Abstract—
Calcium is one of the most important elements for intracellular signaling. Its role is so big and complex that we can distinguish various effects and biochemical cascades involving this ion into a separate signaling system–calcium signaling. This type of cell regulation mechanism is even more important for male gametes. The inability to perform transcription and the low level of translation are the reasons why post-translational processes, many of them being activated/inhibited by calcium or its target proteins, are the main way of regulation of cell function in mature sperm. Intracellular calcium level elevation is an essential step in the processes that precede fertilization, such as capacitation, hyperactivation, and acrosome reaction (AR). Ca2+ is required for progressive and hyperactivated motility; sperm cells incorporate this ion to prevent spontaneous acrosome reaction and to induce AR when the time comes. Huge difference in the impact of the same ion is achieved by the regulation complexity and specific localization of all signaling elements, which regulate Ca2+ influx and efflux, and its target proteins. Successful fertilization is impossible without proper functioning of the calcium signaling system in the male gamete. The achievements of the last decade, mediated by recent technical advances, have significantly improved our knowledge and understanding of the regulation mechanisms of sperm Ca2+ signals in various species, as well as the intracellular effects and spatial-temporal localization of these signals. In this review we have attempted to provide the most complete picture of mammalian sperm calcium signaling and to formulate the questions to be answered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
In spermatozoa, the nucleus is transcriptionally inactive, the level of translation in the cell is insignificant and, hence, the functions of male gametes are regulated mainly due to posttranslational processes; their effect is much more rapid compared to long-term regulation through the effects on gene expression, as it is achieved via modification of the proteins and enzymes already present in the cell. Calcium signaling is an integral part of the system of this rapid regulation of the functional activity of spermatozoa; calcium ions (Ca2+) play the key role in various posttranslational modifications (phosphorylation, nitrosylation, etc.) of enzymes and other proteins, thereby controlling their activity [1]. The main post-ejaculatory processes of spermatozoon such as capacitation, hyperactivation and acrosome reaction (AR) are induced and regulated exactly by changes in the Ca2+ intracellular level ([Ca2+]i) in male gametes. It has been shown that individuals with impaired calcium homeostasis in spermatozoa demonstrate reproductive failure and, hence, the study of Ca2+ signaling mechanisms is important for understanding and subsequent solution of infertility-related problems.
2 PATHWAYS TO INCREASE CALCIUM LEVELS IN THE CYTOSOL
Increase in [Ca2+]i can occur in two ways: by Ca2+ entry from extracellular space or Ca2+ release from intracellular stores (IS). In both cases, calcium ions move against the concentration gradient through ion channels; this route is at least 1000-fold quicker than the transport via ion exchangers and pumps and, hence, allows cells to respond rapidly to the changing conditions [2].
2.1 Calcium Channels of the Outer Plasma Membrane of Spermatozoa
In different years, the plasma membrane (PM) of maturing and mature sperm cells of mammals was shown to have quite a number of Ca2+-permeable ion channels, including voltage-gated Ca2+ channels (CaV), cyclic nucleotide-gated (CNG) channels, TRPC channels (transient receptor potential canonical channels), as well as store operated channels (SOC) ORAI. However, in the past two decades, ample evidence has been obtained indicating that the cation channels of sperm (CatSper) play the key role in providing extracellular Ca2+ influx in mature spermatozoa. At present, these channels are the best studied Ca2+ channels of male gametes. These channels were described in 2001 in mice as sperm-specific ion channels that are localized in the flagellar principal piece and represent a necessary element for cAMP-mediated Ca2+ influx, motility, and fertilization [3]. Further studies have demonstrated that these weakly voltage-dependent pH-sensitive Ca2+ channels are heterotetramers and consist of four major pore-forming subunits, each of them being encoded by an individual gene, and the functioning of all these genes is necessary to provide fertility. In addition, a complex structure of the channel includes auxiliary subunits CatSperβ, CatSperγ, and CatSperδ encoded by at least 7 genes [4], as well as by other, not yet fully studied components [2]. The CatSper genes are expressed only in testicles during spermatogenesis. In humans, it was shown that mutations in the CatSper1 and CatSper2 genes cause infertility in males [4]. A lot of evidence has been obtained for the paramount importance of CatSper channels for hyperactivation: though spermatozoa of mice with knockout of CatSper1–4 gene exhibit normal Ca2+-independent motility, mutant cells are unable to develop hyperactivation. The mice with defective CatSperδ are sterile, while the mutations in CatSper1 and 2 occur in infertile men; however, thus far no such patients with CatSperβ and γ mutations have been revealed. In bovine spermatozoa, it has been shown that the acquisition of hyperactivated motility requires Ca2+ influx induced by the pH increase and apparently occurring through CatSper channels [5], as is confirmed by the absence of pH-induced increase in [Ca2+]i and the inability to develop hyperactivated motility in case of CatSper1 defects in mouse spermatozoa. Nevertheless, the experiments with thimerosal in mice have shown that, even in the medium without Ca2+ and/or in the absence of functioning CatSper channels, in 20–40% of gametes hyperactivation is achieved through Ca2+ mobilization from IS [6]. Later, in human spermatozoa it was shown that the progesterone induced Ca2+ entry through CatSper was followed by Ca2+-induced Ca2+ release (CICR) from IS in the neck region, and Ca2+ mobilization from the stores was necessary for hyperactivation of the gametes [7]. Recent studies have demonstrated that the Ca2+ influx through CatSper, in addition to hyperactivation, is necessary for Ca2+ signal propagation from the tail to the head, followed by the increase in NADH concentration, and the mice with defective CatSper channels show the lower level of ATP compared to wild type. The latter suggests that the Ca2+ influx through CatSper channels can also regulate ATP homeostasis. AR apparently does not require Ca2+ influx from extracellular medium: alkaline depolarization and cGMP addition induced AR in mice with the (–/–) mutations in the CatSper1 and 2 genes [4]. It is interesting that CatSper3 and 4 are found in the acrosome region of human late spermatids and spermatozoa, demonstrating the potential role of these channel proteins in the AR of human male gametes [8].
Many aspects of CatSper sensitivity to different regulators vary among different species, demonstrating evolutionary divergence between the genes of these channels. Regulation of the human CatSper activity in vivo implies activation by the components of follicular fluid, progesterone, and prostaglandins, as well as by the increase in intracellular pH [4]. All these activators are present in the female reproductive tract and are the inducers and necessary conditions of hyperactivation and subsequent AR. CatSper channels of rhesus macaque, like those in humans, demonstrate the sensitivity to progesterone, although this sensitivity is mediated by proteins of the zona pellucida (in contrast to the human CatSper that directly interacts with progesterone) [9]. At the same time, it was shown that the CatSper channels of mouse spermatozoa are not sensitive to progesterone [10]. Similarly, 8-Br-cNMPs (8‑Br-cyclonucleotide monophosphate, 8-Br-cAMP, and 8-Br-cGMP) activate the CatSper channels in human spermatozoa but not in mouse male gametes [4]. Since the CatSper activation requires alkalinization of the cytoplasm, the activity of these channels is also regulated by H+ carriers and other ion channels: NHE (Na+/H+ exchanger), HV1 (voltage-gated H+ channel 1), SLO3K+ (sperm-specific K+ channel). SLO3K+ channels are involved in hyperpolarization of the sperm PM during capacitation. It has been shown in mice that the activity of SLO3K+ is required for CatSper opening; however, defective SLO3K+ does not prevent spermatozoa from successful completion of other stages of capacitation [11]. Male mice with the mutant SLO3K+ and/or CatSper are sterile in vivo and demonstrate considerably lower fertilization ability in vitro [12]. It has been shown that in humans, membrane hyperpolarization involves, in addition to SLO3K+, one more K+ channel, supposedly SLO1K+ [13]. SLO1K+ was also detected in the anterior postacrosomal region, in the midpiece and principal piece of the flagellum in boar sperm; the same study demonstrates that this channel is important for the [Ca2+]i increase and acrosomal exocytosis under the influence of progesterone [14]. HV1 has been identified in the human sperm tail [15], where it can affect the work of pH-dependent CatSper and SLO3K+ channels. Another pathway of pH change, the sperm-specific Na+/H+ exchanger (sNHE), proved to be involved in fertilization ability of mice: in the absence of the functioning sNHE, female mice are sterile [2]. It is interesting that the primary structure of sNHE contains a cyclic nucleotide-binding domain: there are grounds to believe that the function of the exchanger is regulated by cAMP, the concentration of which increases at the initial stages of capacitation [2]. Other regulators of the activity of CatSper channels are neurotransmitters, chemokines, and odorants. In human spermatozoa, it has been shown that the latter are able to activate the Ca2+ flux through CatSper by interacting with the channel directly, without the involvement of either cAMP or G protein-coupled receptor (GPCR) [4]. The activity of CatSper channels can be also influenced by hormones such as pregnenolone sulfate exerting an effect similar to that of progesterone, as well as testosterone and hydrocortisone inhibiting the effect of progesterone. The same authors have shown that steroid-like molecules—in particular, plant triterpenoids pristimerin and lupeol—compete with both progesterone and pregnenolone sulfate and considerably decrease the activation of CatSper by these compounds. In addition, it was shown that pristimerin and lupeol inhibit the hyperactivation of capacitated gametes, which suggests the possibility of using plant triterpenoids as contraceptives [16]. Moreover, it was demonstrated that in humans a broad range of endocrine disrupting chemicals (EGC) contained in foods, household chemistry, and cosmetics, can prematurely activate CatSper channels, making them insensitive to the effects of progesterone and other physiological activators and, consequently, impairing the mechanism of fertilization [17].
2.1.1. ORAI channels (named after the gatekeepers of heaven in Greek mythology [18]) are small protein molecules of 28–33 kDa localized on PM and forming a channel pore by four transmembrane segments [19]. This is a selective Ca2+ channel opening upon IS depletion and being part of the mechanism of store operated Ca2+ entry (SOCE). In somatic cells, ORAI is expressed jointly with stromal interaction molecule (STIM) localized on the membrane of Ca2+ stores. STIM responds to a decrease in the Ca2+ level in the store, moving towards the side adjacent to the cell plasma membrane, where it triggers ORAI or, probably, one of the TRPC channels. There is evidence of the presence of different ORAI and STIM isoforms in human spermatozoa: the immunofluorescence method has shown the localization of ORAI1, ORAI2, and STIM2 in the region of acrosome, the midpiece and principal piece of the flagellum (for ORAI2, the fluorescence of antibodies in the tail was insignificant); STIM1 in the midpiece, with bright fluorescence in the neck region, and ORAI3 in the anterior region of the midpiece and in the neck region of gametes [20]. The authors also demonstrated that the Ca2+ signal triggered by CatSper channels of the tail is transmitted to the head with the involvement of ORAI and SOCE. It has been shown that progesterone, by activating CatSper channels, triggers the biphasic Ca2+ signal: its first phase occurs in the anterior part of the tail and is short-term, followed by a sustained phase of Ca2+ signal in the head and neck areas. In the present study, the pretreatment of cells with 2-APB (2-aminoethoxydiphenyl borate) at a concentration of 5 µM or with loperamide, which stimulated the ORAI activity, in both cases intensified the second sustained phase of progesterone-induced Ca2+ signal in the neck/midpiece area [20]. Another work demonstrates the role of ORAI1 in mouse spermatogenesis, as well as the fact that the males with ORAI1–/– mutations are sterile [21].
2.1.2. TRPC channels are typical of mammals, being homologues of the TRP family of Ca2+-permeable channels found in Drosophila and involved in the activation of fly photoreceptors (for which they got their name) regulated by phospholipase C (PLC) [22]. Usually, TRP channels consist of 6 transmembrane domains, as well as large amino and carboxyl termini localized inside the cell [23]. The TRPC family consists of 7 members, all of them being expressed in testicles [24]. Cationic TRPC channels are permeable for Na+, K+ and Ca2+; they are not voltage-dependent but are voltage-sensitive: their activity can vary depending on the membrane potential [25]. As early as in 2003, it was reported on the presence of TRPC1, 3, 4, and 6 in mature human spermatozoa with localization of TRPC1 in the flagellar principal piece and, in some cells, in the posterior acrosome region, and of TRPC3, 4, and 6, in the midpiece of the flagellum. In addition, TRPC3 is localized in the acrosome region and TRPC6 has pointwise localization in the principal piece of the tail and negligibly in the head [26]. In this work, the role of this channel in ensuring gamete motility was demonstrated using the TRPC inhibitor (SKF96365). There is evidence of the presence of TRPC2 in the acrosome region and in the equatorial segment of mouse spermatozoa and the contribution of TRPC2 to the sustained Ca2+ influx under the influence of proteins of the zona pellucida (ZP3) and to AR. We have also shown the presence of TRPC1 and TRPC5 in the acrosome region in the anterior sperm head and TRPC3 in the posterior head and in the flagellar principal piece in mouse spermatozoa [27]. There is also evidence that TRPC, at least in some types of cells, are store-operated channels that are activated, like ORAI, by Ca2+ IS depletion [28]. The study by Lee et al. demonstrated the interaction between TRPC, STIM, and ORAI; the authors supposed that the SOC mechanism could actually be provided not by separate channel structures (ORAI or TRPC) but by a complex, comprising all of the above components [29]. In human spermatozoa, the treatment with SKF96365, the TRPC and SOCE inhibitor, leads to the inhibition of chemotaxis, suggesting the potential role of TRPC channels and SOCE in this process [30].
2.1.3. Other TRP channels. Spermatozoa proved to have other TRP channels in addition to TRPC. The presence of TRPM (M, melastatin) and TRPV (V, vanilloid) channels has been shown in rat spermatozoa [31], but their role is not quite clear. Bernabò et al. [32] demonstrated that TRPV1 channels shifted during capacitation from the post-acrosomal region to the apical part of the head of boar spermatozoa, as well as the drop of [Ca2+]i and the inhibition of actin polymerization in the acrosome region when the cells were treated with capsazepine (the antagonist of TRPV1 channels). The activation with capsaicin (the agonist of TRPV1 channels) in this work resulted in membrane depolarization, followed by the opening of CaV channels. The data on the localization of TRPV1 channels in the pre-acrosomal, acrosomal, and post-acrosomal regions, as well as in the region of the tail of bovine sperms, have been obtained in one of the recent studies [33]. The authors present unambiguous data on the role of TRPV1 in the functioning of gametes: both inhibition of the channel by capsazepine and its activation by anandamide reduced the rectilinear translational motility and induced hyperactivation and capacitation (as was assessed by the test assay for chlortetracycline). In addition, the presence of TRPM8 channels in human spermatozoa was shown [34]. This channel is activated by menthol and by temperature decrease; De Blas et al. [34] found that menthol activates the AR of gametes; however, this effect is suppressed in the case of pretreatment by capsazepine or BCTC (another TRPM8 inhibitor). An analogous study in mice showed similar results with the difference that, in contrast to human spermatozoa, where the treatment with capsazepine and BCTC had no effect on AR activation by progesterone and ZP3, the inhibition of TRPM8 in mice led to a considerable suppression of AR under the exposure to the above physiological inducers [35].
2.1.4. CNG channels are non-selective cationic channels found in PM of different types of cells. They are activated by cyclic nucleotide (cAMP or cGMP) binding, have low ion selectivity and are weakly voltage dependent [19]. Though it is exactly CNG that was the first sperm ion channel isolated from mouse testicles, its involvement in the functions of mature male gametes has been called in question for a long time [19], and the role of CNG is not quite clear up to now. The high selectivity to Ca2+ in the CNG of mouse spermatozoa, as well as the contribution of this channel to the entry of extracellular Ca2+ into the cytosol of gametes and capacitation, have been shown. The authors have demonstrated that cyclic nucleotides (their analogs 8Br-cAMP and 8Br-cGMP were used) are able to increase macroscopic ion currents in mouse spermatozoa. The inhibition of CNG significantly reduced the cGMP-induced Ca2+ entry and capacitation of male gametes [36].
2.1.5. CaV channels. The role of voltage-gated Ca2+ channels (CaV) in spermatozoa is now widely discussed [37]. The protein structures and mRNA of CaV channels were found in spermatozoa of many mammalian species; in addition, CaV3 channels were shown to function in mouse and human spermatogonia [38] and in mouse testicular sperms [39]. At least two kinds of CaV channels were shown to function in the incapacitated mouse epididymal spermatozoa: according to the data obtained by Wennemuth et al., these are most probably CaV2.2 and CaV2.3 [40]. Interestingly, mouse spermatozoa with defective CaV2.3, in addition to [Ca2+]i reduction in the area of the head under the influence of α-D-mannose-BSA (bovine serum albumin), demonstrate a more rapid and linear motion compared to the wild type [41]. It was supposed that CaV1 and CaV2 channels are significant for AR of human male gametes, as it was shown that some part of the Ca2+ entry required for this process passes through them [42]. However, the mice with the knocked-out CaV3.1 and CaV3.2 genes (the CaV3 channel genes) have normal fertility [43], and not functioning CaV channels were found in epididymal sperm. Nevertheless, the later study in mice provided evidence in support of the opposite: mice with defective CaV3.2 demonstrated the change in the Ca2+ signal, the decrease in the AR capacity, and the percentage of successful IVF (subfertile phenotype). Blocking of CaV3.2 in the wild type (using SNX-482) resulted in a considerable suppression of AR induced by cholera toxin B or ganglioside GM1; the authors showed that membrane rearrangements, such as cholesterol efflux and local increase in GM1 concentration, modulated AR by affecting the activity of CaV3.2 channels [44].
2.2 Calcium Channels of Intracellular Stores of Spermatozoa
The Ca2+ entry from ISs is equally important for implementation of the processes required for fertilization as the influx of this ion from the outside. It was shown that mobilization of deposited Ca2+ is involved in capacitation, AR [45, 46], and hyperactivation [6, 7, 47], while thermotaxis (a type of chemotaxis, the movement of gametes towards the higher temperature) is regulated solely by the Ca2+ signals generated by intracellular stores [48]. The most widespread Ca2+ channels in different types of mammalian cells are inositol-1,4,5-triphosphate (IP3)-sensitive Ca2+ channel (or IP3-receptor, IP3R) and ryanodine-sensitive Ca2+ channel (or Ry-receptor, RyR) localized mainly on the membrane of endoplasmic and sarcoplasmic reticulum (ER). Despite the mass difference (about 260–310 kDa for IP3R and 565 kDa for RyR), these channels, especially their transmembrane domain, have the regions with similar sequences determining their ability to interact with the same compounds but with different, sometimes opposite, effects. Both channels are activated and inhibited by Ca2+: the bell-shaped dependence of the IP3R and RyR activities on [Ca2+]i has been shown, and the maximum probability of channel opening is observed at the following free Ca2+ concentrations: 0.2 µM for IP3R and 1–100 µM, for RyR [49].
2.2.1. IP3 receptor is a protein complex consisting of 8 transmembrane domains, as well as Ca2+, IP3 and ATP-binding sites and Ser residues phosphorylated by protein kinase A (PKA) and protein kinase G (PKG) [50]. In somatic unexcitable cells, IP3R is localized mostly on the membrane of ER and the Golgi complex and on the nuclear envelope [51]. Despite the absence of ER and the Golgi complex in mature sperm, the presence of IP3R in male gametes of many mammalian species has been confirmed, with predominant localization in the region of acrosome, the neck and, sometimes, the midpiece of the tail [52]. It was shown that IP3R present on the acrosomal membrane is involved in the induction of AR in male gametes of mammals [46]. Interestingly, in human spermatozoa, the expression of IP3R1 considerably decreases after AR, while the expression of IP3R3 remains unchanged, probably indicating the involvement of this channel in the events following AR. In addition to the acrosome, IP3R is present in the neck region, on the redundant nuclear envelope (RNE) localized close to the axoneme, and there is evidence of IP3R involvement in hyperactivation [53]. The studies in somatic cells have shown that one of the IP3R functions is the transport of Ca2+ ions to mitochondria (MC), and this process is necessary for mitochondrial respiration and regulation of apoptosis [54]. Together with the data on the [Ca2+]i increase induced by the uncouplers of mitochondrial respiration (2,4-dinitrophenol and carbonylcyanide-4-(trifluorometoxy)phenylhydrazone; as is known, the work of the mitochondrial Ca2+ uniporter (MCU) requires a high membrane potential) presented by Correia et al. [1], all the above suggests that Ca2+ transfer to MC with the involvement of IP3R is functionally significant also for spermatozoa.
Ca2+ mobilization through IP3R is triggered by receptor activation on PM, which leads to IP3 formation via the hydrolysis of phosphatidylinositol-4,5-diphosphate (PIP2) by PLC. Ca2+ is required as a co-antagonist for the opening of an IP3-sensitive Ca2+ channel [50]. In addition, the channel activity can be affected by numerous proteins modulating Ca2+ signals trough IP3R activation or inhibition [51]. Upon IS depletion, IP3R activates SOC [1] and thereby the IP3R of spermatozoon possessing Ca2+ stores of a small volume can provide a sustained Ca2+ influx to the cytosol.
2.2.2. Ry receptor. Another Ca2+ transporter of somatic cells is the Ry-receptor (RyR)—a Ca2+ channel so named because of sensitivity to the alkaloid ryanodine. The presence and role of RyR in spermatozoa is not as well-studied as those of IP3R. However, there is evidence for the presence of RyR in the regions of the neck, the midpiece and, to a lesser extent, the acrosome of human spermatozoa: RyR1 and 2 have been identified in the neck area [55]; there are data on the presence of RyR3 in mature mouse spermatozoa; the study of 2013 detected RyR in the acrosome region [56]. Nevertheless, in some works RyR has not been detected in mature male gametes [52]. In vivo, RyR is activated directly by Ca2+ by the CICR mechanism, and its activation is possible in a broader range of Ca2+ concentrations as compared to IP3R [50]. One more physiological activator of RyR is the secondary mediator cADP ribose synthesized from NAD with the involvement of ADP-ribosyl cyclase (CD-38) [1]. Park et al. have demonstrated that RyR and CD-38 are incorporated in spermatozoa while being mixed with prostasomes (small vesicles secreted by the prostate), whereas the CatSper channels are originally present in mature sperm [57].
The functions of both IP3R and RyR are affected by reactive oxygen (ROS) and nitrogen (RNS) species oxidizing specific sites that contain cysteine radicals [1]. ROS exert both direct activating effect on RyR1 by enhancing the interaction between channel subunits and indirect effect by blocking the Ca2+-calmodulin and apocalmodulin binding; in its turn, nitrogen oxide (NO) blocks the direct effect of ROS and reduces the efficiency of apocalmodulin. In case of IP3R, the process becomes a little more complicated: the low level of cysteine oxidation sensitizes the channel, while enhanced oxidative effect during the treatment with high doses of thimerosal inhibits the activity of IP3R. The IP3R and RyR activities are regulated by different kinases such as PKA, PKG, calmodulin-dependent protein kinase II (CaMK II), protein kinase B (AKT), as well as some tyrosine kinases [1]. It has also been shown that GDP/GTP exchange in the respective domain of Rap from the family of small GTPases provides acrosomal exocytosis in human spermatozoa. At the same time, the biological role of such exchange consists in Ca2+ mobilization from IP3-sensitive IS [58]. The mechanism of this process has been investigated in recent studies: the activation of Epac (cAMP-activated guanine nucleotide-exchange factor) in the presence of cAMP results in GDP/GTP exchange in the small GTPase Rap1, which activates PLC with the formation of IP3, followed by Ca2+ mobilization from the acrosome. In addition, cAMP activates small GTPases Rab3 and 27 completing the exocytotic cascade [59]. In support of the data obtained, another research team has demonstrated that the small GTPase Rab3 may have different effects on the acrosomal exocytosis depending on the type of the bound guanine nucleotide, and GTP hydrolysis on Rab3 is necessary to complete the finals stages of AR [60]. Ghosh et al. obtained the evidence of existence of GTP-activated translocation of Ca2+ between ISs of different types [61]. The authors have experimentally demonstrated for the first time the multidirectional effects of GTP in neuroblastoma and smooth muscle cells: GTP mobilized Ca2+ from IS; however, in the presence of oxalate (capable of stimulating Ca2+ deposition) after the rapid period of mobilization, GTP stimulated Ca2+ loading into IS. According to the hypothesis of Ghosh et al. [61], GTP mediates the process of bond formation between two types of IS, ryanodine- and IP3-sensitive, and provides Ca2+ transition from ryanodine- to IP3-sensitive ISs. The joint effects of GTP and IP3 result in additional Ca2+ release from IS in the cells, indicating the translocation of Ca2+ between the stores. We have also shown the interaction between IP3-sensitive and IP3-insensitive (supposedly Ry-sensitive) Ca2+ stores during capacitation and AR [45]. In the former case, the two stores interaction involves filamentous actin (F-actin) and PKA: the inhibition of microfilaments polymerization by cytochalasin D, as well as the pre-treatment by PKA inhibitor H-89, suppressed both additional mobilization of Ca2+ observed under the exposure to theophylline (increasing the level of cAMP in a cell) together with GTP and capacitation stimulated by this pair of reagents. It is intriguing to suppose that Ca2+ mobilization from IS in the RNE region is accompanied by translocation of membrane vesicles carrying Ca2+ to the acrosomal region via actin-dependent transport based on actin polymerization [62], followed by the fusion of the vesicular membrane with the acrosomal membrane. It is also probable that Ca2+ is transported being bound to calreticulin, and the membrane of cytoplasmic vesicles contains PIP2, the precursor of IP3, and diacylglycerol (DAG), required, inter alia, for inactivation of gelsolin (the F-actin severing protein); this is indirectly confirmed by the fact that Ca2+ and CaMKII are necessary for actin polymerization in the head region [63], i.e., all of the above participants of this process can be transferred to the region of the acrosome jointly. In case of AR, it has been shown that the store interaction under the influence of prolactin/GTP involves microtubules and protein kinase C (PKC): the additional release of Ca2+ and AR stimulation were suppressed in case of pre-treatment with nocodazole (the inhibitor of microtubules polymerization) and the PKC inhibitor Ro 31-8220 [45]. We suppose that, during capacitation of mammalian spermatozoa after Ca2+ release from RyR, IP3R is activated and, in the presence of GDP, Ca2+ is transported from IP3-sensitive into IP3-insensitive IS. Moreover, according to our hypothesis, an opposite process is observed during AR: IP3R activation in the presence of GTP is followed by Ca2+ transit from IP3-insensitive to IP3-sensitive Ca2+ stores. Probably, it is precisely how the Ca2+ signal is propagated from the tail to the head at the initial stages of capacitation; in addition, the transport of Ca2+ between the stores can be necessary in a spermatozoon, where, in spite of the small volume of cytoplasm and the relatively long duration of [Ca2+]i increase, the cell must implement exactly localized Ca2+-induced reactions, as well as avoid Ca2+ overloads in MC and, accordingly, the induction of apoptosis.
3 CALCIUM RELEASE FROM CELLS
Ca2+ pumps, or the pumps that perform ion transport against electrochemical gradient, spending the energy of ATP hydrolysis, make the major contribution to the maintenance of low Ca2+ concentration in the cytosol of eukaryotic cells. Such transporters, the so-called Ca2+-ATPases, are localized both in the outer plasma membrane (PMCA, plasma membrane Ca2+ ATPase) and in the membranes of organelles functioning as Ca2+ IS (SERCA, sarcoplasmic–endoplasmic reticulum ATPase, and SPCA, secretory pathway Ca2+ ATPase). It should be noted that the names of the transporters represent the rule that has some exceptions: e.g., in plants, the SERCA analog occurs also in the plasmalemma and, on the contrary, the characteristic localization of PMCA is the cytoplasmic vacuoles. All 3 types of Ca2+-ATPases belong to the P-type (P is phosphorylation), which is characterized by the change in the E1–E2 conformation with the emergence of a temporarily phosphorylated intermediate state (2 Ca2+E1P → 2 Ca2+E2P) necessary for Ca2+ transport across the membrane [64]. All ATPases of this type consist of 10 transmembrane and 3 cytoplasmic domains: ATP binding domain, phosphorylation domain, and the domain for rearrangement of transmembrane helices during Ca2+ transport.
3.1 Calcium ATPases of the sperm PM
PMCA differs from other representatives of P-type ATPases in the presence of the calmodulin (CaM) binding domain and, hence, the higher molecular mass (130–140 kDa). Calmodulin increases the affinity to Ca2+ and the maximum reaction rate, thereby being an activator of the outgoing Ca2+ current in the spermatozoon. Among about twelve splicing variants of PMCA, the rat and mouse sperms were shown to contain mainly PMCA4: 90% of the total amount, with localization in the flagellar principal piece. It has been shown in mice that PMCA that is the major pathway of Ca2+ removal from the cytosol of spermatozoa [65]. At the same time, the mice with the mutant PMCA4 gene demonstrate infertility in males [66]. It has also been shown that the mechanism of infertility in this case is associated with the inability of cells to effectively reduce the cytosol level of Ca2+: the mice with the mutant PMCA4 gene demonstrated the Ca2+ level at rest equal to 370 nM (instead of 157 nM in the wild type), as well as considerably reduced hyperactivation ability. In bulls, the study with the quantitative PCR test has shown that, out of the two PMCA4 forms, the main splicing variant in the testicle, the head and tail of the epididymis is PMCA4b, while the caudal part of the epididymis exhibits mostly PMCA4a, which is more active with respect to Ca2+ transport [67]. It is supposed that the change of isoform when sperms move to the tail of the epididymis suggests the increasing demand for active Ca2+ transport after getting into female genital tracts. The studies in human sperms have shown the presence of PMCA4 in the area of the acrosome, its internal membrane, as well as in the posterior head, the neck, the midpiece and the proximal section of the principal piece of the flagellum. The authors have also demonstrated the joint localization of and even association between PMCA4 and NO synthases, especially in the capacitated sperms with enhanced Ca2+ levels. The method of co-immunoprecipitation has shown the presence of complexes with Ca2+/CaM-dependent serine kinases (CASK), PMCA4 and NO synthases in the prostasomes of seminal plasma from where, apparently, these complexes get into spermatozoa [66]. The existence of such complexes suggests the role of PMCA4 in the negative regulation of the activity of NO synthases of spermatozoa (activated upon the increase in Ca2+ level in the cytosol), as it has already been shown in embryonic kidney HEK293 and mouse neuroblastoma Neuro-2a cell models. In addition, the data obtained with HEK293 cells demonstrate the interaction between PMCA4 and the Ras signaling pathway via the epidermal growth factor receptor (EGFR) and, hence, the presence of such interaction is quite probable also in spermatozoa, because EGFR was shown to be present in male gametes. In human spermatozoa, phosphorylation of proteins at tyrosine in vivo is triggered aside from the cAMP/kinase induction pathway, also with the interaction between ligands and CPM receptors [68]. Receptor kinases such as EGFR stimulate protein phosphorylation at tyrosine via the Ras-Raf-MEK-ERK-MAP cascade (Ras, membrane-bound proteins, small GTPases; Raf, serine/threonine-specific protein kinases; MEK, MAP-phosphorylating protein kinase) [69]. Thus, we can assume the involvement of PMCA4 in the regulation of protein phosphorylation at tyrosine during capacitation.
Another research team has demonstrated that the maintenance of Ca2+ homeostasis in mouse spermatozoa also requires the balance between JAM-A (Junctional adhesion molecule-A) and PMCA4b: it has been shown that the interaction between CASK and PMCA4b leads to inactivation of ATPase, while JAM-A indirectly performs positive regulation of the pump, also binding CASK. The mice with defective JAM-A exhibited considerable insufficiency of both progressive and particularly hyperactivated motility, electron-dense condensed MCs, and the histopathological phenotype repeating that in the mice with PMCA4b deficiency. Based on the above, the authors have concluded that the insufficiency of JAM-A is accompanied by the impairment of Ca2+ efflux outwards through PMCA4b, as well as the abnormal Ca2+ sequestration by mitochondria [70].
One more pathway for the regulation of PMCA4b activity, which is possible for spermatozoa, is PKC that can partially activate PMCA4b by eliminating pump inhibition via phosphorylation in the area of 20 residues downstream the calmodulin-binding domain. However, calmodulin activates ATPase more effectively by eliminating the inhibition in 2 regions at once, regardless of the PKC-phosphorylated inhibitory site [71].
3.2 Na+/Ca2+ exchanger
In addition to PMCA, which is the main pathway for Ca2+ release into the extracellular space for inexcitable cells, spermatozoa also have a Na+/Ca2+ exchanger (NCX). This transporter releases one Ca2+ molecule against the gradient, simultaneously supplying 3 sodium (Na+) molecules to the cytosol due to the energy of electrochemical gradient. NCX can also supply Ca2+ to a cell under the conditions of membrane depolarization or sodium deficiency in the extracellular medium [65]. There are 2 families of this trasnporter: NCX and K+-dependent NCX (NCKX). The presence of NCX has been shown in both epididymal and ejaculated bovine sperms, and the transporter activity was different: in epididymal spermatozoa, NCX, contrary to its direct function, transported Ca2+ ions to the cytosol; in ejaculated gametes, the exchanger activity was suppressed by the protein of seminal plasma. Rat testicles were shown to contain NCX1.3 and NCX1.7: 2 splicing variants of NCX1; in addition, it was reported on the presence of NCKX3 in mouse testicles. The data on the role of NCX in Ca2+ removal from the cytosol after the increase in its concentration were also obtained in mice; however, the contribution of NCX was considerably less compared to that of PMCA and, consequently, the role of the exchanger consists largely in the reverse transport of Ca2+ to the cytosol at its low levels [65].
In addition, NCX is present in the membrane in the area of the acrosome and the midpiece of human spermatozoa and its inhibition (using bepridil, 3',4'-dichlorobenzamil hydrochloride and KB-R7943) was shown to be accompanied by [Ca2+]i increase and considerable suppression of sperm motility [72]. Later, another research team demonstrated that the incubation of sperms both with the NCX inhibitor KB-R7943 and with the PMCA inhibitor eosin results in the degradation of all motility indicators of CASA (Computer-assisted sperm analysis), while the inhibition of the Na+/H+ exchanger and Na+/K+-ATPase is accompanied by a decrease only in the percentage of forward progressive spermatozoa [73].
3.3 Calcium ATPases of intracellular stores
The replenishment of Ca2+ level in IS and limitation of Ca2+ signals are performed by intracellular Ca2+ pumps: SERCA and SPCA, and their role in the function of mature spermatozoa of mammals is now widely discussed [1]. The major Ca2+ transporter in somatic cells, which transfers this ion from the cytosol to IS, is SERCA, and there are data on its localization on ER vesicles both with IP3R and with RyR. There is also evidence of the presence of ATPases analogous and probably even identical to SERCA on the outer nuclear membrane of rat liver cells. The structure of intracellular Ca2+-ATPase is in many respects similar to that in PMCA, with the difference that SERCA lacks the calmodulin-binding domain [50], which apparently contributes to differentiation of the pumps by the mechanism of regulation of their activity. In mammals, SERCA exists in 3 isoforms (SERCA1, SERCA2 and SERCA3); each of them has numerous splicing forms with different sizes and regulatory properties of the protein [74]. The most widespread isoform is SERCA2, which is present in almost all types of cells [1], including spermatozoa: the presence of SERCA2 has been shown for mature mouse, bovine and human spermatozoa. In the same study, Western blot technique showed the presence of at least two SERCA2 isoforms in male gametes, one of them in the area of the outer acrosomal membrane and the other in the midpiece of the sperm. The SERCA activity can vary under the influence of oxidative stress, and its effect can be both stimulatory and inhibitory depending on the oxidized cysteine residue [75]. Interestingly, it has been shown that phospholamban (the muscle-specific SERCA2 inhibitor) is expressed in mouse testicles and the Ca2+ level in spermatids decreases upon accumulation of this protein, which can lead to the impaired differentiation and function of future gametes [76].
SPCA is another intracellular Ca2+ pump removing Ca2+ from the cytosol to the IS of somatic cells. SPCA exists as 2 isoforms: SPCA1 and SPCA2; SPCA1 is much more widespread. The typical localization of SPCA is the Golgi apparatus. There is evidence that SPCA1 is present in human spermatozoa; it is localized mainly in the area of the sperm neck, where RNE and calreticulin-containing membrane vesicles are localized [77]. It is interesting that immunofluorescence has shown the localization of SPCA in the area of MC in sea urchin spermatozoa [78].
4 CALCIUM SEQUESTRATION BY MITOCHONDRIA
For a long time, MCs have been considered as the major Ca2+-accumulating organelles of cells. Ca2+ was transferred to the matrix via MCU localized on the inner membrane. The nature of MCU as a selective Ca2+ channel with an extremely high affinity to Ca2+ was revealed [79]. Ca2+ ions are transported via MCU by the gradient of potential formed on the membrane during the normal functioning of MC. Ca2+ is released from the mitochondrial matrix through the transporter that releases Ca2+ to the cytosol in exchange for H+ or Na+ (NCX). In 2010, it was shown that the representative of the NCX family, NCLX (Na+(Li+)/Ca2+ exchanger, is localized in mitochondrial cristae and is the main Na+/Ca2+ antiporter of these organelles [80].
It has been shown that Ca2+ begins to be pumped into the MC matrix at a [Ca2+]i increase at rest (i.e., without the signals of its long-term increase) [81], which stimulates ATP synthesis through oxidative phosphorylation. Indeed, it was shown in mouse spermatozoa that the contribution of MC to Ca2+ buffering increased during the inhibition of membrane Ca2+-ATPases, resulting in the [Ca2+]i increase at rest [65]. The Ca2+ intake by MC has a considerable effect on the Ca2+ signals of somatic cells, reducing their amplitude and duration, and any particular “form” of this effect in each type of cells is manifested differently [81]. That is how the Ca2+-activated release of Ca2+ from ISs is avoided. Localization of MC in the midpiece of mature spermatozoa is quite appropriate for the modulation of Ca2+ signals as follows: close to MC in the gamete there is a membrane outgrowth formed as a result of chromatin condensation: RNE that carries, according to different data, RyR and IP3R. However, bovine spermatozoa during Ca2+ mobilization from IS in the tail area, which is recognized to be RNE, did not show enhanced ATP synthesis, suggesting the absence of the involvement of MC in the propagation of Ca2+ signal in this case [47]. The same study showed that hyperactivation promoted by thapsigargin (specific SERCA inhibitor) was not suppressed during NCX inhibition in MC. There are data in support of the fact that MC in human spermatozoa has no effect on Ca2+ fluctuations caused by the activation of IS in the area of the neck under the exposure to NO and progesterone. The authors’ data showed that in the gametes with uncoupled mitochondrial respiration, which were pretreated with NO (activating Ca2+ mobilization from the stores), [Ca2+]i returned to the normal level after the addition of dithiotreitol (the compound efficiently eliminating the effects of S‑nitrosylation) just as it occurred in the cells with intact MC [82]. In all likelihood, spermatozoa must be able to maintain the longer and more intensive Ca2+ signals with the involvement of IS. It should also be remembered that calcium “overload” in MC results in the formation of Ca2+-dependent pores increasing membrane permeability (MPTP, mitochondrial permeability transition pore), which leads to the induction of apoptosis [81]. Probably, for mature spermatozoa, where all post-ejaculatory processes are accompanied by Ca2+ signals, such scenario is highly probable and no less undesired; consequently, the “exclusion” of MC from the chain of regulation of [Ca2+]i fluctuations may have adaptive significance.
5 FUNCTIONAL SIGNIFICANCE OF CALCIUM SIGNALING IN SPERMATOZOA
The role of Ca2+ signaling in all types of cells can hardly be overestimated: the defects in its key elements often lead to severe pathologies and considerable impairments of cell functions, including those of gametes. In spermatozoa, Ca2+ is involved in all post-ejaculatory processes including fertilization. In the cytoplasm of sperms, Ca2+ concentration is very low; posttranslational protein modifications are regulated due to its increase. [Ca2+]i can increase via the influx of this ion from the outside through Ca2+ channels of the outer PM, or via Ca2+ mobilization from IS, where the ion concentration is 4-fold higher compared to the cytoplasm.
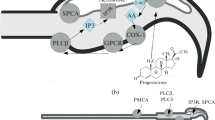
The role of [Ca2+]i fluctuations in providing the motility and chemotaxis of spermatozoa has been shown [83]. The axoneme contains Ca2+-binding sites that regulate tail bending through the modulation of dynein-dependent sliding, and this process involves calmodulin and CaMK II [2]. When spermatozoa leave seminal fluid and pass through cervical mucus, gametes begin to undergo biochemical changes necessary to acquire the AR and fertilization capacity and referred to as capacitation. These changes include intracellular modulation of ion concentration, alkalinization of the cytoplasm, rearrangement of the lipid composition of PM and hyperpolarization of the latter, increase in the PKA activity and tyrosine phosphorylation of proteins, as well as actin polymerization. It has been shown that capacitation (Fig. 1) is accompanied by an increase in [Ca2+]i via Ca2+ influx from extracellular space through CatSper channels under the influence of capacitating agents (such as HCO3– and BSA) [84]. At the same time, researchers observed the rapid increase in [Ca2+]i in the subpopulation of gametes even after 1-min incubation. Ca2+ entering together with HCO3– activates soluble adenylate cyclase (rAC) of a sperm [4], followed by the activation of PKA and the subsequent cascade of reactions with the involvement of this enzyme, which is an integral part of capacitation. In addition, Ca2+ is required for another indispensable attribute of capacitation: actin polymerization. When binding to calmodulin, Ca2+ activates CaMK II, and it has been shown in humans that this enzyme mediates the formation of F-actin in the area of the head, which in turn prevents premature AR; the ability of CaMK II to inhibit spontaneous SR has also been shown in bulls and mice [63]. The data obtained in humans and mice suggest the significance of actin polymerization in the tail area for hyperactivation. It is known that hyperactivation is triggered by the increase in Ca2+ concentration in the tail area; this ion intensifies the filament bending towards one side and, as a result, the movement pattern changes from symmetric to asymmetric [6]. Extracellular Ca2+ is required to maintain hyperactivated motility; however, there is evidence that the induction of hyperactivation also involves Ca2+ from IS. It has been shown that Ca2+ mobilization from RNE and membrane vesicles in the neck area regulates the tail activity and participates in hyperactivation, and the thimerosal-stimulated release of Ca2+ from these stores leads to hyperactivation of some part of mouse [6] and human [7] sperms even in the absence of extracellular Ca2+ and/or functioning CatSper channels. At the subsequent stages of capacitation, [Ca2+]i in the head area increases, actin depolymerization takes place, and AR becomes possible. Exocytosis of acrosomal enzymes after the fusion of the acrosomal outer membrane and the plasmalemma is a necessary stage for overcoming the ovum envelopes. The involvement of Ca2+ mobilization from the acrosome and SOCE during AR induction has been shown in mice. Mobilization of Ca2+ stores proved to be necessary for AR triggering, being its key stage [58]. Physiological AR inducers such as ZP3 and progesterone induce Ca2+ influx through CatSper channels, which results in the activation of PLC that catalyzes the formation of IP3 and diacylglycerol: the former activates IP3R on the outer membrane of the acrosome and the latter stimulates the work of PKC providing AR. Depletion of the acrosomal store leads to the induction of SOCE and a sustained influx of Ca2+, which in turn induces actin depolymerization and triggers AR.
Simplified model of Ca2+ signaling in post-ejaculatory processes of mammalian spermatozoa. The initial stages of capaci-tation are characterized by the entry of hydrocarbonate (\({\text{HCO}}_{3}^{ - }\)) ions to the cytoplasm and pH increase, which is necessary for the activation of soluble adenylate cyclase (sAC), as well as the opening of CatSper channels. Another early event is the generation of reactive oxygen and nitrogen species (ROS and RNS) by membrane oxidase/NO synthase (NOS; it has not yet been accurately identiified ) and/or endogenously (mainly by mitochondria (MC)). ROS oxidizes cholesterol with the formation of oxysterols, which, after contacting albumins of the extracellular space, are released from PM, increasing its fluidity and permeability. In addition, superoxide anion (\({\text{O}}_{2}^{{\centerdot {\kern 1pt} - }}\)) can activate the membrane adenylate cyclase (mAC), the voltage-dependent Ca2+ channel (CaV), as well as phospholipase C (PLC); moreover, ROS and RNS modulate the activity of IP3-sensitive and ryanodine-sensitive Ca2+ channels (IP3R and RyR). It seems that an insignificant increase in [Ca2+]i at the early stages of capaciation leads to the activation of RyR, which is present in the area of redundant nuclear envelope (RNE), while Ca2+ mobilized from the stores activates the sperm-specific potassium channel (SLO3K+) involved in membrane hyperpolarization, which is also necessary for the activation of CatSper and the induction of hyperactivation. Probably, ISs are also directly involved in the induction of hyperactivation; however, the mechanism of this relationship remains to be elucidated. Alkalization of the cytoplasm also activates soluble adenylyl cyclase (sAC), triggering the cAMP/PKA/TK signaling cascade. PKA (protein kinase A) and TK (tyrosine kinase) have numerous substrates; however, one of the most important substrates is gelsolin, the protein that severs F-actin while being in the activated state. Inactivation of gelsolin by tyrosine phosphorylation jointly with the activation of PLD (phospholipase D), followed by the formation of PA (phosphatidic acid) results in the formation of F-actin from G-actin (globular actin), which is necessary to create a barrier between the outer membrane of the acrosome and PM and to prevent spontaneous AR (sAR). Later, after the activation of CatSper channels, Ca2+ signal is transmitted from the tail to the head; probably, this is the biological sense of IS interaction that we have observed in our experiments. In addition, a hypothesis is suggested about the role of store-operated Ca2+ entry (SOCE) in this process. SOCE is activated upon Ca2+ IS depletion and is implemented through store-operated Ca2+ channels (SOC: ORAI and TRPC) mediated by stromal interaction molecule (STIM). PLC activated by ROS and Ca2+ hydrolyzes PIP2 to IP3 (activating IP3R and enhancing Ca2+ signal) and diacylglycerol (DAG involved in the activation of SOC and protein kinase C (PKC)). Probably, IP3R also participates in the Ca2+ transfer to mitochondria (MC) via the mitochondrial calcium uniporter (MCU), which is necessary for ATP production. At the late stages of capacitation, the Ca2+ signal transmitted to the head becomes more intense with the involvement of the acrosomal store: Ca2+ entry through IP3R results in rapid depletion of low-volume stores, which, together with the activation of SOCE, provides a sustained Ca2+ influx. The latter results in gelsolin release and F-actin severing and thereby enables the induced AR (iAR) while contacting ZP3 (a glycoprotein of the zona pellucida). Unspecified abbreviations: PMCA, plasma membrane Ca2+-ATPase; NCX, Na+/Ca2+ exchanger; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase; SPCA; secretory pathway Ca2+-ATPase; cAMP, cyclic AMP.
Over recent years, researchers have made a considerable progress in understanding the molecular biological mechanisms that determine spermatozoa functioning and acquiring fertilization capacity. The key role of Ca2+ in all post-ejaculatory processes in male gametes has been well established and confirmed. However, in spite of the vast array of available data, there is a lot of unsolved problems (including interspecies differences between the biochemical mechanisms providing fertility; the way how Ca2+ signal is transmitted from the tail to the head during capacitation; the pattern of interrelationship between the activation of membrane oxidases and the increase in [Ca2+]i at the initial stages of capacitation; etc.). The answers to these questions will make it possible to understand both the processes that determine fertilization in vivo and in vitro and the mechanisms of the low fertilization capacity of sperm and male infertility.
REFERENCES
Correia J., Michelangeli F., Publicover S. 2015. Regulation and roles of Ca2+ stores in human sperm. Reproduction. 150 (2), R65–R76.
Wang H., McGoldrick L.L., Chung J.J. 2020. Sperm ion channels and transporters in male fertility and infertility. Nat. Rev. Urol. 18 (1), 46–66.
Ren D., Navarro B., Perez G., Jackson A.C., Hsu S., Shi Q., Tilly J.L., Clapham D.E. 2001. A sperm ion channel required for sperm motility and male fertility. Nature. 413 (6856), 603–609.
Sun X.H., Zhu Y.Y., Wang L., Liu H.L., Ling Y., Li Z.L., Sun L.B. 2017. The CatSper channel and its roles in male fertility: A systematic review. Reprod. Biol. Endocrinol. 15 (65), 1–12.
Marquez B., Suarez S.S. 2007. Bovine sperm hyperactivation is promoted by alkaline-stimulated Ca2+ influx. Biol. Reprod. 76 (4), 660–665.
Marquez B., Ignotz G., Suarez S.S. 2007. Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: Release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Dev. Biol. 303, 214–221.
Alasmari W., Barratt C.L., Publicover S.J., Whalley K.M., Foster E., Kay V., Martins da Silva S., Oxenham S.K. 2013. The clinical significance of calcium-signalling pathways mediating human sperm hyperactivation. Hum. Reprod. 28 (4), 866–876.
Jin J.L., O’Doherty A.M., Wang S., Zheng H., Sanders K.M., Yan W. 2005. Catsper3 and catsper4 encode two cation channel-like proteins exclusively expressed in the testis. Biol. Reprod. 73 (6), 1235–1242.
Sumigama S., Mansell S., Miller M., Lishko P.V., Cherr G.N., Meyers S.A., Tollner T. 2015. Progesterone accelerates the completion of sperm capacitation and activates CatSper Channel in spermatozoa from the rhesus macaque. Biol. Reprod. 93 (6), 130.
Lishko P.V., Botchkina I.L., Kirichok Y. 2011. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 471 (7338), 387–391.
Chávez J.C., Ferreira J.J., Butler A., De La Vega Beltrán J.L., Treviño C.L., Darszon A., Salkoff L., Santi C.M. 2014. SLO3 K+ channels control calcium entry through CATSPER channels in sperm. J. Biol. Chem. 289 (46), 32 266–32 275.
Zeng X.H., Yang C., Kim S.T., Lingle C.J., Xia X.M. 2011. Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc. Natl. Acad. Sci. USA. 108 (14), 5879–5884.
Sanchez-Carranza O., Torres-Rodriguez P., Darszon A., Trevino C.L., Lopez-Gonzalez I. 2015. Pharmacology of hSlo3 channels and their contribution in the capacitation-associated hyper-polarization of human sperm. Biochem. Biophys. Res. Commun. 466, 554–559.
Yeste M., Llavanera M., Pérez G., Scornik F., Puig-Parri J., Brugada R., Bonet S., Pinart E. 2019. Elucidating the role of K+ channels during in vitro capacitation of boar spermatozoa: Do SLO1 channels play a crucial role? Int. J. Mol. Sci. 20 (24), 6330.
Lishko P.V., Botchkina I.L., Fedorenko A., Kirichok Y. 2010. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 140, 327–337.
Mannowetz N., Miller M.R., Lishko P.V. 2017. Regulation of the sperm calcium channel CatSper by endogenous steroids and plant triterpenoids. Proc. Natl. Acad. Sci. USA. 114 (22), 5743–5748.
Schiffer C., Müller A., Egeberg D.L., Alvarez L., Brenker C., Rehfeld A., Frederiksen H., Wäschle B., Kaupp U.B., Balbach M., Wachten D., Skakkebaek N.E., Almstrup K., Strünker T. 2014. Direct action of endocrine disrupting chemicals on human sperm. EMBO Rep. 15 (7), 758–765.
Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S.H., Tanasa B., Hogan P.G., Lewis R.S., Daly M., Rao A. 2006. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 441 (7090), 179–185.
Darszon A., Nishigaki T., Beltran C., Treviño C.L. 2011. Calcium channels in the development, maturation, and function of spermatozoa. Physiol. Rev. 91 (4), 1305–1355.
Lefièvre L., Nash K., Mansell S., Costello S., Punt E., Correia J., Morris J., Kirkman-Brown J., Wilson S.M., Barratt C.L., Publicover S. 2012. 2-APB-potentiated channels amplify CatSper-induced Ca2+ signals in human sperm. Biochem. J. 448 (2), 189–200.
Davis F.M., Goulding E.H., D’Agostin D.M., Janardhan K.S., Cummings C.A., Bird G.S., Eddy E.M., Putney J.W. 2016. Male infertility in mice lacking the store-operated Ca2+ channel Orai1. Cell Calcium. 59 (4), 189–197.
Vazquez G., Wedel B.J., Aziz O., Trebak M., Putney J.W. Jr. 2004. The mammalian TRPC cation channels. Biochim. Biophys. Acta. 1742 (1–3), 21–36.
Darszon A., Sánchez-Cárdenas C., Orta G., Sánchez-Tusie A.A., Beltrán C., López-González I., Granados-González G., Treviño C.L. 2012. Are TRP channels involved in sperm development and function? Cell Tissue Res. 349 (3), 749–764.
Kumar P.G., Shoeb M. 2011. The role of TRP ion channels in testicular function. Adv. Exp. Med. Biol. 704, 881–908.
Beech D.J. 2012. Integration of transient receptor potential canonical channels with lipids. Acta Physiol. (Oxf). 204 (2), 227–237.
Castellano L.E., Treviño C.L., Rodríguez D., Serrano C.J., Pacheco J., Tsutsumi V., Felix R., Darszon A. 2003. Transient receptor potential (TRPC) channels in human sperm: Expression, cellular localization, and involvement in the regulation of flagellar motility. FEBS Lett. 541 (1–3), 69–74.
Sutton K.A., Jungnickel M.K., Wang Y., Cullen K., Lambert S., Florman H.M. 2004. Enkurin is a novel calmodulin and TRPC channel binding protein in sperm. Dev. Biol. 274 (2), 426–435.
Yuan J.P., Zeng W., Huang G.N., Worley P.F., Muallem S. 2007. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat. Cell Biol. 9, 636–645.
Lee K.P., Yuan J.P., Hong J.H., So I., Worley P.F., Muallem S. 2010. An endoplasmic reticulum/plasma membrane junction: STIM1/Orai1/TRPCs. FEBS Lett. 584 (10), 2022–2027.
Teves M.E., Guidobaldi H.A., Uñates D.R., Sanchez R., Miska W., Publicover S.J., Morales Garcia A.A., Giojalas L.C. 2009. Molecular mechanism for human sperm chemotaxis mediated by progesterone. PLoS One. 4 (12), e8211.
Li S., Wang X., Ye H., Gao W., Pu X., Yang Z. 2010. Distribution profiles of transient receptor potential melastatin- and vanilloid-related channels in rat spermatogenic cells and sperm. Mol. Biol. Rep. 37 (3), 1287–1293.
Bernabò N., Pistilli M.G., Mattioli M., Barboni B. 2010. Role of TRPV1 channels in boar spermatozoa acquisition of fertilizing ability. Mol. Cell Endocrinol. 323 (2), 224–231.
Kumar A., Mishra A.K., Singh V., Yadav S., Saxena A., Garg S.K., Swain D.K. 2019. Molecular and functional insights into transient receptor potential vanilloid 1 (TRPV1) in bull spermatozoa. Theriogenology. 128, 207–217.
De Blas G.A., Darszon A., Ocampo A.Y., Serrano C.J., Castellano L.E., Hernández-González E.O., Chirinos M., Larrea F., Beltrán C., Treviño C.L. 2009. TRPM8, a versatile channel in human sperm. PLoS One. 4 (6), e6095.
Martínez-López P., Treviño C.L., de la Vega-Beltrán J.L., De Blas G., Monroy E., Beltrán C., Orta G., Gibbs G.M., O’Bryan M.K., Darszon A. 2011. TRPM8 in mouse sperm detects temperature changes and may influence the acrosome reaction. J. Cell Physiol. 226 (6), 1620–1631.
Cisneros-Mejorado A., Hernández-Soberanis L., Islas-Carbajal M.C., Sánchez D. 2014. Capacitation and Ca2+ influx in spermatozoa: Role of CNG channels and protein kinase G. Andrology. 2 (1), 145–154.
Visconti P.E., Krapf D., De la Vega-Beltrán J.L., Acevedo J.J., Darszon A. 2011. Ion channels, phosphorylation, and mammalian sperm capacitation. Asian J. Androl. 13 (3), 395–405.
Lievano A., Santi C.M., Serrano C.J., Treviño C.L., Bellvé A.R. Hernández-Cruz A., Darszon A. 1996. T‑type Ca2+ channels and alpha1E expression in spermatogenic cells, and their possible relevance to the sperm acrosome reaction. FEBS Lett. 388, 150–154.
Martınez-Lopez P., Santi C.M., Trevino C.L., Ocampo-Gutierrez A.Y., Acevedo J.J. Alisio A., Salkoff L.B., Darszon A. 2009. Mouse sperm K+ currents stimulated by pH and cAMP possibly coded by Slo3 channels. Biochem. Biophys. Res. Commun. 381, 204–209.
Wennemuth G., Westenbroek R.E., Xu T., Hille B., Babcock D.F. 2000. CaV2.2 and CaV2.3 (N- and R-type) Ca2+ channels in depolarization-evoked entry of Ca2+ into mouse sperm. J. Biol. Chem. 275 (28), 21210–21217.
Sakata Y., Saegusa H., Zong S., Osanai M., Murakoshi T., Shimizu Y., Noda T., Aso T., Tanabe T. 2002. Ca(v)2.3 (alpha1E) Ca2+ channel participates in the control of sperm function. FEBS Lett. 516 (1–3), 229–233.
José O., Hernández-Hernández O., Chirinos M., González-González M.E., Larrea F., Almanza A., Felix R., Darszon A., Treviño C.L. 2010. Recombinant human ZP3-induced sperm acrosome reaction: Evidence for the involvement of T- and L-type voltage-gated calcium channels. Biochem. Biophys. Res. Commun. 395 (4), 530–534.
Stamboulian S., Kim D., Shin H.S., Ronjat M., De Waard M., Arnoult C. 2004. Biophysical and pharmacological characterization of spermatogenic T-type calcium current in mice lacking the CaV3.1 (alpha1G) calcium channel: CaV3.2 (alpha1H) is the main functional calcium channel in wild-type spermatogenic cells. J. Cell Physiol. 200, 116–124.
Cohen R., Buttke D.E., Asano A., Mukai C., Nelson J.L., Ren D., Miller R.J., Cohen-Kutner M., Atlas D., Travis A.J. 2014. Lipid modulation of calcium flux through CaV2.3 regulates acrosome exocytosis and fertilization. Dev. Cell. 28 (3), 310–321.
Denisenko V.Yu., Boytseva E.N., Kuzmina T.I. 2015. Mobilization of Ca2+ from intracellular stores of spermatozoa of Bos taurus depending on their functional status. Tsitologiya. (Rus.). 57 (3), 233–239.
Li Y.Y., Jia Y.P., Duan L.Y., Li K.M. 2020. Participation of the inositol 1,4,5-trisphosphate-gated calcium channel in the zona pellucida- and progesterone-induced acrosome reaction and calcium influx in human spermatozoa. Asian J. Androl. 22 (2), 192–199.
Ho H.C., Suarez S.S. 2003. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biol. Reprod. 68, 1590–1596.
Bahat A., Eisenbach M. 2010. Human sperm thermotaxis is mediated by phospholipase C and inositol trisphosphate receptor Ca2+ channel. Biol. Reprod. 82 (3), 606–616.
Bezprozvanny I., Watras J., Ehrlich B.E. 1991. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 351 (6329), 751–754.
Zinchenko V.P., Dolgacheva L.P. 2003. Vnutrikletochnaya signalizatsiya: Uchebnoye posobiye (Intracellular Signaling: Tutorial). Pushchino: E-publishing House “Analytical Microscopy”.
Prole D.L., Taylor C.W. 2019. Structure and function of IP3 receptors. Cold Spring Harb. Perspect. Biol. 11 (4), a035063.
Dragileva E., Rubinstein S., Breitbart H. 1999. Intracellular Ca2+-Mg2+-ATPase regulates calcium influx and acrosomal exocytosis in bull and ram spermatozoa. Biol. Reprod. 61 (5), 1226–1234.
Fujinoki M. 2012. Progesterone-enhanced sperm hyperactivation through IP3-PKC and PKA signals. Reprod. Med. Biol. 12 (1), 27–33.
La Rovere R.M., Roest G., Bultynck G., Parys J.B. 2016. Intracellular Ca2+ signaling and Ca2+ microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium. 60 (2), 74–87.
Lefievre L., Chen Y., Conner S.J., Scott J.L., Publico-ver S.J., Ford W.C., Barratt C.L. 2007. Human spermatozoa contain multiple targets for protein S-nitrosylation: An alternative mechanism of the modulation of sperm function by nitric oxide? Proteomics. 7, 3066–3084.
Zhou Y., Ru Y., Wang C., Wang S., Zhou Z., Zhang Y. 2013. Tripeptidyl peptidase II regulates sperm function by modulating intracellular Ca2+ stores via the ryanodine receptor. PLoS One. 8 (6), e66634.
Park K.H., Kim B.J., Kang J., Nam T.S., Lim J.M., Kim H.T., Park J.K., Kim Y.G., Chae S.W., Kim U.H. 2011. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci. Signal. 4 (173), ra31.
Ruete, M.C., Lucchesi O., Bustos M.A., Tomes C.N. 2014. Epac, Rap and Rab3 act in concert to mobilize calcium from sperm’s acrosome during exocytosis. Cell Commun. Signal. 12, 43.
Lucchesi O., Ruete M.C., Bustos M.A., Quevedo M.F., Tomes C.N. 2016. The Signaling module cAMP/epac/ Rap1/PLCƐ /IP3 mobilizes acrosomal calcium during sperm exocytosis. Biochim. Biophys. Acta. 1863 (4), 544–561.
Bustos M.A., Roggero C.M., De la Iglesia P.X., Mayorga L.S., Tomes C.N. 2014. GTP-bound Rab3A exhibits consecutive positive and negative roles during human sperm dense-core granule exocytosis. J. Mol. Cell Biol. 6 (4), 286–298.
Ghosh T.K., Mullaney J.M., Tarazi F.I., Gill D.L. 1989. GTP-activated communication between distinct inositol 1,4,5-trisphosphate-sensitive and -insensitive calcium pools. Nature. 340 (6230), 236–239.
Khaytlina S.Yu. 2014. Intracellular transport based on actin polymerization. Biochemistry (Mosc.). 79 (9), 917–927.
Shabtay O., Breitbart H. 2016. CaMKII prevents spontaneous acrosomal exocytosis in sperm through induction of actin polymerization. Dev. Biol. 415 (1), 64–74.
Jimenez-Gonzalez C., Michelangeli F., Harper C.V., Barratt C.L., Publicover S.J. 2006. Calcium signalling in human spermatozoa: A specialized 'toolkit' of channels, transporters and stores. Hum. Reprod. Update. 12 (3), 253–267.
Wennemuth G., Babcock D.F., Hille B. 2003. Calcium clearance mechanisms of mouse sperm. J. Gen. Physiol. 122, 115–128.
Andrews R.E., Galileo D.S., Martin-DeLeon P.A. 2015. Plasma membrane Ca2+-ATPase 4: Interaction with constitutive nitric oxide synthases in human sperm and prostasomes which carry Ca2+/CaM-dependent serine kinase. Mol. Hum. Reprod. 21 (11), 832–843.
Brandenburger T., Strehler E.E., Filoteo A.G., Caride A.J., Aumüller G., Post H., Schwarz A., Wilhelm B. 2011. Switch of PMCA4 splice variants in bovine epididymis results in altered isoform expression during functional sperm maturation. J. Biol. Chem. 286 (10), 7938–7946.
Gangwar D.K., Atreja S.K. 2015. Signalling events and associated pathways related to the mammalian sperm capacitation. Reprod. Domest. Anim. 50 (5), 705–711.
Roberts P.J., Der C.J. 2007. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 26, 3291–3310.
Aravindan R.G., Fomin V.P., Naik U.P., Modelski M.J., Naik M.U., Galileo D.S., Duncan R.L., Martin-Deleon P.A. 2012. CASK interacts with PMCA4b and JAM-A on the mouse sperm flagellum to regulate Ca2+ homeostasis and motility. J. Cell Physiol. 227 (8), 3138–3150.
Enyedi A., Verma A.K., Filoteo A.G., Penniston J.T. 1996. Protein kinase C activates the plasma membrane Ca2+ pump isoform 4b by phosphorylation of an inhibitory region downstream of the calmodulin-binding domain. J. Biol. Chem. 271 (50), 32461–32467.
Krasznai Z., Krasznai Z.T., Morisawa M., Bazsáné Z.K., Hernádi Z., Fazekas Z., Trón L., Goda K., Márián T. 2006. Role of the Na+/Ca2+ exchanger in calcium homeostasis and human sperm motility regulation. Cell Motil. Cytoskeleton. 63 (2), 66–76.
Peralta-Arias R.D., Vívenes C.Y., Camejo M.I., Piñero S., Proverbio T., Martínez E., Marín R., Proverbio F. 2015. ATPases, ion exchangers and human sperm motility. Reproduction. 149 (5), 475–484.
Michelangeli F., East J.M. 2011. A diversity of SERCA Ca2+ pump inhibitors. Biochem. Soc. Trans. 39 (3), 789–797.
Csordas G., Hajnoczky G. 2009. SR/ER-mitochondrial local communication: calcium and ROS. Biochim. Biophys. Acta. 1787, 1352–1362.
Niemeyer J., Mentrup T., Heidasch R., Müller S.A., Biswas U., Meyer R., Papadopoulou A.A., Dederer V., Haug-Kröper M., Adamski V., Lüllmann-Rauch R., Bergmann M., Mayerhofer A., Saftig P., Wennemuth G., Jessberger R., Fluhrer R., Lichtenthaler S.F., Lemberg M.K., Schröder B. 2019. The intramembrane protease SPPL2c promotes male germ cell development by cleaving phospholamban. EMBO Rep. 20 (3), e46449.
Harper C., Wootton L., Michelangeli F., Lefièvre L., Barratt C., Publicover S. 2005. Secretory pathway Ca2+-ATPase (SPCA1) Ca2+ pumps, not SERCAs, regulate complex [Ca2+]i signals in human spermatozoa. J. Cell Sci. 118 (8), 1673–1685.
Gunaratne H.J., Vacquier V.D. 2006. Evidence for a secretory pathway Ca2+-ATPase in sea urchin spermatozoa. FEBS Lett. 580 (16), 3900–3904.
Kirichok Y., Krapivinsky G., Clapham D.E. 2004. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 427 (6972), 360–364.
Palty R., Silverman W.F., Hershfinkel M., Caporale T., Sensi S.L., Parnis J., Nolte C., Fishman D., Shoshan-Barmatz V., Herrmann S., Khananshvili D., Sekler I. 2010. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. USA. 107 (1), 436–441.
Gunter T.E., Yule D.I., Gunter K.K., Eliseev R.A., Salter J.D. 2004. Calcium and mitochondria. FEBS Lett. 567 (1), 96–102.
Machado-Oliveira G., Lefievre L., Ford C., Herrero M.B., Barratt C., Connolly T.J., Nash K., Morales-Garcia A., Kirkman-Brown J., Publicover S. 2008. Mobilisation of Ca2+ stores and flagellar regulation in human sperm by S-nitrosylation: A role for NO synthesized in the female reproductive tract. Development. 135, 3677–3686.
Guerrero A., Carneiro J., Pimentel A., Wood C.D., Corkidi G., Darszon A. 2011. Strategies for locating the female gamete: The importance of measuring sperm trajectories in three spatial dimensions. Mol. Hum. Reprod. 17 (8), 511–523.
Luque G.M., Dalotto-Moreno T., Martín-Hidalgo D., Ritagliati C., Puga Molina L.C., Romarowski A., Balestrini P.A., Schiavi-Ehrenhaus L.J., Gilio N., Krapf D., Visconti P.E., Buffone M.G. 2018. Only a subpopulation of mouse sperm displays a rapid increase in intracellular calcium during capacitation. J. Cell Physiol. 233 (12), 9685–9700.
ACKNOWLEDGMENTS
The work was supported by the research program according to State Assignment no. 0445-2021-0005 at the All-Russian Research Institute of Genetics and Culture of Agricultural Animals, the branch of the Federal Research Center for Livestock, VIZ named after L. K. Ernst.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by E. Makeeva
Rights and permissions
About this article
Cite this article
Nakidkina, A.N., Kuzmina, T.I. Calcium Homeostasis in Spermatozoa: Regulatory Mechanisms and Biological Significance. Biochem. Moscow Suppl. Ser. A 16, 49–62 (2022). https://doi.org/10.1134/S199074782201007X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S199074782201007X