Abstract
Ca2+ is a universal carrier of biological information: it controls cell life from its origin at fertilization to its end in the process of programmed cell death. Ca2+ is a conventional diffusible second messenger released inside cells by the interaction of first messengers with plasma membrane receptors. However, it can also penetrate directly into cells to deliver information without the intermediation of first or second messengers. Even more distinctively, Ca2+ can act as a first messenger, by interacting with a plasma membrane receptor to set in motion intracellular signaling pathways that involve Ca2+ itself. Perhaps the most distinctive property of the Ca2+ signal is its ambivalence: while essential to the correct functioning of cells, Ca2+ becomes an agent that mediates cell distress, or even (toxic) cell death, if its concentration and movements inside cells are not carefully tuned. Ca2+ is controlled by reversible complexation to specific proteins, which could be pure Ca2+ buffers, or which, in addition to buffering Ca2+, also decode its signal to pass it on to targets. The most important actors in the buffering of cell Ca2+ are proteins that transport it across the plasma membrane and the membrane of the organelles: some have high Ca2+ affinity and low transport capacity (e.g., Ca2+ pumps), others have opposite properties (e.g., the Ca2+ uptake system of mitochondria). Between the initial event of fertilization, and the terminal event of programmed cell death, the Ca2+ signal regulates the most important activities of the cell, from the expression of genes, to heart and muscle contraction and other motility processes, to diverse metabolic pathways involved in the generation of cell fuels.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- apoptosis
- calcium
- calcium buffering proteins
- calcium sensor proteins
- calmodulin
- fertilization
- gene expression
- ion pumps
- mitochondria
- protein dephosphorylation
- protein phosphorylation
- Please cite as: Met. Ions Life Sci. 12 (2013) 119–168
1 Introduction
In the course of evolution, Ca2+ has been selected as a universal carrier of signals. The selection occurred at the time of the transition from unicellular to multicellular life, when the division of labor among cells of the organisms brought with it the necessity of exchanging signals. As a rule, unicellular organisms do not require Ca2+ (although some bacterial functions, e.g., chemotaxis, do require Ca2+ and its manipulation) and do not need to exchange signals, their interplay being restricted to the competition for nutrients. The selection of Ca2+ as carrier of information has been dictated by coordination chemistry, which makes Ca2+ ideally suited to be accommodated within the sites of irregular geometry offered by complex cellular molecules (proteins) [1,2]. A molecule selected to transmit signals within the cell must be tightly regulated. In the case of Ca2+, given its chemical properties, this is optimally achieved by binding it reversibly, and with the appropriate affinity and specificity, to cellular proteins. In the complexing proteins, oxygen is the preferred ligand atom for Ca2+: the introduction of nitrogen in the primary coordination sphere usually decreases the selectivity for Ca2+. In most cases, the coordination number for Ca2+ is 8 (in some cases it may be 6 or 7): by comparison, the coordination number for the other abundant cellular divalent cation, Mg2+, is only 6. The coordination stereochemistry of 6 is that of a regular octahedron, implying that the Mg-O bond distances in the primary coordination sphere vary only little (between 0.200 and 0.216 nm), whereas the Ca-O bond distances vary over a much more extended range (between 0.229 and 0.265 nm). It follows that Ca2+ can accept binding cavities of irregular shape, in which the ligand oxygen atoms can be at considerably variable distances from it.
The facility with which Ca2+ becomes bound permits the lowering of its cell concentration to levels that are too low to trigger its precipitation as an insoluble phosphate salt. This is the extra dividend of the choice of Ca2+ as a cellular signaling agent: if it were not possible to maintain its background concentration very low inside cells, phosphate could not be used as the energy currency. In addition to Ca2+, a number of other metals are essential to cell life, such as iron, zinc, copper, manganese. All of them are active-site metals that participate directly in the mechanism of enzyme catalysis. Ca2+, instead, is not an active-site metal, it is an allosteric metal par excellence, which binds to (enzyme) proteins at sites different from the active site, modulating their activities, namely, activating (in most cases) or inhibiting them. Modulation of enzyme processes is of utmost importance to cells, thus, the control of cellular Ca2+ is of critical importance, as the array of Ca2+-regulated functions covers the entire spectrum of processes that are essential to cell life. The vital importance of the precise control of Ca2+ is reflected in the multitude of systems developed by evolution to fulfill the task. Basically, these systems either transport Ca2+ across membrane boundaries, or complex it reversibly in the cytosol or in the lumen of the organelles.
The transport of Ca2+ across membranes is the ultimate way to buffer it; it is performed by channels, ATPases, exchangers, in which Ca2+ is exchanged for another ion (usually Na+), and by an electrophoretic uniporter in the inner mitochondrial membrane. The control of Ca2+ by non-membrane proteins is performed within the organelles by low-affinity, large-capacity proteins, that, however, may also fulfill other cellular functions [3]. In the cytosol, Ca2+-binding proteins modulate the Ca2+ signal spatially and temporally. Some are pure Ca2+ buffers, e.g., parvalbumin, calbindins, and calreticulin, others are classified as Ca2+ sensors, since in addition to buffering Ca2+, they also process its signal. The most important and versatile Ca2+ sensor protein is calmodulin (CaM), which is expressed ubiquitously in cells, while other Ca2+ sensors are tissue specific, e.g., the neuronal Ca2+ sensor proteins. The distinction between Ca2+ buffering and Ca2+ sensor proteins, while justified in principle, is not absolute, as some cytosolic Ca2+ buffers, e.g, calbindin D28K, may also have signal processing function [4], and on the other hand even the prototypical Ca2+ sensor protein, calmodulin, could under some circumstances act essentially as a Ca2+ buffer [5].
The array of processes that are controlled by Ca2+ begins with the origin of cell life at fertilization, and ends with the process of programmed death that terminates life once cells have reached the end of their vital cycle. Between these two events, Ca2+ controls processes that may be general to all cells, e.g., gene transcription, differentiation, the generation of fuels in a number of metabolic pathways (essentially, by enzyme phosphorylation and dephosphorylation), motility in the cytoplasmic structures, and cell motility and migration in general. Other processes may be cell-specific, e.g., secretion of solutes (of neurotransmitters in neurons), contraction/relaxation of skeletal muscles and heart. Figure 1 offers a comprehensive panorama of the cell processes that are under the control of Ca2+. Some of them demand rapid and transient exposure to large changes of Ca2+ in the environment that may even be accomplished by the generation of repetitive substantial increases in the form of oscillations. Others demand instead a more sustained change of Ca2+ in their vicinity. In all cases, however, it is of utmost importance that the long-term basal concentration of Ca2+ in the bulk cytosol, after the transient elevation demanded by the activation of the target functions, is returned to the low/intermediate nM range. Cells will not tolerate protracted abnormal increases of Ca2+ in the cytosol, where most targets of its signaling function are located. Should this happen, as is frequently the case in disease conditions, the correct functioning of Ca2+-controlled processes becomes compromised and Ca2+ regulation comes to an end. Ca2+ is thus an ambivalent messenger: while essential to the correct functioning of cell life when tightly controlled, it becomes a conveyor of doom when control fails.
2 Distinctive Properties of the Ca2+ Signal
The cellular transmission and processing of signals typically involves the interaction of first messengers, i.e., compounds that interact with receptors on the plasma membrane of cells, e.g., hormones, followed by their processing in a form that activates internal signaling events that are mediated by diffusible molecules, termed second messengers, that are the result of the interaction of first messengers with their own plasma membrane receptors. This is the general rule for the exchange of information among cells, however, cells can communicate with each other in other ways as well, e.g., by direct contacts, in the form of gap junctions, or by means of surface proteins that recognize partner proteins on the surface of adjacent cells. However, first messengers may also bypass the plasma membrane and penetrate directly into cells to interact with receptors in various cell compartments without the intermediation of second messengers. Interesting as they may be, these alternative possibilities are the exception, the typical way to exchange information from cell to cell remaining that based on the first messenger/second messenger pattern of operation. Within this general background, Ca2+ appears to be a typical diffusible second messenger generated within cells in response to the interplay of the plasma membrane with external first messengers. However, in looking at the signaling function of Ca2+ more closely, peculiarities emerge that cannot be reconciled with an exclusive canonical second messenger role.
The canonical processing of the information of first messengers at the plasma membrane through the interaction with G-proteins and the activation of downstream enzymes does not directly “generate” Ca2+. It generates instead another second messenger, e.g., inositol 1,4,5 trisphosphate (InsP3), which then liberates Ca2+ from the endoplasmic reticulum (ER) store. One could thus define Ca2+ as a “third” messenger. But at the same time Ca2+ could also be defined as a bona fide “first” messenger, as it could penetrate directly into cells through a variety of channels, to modulate intracellular systems without the help of other second messengers. In a strict sense, however, the definition of Ca2+ as a first messenger based solely on its direct penetration into the cytoplasm could be questioned, as the opening of the plasma membrane Ca2+ channels demands the intervention of external ligands or of physical events like membrane potential changes, that would be formally equivalent to first messengers. But the first messenger role of Ca2+ is impeccably demonstrated on the plasma membrane by the existence of a growing number of cell types of a classical G-protein-linked seven-transmembrane domain receptor that recognizes Ca2+ as its first messenger [6], to set in motion the conventional chain of phospholipase C (PLC) mediated events that results in the elevation of cytosolic Ca2+. The Ca2+-sensing receptor (CaR) is organized in the plasma membrane in three domains (Figure 2): a large (600 residues) extracellular domain that contains a number of acidic regions similar to those of the low affinity Ca2+-binding proteins, and which are likely to form binding sites for Ca2+, a mid-domain with the canonical 7 transmembrane helices of G-protein-linked receptors, and a 200 residue intracellular C-domain. The receptor, commonly called the “Ca2+ sensor” was first recognized in the parathyroid cells that secrete the calciotropic hormones that regulate the organismic Ca2+ homeostasis, and then discovered also in cells not directly involved in the regulation of organismic Ca2+ homeostasis, e.g., the brain. It modifies the release of hormones in response to changes in extracellular Ca2+ [7], i.e., it depresses the release of parathormone by parathyroid cells, and activates the release of calcitonin by the C-cells of the thyroid [8]. Clearly, then, Ca2+ is not only an intracellular signaling agent, it is also an extracellular carrier of information that transmits signals to cells involved in the production of calciotropic hormones, but possibly to other cell types as well.
Schematic diagram of the 7-transmembrane domain plasma membrane Ca2+-sensing receptor. Symbols are given in the key at the lower left. The diagram highlights the abundance of negative charged residues in the N- and C-terminal portions of the protein. Adapted from [6].
Another distinctive property of the Ca2+ signal, that sets it apart from other carriers of biological information is autoregulation, i.e., Ca2+ itself controls the activity of the actors that transmit its information. Autoregulation occurs at both the transcriptional and post-transcriptional levels. Important early findings on transcriptional regulation are those showing that the long-term survival of cerebellar granule neurons in culture demands a modest increase of Ca2+ in the cytosol. To change the set point of cytosolic Ca2+ to the new modestly increased level, a complete reprogramming occurs in the transcription of its transporters in the plasma membrane and in the membranes of the organelles (see below) [9–12]. The extensive transcriptional re-programming of the transporters to cope with an altogether minor cytosolic Ca2+ increase may at a first glance seem excessive. However, it underlines in a striking way the importance of controlling Ca2+, especially in neurons, with utmost precision, i.e., it demands the concerted work of several systems.
Another important development related to the transcriptional autoregulation of the Ca2+ signal is the control of a plasma membrane Ca2+ transporter (isoform 3 of the Na+/Ca2+-exchanger, NCX3 [13]) which is crucial for the regulation of the homeostasis of Ca2+ in neurons by the downstream regulatory element antagonistic modulator (DREAM) [14]. DREAM (see below) is a Ca2+-binding protein of the EF hand family that binds to a downstream regulatory element (DRE) site in the promoters of a number of genes, silencing them in the absence of Ca2+. Upon binding Ca2+ to the EF hand motifs DREAM leaves the DNA, relieving the genes from inhibition. DREAM is a particularly interesting case of autoregulation of the Ca2+ signal: it is itself Ca2+-regulated and it controls the transcription of an important Ca2+ transporter. More recent work has actually found that another system that controls cellular Ca2+ homeostasis, a plasma membrane voltage-gated channel, is a target of the transcriptional regulation by DREAM [15].
The examples of the post-trancriptional autoregulation of the Ca2+ signal are also numerous. A classical case is the plasma membrane Ca2+ pump, which is regulated by calmodulin [16]. A more recent autoregulation case is that of the neuronal plasma membrane Na+/Ca2+ exchanger, which is cleaved and inactivated by calpain [17]. Calpain itself is Ca2+-dependent, and becomes activated in response to the penetration of Ca2+ induced in the neurons by glutamate to cleave NCX3. It is also worth mentioning that the plasma membrane Ca2+ pump has been shown to modulate the activity of the Ca2+-dependent protein phosphatase calcineurin [18], and that Ca2+ gates the Ca2+ release channels of ER (see below).
3 The Ambivalent Nature of the Ca2+ Signal
As briefly mentioned above, depending on a number of factors Ca2+ can also transmit negative signals, i.e., signals that activate processes that are detrimental to cells, and that can even lead to cell death. This ambivalence is perhaps the most striking distinctive property of Ca2+ as a carrier of information. Its message must be delivered to cells, and processed by them, in an exquisitely controlled way. Its level in the cytoplasm may be allowed to rise to levels above, even much above, the low-middle nM range that characterizes the resting state, but only if this occurs in a carefully controlled spatio-temporal way. This is the essential point: deviations from the physiological Ca2+ concentration at rest, even large deviations, may not only be tolerated, they may even be necessary to satisfy the physiological demands of cell processes, but they must be planned, and shaped by space and time coordinates that, one would be tempted to say, cells have learnt to apply intelligently.
The issue is essentially one of time: for instance, as mentioned, cells could use rapid repetitive Ca2+ transient, i.e., oscillatory signals, as a device to deliver the message to functions that require Ca2+ concentrations much in excess to those of the normal cytosol at rest. The problem of ambivalence sets in when the increase of Ca2+ occurs in a way that is not planned, but induced by the interplay of toxicants with cells. Again, the issue is one of time: abnormal increases of Ca2+ can be coped when their duration is short. The mitochondrial uptake system (see below) can accommodate them, as mitochondria would accumulate the extra Ca2+ together with phosphate, to precipitate insoluble hydroxyapatite within their matrix. Mitochondria are thus safety devices that can buy precious time for the cell, enabling it to survive cytosolic Ca2+ storms. But they can only do it for a short time, as they use the same energy to take up Ca2+, which they use to synthesize essential ATP. If mitochondria are forced to use energy to accumulate Ca2+ for a protracted time a situation of ATP deprivation would ensue, that would even deprive of energy the ATP-dependent Ca2+ pumps that would expel Ca2+ from the cytosol. A negative vicious circle would thus be initiated that would lead to a situation of Ca2+ overload, and would eventually result in cell death. This is so because all Ca2+-controlled stimulated functions would become activated under this condition, including potentially detrimental functions, like proteases, phospholipases, and nucleases. Their uncontrolled activity would damage the cell irreversibly, eventually ending with its death.
In a sense, then, having chosen Ca2+ as a determinant for function, cells are forced to live in a state of permanent controlled risk, in which the possibility of a Ca2+ catastrophe, i.e., of the necrotic cell death resulting from the unwanted global and massive cytosolic Ca2+ overload, is around the corner. But the Ca2+-mediated cell death can also result from the controlled decision of cells to commit suicide. This is the process of programmed cell death (apoptosis), which is one of the meaningful ways in which cells process the Ca2+ signal to control essential processes such as tissue renewal and organ modeling. It has been calculated that a human body of about 70 kg loses (renews) each day a number of cells corresponding to about 1.2 kg. Apoptosis is thus essential to the life of an organism and will be discussed in some more detail later on.
Cell Ca2+, however, may also be deranged in more subtle ways that do not lead to cell death. A number of cell distress conditions exists that may disturb the operation of individual actors in the Ca2+ controlling and signaling operation. Most of these conditions are genetic, and affect proteins (enzymes) that process the Ca2+ signal and/or transport Ca2+ across membrane barriers, thus regulating its homeostasis. These individual defects permit cell life to continue, albeit with various degrees of discomfort that can even be reflected in prominent general disease phenotypes. The area of Ca2+ signaling and disease has now become a popular area of research; a recent book [19] covers it comprehensively.
4 Regulation of the Ca2+ Signal by Ca2+ Buffering and Ca2+ Sensor Proteins
As mentioned, the distinction between Ca2+ buffers and Ca2+ sensors now appears to be less absolute than originally accepted. Distinctive properties of Ca2+ sensor proteins, i.e., the presence of large Ca2+-induced conformational changes, their interaction with specific targets, and the ability to modulate their function as a result of the interaction, are known now to be shared by proteins hitherto classified as pure Ca2+ buffers, e.g, CB-D28k [4,20] and calreticulin [21–24]. Ca2+ buffering proteins are conventionally defined as fast or slow, depending on the rate with which they bind Ca2+. Parvalbumin (PV) is conventionally considered the prototypical slow Ca2+ buffering protein (K d of 4–9 nM), whereas CB-D9k (K d of 200–500 nM) and calreticulin (K d of 2 mM) are routinely classified as fast Ca2+ buffering proteins. The on rate for Ca2+ binding (K on) is 2–3 orders of magnitude faster in CB-D9k than in PV. The specific physiological attitudes and Ca2+ signaling demands of cells determine the expression of slow or fast Ca2+ buffering proteins. Thus, the expression of CB-D9k appears to be restricted to non-excitable cells involved in Ca2+ re-adsorption, e.g., those of various kidney sectors [25], that of PV, in addition to kidneys, to some subsets of neurons [26] and to fast twitch muscles [27]. According to a generally accepted assumption, once Ca2+ gains access to the cytosol it is rapidly buffered by (fast) Ca2+ buffering proteins; the amount that escapes buffering will then activate the targets of the signaling function, for instance, the calmodulin-modulated processes. The list of Ca2+-binding proteins has now grown very impressively. For instance, the superfamily of EF hand proteins, which are the most important Ca2+ sensor proteins, now numbers more than 600 members [28]. Table 1 groups the most important Ca2+ buffering and Ca2+ sensor proteins.
Proteins that are considered as pure Ca2+ sensors may also fulfill an important Ca2+ buffering role, particularly because they are routinely present in cells in high concentrations, on the order of 10 μM or more [4]. Recent work [5] has actually shown that calmodulin, the most important and ubiquitous Ca2+ sensor protein, buffers Ca2+ faster than any other Ca2+ buffering protein. This has led to the proposal that calmodulin would rapidly bind incoming Ca2+, and then pass it on to other, slower, Ca2+ buffers. The proposal is at sharp variance with the common conception according to which calmodulin would instead sense the lower Ca2+ left free by the other Ca2+ buffers. Thus, according to the proposal, slow Ca2+ buffering proteins like PV would regulate the amount of Ca2+ bound to calmodulin, and in this way contribute directly to the regulation of the Ca2+ signal.
However, even if the buffering of cell Ca2+ by Ca2+ sensor proteins may be quantitatively significant, it is not their most important role; as stated above, their primary role is the processing of the Ca2+ signal. The transformation process has been studied in great molecular detail only in the most important sensor protein, calmodulin, but its general principles are likely to be valid for at least the hundreds of sensor proteins of the calmodulin family, the EF-hand proteins [29].
Calmodulin is an elongated protein, in which two terminal lobes, each containing two Ca2+-binding helix-loop-helix motifs, are separated by a long 25-residue α helix. When Ca2+ becomes bound, the protein undergoes a first conformational change that exposes hydrophobic patches, mostly methionine pockets, on the surface of the two lobes. The protein at this stage still maintains its length of 62 Å, but collapses instead around the binding domains of target proteins that have come in contact with it (Figure 3). At this point, the extended dumbbell-shaped calmodulin molecule has the conformation of a hairpin, and the Ca2+ information it originally carried is transferred to the target protein. Calmodulin processes and transmits the Ca2+ information to dozens of targets, i.e., it is not a Ca2+ sensor committed to a single target partner. It becomes temporarily associated with them in its Ca2+-bound form as a separate subunit, and in a small number of cases the association may occur and persist even in the absence of Ca2+ (see below).
The mechanism of the decoding of the Ca2+ message by calmodulin. (a) The binding of Ca2+ induces a conformational change of the calmodulin molecule that exposes hydrophobic patches on its surface (methionine pockets) without changing the overall shape of the molecule. (b) Ca2+ saturates calmodulin which approaches its binding site of a target molecule (the red helix is the calmodulin-binding domain of myosin light chain kinase) collapsing hairpin-like around it. At this point the transmission of the Ca2+ message to the target protein is complete.
Other sensor proteins of the EF hand group, e.g., recoverin or troponin-C, are instead committed to the modulation of the activity of a single target or of a limited number of them. Ca2+ sensor proteins not belonging to the EF hand family, e.g., annexins, gelsolin, proteins containing C2 domains, are generally also committed, i.e., they transform Ca2+ information for the benefit of a single target. The rule, however, is not absolute: important Ca2+ sensor proteins exist that process the Ca2+ signal directly and then act on numerous interacting targets, e.g., by phosphorylating them. A prominent example of Ca2+ sensors of this type is protein kinase C (PKC).
Coming briefly back to calmodulin and the EF hand proteins, it is interesting that some target proteins of the signaling function of Ca2+ possess their own “calmodulin” covalently integrated in the sequence (this is the case, for instance, for calpain). In these cases, the “calmodulin-like” processing of the Ca2+ signal occurs directly within the target protein itself. The pattern of transmission and processing of Ca2+ information is even more complex in other EF hand proteins, such as calcineurin. This phosphatase, in addition to having its own “calmodulin” as a separate subunit, also has a conventional calmodulin binding domain that senses real exogenous calmodulin with the larger subunit and it is, thus, under dual regulation by the Ca2+ signal.
5 Regulation of the Ca2+ Signal by Membrane Transport Systems
5.1 Ca2+ Channels
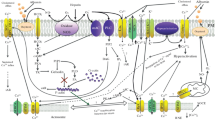
Several plasma membrane Ca2+ channels have been identified and in recent years the focus has moved towards the identification of their distinctive function. The channels have been historically divided in three major groups: the voltage-gated channels (VOCs), the receptor-operated channels (ROCs), and the store-operated Ca2+ entry channels (SOCEs) (Figure 4).
Schematic representation of the different Ca2+ transport proteins of the plasma membrane and the main intracellular stores. For details see the text. TK, tyrosine kinase-coupled receptor; GPCR, G-protein-coupled receptor; PLC, phospholipase C, PIP2, phosphatidyl inositol 4,5-biphosphate, DAG, diacylglycerol.
5.1.1 The Voltage-Gated Channels
The VOCs are key transducers of membrane potential changes into intracellular Ca2+ transients. They are the best characterized and are divided in subfamilies that have distinct roles in biological processes. Their Ca2+ selectivity is high, thus making Ca2+ the preferred permeating species even in the presence of other abundant cations, i.e., Na+ and K+, in the extracellular ambient. They are complexes of 5 distinct subunits (α1, α2, β, γ, δ) encoded by different genes (Figure 5). α1 is the largest subunit and forms the pore: it is organized in four repeated modules of six transmembrane domains (S1-S6), the fourth of which contains the voltage sensor, in analogy with the S4 domain of Na+ and K+ voltage-gated channels [30]. A membrane-associated loop between the 4 S5 and S6 domains forms the channel properly. The β subunit has no transmembrane segments, whereas the γ subunit is a glycoprotein with four transmembrane segments. The α2 subunit is an extracellular extrinsic glycoprotein, bound to the δ subunit through a disulfide linkage that is possibly linked to the membrane through a phosphatidylinositol anchor.
The membrane topology of the 5 subunits of the voltage-dependent plasma membrane Ca2+ channels. Details are found in the text. The positive residues in transmembrane helix 4 in each of the 6-helix transmembrane domain 4 of the α1 subunit are the voltage sensor. Adapted from [31].
Based on physiological and pharmacological properties of the type of current carried, the VOCs can be divided in six classes, termed L, N, P, Q, R and T, distinguished on the basis of the α1 type subunit. In turn, they can be divided in three structurally and functionally related subfamilies: Cav1, Cav2, and Cav3. The Cav1 subfamily initiates muscle contraction, secretion, regulation of gene expression, integration of synaptic signals, and mediates the L-type current. Cav2 subunits conduct N-type, P/Q and R currents, and are mainly responsible for the initiation of synaptic transmission at fast synapses. The Cav3 subfamily is important for the repetitive firing of action potentials in cardiac myocytes and thalamic neurons and is responsible for the T-type current [31].
The diversity of channels structure and function is further enhanced by the presence of multiple β subunits, that are encoded by four different genes [32,33]. Cav1 channels are more abundant in the cell bodies and proximal dendrites of neurons than Cav2 and Cav3 channels, which are instead predominant in nerve terminals and dendrites, respectively. Their preferential locations, coupled with the selective local regulation of Ca2+ by specific buffers, confers specificity to the processes regulated by Ca2+ entry, e.g., it confers to the L-type channels a privileged role in the regulation of gene transcription. Calmodulin binding to the proximal C-terminal domain of Cav1.2 channel is required for the regulation of transcription in neurons [34,35], and calcineurin binding to the distal C-terminus acts as a potential transcriptional regulator as well. The distal C-terminus itself has also been proposed as transcriptional regulator [36].
Cav1 channel activity is also involved in the secretion of hormones from endocrine cells, and is specifically required for some type of synaptic transmission, i.e., in photoreceptors, when continuous neurotransmitter release is necessary.
Cav2 channels are the predominant pathways for Ca2+ entry initiating synaptic transmission by the release of classical neurotransmitters like glutamate, acetylcholine and GABA. Ca2+ entry through presynaptic P/Q and N-type channels initiates exocytosis by triggering the fusion of secretory vesicles with the plasma membrane through the action of the SNARE protein complex of syntaxin, SNAP-25 and VAMP/synaptobrevin [37]. Presynaptic Cav2.1 and Cav2.2 channels interact with SNARE, the interaction being regulated by Ca2+ and protein phosphorylation. The interaction has a dual role. It favors the coupling of Ca2+ channels with secretory vesicles, and it regulates channels activity.
Interestingly, N-type and P/Q-type Ca2+ currents are also regulated by G-proteins, possibly through the activity of Gβγ subunits that shift the voltage dependence of Ca2+ channel activation to more positive values of membrane potential through a mechanism of protein-protein interactions, thus slowing channel activation [38].
Cav3 channels conduct a T-type current, which is activated in the same range of negative membrane potential of the Na+ channels, and it thus well suited to sustain the rhythmic firing of action potential.
5.1.2 The Receptor-Operated Channels
The second class of Ca2+ channels is activated by the interaction with ligands. Most prominent among them is l-glutamate, which is the most important excitatory transmitter in mammalian brain. Glutamate activates two classes of receptors, the ionotropic receptors (iGluRs) and the metabotropic receptors (mGluRs). The iGluRs are ligand-gated non-selective cation channels and are divided in three groups on the basis of the activity of specific agonist: AMPA (2-amino-3-hydroxy-5-lethyl-4-isoxazolepropionic acid), NMDA (N-methyl-D-aspartate), and kainate (KA) [39]. iGluRs are macromolecular complexes composed of four or five subunits, and are predicted to have a bilobar structure, four membrane-spanning helices (however, M2 is not a bonafide transmembrane domain but rather a hairpin loop), with a large extracellular N-terminal domain, and an intracellular C-terminal domain. They depend on ATP for full activity: phosphorylation of their C-terminal domain by PKA, but also PKC and CaMKII, increases currents in all types of glutamate receptors [40–43]. An interesting post-transcriptional mechanism regulates the Ca2+ permeability of iGluRs. RNA editing occurs in the GluR2 subunit of AMPA receptors, as well as in subunit GluR5 and GluR6 of KA receptors, leading to a Gln to Arg substitution in the M2 hairpin loop. The replacement of Gln with a positively charged amino acid is evidently essential to confer Ca2+ impermeability to the channels. The efficiency of editing is higher in the GluR2 subunit than in the GluR5 and GluR6 subunits, and, in the case of GluR6, the editing of other two residues in the M1 transmembrane domain also controls the Ca2+ permeability.
KA and AMPA receptors are the primary receptors for rapid excitatory transmission in the central nervous system and, following glutamate activation, they are primarily permeable to Na+ and K+. However, AMPA and KA receptors may also be permeable to Ca2+. NMDA receptors respond to glutamate more slowly than AMPA and KA receptors, possibly because Mg2+ inhibits them in a voltage-dependent manner: membrane depolarization following AMPA and KA receptor activation relieves the Mg2+ inhibition of NMDA receptors.
The mGluRs, instead, are coupled to G-proteins. Accordingly, they are organized with the canonical seven transmembrane domains. They are encoded by 8 genes (mGluR1-8) and exist as homodimers that generate Ca2+ signals through the activation of distinct downstream signaling cascades that activate PLC and activate, or inhibit, adenylyl cyclase. They are expressed in neuronal and glial cells within the brain, spinal cord, and peripheral neurons and are involved in the pathophysiology of a number of diseases.
mGluR1 is the most abundantly expressed metabotropic receptor in the mammalian central nervous system, with highest expression in the Purkinje cells of the cerebellum. mGluR1 produces two types of neuronal depolarization, a rapid transient depolarization related to the release of Ca2+ from intracellular stores, and a prolonged and larger depolarization resulting from the activation of transient receptor potential (TRP) channels (see below).
5.1.3 The Store-Operated Ca2+ Entry channels
The third class of Ca2+ channels is that of the store-operated Ca2+ entry channels (SOCEs), which are activated by the release of Ca2+ from the ER. They were initially described in non-excitable cells, but they have now been documented in neurons and skeletal muscle cells. The idea that ER Ca2+ depletion could represent a signal for Ca2+ entry can be traced back to early work that had proposed that the biphasic nature of agonist-activated Ca2+-mobilization was due to an initial emptying of the intracellular Ca2+ pool, e.g., by InsP3, followed by the rapid entry of Ca2+ into the cytosol in the continued presence of InsP3. The rapid entry of Ca2+ from outside the cell continued until the Ca2+ content of the store pool reached a level that inactivated it [44].
Later on, it was reported that the depletion of intracellular stores induced by thapsigargin (TG), an inhibitor of the SERCA pump (see below) was per se able to induce Ca2+ entry in different cell types. The identification of a small store-operated Ca2+current (the Ca2+-release activated current, CRAC) [45] activated in mast and T cells independently of the occupancy of surface receptors or of changes in cytosolic Ca2+ enhanced the interest in the topic, but the pathway involved in the process remained obscure for a long time. Different mechanisms were proposed ranging from the existence of free diffusible messengers to a conformational coupling of CRAC channels and InsP3 receptors. A number of candidate genes were proposed as the putative messenger, among them those of the TRP channels, but the molecules involved in the pathway were identified only recently: a Ca2+-binding transmembrane protein of the EF-hand family (STIM proteins) serves as sensor of Ca2+ within the ER. The protein communicates with the plasma membrane store-operated channel that is composed of ORAI subunits. 2 STIM isoforms (1 and 2) were identified and the ORAI protein family was found to be composed of three isoforms [46], in which ORAI1 was demonstrated to be the pore-forming subunit of the channels [47]. Under resting condition, with the STIM proteins fully occupied by Ca2+, STIM1 and ORAI1 would be diffusely localized at the ER and plasma membrane (PM) sites, respectively. When store depletion occurs, STIM1 undergoes a conformational change that redistributes it to specific districts forming “puncta” structures, that correspond to ER-PM junction, i.e., to specialized regions of the ER positioned within 10–20 nm of the PM. At the same time, ORAI1 accumulates at the corresponding PM sites, thus coupling with STIM1 and allowing the opening of the CRAC channels and the generation of localized Ca2+ hot spots [48]. In a second phase, store refilling causes the return of STIM1 and ORAI1 proteins to the original states, thus dissolving the “puncta”. This model, based on distinctive rearrangements of STIM1 and ORAI1 in the cell, would require seconds for the activation of the channels. This timing has functional consequences, e.g., in T cells the “puncta” assembling and disassembling may generate Ca2+ oscillations [49] that would in turn drive gene expression through NFAT and other transcription factors [50,51] (see below).
5.1.4 Transient Receptor Potential Channels
Another class of channels, which had been originally related to the SOCE channels, and which can also generate changes in intracellular Ca2+ concentration by mediating its entry across the PM are the TRP channels.
They constitute a large and functionally versatile family of cation-conducting channels, and are generally considered cell sensors. They are expressed in a large number of tissues and cell types (excitable and non-excitable) and, when activated, cause a cell depolarization that in turn may trigger the activation of different voltage-dependent ion channels. In mammals, 28 TRP channels have so far been found, classified according to their homology in 6 different subtypes: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRPML (mucolipin), and TRPP (polycistin). The literature on TRP channels has now grown very impressively and only a short overview can be given here. More detailed information can be found in a number of comprehensive reviews, e.g., [52,53].
A common theme that links the TRP channels is their activation or modulation by phosphatidylinositol phosphates, such as phosphatidylinositol 4,5-bisphosphate (PIP2) [54]. However, they are also modulated by Ca2+, which is responsible for generating both a positive and a negative feedback. They are organized with six transmembrane domains, and most probably assemble into tetramers to form non-selective cationic channels. The TRP channels can contribute to change the intracellular Ca2+ concentration either directly by acting as a Ca2+ entry pathway (even if their selectivity for Ca2+ differs in the different subtypes), or indirectly by changing the membrane polarization. The TRPC-type channels have been claimed to have a special relationship with the SOCEs (and their constituents STIM1 and ORAI1); the issue has been a matter of vigorous discussion in the field of Ca2+ signaling. It is now generally accepted that ORAI1 may interact with TRPCs and act as regulatory subunit that confers STIM1-mediated store depletion sensitivity to them [55–57]. Thus, in a sense, the TRPC channels might act as SOCEs, even if they are a distinct type of channel with their own properties: high Ca2+ selectivity, very small single channel conductance, and different Ca2+ modulation.
A final comment is necessary on TRP channels: even if it is clear that they have different functional effects depending on their strategic localization on the plasma membrane, most of them are also localized in the membrane of the intracellular organelles. Thus, TRPV and TRPP channels have been found on ER and Golgi membranes, and TRPMLs have been proposed to mediate a NAADP-activated intracellular Ca2+ release from endosomes and lysosomes (see below).
5.1.5 The Intracellular Ca2+ Channels
The endo/sarcoplasmic reticulum (ER/SR) and more recently, the Golgi apparatus (GA), are recognized as the main intracellular Ca2+ stores. Two types of Ca2+ receptors/channels essentially operate Ca2+ mobilization from them, the ubiquitous inositol 1,4,5-trisphosphate receptors (InsP3R) and the ryanodine receptor (RyR), which is not present in all cell types. InsP3R and RyR are channels with large conductance. They are only relatively selective for Ca2+, at variance with the voltage-gated and store-operated plasma membrane Ca2+ channels that are more selective. However, considering that Ca2+ is probably the only cation with an appreciable electrochemical gradient across the ER/SR membrane the lack of selectivity does not represent a problem.
The InsP3Rs are encoded by three different genes that have distinct patterns of tissue expression (however, some overlapping occurs, especially during cell differentiation) and contribute to shaping different Ca2+-linked signaling pathways. The channels are constituted by homo- or hetero-tetramers of a large (2700 residues) protein spanning the membrane with a hydrophobic region containing six helices. A partial 3D structure of the channel has recently become available [58]. The N-terminus and the C-terminus of the protein are in the cytosolic region, the N-terminal region representing the portion of the protein, which contains the InsP3 binding domain and the “regulatory” domain. The opening of the InsP3R is controlled by the binding of the second messenger InsP3 (generated by activation of PLC enzymes), mainly PLC β-stimulated by G-protein-coupled receptors, and PLCγ by tyrosine kinase receptors.
A flexible linker region, connecting the InsP3 binding domain with the first 200 amino acids (residues 1–223 of isoform 1) of the protein, is essential for the modulation of pore opening, possibly by decreasing InsP3 affinity. Several molecules interact with the InsP3R and modulate its activity, e.g., homer family adaptor proteins, protein phosphatases (i.e., calcineurin), PKA, PKC, and CaMKII, the tacrolimus-binding immunophilin FKBP12, IRBIT, ATP, and Ca2+. Ca2+ is probably the most important interactor as it has both stimulatory and inhibitory effects, however, the structural basis of its regulation is still not understood [59]. Cytosolic Ca2+ is a co-agonist of the InsP3Rs, as it strongly increases its activity at concentrations up to about 300 nM. By contrast, at higher concentrations it inhibits the receptor. Luminal Ca2+ also sensitizes the InsP3Rs, possibly by tuning its sensitivity to cytosolic InsP3. A Ca2+-mediated inhibition of the receptor is assumed to contribute to the termination of local cytosolic Ca2+ signals. However, it is not clear whether this effect depends on the binding of Ca2+ to the receptor or to an associated protein [60]. Calmodulin had been initially suggested as a candidate protein, but the suggestion has now lost momentum.
The RyRs are also encoded by three distinct genes with different tissue expression pattern. RyR1 is expressed in skeletal muscle, RyR2 in heart, cerebellum (Purkinije neurons), and cerebral cortex, and RyR3 is more ubiquitous, even if with low levels of expression. The RyRs are formed by homo-tetramers that associate to form the largest known channel (>2 MDa). Cryoelectron microscopy studies have contributed to the understanding of the functioning of this gigantic molecule (reviewed in [61]). The C-terminal portion of the protein forms the pore and the large cytoplasmic region contains the sites where most RyR modulators interact. The major gating mechanism is the excitation-contraction (E-C) mediated coupling with the voltage-dependent Ca2+ channel dihydropyridine receptor (DHPR) located in the T-tubules. The molecular mechanism of E-C coupling differs between skeletal and cardiac muscle [62]. In skeletal muscles a physical interaction (electromechanical coupling) between the Cav1.1 DHPR and RyR1 is required; in cardiac muscle Ca2+ release by the RyR2 is initiated by Ca2+ influx via Cav1.2, i.e., by the Ca2+-induced Ca2+ release (CICR). In heart, then, the interaction is functional rather than physical as in the case of RyR1. CIRC can also originate from the flickering of ER Ca2+ channels and, even if originally described for the gating of RyR2, is now recognized as the major gating mechanism for RyR3. Other agents can gate RyR2 and 3, i.e., cyclic ADP ribose, cADPR, generated by ADP-ribosyl cyclases, in particular by their ectoenzyme form [63]. cADPR appears to act mainly in smooth muscle cells [64], pancreatic acinar cells [65], and in the nervous system [66].
The InsP3R the channel activity of the RyR is also modulated by a number of molecules, e.g., PKA, FK506 binding proteins (FKBP12 and 12.6), calmodulin, Ca2+/calmodulin-dependent protein kinase II, calsequestrin, triadin, junctin, Mg2+, ATP, and Ca2+ itself.
5.2 Ca2+ Pumps
Animal cells express three Ca2+ ATPases (pumps) in the PM (PMCAs), in the ER/SR (SERCAs), and in the Golgi membranes (SPCAs). They lower the concentration of cytosolic Ca2+ by exporting it to the external medium, or to the internal space of the vesicles of the reticulum and of the Golgi system. The three pumps, like additional Ca2+ pumps in plant cells and in cells of lower eukaryotes, which will not be discussed here, belong to the superfamily of P-type ATPases [67] which conserve temporarily the energy liberated by the splitting of ATP in the form of an aspartyl phosphate in their reaction center. The superfamily now contains hundreds of members, sub-grouped in at least 8 subfamilies (Figure 6).
Phylogenetic tree of Ca2+ transporting ATPases (pumps). The sequences were aligned with ClustalW software, and the tree was generated using Tree View. The 3 branches represent the 3 Ca2+- ATPases discussed in the text. Adapted from [71].
The mammalian ATPases belong to sub-groups II A (the SERCA and SPCA ATPases) and IIB (the PMCA ATPases). They display significant sequence differences in regions not directly related to the catalytic mechanism, i.e., in areas related to regulation and interaction with inhibitors and other partners, but share essential properties, e.g., membrane topography and the general reaction mechanism. The reaction scheme of the three pumps (Figure 7) had initially predicted two functional/conformational states: in the E1 state the pumps would have high affinity for Ca2+ and would interact with it at one membrane side, and in the E2 state the affinity for Ca2+ would become much lower, causing its release to the opposite side of the membrane [68]. The solution of the 3D structure of the SERCA pump at the atomic level 12 years ago [69,70] has confirmed the basic principle of the E1-E2 reaction scheme, but has greatly increased the complexity of the catalytic mechanism, showing that the binding of Ca2+ at one side of the membrane induces a series of large conformational transformations that switch the extra-membrane portion of the pump from a compact to a more open structure. The conformational changes, however, also involve the transmembrane domains of the pump, leading to the phosphorylation of the catalytic aspartic acid by ATP and, in a series of documented conformational transitions, to the change of the high affinity phosphorylated E1 pump to a lower Ca2+ affinity state that leads to the dissociation of Ca2+, regenerating the Ca2+-free E2 enzyme.
The cartoon of Figure 8 [71], which is reproduced with minor modifications from a review by Toyoshima [72], offers a pictorial view of the atomic path by which Ca2+ crosses the membrane of the SR on its way from the cytosol to its lumen. It contains details on the atomic aspects of the transfer that cannot be described and explained in the context of this review. A full discussion of them can be found in [72]. The 3D structure has confirmed the existence, in the SERCA pump, of the two Ca2+ binding sites that had been predicted by mutagenesis work [73,74]. The two sites are a peculiarity of the SERCA pump; the PMCA pump has only one, corresponding to site 2 of the SERCA pump, as it lacks an essential acidic residue in the transmembrane domain 5 [75]. This residue is also absent in the SPCA pump, which also has only one Ca2+ binding site.
A cartoon illustrating the conformational changes of the main domain of SERCA pump during the reaction cycle. The model is based on the three dimensional structure of the SERCA pump. Adapted from [72].
The three mammalian pumps are all inhibited by the general inhibitors of P-type ATPases La3+ and orthovanadate [(VO3(OH)]\( {}_{2}^{-}\), although mechanistic differences in the case of La3+ exist for the case of the PMCA pumps. The SERCA pump is also specifically inhibited by compounds that are inactive against the other two pumps, e.g., TG. Inhibitors of similar specificity and potency are not available for the other two pumps. Interestingly, however, 2 peptides of the caloxin family (caloxin 2A1 and caloxin 1A1) have been claimed to inhibit the PMCA pump by interacting with its extracellular domains 2 and 1, respectively [76,77].
All Ca2+ pumps interact with Ca2+ with high affinity [71], and are thus the fine tuners of cell Ca2+. Their K m(Ca2+) is well below 1 μM. The affinity for Ca2+ is particularly high in the SPCA pumps, whose K d is about 10 nM in the SPCA1 isoform, and even lower in the SPCA2 isoform [78,79]. The extremely high Ca2+ affinity of the SPCA pumps, which have K ds well below the concentration of Ca2+ in the cytosol at rest, ensures that the Golgi vesicles will be always filled with Ca2+ even in the absence of agonist-induced cytosolic transients. This is crucial, since Ca2+ is required for the activity of enzymes within the Golgi vesicles, most notably the endoproteases that process the pro-hormones. Importantly, the SPCAs also transport Mn2+, which is essential inside the Golgi vesicles for the O- and N-glycosylations of a number of proteins [80,81]. The Ca2+ affinity of the PMCA pump requires another comment. The pump is a classical target of calmodulin regulation (see above). In its absence it can still interact with Ca2+, but only with very low affinity, i.e., with a K d between 10 and 30 μM [82]. The K d drops to about 0.5 μM in the presence of calmodulin [82] which interacts with a C-terminal domain of the pump [83], with a K d in the nM range [84].
This domain, however, also plays another role in the regulation of the activity of the pump. In the absence of calmodulin it folds over, binding to two sites in the main body of the enzyme. It keeps the pump auto-inhibited until calmodulin removes it from the binding sites, relieving the autoinhibition [85,86]. Of the 4 basic PMCA isoforms (see below), one, PMCA2, behaves peculiarly in its reaction to calmodulin [87,88]; it expresses very high activity even in its absence. Since the PMCA2 calmodulin-binding domain does not differ from that of the other 3 basic PMCA isoforms, it is likely that the high activity in the absence of calmodulin reflects the suboptimal ability of the calmodulin-binding domain of PMCA2 to interact with the autoinhibitory site(s) in the main body of the pump. Another important aspect of the Ca2+ affinity of the PMCA pump is its stimulation by acidic phospholipids, which decreases the K d to values even lower than those achieved with optimal calmodulin (about 0.2 μM [89]). The significance of the activation by acidic phospholipids, which bind to the basic calmodulin domain, but also to another domain in the first large cytosolic loop [90,91] is still obscure, but it appears possible that phospholipids could activate the pump in vivo in alternative to calmodulin.
The three mammalian Ca2+ pumps are the products of multigene families: separate genes express three basic isoforms of the SERCA pump, four of the PMCA pump, two of the SPCA pump. The basic isoforms of each pump type share reaction mechanism and membrane topology, but differ in tissue distribution, regulation properties, and in some details of activity, e.g., the affinity for Ca2+. Their number is greatly increased by the alternative splicing of the primary transcripts of all pumps (SPCA2 is the only exception), the functional differences among the splicing products being in general greater than those among the original basic gene products. A detailed discussion of all splicing variants of the pumps would be out of the scope of this contribution; the short description offered here will thus only underline aspects and variants that are particularly significant.
The transcripts of the SERCA genes are subjected to alternative processing at their 3’ end, generating a number of splice variants with specific tissue distribution and activity. That of SERCA1 is spliced to generate the SERCA1a and 1b variants, which are expressed in adult and neonatal fast-twitch skeletal muscles, respectively. The change in expression pattern during development and tissue differentiation indicates that each isoform is adapted to specific functions. The transcript of the SERCA2 gene is alternatively processed to generate the SERCA2a variant, which is expressed selectively in heart, slow-twitch skeletal muscles, and smooth muscle, and to the SERCA2b variant which is expressed ubiquitously and is thus considered as the housekeeping SERCA pump [92]. Interestingly, the extended, 49 amino acid long C-terminal portion of the SERCA2b pump, which contains a highly hydrophobic segment that forms an additional transmembrane domain (11th) [93], confers to the variant higher affinity for Ca2+ and lower catalytic turnover rate [92,94]. Both SERCA2a and 2b are sensitive to the membrane protein phospholamban, which regulates their activity by becoming reversibly bound to them in a process that depends on its phosphorylation by PKA (possibly, also by a calmodulin-dependent protein kinase). Unphosphorylated phospholamban binds to the pump maintaining it inhibited, phosphorylated phospholamban leaves the binding site(s), restoring pump activity, for instance during β-adrenergic stimulation [95–97]. SERCA3 is expressed in a limited number of non-muscle cells, and the splicing pattern of the transcript of its gene is complex. All documented variants have lower Ca2+ affinity than the other basic isoforms. SERCA3 seems to be specialized for the control of vascular and tracheal smooth muscles, its low Ca2+ affinity suggesting that it would only become activated when cytosolic Ca2+ reaches abnormally high levels.
The two basic products of the SPCA genes have different tissue distribution. The SPCA1 pump is ubiquitous, and is thus considered the housekeeping isoform. Its expression level varies with the tissue, and is particularly high in human epidermal keratinocytes [98]. The expression of SPCA2 is much more tissue restricted. Its transcript has been found in particularly high amounts in the mucus-secreting goblet cells of human colon [99] indicating its possible role in the regulation of the secretion of mucus. Alternative splicing has only been documented in the primary transcript of SPCA1, resulting in the generation of four transcripts. Very little is known on their possible differential properties, although some kinetic differences have been described.
The four basic PMCA gene products differ in tissue distribution and calmodulin affinity. Pumps 1 and 4 are ubiquitous and have poorer calmodulin affinity than isoforms 2 and 3. They were both considered as housekeeping pumps, but recent work has indicated that PMCA4 could have more specialized roles, e.g., in the testis where it represents more than 90% of the total PMCA protein [100]. The ablation of the PMCA4 gene causes male infertility, due to the inability of the sperms to achieve hyperactivated motility, and thus to reach the eggs to perform the fertilization [101]. PMCA2 and 3 have higher calmodulin affinity and their expression is restricted to a limited number of tissues; PMCA2 is expressed prominently in the nervous system and in the mammary gland, and PMCA3 in the nervous system and skeletal muscles [102]. The transcripts of all PMCA genes undergo alternative processing at two sites. Site A corresponds to the portion of the pump located upstream of the phospholipid binding domain in the first cytosolic loop. Site A insertions lead to a number of variants depending on the number of exons inserted; the most important is variant w in which the insertion of 3 exons directs the pump (the isoform tested was PMCA2) to the apical plasma membrane of polarized cells, whereas smaller inserts sort the variants to the basolateral domain [103]. Site C corresponds to the C-terminal calmodulin binding domain of the pump and generates a plethora of variants depending on the type of alternative processing, in which portions of exons can be inserted piecemeal. In most cases the result of the site C insert(s) is a change of the reading frame, and the creation of a premature stop codon that truncates the resulting pump protein. Pumps in which no site C inserts occur are designated as b variants, whereas those with various inserts are designated as c, d, e, f, and a. The most important is variant a, in which one full exon (PMCA1, 3, and 4), or two full exons (PMCA2) are inserted.
The alternative processing of the transcripts of the PMCA1, 3, and 4 genes occurs in an essentially similar way. That of the gene of PMCA2, however, has peculiar complexities. Together with other properties, for instance the ability of the pump to function at a very high rate in the absence of calmodulin (see above), the complexity of alternative processing singles out PMCA2 from the other 3 basic PMCA isoforms.
5.3 The Plasma Membrane Na+/Ca2+ Exchanger
The plasma membrane of most animal cells contains a system with lower Ca2+ affinity than the PMCA pump that ejects Ca2+ in exchange with Na+ (NCX). The system belongs to the SLC8 (solute carrier family 8) superfamily of Na+/Ca2+ exchangers. The superfamily also contains SLC24 that transports K+ as well; a cluster of 23 orthologous genes (COG0530) are named Ca2+/Na+ antiporters, even if no functional data have so far been produced. The NCX is particularly active in the cells of excitable tissues, and uses the energy of the electrochemical Na+ gradient to allow Na+ to flow into the cell across the PM in exchange for the export of Ca2+, with a transport stoichiometry of 3 Na+ for 1 Ca2+. It has a much larger transport capacity than the PMCA, transporting thousands of Ca2+ ions per second [104], a property that, coupled to its low Ca2+ affinity, allows it to return the cytosolic Ca2+ concentration to its normal resting low levels after large physiological increases, for instance those produced by the neuronal action potentials. Since the operation of the NCX is electrogenic and voltage-sensitive, it can reverse during cell activation and lead to the intake of Ca2+ into the cell [105]. The reversal of the canonical direction of the exchanger is also induced by changes in the concentration, i.e., the gradient, of the transported species. This occurs, for instance, in the case of heart cells exposed to the action of digitalis: the inhibition of the Na+/K+-pump increases Na+ in the sarcoplasm, reversing the operation of the exchanger and inducing the well-known positive inotropic effect linked to the influx of Ca2+.
Three genes code for distinct isoforms of the NCX in mammalian cells (NCX 1-3). They have variable tissue distribution and regulatory properties. NCX1 is distributed ubiquitously, whereas the expression of NCX2 and NCX3 is restricted to brain and skeletal muscles [106,107]. All NCXs are predicted to contain nine membrane-spanning domains, separated in two parts of the sequence by a 500-residue intracellular loop [108,109]. Interestingly, the loop can be removed still leaving behind an active exchanger [110], showing that the transmembrane portion of the exchanger is the basic functional transport unit (the 3D structure of a prokaryotic exchanger has recently been published, showing 10 transmembrane domains instead of the 9 of the eukaryotic exchangers [111]). Each exchanger protein contains two conserved homologous α repeats, one on each of the two transmembrane domains, arising from a gene duplication event, that are important for the binding and the translocation of ions [109]. The two motifs are also present in other members of the Na+/Ca2+ exchanger superfamily, e.g., the NCKX, that also exchanges K+ and was first identified in the retina [112], and in microbial exchangers that lack the large intracellular loop [109,113]. They are not present, however, in NCLX, a philogenetically ancestral branch of the Na+/Ca2+ exchanger superfamily which also exchanges Li+ [114,115], and which has recently been identified as the long sought mitochondrial Na+/Ca2+ exchanger (see below). The number of exchanger isoforms is increased by alternative processing of the transcripts in a region corresponding to the C-terminal portion of the large cytosolic loop. The processing of the primary transcript of NCX1 gives rise to a complex set of splice variants that differ in the 561–681 stretch of the protein. The transcript of NCX3 also undergoes alternative splicing in a region corresponding to a similar location in the protein as NCX1, whereas no splicing products have so far been described for NCX2.
Operationally, the NCX is activated by the binding of intracellular Ca2+ to a Ca2+-binding domain 1 (CBD1) in the main cytosolic loop that triggers a conformational change that transforms the NCX into an activated state. The 3D crystal [116] and NMR [117] structures of CBD1 have recently been solved, and shown to bind 4 Ca2+ to an immunoglobulin-like fold. A second Ca2+-binding domain was also identified [117] (CBD2), and a structural model was built of the entire regulatory loop which is given in the cartoon of Figure 9. A detailed discussion of the model is out of the scope of this contribution, but can be found in [117] and [118]. Basically, in the model CBD1 and CBD2 are arranged in antiparallel fashion, the Ca2+-binding region of CBD1 unfolding when Ca2+ is removed, possibly moving the exchanger into an inactive state. Hilge et al. [117] have presented a structure for each of the two main splicing variants of the NCX, which contain the mutually exclusive CBD2 encoding exons A and B. At variance with CBD2A, CBD2B has unstructured Ca2+-binding sites under physiological conditions, suggesting lower Ca2+ fluxes in non-excitable cells that contain exon B compared with excitable cells that contain exon A.
A model for the structure of the plasma membrane Na+/Ca2+ exchanger. The cartoon is a modified version of the model proposed by Hilge et al. [117].
An interesting recent development relates NCX (or, rather, NCKX) to the color of the skin. The allelic frequency of a single nucleotide polymorphism (SNP) in the coding region of member 5 of SLC24A family (which contains 6 members) varied considerably between populations of Caucasian and African ancestry [119]. The study was extended to zebrafish [120] and other human groups, and has shown that the SLCA24A5 encodes a protein similar to the K+-dependent members of the Na+/Ca2+ exchanger superfamily.
6 Intracellular Organelles
6.1 Mitochondria
Mitochondria are intracellular organelles endowed with two membrane barriers with decreasing ion permeability properties: the outer mitochondria membrane (OMM) is freely permeable to ions and small molecules, and the inner mitochondrial membrane (IMM), which is folded into the internal invagination called “cristae”, contains the multi-enzymatic complexes of the respiratory chain, of the ATP synthase and the Ca2+ transport systems.
Mitochondrial Ca2+ transport has unique characteristics. The uptake does not need ATP hydrolysis for Ca2+ entry, but utilizes a uniporter and the membrane potential (ΔY, negative inside) maintained across the inner membrane by the respiratory chain as the driving force. The uniporter has been proposed to be a gated and highly selective ion channel [121,122]. For Ca2+ efflux, mitochondrial exchangers use the concentration gradient of Na+ (H+ as well in some mitochondrial types) across the inner membrane to cause the release of Ca2+ back into the cytosol [123]. The cycle is then completed thanks to the efflux of Na+ via the Na+/H+ exchanger (NHE) (Figure 10) [124]. Under resting conditions the rates of Ca2+ influx and efflux are slow and ensure the maintenance of a low matrix Ca2+ concentration. The kinetic equilibrium between influx and efflux thus results in a futile (energy consuming) cycle of Ca2+ across the mitochondrial inner membrane [125]. When cytoplasmic Ca2+ increases above a given threshold (>10 μM) a rapid Ca2+ accumulation by mitochondria is initiated and matrix Ca2+ increases dramatically. Finally, excess Ca2+ accumulation by mitochondria (mitochondrial Ca2+ overload, MCO) may result in the opening of a large non-selective channel in the inner mitochondrial membrane, the mitochondrial permeability transition pore (mPTP) that collapses the membrane potential, induces swelling of the inner membrane and rupture of the outer one, and releases proteins of the intermembrane space (IMS) into the cytoplasm.
Schematic representation of Ca2+-handling organelles and their molecular toolkit. Mitochondria, endoplasmic reticulum (ER), Golgi apparatus, and acidic stores are shown. For details see the text. ER mitochondria contact sites are shown by the juxtaposition of the MCU and InsP3 receptor. Interestingly, the voltage-dependent cation channels (VDAC) of the OMM clustered at the ER/mitochondrial contact sites play a key role in the rapid transfer of the high Ca2+ microdomain from the surface of the mitochondria to the intermembrane space to which the MCU is exposed [228].
After the discovery that isolated respiring mitochondria were capable to sustain Ca2+ accumulation [126,127], many aspects of the mitochondrial Ca2+ uptake and extrusion mechanisms were clarified. Thus, it was established that Ca2+ uptake was an electrogenic process, which was countered by Ca2+ efflux so that electrochemical gradient equilibrium did not occur [128].
However, the finding that the uptake system operated with very low Ca2+ affinity appeared difficult to reconcile with its function in the extremely low cytosolic Ca2+ concentration. For a while, then, the idea that mitochondria could efficiently control the homeostasis of Ca2+ in the cell lost favor, even if research on the topic continued to produce information. Thus, patch clamp experiments on mitoplasts (swollen mitochondria without the OMM) showed that the uniporter (MCU) is a highly selective hardly saturable Ca2+ channel with an activation domain and a transport site [121]. Pharmacological studies led to the identification of compounds able to either inhibit or activate the MCU, e.g., ions like lanthanides, Mg2+, ruthenium red (RR) and its derivate Ru360 (reviewed in [129]), and the plasma membrane Na+/Ca2+ exchanger inhibitor KB-R7943 [130]. Physiological concentrations of polyamines, such as spermine and related compounds [131], were instead shown to activate the MCU at Ca2+ concentrations that would otherwise be too low to allow the uniporter to operate efficiently: they could thus have a physiological role in intracellular Ca2+ handling.
The efflux route of Ca2+ from mitochondria was documented as Na+-dependent pathway by the observation that the addition of Na+ to isolated mitochondria promoted the efflux of Ca2+ [123]. Further work then characterized the pathway as a Na+/Ca2+ antiporter (NCLX) [132]. The transport was later found to be electrogenic with a probable transport stoichiometry of 3 to 1, as in the case of the plasma membrane NCX [133,134]. The NCLX was inhibited competitively by Sr2+, Ba2+, Mg2+ or Mn2+, and by many compounds of pharmacological interest including diltiazem, clonazepam, verapamil, tetraphenyl-phosphonium, trifluoperazine amiloride and its derivatives. In particular, the chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3 H)-one (CGP 37157) inhibited it with high specificity and it is now widely used [135]. In the exchange process Na+ could be replaced by Li+, an observation that was later used in the work that identified the exchanger protein (see below).
A Na+-independent Ca2+ extrusion mechanism has also been described in liver [136] and some other mitochondrial types. It transports Ca2+, but also Sr2+, or Mn2+ from the matrix to the intermembrane space against the Ca2+ electrochemical gradient. The rate of efflux via this mechanism decreases with increasing ΔpH (internally alkaline) [137]. The transport is electroneutral and it has been characterized as a 1 Ca2+ for 2 H+ exchanger [138]. Cyanide, low levels of uncouplers, and very high levels of RR inhibit it [139]. In spite of the large mass of information that was becoming available, the low Ca2+ affinity problem led to the general assumption that Ca2+ sequestration by mitochondria in living cells had no important role in the regulation of Ca2+ homeostasis, unless in extreme conditions of Ca2+ overload [140]. The very limited Ca2+ transport activity (assumed to occur in vivo) was essentially only considered important for the activation of 3 matrix deydrogenases that have been found to be controlled by Ca2+ [141]. Thus, even if information had become available that mitochondrial Ca2+ transport did occur in vivo in spite of the insufficient affinity of the system, skepticism prevailed. At the beginning of 1990s the conundrum was solved by specifically targeting a recombinant Ca2+ sensor to the mitochondrial matrix. The work clearly demonstrated that in intact cells mitochondria promptly accumulated Ca2+ following cell stimulation [142]. The problem of the low affinity of the mitochondrial uptake system was overcome by demonstrating that mitochondria could sense localized microdomains at high Ca2+ concentration generated close to the mouth of the ER Ca2+ channels [143] in close proximity to mitochondrial Ca2+ uptake sites [144], and presumably also by functional coupling with Ca2+ entry channels at the plasma membrane [145,146]. The finding renewed interest in mitochondrial Ca2+ and on its physiological role and sparked new research aimed at identifying the mitochondrial Ca2+ transporters.
Their molecular identity had remained elusive for a long time (for comprehensive reviews see [147,148]). Different actors had been proposed but none of them had survived conclusive tests. Only very recent research has eventually identified molecularly the NCLX and the MCU [149–151]. A uniporter component (named MICU1) that may have a role in Ca2+ sensing rather than in Ca2+ transport has been identified in silico by developing a MitoCarta database [152] as a 54 kDa protein, associated with the mitochondrial inner membrane. It has one putative transmembrane domain and two canonical EF hands.
Using the same database, two independent groups then identified an integral inner membrane protein that satisfies the criteria for being the pore-forming subunit of the uniporter MCU [149,150]. The 40 kDa protein is ubiquitously expressed in mammals, but missing in yeast (yeast mitochondria do not have a uniporter [153]). It is predicted to have two transmembrane regions connected by an acidic loop, and it forms oligomers in the inner membrane. Its downregulation drastically reduced mitochondrial Ca2+ uptake and its overexpression enhanced it in intact cells. Most importantly, the channel activity of purified MCU reconstituted in a planar lipid bilayer revealed properties previously reported for the uniporter, thus definitively demonstrating that MCU represents its pore-forming channel [149].
The Na+/Ca2+ exchanger (NCLX) has also been identified [151] as a mammalian member of the phylogenetically ancestral Ca2+/anion exchanger family that catalyzes Na+ (or Li+) dependent Ca2+ transport [113]. NCLX was found to be enriched in the mitochondrial cristae. As expected, it transported Li+ in addition to Ca2+ and was sensitive to CGP-35137. Its size was very similar to that of a mitochondrial protein that, when purified and reconstituted, exhibited Na+/Ca2+ exchange activity [154,155].
In addition to the antiporters, the other mechanism of Ca2+ transport that may play a role in the mitochondrial Ca2+ efflux, especially in conditions in which mitochondrial Ca2+ concentration in the matrix reaches threshold levels, is still molecularly unknown; this is the mPTP [156]. In addition to Ca2+, factors such as pH, adenine nucleotides, free radicals, and the mitochondrial membrane potential (ΔΨm) modulate its opening. Mitochondrial Ca2+ overload and excess increases in reactive oxygen species (ROS) in the matrix would be the “point of no return” that causes permeabilization of the inner membrane, proton electrochemical gradient dissipation, ATP depletion, further ROS production and organelle swelling. These events are collectively termed “mitochondrial permeability transition” (MPT), a process that, in turn, causes the release of cytochrome c and culminates in cell death.
6.2 The Acidic Compartments
In addition to the ER/SR and the GA, other acidic organelles, such as the acidic endosomes, lysosomes, and secretory granules are now also considered as possible Ca2+ stores in mammalian cells. Their Ca2+ transport functions are not yet well characterized and the literature on the “acidic Ca2+ stores” has controversial aspects, especially on the possibility of a still unknown, ATP-dependent Ca2+ uptake mechanism, at least in lysosomes. The Ca2+ uptake through this system appears to rely on the large proton gradient established by the vacuolar proton V-ATPase. A Ca2+/H+ exchanger has been proposed, but Ca2+/H+ exchangers have so far only been found in protist, yeast and plant vacuoles [157]. The total Ca2+ content in the acidic Ca2+ stores changes with the organelle type, but has been claimed to be in the mM range. However, direct measurements of free Ca2+ in the lumen of the organelles have reported values in the μM range, the discrepancy being possibly due to the presence of Ca2+-binding proteins, such as chromogranins and secretogranins, with a large Ca2+-buffering capacity [158]. A direct measurement of lysosomal luminal Ca2+ is difficult, due to the very acidic environment and to the presence of proteolytic enzymes, however, a specific localized probe has revealed a very high Ca2+ concentration (about 500 μM [159]).
The acidic organelles have also been reported to be able to release Ca2+, supporting a possible physiological role in modulating specific cell function such as secretion, endosome-lysosome fusion and, possibly, maintenance of osmoregulation. The release of Ca2+ has been reported to be promoted by all canonical second messengers described for the ER/SR and the Golgi, i.e., InsP3, ryanodine, caffeine, and cADPR. Importantly, the most efficient Ca2+-releasing agent is the novel Ca2+-linked messenger NAADP (nicotinic acid adeninedinucleotide phosphate) [160], as mentioned, the ectoenzyme ADP-ribosyl cyclase, produces cADPR, but also produces NAADP from NADP and nicotinic acid. NAADP-sensitive Ca2+ release has been reported from endosomes [161], lysosomes [162], and secretory granules [163], but the existence of a specific NAADP receptor in the acidic organelles is still not conclusively established. cADPR and NAADP would operate on a non-selective cation channel, the transient receptor potential mucolipin 1 channel (TRPML1) which is present in lysosomes [164]. A new family of channels, called “two pore channels” (TPC) has also been proposed to operate in the membrane of acidic organelles. They are present ubiquitously in mammalian cells and can be divided in three subtypes according to their specific localization: TPC1 and 3 are found in endosomes, TPC1 mainly in lysosomes [165]. An interesting aspect of these channels is that the release of Ca2+ by TPC1 generally leads to a spatially restricted Ca2+ signal, whereas that operated by TPC2 triggers ER Ca2+ release by activating InsP3/ryanodine receptors, enhancing the propagation of a global signal [166].
6.3 Ca2+ Regulation in the Nucleus: An Open Problem
The nucleus is the seat of numerous functions that are regulated by Ca2+. Some are specific of the organelle, beginning with the expression of some genes, and which will be discussed in more detail later on. The presence of specific Ca2+ regulated functions would prima facie demand that Ca2+ in the nucleoplasm be regulated independently of the cytosol. The problem is that the nuclear envelope, which is an extension of the ER that separates the nucleoplasm from the cytosol, is interrupted by numerous nuclear pores, complex structures that form openings with a diameter of about 9 nm that allow the traffic of nucleic acids, proteins, and other macromolecules. If no mechanism existed to temporarily occlude the pores, nuclear Ca2+ would thus instantaneously equilibrate with Ca2+ in the cytosol. In line with this idea, numerous experiments with various Ca2+ indicators, including some in which the indicator was selectively targeted to the nucleoplasm [167,168], have indeed shown that the kinetics of cytosolic and nuclear Ca2+ increases induced by cell stimulation were temporally indistinguishable, suggesting that the envelope did not represent a barrier to the free diffusion of Ca2+. Others, however, using the same technique found that the Ca2+ signals evoked by the stimulation of cells were invariably lower in the nucleus [169]. Persistent gradients of Ca2+ between the nucleus and the cytosol were also observed by directly injecting Ca2+ dyes into the nucleus of starfish oocytes [170]. Patch clamp experiments on the envelope of isolated nuclei [171], and even on the nuclear envelope in situ [172] are also difficult to reconcile with the idea of free diffusion of Ca2+ between the cytosol and the nucleoplasm. They showed no flow of current during long recording periods in spite of the presence of hundreds of pores in the patch, but recorded instead the activity of selective K+ channels with multiple conductance states. The logical conclusion of this electrophysiological work would be that for significant periods of time the pore would remain sealed to ions, including Ca2+. The mechanism of the putative gating of the pores is unknown, but atomic force microscopy work has shown that most pores contain a “plug” that could be part of the gating mechanism [173], and that the conformation of the pores is altered by extranuclear Ca2+ and ATP [174]. Thus, the matter of the Ca2+ permeability of the nuclear envelope is still an open issue. Perhaps, a conciliatory view could propose that the pores would exist in freely permeable or gated states depending on physiological conditions and demands (see some comprehensive reviews for a full discussion of the issue [104,175,176]).
A recent development on the matter of the role of Ca2+ in the nucleus and on its release to it is the demonstration that the envelope folds inside the nucleoplasm forming invaginations (a “nucleoplasmic reticulum”) [176–179]. Earlier work had shown that the nuclear envelope contains InsP3Rs and RyRs [180,181] and a Ca2+ pump predictably identical to that of the ER [182]. Early work had also found most enzymes of the phosphoinositide cycle in the nuclear envelope [183–185]. A problem, here, is to understand how plasma membrane agonists that are known to initiate the phosphatidylinositol cycle would become activated in the nuclear envelope. Irrespective of this problem, however, the structural arrangement of the invaginations would facilitate the agonist-induced delivery of Ca2+ to selective sub-compartments of the nucleoplasm.
7 Physiology of the Ca2+ Signal: A Selection of Cellular Processes Controlled by Ca2+
As repeatedly underlined above, Ca2+ controls a very large number of the processes that are essential to cell life. A detailed and comprehensive discussion of the physiology of the signal would evidently be out of the scope of this contribution; here, only a succinct description of the most significant Ca2+ regulated functions will thus be presented.
The discussion could be initiated with the process of fertilization, which originates new cell life. Vertebrate eggs remain arrested at the metaphase of the second meiotic division until sperm interacts with them to generate an increase of Ca2+ that initiates at the sperm interaction site [186]. This triggers the exit from metaphase II arrest, and initiates the cell divisions which will eventually produce the multicellular organisms. In many invertebrates and non-mammalian vertebrates the Ca2+ increase takes the form of a single transient, but in mammals the fertilizing Ca2+ signal consists of repetitive transients [187,188]. The mechanism by which the Ca2+ increase is generated has been controversial. One proposal suggested the direct flow of Ca2+ into the egg during gamete fusion, another the role of a surface receptor activated by a sperm factor that would set in motion an intracellular signaling pathway linked to PLC and InsP3. A third proposal, which has now become generally accepted, suggests instead that the fusion of the sperm with the egg delivers into the latter a sperm-specific new isoform of PLC (PLCζ) which initiates the hydrolysis of PIP2 to produce InsP3 [189,190]. That InsP3 is involved in the Ca2+ release in the fertilized egg is now broadly accepted. However, recent evidence suggests that the InsP3-mediated global increase in Ca2+, at least in echinoderm eggs, could be preceded by a localized increase of Ca2+ promoted by the recently discovered Ca2+ messenger NAADP, that would be followed by the globalization of the Ca2+ wave [191]. It should also be mentioned that recent work has underlined the importance of the dynamic rearrangement of the actin cytoskeleton produced by the increase of Ca2+ at fertilization in guiding sperm entry and in modulating the intracellular Ca2+ signaling [192].
A second process in which Ca2+ regulation is acquiring increasing importance is gene expression. A seminal report by Greenberg and coworkers in 1986 [193] had shown that acetylcholine receptor agonists induced the rapid transcription of the c-fos protooncogene in PC12 pheochromcytoma cells in a process that required Ca2+ influx. The work was then extended to neurons, and to numerous other genes involved in neuronal activity [194,195] underlining the special importance of the regulation of gene transcription by Ca2+ to neurons (see [196] for a review). Early work showed that the Ca2+ regulation of the transcription of (immediate early) genes could be mediated by phosphorylation/dephosphorylation reactions catalyzed by calmodulin-dependent kinases and the also calmodulin-dependent phosphatase calcineurin [197]. The most interesting extension of the work on gene regulation by Ca2+ is that on the EF-hand protein DREAM (see above) which acted as a gene silencer on the dynorphin gene [14]. As briefly mentioned above, Ca2+-free DREAM binds to a tandem of DRE sites in the promoter of the gene, repressing its transcription. Binding of Ca2+ to the EF motifs of DREAM promotes its detachment from the DRE sites, reactivating transcription. The list of genes controlled by DREAM has now increased substantially, and includes some that code for Ca2+ regulating/regulated systems, e.g., one of the Na+/Ca2+ exchangers (NCX3) [13], the L-type Ca2+ channels [15], and a nucleotidase that plays a role in the protein folding pathway [198].
That Ca2+ plays a role in the contraction of muscles has been known for 130 years. It was the finding that Ca2+ promoted the contraction of heart cells [199] that officially inaugurated the topic of Ca2+ signaling. The story of the role of Ca2+ in the regulation of heart, and then skeletal muscle, has progressed from the original days of Ringer through other seminal findings such as, to name only some, the discovery of the Ca2+ receptor in the myofibrils (the EF-hand protein troponin C), the findings on the Ca2+ fluxes in SR mediated by a Ca2+ pump and by ligand-gated channels, the characterization of the regulatory roles of phospholamban and sarcolipin in the uptake of Ca2+ in the ER/SR. Some of these aspects of the function of Ca2+ in the regulation of muscle contraction have been already discussed in the sections above; appropriate details can be found in a number of recent comprehensive reviews [200].
Protein phosphorylation/dephosphorylation is a universal mechanism by which the activity of enzymes is regulated. The large group of protein kinases and phosphatases includes important members that are activated by Ca2+-calmodulin [201]. While several calmodulin kinases are known, only one protein phosphatase (calcineurin, also known as protein phosphatase 2B [202]) is regulated by Ca2+-calmodulin. Calmodulin (CaM) kinases phosphorylate Ser-Thr residues, however, calcineurin also dephosphorylates phosphorylated Tyr residues. The CaM kinases can have narrow specificity, i.e., they only phosphorylate one substrate. Myosin light chain kinase (MLCK) [203] phosphorylates the light chain of myosin to initiate smooth muscle contraction and potentiate the contraction of skeletal muscles. It exists in two gene products, one only expressed in skeletal muscles and one, termed smooth muscle MLCK, expressed in a number of tissues. Phosphorylase kinase [204] phosphorylates and activates glycogen phosphorylase, thus accelerating glycogen degradation to contributing to blood glucose homeostasis and providing an energy source for muscle contraction. The enzyme consists of 4 catalytic γ subunits which form a holoenzyme complex with α, β, and δ regulatory subunits, each present in 4 copies: the 4 δ subunits are calmodulin molecules which, interestingly, remain stably associated with the holoenzyme even when the concentration of Ca2+ in the ambient is very low. The binding of Ca2+ to the δ subunits activates the enzyme, which is further activated by the phosphorylation of the α and β subunits by PKA. The elongation factor 2 kinase (also known as CaMK III [205]) translocates along mRNA during translation and is inhibited by phosphorylation.
CaMK I [206] is a ubiquitous cytosolic enzyme which exists in 3 isoforms, α, β, and γ, which are the product of separate genes, which are processed alternatively to generate additional isoforms (a kinase originally termed CaMK V is actually a spliced variant of CaMK I). CaMK I is initially activated by the binding of calmodulin, and further activated by an upstream kinase, the calmodulin-dependent kinase kinase (CaMKK). Not much is known on the substrates phosphorylated by CaMK I, but in vitro experiments have described phosphorylation of synapsin I and of CREB, to activate CREB-dependent gene transcription.
The activity of CaM kinases is not restricted to the phosphorylation of only one substrate. For instance, CaMK II is a ubiquitous enzyme that has been shown to phosphorylate over 50 protein substrates in vitro (only relatively few of them, however, have been shown to be phosphorylated within cells under physiological conditions). It regulates diverse important physiological processes, among them neuronal plasticity, gene transcription, learning and memory, and exocytosis [207]. One of the best characterized substrates of CaMK II is the AMPA ionotropic glutamate receptor; its phosphorylation at Ser 831 plays an important role in synaptic transmission. Four genes encode α, β, γ, and δ isoforms of the kinase, and the alternative processing of the transcripts gives rise to nearly 30 variants of the enzyme, many of them with specific tissue distribution. Some tissues contain very high amounts of CaMK II, for instance the brain, where the kinase accounts for 1–2% of the total protein. All CaMK II isoforms contain a catalytic domain, an autoinhibitory domain, a variable segment, and a self-association domain [207]. The autoinhibitory domain binds to the catalytic domain, blocking its activity [208]. Auto-phosphorylation of the autoinhibitory domain (Thr 286) in the presence of Ca2+ and calmodulin removes the block, leading to persistent activation of the enzyme [209–212]. The concentration of Ca2+ in the vicinity determines the number of subunits that become autophosphorylated on Thr 286, i.e., CaMK II is able to decode the frequency and amplitude of the Ca2+ transients. This property may prolong the effects of the signaling after transient Ca2+ changes, as could for instance occur in learning and memory. The splice variants of the kinase could have specific intracellular localization. For example, one splice variant of CaMK II δ contains a nuclear localization signal and has been shown to regulate gene transcription.
α CaMK IV [197] is a monomeric enzyme expressed in the nervous tissue, in the testis, and in T-cells, while its β splice variant is expressed in the cerebellum during development. Like CaMK I, it is initially activated by calmodulin binding and further activated by phosphorylation by CaMKK. Combined with N-terminal autophosphorylation, this leads to Ca2+-independent activity of CaMK IV. CaMK IV contains a nuclear localization sequence and is thought to phosphorylate numerous transcription factors.
CaMKK exists in two isoforms (α and β) and increases the activity of CaMK I and IV in the presence of calmodulin. Unlike all other CaM kinases, CaMKK does not contain acidic residues that recognize basic residues close to its preferred phosphorylation site.
Calcineurin, also called protein phosphatase 2B, is the only protein phosphatase whose activity is regulated by Ca2+. It was first identified in extracts of mammalian brain (hence its name) but was later found to be expressed in most tissues of eukaryotes [202]. As mentioned above, it is a heterodimer of a catalytic subunit (CnA) tightly bound to a smaller, calmodulin-like regulatory subunit (CnB). Three basic isoforms of CnA (α, β, γ) and two of CnB (CnB1 and CnB2) exist as the products of separate genes. Splicing variants, however, have only been detected at the transcript level. Structurally, the catalytic domain in the N-terminal two thirds of calcineurin, which contains a binuclear iron-zinc active center, is followed down in the sequence by a CnB-binding, a calmodulin-binding, and an autoregulatory domain. CnB binds 4 Ca2+ to canonical EF hand motifs. The activity of CnA requires the binding of CnB, which in turn only occurs if the latter has bound Ca2+. The binding of calmodulin to CnA increases the activity of the phosphatase 50-100 fold.
Importantly, calcineurin is the target of the immunosuppressive drugs cyclosporin A and tacrolimus (FK506) bound to their respective immunophilins [213] and has thus a key role in the transduction pathway from the plasma membrane to the nucleus leading to T-cell activation [214]. This occurs by dephosphorylation of the transcription factor NFAT following Ca2+ increase induced by the occupancy of the plasma membrane T-cell receptor. The activation of calcineurin promotes the dephosphorylation and the exposure of a nuclear localization signal in NFAT, promoting its translocation together with calcineurin to the nucleus, where NFAT can then perform its gene regulation tasks. As mentioned, calcineurin was originally discovered in the brain, where it represents about 1% of the total proteins. In the brain, calcineurin dephosphorylates two inhibitors of protein phosphatase-1 (Inhibitor 1 and DARPP32), inhibiting them. This triggers a phosphatase cascade that opposes the effects of cAMP and Ca2+-activated kinases, explaining for instance the antagonistic effects of Ca2+ release induced by the occupancy of some receptors, e.g., the NMDA glutamate and dopamine receptors. Calcineurin, however, dephosphorylates a number of other substrates involved in the regulation of important neuronal processes, including the expression and activity of ion channels, the release of neurotransmitters, and the outgrowth of neurites.
The mention of calcineurin’s role in the release of neurotransmitters introduces the secretion process, in which Ca2+ has a crucial role, which was first described nearly 50 years ago by Katz and Miledi [215,216] for the release of neurotransmitters at the neuromuscular junctions. The role was then extended to release processes in endocrine cells and in other cell types [217]. The basic mechanistic principle of the release process is the storage of the substance to be released in membrane vesicles that will eventually fuse with the plasma membrane in a process mediated by Ca2+ penetrating through activated plasma membrane channels, discharging their content, be it a neurotransmitter or a hormone, in the extracellular space; in the case of neurotransmitters, this is the synaptic cleft. The difference between the release by the synaptic terminals, and the release, for instance, by endocrine cells, is essentially one of time; the secretion by endocrine cells is a much slower process, with long latencies [218,219]. Differences in the concentrations of Ca2+-buffering proteins may explain the delayed response in endocrine cells, but the distance between the plasma membrane Ca2+ channels and the storage vesicles may have a greater influence on the secretory response.
It seems appropriate to close the description of the cellular processes controlled by Ca2+ with a brief description of the role of Ca2+ in the controlled termination of cell life. There is growing consensus that the various forms of cell death (necrosis, apoptosis, and autophagy) do not occur through entirely separate pathways, but share molecular effectors and signaling routes. Among them, Ca2+ plays a clear role. Apoptosis is the best characterized form of cell death from the standpoint of its relationship to Ca2+ signaling.
Apoptosis (programmed cell death) involves the suicide of individual cells to guarantee normal tissue development and homeostasis in both vertebrate and invertebrate species. However, it can also contribute to many forms of pathological cell loss as it can degenerate into necrotic death. Apoptosis probably plays a role in many chronic degenerative processes, for instance in neuron conditions like Alzheimer’s and Parkinson’s diseases and in heart failure. By contrast, inhibition of apoptosis can be at the basis of the abnormal cell growth in tumors. Apoptotic cells are classified on morphological characteristics that include condensation and margination of chromatin, cytoplasmic vacuolization, cellular shrinkage, increase in cellular density, nuclear fragmentation, and apoptotic body formation [220].
The Ca2+ link with the apoptotic pathways is now a large topic, which cannot be covered in detail in this contribution; a number of comprehensive reviews offer a more complete panorama of the topic [221,222]. The idea of the involvement of Ca2+ initiated with the in vitro demonstration that Ca2+ ionophores, i.e., molecules capable of transporting Ca2+ across membranes down its electrochemical gradient, are highly toxic to cells, and by the finding that the neurotransmitter glutamate, or related compounds, have the ability to induce neuronal death as a result of excess Ca2+ penetration due to receptor overstimulation. Later on, both Ca2+ release from the ER and capacitative Ca2+ influx through Ca2+ release-activated Ca2+ channels were shown to be apoptogenic [223–225]. The discovery that important regulators of apoptosis, namely the proteins of the Bcl-2 family, are localized in organelles deeply involved in Ca2+ handling (the mitochondria and the ER), and may modulate the ER content/release of Ca2+, definitely established the Ca2+ link to apoptosis. The current view is that Ca2+ can sensitize cells to apoptotic challenges, acting on the mitochondrial “checkpoint”. Mitochondria are the site of several proapoptotic proteins like Smac/DIABLO, Omi/HtrA2, AIF, and EndoG, which are maintained in equilibrium with antiapoptotic proteins like XIAP, cIAP-1, and cIAP-2 to finely regulate the balance between cell death and life. Thus, the role of mitochondria and Ca2+ appears to be a key determinant in the molecular events leading to cell death. Ca2+ loads in the mitochondrial matrix have been shown to sensitize the mPTP to apoptotic stimuli, inducing its opening, causing mitochondrial changes in morphology and the release of cytochrome c [226], followed by caspase activation [221,227].
The overall picture that emerges from a large number of contributions is that the release of Ca2+ from the ER and its uptake into mitochondria are pivotal in initiating apoptotic signals, and that a mechanism through which the overexpression of antiapoptotic proteins (or the ablation of proapoptotic proteins) counteracts cell death, is the reduction in the amount of Ca2+ available in the ER for the release process and the uptake into mitochondria. The amount of releasable Ca2+ – rather than the Ca2+ concentration of the ER – appears to be the important parameter for the transduction of the death signal, as it eventually controls the “amplitude” of the signal that reaches mitochondria.
8 Concluding Remarks
Ca2+ signaling has become a topic too large to be covered comprehensively in a normal review. This contribution has thus singled out aspects of the Ca2+ signal that distinguish it from all the carriers of biological information. Among them are the autoregulatory property, its ability to function both as a second and a first messenger, and, especially, its ambivalence: Ca2+ is not only a messenger without which correct cell life would not be possible, it also conveys negative signals, or even death signals, if its concentration and movements within cells are not carefully controlled. However, if correctly controlled and delivered, the Ca2+ signal modulates essentially all important aspects of cell life, from its origin at fertilization, to its end in the process of apoptosis.
Abbreviations
- AMPA:
-
2-amino-3-hydroxyl-5-ethyl-4-isoxazolepropionic acid
- ATP:
-
adenosine 5’-triphosphate
- cADPR:
-
cyclic adenosine diphosphate ribose
- CaM:
-
calmodulin
- CaMK:
-
calmodulin dependent kinase
- cAMP:
-
cyclic adenosine monophosphate
- CaR:
-
calcium receptor
- CBD:
-
Ca2+-binding domain
- CICR:
-
Ca2+-induced Ca2+ release
- CRAC:
-
Ca2+-release activated current
- CREB:
-
cAMP response element binding
- DHPR:
-
dihydropyridine receptor
- DRE:
-
downstream regulatory element
- DREAM:
-
downstream regulatory element
- ER:
-
endoplasmic reticulum
- GA:
-
Golgi apparatus
- GABA:
-
γ-amino butyric acid
- GluR:
-
glutamate receptor
- IMM:
-
inner mitochondrial membrane
- IMS:
-
intermembrane space
- InsP3:
-
inositol 1,4,5-trisphosphate
- InsP3R:
-
inositol 1,4,5-trisphosphate receptor
- KA:
-
kainate
- MCO:
-
mitochondrial Ca2+ overload
- MCU:
-
mitochondrial Ca2+ uniporter
- MICU1:
-
mitochondrial calcium uptake 1
- MPT:
-
mitochondrial permeability transition
- NAADP:
-
nicotinic acid adenine dinucleotide phosphate
- NADP:
-
nicotinamide adenosine diphosphate
- NCLX:
-
mitochondrial Na+/Ca2+ exchanger
- NCX:
-
Na+/Ca2+ exchanger
- NFAT:
-
nuclear factor of activated T cells
- NHE:
-
Na+/H+ exchanger
- NMDA:
-
N-methyl-D-aspartate
- OMM:
-
outer mitochondrial membrane
- PC12:
-
pheochromcytoma cells
- PIP2:
-
phosphatidylinositol 4,5-bisphosphate
- PKA:
-
protein kinase A
- PKC:
-
protein kinase C
- PLC:
-
phospholipase C
- PM:
-
plasma membrane
- PMCA:
-
plasma membrane Ca2+-ATPase
- PTP:
-
permeability transition pore
- PV:
-
parvalbumin
- ROC:
-
receptor operated Ca2+ channels
- ROS:
-
reactive oxygen species
- RR:
-
ruthenium red
- RyR:
-
ryanodine receptor
- SERCA:
-
sarco/endoplasmic reticulum Ca2+-ATPase
- SLC:
-
solute carrier
- SNAP-25:
-
synaptosomal-associated protein 25
- SNARE:
-
soluble NSF attachment protein receptor
- SNP:
-
single nucleotide polymorphism
- SOCE:
-
store operated Ca2+ entry channels
- SPCA:
-
secretory pathway Ca2+ ATPase
- SR:
-
sarcoplasmic reticulum
- STIM:
-
sensors stromal interaction molecule
- TG:
-
thapsigargin
- TPC:
-
two pore channel
- TRP:
-
transient receptor potential channels
- VAMP:
-
vesicle associate membrane protein
- VOC:
-
voltage operated Ca2+-channels
References
E. Carafoli, Proc. Natl. Acad. Sci. USA 2002, 99, 1115–1122.
E. Carafoli, Nat. Rev. Mol. Cell. Biol. 2003, 4, 326–332.
D. Prins, M. Michalak, Cold Spring Harbor Perspect. Biol. 2011, 3.
B. Schwaller, Cold Spring Harbor Perspect. Biol. 2010, 2, a004051.
G. C. Faas, S. Raghavachari, J. E. Lisman, I. Mody, Nat. Neurosci. 2011, 14, 301–304.
E. M. Brown, S. M. Quin, P. M. Vassilev, in Calcium as a Cellular Regulator, Eds E. Carafoli, C. Klee, Oxford University Press, New York, Oxford, 1999, pp. 295–310.
E. F. Nemeth, J. Wallace, A. Scarpa, J. Biol. Chem. 1986, 261, 2668–2674.
D. S. McGehee, M. Aldersberg, K. P. Liu, S. Hsuing, M. J. Heath, H. Tamir, J. Physiol. 1997, 502, 31–44.
D. Guerini, E. Garcia-Martin, A. Gerber, C. Volbracht, M. Leist, C. G. Merino, E. Carafoli, J. Biol. Chem. 1999, 274, 1667–1676.
D. Guerini, X. Wang, L. Li, A. Genazzani, E. Carafoli, J. Biol. Chem. 2000, 275, 3706–3712.
L. Li, D. Guerini, E. Carafoli, J. Biol. Chem. 2000, 275, 20903–20910.
A. A. Genazzani, E. Carafoli, D. Guerini, Proc. Natl. Acad. Sci. USA 1999, 96, 5797–5801.
R. GomezVillafuertes, B. Torres, J. Barrio, M. Savignac, N. Gabellini, F. Rizzato, B. Pintado, A. Gutierrez-Adan, B. Mellstrom, E. Carafoli, J. R. Naranjo, J. Neurosci. 2005, 25, 10822–10830.
A. M. Carrion, W. A. Link, F. Ledo, B. Mellstrom, J. R. Naranjo, Nature 1999, 398, 80–84.
J. J. Ronkainen, S. L. Hanninen, T. Korhonen, J. T. Koivumaki, R. Skoumal, S. Rautio, V. P. Ronkainen, P. Tavi, J. Physiol. 2011, 589, 2669–2686.
E. Carafoli, FASEB J. 1994, 8, 993–1002.
D. Bano, K. W. Young, C. J. Guerin, R. Lefeuvre, N. J. Rothwell, L. Naldini, R. Rizzuto, E. Carafoli, P. Nicotera, Cell 2005, 120, 275–285.
K. Schuh, S. Uldrijan, M. Telkamp, N. Rothlein, L. Neyses, J. Cell Biol. 2001, 155, 201–205.
Calcium Signalling and Disease, Ed J. R. Harris, Springer, New York, 2007.
B. Schwaller, Cell Mol. Life Sci. 2009, 66, 275–300.
E. E. Saftenku, Cerebellum 2012, 11, 102–120.
M. J. Hubbard, N. J. McHugh, FEBS Lett. 1995, 374, 333–337.
N. J. Hack, M. C. Wride, K. M. Charters, S. B. Kater, T. N. Parks, J. Neurosci. 2000, 20, RC67.
B. Schwaller, I. Durussel, D. Jermann, B. Herrmann, J. A. Cox, J. Biol. Chem. 1997, 272, 29663–29671.
G. S. Lee, K. C. Choi, E. B. Jeung, Am. J. Physiol. Endocrinol. Metab. 2006, 290, E299–307.
M. R. Celio, Science 1986, 231, 995–997.
C. W. Heizmann, M. W. Berchtold, A. M. Rowlerson, Proc. Natl. Acad. Sci. USA 1982, 79, 7243–7247.
S. K. Nakayama, H., Kretsinger, R., in Calcium Homeostasis, Eds E. Carafoli, C. Klee, Springer, Vol. 3, 2000, pp. 29–58.
H. Kawasaki, S. Nakayama, R. H. Kretsinger, Biometals 1998, 11, 277–295.
W. A. Catterall, Neuron 2010, 67, 915–928.
W. A. Catterall, Cold Spring Harbor Perspect. Biol. 2011, 3, a003947.
F. Hofmann, M. Biel, V. Flockerzi, Annu. Rev. Neurosci. 1994, 17, 399–418.
A. C. Dolphin, J. Bioenerg. Biomembr. 2003, 35, 599–620.
H. Bito, K. Deisseroth, R. W. Tsien, Cell 1996, 87, 1203–1214.
R. E. Dolmetsch, U. Pajvani, K. Fife, J. M. Spotts, M. E. Greenberg, Science 2001, 294, 333–339.
N. Gomez-Ospina, F. Tsuruta, O. Barreto-Chang, L. Hu, R. Dolmetsch, Cell 2006, 127, 591–606.
Z. P. Pang, T. C. Sudhof, Curr. Opin. Cell Biol. 2010, 22, 496–505.
K. D. Wickman, D. E. Clapham, Curr. Opin. Neurobiol. 1995, 5, 278–285.
S. P. H. Alexander, A. Mathie, J. A. Peters, Br. J. Pharmacol. 2009, 158, S1–254.
E. R. Liman, A. G. Knapp, J. E. Dowling, Brain Res. 1989, 481, 399–402.
C. Rosenmund, D. W. Carr, S. E. Bergeson, G. Nilaver, J. D. Scott, G. L. Westbrook, Nature 1994, 368, 853–856.
L. Y. Wang, M. W. Salter, J. F. MacDonald, Science 1991, 253, 1132–1135.
I. M. Raman, G. Tong, C. E. Jahr, Neuron 1996, 16, 415–421.
J. W. Putney, Jr., Cell Calcium 1986, 7, 1–12.
M. Hoth, R. Penner, Nature 1992, 355, 353–356.
Y. Gwack, S. Srikanth, S. Feske, F. Cruz-Guilloty, M. Oh-hora, D. S. Neems, P. G. Hogan, A. Rao, J. Biol. Chem. 2007, 282, 16232–16243.
M. Prakriya, S. Feske, Y. Gwack, S. Srikanth, A. Rao, P. G. Hogan, Nature 2006, 443, 230–233.
R. M. Luik, M. M. Wu, J. Buchanan, R. S. Lewis, J. Cell Biol. 2006, 174, 815–825.
R. E. Dolmetsch, R. S. Lewis, J. Gen. Physiol. 1994, 103, 365–388.
R. E. Dolmetsch, R. S. Lewis, C. C. Goodnow, J. I. Healy, Nature 1997, 386, 855–858.
R. E. Dolmetsch, K. Xu, R. S. Lewis, Nature 1998, 392, 933–936.
C. Montell, Science Signalling, The Transduction Knowledge Environment 2005, 2005, re3.
M. Gees, B. Colsoul, B. Nilius, Cold Spring Harbor Perspect. Biol. 2010, 2, a003962.
T. Rohacs, Pflugers Arch. 2007, 453, 753–762.
I. S. Ambudkar, H. L. Ong, X. Liu, B. C. Bandyopadhyay, K. T. Cheng, Cell Calcium 2007, 42, 213–223.
M. Lu, R. Branstrom, E. Berglund, A. Hoog, P. Bjorklund, G. Westin, C. Larsson, L. O. Farnebo, L. Forsberg, J. Mol. Endocrinol. 2010, 44, 285–294.
Y. Liao, C. Erxleben, E. Yildirim, J. Abramowitz, D. L. Armstrong, L. Birnbaumer, Proc. Natl. Acad. Sci. USA 2007, 104, 4682–4687.
J. Chan, H. Yamazaki, N. Ishiyama, M. D. Seo, T. K. Mal, T. Michikawa, K. Mikoshiba, M. Ikura, J. Biol. Chem. 2010, 285, 36092–36099.
C. W. Taylor, S. C. Tovey, Cold Spring Harbor Perspect. Biol. 2010, 2, a004010.
C. W. Taylor, A. J. Laude, Cell Calcium 2002, 32, 321–334.
L. Kimlicka, F. Van Petegem, Sci. China Life Sci. 2011, 54, 712–724.
J. T. Lanner, D. K. Georgiou, A. D. Joshi, S. L. Hamilton, Cold Spring Harbor Perspect. Biol. 2010, 2, a003996.
A. De Flora, L. Franco, L. Guida, S. Bruzzone, E. Zocchi, Cell Biochem. Biophys. 1998, 28, 45–62.
N. Bai, H. C. Lee, I. Laher, Pharmacol. Ther. 2005, 105, 189–207.
P. Thorn, O. Gerasimenko, O. H. Petersen, EMBO J. 1994, 13, 2038–2043.
H. Higashida, A. B. Salmina, R. Y. Olovyannikova, M. Hashii, S. Yokoyama, K. Koizumi, D. Jin, H. X. Liu, O. Lopatina, S. Amina, M. S. Islam, J. J. Huang, M. Noda, Neurochem. Int. 2007, 51, 192–199.
P. Pedersen, E. Carafoli, Trends Biochem. Sci. 1987, 12, 146–150.
L. de Meis, A. L. Vianna, Annu. Rev. Biochem. 1979, 48, 275–292.
C. Toyoshima, M. Nakasako, H. Nomura, H. Ogawa, Nature 2000, 405, 647–655.
C. Toyoshima, H. Nomura, Nature 2002, 418, 605–611.
M. Brini, E. Carafoli, Physiol. Rev. 2009, 89, 1341–1378.
C. Toyoshima, Arch. Biochem. Biophys. 2008, 476, 3–11.
D. M. Clarke, T. W. Loo, G. Inesi, D. H. MacLennan, Nature 1989, 339, 476–478.
D. M. Clarke, T. W. Loo, D. H. MacLennan, J. Biol. Chem. 1990, 265, 6262–6267.
D. Guerini, A. Zecca-Mazza, E. Carafoli, J. Biol. Chem. 2000, 275, 31361–31368.
J. Pande, M. M. Szewczyk, I. Kuszczak, S. Grover, E. Escher, A. K. Grover, J. Cell Mol. Med. 2008, 12, 1049–1060.
J. Chaudhary, M. Walia, J. Matharu, E. Escher, A. K. Grover, Am. J. Physiol. Cell Physiol. 2001, 280, C1027–1030.
L. Dode, J. P. Andersen, L. Raeymaekers, L. Missiaen, B. Vilsen, F. Wuytack, J. Biol. Chem. 2005, 280, 39124–39134.
L. Dode, J. P. Andersen, J. Vanoevelen, L. Raeymaekers, L. Missiaen, B. Vilsen, F. Wuytack, J. Biol. Chem. 2006, 281, 3182–3189.
R. J. Kaufman, M. Swaroop, P. Murtha-Riel, Biochemistry 1994, 33, 9813–9819.
A. Varki, Trends Cell Biol. 1998, 8, 34–40.
V. Niggli, E. S. Adunyah, J. T. Penniston, E. Carafoli, J. Biol. Chem. 1981, 256, 395–401.
P. James, M. Maeda, R. Fischer, A. K. Verma, J. Krebs, J. T. Penniston, E. Carafoli, J. Biol. Chem. 1988, 263, 2905–2910.
A. Enyedi, A. K. Verma, R. Heim, H. P. Adamo, A. G. Filoteo, E. E. Strehler, J. T. Penniston, J. Biol. Chem. 1994, 269, 41–43.
R. Falchetto, T. Vorherr, J. Brunner, E. Carafoli, J. Biol. Chem. 1991, 266, 2930–2936.
R. Falchetto, T. Vorherr, E. Carafoli, Protein Sci. 1992, 1, 1613–1621.
N. L. Elwess, A. G. Filoteo, A. Enyedi, J. T. Penniston, J. Biol. Chem. 1997, 272, 17981–17986.
H. Hilfiker, D. Guerini, E. Carafoli, J. Biol. Chem. 1994, 269, 26178–26183.
A. Enyedi, T. Vorherr, P. James, D. J. McCormick, A. G. Filoteo, E. Carafoli, J. T. Penniston, J. Biol. Chem. 1989, 264, 12313–12321.
E. Zvaritch, P. James, T. Vorherr, R. Falchetto, N. Modyanov, E. Carafoli, Biochemistry 1990, 29, 8070–8076.
P. Brodin, R. Falchetto, T. Vorherr, E. Carafoli, Eur. J. Biochem. 1992, 204, 939–946.
J. Lytton, M. Westlin, S. E. Burk, G. E. Shull, D. H. MacLennan, J. Biol. Chem. 1992, 267, 14483–14489.
H. Verboomen, F. Wuytack, L. Van den Bosch, L. Mertens, R. Casteels, Biochem. J. 1994, 303, 979–984.
L. Dode, J. P. Andersen, N. Leslie, J. Dhitavat, B. Vilsen, A. Hovnanian, J. Biol. Chem. 2003, 278, 47877–47889.
A. M. Katz, Ann. NY Acad. Sci. 1998, 853, 9–19.
D. H. MacLennan, E. G. Kranias, Nat. Rev. Mol. Cell Biol. 2003, 4, 566–577.
P. James, M. Inui, M. Tada, M. Chiesi, E. Carafoli, Nature 1989, 342, 90–92.
Z. Hu, J. M. Bonifas, J. Beech, G. Bench, T. Shigihara, H. Ogawa, S. Ikeda, T. Mauro, E. H. Epstein, Jr., Nat. Genet. 2000, 24, 61–65.
J. Vanoevelen, L. Dode, K. Van Baelen, R. J. Fairclough, L. Missiaen, L. Raeymaekers, F. Wuytack, J. Biol. Chem. 2005, 280, 22800–22808.
V. Prasad, G. W. Okunade, M. L. Miller, G. E. Shull, Biochem. Biophys. Res. Commun. 2004, 322, 1192–1203.
K. Schuh, E. J. Cartwright, E. Jankevics, K. Bundschu, J. Liebermann, J. C. Williams, A. L. Armesilla, M. Emerson, D. Oceandy, K. P. Knobeloch, L. Neyses, J. Biol. Chem. 2004, 279, 28220–28226.
E. E. Strehler, D. A. Zacharias, Physiol. Rev. 2001, 81, 21–50.
M. C. Chicka, E. E. Strehler, J. Biol. Chem. 2003, 278, 18464–18470.
E. Carafoli, L. Santella, D. Branca, M. Brini, Crit. Rev. Biochem. Mol. Biol. 2001, 36, 107–260.
M. P. Blaustein, W. J. Lederer, Physiol. Rev. 1999, 79, 763–854.
K. D. Philipson, D. A. Nicoll, S. Matsuoka, L. V. Hryshko, D. O. Levitsky, J. N. Weiss, Ann. NY Acad. Sci. 1996, 779, 20–28.
S. L. Lee, A. S. Yu, J. Lytton, J. Biol. Chem. 1994, 269, 14849–14852.
D. A. Nicoll, M. Ottolia, K. D. Philipson, Ann. NY Acad. Sci. 2002, 976, 11–18.
K. D. Philipson, D. A. Nicoll, Annu. Rev. Physiol. 2000, 62, 111–133.
D. W. Hilgemann, Nature 1990, 344, 242–245.
J. Liao, H. Li, W. Zeng, D. B. Sauer, R. Belmares, Y. Jiang, Science 2012, 335, 686–690.
H. Reilander, A. Achilles, U. Friedel, G. Maul, F. Lottspeich, N. J. Cook, EMBO J. 1992, 11, 1689–1695.
X. Cai, J. Lytton, Mol. Biol. Evol. 2004, 21, 1692–1703.
R. Palty, E. Ohana, M. Hershfinkel, M. Volokita, V. Elgazar, O. Beharier, W. F. Silverman, M. Argaman, I. Sekler, J. Biol. Chem. 2004, 279, 25234–25240.
J. Lytton, Biochem. J. 2007, 406, 365–382.
D. A. Nicoll, M. R. Sawaya, S. Kwon, D. Cascio, K. D. Philipson, J. Abramson, J. Biol. Chem. 2006, 281, 21577–21581.
M. Hilge, J. Aelen, G. W. Vuister, Mol. Cell 2006, 22, 15–25.
D. Noble, A. Herchuelz, EMBO Rep. 2007, 8, 228–232.
The International HapMap Consortium, A Haplotype Map of the Human Genome, Nature 2005, 437, 1299–1320.
R. L. Lamason, M. A. Mohideen, J. R. Mest, A. C. Wong, H. L. Norton, M. C. Aros, M. J. Jurynec, X. Mao, V. R. Humphreville, J. E. Humbert, S. Sinha, J. L. Moore, P. Jagadeeswaran, W. Zhao, G. Ning, I. Makalowska, P. M. McKeigue, D. O’Donnell, R. Kittles, E. J. Parra, N. J. Mangini, D. J. Grunwald, M. D. Shriver, V. A. Canfield, K. C. Cheng, Science 2005, 310, 1782–1786.
Y. Kirichok, G. Krapivinsky, D. E. Clapham, Nature 2004, 427, 360–364.
N. E. Saris, A. Allshire, Methods Enzymol. 1989, 174, 68–85.
E. Carafoli, R. Tiozzo, G. Lugli, F. Crovetti, C. Kratzing, J. Mol. Cell Cardiol. 1974, 6, 361–371.
D. G. Nicholls, Cell Calcium 2005, 38, 311–317.
E. Carafoli, FEBS Lett. 1979, 104, 1–5.
F. D. Vasington, J. V. Murphy, J. Biol. Chem. 1962, 237, 2670–2677.
H. F. Deluca, G. W. Engstrom, Proc. Natl. Acad. Sci. USA 1961, 47, 1744–1750.
D. G. Nicholls, M. Crompton, FEBS Lett. 1980, 111, 261–268.
R. Rizzuto, P. Bernardi, T. Pozzan, J. Physiol. 2000, 529 Pt 1, 37–47.
J. Santo-Domingo, L. Vay, E. Hernandez-Sanmiguel, C. D. Lobaton, A. Moreno, M. Montero, J. Alvarez, Br. J. Pharmacol. 2007, 151, 647–654.
I. Rustenbeck, G. Eggers, H. Reiter, W. Munster, S. Lenzen, Biochem. Pharmacol. 1998, 56, 977–985.
M. C. Crompton, E. Carafoli, Eur. J. Biochem. 1976, 69, 435–462.
D. W. Jung, K. Baysal, G. P. Brierley, J. Biol. Chem. 1995, 270, 672–678.
R. K. Dash, D. A. Beard, J. Physiol. 2008, 586, 3267–3285.
D. A. Cox, L. Conforti, N. Sperelakis, M. A. Matlib, J. Cardiovasc. Pharmacol. 1993, 21, 595–599.
G. Fiskum, A. L. Lehninger, J. Biol. Chem. 1979, 254, 6236–6239.
T. E. Gunter, J. H. Chace, J. S. Puskin, K. K. Gunter, Biochemistry 1983, 22, 6341–6351.
T. E. Gunter, L. Buntinas, G. Sparagna, R. Eliseev, K. Gunter, Cell Calcium 2000, 28, 285–296.
D. E. Wingrove, T. E. Gunter, J. Biol. Chem. 1986, 261, 15166–15171.
A. P. Somlyo, M. Bond, A. V. Somlyo, Nature 1985, 314, 622–625.
R. M. Denton, J. G. McCormack, Nature 1985, 315, 635.
R. Rizzuto, A. W. Simpson, M. Brini, T. Pozzan, Nature 1992, 358, 325–327.
R. Rizzuto, M. Brini, M. Murgia, T. Pozzan, Science 1993, 262, 744–747.
R. Rizzuto, P. Pinton, W. Carrington, F. S. Fay, K. E. Fogarty, L. M. Lifshitz, R. A. Tuft, T. Pozzan, Science 1998, 280, 1763–1766.
M. Hoth, C. M. Fanger, R. S. Lewis, J. Cell Biol. 1997, 137, 633–648.
R. Malli, M. Frieden, K. Osibow, W. F. Graier, J. Biol. Chem. 2003, 278, 10807–10815.
G. Hajnoczky, G. Csordas, Curr. Biol. 2010, 20, R888–891.
I. Drago, P. Pizzo, T. Pozzan, EMBO J. 2011, 30, 4119–4125.
D. De Stefani, A. Raffaello, E. Teardo, I. Szabo, R. Rizzuto, Nature 2011, 476, 336–340.
J. M. Baughman, F. Perocchi, H. S. Girgis, M. Plovanich, C. A. Belcher-Timme, Y. Sancak, X. R. Bao, L. Strittmatter, O. Goldberger, R. L. Bogorad, V. Koteliansky, V. K. Mootha, Nature 2011, 476, 341–345.
R. Palty, W. F. Silverman, M. Hershfinkel, T. Caporale, S. L. Sensi, J. Parnis, C. Nolte, D. Fishman, V. Shoshan-Barmatz, S. Herrmann, D. Khananshvili, I. Sekler, Proc. Natl. Acad. Sci. USA 2010, 107, 436–441.
F. Perocchi, V. M. Gohil, H. S. Girgis, X. R. Bao, J. E. McCombs, A. E. Palmer, V. K. Mootha, Nature 2010, 467, 291–296.
E. Carafoli, W. X. Balcavage, A. L. Lehninger, J. R. Mattoon, Biochim. Biophys. Acta. 1970, 205, 18–26.
W. Li, Z. Shariat-Madar, M. Powers, X. Sun, R. D. Lane, K. D. Garlid, J. Biol. Chem. 1992, 267, 17983–17989.
P. Paucek, M. Jaburek, Biochim. Biophys. Acta 2004, 1659, 83–91.
A. Rasola, P. Bernardi, Cell Calcium 2011, 50, 222–233.
S. Patel, R. Docampo, Trends Cell Biol. 2010, 20, 277–286.
S. H. Yoo, S. Y. Chu, K. D. Kim, Y. H. Huh, Biochemistry 2007, 46, 14663–14671.
E. Lloyd-Evans, A. J. Morgan, X. He, D. A. Smith, E. Elliot-Smith, D. J. Sillence, G. C. Churchill, E. H. Schuchman, A. Galione, F. M. Platt, Nat. Med. 2008, 14, 1247–1255.
A. H. Guse, H. C. Lee, Sci. Signal 2008, 1, re10.
A. Menteyne, A. Burdakov, G. Charpentier, O. H. Petersen, J. M. Cancela, Curr. Biol. 2006, 16, 1931–1937.
M. Yamasaki, R. Masgrau, A. J. Morgan, G. C. Churchill, S. Patel, S. J. Ashcroft, A. Galione, J. Biol. Chem. 2004, 279, 7234–7240.
K. J. Mitchell, F. A. Lai, G. A. Rutter, J. Biol. Chem. 2003, 278, 11057–11064.
F. Zhang, P. L. Li, J. Biol. Chem. 2007, 282, 25259–25269.
M. X. Zhu, J. Ma, J. Parrington, A. Galione, A. M. Evans, FEBS Lett. 2010, 584, 1966–1974.
M. X. Zhu, J. Ma, J. Parrington, P. J. Calcraft, A. Galione, A. M. Evans, Am. J. Physiol. Cell Physiol. 2010, 298, C430–441.
M. Brini, M. Murgia, L. Pasti, D. Picard, T. Pozzan, R. Rizzuto, EMBO J. 1993, 12, 4813–4819.
N. L. Allbritton, E. Oancea, M. A. Kuhn, T. Meyer, Proc. Natl. Acad. Sci. USA 1994, 91, 12458–12462.
M. N. Badminton, J. M. Kendall, C. M. Rembold, A. K. Campbell, Cell Calcium 1998, 23, 79–86.
L. Santella, K. Kyozuka, Biochem. Biophys. Res. Commun. 1994, 203, 674–680.
M. Mazzanti, L. J. DeFelice, J. Cohn, H. Malter, Nature 1990, 343, 764–767.
M. Mazzanti, B. Innocenti, M. Rigatelli, FASEB J. 1994, 8, 231–236.
C. Perez-Terzic, L. Stehno-Bittel, D. E. Clapham, Cell Calcium 1997, 21, 275–282.
A. Rakowska, T. Danker, S. W. Schneider, H. Oberleithner, J. Membr. Biol. 1998, 163, 129–136.
L. Santella, E. Carafoli, FASEB J. 1997, 11, 1091–1109.
W. Echevarria, M. F. Leite, M. T. Guerra, W. R. Zipfel, M. H. Nathanson, Nat. Cell Biol. 2003, 5, 440–446.
P. Marius, M. T. Guerra, M. H. Nathanson, B. E. Ehrlich, M. F. Leite, Cell Calcium 2006, 39, 65–73.
C. Cardenas, J. L. Liberona, J. Molgo, C. Colasante, G. A. Mignery, E. Jaimovich, J. Cell Sci. 2005, 118, 3131–3140.
S. Guatimosim, M. J. Amaya, M. T. Guerra, C. J. Aguiar, A. M. Goes, N. L. Gomez-Viquez, M. A. Rodrigues, D. A. Gomes, J. Martins-Cruz, W. J. Lederer, M. F. Leite, Cell Calcium 2008, 44, 230–242.
L. Santella, K. Kyozuka, Cell Calcium 1997, 22, 11–20.
D. J. Hennager, M. J. Welsh, S. DeLisle, J. Biol. Chem. 1995, 270, 4959–4962.
L. Lanini, O. Bachs, E. Carafoli, J. Biol. Chem. 1992, 267, 11548–11552.
N. Divecha, H. Banfic, R. F. Irvine, Cell 1993, 74, 405–407.
A. M. Martelli, L. M. Neri, R. S. Gilmour, P. J. Barker, N. S. Huskisson, F. A. Manzoli, L. Cocco, Biochem. Biophys. Res. Commun. 1991, 177, 480–487.
A. M. Martelli, L. Cocco, R. Bareggi, G. Tabellini, R. Rizzoli, M. D. Ghibellini, P. Narducci, J. Cell Biochem. 1999, 72, 339–348.
S. A. Stricker, Dev. Biol. 1999, 211, 157–176.
D. Kline, J. T. Kline, Dev. Biol. 1992, 149, 80–89.
S. Miyazaki, H. Shirakawa, K. Nakada, Y. Honda, Dev. Biol. 1993, 158, 62–78.
K. Swann, C. M. Saunders, N. T. Rogers, F. A. Lai, Semin. Cell Dev. Biol. 2006, 17, 264–273.
C. M. Saunders, M. G. Larman, J. Parrington, L. J. Cox, J. Royse, L. M. Blayney, K. Swann, F. A. Lai, Development 2002, 129, 3533–3544.
L. Santella, D. Lim, F. Moccia, Trends Biochem. Sci. 2004, 29, 400–408.
L. Santella, J. T. Chun, Sci. China Life Sci. 2011, 54, 733–743.
M. E. Greenberg, E. B. Ziff, L. A. Greene, Science 1986, 234, 80–83.
L. B. Rosen, D. D. Ginty, M. E. Greenberg, Adv. Second Messenger Phosphoprotein Res. 1995, 30, 225–253.
A. Lanahan, P. Worley, Neurobiol. Learn Mem. 1998, 70, 37–43.
C. P. Bengtson, H. Bading, Adv. Exp. Med. Biol. 2012, 970, 377–405.
H. Enslen, P. Sun, D. Brickey, S. H. Soderling, E. Klamo, T. R. Soderling, J. Biol. Chem. 1994, 269, 15520–15527.
T. Cali, L. Fedrizzi, D. Ottolini, R. Gomez-Villafuertes, B. Mellstrom, J. R. Naranjo, E. Carafoli, M. Brini, J. Biol. Chem. 2012.
S. Ringer, J. Physiol. 1883, 4, 29–42 23.
S. E. Ebashi, M. Endo, I. Ohtsuki, in Calcium as a Cellular Regulator, Eds E. Carafoli, C. Klee, Oxford University Press, New York, Oxford, 1999, pp. 579–595.
A. P. Braun, H. Schulman, Annu. Rev. Physiol. 1995, 57, 417–445.
J. Aramburu, A. Rao, C. B. Klee, Curr. Top. Cell Regul. 2000, 36, 237–295.
K. E. Kamm, J. T. Stull, J. Biol. Chem. 2001, 276, 4527–4530.
R. J. Brushia, D. A. Walsh, Front Biosci. 1999, 4, D618–641.
K. S. Pavur, A. N. Petrov, A. G. Ryazanov, Biochemistry 2000, 39, 12216–12224.
J. Goldberg, A. C. Nairn, J. Kuriyan, Cell 1996, 84, 875–887.
A. Hudmon, H. Schulman, Annu. Rev. Biochem. 2002, 71, 473–510.
T. Kanaseki, Y. Ikeuchi, H. Sugiura, T. Yamauchi, J. Cell Biol. 1991, 115, 1049–1060.
L. C. Griffith, C. S. Lu, X. X. Sun, Mol. Interv. 2003, 3, 386–403.
J. Lisman, Trends Neurosci. 1994, 17, 406–412.
R. D. Blitzer, T. Wong, R. Nouranifar, R. Iyengar, E. M. Landau, Neuron 1995, 15, 1403–1414.
K. P. Giese, N. B. Fedorov, R. K. Filipkowski, A. J. Silva, Science 1998, 279, 870–873.
J. Liu, J. D. Farmer, Jr., W. S. Lane, J. Friedman, I. Weissman, S. L. Schreiber, Cell 1991, 66, 807–815.
N. A. Clipstone, G. R. Crabtree, Nature 1992, 357, 695–697.
B. Katz, R. Miledi, J. Physiol. 1965, 181, 656–670.
B. Katz, R. Miledi, J. Physiol. 1967, 189, 535–544.
R. P. Rubin, Calcium and the Secretory Process, Plenum Press, New York, 1974.
G. J. Augustine, M. P. Charlton, S. J. Smith, J. Physiol. 1985, 367, 163–181.
R. Llinas, I. Z. Steinberg, K. Walton, Biophys. J. 1981, 33, 323–351.
J. F. Kerr, A. H. Wyllie, A. R. Currie, Br. J. Cancer 1972, 26, 239–257.
P. Pinton, C. Giorgi, R. Siviero, E. Zecchini, R. Rizzuto, Oncogene 2008, 27, 6407–6418.
R. Rizzuto, P. Pinton, D. Ferrari, M. Chami, G. Szabadkai, P. J. Magalhaes, F. Di Virgilio, T. Pozzan, Oncogene 2003, 22, 8619–8627.
I. E. Wertz, V. M. Dixit, J. Biol. Chem. 2000, 275, 11470–11477.
P. Pinton, D. Ferrari, E. Rapizzi, F. Di Virgilio, T. Pozzan, R. Rizzuto, EMBO J. 2001, 20, 2690–2701.
S. Jiang, S. C. Chow, P. Nicotera, S. Orrenius, Exp. Cell Res. 1994, 212, 84–92.
P. Pacher, G. Hajnoczky, EMBO J. 2001, 20, 4107–4121.
G. Kroemer, J. C. Reed, Nat. Med. 2000, 6, 513–519.
G. Szabadkai, K. Bianchi, P. Varnai, D. De Stefani, M. R. Wieckowski, D. Cavagna, A. I. Nagy, T. Balla, R. Rizzuto, J. Cell Biol. 2006, 175, 901–911.
Acknowledgments
The original work by the authors has been supported over the years by grants from the Italian Ministry of University and Research (FIRB2001 to E.C., PRIN 2003, 2005 and 2008 to M.B), the Telethon Foundation (Project GGP04169 to M.B.), the FP6 program of the European Union (FP6 Network of Excellence NeuroNe, LSH-2003-2.1.3-3 to E.C. and Integrated Project Eurohear to E.C.), the Human Frontier Science Program Organization to E.C., the ERANet- Neuron (nEUROsyn), and CARIPARO Foundation to E.C, the Italian National Research Council (CNR) and the University of Padova (Progetto di Ateneo 2008 CPDA082825) to M.B.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Brini, M., Calì, T., Ottolini, D., Carafoli, E. (2013). Intracellular Calcium Homeostasis and Signaling. In: Banci, L. (eds) Metallomics and the Cell. Metal Ions in Life Sciences, vol 12. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5561-1_5
Download citation
DOI: https://doi.org/10.1007/978-94-007-5561-1_5
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5560-4
Online ISBN: 978-94-007-5561-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)