Abstract
Plants growing under canopy shade or in near-neighboring proximity of taller vegetation are the receivers of shade light conditions. The effect of light irradiance (photosynthetically active radiation [PAR]), one of the main components of shade light, on the growth of various tissues of sunflower seedlings and the possible role of auxin were investigated. Gradual reductions in PAR irradiance level from near-normal to low and very low result in significant and gradual increases in sunflower hypocotyl growth and endogenous auxin content. Similar reductions in PAR level resulted in significant and gradual decreases in sunflower cotyledon and leaf growth, and endogenous auxin content. Exogenously applied auxin increased hypocotyl elongation under near-normal PAR, where IAA levels are below optimum, but decreased elongation under very low PAR, where IAA levels are already at optimum. These results suggests that auxin acts as positive growth regulator of sunflower hypocotyls subjected to low light irradiance stress. This is further supported by the transfer experiments where seedlings transferred, for example, from near-normal PAR to very low PAR showed increased elongation associated with increased IAA levels. Therefore, it is reasonable to conclude that light irradiance-mediated changes in hypocotyl elongation of young sunflower seedlings are regulated by endogenous auxin levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants growing under canopy or in the proximity of neighboring vegetation often increase stem elongation at the expense of leaf growth (Ballare 1999; Smith 2000; Franklin and Whitelam 2005; Vandenbussche et al. 2005; Kurepin et al. 2007b). This phenomenon is regulated by interactions between shade light and numerous plant hormones, as was demonstrated for sunflower hypocotyls (Kurepin et al. 2007a, c, 2010a, b; Walton et al. 2010). The two key components of shade light, lower red to far red (R/FR) ratio and reduced photosynthetically active radiation (PAR) (Ballare et al. 1990; Ballare 1999; Smith 2000), when uncoupled, can have different effect not only on plant growth, but also on endogenous levels of multiple plant hormones (Kurepin et al. 2007a, c, 2010b).
Moreover, since shaded plants often increase stem elongation at the expense of leaf area expansion (Kurepin et al. 2007b), there is also a differential hormonal regulation of stem and leaf growth. For sunflower seedlings growing under lower PARs, ethylene, found at lower endogenous concentrations in hypocotyls, but at higher concentrations in cotyledons and leaves, promotes hypocotyl elongation, but inhibits cotyledon and leaf expansion (Kurepin et al. 2010b). This can be explained by reports showing that ethylene, like auxin, demonstrates a biphasic dose–response curve. Lower doses producing stimulation of various growth and developmental events, but higher doses producing the inhibitory responses, see Zobel and Roberts (1978), Reid et al. (1985), Lee and Reid (1997).
The increase in shoot elongation growth seen under reduced R/FR ratio or low PAR appears to be mediated by at least four classes of plant growth-regulating phytohormones, gibberellins, cytokinins, auxin (IAA [indole-3-acetic acid]) and ethylene (Kurepin et al. 2007a, b, c, 2010a). End-of-day FR light enrichment increases endogenous IAA levels in the third internode of Pisum sativum seedlings (Behringer and Davies 1992). Arabidopsis hypocotyl elongation under reduced R/FR ratio coupled with low PAR is IAA-dependent (Steindler et al. 1999). Also, a low PAR coupled with a normal R/FR ratio induces auxin activity (IAA-mediated gene expression) in rosette leaves of Arabidopsis (Vandenbussche et al. 2003). For sunflower hypocotyls, internodes and leaves, gradual changes in R/FR ratio level from high to low significantly increased endogenous IAA levels, whereas the change in PAR level from low (138 μmol m−2 s−1) to near-normal (510 μmol m−2 s−1) had no effect on IAA levels in these tissues (Kurepin et al. 2007a, b).
In this study, the interaction of PAR levels (10, 100 and 1,000 μmol m−2 s−1) with IAA in mediating hypocotyl elongation at the expense of leaf area expansion in young sunflower (Helianthus annuus L.) seedlings was investigated to better understand the role of IAA in growth and development under low PAR conditions which is usually associated with shade.
Materials and methods
Plants and experimental system
Sunflower seeds (6946, Pioneer Seeds, USA) were germinated in a soil mix (2 parts of peat moss, 1—Perlite, 1—Vermiculate and 0.25—Terragreen [a crushed baked clay medium] from Professional Gardener, Calgary, Alberta, Canada). Plants were grown in growth chambers (Conviron, Manitoba) equipped with fluorescent (Sylvania cool white 160 W) and incandescent lights (Philips 60 W). Temperature was maintained at 20°C during the 16 h light period and at 16°C during the 8 h dark period. Sunflower seedlings were watered each day with 25% strength Hoagland’s solution (Hoagland and Arnon 1950). Changing the distance between the light sources and the pot soil level, as well as the use of commercial shade cloth was used to alter PAR. The values of PAR levels were measured with a LI-COR LI-1800/22 quantum sensor (LI-COR, Inc., Lincoln, NE, USA). The Tukey’s ANOVA tests for analysis of significance (at P ≤ 0.05) were run on SPSS software version 15. Each experiment was repeated at least 3 times.
Measurement of endogenous IAA levels
Hypocotyl, cotyledon and leaf tissue was collected and immediately frozen in liquid N2, then freeze-dried in a Freezemobile 12EL (Virtis Inc., Cardiner, NY, USA). Approximately 0.2–0.5 g dw of each tissue sample was ground with liquid N2 and washed sea sand (Fisher Scientific, NJ 07410, USA), then extracted in 80% MeOH (H2O:MeOH = 20:80, v/v). Following this, 200 ng of [13C6] IAA (gift from Dr. J. Cohen, available from Cambridge Isotope Laboratories, Inc.) were added to the aqueous MeOH extraction solvent as internal standards. The 80% MeOH extract was filtered through Whatman #2 filter paper (55 mm, Whatman International Ltd, Maidstone, UK) and then purified with a C18 preparative column (C18-PC) made of a syringe barrel (inside diameter 2 cm) filled with 3 g of C18 preparative reversed-phase material (Waters Ltd) (Koshioka et al. 1983). The 80% MeOH eluate from this column was dried in vacuo at 35°C.
The dry sample was dissolved in 1 ml of 10% MeOH with 1% acetic acid and injected into the HPLC using the method described initially by Koshioka et al. (1983). The HPLC (Waters Ltd) apparatus consisted of two pumps (model M-45), an automated gradient controller (model 680), and a Rheodyne injector (model 7125). The solvents were, pump A: 10% MeOH in 1% acetic acid [H2O:MeOH:acetic acid = 89:10:1 (v/v)], pump B: 100% MeOH. A reversed phase C18 Radial-PAK (μ-Bondapak column (8 mm × 10 cm) was used with a manually implemented 10–73% gradient programme at a flow rate of 2 ml min−1, i.e. 0–10 min (pump A, 100%; pump B, 0%), 10–50 min (pump A, 30%; pump B, 70%), 50–80 min (pump A, 0%; pump B, 100%), 80–90 min (pump A, 100%; pump B, 0%). The HPLC fractions (4 ml) were dried in vacuo at 35°C. Fractions from the C18 HPLC which were expected to contain the plant hormone were transferred with 100% MeOH to 2 ml glass vials, dried in vacuo and methylated by ethereal CH2N2 for 20 min. Then, the methylated sample was trimethylsilylated by N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (TMCS) (Hedden 1987; Gaskin and MacMillan 1991).
The identification and quantification of IAA was carried out using a gas chromatograph connected to a mass spectrometer (GC-MS) using the -selected ion monitoring (-SIM) mode. The derivatized sample was injected into a capillary column installed in an Agilent 6890 GC with a capillary direct interface to the Agilent 5973 mass selective detector (MSD). The dimensions of the capillary column were 0.25 μm film thickness, 0.25 mm internal diameter, 30 m DB-1701 (Model No.: J&W122-0732, J&W Scientific, Inc.). The GC temperature programme was: 1 min at 60°C, followed by an increase to 240°C at 25°C min−1, then an increase at 5°C min−1 to 280°C where it remained constant for 15 min before returning to 60°C. The interface temperature was maintained at 280°C. The dwell time was 100 ms and data was processed using HP G1034C MS ChemStation Software.
For identification of the endogenous IAA we utilized a comparison of GC-retention times (Rts) of the IAA and [13C6]-IAA, as well as a comparison of the relative intensities of the molecular ion (M+) pairs. We also compared the relative intensities of at one more characteristic m/z ion pair for each endogenous hormone and its deutero standard. Quantification was accomplished by reference to the stable isotope-labelled internal standard using equations for isotope dilution analysis, adapted by DW Pearce (see Jacobsen et al. 2002) from Gaskin and MacMillan (1991).
Chemicals
The indole-3-acetic acid (IAA, from Sigma Inc., Oakville, ON, Canada) was applied at concentrations of 10−4, 10−5 and 10−6 M. The final IAA solutions had 0.1% ethanol in H2O, as the original weighted IAA was dissolved in 95% ethanol. Thus, the Control seedlings were sprayed with a 0.1% ethanol in H2O solution. Sunflower seedlings were sprayed to drip-off on days 4, 5 and 6 after planting and measured on day 7. On each of these days, each seedling received 5 sprays from a 500 ml spray bottle.
Results and discussion
Growth and endogenous IAA levels of 7-day old sunflower seedlings grown under varying PARs
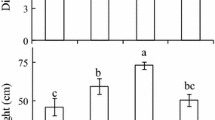
Decrease in PAR levels from near-normal PAR of 1,000 μmol m−2 s−1 to low PAR of 100 μmol m−2 s−1 and very low PAR of 10 μmol m−2 s−1 resulted in significant and gradual increases in hypocotyl dry biomass accumulation (Fig. 1a), as well as hypocotyl elongation (Kurepin et al. 2010a). These gradual changes in hypocotyl lengths and dry biomass accumulation were significantly and positively correlated with hypocotyl endogenous free IAA content (Fig. 1b). The shorter and smaller hypocotyls obtained under near-normal PAR had the lowest levels of endogenous IAA compared to other PAR treatments, whereas longer and larger hypocotyls under very low PAR had the highest levels of endogenous IAA (Fig. 1b). In previous study (Kurepin et al. 2007a), where endogenous IAA levels were measured from the 7-day old sunflower hypocotyls under PAR irradiances of 138 and 510 μmol m−2 s−1, no significant differences in IAA content were reported. Therefore, it appears that only relatively large (or logarithmical, Fig. 1) differences in PAR levels are translated into significant differences in hypocotyl endogenous IAA content.
Tissue specific dry weights (a, mg) and endogenous IAA levels (b, ng gDW−1) of 7-days old sunflower seedlings grown under normal (1,000 μmol m−2 s−1), low (100 μmol m−2 s−1) and very low (10 μmol m−2 s−1) PAR irradiances. The error bars represent one SE of mean. The mean values with the same letter are not significantly different based on Tukey’s ANOVA test at P ≤ 0.05. Separate ANOVA tests were performed for each type of the tissue under varying PAR irradiances
Cotyledon and leaf tissues exhibited opposite trends in dry biomass accumulation when compared to hypocotyl tissues of 7-day old sunflower seedlings (Fig. 1a; Kurepin et al. 2010a). Sunflower cotyledons and leaves had significantly higher dry biomass accumulation under near-normal PAR than under low or very low PAR (Fig. 1a). These differences in dry biomass accumulation by cotyledon and leaf tissues were accompanied by similar differences in endogenous IAA content (Fig. 1b). However, the significant difference in endogenous IAA levels was accomplished only between the two extreme levels of PAR irradiance, 10 and 1,000 μmol m−2 s−1 (Fig. 1b). In previous study (Kurepin et al. 2007b), where endogenous IAA levels were measured from the 17-day old sunflower leaves under PAR irradiances of 138 and 510 μmol m−2 s−1, no significant differences in IAA content were reported. Therefore, as is the case with hypocotyls, it appears that only relatively large (or logarithmical, Fig. 1) differences in PAR levels are translated into significant differences in cotyledon and leaf endogenous IAA content.
Lower PAR levels (from 15 to 125 μmol m−2 s−1) coupled with a normal R/FR ratio have been shown to induce auxin activity (IAA-mediated gene expression) in rosette leaves of Arabidopsis (Vandenbussche et al. 2003), plants which normally occupy near-ground niche in plant communities. Cultivated sunflower plants which normally grow in agricultural fields and are subjected to full sun light spectrum, can only receive a very low PAR levels comparable to the range between 10 and 100 μmol m−2 s−1 in earlier (seedling) stages of growth and development. Sunflower seedlings subjected to lower PAR levels of 10 and 100 μmol m−2 s−1 significantly increased endogenous IAA content in hypocotyls compared to near-normal PAR level of 1,000 μmol m−2 s−1. Low R/FR ratio, compared to normal and high R/FR ratios, was shown to significantly increase endogenous IAA levels in 7-day old sunflower hypocotyls (Kurepin et al. 2007a). Thus, it appears that both components of shade light, low R/FR ratio and low PAR not only have similar impact of hypocotyl (and stem) elongation, but also are both capable of significantly increasing endogenous IAA levels. Gibberellin and ethylene levels changed gradually as R/FR ratio decreased from high to low or PAR irradiance level was lowered from near-normal, to low and very low in sunflower seedling tissues (Kurepin et al. 2007a, b, c). However, unlike these later plant hormones, auxin levels are modified only or mainly by the extreme reduction in either R/FR ratio or PAR level (Fig. 1b; Kurepin et al. 2007a, b).
Growth response of 7-day old sunflower seedlings to exogenous IAA application under varying PARs
Application of IAA at concentration of 10−4 M significantly inhibited hypocotyl elongation under very low PAR, had no effect on hypocotyl elongation under low PAR and significantly increased hypocotyl elongation under near-normal PAR (Fig. 2a), all relative to the control plants. Applied IAA also significantly increased hypocotyl dry biomass accumulation under near-normal PAR (Fig. 2b). However, as would be expected based on hypocotyl elongation data (Fig. 2a), had no effect on dry biomass accumulation under very low PAR (Fig. 2b). Application of IAA at other concentrations (10−5 and 10−6 M) had no significant effect on hypocotyl elongation or dry biomass accumulation under all three varying PAR levels (data not shown). Application of exogenous IAA to sunflower seedlings at concentrations ranging from 10−4 to 10−6 M had no significant effect on either cotyledon or leaf growth under all three varying PAR levels (data not shown).
Hypocotyl lengths (a, mm) and dry weights (b, mg) of 7-days old sunflower seedlings treated with either control solution or 10−4 M IAA and grown under normal (1,000 μmol m−2 s−1), low (100 μmol m−2 s−1) and very low (10 μmol m−2 s−1) PAR irradiances. The error bars represent one SE of mean. The mean values with the same letter are not significantly different based on Tukey’s ANOVA test at P ≤ 0.05. Separate ANOVA tests were performed for each PAR irradiance level
The decrease in hypocotyl elongation coupled with no change in dry biomass accumulation observed for sunflower hypocotyls treated with 10−4 M IAA under very low PAR of 10 μmol m−2 s−1 can probably be explained by typical auxin-ethylene interaction. In this type of interaction the increased auxin (higher than the optimum level) can cause increased ethylene production (reviewed in McKeon and Yang 1987). This is further supported by a previous study, where applied ethephon, a substance which releases ethylene at a physiological pH, significantly (and to the same degree) inhibited 7-day old sunflower hypocotyl elongation without significantly affecting the dry biomass accumulation (Kurepin et al. 2010a).
The results presented in Figs. 1b and 2a suggest that auxin does play a significant role in PAR-mediated hypocotyl elongation. Further, its mode of action is, most likely, based on optimum level of concentration. The low levels of endogenous IAA (near-normal PAR) maintain a low rate of hypocotyl elongation. The near-optimum levels of IAA (low PAR) increase hypocotyl elongation. However, the optimum levels of IAA (very low PAR) cause a rapid and maximal hypocotyl elongation which can be overturn if endogenous IAA content is further (over-optimum) increased, as is evident from Fig. 2a.
Growth and endogenous IAA levels of sunflower seedling grown under varying and changing PARs
Transfer of 7-day old sunflower seedlings from very low PAR (10 μmol m−2 s−1) to low PAR (100 μmol m−2 s−1) yielded significant increase in hypocotyl elongation by time 72 h (Fig. 3a). Similar and significant results were also observed for sunflower hypocotyls which remained at very low PAR for the next 72 h (Fig. 3a). These significant changes in hypocotyl elongation over 72 h period for both transferred and remained seedlings did not result in significant changes in endogenous IAA content (Fig. 3b). However, transfer of sunflower seedlings from very low PAR to near-normal PAR resulted in complete arrest of hypocotyl elongation (Fig. 3a). Further, this arrest of hypocotyl elongation was accompanied by significantly reduced endogenous IAA levels (Fig. 3b).
Hypocotyl lengths (a, c, e [mm]) and hypocotyl endogenous IAA levels (b, d, f [ng gDW−1]) of sunflower seedlings at time 0 (day 7) and 72 h after they were divided to three groups: one group was left to grow under the initial lighting condition, whereas the other two groups were transferred to two other lighting conditions. The number in brackets by capital letter in each figure indicates the initial lighting condition in μmol m−2 s−1. Therefore, the initial lighting conditions for results presented in figures a and b were 10 μmol m−2 s−1, for figures c and d they were 100 μmol m−2 s−1, and for figures e and f they were 1,000 μmol m−2 s−1. The error bars represent one SE of mean. The mean values with the same letter are not significantly different based on Tukey’s ANOVA test at P ≤ 0.05. Separate ANOVA tests were performed for each light treatment
Transfer of 7-day old sunflower seedlings from low PAR (100 μmol m−2 s−1) to very low PAR (10 μmol m−2 s−1) yielded significant increase in hypocotyl elongation by time 72 h (Fig. 3c). Similar and significant results were also observed for sunflower hypocotyls which remained at low PAR for the next 72 h (Fig. 3c). These significant changes in hypocotyl elongation over 72 h period for both transferred and remained seedlings did not result in significant changes in endogenous IAA content (Fig. 3d). However, transfer of sunflower seedlings from low PAR to near-normal PAR resulted in complete arrest of hypocotyl elongation (Fig. 3c). Further, this arrest of hypocotyl elongation was accompanied by significantly reduced endogenous IAA levels (Fig. 3d).
Transfer of 7-day old sunflower seedlings from near-normal PAR (1,000 μmol m−2 s−1) to very low PAR (10 μmol m−2 s−1) or low PAR (100 μmol m−2 s−1) yielded significant increases in hypocotyl elongation by time 72 h (Fig. 3e) for both low PAR treatments. However, the transfer to very low PAR, produced a higher effect than the transfer to low PAR. These significant changes in hypocotyl elongation (over 72 h period) for seedlings transferred to very low and low PARs resulted also in significant and similar increases in endogenous IAA levels (Fig. 3f). Sunflower hypocotyls which remained at near-normal PAR for the next 72 h also increased in length with time passed (Fig. 3e), but their endogenous IAA levels remained the same following the 72 h period (Fig. 3f).
Transfer of 7-day old sunflower seedlings from very low PAR (10 μmol m−2 s−1) to low PAR (100 μmol m−2 s−1) yielded no significant differences in dry biomass accumulation of cotyledon and leaf tissues by time 72 h (Fig. 4a). Similarly, sunflower seedlings which remained at very low PAR for the next 72 h showed no significant change in dry biomass accumulation of cotyledon and leaf tissues (Fig. 4a). This lack of change in dry biomass accumulation of cotyledon and leaf tissues grown under lower PARs was correlated with no significant changes in endogenous IAA levels in these tissues (Fig. 4b). However, transfer of sunflower seedlings from very low PAR to near-normal PAR resulted in significant increase in dry biomass accumulation of cotyledon and leaf tissues (Fig. 4a). This increase was accompanied by a significant increase in endogenous IAA levels in cotyledon and leaf tissues (Fig. 4b).
Cotyledon + leaf dry weights (a, c, e [mg]) and cotyledon + leaf endogenous IAA levels (b, d, f [ng g DW−1]) of sunflower seedlings at time 0 (day 7) and 72 h after they were divided to three groups: one group was left to grow under the initial lighting condition, whereas the other two groups were transferred to two other lighting conditions. The number in brackets by capital letter in each figure indicates the initial lighting condition in μmol m−2 s−1. Therefore, the initial lighting conditions for results presented in figures a and b were 10 μmol m−2 s−1, for figures c and d they were 100 μmol m−2 s−1, and for figures e and f they were 1,000 μmol m−2 s−1. The error bars represent one SE of mean. The error bars represent one SE of mean. The mean values with the same letter are not significantly different based on Tukey’s ANOVA test at P ≤ 0.05. Separate ANOVA tests were performed for each treatment
Transfer of 7-day old sunflower seedlings from low PAR (100 μmol m−2 s−1) to very low PAR (10 μmol m−2 s−1) yielded no significant differences in dry biomass accumulation of cotyledon and leaf tissues by time 72 h (Fig. 4c). Similarly, sunflower hypocotyls which remained at low PAR for the next 72 h showed no significant change in dry biomass accumulation of cotyledon and leaf tissues (Fig. 4c). This lack of change in dry biomass accumulation of cotyledon and leaf tissues grown under lower PARs was correlated with no significant changes in endogenous IAA levels in these tissues (Fig. 4d). However, transfer of sunflower seedlings from low PAR to near-normal PAR resulted in significant increase in dry biomass accumulation of cotyledon and leaf tissues (Fig. 4c). This increase was accompanied by a significant increase in endogenous IAA levels in cotyledon and leaf tissues (Fig. 4d).
Transfer of 7-day old sunflower seedlings from near-normal PAR (1,000 μmol m−2 s−1) to very low PAR (10 μmol m−2 s−1) or low PAR (100 μmol m−2 s−1) yielded significant increases in dry biomass accumulation of cotyledon and leaf tissues by time 72 h (Fig. 4e) for both low PAR treatments. These significant changes in dry biomass accumulation of cotyledon and leaf tissues (over 72 h period) for seedlings transferred to very low and low PARs resulted also in significant decreases in endogenous IAA levels (Fig. 4f). Sunflower seedlings which remained at near-normal PAR for the next 72 h also increased in dry biomass accumulation of cotyledon and leaf tissues with time passed (Fig. 4e), but their endogenous IAA levels remained the same following the 72 h period (Fig. 4f).
Conclusions
Gradual and logarithmic reductions in PAR irradiance level from near-normal to low and very low results in significant and gradual increases in sunflower hypocotyl elongation and dry biomass accumulation. On the other hand, it results in gradual decreases in sunflower cotyledon and leaf tissue dry biomass accumulation. These morphological changes are correlated with endogenous auxin content: as PAR level is reduced, IAA levels increase in hypocotyls and decrease in cotyledon and leaf tissues. Further, exogenous auxin application results in increased elongation of hypocotyls grown under near-normal PAR, where IAA levels are below optimum, but decreased elongation under very low PAR, where IAA levels are already at optimum. Applied auxin did not modify cotyledon and leaf biomass accumulation. Moreover, the ability of changing PAR levels to modify endogenous auxin content and the interaction of light irradiance with auxin in regulating at least hypocotyl elongation of sunflower seedlings is further supported by the numerous transfer experiment. Therefore, it is reasonable to conclude that light irradiance-mediated changes in hypocotyl elongation of young sunflower seedlings are regulated by endogenous auxin levels. Also, based on previous study (Kurepin et al. 2010a), where ethylene regulation of growth of sunflower seedlings in response to changing PAR levels was presented, and conclusions from current study, it seems likely that both ethylene and auxin are involved in light irradiance-mediated elongation of sunflower hypocotyls. However, they act at opposite direction: auxin acts as positive regulator of elongation, whereas ethylene—negative. Similar conclusions were obtained for the roles of ethylene and auxin in regulating sunflower seedling growth in response to changing R/FR ratios (Kurepin et al. 2007a, b, c). Therefore, both components of shade light, PAR and R/FR ratio use similar hormonal mechanisms to alter plant growth.
References
Ballare CL (1999) Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plant Sci 4:97–102
Ballare CL, Scopel AL, Sanchez RA (1990) Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247:329–331
Behringer FJ, Davies PJ (1992) Indole-3-acetic acid levels after phytochrome-mediated changes in the stem elongation rate of dark-grown and light-grown Pisum seedlings. Planta 188:85–92
Franklin KA, Whitelam GC (2005) Phytochromes and shade-avoidance responses in plants. Ann Bot (Lond) 96:169–175
Gaskin P, MacMillan J (1991) GC-MS of the gibberellins and related compounds. Methodology and a library of spectra. University of Bristol (Cantock’s Enterprises), Bristol, UK
Hedden P (1987) Gibberellins. In: Rivier L, Crozier A (eds) Principles and practice of plant hormone analysis, vol 1. Academic Press, London, pp 9–110
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Cal Agr Exp Stat Circ 347:1–32
Jacobsen JV, Pearce DW, Poole AT, Pharis RP, Mander LN (2002) Abscisic acid, phaseic acid and gibberellin contents associated with dormancy and germination in barley. Physiol Plant 115:428–441
Koshioka M, Takeno K, Beall FD, Pharis RP (1983) Purification and separation of gibberellins from their precursors and glucosyl conjugates. Plant Physiol 73:398–406
Kurepin LV, Walton LJ, Reid DM (2007a) Interaction of red to far red light ratio and ethylene in regulating stem elongation of Helianthus annuus. Plant Growth Regul 51:53–61
Kurepin LV, Emery RJN, Pharis RP, Reid DM (2007b) The interaction of light quality and irradiance with gibberellins, cytokinins and auxin in regulating growth of Helianthus annuus hypocotyls. Plant Cell Environ 30:147–155
Kurepin LV, Emery RJN, Pharis RP, Reid DM (2007c) Uncoupling light quality from light irradiance effects in Helianthus annuus shoots: putative roles for plant hormones in leaf and internode growth. J Exp Bot 58:2145–2157
Kurepin LV, Walton LJ, Reid DM, Chinnappa CC (2010a) Light regulation of endogenous salicylic acid levels in Helianthus annuus hypocotyls. Botany 88:668–674
Kurepin LV, Walton LJ, Yeung EC, Chinnappa CC, Reid DM (2010b) The interaction of light irradiance with ethylene in regulating growth of Helianthus annuus shoot tissues. Plant Growth Regul 62:43–50
Lee SH, Reid DM (1997) The role of endogenous ethylene in the expansion of Helianthus annuus leaves. Can J Bot 78:501–508
McKeon T, Yang SF (1987) Biosynthesis and metabolism of ethylene. In: Davies PJ (ed) Plant hormones and their role in plant growth and development. Martinus Nijhoff, Boston, pp 94–112
Reid DM, Sheffer MG, Pierce RC, Bezdicek DF, Linzon SN, Revven T, Spenser MS, Vena F (1985) Ethylene in the environment: scientific criteria for assessing its effects on environmental quality. National Research Council of Canada, Ottawa, p 110 (publication 22497)
Smith H (2000) Phytochromes and light signal perception by plants—an emerging synthesis. Nature 407:585–591
Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, Aoyama T, Morelli G, Ruberti I (1999) Shade avoidance responses are mediated by the ATHB-2 HD-Zip protein, a negative regulator of gene expression. Development 126:4235–4245
Vandenbussche F, Vriezen WH, Smalle J, Laarhoven LJ, Harren FJ, Van Der Straeten D (2003) Ethylene and auxin control the Arabidopsis response to decreased light irradiance. Plant Physiol 133:517–527
Vandenbussche F, Pierik R, Millenaar FF, Voesenek LA, Van Der Straeten D (2005) Reaching out of the shade. Curr Opin Plant Biol 8:462–468
Walton LJ, Kurepin LV, Reid DM, Chinnappa CC (2010) Narrow-band light regulation of ethylene and gibberellin levels in hydroponics-grown Helianthus annuus hypocotyls and roots. Plant Growth Regul 61:53–59
Zobel RW, Robert LW (1978) Effect of low concentration of ethylene on cell division and cell differentiation in lettuce pit explants. Can J Bot 56:987–990
Acknowledgments
We would like to thank Ms. Bonnie Smith and Mr. Ken Girard for excellent greenhouse assistance. This work was funded by NSERC (Canada) grant to DMR and ECY.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurepin, L.V., Walton, L.J., Yeung, E.C. et al. The interaction of light irradiance with auxin in regulating growth of Helianthus annuus shoots. Plant Growth Regul 65, 255–262 (2011). https://doi.org/10.1007/s10725-011-9596-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-011-9596-8