Abstract
Salicylic acid (SA), a naturally occurring plant hormone, is primarily associated with the induction or activation of defence mechanism responses by higher plants when they are attacked by pathogens. Attack of these plants by pathogens rapidly triggers changes in a wide range of the plant’s metabolic pathways which in turn are followed by modifications in the plant’s growth and development. There are a number of references in the recent literature where SA was applied to plants that are being subjected to changes in environmental signaling without the involvement of pathogens. In these examples, SA appears to be functioning as a hormone. Significant changes (usually positive) in shoot growth and photosynthesis occur when SA is applied at low concentrations to plants subjected to environmental stresses. In this review we focused on the interplay between changes in endogenous SA concentrations and key environmental signals, i.e. light intensity and quality, temperature, soil water availability and carbon dioxide levels. In doing so, we evaluated the concept that endogenous SA functions as an important signaling hormone in the plant’s growth response to a changing environment, even in the absence of pathogen attack.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
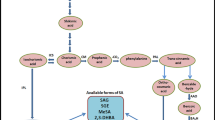
Salicylic acid (SA) has only recently been recognized to have a role in the plant’s ability to mount a defence against pathogen attack. In contrast, the human therapeutic properties of SA (2-hydroxy-benzoic acid) have been known for over 25 centuries. In traditional medicine pieces of bark from willow trees (Salix spp.) were chewed to provide relief from pain and inflammation, as described in writings by the Greek physician, Hippocrates (Fifth Century B.C.) and the physician and botanist Pedanius Dioscorides (First Century A.D.). The therapeutic component in willow bark, SA, was first identified and isolated during the 19th century. Toward the end of the 19th century it was chemically modified into what is known today as aspirin or acetylsalicylic acid (Delaney 2010). In plants, SA is biosynthesized by the shikimic acid pathway, which can produce SA in two ways: chorismic acid → phenylalanine → trans-cinnamic acid → benzoic acid → SA, or chorismic acid → isochorismic acid → SA (Delaney 2010).
The role of SA as an endogenous signaling molecule in the plant’s defence mechanism against pathogens has now been extensively characterized (Raskin 1992). In a similar manner, the effect of SA, when applied exogenously, on the physiology, metabolism and reproductive development of some higher plants was also reported (Raskin 1995). Exogenously applied SA can promote fresh and dry biomass accumulation at low (near-physiological) concentrations (Hayat et al. 2010). However, at higher exogenous concentrations, SA often shows an inhibition of dry mass accumulation (Schettel and Balke 1983). Applications of SA can also induce stomatal closure (Larque-Saavedra 1979) and influence ion uptake and transport (Harper and Balke 1981), as well as regulate chlorophyll accumulation and the rate of photosynthesis (Hayat et al. 2010). Applications of low concentrations of SA have thus been shown to increase chlorophyll content, though higher concentrations were inhibitory (Ghai et al. 2002; Fariduddin et al. 2003; Hayat et al. 2005). Low concentrations of exogenously applied SA also gave a slightly elevated photosynthetic efficiency of photosystem (PS) II in both Arabidopsis thaliana (Heynh.) wild type (WT) plants and A. thaliana SA non-responsive mutant plants, although higher concentrations of SA reduced PS II photosynthetic efficiency (Chen et al. 2009). Consistent with these results, an A. thaliana SA-overproducing mutant exhibited decreased photosynthetic efficiency of PS II when SA was applied across wide range (low and high) of concentrations (Chen et al. 2009).
Responses by barley (Hordeum vulgare L.) leaves to applied SA at relatively high concentrations included a decreased rate of leaf expansion as well as a decrease in overall leaf area growth and decreased thickness of all leaf tissue components (Pancheva et al. 1996; Pancheva and Popova 1998; Uzunova and Popova 2000). Further, these morphological responses were associated with decreases in both the rate of photosynthesis and chlorophyll content (Pancheva et al. 1996; Pancheva and Popova 1998; Uzunova and Popova 2000). Thus, as appears to be the case with many of the plant hormone classes, exogenously applied SA may promote photosynthesis and growth at lower concentrations, but be inhibitory when applied at high concentrations. Further, the growth-promotive or inhibitory effects of exogenous SA application appear to depend on the environmental conditions at the time of application (Hayat et al. 2010). Based on the above literature, we think it is time to evaluate the effects of environmental signaling on endogenous SA levels in the context of a range of plant growth mechanisms. Such an approach should allow us to better understand how exogenously applied SA alleviates stress symptoms in many higher plants.
2 Assessing Endogenous Salicylic Acid Levels
Endogenous SA levels in plant tissues can be precisely identified and accurately quantified using the stable isotope dilution method (Gaskin and MacMillan 1991). This is accomplished by selected ion monitoring (SIM) using capillary gas chromatography (GC)—mass spectrometry (MS) or liquid chromatography (LC)—MS (Scott et al. 2004; Kurepin et al. 2010a). The first stage, consisting of extraction, addition of stable isotope internal standards and purification is the same for both SIM-GC–MS and LC–MS methods. Plant tissue is collected and immediately frozen in liquid N2, then freeze-dried or stored at −80 ºC until extraction. Extraction consists of grinding in a mortar and pestle with liquid N2, followed by use of 80 % aqueous methanol (H2O–MeOH = 20:80, v/v) as an extraction solvent. A known amount of high specific activity deuterated SA (usually 2H6-SA) is added to the 80 % MeOH extract as a quantitative internal standard. The 80 % MeOH extract is then purified with a C18 preparative column (using reversed phase C18 material) and dried in vacuo at 35 ºC. For the LC–MS method, the dried residue is reconstituted in 0.05 % HOAc in H2O–MeCN (85:15, v/v) and then filtered with a 0.45 μm filter prior to the injection into the LC–MS. The characteristic m/z ions that are usually used for quantification are (m/z 141 for d6SA and m/z 137 for the endogenous protio SA). The quantification of endogenous SA is then accomplished based on the relative intensities (peak areas) of these characteristic m/z ions, the known amount of d6-labeled SA internal standard added and the recorded fresh or dry tissue weight (Scott et al. 2004). For the SIM-GC–MS method, the dried residue is reconstituted in 1 % HOAc in H2O–MeOH (90:10, v/v) and injected into the high performance LC (HPLC). In one example (Kurepin et al. 2010a) the HPLC used a manually implemented 10–73 % linear gradient program for separation of SA from other plant metabolites. The eluted fractions that are expected to contain SA (based on previous HPLC runs with a SA standard) are dried in vacuo at 35 ºC and then methylated using ethereal CH2N2 at room temperature as described in Kurepin et al. (2010a). The methylated SA sample is then injected into the GC–MS and the SIM program is set to monitor characteristic m/z ions for both the deuterated SA-Me standard (124, 96 and 156 for d6SA) and endogenous protio SA-Me (120, 92 and 152). For identification of endogenous SA a comparison of the relative intensities of the three m/z ion pairs, i.e. 124/120, 96/92 and 156/152, is accomplished. The amount of endogenous (protio) SA is then quantified by the reference to the stable isotope-labeled internal standard using equations for isotope dilution analysis using the 124/120 ion pair (see Gaskin and MacMillan 1991; Jacobsen et al. 2002).
As with other classes of plant hormones, large variations in endogenous SA levels between species, ecotypes, tissues, and, more importantly in response to various environmental signals are not uncommon. Species-related difference in endogenous SA levels can be very significant. In two-week old A. thaliana and sunflower (Helianthus annuus L.) shoots grown under exactly the same conditions, they can vary from a few ng per gDW in A. thaliana to several thousand ng per gDW in sunflower (Kurepin et al. 2010a). Ecotype-related differences can also be large. For example, alpine plants of Stellaria longipes [(L.) Goldie] have ca. half the amount of endogenous SA, relative to plants of a prairie ecotype of the same species, both ecotypes being grown under the same conditions and also being of the same age (Kurepin et al. 2012a). Tissue-related difference in SA can be highlighted by comparing sunflower internodes (which contain several hundred ng per gDW) with leaves (which have several thousand ng per gDW) above that internode. Although species-, ecotype- and tissue-related differences are “interesting”, environment-related differences in endogenous SA will have the most substantial impact on plant performance and, even survival (Scott et al. 2004; Abreu and Munne-Bosch 2008; Kurepin et al. 2010a)—see also evidence from numerous SA application studies (Hayat et al. 2010).
3 Light Signaling and Endogenous Salicylic Acid Levels
Among the many environmental factors, light plays a key role in plant growth and development. The irradiance levels of “visible” light (ca. 400–800 nm) received by a plant can control both photosynthesis and growth, including etiolation and de-etiolation. In contrast, it is the quality or specific wavelengths of visible light that regulates many growth and developmental events (Smith 2000). Additionally, invisible light, such as ultraviolet (UV) light (ca. 100–400 nm), can both influence growth and cause damage to plants (Jenkins 2009). For example, UV-C irradiance of tobacco (Nicotiana tabacum L.) plants caused a significant accumulation of SA (Yalpani et al. 1994). Silencing the isochorismate (a SA biosynthetic precursor) synthase (ICS) gene using a virus-induced technique prevented an accumulation in endogenous SA in tobacco plants inoculated with a pathogen (Catinot et al. 2008). Exposure of these transgenic plants to UV-C light also significantly decreased the accumulation of endogenous SA (Catinot et al. 2008). This implies that a de novo synthesis of SA may occurr in response to UV light stress. Additionally, exogenously applied SA is reported to alleviate the UV-B irradiance-related stress in swards of both Kentuky bluegrass (Poa pratensis L.) and tall fescue (Festuca arundinacea Schreb.) (Ervin et al. 2004).
In A. thaliana, a plant that is often grown for experimental use at light irradiance levels well below those of natural sun light, endogenous SA levels were higher by ca. 50 % at low light irradiance relative to dark (Genoud et al. 2002). However, a further increase in light irradiance had no significant effect on endogenous SA levels (Zeier et al. 2004). For sunflower (Helianthus annuus L.), a plant that is adapted to grow best at full sun light, endogenous SA levels in hypocotyl tissue showed consistent and appreciable (ca. 10-fold) increases as light irradiance levels increased from very low to low and then to full sunlight (Kurepin et al. 2010a). These increases in endogenous SA levels of sunflower shoot tissue at the increased light irradiance levels were associated with significant decreases in hypocotyl elongation and biomass accumulation (Kurepin et al. 2010a). Thus, the difference in the endogenous SA response and its magnitude in response to changes in light irradiance levels, vary between A. thaliana and sunflower. The changes in growth and endogenous SA levels seen in the plant’s response to light irradiance level can also be ecotype-specific. The Stellaria longipes sun ecotype grows in an alpine habitat characterized by very short plants and distant surrounding vegetation. In contrast, the S. longipes shade ecotype grows in nearby low elevation prairie grasslands, where the habitat is characterized by tall neighbouring vegetation which causes canopy shading and/or shading by neighbours. These two ecotypes grow just a few kilometres apart, but the habitat differences caused by elevation make them an excellent model for light signaling research (Emery et al. 1994). Plants of both ecotypes decrease their shoot growth in response to increase in light irradiance levels and endogenous SA levels increase coincidentally (Kurepin et al. 2012a). This decrease in shoot growth in response to an increase in light irradiance was proportionally similar for both sun and shade ecotypes of S. longipes. However, the magnitude of changes in endogenous SA levels varied between the two ecotypes. The sun ecotype plants showed less than a 2-fold increase in endogenous SA levels. Yet shade ecotype plants had more than a 3-fold increase in endogenous SA levels (Kurepin et al. 2012a). Thus, the magnitude of the SA concentration in response to increasing irradiance levels can vary depending on species and ecotype. Even so, the high light irradiance-mediated increase in endogenous SA levels may have ecological significance. For example, it is a well-known fact that there is a higher susceptibility of younger trees to pathogen attack in understory environments. This may be because the fungal activity in understory environments is greater than in forest openings (Kitajima and Augspurger 1989). Thus, when seeds of Betula papyrifera Marsh., (a species of birch native to North America) were planted in a range of habitats differing in light availability (from understory to open forest), the application of a fungicide reduced losses in a habitat-dependent manner (O’Hanlon-Manners and Kotanen 2004). Thus, the fungicide was more effective in understory than in open habitats. One possible explanation is that low light irradiance in the understory forest habitat prevents the establishment of B. papyrifera plants because their ability to modify endogenous SA levels in response to pathogen attack is depressed.
In both sunflower and S. longipes plants the high light irradiance-mediated increase in endogenous SA levels was correlated with a decrease in shoot growth. Simplistically, then, the role of SA could be classified as growth inhibitory for light irradiance-mediated responses. However, A. thaliana mutants with constitutively high endogenous SA levels (cpr1-1, cpr5-1 and cpr6-1; Bowling et al. 1994, 1997; Clarke et al. 1998) exhibit the dwarf growth phenotype at low, but not at higher light irradiances, relative to wild type line Col-0 (Mateo et al. 2006). Further, these A. thaliana mutants with high SA levels, when grown at a lower light irradiance, had lower maximum efficiency and quantum yield of PSII, as well as having reduced stomatal conductance and a lower accumulation of photo-assimilates (Mateo et al. 2006). Thus, the negative effects of possessing high SA levels are only expressed, in these mutant lines, under low irradiance levels. Further, a mutant with constitutively reduced endogenous SA levels (sid2-2; Nawrath and Metraux 1999) did not show a dwarfing response, nor did it exhibit reduced metabolic responses to low light irradiance levels (Mateo et al. 2006). Therefore, low light irradiance-induced growth promotion is associated with low SA levels and plants with high endogenous SA levels fail to etiolate under low light irradiances, yet they have the same phenotype as wild type plants under high irradiance light.
In tobacco (Nicotiana benthamiana L.) plants inoculated with Turnip mosaic virus (TuMV), both low light irradiance and photosystem impairment can increase the susceptibility of the host to TuMV infection (Manfre et al. 2011). Although endogenous SA levels were not measured, exogenous SA application had no effect on TuMV infection. Further, the expression of pathogen response-1 (pr-1) gene was not affected by lower light irradiances or photosystem impairment (Manfre et al. 2011). By implication, the reduction in light irradiance and associated increased susceptibility of host to pathogen may not be mediated by lowered endogenous SA levels. Such a conclusion is also supported by an earlier observation where the inoculation of A. thaliana with Pseudomonas syringae pv. maculicola resulted in systemic acquired resistance (SAR) for plants grown at both low and high light irradiances (Zeier et al. 2004). However, the higher light irradiance did not result in an accumulation of endogenous SA or PR-1 protein (Zeier et al. 2004).
In addition to light irradiance regulation of endogenous SA levels in plant tissues, the quality of light perceived by a plant, via plant photoreceptors such as phytochromes and cryptochromes, can also influence endogenous SA levels (Kurepin et al. 2010a, 2012a). For example, narrow-band far red (FR) light [supplied by light emitting diodes (LED) as the dark period began] yielded up to a 5-fold increase in endogenous SA levels of sunflower hypocotyls, relative to white (W) light or dark (D) treatments (Kurepin et al. 2010a). Here, the FR-induced increase in endogenous SA levels was positively associated with increased hypocotyl elongation. In contrast, LED-produced red (R) light and blue (B) light inhibited hypocotyl elongation relative to W or D treatments (Kurepin et al. 2010a). That inhibition was associated with a decrease in endogenous SA levels, relative to a W light treatment (but not a D treatment). Thus, it appears that the endogenous SA content can also be regulated by light quality. That conclusion is further supported by experiments where the effect of a change in R/FR ratio was tested on endogenous SA accumulation. There, sunflower plants were grown at both low and high light irradiances, each with varying R/FR ratios, and effects on hypocotyl growth and endogenous SA concentrations were measured. A decrease in the R/FR ratio (i.e. an increase in FR radiation relative to R irradiance) from a high R/FR ratio to a normal R/FR ratio, and then to a low R/FR ratio yielded a gradual increase in endogenous SA levels. This, in turn, was positively associated with increased hypocotyl elongation (Kurepin et al. 2010a). For another species, S. longipes, using both sun and shade ecotypes, decreasing the R/FR ratio from high to normal had no effect on endogenous SA levels. However, the further decrease in R/FR ratio from normal to a low R/FR ratio significantly increased endogenous SA levels in the shade ecotype plants (Kurepin et al. 2012a). Again, there was a clear positive association between the changes in endogenous SA levels and shoot growth, as only shade (but not sun) ecotype plants increased their shoot growth in response to decreases in the R/FR ratio (Kurepin et al. 2012a).
Therefore, light irradiance (a sum of total light that a plant can absorb, i.e. photosynthetically active radiation) and light quality (a manipulation of individual light wavelengths, especially R and FR) have different effects on endogenous SA levels (and also on growth). Further, SA levels are associated with growth changes in a different manner, i.e. a decrease in light irradiance causes an increase in shoot elongation, but that increased growth is associated with decreased endogenous SA levels. However, when shoot elongation is induced by a decrease in R/FR ratio, endogenous SA levels rise. Does endogenous SA, then, play any role in these light-induced growth responses? There are reports that exogenously applied SA promotes stem elongation in bean (Phaseolus vulgaris L.; Hegazi and El-Shraiy 2007) and applied SA also promotes the growth of isolated stem segments of Ullucus tuberosus (Caldas) plants (Handro et al. 1997). In contrast, there are numerous examples where high concentrations of exogenously applied SA have inhibited shoot growth, while lower doses of SA promoted it (Hayat et al. 2010). For sunflower hypocotyls, exogenous SA applied at higher concentrations inhibited growth at lower light irradiances, whereas lower concentrations had no effect on growth (Kurepin et al. 2010a). Similar results (different responses from low versus high SA concentrations) were obtained when sunflower hypocotyl growth was measured under different R/FR ratios across a range of exogenously applied SA doses (Kurepin et al. 2010a).
To summarize, the light irradiance and light quality effects on endogenous SA levels are generally quite different than is seen for other classes of plant hormones that have a long and proven history of causally regulating plant shoot growth. In fact, for each of sunflower and A. thaliana, both light irradiance and light quality signaling are known to modify endogenous hormone levels. More specifically, they increase gibberellin, auxin and cytokinin levels, decrease ethylene evolution and generally have a nil effect on abscisic acid and brassinosteroid levels (Kurepin et al. 2007a, b, c, 2010b, 2011a, b, 2012b, c). Additionally, only gibberellins, auxin and ethylene have been shown to directly regulate stem elongation growth increases in response to low light irradiance and low R/FR ratio signals for both sunflower and A. thaliana (Kurepin et al. 2007a, b, c, 2011a, b, 2012b, c). Since a low R/FR ratio increases endogenous SA levels in sunflower hypocotyls, while low light irradiance has the exact opposite effect, it does not seem reasonable to assign a direct role to SA in light signaling. Rather, we conclude that the observed changes in endogenous SA levels are more likely to be an indirect result of changes in endogenous levels of other hormones.
4 Temperature and Endogenous Salicylic Acid Levels
Plants can experience a significant stress from exposure to temperatures that are either lower or higher than optimal. Freezing or heat stress administered over a brief period of time will usually result in irreversible damage to plant tissues, and thus to growth and metabolism. However, cold stress, when the temperature does not drop below freezing, is often a reversible event in terms of both plant growth and metabolism. There are many examples in the literature where pre-treatment applications with SA increased cold stress tolerance across a range of plant species. In hydroponically-grown corn (Zea mays L.) plants, pre-treatments with exogenous SA or acetyl-SA decreased net photosynthesis, stomatal conductivity and transpiration prior to cold stress, but had no effect on Fv/Fm chlorophyll fluorescence ratio (Janda et al. 1999, 2000). However, upon transfer of these plants to cold temperature conditions, the SA-treated plants fared significantly better than the control, untreated corn plants, in terms of growth and metabolism (Janda et al. 1999, 2000). Pre-treatment of tomato (Lycopersicon esculentum L.) fruits with lower concentrations of exogenous methyl salicylate (MeSA) increased their resistance to cold stress and there was also a decreased incidence of decay in low-temperature storage and an increase in the synthesis of PR proteins (Ding et al. 2002). However, pre-treatment of tomato fruits with high concentrations of MeSA had just the opposite effect (Ding et al. 2002). Pre-treatment of germinating radicals of corn, cucumber (Cucumis sativus L.) and rice (Oryza sativa L.) plants with exogenously applied SA improved leaf and hypocotyl tolerance to cold stress, but had no effect on the radicles tolerance to the stress (Kang and Saltveit 2002). Further, the cold stress-induced electrolyte leakage was lower in SA-treated leaves and hypocotyls, but there was no change in electrolyte leakage from the radicles for all three species (Kang and Saltveit 2002). Pre-treatment of banana (Musa acuminate L.) seedlings with exogenous SA (as a foliar spray or via soil irrigation) increased seedling tolerance to subsequent cold stress treatments (Kang et al. 2003). Pre-treatment of potato (Solanum tuberosum L.) plants with exogenous SA applied at low doses increased tolerance to cold stress in cultivars with a wide range of cold stress-susceptibility, whereas high doses of SA were not as effective in doing this (Mora-Herrera et al. 2005). Pre-treatment of mature peach [Prunus persica (L.) Batch.] fruits with exogenous SA, followed by storage at 0 ºC for a four week-period, resulted in higher fruit firmness, lower decay and reduced chilling stress injury than was seen for untreated fruits (Wang et al. 2006). Pre-treatment of hybrid corn seeds with exogenous SA considerably improved seedling emergence, root and shoot length, and seedling fresh and dry weight, all compared with the controls, both at optimal temperatures and under cold stress temperatures (Farooq et al. 2008). Finally, exogenously applied SA alleviated the cold stress effect on growth of cucumber seedlings (Lei et al. 2010). Thus, there is a plethora of examples where a pre-treatment with exogenous SA has reduced the negative effects of cold stress on plant growth and metabolism.
A.thalianaCol-0 plants grown at 23 ºC and then transferred to 5 ºC for several weeks, had significantly increased endogenous SA levels when compared to plants that remained at 23 ºC for the same time period (Scott et al. 2004). A. thaliana mutant lines with reduced SA levels (NahG; Larkindale and Knight 2002), deficient in SA levels (eds5; Nawrath and Metraux 1999) and possessing elevated SA levels (cpr1; Bowling et al. 1994, 1997) were compared to wild type Col-0 plants at both 23 and 5 ºC. Under cold stress conditions, the plants of NahG line showed no increase in endogenous SA levels and accumulated considerably more dry weight than did Col-0 plants (though at 23 ºC the NahG plants accumulate less dry weight than Col-0 plants) (Scott et al. 2004). Further, the cold-stressed NahG plants also had larger leaves as a result of increases in cell size, but there was no change to leaf number. Although there was no cold stress-related damage in photosystem II in either Col-0 or NahG plants, net assimilation rate after the cold stress treatment was higher for NahG plants (Scott et al. 2004). Cold-stressed eds5 plants had a phenotype similar to NahG plants, but possessed even lower endogenous SA levels. Another mutant, cpr1, whose plants have a dwarf phenotype at 23 ºC relative to Col-0, showed even higher dwarfism at low temperatures, and this dwarfism change was associated with ca. 2-fold higher levels of SA, relative to cold-stressed Col-0 plants (Scott et al. 2004). Finally, canola (Brassica napus L.) plants grown at 5 ºC for four weeks after germination had significantly higher endogenous SA levels than plants grown at 20 ºC.
The application of low concentrations of SA can also influence (increase) the tolerance of plant tissues to short-term heat stress, whereas at higher concentrations SA had either the opposite or nil effect. There are numerous examples of this phenomenon, i.e. mustard (Sinapis alba L.; Dat et al. 1998a), tobacco (Nicotiana tabacum L.; Dat et al. 2000), A. thaliana (Larkindale and Knight 2002; Clarke et al. 2004), Kentucky bluegrass (Poa pratensis L.; He et al. 2005), creeping bentgrass (Agrostis stolonifera L.; Larkindale and Huang 2005) and pea (Pisum sativum L.; Pan et al. 2006). Exogenous SA pre-treatment also improves net photosynthetic rate of leaves of heat-stressed grape (Vitis vinifera L.) plants, apparently by maintaining a higher Rubisco activation state and accelerating the recovery of PSII (Wang et al. 2010). Short-term heat stress caused increases in endogenous SA levels, likely as a result of its de novo synthesis (Pan et al. 2006), in mustard (Dat et al. 1998b), A. thaliana (Kaplan et al. 2004) and pea (Liu et al. 2006) plants during the first 30 min of the stress. The heat-stressed pea leaves also had parallel (with SA) increases in the activities of phenylalanine ammonia lyase (PAL) and benzoic acid 2-hydroxylase (BA2H) (Pan et al. 2006). However, as occurred with grape plants, the initial and substantial increase in endogenous SA following the administration of short-term heat stress diminishes over time. Thus, after 24 h the endogenous SA levels in control and heat-stressed plants were essentially the same (Wang et al. 2004, 2005). Interestingly, it appears that the source of this increased SA in heat-stressed grape stem and leaf tissue is the roots, as progressive increases in labelled SA transported in xylem from the roots to the shoot occurred coincidentally with the time of the heat treatment (Liu et al. 2008). Other support for high temperature induction of SA synthesis comes from studies with A. thaliana mutant lines which have low (NahG) or high (cpr5) endogenous SA levels. Thus, cpr5 plants (high SA) were more tolerant of heat stress, whereas NahG (low SA) were less tolerant, both relative to Col-0 plants (Clarke et al. 2004). Also, unlike Col-0, the NahG plants did not show a short-term spike in endogenous SA levels following the administration of heat stress (Clarke et al. 2004).
Heat stress also causes an accumulation of endogenous abscisic acid (ABA) levels within the first hour in grape (Abass and Rajashekar 1993) and also other plant species (Chandler and Robertson 1994). It was postulated that this increase in endogenous ABA may help plants cope with the excessive transpiration caused by heat stress, e.g., by closing the stomata (Assmann 2010). Also, endogenous ABA levels are reported to increase in plants grown at low temperatures (Assmann 2010). Young grape leaves subjected to short-term heat stress showed rapid increases in endogenous SA and ABA accumulation during the first hour, with a rapid decline over the next 24 h (Wang et al. 2005). Pre-treatment of the grape plants with exogenous SA caused the same increase (in terms of both absolute levels and trend over time) of endogenous ABA (Wang et al. 2005). In a similar manner, heat-stressed young pea leaves showed rapid increases in endogenous SA and ABA (with a peak occurring at about the 25 min mark). Then, levels of both of these hormones declined before the end of the first hour to levels found in unstressed plants (Liu et al. 2006). Pre-treatment of pea leaves with abamine, an ABA-specific biosynthesis inhibitor (Han et al. 2004), gave similar (to control) low baseline endogenous SA and ABA levels in heat-stressed plants, i.e. there was no heat-induced increase in ABA or SA levels (Liu et al. 2006).
Paclobutrazol is a known gibberellin biosynthesis inhibitor (Rademacher 2000) which has also been shown to inhibit the activity of benzoic acid 2-hydroxylase (BA2H), a key enzyme in the final step of SA biosynthesis (Leon et al. 1995). Pre-treatment of pea leaves with Paclobutrazol did not, however, influence the increase in endogenous SA and ABA levels seen when the pea plants were heat-stressed (Liu et al. 2006). Heat-stressed pea plants also showed increased SAG (SA 2-O-β-d-glucose, a major conjugated form of SA) levels, peaking around the 50 min mark of the heat stress treatment. However, SAG levels showed no change in Paclobutrazol-treated heat stressed pea plants (Liu et al. 2006). Finally, A. thaliana mutants with lower endogenous ABA (aba1) (Robertson et al. 1994), or reduced SA (NahG) levels were more susceptible to heat stress than wild type lines (Larkindale et al. 2005).
Numerous studies show that there are optimal concentrations for exogenously applied SA, concentrations which can alleviate the symptoms (growth inhibition and reduced metabolism) of both low temperature and short-term heat stress. Additionally, there is evidence that both low temperature and heat stress can increase endogenous SA levels in several plant species. However, studies with SA biosynthesis mutant lines of A. thaliana indicate that while endogenous SA levels are important for increasing tolerance to short-term heat stress, this same conclusion is not applicable to gaining tolerance to low temperature stress. Rather, SA accumulation was associated with increased susceptibility to low temperature stress. Further, based on studies with ABA and SA biosynthesis inhibitors, it seems that the heat-induced increases in endogenous SA levels are not the result of a direct interaction. Rather, there is an indirect interaction, one where heat stress increases ABA accumulation, which then acts to protect the plant from heat shock. Coincidental with elevated ABA, an accumulation of SA also occurs, cause unknown. A similar scenario also seems likely for the apparent ABA-SA interaction in plants subjected to low temperature stress.
5 Water Stress and Endogenous Salicylic Acid Levels
There is good evidence that exogenously applied SA can improve the growth and productivity of plants subjected to drought stress. For example, pre-treatment of bean (Phaseolus vulgaris L.; Senaratna et al. 2000), chickpea (Cicer arietinum L.; Kumar Patel et al. 2011), rice (Farooq et al. 2010), tomato (Senaratna et al. 2000; Hayat et al. 2008) and wheat (Triticum aestivum L.; Singh and Usha 2003) plants with SA by seed imbibition, soil drenching or foliar spray improved the ability of these plants to tolerate drought stress. These SA-treated plants accumulated increased biomass and exhibited a higher photosynthesis rate relative to drought-stressed plants which did not receive SA pre-treatment. Currently, the decreases in plant growth and photosynthesis that occur in response to drought stress are generally attributed to increases in stress-induced biosynthesis of ABA which then functions to reduce water stress by enhancing stomatal closure (Assmann 2010). However, Rai et al. (1986) demonstrated that subsequent application of SA actually antagonizes the stomatal closure that is induced by applied ABA. While this may be correct when both of ABA and SA are applied exogenously, we would postulate that an antagonism between endogenous ABA and endogenous SA, in the regulation of stomatal closure, is unlikely. This conclusion is supported by studies where application of SA at low (optimal) concentrations increased endogenous ABA levels (Shakirova et al. 2003; Bandurska and Stroinski 2005). Further, as presented below for SA (Munne-Bosch and Penuelas 2003; Abreu and Munne-Bosch 2008; Bechtold et al. 2010; Fig. 1) and from a vast literature on ABA (Assmann 2010), moderate drought stress increases endogenous levels of both ABA and SA. Finally, numerous studies using a range of plant species have observed a closure of stomata in response to the exogenous application of SA (Manthe et al. 1992; Chen et al. 1993; Rao et al. 1997; Shirasu et al. 1997; Mateo et al. 2004).
Munne-Bosch and Penuelas (2003) have worked with field-grown Phillyrea angustifolia (L.), a member of the Oleaceae, which is an evergreen bush or a small tree and native to the Mediterranean region. They reported that drought stress of these plants in their natural habitat resulted in an increased accumulation of endogenous SA in leaves, relative to SA levels seen for well-irrigated plants. However, if drought-stressed P. angustifolia plants were allowed to recover by providing irrigation, their endogenous SA levels quickly decreased to the levels of unstressed plants. Further, they demonstrated a significant linear negative correlation between endogenous SA levels of the leaves and the leaf relative water content. That is, as leaf water content increased the endogenous SA levels (per gram DW) decreased. Thus, a direct link between plant water balance and endogenous SA levels was established. Munne-Bosch and Penuelas (2003) also tested the association of photosynthesis with endogenous SA levels. They took this approach since previous work (Singh and Usha 2003) suggested an increase in photosynthetic activity by drought-stressed plants that had been pre-treated with SA. However, Munne-Bosch and Penuelas (2003) found that the drought-induced increases in endogenous SA of P. angustifolia plants were associated with decreases in the maximum efficiency of PSII (Fv/Fm), though leaf chlorophyll levels were not affected. Also, irrigating the drought-stressed P. angustifolia plants restored the maximum photochemical efficiency of PSII to levels seen for non-stressed, irrigated plants. A later study by Abreu and Munne-Bosch (2008), this time with field-grown plants of common sage (Salvia officinalis L.), demonstrated that drought stress increased endogenous SA levels, though coincidentally decreases were seen for total chlorophyll content. Finally, the same study showed that MeSA applied to the drought-stressed sage plants caused decreases in total chlorophyll content. Thus, while pre-treatment with SA can improve growth and photosynthesis of plants subjected to drought stress, plants that are not treated with SA show increased endogenous SA levels that coincide with a decreased growth and reduced photosynthesis.
In experiments with canola (Brassica napus L.) plants, a one week drought stress given to two-week old seedlings caused a ca. 3-fold increase in endogenous SA levels (Fig. 1). Interestingly, flooding also increased SA levels, up to 2-fold, relative to well-watered control plants (Fig. 1). It should be noted that leaf biomass growth of the canola seedlings was unaffected by the drought stress (though the plants did wilt). In contrast, flooding caused a ca. 2-fold increase in leaf biomass accumulation relative to the well-watered control plants. The fact that both drought and flooding stresses increased endogenous SA concentrations suggest that the SA increases are essentially a general “stress-related” phenomenon. Therefore, SA may not be causally or directly involved in many of the plant’s physiological responses to water stress. Finally, as expected, endogenous ABA levels in these canola leaves increased for drought-stressed seedlings relative to well-watered control. However, there was no increase in endogenous ABA levels in leaves of canola seedlings subjected to flooding relative to well-watered control (data not shown).
Leaf endogenous SA levels (ng gFW-1) in three week-old canola seedlings subjected to “normal” watering conditions (watered bi-daily for all growth period), drought stress (watered bi-daily for the initial two weeks of growth) and flooding (watered bi-daily for the initial two weeks of growth and then moved to a tray with water cover above soil level). The harvested leaf tissue was analyzed on LC-MS using stable isotope dilution technique (see above). The error bars indicate one ±SE of the mean and the mean was calculated from three independent biological experiments
An examination of drought stress responses was accomplished for two A. thaliana genotypes (cpr6-1 and C24) which possess inherently elevated endogenous SA levels (Bechtold et al. 2010). The A. thaliana genotype cpr6-1 is a mutant line with constitutive expression of PR1-6 and both of genotypes cpr6-1 and C24 are known to be highly resistant to biotrophic pathogens (Clarke et al. 1998; Ton et al. 1999). Here, a biotrophic pathogen is defined as a fungus which establishes a long-term feeding relationship with the living cells of a host, rather than killing the host (Zeier et al. 2004). The endogenous SA levels of these two mutant genotypes are ca. 6-fold higher than wild type lines such as Col-0 and Ws-0 lines (Bechtold et al. 2010). Finally, plants of the C24 genotype have the same seed yield as Col-0 and Ws-0. In contrast, seed yield of cpr6-1 plants is ca. 6-fold lower than seed yield of Col-0 and Ws-0 plants. Further, when cpr6-1 and C24 plants were subjected to drought stress, both lines retained their respective seed yield characteristics, whereas the seed yield of drought-stressed Col-0 and Ws-0 plants was appreciably reduced (Bechtold et al. 2010). Therefore, under drought stress conditions, the seed yield of C24 genotype remained high, unlike Col-0 and Ws-0 plants (and cpr6-1 plants), all of which had very low seed yield under drought stress. Thus, cpr6-1 plants fit a “working hypothesis” whereby enhanced drought resistance and enhanced biotrophic pathogen resistance is a result of higher endogenous SA accumulation. In contrast, C24 plants have a “disconnect”, where elevated SA levels ensure high seed yields, as well as higher drought and biotrophic fungal resistance.
6 Carbon Dioxide and Endogenous Salicylic Acid Levels
Based on Intergovernmental Panel on Climate Change (IPCC 2007) predictions, the atmospheric carbon dioxide (CO2) concentration is expected to be double (to ca. 700 ppm) by the end of 21st century. Thus, it is important to understand what impact this change will have on plant growth and survival. At present, the effect of elevating CO2 levels on endogenous SA biosynthesis is poorly understood. In plants of tomato that were grown at each of ambient and elevated CO2 levels, an infection with root rot (Phytophthora parasitica) gave significant increases in endogenous SA levels (Jwa and Walling 2001). However, there was no difference detected in SA levels between infected plants grown at ambient or elevated CO2 levels. Even so, plants grown at elevated CO2 showed an increased degree of tolerance to inoculation with the pathogen (Jwa and Walling 2001). In another system elevated CO2 levels increased the total (but not free) endogenous SA levels in tobacco plants and also led to increased resistance against infection by potato virus Y (Matros et al. 2006). Elevated CO2 levels significantly decreased the endogenous levels of compounds related to SA metabolism, i.e. myo-Inositol and two hydroxybenzoic acids (m-hydroxybenzoate and p-hydroxybenzoate) in leaves of rice (Prins et al. 2011). However, elevating CO2 had no effect on photosynthesis, photorespiration, leaf C/N ratios or anthocyanin contents (Prins et al. 2011). Finally, growing canola plants at elevated levels of CO2 significantly decreased the endogenous SA concentrations, relative to canola plants grown at ambient CO2 levels (Fig. 2). Furthermore, the decrease in SA levels of the high CO2-grown plants was associated with increased photosynthetic efficiency as well as photosynthetic capacity, both relative to plants grown at ambient CO2 levels (Dahal et al. 2012). Elevated ambient CO2 levels also decreased endogenous ABA levels in canola leaves (Fig. 2), though a flush with very high (ca. 50-fold relative to ambient) CO2 levels had no effect on endogenous ABA levels in grape leaves (Loveys et al. 1973). Growth of Chinese red pine (Pinus tabuliformis Carr.) plants for prolonged period under elevated CO2 levels (ca. 2-fold higher than ambient) significantly decreased endogenous ABA levels in needle tissue (Li et al. 2011). Although decreases in endogenous ABA of plants growing at elevated CO2 may be beneficial for growth, the decrease in endogenous SA levels could potentially make plants more susceptible to pathogen attack.
Leaf endogenous SA and ABA levels in three week-old canola seedlings grown at ambient (340 ppm) or elevated (700 ppm) CO2 levels from the germination stage. The endogenous SA levels are expressed per fresh weights (ng gFW-1), dry weights (ng gDW-1), leaf area (µg gFW-1) and total chlorophyll content (ng mg-1). The harvested leaf tissue was analyzed on LC-MS using stable isotope dilution technique (see above). The error bars indicate one ±SE of the mean and the mean was calculated from three independent biological experiments
7 Conclusion
To conclude, fluctuations in environmental signals such as light, temperature, water availability and carbon dioxide concentrations can all influence endogenous SA levels (Table 1)
. However, it does not seem likely, based on the literature we have reviewed, that environmentally induced changes in plant SA content are a direct (per se) effect of most environmental fluctuations. Nor does it seem likely that the observed changes in endogenous SA levels play a direct role in adjusting plant growth in response to fluctuating environmental signals. Rather, as is evident from application studies with exogenous SA, it appears more likely that changes in endogenous SA levels result from cross-talk with other endogenous plant hormones. Thus, it will be changes in concentration of the other hormones (not SA) that are responsible for adjusting the plant’s growth in response to the fluctuating environmental signal. For example, another class of plant hormones, brassinosteroids, when applied exogenously, can alleviate many plant abiotic stress responses (Khripach et al. 1999; Kang and Guo 2011). However, this stress alleviation is likely a result of the brassinosteroid influencing endogenous levels of other plant hormones, which then play a direct role in the alleviation of symptoms of plant stress (Kurepin et al. 2008, 2012b).
References
Abass, M., & Rajashekar, C. B. (1993). Abscisic acid accumulation in leaves and cultured cells during heat acclimation in grapes. HortScience, 28, 50–52.
Abreu, M. E., & Munne-Bosch, S. (2008). Salicylic acid may be involved in the regulation of drought-induced leaf senescence in perennials: A case study in field-grown Salvia officinalis L. plants. Environmental and Experimental Botany, 64, 105–112.
Assmann, S. M. (2010). Abscisic acid signal transduction in stomatal responses. In P. J. Davies (Ed.), Plant hormones: Biosynthesis, signal transduction and action! (3rd ed., pp. 399–426). Dordrecht, Heidelberg: Springer.
Bandurska, H., & Stroinski, A. (2005). The effect of salicylic acid on barley response to water deficit. Acta Physiologiae Plantarum, 27, 379–386.
Bechtold, U., Lawson, T., Mejia-Carranza, J., Meyer, R. C., Brown, I. R., Altmann, T., et al. (2010). Constitutive salicylic acid defences do not compromise seed yield, drought tolerance and water productivity in the Arabidopsis accession C24. Plant, Cell and Environment, 33, 1959–1973.
Bowling, S. A., Guo, A., Cao, H., Gordon, A. S., Klessig, D. F., & Dong, X. (1994). A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell, 6, 1845–1857.
Bowling, S. A., Clarke, J. D., Liu, Y., Klessig, D. F., & Dong, X. (1997). The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell, 9, 1573–1584.
Catinot, J., Buchala, A., Abou-Mansour, E., & Metraux, J.-P. (2008). Salicylic acid production in response to biotic and abiotic stress depends on isochorismate in Nicotiana benthamiana. FEBS Letters, 582, 473–478.
Chandler, P. M., & Robertson, M. (1994). Gene expression regulated by abscisic acid and its relation to stress tolerance. Annual Review of Plant Physiology and Plant Molecular Biology, 45, 113–141.
Chen, Z., Silva, H., & Klessig, D. F. (1993). Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science, 262, 1883–1886.
Chen, Z. Q., Chen, W. L., & Yang, C. W. (2009). Effects of exogenous salicylic acid on photosynthesis in Arabidopsis leaves based on fluorescence spectra and delayed fluorescence technique. Spectroscopy and Spectral Analysis, 29, 2208–2212.
Clarke, J. D., Liu, Y., Klessig, D. F., & Dong, X. (1998). Uncoupling PR-gene expression from NPR1 and bacterial resistance: characterization of the dominant Arabidopsis cpr6 mutant. Plant Cell, 10, 557–567.
Clarke, S. M., Mur, L. A. J., Wood, J. E., & Scott, I. M. (2004). Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant Journal, 38, 432–447.
Dahal, K., Kane, K., Gadapati, W., Webb, E., Savitch, L. V., Singh, J., et al. (2012). The effects of phenotypic plasticity on photosynthetic performance in winter Rye, winter wheat and Brassica napus. Physiologia Plantarum, 144, 169–188.
Dat, J. F., Lopez-Delgado, H., Foyer, C. H., & Scott, I. M. (1998a). Parallel change in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiology, 116, 1351–1357.
Dat, J. F., Foyer, C. H., & Scott, I. M. (1998b). Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiology, 118, 1455–1461.
Dat, J. F., Lopez-Delgado, H., Foyer, C. H., & Scott, I. M. (2000). Effects of salicylic acid on oxidative stress and thermotolerance in tobacco. Journal of Plant Physiology, 156, 659–665.
Delaney, T. P. (2010). Salicylic acid. In P. J. Davies (Ed.), Plant hormones: Biosynthesis, signal transduction and action! (3rd ed., pp. 681–699). Dordrecht, Heidelberg: Springer.
Ding, C. K., Wang, C. Y., Gross, K. C., & Smith, D. L. (2002). Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta, 214, 895–901.
Emery, R. J. N., Chinnappa, C. C., & Chmielewski, J. G. (1994). Specialization, plant strategies, and phenotypic plasticity in populations of Stellaria longipes along an elevational gradient. International Journal of Plant Sciences, 155, 203–219.
Ervin, E. H., Zhang, X. Z., & Fike, J. H. (2004). Ultraviolet-B radiation damage on Kentucky Bluegrass II: Hormone supplement effects. HortScience, 39, 1471–1474.
Fariduddin, Q., Hayat, S., & Ahmad, A. (2003). Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity and seed yield in Brassica juncea. Photosynthetica, 41, 281–284.
Farooq, M., Wahid, A., Lee, D.-J., Cheema, S. A., & Aziz, T. (2010). Comparative time course action of the foliar applied glycinebetaine, salicylic acid, nitrous oxide, brassinosteroids and spermine in improving drought resistance of rice. Journal of Agricultural Crop Science, 196, 336–345.
Farooq, M., Aziz, T., Basra, S. M. A., Cheema, M. A., & Rehman, H. (2008). Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. Journal of Agricultural Crop Science, 194, 161–168.
Gaskin, P., & MacMillan, J. (1991). GC-MS of the gibberellins and related compounds. Methodology and a library of spectra. Bristol: University of Bristol (Cantock’s Enterprises).
Genoud, T., Buchala, A.J., Chua, N.H., & Metraux, J.P. (2002). Phytochrome signalling modulates the SA-perceptive pathway in Arabidopsis. Plant Journal, 31, 87–95.
Ghai, N., Setia, R. C., & Setia, N. (2002). Effects of paclobutrazol and salicylic acid on chlorophyll content, hill activity and yield components in Brassica napus L. (cv. GSL-1). Phytomorph, 52, 83–87.
Han, S. Y., Kitahata, N., Sekimata, K., Saito, T., Kobayashi, M., Nakashima, K., et al. (2004). A novel inhibitor of 9-cis-epoxycarotenoid dioxygenase in abscisic acid biosynthesis in higher plants. Plant Physiology, 135, 1574–1582.
Handro, W., Mello, C. M., Manzano, M. A., & Floh, E. I. S. (1997). Enhancement of stem elongation and flower bud regeneration by salicylic acid. Revista Brasileira de Fisiologia Vegetal, 9, 139–142.
Harper, J. R., & Balke, N. E. (1981). Characterization of the inhibition of K+ absorbtion in oats roots by salicylic acid. Plant Physiology, 68, 1349–1353.
Hayat, S., Fariduddin, Q., Ali, B., & Ahmad, A. (2005). Effect of salicylic acid on growth and enzyme activities of wheat seedlings. Acta Agronomica Hungarica, 53, 433–437.
Hayat, S., Hasan, S. A., Fariduddin, Q., & Ahmad, A. (2008). Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. Journal of Plant International, 3, 297–304.
Hayat, Q., Hayat, S., Irfan, M., & Ahmad, A. (2010). Effect of exogenous salicylic acid under changing environment: A review. Environmental and Experimental Botany, 68, 14–25.
He, Y., Liu, Y., Cao, W., Huai, M., Xu, B., & Huang, B. (2005). Effects of salicylic acid on heat tolerance associated with antioxidant metabolism in Kentucky bluegrass. Crop Science, 45, 988–995.
Hegazi, A. M., & El-Shraiy, A. M. (2007). Impact of salicylic acid and paclobutrazol exogenous application on the growth, yield and nodule formation of common bean. Australian Journal of Basic and Applied Science, 1, 834–840.
IPCC. (2007). Climate change 2007: The physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press.
Jacobsen, J. V., Pearce, D. W., Poole, A. T., Pharis, R. P., & Mander, L. N. (2002). Abscisic acid, phaseic acid and gibberellin contents associated with dormancy and germination in barley. Physiologia Plantarum, 115, 428–441.
Janda, T., Szalai, G., Tari, I., & Paldi, E. (1999). Hydroponic treatment with salicylic acid decreases the effect of chilling injury in maize (Zea mays L.) plants. Planta, 208, 175–180.
Janda, T., Szalai, G., Antunovics, Z. S., Horvath, E., & Paldi, E. (2000). Effect of benzoic acid and aspirin on chilling tolerance and photosynthesis in young maize plants. Maydica, 45, 29–33.
Jenkins, G. I. (2009). Signal transduction in responses to UV-B radiation. Annual Review of Plant Biology, 60, 407–431.
Jwa, N. S., & Walling, L. L. (2001). Influence of elevated CO2 concentration on disease development in tomato. New Phytologist, 149, 509–518.
Kang, H. M., & Saltveit, M. E. (2002). Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiologia Plantarum, 115, 571–576.
Kang, G. Z., Wang, C. H., Sun, G. C., & Wang, Z. X. (2003). Salicylic acid changes activities of H2O2-metabolizing enzymes and increases the chilling tolerance of banana seedlings. Environmental and Experimental Botany, 50, 9–15.
Kang, Y. Y., & Guo, S.R. (2011). Role of Brassinosteroids on horticultural crops. In S. Hayat & A. Ahmad (Eds.), Brassinosteroids: A class of plant hormones (pp. 269–288). Springer Science and Business Media B.V. http://springerlink.bibliotecabuap.elogim.com/book/10.1007/978-94-007- 0189-2/page/1
Kaplan, F., Kopka, J., Haskell, D. W., Zhao, W., Schiller, K. C., Gatzke, N., et al. (2004). Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiology, 136, 4159–4168.
Khripach, V. A., Zhabinskii, V. N., & de Groot, A. E. (1999). Brassinosteroids—a new class of plant hormones (pp. 325–346). San Diego: Academic Press.
Kitajima, K., & Augspurger, C.K. (1989). Seed and seedling ecology of a monocarpic tropical tree, Tachigalia versicolor. Ecology, 70, 1102–1114.
Kumar Patel, P., Hemantaranjan, A., Sarma, B. K., & Singh, R. (2011). Growth and antioxidant system under drought stress in Chickpea (Cicer arietinum L.) as sustained by salicylic acid. Journal of Stress Physiology Biochemistry, 7, 130–144.
Kurepin, L. V., Emery, R. J. N., Pharis, R. P., & Reid, D. M. (2007a). Uncoupling light quality from light irradiance effects in Helianthus annuus shoots: putative roles for gibberellins, auxin, cytokinins, abscisic acid and ethylene in leaf and internode growth. Journal of Experimental Botany, 58, 2145–2157.
Kurepin, L. V., Emery, R. J. N., Pharis, R. P., & Reid, D. M. (2007b). The interaction of light quality and irradiance with gibberellins, cytokinins and auxin in regulating growth of Helianthus annuus hypocotyls. Plant, Cell and Environment, 30, 147–155.
Kurepin, L. V., Walton, L. J., & Reid, D. M. (2007c). Interaction of red to far red light ratio and ethylene in regulating stem elongation of Helianthus annuus. Plant Growth Regulation, 51, 53–61.
Kurepin, L. V., Qaderi, M. M., Back, T. G., Reid, D. M., & Pharis, R. P. (2008). A rapid effect of applied brassinolide on abscisic acid levels in Brassica napus leaf tissue subjected to short-term heat stress. Plant Growth Regulation, 55, 165–167.
Kurepin, L. V., Walton, L. J., Reid, D. M., & Chinnappa, C. C. (2010a). Light regulation of endogenous salicylic acid levels in hypocotyls of Helianthus annuus seedlings. Botany, 88, 668–674.
Kurepin, L. V., Walton, L. J., Yeung, E. C., Chinnappa, C. C., & Reid, D. M. (2010b). The interaction of light irradiance with ethylene in regulating growth of Helianthus annuus shoot tissues. Plant Growth Regulation, 62, 43–50.
Kurepin, L. V., Walton, L. J., Pharis, R. P., Emery, R. J. N., & Reid, D. M. (2011a). Interactions of temperature and light quality on phytohormone-mediated elongation of Helianthus annuus hypocotyls. Plant Growth Regulation, 64, 147–154.
Kurepin, L. V., Walton, L. J., Yeung, E. C., & Reid, D. M. (2011b). The interaction of light irradiance with auxin in regulating growth of Helianthus annuus shoots. Plant Growth Regulation, 65, 255–262.
Kurepin, L. V., Walton, L. J., Hayward, A., Emery, R. J. N., Reid, D. M., & Chinnappa, C. C. (2012a). Shade light interaction with salicylic acid in regulating growth of sun (alpine) and shade (prairie) ecotypes of Stellaria longipes. Plant Growth Regulation, 68, 1–8.
Kurepin, L. V., Hwan-Joo, S., Kim, S.-K., Pharis, R. P., & Back, T. G. (2012b). Interaction of brassinosteroids with light quality and plant hormones in regulating shoot growth of young sunflower and Arabidopsis seedlings. Journal of Plant Growth Regulation, 31, 156–164.
Kurepin, L. V., Walton, L. J., Hayward, A., Emery, R. J. N., Pharis, R. P., & Reid, D. M. (2012c). Interactions between plant hormones and light quality signaling in regulating the shoot growth of Arabidopsis thaliana seedlings. Botany, 90, 237–246.
Larkindale, J., & Knight, M. R. (2002). Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiology, 128, 682–695.
Larkindale, J., & Huang, B. R. (2005). Effects of abscisic acid, salicylic acid, ethylene and hydrogen peroxide in thermotolerance and recovery for creeping bentgrass. Plant Growth Regulation, 47, 17–28.
Larkindale, J., Hall, J. D., Knight, M. R., & Vierling, E. (2005). Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiology, 138, 882–897.
Larque-Saavedra, A. (1979). Stomatal closure in response to acetylsalicylic acid treatments. Zeitschrift fur Physiologische, 93, 371–375.
Lei, T., Feng, H., Sun, X., Dai, Q.-L., Zhang, F., Liang, H.-G., et al. (2010). The alternative pathway in cucumber seedlings under low temperature stress was enhanced by salicylic acid. Plant Growth Regulation, 60, 35–42.
Leon, J., Shulaev, V., Yalpani, N., Lawton, M. A., & Raskin, I. (1995). Benzoic acid 2-hydroxylase, a soluble oxygenase from tobacco, catalyzes salicylic acid biosynthesis. Proceedings of National Academy of Science USA, 92, 10413–10417.
Li, X. M., Zhang, L. H., Ma, L. J., & Li, Y. Y. (2011). Elevated carbon dioxide and/or ozone concentrations induce hormonal changes in Pinus tabulaeformis. Journal of Chemical Ecology, 37, 779–784.
Liu, H. T., Liu, Y. Y., Pan, Q. H., Yang, H. R., Zhan, J. C., & Huang, W. D. (2006). Novel interrelationship between salicylic acid, abscisic acid, and PIP2-specific phospholipase C in heat acclimation-induced thermotolerance in pea leaves. Journal of Experimental Botany, 57, 3337–3347.
Liu, H. T., Liu, Y. P., & Huang, W. D. (2008). Root-fed salicylic acid in grape involves the response caused by aboveground high temperature. Journal of Integrative Plant Biology, 50, 761–767.
Loveys, B. R., Kriedemann, P. E., & Torokfalvy, E. (1973). Is abscisic acid involved in stomatal response to carbon dioxide? Plant Science Letters, 1, 335–338.
Manfre, A., Glenn, M., Nunez, A., Moreau, A. R., & Dardick, C. (2011). Light quantity and photosystem function mediate host susceptibility to Turnip mosaic virus via a salicylic acid–independent mechanism. Molecular Plant-Microbe Interactions, 24, 315–327.
Manthe, B., Schulz, M., & Schnable, H. (1992). Effects of salicylic acid on growth and stomatal movements on Vicia faba L: evidence for salicylic acid metabolism. Journal of Chemical Ecology, 18, 1525–1539.
Mateo, A., Muhlenbock, P., Rusterucci, C., Chang, C. C., Miszalski, Z., Karpinska, B., et al. (2004). LESION SIMULATING DISEASE 1 is required for acclimation to conditions that promote excess excitation energy. Plant Physiology, 136, 2818–2830.
Mateo, A., Funck, D., Muhlenbock, P., Kular, B., Mullineaux, P. M., & Karpinski, S. (2006). Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. Journal of Experimental Botany, 57, 1795–1807.
Matros, A., Amme, S., Kettig, B., Buck-Sorlin, G. H., Sonnewald, U., & Mock, H. P. (2006). Growth at elevated CO2 concentrations leads to modified profiles of secondary metabolites in tobacco cv. SamsunNN and to increased resistance against infection with potato virus Y. Plant Cell Environmental, 29, 126–137.
Mora-Herrera, M. E., Lopez-Delgado, H., Castillo-Morales, A., & Foyer, C. H. (2005). Salicylic acid and H2O2 function by independent pathways in the induction of freezing tolerance in potato. Physiologia Plantarum, 125, 430–440.
Munne-Bosch, S., & Penuelas, J. (2003). Photo- and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta, 217, 758–766.
Nawrath, C., & Metraux, J. P. (1999). Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell, 11, 1393–1404.
O’Hanlon-Manners, D.L., & Kotanen, P.M. (2004). Evidence that fungal pathogens inhibit recruitment of a shade-intolerant tree, White Birch (Betula papyfifera), in understory habitats. Oecologia, 140, 650–653.
Pan, Q., Zhan, J., Liu, H., Zhang, J., Chen, J., Wen, P., et al. (2006). Salicylic acid synthesized by benzoic acid 2-hydroxylase participates in the development of thermotolerance in pea plants. Plant Science, 171, 226–233.
Pancheva, T. V., Popova, L. P., & Uzunova, A. N. (1996). Effects of salicylic acid on growth and photosynthesis in barley plants. Journal of Plant Physiology, 149, 57–63.
Pancheva, T. V., & Popova, L. P. (1998). Effect of salicylic acid on the synthesis of ribulose-1,5-bisphosphate carboxylase/oxygenase in barley leaves. Plant Physiology, 152, 381–386.
Prins, A., Mukubi, J. M., Pellny, T. K., Verrier, P. J., Beyene, G., Lopes, M. S., et al. (2011). Acclimation to high CO2 in maize is related to water status and dependent on leaf rank. Plant Cell Environmental, 34, 314–331.
Rademacher, W. (2000). Growth retardants: Effects on gibberellin biosynthesis and other metabolic pathways. Annual Review Plant Physiology Plant Molecular Biology, 51, 501–531.
Rai, V. K., Sharma, S. S., & Sharma, S. (1986). Reversal of ABA-induced stomatal closure by phenolic compounds. Journal of Experimental Botany, 37, 129–134.
Rao, M. V., Paliyath, G., Ormrod, D. P., Murr, D. P., & Watkins, C. B. (1997). Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Salicylic acid-mediated oxidative damage requires H2O2. Plant Physiology, 115, 137–149.
Raskin, I. (1992). Role of salicylic acid in plants. Annual Review Plant Physiology Plant Molecular Biology, 43, 439–463.
Raskin, I. (1995). Salicylic acid. In P. J. Davies (Ed.), Plant hormones, physiology, biochemistry and molecular biology (pp. 188–205). Dordrecht: Kluwer Academic Publishers.
Robertson, A. J., Ishikawa, M., Gusta, L. V., & MacKenzie, S. L. (1994). Abscisic acid-induced heat tolerance in Bromus inermis Leyss cell-suspension cultures. Heat-stable, abscisic acid-responsive polypeptides in combination with sucrose confer enhanced thermostability. Plant Physiology, 105, 181–190.
Schettel, N. L., & Balke, N. E. (1983). Plant growth response to several allelopathic chemicals. Weed Science, 31, 293–298.
Scott, I. M., Clarke, S. M., Wood, J. E., & Mur, L. A. J. (2004). Salicylate accumulation inhibits growth at chilling temperature in Arabidopsis. Plant Physiology, 135, 1040–1049.
Senaratna, T., Touvhell, D., Bunn, E., & Dixon, K. (2000). Acetyl salicylic acid (aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plant. Plant Growth Regulation, 30, 157–161.
Shakirova, F. M., Sakhabutdinova, A. R., Bezrukova, M. V., Fatkhutdinova, R. A., & Fatkhutdinova, D. R. (2003). Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Science, 164, 317–322.
Shirasu, K., Nakajima, H., Rajasekhar, V. K., Dixon, R. A., & Lamb, C. (1997). Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell, 9, 261–270.
Singh, B., & Usha, K. (2003). Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regulation, 39, 137–141.
Smith, H. (2000). Phytochromes and light signal perception by plants—an emerging synthesis. Nature, 407, 585–591.
Ton, J., Pieterse, C. M. J., & Van Loon, L. C. (1999). Identification of a locus in Arabidopsis controlling both the expression of rhizobacteria-mediated induced systemic resistance (ISR) and basal resistance against Pseudomonas syringae pv. tomato. Molecular Plant–Microbe Interaction, 12, 911–918.
Uzunova, A. N., & Popova, L. P. (2000). Effect of salicylic acid on leaf anatomy and chloroplast ultrastructure of barley plants. Photosynthetica, 38, 243–250.
Wang, L. J., Huang, W. D., & Zhan, J. C. (2004). The response of 14C-salicylic acid to heat stress in young plants of Vitis vinifera. Russian Journal of Plant Physiology, 51, 194–197.
Wang, L. J., Huang, W. D., Liu, Y. P., & Zhan, J. C. (2005). Changes in salicylic and abscisic acid contents during heat treatment and their effect on thermotolerance of grape plants. Russian Journal of Plant Physiology, 52, 516–520.
Wang, L., Chen, S., Kong, W., Li, S., & Archbold, D. D. (2006). Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peaches during cold storage. Postharvest Biology and Technology, 41, 244–251.
Wang, L.-J., Fan, L., Loescher, W., Duan, W., Liu, G.-J., Cheng, J.-S., et al. (2010). Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biology, 10, 34–43.
Yalpani, N., Enyedi, A. J., Leon, J., & Raskin, I. (1994). Ultraviolet light and ozone stimulate accumulation of salicylic acid, pathogenesis-related proteins and virus resistance in tobacco. Planta, 193, 372–376.
Zeier, J., Pink, B., Mueller, M. J., & Berger, S. (2004). Light conditions influence specific defence responses in incompatible plant–pathogen interactions: uncoupling systemic resistance from salicylic acid and PR-1 accumulation. Planta, 219, 673–683.
Acknowledgments
This work was sponsored by Ballance Agri-Nutrients, Ltd., New Zealand.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Kurepin, L.V., Dahal, K.P., Zaman, M., Pharis, R.P. (2013). Interplay Between Environmental Signals and Endogenous Salicylic Acid Concentration. In: Hayat, S., Ahmad, A., Alyemeni, M. (eds) SALICYLIC ACID. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6428-6_5
Download citation
DOI: https://doi.org/10.1007/978-94-007-6428-6_5
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6427-9
Online ISBN: 978-94-007-6428-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)