc.) in sugar beet (Beta vulgaris L.). Theor Appl Genet 100:1176–1182
Simpson GG (2004) The autonomous pathway: epigenetic and post-transcriptional gene regulation in the control of Arabidopsis flowering time. Curr Opin Plant Biol 7:570–574
Song YH, Lee I, Lee SY et al (2012) CONSTANS and ASYMMETRIC LEAVES 1 complex is involved in the induction of FLOWERING LOCUS T in photoperiodic flowering in Arabidopsis. Plant J 69:332–342
Sorce C, Stevanato P, Biancardi E, Lorenzi R (2002) Physiological mechanisms of floral stem elongation (bolting) control in sugar beet (Beta vulgaris ssp. vulgaris L.). Agroindustria 1:87–91
Srikanth A, Schmid M (2011) Regulation of flowering time: all roads lead to Rome. Cell Mol Life Sci 68:2013–2037
Suarez-Lopez P, Wheatley K, Robson F et al (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410:1116–1120
The Angiosperm Phylogeny Group (2009) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 161:105–121
Turck F, Fornara F, Coupland G (2008) Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 59:573–594
Turner A, Beales J, Faure S et al (2005) The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310:1031–1034
Van Dijk H (2007) Artificial selection for flowering date in the iteroparous perennial plant Beta vulgaris subsp. maritima (sea beet): an analysis of the underlying mechanisms. In: Conference of the European Society for Evolutionary Biology, Uppsala
Van Dijk H, Boudry P, McCombie H, Vernet P (1997) Flowering time in wild beet (Beta vulgaris ssp. maritima) along a latitudinal cline. Acta Oecol 18:47–60
Weisshaar B, Dohm JC, Minoche A et al (2011) The draft genome sequence of sugar beet (Beta vulgaris L.). In: Plant & Animal Genome Conference XIX, San Diego
Wenkel S, Turck F, Singer K et al (2006) CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell 18:2971–2984
Wilson RN, Heckman JW, Somerville CR (1992) Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Phys 100:403–408
Würschum T, Maurer HP, Schulz B et al (2011) Genome-wide association mapping reveals epistasis and genetic interaction networks in sugar beet. Theor Appl Genet 123:109–118
Zeevaart JAD (1983) Gibberellins and flowering. Praeger, New York
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Melzer, S., Müller, A., Jung, C. (2014). Genetics and Genomics of Flowering Time Regulation in Sugar Beet. In: Tuberosa, R., Graner, A., Frison, E. (eds) Genomics of Plant Genetic Resources. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7575-6_1
Download citation
DOI: https://doi.org/10.1007/978-94-007-7575-6_1
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7574-9
Online ISBN: 978-94-007-7575-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)