Abstract
Shade light found in ecological niches where plants are growing under a canopy or in proximity of taller neighbouring vegetation consist mainly of two separate light signals: low red to far-red ratio and low photosynthetically active radiation (PAR). The effect of the latter on the growth of 7-day old sunflower shoots was examined by assessing hypocotyl, cotyledon and leaf tissue growth under three varying PAR levels: near-normal of 1,000 μmol m−2 s−1, low of 100 μmol m−2 s−1 and very low of 10 μmol m−2 s−1. Then, the possible interaction between PAR signaling and ethylene in regulating growth of these sunflower tissues was investigated. The results showed that gradual decrease in PAR level increases hypocotyl elongation and decreases ethylene evolution. However, gradual decrease in PAR level decreases cotyledon and leaf growth and increases ethylene evolution. Thus it seems possible that PAR regulation of shoot growth is mediated by changes in ethylene evolution in tissue specific manner. This hypothesis was supported by experiments with the ethylene releasing factor, ethephon, and the ethylene biosynthesis inhibitor, AVG, as well as by transfer experiments where sunflower seedlings were transferred from one PAR regime to another with subsequent growth and ethylene measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants growing under canopy shade or in the shade of neighbouring vegetation often have etiolated stems and frequently exhibit reduced leaf area gowth (Ballare 1999; Smith 2000; Franklin and Whitelam 2005; Vandenbussche et al. 2005). These phenotypic traits are regulated by shade light, i.e. lower red to far red (R/FR) ratio and reduced photosynthetically active radiation (PAR), the key components of vegetative shade (Ballare et al. 1990; Ballare 1999; Smith 2000), and by several classes of plant hormones (Franklin and Whitelam 2005; Vandenbussche et al. 2005).
Ethylene has been shown to regulate both stem elongation and leaf expansion, often acting as an inhibition factor (Abeles et al. 1992). However, in a few cases a growth-stimulatory role for endogenous ethylene has been reported (Raskin and Kende 1984; Jackson 1985; Rijnders et al. 1997). These apparently contradictory results can be explained by reports showing that ethylene, like auxin, demonstrates a biphasic dose–response curve. Lower doses producing stimulation of various growth and developmental events, but higher doses producing the inhibitory responses, see Zobel and Roberts (1978), Reid et al. (1985), Lee and Reid (1997).
Interaction between ethylene and phytochrome, the R and FR light plant photoreceptor, has been extensively investigated in the number of plant species (Goeschl et al. 1967; Imaseki et al. 1971; Finlayson et al. 1998; 1999; Pierik et al. 2003). In etiolated pea (Pisum sativum) seedlings the short-term exposure to broad-band R and FR light decreased ethylene evolution and increased plumular growth, with R light inducing double the plumular growth with half the ethylene content observed under FR light (Goeschl et al. 1967). In excised apical segments of etiolated rice (Oriza sativa), coleoptiles produced more ethylene when exposed to short-term FR light and less ethylene when exposed to short-term R light (Imaseki et al. 1971). Further, FR light reversed the inhibition of ethylene evolution observed in rice coleoptiles grown under R light (Imaseki et al. 1971). Increased ethylene under low R/FR ratio has been reported for sorghum (Sorghum bicolor) plants containing a null mutation in the gene encoding phytochrome B (phyB-1; Finlayson et al. 1998). These plants exhibited a constitutive phenotype similar to plants grown in shade and had higher ethylene levels under dim, FR enriched light (Finlayson et al. 1998, 1999). In a different study using mature tobacco (Nicotiana tabacum) plants, application of ethylene in low concentration resulted in increased stem elongation under FR enriched light (Pierik et al. 2003). Ethylene-insensitive transgenic tobacco plants failed to elongate to the same extent as wild type plants in response to a low R/FR ratio (Pierik et al. 2003). Finally, low PAR was shown to increase ethylene evolution in A. thaliana rosette-stage plants (Vandenbussche et al. 2003).
In this study, the interaction of PAR level with ethylene in regulating early stages of shoot growth in young sunflower (Helianthus annuus L.) seedlings was investigated to better understand the role of ethylene in growth and development under low PAR conditions which is usually associated with shade.
Materials and methods
Plants and experimental system
Sunflower seeds (6946, Pioneer Seeds, USA) were germinated in a soil mix (2 parts of peat moss, 1—Perlite, 1—Vermiculate and 0.25—Terragreen [a crushed baked clay medium] from Professional Gardener, Calgary, Alberta, Canada). Plants were grown in growth chambers (Conviron, Manitoba) equipped with fluorescent (Sylvania cool white 160 W) and incandescent lights (Philips 60 W). Temperature was maintained at 20°C during the 16 h light period and at 16°C during the 8 h dark period. Sunflower seedlings were watered each day with 25% strength Hoagland’s solution (Hoagland and Arnon 1950). Changing the distance between the light sources and the pot soil level, as well as the use of commercial shade cloth was used to alter PAR. The values of PAR levels were measured with a LI-COR LI-1800/22 quantum sensor (LI-COR, Inc., Lincoln, Nebraska, USA). The Tukey’s ANOVA tests for analysis of significance (at P ≤ 0.05) were run on SPSS software version 15. Each experiment was repeated at least three times.
Measurement of ethylene evolution
Ethylene evolution by various sunflower tissues was measured by incubating the tissue in a 3 mL syringe (1.5 mL volume) for 15 min. A 1 mL gas sample was collected and injected into a Photovac 10Splus GC (Photovac Inc., Markham, Ontario) with a photoionization detector and a 40/60 Carbopack B column (Supelco Canada, Oakville, Ontario).
Chemicals
The ethylene biosynthesis inhibitor, aminoethoxyvinylglycine (AVG, from Sigma Inc., Oakville, Ontario, Canada) was applied at concentrations of 10−3, 10−4 and 10−5 M. The ethylene-releasing chemical, ethephon (2-chloroethylphosphonic acid, from Sigma Inc., Oakville, Ontario, Canada) was prepared on the day of use at concentrations of 10−3, 10−4 and 10−5 M. Sunflower seedlings were sprayed to drip-off on days 4, 5 and 6 after planting and measured on day 7.
Results and discussion
Growth and ethylene evolution of 7-day old sunflower seedlings grown under varying PARs
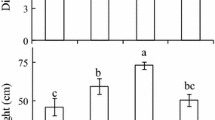
Decrease in PAR levels from near-normal PAR of 1,000 μmol m−2 s−1 to low PAR of 100 μmol m−2 s−1 and very low PAR of 10 μmol m−2 s−1 resulted in significant increases in hypocotyl lengths from 25 (±0.99) to 77.93 (±1.19) mm and to 114.5 (±1.68) mm, respectively (see also Fig. 1a for visual comparisons). Increase in stem elongation as PAR level decreases have previously been reported (Ballare 1999; Smith 2000; Franklin and Whitelam 2005; Vandenbussche et al. 2005). Similar significant results were obtained for fresh (Fig. 2a) and dry (data not shown) hypocotyl weights. These gradual changes in hypocotyl lengths and biomass accumulation were significantly and negatively correlated with hypocotyl ethylene evolution, i.e. shorter and smaller hypocotyls obtained under near-normal PAR had the highest level of ethylene evolution compared to other PAR treatments, whereas longer and larger hypocotyls under very low PAR had the lowest level of ethylene evolution, as shown in Fig. 2a and b. Cotyledon and leaf tissues exhibited opposite trends in biomass accumulation and ethylene evolution when compared to hypocotyl tissues of 7-day old sunflower seedlings (Fig. 2). Sunflower cotyledons and leaves had significantly higher fresh (Fig. 2a) and dry (data not shown) weights under near-normal PAR than under low or very low PAR. Ethylene evolution from cotyledon and leaf tissues, however, was significantly higher under very low PAR than under low PAR, and significantly higher under low PAR than under near-normal PAR (Fig. 2b).
The 7-days old sunflower seedlings (a) grown under normal PAR of 1,000 μmol m−2 s−1 (three plants on left), low PAR of 100 μmol m−2 s−1 (three plants in center) and very low PAR of 10 μmol m−2 s−1 (three plants on right) from germination. Cotyledons and leaves (b) of 7-days old sunflower seedlings grown under normal PAR of 1,000 μmol m−2 s−1 (top) and low PAR of 100 μmol m−2 s−1 (bottom). Cotyledons and leaves (c) of 7-days old sunflower seedlings grown under very low PAR of 10 μmol m−2 s−1. Scale: the distance between each two lines on the scale equals to 1 mm and numbers on scales are in cm
Tissue fresh weight (a, mg) and ethylene levels (b, pmol gFW−1 h−1) of 7-days old sunflower seedlings grown under normal (1,000 μmol m−2 s−1), low (100 μmol m−2 s−1) and very low (10 μmol m−2 s−1) PAR irradiances. The error bars represent one SE of mean. The mean values with the same letter are not significantly different based on Tukey’s ANOVA test at P ≤ 0.05. Separate ANOVA tests were performed for each type of the tissue under varying PAR irradiances
Low PAR has been shown to increase ethylene evolution in young A. thaliana rosette-stage plants (Vandenbussche et al. 2003). In sunflower seedlings, reduction in PAR level was positively correlated with cotyledon and leaf ethylene evolution. Because young Arabidopsis seedlings consist mostly of cotyledonary and leaf tissues, these sunflower results fit well with the previously reported data (Vandenbussche et al. 2003). However, for sunflower hypocotyls, reduction in PAR level was significantly and positively correlated with growth, but significantly and negatively correlated with ethylene evolution. Previously, reduction in R/FR ratio was shown to be significantly and positively correlated with growth, but significantly and negatively correlated with ethylene evolution for the same 7-day old sunflower hypocotyls (Kurepin et al. 2007a). Thus, it appears that both components of shade light, low R/FR ratio and low PAR not only have similar impact of hypocotyl (and stem) elongation, but also are both capable of significantly decreasing ethylene evolution.
Growth response of 7-day old sunflower seedlings to ethylene-releasing agent ethephon and biosynthesis inhibitor under varying PARs
Application of ethephon at concentration of 10−3 M significantly inhibited hypocotyl elongation under very low PAR (ca 90%) and under low PAR (ca 30%), whereas application of ethephon had no effect on hypocotyl elongation under near-normal PAR (Fig. 3a), all relative to the control plants. Applied ethephon also significantly reduced hypocotyl fresh weights under low and very low PAR, but not under near-normal PAR (Fig. 3b). Hypocotyl dry weights were not significantly affected by applied ethephon at concentration of 10−3 M under either PAR level (Fig. 3c). Inhibition of hypocotyl growth by applied ethephon for plants grown under low and very low PAR, but not for plants grown under near-normal PAR (Figs. 3a–c) correlates well with the growth and ethylene evolution data (Fig. 2) and further implies an inhibitory role for ethylene in PAR-mediated hypocotyl growth of sunflower seedlings. This role for ethylene is additionally supported by the AVG data. Application of AVG at concentration of 10−3 M significantly increased hypocotyl elongation (Fig. 3a) and hypocotyl fresh (Fig. 3b) and dry (Fig. 3c) weight accumulation under near-normal PAR, all relative to the control plants. Applied AVG had no effect on hypocotyl elongation or hypocotyl biomass accumulation under low or very low PARs (Fig. 3a–c).
Hypocotyl lengths (a, mm), fresh (b, mg) and dry weights (c, mg), cotyledons +leaves fresh (d, mg) and dry weights (e, mg) of 7-days old sunflower seedlings treated with either ethephon or AVG at concentration of 10−3 M and grown under normal (1,000 μmol m−2 s−1), low (100 μmol m−2 s−1) and very low (10 μmol m−2 s−1) PAR irradiances. The error bars represent one SE of mean. The mean values with the same letter are not significantly different based on Tukey’s ANOVA test at P ≤ 0.05. Separate ANOVA tests were performed for each PAR irradiance level
The effect of applied ethephon at concentration of 10−3 M on leaf and cotyledon fresh and dry weights under varying PARs was different in direction from hypocotyl tissue responses. Lee and Reid (1997) were the first to report the involvement of ethylene in regulation of leaf growth in sunflower plants, where lower doses of ethylene increased leaf expansion, whereas higher doses inhibited it. Here, applied ethephon significantly reduced both fresh and dry weights of leaf and cotyledon tissues under low and near-normal PAR, but not under very low PAR (Fig. 3d, e). Thus, again, cotyledon and leaf tissues which had the lowest ethylene evolution (i.e. grown under near-normal and low PAR) responded to applied ethephon with a decrease in growth, whereas cotyledon and leaf tissues which had the highest ethylene evolution (i.e. grown under very low PAR) showed no significant response to applied ethephon. Therefore suggesting that the growth-inhibitory effect of ethylene on cotyledon and leaf growth under very low PAR was already saturated by the light signal and the concentration of ethephon which was potent in inhibiting growth under two other PARs was no longer high enough to have its growth-inhibitory impact.
Application of ethephon at other concentrations either had no effect on shoot growth (10−5 M) or had a similar (10−4 M), but much lower effect then 10−3 M (data not shown). Application of AVG at concentration of 10−3 M to cotyledon and leaf tissues yielded no significant changes in their biomass accumulation (Fig. 3d, e). Application of AVG at concentrations of 10−4 and 10−5 M had no effect on either parameter of shoot growth (i.e. hypocotyl elongation, tissue expansion, dry and fresh biomass accumulation) under all PAR levels tested (data not shown).
Short-term growth and ethylene evolution response of sunflower seedling grown under varying and changing PARs
Transfer of 7-day old sunflower seedlings from very low PAR to low PAR (time 0) yielded no significant changes in hypocotyl elongation (Fig. 4a) or in cotyledon and leaf tissues dry weight accumulation (Fig. 4d) at either time point measured. However, transfer of sunflower seedlings from very low PAR to near-normal PAR significantly decreased hypocotyl elongation 24 h after transfer (Fig. 4a) and significantly increased cotyledon and leaf tissues dry weights 72 h after transfer (Fig. 4d). These significant changes in very low PAR-grown seedling tissues after transfer to near-normal PAR were accompanied by a significant increase in ethylene evolution by hour 24 for hypocotyls (Fig. 5a) and by a significant decrease in ethylene evolution by hour 72 for cotyledons and leaves (Fig. 5d). Although the transfer of seedlings from very low PAR to low PAR did not result in significant changes in hypocotyl elongation (Fig. 4a) or cotyledon and leaf dry biomass accumulation (Fig. 4d), ethylene evolution of these seedlings was significantly higher for hypocotyl tissues at hour 24 (but not at hour 72, see Fig. 5a) and significantly lower for cotyledon and leaf tissues by hour 72 (Fig. 5d).
Hypocotyl lengths (a, b, c [mm]) and dry weights (d, e, f [mg]) of cotyledons + leaves of sunflower seedlings at time 0 (day 7), 24 and 72 h after they were divided to three groups: one group was left to grow under the initial lighting condition, whereas the other two groups were transferred to two other lighting conditions. The number in brackets by capital letter in each figure indicates the initial lighting condition in μmol m−2 s−1. Therefore, the initial lighting conditions for results presented in figures a and d were 10 μmol m−2 s−1, for figures b and e they were 100 μmol m−2 s−1, and for figures c and f they were 1,000 μmol m−2 s−1. The closed circles in each figure indicate initial lighting condition (see above) plus the following treatment (from day 0 and for the next 72 h) of 10 μmol m−2 s−1. The open circles in each figure indicate initial lighting condition plus the following treatment of 100 μmol m−2 s−1. The closed triangles in each figure indicate initial lighting condition plus the following treatment of 1,000 μmol m−2 s−1. The error bars represent one SE of mean. The mean values with the same letter are not significantly different based on Tukey’s ANOVA test at P ≤ 0.05. Separate ANOVA tests were performed for each time point
Hypocotyl (a, b, c) and cotyledons + leaves (d, e, f) ethylene levels (pmol gFW−1 h−1) at time 0 (day 7), 24 and 72 h after they were divided to three groups: one group was left to grow under the initial lighting condition, whereas the other two groups were transferred to two other lighting conditions. The number in brackets by capital letter in each figure indicates the initial lighting condition in μmol m−2 s−1. Therefore, the initial lighting conditions for results presented in figures a and d were 10 μmol m−2 s−1, for figures b and e they were 100 μmol m−2 s−1, and for figures c and f they were 1,000 μmol m−2 s−1. The closed circles in each figure indicate initial lighting condition (see above) plus the following treatment (from day 0 and for the next 72 h) of 10 μmol m−2 s−1. The open circles in each figure indicate initial lighting condition plus the following treatment of 100 μmol m−2 s−1. The closed triangles in each figure indicate initial lighting condition plus the following treatment of 1,000 μmol m−2 s−1. The error bars represent one SE of mean. The mean values with the same letter are not significantly different based on Tukey’s ANOVA test at P ≤ 0.05. Separate ANOVA tests were performed for each time point
Transfer of 7-day old sunflower seedlings from low PAR to very low PAR yielded no significant change in hypocotyl elongation (Fig. 4b) or in cotyledon and leaf tissue dry weight accumulation (Fig. 4e) at either time point measured. However, transfer of sunflower seedlings from low PAR to near-normal PAR significantly decreased hypocotyl elongation by hour 24 after transfer (Fig. 4b) and significantly increased cotyledon and leaf tissue dry weights by hour 72 after transfer (Fig. 4e). The significant decrease in hypocotyl elongation of low PAR-grown seedling tissues after transfer to near-normal PAR were accompanied by a significant increase in ethylene evolution by hour 24 (Fig. 5b). However, the significant increase in cotyledon and leaf tissues dry weights by hour 72 after transfer (Fig. 4e) resulted in a significant increase in ethylene evolution by these tissues at hour 4, but not at hours 24 or 72 (Fig. 5e). The transfer of low PAR-grown seedlings to very low PAR resulted in non-significant, but consistently lower hypocotyl ethylene evolution at all time points, but especially at hours 4 and 24 (Fig. 5b), and significantly increased ethylene evolution by cotyledons and leaves by hour 24 (Fig. 5e).
Transfer of 7-day old sunflower seedlings from near-normal PAR to low and very low PARs yielded significant and gradual increases in hypocotyl elongation starting at hour 24 and ending at hour 72 after transfer (Fig. 4c). A similar trend, but in the opposite direction, was observed for cotyledon and leaf tissues of these seedlings, although it was largely non-significant (Fig. 4F). Transfer of near-normal PAR-grown seedlings to very low PAR resulted in significantly decreased hypocotyl ethylene evolution at hours 4 and 72, and a non-significant decrease at hour 24 (Fig. 5c). There was no significant difference in hypocotyl ethylene evolution between continuously near-normal PAR-grown seedlings and near-normal PAR-grown seedlings which were transferred to low PAR (Fig. 5c). Transfer of near-normal PAR-grown seedlings to low or very low PAR did not result in any significant changes in ethylene evolution by cotyledon and leaf tissues at time 4 or 24 h, but at hour 24 the ethylene evolution of seedlings transferred to low and very low PAR was significantly higher than of seedlings grown continuously under near-normal PAR (Fig. 5f).
Conclusions
The gradual change in both main components of shade light, R/FR ratio and PAR, has a similar effect on hypocotyl growth of 7-day old sunflower seedlings: reduction in either R/FR ratio (Kurepin et al. 2007a) or PAR (Figs. 1, 2) significantly and gradually increases elongation and significantly and gradually decreases ethylene evolution. This suggests a growth inhibitory role for ethylene in hypocotyl elongation under shade light. Experiments with exogenously-induced ethylene induction and inhibition (Kurepin et al. 2007a and Fig. 3) and by experiments with transfer of plants from one PAR regime to another with subsequent ethylene measurement (Figs. 4, 5) support this idea. Thus, plants growing under cover of a canopy or in proximity to taller neighbouring vegetation can increase stem elongation in an attempt to reach more sunlight via both main shade light components, low R/FR ratio and low PAR, and both are equally capable of upregulating this stem elongation by directly decreasing ethylene evolution in stem tissues.
A gradual decrease in PAR level significantly decreased growth and increased ethylene evolution in cotyledon and leaf tissues of 7-day old sunflower hypocotyls (Figs. 1, 2), suggesting a growth inhibitory role for ethylene in leaf growth in response to PAR signaling. This interpretation is corroborated by experiments in which we studied the effects of ethephon and AVG (Fig. 3), and by experiments with transfer of plants from one PAR to another with subsequent ethylene measurement (Figs. 4, 5). For 17-day old sunflower seedlings, the decrease of PAR level from normal to low also significantly decreased leaf area growth, but the decrease in R/FR ratio from normal to low had no effect on leaf area growth (Kurepin et al. 2007b). Further, while the decrease of PAR level from normal to low significantly increased leaf ethylene evolution, the decrease in R/FR ratio from normal to low had no effect on leaf ethylene evolution (Kurepin et al. 2007b). Therefore, unlike the hypocotyl (stem) case, where both R/FR ratio and PAR signals elicited similar growth responses, for leaf tissues (of at least the sunflower plant) it is the PAR signal which is responsible for minimizing leaf area growth at the time when the majority of plant building resources are dedicated for upward growth allowing the plant to better grow into the sunlight. This PAR-regulated decrease in leaf area growth appears to be directly mediated by changes in leaf ethylene evolution.
References
Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in plant biology, 2nd edn. Academic Press, New York
Ballare CL (1999) Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plant Sci 4:97–102
Ballare CL, Scopel AL, Sanchez RA (1990) Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247:329–331
Finlayson SA, Lee IJ, Morgan PW (1998) Phytochrome B and the regulation of circadian ethylene production in sorghum. Plant Physiol 116:17–25
Finlayson SA, Jung IJ, Mullet JE, Morgan PW (1999) The mechanism of rhythmic ethylene production in Sorghum. The role of phytochrome B and simulated shading. Plant Physiol 119:1083–1089
Franklin KA, Whitelam GC (2005) Phytochromes and shade-avoidance responses in plants. Ann Bot (Lond) 96:169–175
Goeschl JD, Pratt HK, Bonner BA (1967) An effect of light on the production of ethylene and the growth of the plumular portion of etiolated pea seedlings. Plant Physiol 42:1077–1080
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Cal Agr Exp StatCirc 347:1–32
Imaseki H, Pjon CJ, Furuya M (1971) Phytochrome action in Oryza sativa L.IV. Red and far-red reversible effect on the production of ethylene in excised coleoptiles. Plant Physiol 48:241–244
Jackson MB (1985) Ethylene and responses of plants to soil waterlogging and submergence. Ann Rev Plant Physiol 36:145–174
Kurepin LV, Walton LJ, Reid DM (2007a) Interaction of red to far red light ratio and ethylene in regulating stem elongation of Helianthus annuus. Plant Growth Regul 51:53–61
Kurepin LV, Emery RJN, Pharis RP, Reid DM (2007b) Uncoupling light quality from light irradiance effects in Helianthus annuus shoots: putative roles for plant hormones in leaf and internode growth. J Exp Bot 58(8):2145–2157
Lee SH, Reid DM (1997) The role of endogenous ethylene in the expansion of Helianthus annuus leaves. Can J Bot 78:501–508
Pierik R, Visser EJW, De Kroon H, Voesenek LACJ (2003) Ethylene is required in tobacco to successfully compete with proximate neighbours. Plant Cell Environ 26:1229–1234
Raskin I, Kende H (1984) The role of gibberellin in the growth response of submerged deep water rice. Plant Physiol 76:947–950
Reid DM, Sheffer MG, Pierce RC, Bezdicek DF, Linzon SN, Revven T, Spenser MS, Vena F (1985) Ethylene in the environment: scientific criteria for assessing its effects on environmental quality. National Research Council of Canada, Ottawa, publication 22497, p 110
Rijnders JGHM, Yang YY, Kamiya Y, Takahashi N, Barendse GWM, Blom CWPM, Voesenek LACJ (1997) Ethylene enhances gibberellin levels and petiole sensitivity in flooding-tolerant Rumex palustris but not in flooding-intolerant R. acetosa. Planta 203:20–25
Smith H (2000) Phytochromes and light signal perception by plants-an emerging synthesis. Nature 407:585–591
Vandenbussche F, Vriezen WH, Smalle J, Laarhoven LJ, Harren FJ, Van Der Straeten D (2003) Ethylene and auxin control the Arabidopsis response to decreased light irradiance. Plant Physiol 133:517–527
Vandenbussche F, Pierik R, Millenaar FF, Voesenek LA, Van Der Straeten D (2005) Reaching out of the shade. Curr Opin Plant Biol 8:462–468
Zobel RW, Robert LW (1978) Effect of low concentration of ethylene on cell division and cell differentiation in lettuce pit explants. Can J Bot 56:987–990
Acknowledgments
We would like to thank Ms. Bonnie Smith and Mr. Ken Girard for excellent greenhouse assistance. This work was funded by NSERC (Canada) grants to DMR, ECY and CCC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurepin, L.V., Walton, L.J., Yeung, E.C. et al. The interaction of light irradiance with ethylene in regulating growth of Helianthus annuus shoot tissues. Plant Growth Regul 62, 43–50 (2010). https://doi.org/10.1007/s10725-010-9483-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-010-9483-8