Abstract
This chapter reviews the molecular structure, synthesis, interfacial activity, bulk aqueous solution behavior, and commercial applications of silicone surfactants. While providing a historical overview of the technology, the focus is on scientific and technological development in the last ten years. Particular attention is paid to carbohydrate-functional silicones. Silicone surfactants have the intriguing and commercially viable ability to reduce the surface tension of polar and non-polar liquids to values 15–20 mN/m lower than commonly achieved with organic-based surfactants. The latest developments on understanding and commercially exploiting the phenomenon of superwetting are reviewed. Silicone surfactants demonstrate a marked tendency to form aggregate structures featuring surfactant bilayers including vesicles and lamellar liquid crystals. This tendency has been recently applied in the development of silicone vesicles as nanoscale delivery “vehicles” and in the templating of lamellar metal oxide structures. The utilization of these properties is reviewed, including applications as diverse as oil and gas, performance coatings and personal care products.
An erratum to this chapter can be found at http://dx.doi.org/10.1007/978-94-007-3876-8_14
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Silicone surfactants
- Carbohydrate-functional silicones
- Superwetting
- Surfactant bilayers
- Silicone vesicles
- Templating of lamellar metal oxide structures
9.1 Introduction
The energetic and commercially relevant topic of silicone surfactants was extensively reviewed by Hill in 1999 [1] with a series of follow-up, more specialized reviews between 2001–2011 by Henning et al. [2]; Hill [3]; Ruiz et al. [4]; Fleute-Schlachter and Feldmann-Krane [5]; Long and Wang [6]; Kamei [7]; Huang [8]; Hill [9]; Huang et al. [10]; O’Lenick and O’Lenick [11]; Han et al. [12]; Han et al. [13]; Huang et al. [14]; Somasundaran et al. [15]; Huang [16]; and Rodriguez-Abreu and Esquena [17]. With this in mind, and within the context of being part of the larger topic of silicon-based surface science, this chapter aims to carry out the following:
-
Give an overview of the molecular structures, synthetic chemistry, interfacial activity and solution aggregation behavior of silicone surfactants.
-
Make reference to the previously published reviews.
-
Cover new developments in the field in the last twelve years.
-
Discuss how these properties tie into the application science for these materials.
Silicone surfactants were first introduced into the marketplace in the 1950’s as stabilizing agents for polyurethane foam [18]. This application was unusual in that more traditional surfactants, based on hydrocarbon residues as the “hydrophobic” portion of the molecule, did not act as effective stabilizers in this media. The experimental verity of surface activity in a non-aqueous media suggested that silicone surfactants would have some significant differences in physico-chemical behavior from their hydrocarbon analogues.
Over the next 60 years, both striking similarities and differences were observed between the behavior of silicone surfactants and their hydrocarbon analogues. Table 9.1 lists the similarities and differences between siloxane-based surfactants and hydrocarbon-based surfactants.
The physical behavior of silicone surfactants, as outlined in Table 9.1, has been the basis for a rich variety of commercial applications. Along with the fore-mentioned example of the stabilization of polyurethane foams, the following applications have achieved significant commercial success:
-
Process aids in fiber manufacturing.
-
Spreading agents and emulsifiers in personal care and cosmetic formulations.
-
Wetting agents, flow promoters, lubricants and foam control agents in paint and coating products/processes.
Therefore, this review of the field of silicone surfactants focuses on the following topics:
-
The molecular structures of silicone surfactants and how they are synthesized.
-
The interfacial activity of silicone surfactants.
-
The aqueous aggregation behavior of silicone surfactants.
-
The commercial applications of silicone surfactants.
This review also highlights areas of high activity which have begun in the last 12 years or have greatly intensified during this period:
-
All aspects of research and product development of carbohydrate-functional silicone surfactants including synthesis, characterization, interfacial science, aggregation in aqueous solution and product conceptualization and development. This work is driven by environmental concerns, specifically the desire to work with amphiphiles based on “natural” products, such as sugars/carbohydrates. This focus is highly relevant for the potential application of these materials into personal care, household care and health care markets.
-
The intense work carried out on the bulk solution aggregation properties of silicone surfactants has yielded a number of exciting avenues of research and development. These areas lie in the exploding field of nanoscience and technology. For example:
-
The science and technology of nanoscale silicone surfactant vesicles has been extensively developed in the subsequent years. These materials are the first robust alternative to the highly established field of organic-surfactant-based vesicles/liposomes which are well-established in the personal care product and health care fields. By contrast to these organic materials, silicone vesicles are formed by a wide range of materials, under mild conditions and bring the benefit of silicone aesthetics to the skin care market.
-
The concentrated interest in and application of silicone emulsifiers in the personal care market has driven the discovery of novel methods of dispersion stabilization employing silicone surfactants. Specifically, a number of silicone surfactants act as nanoparticulate stabilizers at interfaces.
-
Silicone surfactant aggregates have been employed to “template” the formation of specific nanoscopic structures of metal oxides.
-
9.2 Molecular Structure
Silicone surfactants feature an amphiphilic molecular structure consisting of a non-polar/hydrophobic moiety, silicone, and various polar/hydrophilic moieties. The silicone moiety can vary from a linear to a highly branched (network) structure. The hydrophilic groups, including both non-ionic (polyether and carbohydrate) and ionic (cationic, anionic and zwitterionic) species can attach to the siloxanes in a wide variety of ways.
9.2.1 Silicone Structure
As discussed extensively in this book, “silicones” contain non-polar, hydrophobic groups composed of combinations of the monomer species R1R2R3SiO1/2, R1R2SiO, RSiO3/2, and SiO2. Common, minimally polar R groups bonded to silicon include methyl, longer chain alkyl, phenyl and γ,γ,γ-trifluoropropyl, with (by far) the most common R group being methyl. In order to satisfy the requirements of amphiphilicity, some of the R groups are highly polar and hydrophilic. These groups are discussed below.
9.2.1.1 Linear Silicone Structures
Many silicone surfactants are based upon a linear silicone structure featuring the oligomeric silicone group, -(R2SiO) x -. The typical R group is methyl and x can range from one to multiple hundreds. Hydrophilic groups can be attached to one (an “AB” structure) or both (an “ABA” structure) ends of this linear silicone. One can consider a linear structure BAB, where the hydrophilic group is in the middle. Examples of all three linear type structures are shown below, where the A group is a poly(oxyethylene) group. These have either a C-O-Si bond (hydrolytically unstable) or a O-C-C-C-Si bond (hydrolytically stable) as the linkage between the polyether portion and the silicone portion.
AB Structures:
ABA Structures:
BAB Structures:
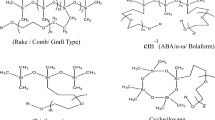
9.2.1.2 Branched Silicone Structures
Silicone branched structures can be further grouped according to the “degree” of branching. Examples with a lower degree of branching include “graft”, “comb” or “rake” structures. Examples with higher degrees of branching usually feature some silicon atoms of the general formulas RSiO3/2, and SiO2, where R can be the hydrophilic moiety. These examples have been further grouped into the categories “resins”, “dendrimeric structures” and “surface-active nanoparticles”.
-
(a)
“Graft”, “Comb” or “Rake” branched silicone structures
The general representation of a “graft”- or “rake”-structured silicone surfactant is displayed in Structure 9.7. As in Structures 9.1–9.6, the hydrophilic group of choice is the poly(oxyethylene) group.
One specific case of the general formula in Structure 9.7 is worthy of further mention; specifically, the case where x=0, y=1 and z=0. This specific example, often referred to as a “branched” trisiloxane structure, appears frequently in the literature on silicone surfactants.
Another variation of structure on the theme of “graft” structure is where the siloxane backbone is in a cyclic, rather than a linear form. This is shown as Structure 9.8.
-
(b)
Network silicone structures
The term “branching” within a silicone surfactant can also refer to materials containing RSiO3/2 and/or SiO2 units within their structure. The R group in the RSiO3/2 unit can feature either a hydrophobic (typically methyl) or hydrophilic group. Gentle and Bassindale reported [19] a series of materials of the molecular formula [(polyether)SiO3/2]8. These materials featured a cubic cage structure as depicted in Structure 9.9, also known as a polyhedral oligomeric silsesquioxane, POSS (see also Chaps. 6 and 7), where the polyether groups are the “R” groups attached to each corner of the cube. The interfacial activity of these compounds was not reported; however, interfacial activity was observed in cases where the R groups were a mixture of alkyl and polyether [20]. In a related study Deng and coworkers reported [21] that the species [(isobutyl)SiO3/2]8 was not amphiphilic in behavior; however, they observed amphiphilic behavior in the case of the open cage species [(isobutyl)SiO3/2]4[(isobutyl)SiO(OH)]3.
In Structure 9.9, R represents a polyether group. For simplicity of presentation, one vertex Si atom, the one behind the plane of paper, is not shown.
Most investigators consider the [RSiO3/2]8-based structures to be “molecular” surfactants. However, as the highly branched siloxane structure attains a much greater size and molecular weight, typically through incorporation of SiO2 units, one might consider them to function as surface-active nanoparticles. Commercial materials are available which are best considered to be silica nanoparticles whose surfaces are covered with a mixture of organic and OH (silanol) groups. For example, amphiphilic behavior has been observed in the case where the organic group was methyl [22].
9.2.2 Silicon-Centered Hydrophobic Groups Other than Silicone
There are two such cases worthy of mention, both of which are structurally related to the siloxane (Si-O-Si-O-) backbone. The first example is a polysilane, where the backbone is Si-Si-Si and a carbosilane, where the backbone is Si-C-Si-C-. A small number of surfactants have been prepared from this backbone, as reviewed previously [23]. Since this review, one study reported [24] the synthesis and characterization of mixed fluoroalkyl/hydroxyl functional carbosilane dendrimers which demonstrated amphiphilic behavior. In a related study, Krska and Seyferth [25] reported the synthesis, characterization and amphiphilic behavior of carbosilane dendrimers decorated with a variety of hydrophilic groups. Kim et al. reported [26] the synthesis, characterization and amphiphilicity of dendrimers build around a cyclic siloxane core with silyl ether-based linkages (comprising the “generations” of the dendrimer structure) and capped with hydroxyl groups. For more detail about these and other silicon-containing dendrimers see Vol. 2 of this book series.
9.2.3 Hydrophilic Group Structure
Within the category of “silicone surfactants” one can also make distinctions based on the structural classification of the hydrophilic group. A first point of distinction would be between “non-ionic” and “ionic” hydrophilic groups. The two major categories of non-ionic groups are polyethers and carbohydrates. Within the category of ionic groups there are cationic, anionic and zwitterionic members.
9.2.3.1 Non-ionic Hydrophilic Groups
The vast majority of silicone surfactants feature the polyether group. Typically the polyether group is that of a poly(ethylene oxide)-PEO. However, a substantial number of silicone surfactants contain some poly(propylene oxide)-PPO in their polyether portion. PPO is generally used for non-aqueous applications of silicone surfactants [18] as it would be essentially a slightly polar hydrophobic material. For an alternative hydrophilic group, much work has been carried out on silicones containing hydrophilic carbohydrate moieties [27–49]. A small amount of work has also been carried out using phosphine oxides as the hydrophilic moiety [50].
9.2.3.2 Ionic Hydrophilic Groups
Although silicone surfactants containing ionic hydrophilic groups have not attained the commercial significance of their polyether-based, non-ionic cousins, there has been effort expended to synthesize, characterize and develop applications for these materials. A number of aspects of this field were covered in the afore-mentioned treatise on silicone surfactants [50]; however, this effort had a rather limited scope.
9.2.3.2.1 Cationic Silicone Surfactants
Silicone surfactants with a range of cationic hydrophilic moieties have been synthesized, characterized and their application potential assessed. The great majority of these materials have a quaternary ammonium moiety for the cationic group [51–60].
9.2.3.2.2 Anionic Silicone Surfactants
Silicone surfactants with a range of anionic hydrophilic moieties have been synthesized, characterized and their application potential assessed [61–66]. A wide variety of hydrophilic groups were investigated including sulfate, sulfonate, sulfosuccinate, carboxylate and phosphonate.
9.2.3.2.3 Zwitterionic Silicone Surfactants
A small number of silicone surfactants featuring zwitterionic hydrophilic groups have been prepared. Those reported included both betaine [67] and sulfobetaine [68] moieties.
9.3 The Synthesis of Silicone Surfactants
Because of the amphiphilic nature of silicone surfactants, their hydrophobic and hydrophilic parts are usually separately synthesized and subsequently linked together. This section mirrors that logic as we cover (1) Silicone synthesis, and (2) Linkage of the hydrophilic group to the silicone. In some cases the hydrophilic group is directly linked to the siloxane in one step. In other cases, the silicone is converted to an intermediate organofunctional silicone, which is then converted into the surfactant via traditional organic chemistry routes.
9.3.1 Silicone Synthesis
The broad topic of silicone synthesis has been extensively reviewed in many publications and the reader is advised to consult some of the classic publications in this field [69–71]. Nevertheless, we briefly outline here some of the key steps:
-
The key raw material for the synthesis of silicones is silicon metal. The metal is treated with mixtures of methyl chloride and hydrogen chloride producing chlorosilanes such as Me3SiCl, Me2SiCl2, MeSiCl3, HSiCl3 and MeSiHCl2.
-
As can be seen in Schemes 9.1, 9.2 and 9.3, chlorosilanes readily hydrolyze producing silanol species such as Me3Si(OH), Me2Si(OH)2, MeSi(OH)3 and MeHSi(OH)2. As Scheme 9.4 shows, these silanol species, with the concurrent elimination of water, readily condense together to produce silicones. Within the silicone (Scheme 9.4), the Si-H functional group is introduced in order to provide an attachment point for a hydrophilic moiety.
-
In some cases, in an intermediate step, an organic moiety is directly attached to the siloxane backbone via hydrosilylation reaction (the addition of an Si-H bond to an olefin). This moiety will be used as a synthon to link a hydrophilic group to the siloxane moiety. An example [72, 73] is shown in Scheme 9.5. This reaction, like the great majority of hydrosilylations, is catalyzed by a platinum-based catalyst.
9.3.2 Linkage of the Hydrophilic Group to the Silicone
In the final step to produce the silicone surfactant, there are two general synthetic approaches. First, one can directly convert an Si-X-functional silicone (X = H, Cl) to the surfactant (Schemes 9.6 and 9.7). Secondly, one can convert an organofunctional silicone in one step to the surfactant (Scheme 9.8).
9.3.2.1 Direct Linkage of the Hydrophilic Group to an Si-H Functional Silicone
There are two general methods to directly link a hydrophilic group to an Si-H functional silicone. First, as shown in Scheme 9.6, is the reaction between the hydroxyl group at the end of a polyether and the Si-R (R = H, Cl) group on a siloxane [74]. This silylation process is catalyzed by platinum compounds and yields H2 gas as a by-product if R is H. Handling of the evolved hydrogen gas must be considered as part of the safety assessment of any material produced via this route. The resulting surfactants are typically used in non-aqueous applications as the Si-O-C linkage formed by the reaction is hydrolytically unstable.
The second method to directly link a hydrophilic group to a silicone is through hydrosilylation chemistry [74]. A seen in Scheme 9.7, an Si-H functional silicone is exposed to an olefin containing hydrophilic functionality, in this case an ethoxy polyether, in the presence of a platinum-based catalyst compound. This is a highly exothermic reaction (+28 kcal mole−1), so in many cases care must be taken to ensure adequate dissipation of the heat generated. This can be done via external heat exchangers, or by the controlled/metered addition of one of the reactants. Additionally, the SiH groups can unintentionally react with terminal hydroxyl groups or water to liberate hydrogen gas. Care must be taken to minimize this during the reaction and with any residual SiH groups present after the reaction is deemed to be complete [75].
The resultant linkage, a propyl group, is hydrolytically stable; therefore, the product can function effectively as a surfactant in aqueous media. This strategy is particularly useful for the attachment of non-ionic hydrophilic groups, such as polyethers and carbohydrates, to the silicone backbone. The authors are not aware of any examples where ionic hydrophilic groups have been directly attached to the silicone backbone through this methodology. In those cases, the hydrophilic groups is usually generated by chemistry performed on an organofunctional silicone such as that seen in Scheme 9.5 and described in more detail below.
9.3.2.2 Conversion of an Intermediate Organofunctional Silicone into a Surfactant
A general method of converting organofunctional silicones (specifically amino-, phosphino- and sulfido-functional silicones) into surfactants is via traditional, carbon-based nucleophilic displacement types of chemistry. For example, as shown in Scheme 9.8, treatment of a tertiary aminofunctional silicone with standard quaternizing agents (R = alkyl, hydroxyalkyl, benzyl, alkylcarboxylate, alkylsulfonate or carbohydrate; X = halogen, alkylsulfonate, fluoroalkylsulfonate) results in the formation of cationic or zwitterionic silicone surfactants [76–83].
For cationic silicone surfactants, families of analogous species can be generated by standard anion exchange chemistries [84]. For example, as shown in Scheme 9.9, the chloride salt of the silicone surfactant can be converted into the nitrate salt. These anion exchanges produce surfactants with markedly different properties.
Anionic silicone surfactants have been synthesized by a variety of routes, most of which have their origins in the preparation of their hydrocarbon analogs. Many of the examples of anionic silicone surfactants are those containing sulfate or sulfonate anionic groups. For example, as shown in Scheme 9.10, the ring-opening of an epoxy-functional silicone by sodium bisulfate yields the sulfate-functional surfactant [85].
Although the authors are not aware of literature describing the synthesis of analogous anionic silicone surfactants, as generated by standard cation exchange chemistries, they postulate that this would be a feasible synthetic methodology.
9.4 Interfacial Behavior of Silicone Surfactants
Silicone surfactants are widely acclaimed for their exceptional activity at a wide range of interfaces. This activity is manifested by (1) the reduction of equilibrium surface tension, (2) the orientation of the surfactant at an interface, (3) interfacial viscosity, dispersion stability and lubrication, (4) dynamic interfacial tension, and (5) the “superwetting” behavior of silicone surfactant solutions.
9.4.1 The Reduction of Equilibrium Interfacial Tension
Regarding the activity of silicone surfactants at the air/water interface, a defining feature of this behavior is the extraordinarily low equilibrium/static surface tensions that are routinely measured. Many silicone surfactants can reduce the surface tension of water down to 21–30 mN m−1 [86–92]. This value is significantly lower (by ca. 10 mN m−1) than those commonly achieved with hydrocarbon surfactants and is generally only bettered by fluorocarbon-containing surfactants.
To a first approximation, the minimal tension achieved by surfactants at the air/water interface reflects the nature of the cohesive forces existing between the hydrophobic portions of the molecule. The higher the cohesive forces, the higher the degree of tension at the interface (or, alternatively, the energy required to stretch/extend the interface). As discussed extensively throughout this treatise silicones, particularly methyl silicones, have low intermolecular cohesive forces. Furthermore, the extraordinarily low energy barrier to rotation of the siloxane backbone allows the methyl groups of the silicone to adopt the lowest energy conformation possible [93]. This is also a reasonable explanation as to why methylated silicone surfactants with significant degrees of branching in the siloxane backbone generally have higher surface tension values than their linear analogs; the barrier to rotation of the siloxane network is much higher due to steric hindrance [93].
One result of the low interfacial tension of silicone surfactants is their vigorous adsorption at the organic/air interface [94–98].
9.4.2 The Orientation of Siloxane Surfactants at the Interface
As mentioned in the previous section, it has been well-established that silicone surfactants robustly adsorb at a variety of interfaces, including the following:
-
Water/air.
-
Organic liquid/air.
-
Water/organic liquid.
-
Many solid/liquid interfaces.
The adsorption of branched trisiloxane surfactants at the air/water interface has been extensively documented. A key principle underlying many of these studies is that the packing of the surfactant molecules at the air/liquid interface is a function of the nature of the hydrophilic group [99]. This principle is probably operative given that the siloxane moiety is relatively small in area. Within this category, one can separate the relative influences of ionic and non-ionic hydrophilic groups. For the ionic trisiloxane surfactants, the area per molecule at the interface is strongly determined by shielded electrostatic interactions between the solvated hydrophilic group and its counter ion. For non-ionic species, specifically the polyether-based trisiloxane surfactants, in the absence of strong electrostatic interactions, a much more complicated picture comes into play. Generally, the area/molecule scales with the length of the polyether chain [100]. For non-ionic carbohydrate-functional silicone surfactants the size of the carbohydrate groups strongly influences the area/molecule at the interface [101, 102]. Unlike for small trisiloxane surfactants, for polymeric silicone surfactants both the silicone and hydrophilic moieties have an effect on the area/molecule at the interface [103].
The case where the area per molecule at the interface is strongly determined by the dimension of the siloxane portion of the amphiphile is the general case for silicone surfactants based on [RSiO3/2]4[RSiO(OH)]3 [104] or (R3SiO) x (SiO3/2OH) y (SiO2) z [105] (R = short chain alkyl) molecules. In these cases, the hydrophilic group is OH, specifically the Si-OH (silanol) group. The [RSiO3/2]4[RSiO(OH)]3 (R = isobutyl) species [a “pseudo-cube” (a cube missing one vertex) structure with Si atoms at each vertex of the pseudo-cube] was demonstrated to adsorb at the air/water interface with a near-saturation area/molecule of 1.35 nm2 (approx. 180 Å2) consistent with the size of the isobutyl-substituted POSS molecule.
9.4.3 Interfacial Viscosity, Dispersion Stability and Lubrication
Along with extraordinarily low liquid/air interfacial tensions, another result of the presence of the low energy cohesive forces between the methylated siloxane chains is low interfacial (air/liquid) viscosity. Measurements of the surface viscosity of spread polydimethylsiloxane monolayers put this value close to zero. In some cases, surface viscosity can be built up at a silicone surfactant-adsorbed interface by interactions of the hydrophilic groups with either the underlying liquid or with each other. However, typically the surface viscosity values are nevertheless quite low.
One example of this was observed in the stabilization of polyurethane foam. Model studies [106–108] of the stabilization process, employing free-standing silicone surfactant-containing polyol films (“soap films”), showed that the foam drainage process was governed by the processes of “marginal regeneration” and highly turbulent surface flows. These phenomena are signatures of a low surface viscosity film [109, 110].
Conversely, highly viscous surface films have also been produced employing silicone surfactants. For example, surface films of the (R3SiO) x (SiO3/2OH) y (SiO2) z species. These films, featuring a highly packed surface layer of amphiphilic silica nanoparticles, have a high surface viscosity [109, 110]. In accordance, polyurethane foams produced using this material as a surfactant were extraordinarily stable.
In a related study, Hill and coworkers employed an interfacial stress rheometer to study the rheological properties of a silicone oil/water interface in the presence of siloxane surfactants that are used in the personal care industry as water-in-silicone-oil emulsifiers [111]. They appeared to stabilize water-in-silicone-oil emulsions in a fashion similar to that of Pickering emulsions, in which solid particles, such as silica or clay, accumulate at the oil/water interface forming a solid like “eggshell” that resists coalescence.
Regarding using silicone surfactants as dispersion stabilizers, along with the considerations of interfacial viscosity, one might consider the other traditional mechanisms of stabilization as well. These include bulk visco-elastic effects, electrostatic (double layer) stabilization and steric repulsion. Regarding bulk viscoelastic effects, Mehta and Somasundaran [112] studied the mechanism of emulsion stabilization where ionic silicone surfactants were employed as the stabilizer. Non-Newtonian behavior with viscosities an order of magnitude higher than that measured with non-ionic silicone emulsifiers was observed. This was explained by network formation at the droplet interface by weak interactions between the ionic functional groups. Bulk viscoelastic stabilization effects were also reported by Brook et al. in their investigation of the silicone surfactant stabilization of elastomeric silicone foams [113]. Liu and coworkers investigated the adsorption of “comb”-type silicone polyether (SPE) surfactants at the interface between water and a hydrophobic, self-assembled monolayer [114, 115]. They concluded that SPEs showed significant oleophobic behavior and were therefore capable of stabilizing dispersions in organic media. They postulated that the stabilizing behavior was the result of a steric repulsion mechanism. This stabilization mechanism might also be operative in the case of castor oil-in-silicone emulsions stabilized by cyclomethicone/dimethicone copolyether surfactants [116] (see Chap. 13 for explanation of this therminology).
The adsorption and interfacial rheology of silicone surfactants adsorbed onto a solid surface is an active area of investigation, particularly in terms of the resulting lubricity of the solid surface. For example, Liu and coworkers [117] reported on interactions of an amphiphilic block copolymer of polyalkylene oxide-modified polydimethylsiloxane with thin films of polypropylene (PP), poly(ethylene terephthalate) (PET), and nylon, as well as with reference hydrophilic silica surfaces. They found that the silicone surfactant adsorbed following a Langmuir isotherm and that the adsorbed layers significantly improved fiber wettability and lowered friction.
9.4.4 Dynamic Interfacial Tension
Historically, the dynamic interfacial tension (air/water interface) or alternatively, the rate of interfacial tension reduction, has been a subject of intense interest in the silicone surfactant field. A likely reason for this interest is that dynamic interfacial tension plays a significant role in many processes and surfactant applications including wetting and dispersion stabilization, which are of special interest in the silicone surfactant field.
Generally, as expected, the rate of interfacial tension reduction of silicone surfactants scales inversely with the molecular weight (as expressed by the solvodynamic radius) of the surfactant. This conclusion is consistent with a model where the rate-determining step(s) of the interfacial adsorption process is the diffusion of the surfactant through solution or its rate of orientation at the interface. This has been recently confirmed in two independent studies [118, 119].
9.4.5 The “Superwetting” Behavior of Silicone Surfactant Solutions
For the last 30 years one of the most investigated phenomena regarding silicone surfactants has been that of “superwetting” of aqueous mixtures of certain low molecular weight, trisiloxane-based, silicone polyethers. This fascinating topic has been reviewed in a number of publications [120, 121]. “Superwetting” refers to the extraordinarily rapid wetting of low energy, hydrophobic surfaces (such as parafilm) by these aqueous surfactant mixtures. This phenomenon has been correlated to a number of the physical properties of the surfactant including:
-
Low equilibrium interfacial tension. As seen below in Fig. 9.1.
-
Low dynamic interfacial tension (high rate of interfacial tension lowering).
-
The presence of lamellar phases of surfactant bilayer aggregates (more information on surfactant aggregation is presented in Sect. 9.5.2).
-
Marangoni flow.
Key to understanding superwetting is the assumption of rapid surfactant transport and surface reorientation. There is a strong correlation between a high rate of interfacial tension reduction (low dynamic surface tension) and superwetting [122]. Furthermore, in order to achieve a high rate of interfacial tension reduction, one should be using a relatively small surfactant, which as a result of its compact size will have high linear transport- and rotational-diffusion coefficients. This hypothesis has been quite robustly confirmed including in a recent study that demonstrated that for trisiloxane surfactants of the general formula (Me3SiO)2Si(Me)-(CH2)3(EO) x OH (where EO is (CH2)2O), the highest initial spreading rate and largest spreading area were measured for the x=6 derivative [122].
Regarding the presence of lamellar phases of surfactant bilayer aggregates in superwetting, informal observations made in the authors’ laboratories over 20 years ago showed that the superwetting solutions were often “cloudy”, hinting at some type of two-phase system and the presence of lamellar surfactant aggregates [123]. More recently, a correlation between the ability of a silicone surfactant in aqueous media to aggregate into bilayer structures and a tendency to act as a “superwetter” was reported [124]. However, regarding this conceptual linkage of bilayer formation and superwetting, there is a significant amount of controversy with other authors disputing it [125, 126].
The role of surface tension gradient-stimulated flows (i.e. “Marangoni flows) in the superwetting phenomenon has also been discussed [127]. For example, the spreading front of the surfactant solution droplet causes the formation of a surface tension gradient which stimulates Marangoni flow. Furthermore, experiments [128] showed a correlation between the value of the gradient and the rate of flow.
9.5 Aqueous Solution Behavior—Hydrolysis and Aggregation
9.5.1 Hydrolytic Stability
An early and enduring observation about silicone surfactants was that they had a tendency to hydrolyze in aqueous solutions, a process that was quite slow at neutral pH and rapid at pH values below 4 and above 9. The hydrolysis process can be viewed as being the “reverse” chemical reaction to the siloxane condensation depicted in Scheme 9.4. The pH dependence of this phenomenon is explained by the consideration that acids and bases are catalysts for siloxane bond condensation and hydrolysis.
Hence, many studies and inventions in this field have been concerned with lessening the rate of hydrolysis of these surfactants. A common approach to this problem has been to consider the substitution of carbosilane (-Si-C-Si-)-based surfactants for their siloxane counterparts. However, carbosilane species are expensive and often do not have the favorable interfacial behavior of silicones.
Alternative approaches to diminish the rate of hydrolysis of silicone surfactants have been proposed by a number of authors. For example, Peng and coworkers demonstrated [129], while investigating the behavior of novel double-tail polyether-functional trisiloxanes, that some of the species were stable for more than 270 days in a neutral environment (pH 7.0). They concluded that the hydrolysis resistance of the double-tail trisiloxane surfactants can be improved by a weaker hydrophilicity of the surfactant molecule, and a larger volume of the hydrophobic groups. Another approach to lowering the rate of hydrolysis was reported by Pollicello and coworkers [130]. They claimed that the use of (presumably bulkier) alternative groups on both the siloxane backbone and the hydrophilic group achieved the purpose.
A less documented aspect of hydrolysis of silicone surfactants is the hydrolysis of silicone polyethers where the silicone-to-polyether linkage was a Si-O-C bond. Recently this hydrolytic “instability” was exploited by Lin and coworkers in order to prepare deliberately “cleavable” silicone surfactants [131–133]. The hydrolysis of these surfactants was, in some cases, accelerated by exposure to catalysts such as TiO2, radiation or plasma sources. In these applications the siloxane fragment with silanol (Si-OH) functionality resulting from the cleavage, could be profitably applied as water-proofing and anti-bacterial coatings.
9.5.2 Aggregation
Due to the well-known “hydrophobic effect” [134], silicone surfactants exhibit a pronounced tendency, in aqueous media, to self-assemble into various aggregates including micelles, vesicles and liquid crystalline phases. An extraordinary number of these aggregates are characterized by the presence of surfactant bilayers. These aggregates include non-spherical, oblate-ellipsoidal, disk- or plate-like micelles [135–138], vesicles, lamellar liquid crystal phase [139] and microemulsion “middle” phase.
For example, since the original reports [140–147], many workers [148–153] have documented the ubiquitous tendency of silicone surfactants (different siloxane structures, different hydrophilic groups) to form bilayer vesicles in aqueous mixtures. This formation, properties and application potential of silicone vesicles has been extensively investigated and the following key features have been identified:
-
In some cases, silicone vesicles spontaneously form upon the mixing of the specified silicone surfactant with the correct amount of water.
-
The bilayers of silicone vesicles are quite fluid and do not show the gel-to-fluid phase transition ubiquitous to hydrocarbon-based vesicles/liposomes.
-
A variety of materials can be encapsulated into silicone vesicles [154–158].
-
The width of the bilayer corresponds to the length of the siloxane portion of the silicone surfactant [159, 160].
Other studies involving silicone surfactant bilayers include:
-
The presence of an AB-structured silicone polyether surfactant, as a co-surfactant with an alkyl ethoxylate, in a surfactant/dodecane/water mixture, causes a striking increase in the solubilization power of either a lamellar liquid crystal (LC) phase or else a microemulsion “middle” phase [161]. This increase in solubilization capacity correlated with an increase in the structural length scale of the microemulsion.
-
The bilayer-forming tendency of silicone surfactants has also been exploited in the fabrication of surfactant-templated, mesostructured metal oxide phases [162–168]. In a series of intriguing studies the investigators found that the templating process often leads to the formation of lamellar phases with long-range order. In one case, a silica mesophase was prepared featuring the largest lattice constant reported for lamellar materials to that date [169]. The authors concluded, consistent with other studies, that the lamellar structuring was the result of the virtually unrestricted chain mobility within the silicone surfactant.
This “flexibility factor” favoring the self-assembly of silicone surfactants into bilayer structures has been mentioned by a number of investigators in the field. For example, siloxane chain conformations with surfactant bilayer aggregates have been proposed including coiled (based on micelle aggregation numbers and small angle neutron scattering (SANS) data) [170], flexible [171], and folded ones (Hill and colleagues have also found that the bilayer thickness of vesicles formed by comb-type silicone surfactants is significantly smaller than their extended molecular length) [172].
Finally, we also wish to cite the following:
-
As expected, the cloud point of SPE surfactants scales with the degree of hydration of the EO chain. This was confirmed in a recent study [173].
-
For a series of AB-structured silicone polyether surfactants in water, a number of unusual aggregate structures were observed including reverse discontinuous cubic phase (I 2), reverse hexagonal phase (H2), and discontinuous cubic (I 1) phases along with the common lamellar (L α ) and hexagonal (H1) phases [174]. The authors rationalized the correlation of the appearance of these phases by considerations of the entropy gain/loss of the silicone chain. Specifically, the entropy loss of a long hydrophobic chain (such as the silicone chain) would be largely increased when it is stretched, and thus, long hydrophobic chains tend to be in a shrunk-bulky state. This shrinkage affects the surfactant parameter, which heavily influences the state of aggregation.
9.6 Applications
The unique combination of physical and surface properties of ethoxylated siloxane copolymers (or silicone polyethers (SPEs)) results in the specification of these materials in numerous industrial applications. Inherently the SPEs provide a means to deliver silicone properties to an aqueous system. These properties can be expressed in terms of surfactancy by delivering the low surface energies, as described earlier, to the end application. This can make the materials effective as emulsifiers, wetting agents, foam control agents, or surface modifiers. The flexibility in the product chemistries allows these properties to be tailored to specific end uses. Materials within the SPE family can be effective at emulsifying water in oil or oil in water emulsions. Alternatively the structure can be tailored to demulsify these same two-phase systems. Similarly, the SPEs can be designed to be pro-foaming and stabilize foam systems, or can be designed to be effective foam control agents.
However, the low surface energies are not the only feature that makes these materials of commercial interest in so many applications. The inclusion of polyether groups onto a siloxane backbone allows these materials to treat surfaces and render them hydrophilic. The functionality present on the terminal end of the polyethers can make the SPE copolymer reactive with other cure chemistries. The siloxane portion of the copolymer allows the delivery of silicone feel into aqueous systems. In total, the combinations of these properties offer multiple benefits to the individual application.
Each of these end uses for silicone surfactants is unique and is summarized in the next few sections of this chapter. Many of these applications have been reviewed in other publications. The intent of this portion of the chapter is not to further review, but to summarize these applications and how the surface behavior of the silicone surfactants enables them to provide benefits in these applications. In addition, new developments are also highlighted.
9.6.1 Personal Care
Silicone surfactants have been used in the personal care industry for a number of years, beginning primarily in the mid-1980s. Mainly, these have been silicone polyether copolymer-based products although many other functional compositions have been developed and commercialized. The unique surface properties have allowed these products to be incorporated into a broad spectrum of personal care uses. These include antiperspirant formulations, skin care, facial care, as well as hair care applications. The primary functions of these materials are to provide emulsification, humectancy, and overall aesthetics to the personal care formulations.
Several review articles have been written on the use of silicone surfactants in personal care. Floyd described a range of silicone surfactant structures and their ties to specific applications and the resulting intellectual property in a review in Hill’s book on silicone surfactants [175]. He describes the foundational patents for this application and considers specific segments which utilize these materials and the claims they provide in these segments. O’Lenick has also published extensively in this field. He has described the use of silicone surfactants in various personal care formulations [176]. He has also published a review that describes the nomenclature system used in this industry and summarizes many of the properties important to various personal care applications [177]. This review details his work not only with non-ionic silicone polyethers, but also with other amphiphilic silicone copolymers that he has developed. Many of the patents on these compositions are described in Floyd’s review [181].
One of the major uses of silicone surfactants in personal care is in the area of emulsification. They have been used as emulsifiers for several years, beginning in the early 1980s. The initial work was in the antiperspirant segment where a series of unique silicone polyethers were utilized to stabilize water in silicone emulsions. Most specifically these were emulsions in which the external phase was based on cyclosiloxanes. The initial use of silicone surfactants as emulsifiers for water in silicone emulsions was described by Keil and Starch [178, 179]. The low surface energy of the siloxane external phase, as well as solubility parameters, did not allow the conventional hydrocarbon-based emulsifiers to stabilize this form of emulsion. The siloxane portion of a silicone surfactant is sufficiently low in surface energy, and is sufficiently compatible with the external phase to prevent coalescence of internal aqueous phase droplets. Key to this stabilization is managing the structural architecture of the silicone surfactant by balance of the dimethylsiloxane segments to the methyl/polyether siloxane segments of the copolymer. This provides the right solubility parameter of the silicone surfactant. Gruning and Bungard describe the use of hydrophile lipophile balance (HLB) methods to help predict the silicone surfactant composition best suited for stabilizing a water in silicone, as well as other types of emulsion (water-in-oil (W/O), oil-in-water (O/W), multiple emulsions) in their review article on silicone emulsifiers [180]. O’Lenick gives an additional perspective with a method called the three dimensional HLB system. This takes into account the consideration of the siloxane’s unique solubility parameters and the fact that the silicones are hydrophobic, and yet not lipophilic [181]. Dahms and Zombeck collaborated on a series of papers in which they described how these molecular architectures are varied and used to prepare a range of personal care formulations, creams, and emulsions [182, 183].
Modifications of the basic structures of silicone oxyalkylene copolymers to include grafts of various alkyl functionalities and chain lengths have also been found to stabilize water-in-oil emulsions, where the oil phase is hydrocarbon oil. Because of the non-lipophilic nature of the siloxane hydrophobe, there is little compatibility of the traditional silicone polyether graft copolymer with an oil phase, and these are not suitable for stabilizing W/O formulations. However, the added alkyl functionality to the composition provides for a proper solubility balance to effectively stabilize these systems [184].
Utilizing silicone surfactants as a primary surfactant in O/W formulations is not as common, as many organic-based surfactants are suitable for stabilizing these emulsions. Nevertheless, silicone surfactants can effectively perform in these formulations as well. Generally, the higher HLB silicone surfactants (HLB 10–18) are required to stabilize these formulations. More recently, non-polyether-based silicone modified carbonic acid surfactants have been shown to effectively stabilize O/W emulsions as well [185]. Further, efforts have shown how O/W emulsions containing pigment dispersants can be formed utilizing silicone surfactants with glycoside radicals as the key hydrophilic component in the surfactant composition. These are particularly useful in sun care products where the pigments help block harmful UV radiation [186].
Other recent developments in the field of silicone emulsifiers have focused on different architectures and improved understanding of emulsion fundamentals behind the preparations of various emulsions. Dimitrova et al. published two papers in which they describe the use of (AB) n type silicone polyether copolymers as emulsifiers for water in oil systems, and compare these with the more traditional graft or rake type structures [187]. These (AB) n materials are shown below as Structure 9.10 where the dimethylsiloxane block is the “A” component of the block copolymer and the “B” block is the combined EO and PO segments. These are prepared by the platinum catalyzed hydrosilylation of a silicon hydride end-blocked siloxane and an allyl end-blocked polyether. The EO/PO portion is prepared independently. The R1 simply signifies an end-blocking group.
The data suggested that these (AB) n type emulsifiers require less shear energy to produce W/O emulsions vs. rake type SPEs of similar solubility parameters. These conclusions were further supported in a second paper in which a variety of personal care formulations were prepared and the fundamental stability studied [188]. Broader descriptions of how to use these fundamental properties in the preparation of stable formulations have also been described in a series of Society of Cosmetic Chemists papers [189, 190]. These papers describe how to prepare a range of formulations including glycerin in silicone, glycerin + water in silicone, and water in silicone emulsions and their stability and aesthetic properties.
Beyond the use as emulsifiers, the silicone-based surfactants have found utility as humectants, surface modifiers, and conditioning agents. In hair care applications, a range of silicone surfactant structures has been shown to provide conditioning effects. The non-ionic polyether type surfactants confer a light conditioning. The lower HLB value silicone polyethers tend to be more effective in 2-in-1 conditioners [191]. In other cases, higher HLB surfactants improve conditioning on the hydrophilic portions of hair strands. Cationic silicone surfactants, like silicone quaternary amine compounds, give excellent conditioning properties as well as an improvement in hair body and curl retention [192]. The non-ionic polyether materials provide benefits to skin care and shave formulations by contributing humectancy and lubrication properties to the formulations [193].
9.6.2 Coatings
A second major application area for silicone-based surfactants is in the coatings industry. Here, they are primarily used as additives to various coating formulations to improve surface properties. The low surface energies provide for good leveling, wetting, spreading and gloss properties of the coating. The type of coatings where these materials are utilized is far reaching and includes: architectural, industrial protective coatings, wood, marine, cookware, and coil coatings. They are also used in many printing inks. The use of these additives significantly increased with the advent of more water borne systems which have inherently higher surface energies than solvent-based systems. Many of the desired coating properties were compromised with aqueous formulations without the use of additives. Easton provided an overview of silicone surfactants and their uses in waterborne coatings describing how they impacted the coating performance [194]. Perry published an updated review of these properties and discussed how dynamic surface tension and the rapid wet out provided by trisiloxane-based superwetters impact primarily aqueous-based coating formulations [195]. Her work also showed higher molecular weight copolymers tend to improve the slip and mar resistance of the coatings. This can be attributed to the orientation of the polysiloxane portion of the copolymer segments to the polymer/air interface yielding a lower energy surface. Ferritto et al. further developed this approach by extending the technology of branched silicone polyether copolymers into coatings [196]. These polymers utilized branched polyethers in the composition in place of pure linear ethoxylated or propoxylated ethers. The branching is derived from glycidol being utilized in the preparation of the polyether intermediates. These were shown to impact overall dirt pick up when formulated into wood coatings.
Another parameter that these materials provide is in reducing the propensity of these formulations to foam and to eliminate the appearance of microfoams in the resulting coatings, which can significantly impact end coating quality as well as processing when a coating is being applied. Semmler describes how the use of specialty silicone polyether additives in an overall formulation of a silicone antifoam helped to reduce the occurrence of microfoam when the coating was spray applied [197]. Van Dam describes the use of antifoams containing silicone polyether additives in printing inks [198]. These additives were more defoaming at the ink surface and had good dynamic properties to eliminate foam build up during the printing process. Further, they had good durability and persistency in the process. O’Neil reviews the various mechanisms of foam destabilization by silicone surfactants and antifoam compounds. He describes differences between rake and ABA type silicone surfactants and their performance, as well as their uses in clear overprint varnishes, flexographic inks, wood parquet lacquers and automotive clear basecoats [199].
9.6.3 Household Care
The primary use of silicone surfactants in household care applications is again related to the control of foam. Unlike the applications in coatings where the target is to prevent the formation of foam, or to defoam a system in which foam has been created, the goal here is to control the foam to fit specific profiles. This is especially true in laundry applications. Fey has described the mechanism of foam control provided for with silicone fluids and silicone polyethers [200]. He suggested three main requirements for a material to behave as an effective antifoaming agent: (1) it must be insoluble in the foaming medium, (2) it must be readily dispersible in the foaming medium, and (3) it must have a lower surface energy than the foaming medium. This allows for the fluid to enter the air/liquid interface and spread over that interface. This allows for bridging of particles and eventual rupture (see Chap. 13).
In most cases, the silicone surfactant is used as part of an overall antifoam compound or composition. They are normally of the non-ionic polyether type and their role is to assist in the dispersion of the antifoam compound into the foaming media and to aid in the reduction and/or control of the foam. McGee et al. first showed this effect and described the impact of the incorporation of the silicone surfactant in combinations with silicone resins, fluids, silica particles and catalyst [201, 202].
Much of this knowledge has been applied to the development of antifoams specific for control of foam in liquid and powdered laundry detergents. In these products it has been found that branching in the polymer composition aids in the overall control of the foam. This branching can be introduced into the polymer chain by hydrosilylation of vinyl terminated polydimethylsiloxanes with a silicone polyether containing residual silicon hydride groups. Alternately, the crosslinking can be conducted in advance of the introduction of the allyl polyether to the polymer in the formation of the silicone polyether [203]. Elms et al. further developed this concept with formulated antifoam compounds containing linear and branched silicone polyether copolymers. These were shown to be more effectively dispersed in the detergent medium and to provide improved foam control profiles [204].
Beyond antifoaming there have been other uses of silicone surfactants in household care applications. Henning described the use of a range of silicone materials, especially silicone polyethers, in polishes and household cleaning products [205]. These included uses in car, furniture and shoe polishes, and in household and industrial cleaning applications. In some cases these uses are limited due to the inherent hydrolytic instability of silicone polyether copolymers under acidic or basic conditions. Panandiker described the use of low HLB type silicone polyethers in liquid detergent formulations [206, 207]. These low HLB polymers were part of an overall formulation and were designed to deposit onto fabric in the washing cycle to improve fabric feel and hand.
9.6.4 Textiles
Silicone surfactants are used in numerous textile applications to impart hydrophilic properties to the textile. In this sense they are not specifically being utilized for their surfactancy properties; rather, their bulk structural properties allow them to orient at the interface of the textile to impact the feel. Often, these silicone materials contain amino groups to provide for excellent anchoring and hand to the fabric, and also polyether functionality to impart hydrophilicity [208]. More recently efforts have been focused on linear (AB) n type block copolymers containing both amino and polyether functionalities. Czech published the initial work in this area [215]. The polymers were block copolymers prepared from the addition of end-blocked diepoxysiloxane fluids to various amines and diepoxy end-blocked polyethers. They imparted good softening and hydrophilicity to cotton [209]. Favresse prepared versions of these via different routes based on grafting functional groups on to (AB) n block silicone polyether compounds utilizing free radical polymerizations. These showed improvements in hand on cotton, non-wovens, as well as synthetic fibers [210]. Kennan prepared different materials based on a process where an epoxy terminated (AB) n silicone polyether was initially prepared and then aminated. These too showed excellent hand and provided improved hydrophilicity [211].
Silicone polyethers and silicone polyether terpolymers with other functionalities have also been used to treat non-woven textiles. Non-woven fabrics are traditionally made from polypropylene and are very hydrophobic. They are used as backing on many textile substrates as well as synthetic leathers, feminine care products and baby diapers. Improving the hydrophilicity of these non-woven products can significantly improve end performance. Sabia discussed the use of standard graft silicone polyethers to improve the finish of non-woven fabrics [212]. One detriment has been the durability of the treatment. Since there is no reactive functionality on the silicone polyether copolymers, they tend to wash off if exposed to water and then render the fabric hydrophobic once again. This is particularly of concern in diaper applications where it results in leakage. To address this durability issue, multiple functionalities are often incorporated onto the silicone. Most common are epoxy groups and polyethers. The epoxy can open and provide for improved durability to the non-woven substrate.
Standard silicone grafts and ABA type silicone polyethers are also used as fabric treatments to aid ironing. For example, the use of these materials in iron spray solutions for cotton and synthetic fabrics has been shown to significantly reduce the difficulty in removing wrinkles from fabric [213].
9.6.5 Oil and Gas
Applications of silicone surfactants in the oil and gas industry primarily center on demulsification and foam control. One concern that dictates the type of silicone and application of silicones in the oil recovery, transportation and refining processes is the potential of silicones to foul the catalysts used in the hydrocracking process [214]. However, this is somewhat limited to more oil soluble silicones and not necessarily silicone surfactants. It is critical that the silicone does not enter the oil phase in the refinery where it can be converted into silica upon heating in the cracking process thereby impacting the catalyst activity.
Most of the references relating to the use of silicone foam control agents in the oil and gas industry are centered on pure polydimethylsiloxane- (PDMS) based technologies. Foams in the oil and gas industry are primarily non-aqueous foams stabilized by naturally occurring asphaltenes. The gas phases are generally low molecular weight hydrocarbons. High molecular weight PDMS fluids tend to lower surface energies and allow for coalescence of the gas phase droplets resulting in foam rupture [215]. Because of the need for high oil solubility, silicone surfactants are not generally utilized in foam control formulations in the oil and gas industry, although there have been some efforts with low HLB-based silicone polyethers, but this is not widely practiced [216].
Silicone surfactants are more broadly utilized in the field of crude oil demulsification. In the recovery and transportation of crude oils, there is an undesired formation of various emulsions of oil in water and water in oil. The exact type is dictated by many factors, but depends heavily on the type of crude, salinity of the aqueous/brine phase, level of stabilizing asphaltenes, age of the well, and extraction process. The emulsions are generally formed as part of the oil extraction due to high turbulence in the production process [217]. Before the recovered oil is sent to a refinery for further processing the emulsion must be broken, oil recovered, and the produced water treated and then generally re-injected back into the recovery process or treated/cleaned and released. Many types of surface-active material are used in this demulsification process. Most are organic and include polymers of EO, PO phenols, and nonylphenols. Oil service companies formulate demulsification cocktails and tailor them to specific field conditions. The demulsifiers are generally formulated into organic solvents such as aromatic naphtha. By far, the greatest volumes of demulsifiers utilized in the industry are of these types.
Silicone polyethers are also utilized and a wide range of product offerings and technologies are available. Early investigations and use in this application for silicone surfactants dates back to the early 1970s [218]. However, widespread use has not resulted, primarily due to cost considerations as the silicone materials typically have a higher in-use cost than organic-based options. In some cases efforts have been made to overcome this issue by blending or by the incorporation of solid particles such as silica [219, 220]. The silicone materials do find utility when the crude oil/water emulsions are difficult to break, such as heavy crudes, or under cold conditions [221]. This has not deterred further development of new silicone surfactant compositions and improved understanding of the mechanism of how they behave as emulsion breakers. David et al. described efforts to better understand destabilization mechanisms and proposed two possible scenarios: dissolution of the stabilizing asphaltene aggregates thereby removing the natural occurring surfactant, and displacement of these asphaltenes with a more surface-active silicone component. Further, these components are inherently not designed to be stabilizing, thereby allowing coalescence [222]. More recently, Phukan et al. described a new composition type of a silicone demulsifier that contains multiblocks of silicone and polyether with amino groups in the backbone that introduce further silicone branching sites [223].
9.6.6 Pulp and Paper Applications
Within the pulp and paper industry silicone surfactants are used as various process aides. These applications include pulp drainage, pulp digestion, antifoaming/defoaming, cleaning, and paper deinking. Silicone polyethers are used in many of these applications either directly or as part of an overall formulation component.
One of the first steps in the papermaking process is cooking which liberates the cellulosic fibers from the wood chips. This is done under highly alkaline conditions and is referred to as the Kraft process. In a simplified description of this process the wood chips are hydrated and impregnated with pulping chemicals (mainly highly alkaline sodium hydroxide and sodium sulfide) called white liquor. These are cooked to remove the lignin and recover the individual cellulosic fibers [224]. The used white liquor is referred to as the ‘black liquor’. It is separated from the fibers by washing and recycled. The fibers are then screened and the resulting brownstock pulp is washed and bleached before the final conversion to paper. Process aids, surfactants, and additives are added throughout this process to aid in the paper production.
In the first part of this cooking, digester additives are commonly added to aid delignification. Silicone polyethers have been used in this process to reduce the digestion/cook time, to increase the yield of the pulp, and to reduce the level of undigested fibrous materials [225]. The low surface energies provided by the inclusion of the silicone surfactant allow the cooking chemicals to better impregnate the wood chips, thereby increasing their effectiveness.
Silicone defoaming agents are used in the washing of the brownstock. During delignification, many types of surface-active agent are formed. This causes severe foam control issues downstream during the pulp washing steps. Silicone-based defoaming agents are highly effective due to their inherent low surface activity and spreadability at the air/liquid interface. They exhibit good foam knock-down and persistence and are used at very low concentrations [226]. Silicone polyethers are included in many of these antifoam formulations to aid in the delivery of the primary silicone active, or to act as secondary antifoam fluids. The low surface energies have also been shown to aid in pulp drainage [227]. Compositions with branching in the siloxane backbone have also shown effectiveness in this application [228].
Silicone polyethers aid in the process of treating paper making equipment which helps to eliminate the need for frequent shut-down and cleaning of the equipment. In this stage of the paper making process, dilute cleaned pulp is applied to a forming fabric and begins to drain and dry. The resulting paper web comes into contact with felt presses to further dry the pulp web to the desired moisture content. These felts become fouled with various deposits from the pulp drying process which can significantly decrease the process on-line time as a result of frequent cleaning. Solutions of silicone polyethers have been shown to reduce the amount of time required to clean when spray applied on these press felts [229].
Another segment in the pulp and paper industry that is growing in significance is in the area of paper deinking as part of the overall paper recycling process. Existing technologies often lead to inferior quality pulp that limits the applications for the recycled pulp to low value forms of paper or cardboard. Hence, removal of the ink from the recycled paper is critical in achieving a high quality recycled pulp that can be used for higher end paper products. Silicone polyethers have been shown to be effective in deinking flotation processes [230]. In this process the ink is removed utilizing more mild conditions vs. caustic treatments. SPEs help to emulsify the ink and are then skimmed off from the deinking bath [231].
9.6.7 Other Foam Control Applications
In addition to the foam control applications that are described in the sections on detergent foam control, pulp and paper, and in defoaming and antifoaming of coating formulations, silicone polyethers are also used in other foam control applications such as defoaming of diesel fuel. Silicone polyether copolymers that had the terminal carbinol functionality present on the polyether capped with succinic anhydride provide for excellent foam control in diesel and jet fuels. These polymers had good stability in the hydrocarbon liquid and retained their defoaming ability during storage [232]. Adding branching or crosslinking to the siloxane portion of the silicone polyether was also shown to impart improved foam control properties [233].
9.6.8 Agriculture
The use of silicone surfactants in the agricultural industry is mainly based on the wetting and penetration behavior of the trisiloxane-based silicone polyethers. As described earlier, the trisiloxane polyether copolymers are commonly referred to as superwetters. The unique, spontaneous wetting achieved by the use of these materials allows them to be very effective wetting agents and adjuvants in aqueous-based pesticide formulations. Penner et al. provided a comprehensive review of this application in Hill’s book on silicone surfactants [234]. They described how the ultra low surface tension allows the aqueous pesticide solution to spontaneously wet the surface of leaves and aids in the penetration of the pesticide solution through the leaf cuticle into the active cell structure. This can impact the rate of pesticide applied and can also provide for the pesticide to be resistant to wash off by rain, as the rate of pesticide uptake is significantly increased.
A common issue with trisiloxane-based superwetters is the inherent hydrolytic instability of the siloxane bond when solubilized into acidic or basic formulations. Under these conditions the siloxane bond will rearrange and surfactancy and superwetting behavior is lost. To combat this issue, non-siloxane-based Si containing materials have been suggested. Letherman et al. proposed a series of M–M′ disiloxanes modified with ionic functionalities to combat the hydrolysis [235]. Additionally, they also proposed t-butyl substitutions on the terminal silicon groups to stabilize the trisiloxane groups to hydrolysis [236]. Klein et al. also addressed this issue by developing polyether-modified trimethylsilanes [237].
9.6.9 Polyurethane Foams
The final application in this review is the use of silicone surfactants as stabilizers for polyurethane foams. This was the original use of these materials and was the initial reason for their development. It still remains today the largest use of silicone surfactants although other applications discussed in this review are of growing importance. The primary functions these materials play in this application is to compatibilize the polyol and isocyanate intermediates, blowing agents and catalysts, and then to aid in the stabilization of the bubbles/cells during the foam formation until the final cure of the foam. This prevents coalescence of the cells and provides for the desired foam density and cell structure. Snow and Stevens have provided a thorough review of the types of silicone surfactant used to stabilize flexible, rigid and molded foams [18].
9.7 Conclusions
Silicone surfactants are a broad class of surface-active silicone compounds. The unique surface properties contributed by the siloxane structure are the result of the siloxane bonds and the free rotation of functional groups grafted onto the silicon atoms. This allows these materials to uniquely orient at interfaces, drive surface energies to very low values, and to form structured systems in aqueous formulations and solutions. This gives them a set of unique properties that allows them to be utilized in a diverse set of end applications ranging from polyurethane foam stabilization to personal care emulsifiers. The structural variations are many and will continue to develop and expand as new applications continue to evolve.
References
Hill RM (1999) Silicone surfactants. Surfactant science series, vol 86. Marcel Dekker, New York
Henning J, Muller F, Peggau J (2001) Silicone surfactants—multitalented with backbone. SOFW J 127(1/2):38–43
Hill R (2002) Silicone surfactants-new developments. Curr Opin Colloid Interface Sci 7(5/6):255–261
Ruiz MA, Hernandez A, Llacer JM, Gallardo V (2003) The development of silicone chemistry II Hydrophilic silicones. J Appl Cosmetol 21(4):147–157
Fleute-Schlachter I, Feldmann-Krane G (2003) Silicone surfactants. In: Novel surfactants. Surfactant science series, vol 114. Marcel Dekker, New York, pp 585–622
Long B, Wang H (2004) Synthesis and application of polysiloxane-polyether surfactant. Xiangliao Xiangjing Huazhuangpin 2:31–35
Kamei M (2005) High performance trend of silicone surfactants. Fragr J 33(6):28–34
Huang W (2005) Silicone surfactant with special structure (continued). Youjigui Cailiao 19(3):48–51
Hill RM (2006) Other types of surfactants—silicone surfactants. In: Farn RJ (ed) Chemistry and technology of surfactants. Blackwell Publishing, Oxford
Huang L, Yang J, Lu B, Li G, An Q (2008) Development of amino polyether organic silicone surfactants. Riyong Huaxuepin Kexue 31(9):21–24
O’Lenick AJ, O’Lenick KA (2008) Silicone amphiphiles; getting the best of all worlds. Househ Pers Care Today 2:xxiv–xxvii
Han F, Liu Z, Zhou Y, Xu B (2009) Special surfactants and functional surfactants (III)—preparation and properties of organic silicone surfactants. Riyong Huaxue Gongye 39(2):133–137
Han F, Liu Z, Zhou Y, Xu B (2009) Special surfactants and functional surfactants (IV)—application of organic silicone surfactants. Riyong Huaxue Gongye 39(3):200–206, 212
Huang L, Hao L, Yuan J, Liu Y, An Q (2010) Research progress on preparation and application of silicone surfactants for pesticide adjuvants. Youjigui Cailiao 24(1):59–64
Somasundaran P, Purohit P, Gokarn N, Kulkarni R (2010) Silicone emulsions: interfacial aspects and applications. Househ Pers Care Today 3:35–39, 42
Huang W (2010) Silicone nonionic surfactant. Youjigui Cailiao 24(1):65–66
Rodriguez-Abreu C, Esquena J (2011) Preparation of mesoporous materials with nonhydrocarbon surfactants. In: Tadros TF (ed) Self-organized surfactant structures. Wiley, Weinheim, pp 213–238
Snow S, Stevens R (1999) The science of silicone surfactant application in the formation of polyurethane foam. In: Hill RM (ed) Silicone surfactants. Surfactant science series, vol 86. Marcel Dekker, New York, Chap 5
Bassindale AR, Gentle TE, Taylor PG, Watt A (1996) Octopus molecules based on silsesquioxane cores Tailor-made silicon-oxygen compd. In: Corriu R, Jutzi P (eds) Lect workshop, meeting date 1995. Vieweg, Wiesbaden, pp 171–176
Gentle TE, private communication
Deng J, Polidan JT, Hottle JR, Farmer-Creely CE, Viers BD, Esker AR (2002) Polyhedral oligomeric silsesquioxanes: a new class of amphiphiles at the air-water interface. J Am Chem Soc 124(51):15194–15195
Snow SA, Pernisz UC, Nugent BM, Stevens RE, Braun RJ, Naire S (2001) Modeling the stabilizing behaviour of silicone surfactants during the processing of polyurethane foam: the use of thin liquid films. In: Klempner D, Frisch KC (eds) Advances in urethane science and technology. In this document the material of interest is referred to as the Trimethylsilyl Capped Polysilicate (TCP), Chap 5
Hill RM (1999) Siloxane surfactants. In: Hill RM (ed) Silicone surfactants. Surfactant science series, vol 86. Marcel Dekker, New York, Chap 1
Omotowa BA, Shreeve JM (2003) Preparation, characterization, and thermal and surfactant studies of polyfluorinated amphiphilic carbosilane dendrimers. Macromolecules 36(22):8336–8345
Krska SW, Seyferth D (1998) Synthesis of water-soluble carbosilane dendrimers. J Am Chem Soc 120(15):3604–3612
Kim C (2009) Silyl ether containing dendrimers with cyclic siloxane cores. In: Dvornic PR, Owen MJ (eds) Silicon-containing dendritic polymers. Springer, Berlin, Chap 6
Jonas G, Stadler R (1991) Polysiloxanes with statistically distributed glucose and galactose units. I. Synthesis and thermal characterization. Makromol Chem, Rapid Commun 12(11):625–632
Jonas G, Stadler R (1994) Carbohyrate modified polysiloxanes. II. Synthesis via hydrosilylation of mono-, di- and oligosaccharide allylglycosides. Acta Polym 45(1):14–20
Akimoto T, Kawahara K, Nagase Y, Aoyagi T (2000) Preparation of oligodimethylsiloxanes with sugar moiety at a terminal group as a transdermal penetration enhancer. Macromol Chem Phys 201:2729–2734
Loos K, Jonas G, Stadler R (2001) Carbohydrate modified polysiloxanes, 3 solution properties of carbohydrate-polysiloxane conjugates in toluene. Macromol Chem Phys 202(16):3210–3218
Boysen MMK, Lindhorst TK (2003) Sugaring’ carbosilane dendrimers via hydrosilylation. Tetrahedron 59(22):3895–3898
Ogawa T (2003) Simplified synthesis of carbohydrate-functional siloxanes via transacetalation. I. Glucose-functional siloxanes. J Polym Sci A Polym Chem 41(21):3336–3345
Ogawa T (2003) Simplified synthesis of amphiphilic siloxanes with methyl gluconyl glycinate functionalities via transacetalation. Macromolecules 36(22):8330–8335
Brandstadt KF, Gross RA, Lane TH (2004) New organosilicon carbohydrate compound for use in forming gels, fibers, films, or coatings. US Patent 7,078,519
Gross RA, Kalra B, Kumar A (2004) Enzymatic condensation polymerization used to prepare polyester-containing polymers, comprises combining enzyme, compound consisting of diols and polyols, and diacid in reaction vessel, and heating vessel to preselected temperature. US Patent 6,972,315
Henkensmeier D, Abele BC, Candussio A, Thiem J (2004) Synthesis and characterization of terminal carbohydrate modified poly(dimethylsiloxane)s. Macromol Chem Phys 205(14):1851–1857
Sahoo B, Brandstadt KF, Lane TH, Gross RA (2005) Sweet silicones: biocatalytic reactions to form organosilicon carbohydrate macromers. Organic Lett 7(18):3857–3860
Henkensmeier D, Abele BC, Candussio A, Thiem J (2005) Synthesis of carbohydrate-segmented polydimethylsiloxanes by hydrosilylation. J Polym Sci A Polym Chem 43(17):3814–3822
Racles C, Hamaide T (2005) Synthesis and characterization of water-soluble saccharide functionalized polysiloxanes and their use as polymer surfactants for the stabilization of polycaprolactone nanoparticles. Macromol Chem Phys 206:1757–1768
Carillo FV, Costello M, Creutz SFA, Deklippel L, Henault B, Joffre EJ, McAuliffe JC, O’Neil VK, Simon C (2006) Surface treatment composition use as fabric treatment composition, comprises saccharide-siloxane copolymer(s), which is reaction product of functionalized organosiloxane polymer and hydroxyfunctional saccharide(s). Patent applications WO2006127882, US0683589, US0915007
Joffre EJ, Johnson BK, Starch MS, Swanton BJ (2006) Personal care composition for personal care product for hair, and skin, i.e. antiperspirant, comprises saccharide-siloxane copolymer(s) having a saccharide component and an organosiloxane component and linked by linking group. Patent applications WO2006127883, US0683590, US0915051
Joffre EJ, McAuliffe JC (2006) New ionically-modified saccharide siloxane copolymer useful in e.g. personal care product comprises saccharide and organosiloxane component, and is prepared by reaction of saccharide siloxane copolymer with ionic monomer/oligomer. Patent applications WO2006127924, US0683718 US0915077
Joffre EJ, Kollar C, McAuliffe JC (2006) Cross-linkable composition for use as adhesive release coatings on paper, as wood water repellents or as wound dressings, comprises saccharide-siloxane copolymer, cross-linking agent and optionally solvent. Patent applications WO2006071772, US0638871, US0793067
McAuliffe JC, Smith WC, Starch MS (2006) Saccharide compound for cosmetic and personal care formulations comprises ester derivative of ascorbic acid or 2-keto acid saccharide, where ester has been introduced by ester bond formation between ascorbic acid or 2-keto acid saccharide. Patent applications WO2006066227, US0636567, US0636567, US0792460
Canfield L, Debdi N, Lavaux V, Starch MS, Van Reeth I (2006) Cosmetic, veterinary, pharmaceutical or therapeutic composition comprises ionic cross-linked polymer as thickening agent, water-in-oil emulsifying agent, silicone material, active agent, water and silicone-based emulsifying agent. Patent GB2422605
Racles C, Hamaide T, Ioanid A (2006) Siloxane surfactants in polymer nanoparticles formulation. Appl Organomet Chem 20:235–245
Wang GY, Du ZP, Li QX, Zhang W (2010) Carbohydrate modified siloxane surfactants and their adsorption and aggregation behavior in aqueous solution. J Phys Chem B 114:6872–6876
Han F, Zhang G (2003) New family of siloxane surfactants having glucosamide. Tenside Surfactants Deterg 40(6):332–337
Haupt M, Knaus S, Rohr T, Gruber H (2000) Carbohydrate modified polydimethylsiloxanes. Part 1. Synthesis and characterization of carbohydrate silane and siloxane building blocks. J Macromol Sci, Part A, Pure Appl Chem A37(4):323–341
Schmaucks G (1999) Novel siloxane surfactant structures. In: Hill RM (ed) Silicone surfactants. Surfactant science series, vol 86. Marcel Dekker, New York, Chap 3
Maki H, Horiguchi Y, Suga T, Komori S (1970) Syntheses and properties of surfactants containing organometallic compounds. VI. Syntheses and properties of surfactants with three-chained hydrophobic groups containing organotin and organosilicon compounds. Yukagaku 19(4):245
Maki H, Horiguchi Y, Suga T, Komori S (1970) Syntheses and properties of organometallic surfactants. VII. Cationic surfactants containing polydimethylsiloxane. Yukagaku 19(11):1029
Azechi S, Meguriya N, Tanaka M (1989) Cationic silicone surfactant and method of its manufacture. US Patent 5,124,466
Schmaucks G, Sonnek G, Wuestneck R, Herbst M, Ramm M (1992) Effect of siloxanyl groups on the interfacial behavior of quaternary ammonium compounds. Langmuir 8(7):1724
Snow SA (1993) Synthesis and characterization of cationic siloxane surfactants (Me3SiO)2Si(Me)-(CH2)3NMe2(CH2)2OR+ X. Langmuir 9(2):424
Hill RM, Snow SA (1993) Cationic diquaternary ammonium salt functional silicones. US Patent 5,235,082
Wagner R, Sonnek G (1994) Dicyclopentadienyl units containing silicone surfactants. In: Auner N, Weis J (eds) Organosilicon chem. VCH, Weinheim, pp 267–268
Kuo P-L, Hou S-S, Teng C-K, Liang W-J (2001) Function and performance of silicone copolymer (VI). Synthesis and novel solution behavior of water-soluble polysiloxanes with different hydrophiles. Colloid Polym Sci 279(3):286–291
Cheng J, Wang X, Wu Q, Gan G (2002) Synthesis of polyether-modified silicone quaternary ammonium salt and its properties. Youjigui Cailiao 16(2):10–13
Coo-Ranger JJ, Zelisko PM, Brook MA (2004) Ionic silicone surfactants in water-in-silicone oil emulsions containing proteins. Papers Present Meet - Am Chem Soc, Div Polym Chem 45(1):674–675
Klein KD, Schaefer D, Lersch P (1994) Anionic silicone surfactants. Tenside Surfactants Deterg 31(2):115–119
Renauld F, Colas AR (1988) Organosilicon sulfosuccinate(s) preparation by reaction of organosilicon compounds with base and sodium bisulfite, useful as surfactants. US Patent 4,777,277
Renauld F, Colas AR, Sawick GC (1988) Surface active silicon compound. GB Patent 2,203,152
Renauld F, Colas AR (1988) Preparation of organo-silicon compounds having sulphoxide-containingt hydrocarbon groups from sodium periodate and silane or organo-siloxane, used for textile softeners and antistatic treatments. GB Patent 2,223,232
Azechi S, Meguriya N, Tanaka M (1989) Anionic silicone surfactant and method of its manufacture. US Patent 5,068.380
Huang W (2005) Silicone surfactant with special structure. Youjigui Cailiao 19(3):48–51
Snow SA, Fenton WN, Owen MJ (1991) Zwitterionic organofunctional siloxanes as aqueous surfactants: synthesis and characterization of betaine functional siloxanes. Langmuir 7(5):868
Snow SA, Fenton WN, Owen MJ (1990) Synthesis and characterization of zwitterionic silicone sulfobetaine surfactants. Langmuir 6(2):385
Eaborn C (1960) Organosilicon compounds. Butterworth Publications. The authors were able to obtain a copy form the service. “Out of Print Books on Demand” from University Microfilms International
Noll W (1968) Chemistry and technology of silicones
Brook MA (2000) Silicon in organic, organometallic and polymer chemistry
Snow SA, Fenton WN, Owen MJ (1990) Synthesis and characterization of zwitterionic silicone sulfobetaine surfactants. Langmuir 6(2):385
Pricop L, Hamciuc V, Marcu M (2002) Siloxane surfactants. Mater Plast 39(4):213–216
LeGrow GE, Petroff LJ (1999) Silicone polyether copolymers: synthetic methods and chemical compositions. In: Hill RM (ed) Silicone surfactants. Surfactant science series, vol 86. Marcel Dekker, New York, Chap 2
The silicones environmental, health and safety council of north America, materials handling guide: hydrogen-bonded silicon compounds (http://www.sehsc.com/PDFs/SiHManual Revised 01 Aug 07.pdf)
Maki H, Horiguchi Y, Suga T, Komori S (1970) Syntheses and properties of surfactants containing organometallic compounds. VI. Syntheses and properties of surfactants with three-chained hydrophobic groups containing organotin and organosilicon compounds. Yukagaku 19(4):245
Maki H, Horiguchi Y, Suga T, Komori S (1970) Syntheses and properties of organometallic surfactants VII. Cationic surfactants containing polydimethylsiloxane. Yukagaku 19(11):1029
Snow SA, Fenton WN, Owen MJ (1990) Synthesis and characterization of zwitterionic silicone sulfobetaine surfactants. Langmuir 6(2):385
Snow SA, Fenton WN, Owen MJ (1991) Zwitterionic organofunctional siloxanes as aqueous surfactants: synthesis and characterization of betaine functional siloxanes. Langmuir 7(5):868
Schmaucks G, Sonnek G, Wuestneck R, Herbst M, Ramm M (1992) Effect of siloxanyl groups on the interfacial behavior of quaternary ammonium compounds. Langmuir 8(7):1724
Snow SA (1993) Synthesis, characterization of cationic siloxane surfactants (Me3SiO)2Si(Me)-(CH2)3NMe2(CH2)2OR+ X. Langmuir 9(2):424
Schmaucks G (1999) Novel siloxane surfactant structures. In: Hill RM (ed) Silicone surfactants. Surfactant science series, vol 86. Marcel Dekker, New York, Chap 3
Cheng J, Wang X, Wu Q, Gan G (2002) Synthesis of polyether-modified silicone quaternary ammonium salt and its properties. Youjigui Cailiao 16(2):10–13
Snow SA (1993) Synthesis, characterization of cationic siloxane surfactants (Me3SiO)2Si(Me)-(CH2)3NMe2(CH2)2OR+ X. Langmuir 9(2):424
Colas AR, Renauld FA (1988) Organosilicon sulfosuccinate(s) preparation by reaction of organosilicon compounds with base and sodium bisulfite, useful as surfactants. US Patent 4,777,277
Hoffmann H, Ulbricht W (1999) Surface activity and aggregation behavior of siloxane surfactants. In: Hill RM (ed) Silicone surfactants. Surfactant science series, vol 86. Marcel Dekker, New York, Chap 4
Kuo P-L, Hou S-S, Teng C-K, Liang W-J (2001) Function and performance of silicone copolymer (VI). Synthesis and novel solution behavior of water-soluble polysiloxanes with different hydrophiles. Colloid Polym Sci 279(3):286–291
Wang G, Qu W, Du Z, Cao Q, Li Q (2011) Adsorption and aggregation behavior of tetrasiloxane-tailed surfactants containing oligo(ethylene oxide) methyl ether and a sugar moiety. J Phys Chem B 115(14):3811–3818
Wang GY, Du ZP, Li QX, Zhang W (2010) Carbohydrate modified siloxane surfactants and their adsorption and aggregation behavior in aqueous solution. J Phys Chem B 114:6872–6876
Kim D, Lim C, Choi J, Noh S (2004) Surface active properties and LCST behavior of oligo(propylene oxide-blockethylene oxide) allyl ether siloxane surfactants in aqueous solution. Bull Korean Chem Soc 25(8):1182–1188
Kim D, Noh S, Jo B (2006) Effect of salt and pH on surface active properties of comb rake-type polysiloxane surfactants. Colloids Surf A 287(1–3):106–116
Wang W, Lu Y, Cai Z (2010) Surface characters of methylsiloxane-oxyalkylene copolymers. Jingxi Huagong 27(3):229–233
Owen MJ (1980) The surface activity of silicones: a short review. Ind Eng Chem Prod Res Dev 19:97
Owen MJ (1980) The surface activity of silicones: a short review. Ind Eng Chem Prod Res Dev 19:97
Hoffmann H, Ulbricht W (1999) Surface activity and aggregation behavior of siloxane surfactants. In: Hill RM (ed) Silicone surfactants. Surfactant science series, vol 86. Marcel Dekker, New York, Chap 4
Snow SA, Stevens RE (1999) The science of silicone surfactant application in the formation of polyurethane foam. In: Hill RM (ed) Silicone surfactants. Surfactant science series, vol 86. Marcel Dekker, New York, Chap 5
Snow SA, Pernisz UC, Braun RJ (2006) Tying up loose ends silicone surfactants as stabilizing agents for flexible polyurethane foam. Silicon Chem 3(1/2):1–10
Fawcett AS, So HY, Brook MA (2010) Silicone foams stabilized by surfactants generated in situ from allyl-functionalized PEG. Soft Matter 6:1229–1237
Hoffmann H, Ulbricht W (1999) Surface activity and aggregation behavior of siloxane surfactants. In: Hill RM (ed) Silicone surfactants. Surfactant science series, vol 86. Marcel Dekker, New York, Chap 4
Gentle TE, Snow SA (1995) Adsorption of small silicone polyether surfactants at the air/water interface. Langmuir 11:2905–2910
Wang G, Qu W, Du Z, Cao Q, Li Q (2011) Adsorption and aggregation behavior of tetrasiloxane-tailed surfactants containing oligo(ethylene oxide) methyl ether and a sugar moiety. J Phys Chem B 115(14):3811–3818
Wang GY, Du ZP, Li QX, Zhang W (2010) Carbohydrate modified siloxane surfactants and their adsorption and aggregation behavior in aqueous solution. J Phys Chem B 114:6872–6876
Kuo P-L, Hou S-S, Teng C-K, Liang W-J (2001) Function and performance of silicone copolymer (VI). Synthesis and novel solution behavior of water-soluble polysiloxanes with different hydrophiles. Colloid Polym Sci 279(3):286–291
Deng J, Polidan JT Hottle JR, Farmer-Creely CE, Viers BD, AR Esker (2002) Polyhedral oligomeric silsesquioxanes: a new class of amphiphiles at the air/water. Interface (J Am Chem Soc) 124(51):15194–15195
Snow SA, Pernisz UC, Nugent BM, Stevens RE, Braun RJ, Naire S (2001) Modeling the stabilizing behaviour of silicone surfactants during the processing of polyurethane foam: the use of thin liquid films. In: Klempner D, Frisch KC (eds) Advances in urethane science and technology. In this document the material of interest is referred to as the trimethylsilyl capped polysilicate (TCP). Chap 5
Snow SA, Pernisz UC, Nugent BM, Stevens RE, Braun RJ, Naire S (2001) Modeling the stabilizing behaviour of silicone surfactants during the processing of polyurethane foam: the use of thin liquid films. In: Klempner D, Frisch KC (eds) Advances in urethane science and technology. Chap 5
Snow SA, Pernisz UC, Braun RJ (2006) Tying up loose ends silicone surfactants as stabilizing agents for flexible polyurethane foam. Silicon Chem 3(1/2):1–10
Snow SA, Pernisz UC, Stevens RE (1998) Thin liquid model polyurethane films. In: Polyurethanes world congress, pp 1–10
Braun RJ, Snow SA, Naire S (2002) Models for gravitationally-driven free-film drainage. J Eng Math 43:281–314
Mysels KJ, Shinoda K, Frankel S (1959) Soap films and studies of their thinning. Pergamon, Elmsford
Anseth JW, Bialek A, Hill RM, Fuller GG (2003) Interfacial rheology of graft-type polymeric siloxane surfactants. Langmuir 19(16):6349–6356
Mehta SC, Somasundaran P (2007) Modification in rheological properties due to charged network of ionic silicone surfactants at water-oil interface. Abstracts of papers. In: 233rd ACS national meeting, Chicago, IL, United States
Fawcett AS, So HY, Brook MA (2010) Silicone foams stabilized by surfactants generated in situ from allyl-functionalized PEG. Soft Matter 6(6):1229–1237
Wang A, Jiang L, Mao G, Liu Y (2001) Direct force measurement of comb silicone surfactants in alcoholic media by atomic force microscopy. J Colloid Interface Sci 242(2):337–345
Wang A, Jiang L, Mao G, Liu Y (2002) Direct force measurement of silicone and hydrocarbon-based ABA triblock surfactants in alcoholic media by atomic force microscopy. J Colloid Interface Sci 256(2):331–340
Suitthimeathegorn O, Jaitely V, Florence T (2005) Novel anhydrous emulsions: formulation as controlled release vehicles. Int J Pharm 298(2):367–371
Liu X, Song J, Wu D, Genzer J, Theyson T, Rojas OJ (2010) Surface and friction behavior of a silicone surfactant adsorbed on model textiles substrates. Ind Eng Chem Res 49(18):8550–8557
Snow SA, Pernisz UC, Nugent BM, Stevens RE, Braun RJ, Naire S (2001) Modeling the stabilizing behaviour of silicone surfactants during the processing of polyurethane foam: the use of thin liquid film. In: Klempner D, Frisch KC (eds) Advances in urethane science and technology. Chap 5
Kuo P-L, Hou S-S, Teng C-K, Liang W-J (2001) Function and performance of silicone copolymer (VI). Synthesis and novel solution behavior of water-soluble polysiloxanes with different hydrophiles. Colloid Polym Sci 279(3):286–291
Stoebe T, Hill RM, Ward MD, Scriven LE, Davis HT (1999) Surfactant-enhanced spreading. In: Hill RM (ed) Silicone surfactants. Surfactant science series, vol 86. Marcel Dekker, New York, Chap 11
De Ruijter MJ (2000) The role of surfactants in dynamic wetting. Annu Surfactants Rev 3(Surface Active Behaviour of Performance Surfactants):169–188
Wagner R, Wu Y, Czichocki G, Berlepsch HV, Weiland B, Rexin F, Perepelittchenko L (1999) Silicon-modified surfactants and wetting: I. Synthesis of the single components of Silwet L77 and their spreading performance on a low-energy solid surface. Appl Organomet Chem 13(9):611–620
Unpublished data of the Dow Corning Corporation
Venzmer J, Wilkowski SP (2000) Trisiloxane surfactants-mechanisms of wetting and spreading. In: Auner N, Weis J (eds) Organosilicon chem IV lect poster contrib muenchner silicontage meeting 1998. Wiley-VCH, Weinheim, pp 690–698
Nikolov AD, Wasan DT, Chengara A, Koczo K, Policello GA, Kolossvary I (2002) Superspreading driven by Marangoni flow. Adv Colloid Interface Sci 96(1-3):325–338
Wagner R, Wu Y, Berlepsch HV, Rexin F, Rexin T, Perepelittchenko L (1999) Silicon-modified surfactants and wetting: III. The spreading behavior of equimolar mixtures of nonionic trisiloxane surfactants on a low-energy solid surface. Appl Organomet Chem 13(9):621–630
Peng Z, Lu C, Xu M (2010) Influence of substructures on the spreading ability and hydrolysis resistance of double-tail trisiloxane surfactants. J Surfactants Deterg 11(1):75–81
Nikolov AD, Wasan DT, Chengara A, Koczo K, Policello GA, Kolossvary I (2002) Superspreading driven by Marangoni flow. Adv Colloid Interface Sci 96(1-3):325–338
Peng Z, Lu C, Xu M (2010) Influence of substructures on the spreading ability and hydrolysis resistance of double-tail trisiloxane surfactants. J Surfactants Deterg 13(1):75–81
Policello GA, Leatherman MD, Peng W, Rajaraman SK, Xia Z (2007) Hydrolysis-resistant organo modified trisiloxane surfactants and aqueous emulsion incorporating surfactants. US Patent Application, Publ 17 pp
Lin L, Chen K (2006) Surface activity and water repellency properties of cleavable-modified silicone surfactants. Colloids Surf A 275(1–3):99–106
Lin L, Wang C, Chen K (2006) Water-repellency and antibacterial activities of plasma-treated cleavable silicone surfactants on nylon fabrics. Surf Coat Technol 201(3–4):674–678
Lin L, Wang C, Chen C, Chen K (2006) Water-repellency and antibacterial activities of plasma-treated cleavable silicone surfactants on nylon fabrics. Surf Coat Technol 201(3–4):674–678
Tanford C (1980) The hydrophobic effect. Wiley, New York
Soni SS, Sastry NV, Aswal VK, Goyal PS (2002) Micellar structure of silicone surfactants in water from surface activity, SANS and viscosity studies. J Phys Chem B 106(10):2606–2617
Soni SS, Sastry NV, Joshi JV, Seth E, Goyal PS (2003) Study on the effects of nonelectrolyte additives on the phase, thermodynamics, and structural changes in micelles of silicone surfactants in aqueous solutions from surface activity, small angle neutron scattering, and viscosity measurements. Langmuir 19(17):6668–6677
Soni SS, Sastry NV, George J, Bohidar HB (2003) Dynamic light scattering and viscosity studies on the association behavior of silicone surfactants in aqueous solutions. J Phys Chem B 107(22):5382–5390
Lin Y, Alexandridis P (2003) Association of siloxane polymeric surfactants in aqueous solution. In: Synthesis and properties of silicones and silicone-modified materials. ACS symposium series, vol 838, pp 222–234
Ahn S, Alexandridis P (2001) Phase behavior and structural characterization of trisiloxane surfactant—water-silicone oil systems. Papers Present Meet - Am Chem Soc, Div Polym Chem 42(1):169–170
Lin Z, He M, Scriven LE, Davis HT, Snow SA (1993) Vesicle formation in electrolyte solutions of a new cationic siloxane surfactant. J Phys Chem 97:3571
Hill RM, Lin Z, He M, Scriven LE, Davis HT (1993) Lyotropic liquid crystal phase behavior of polymeric siloxane surfactants. Langmuir 9(11):2789–2798
Lin Z, He M, Scriven LE, Davis HT, Snow SA (1994) Aggregation behavior and microstructures of cationic trisiloxane surfactants in aqueous solutions. J Phys Chem 98:6148
Hoffmann H, Munkert U, Thunig C, Valiente M (1994) Altering the rheological properties of silicone surfactant vesicles. J Colloid Interface Sci 163:217
Hill RM, Lin Z, He M, Scriven LE, Davis HT, Talmon Y (1994) Cryo transmission electron microscopy study of vesicles and micelles in siloxane surfactant aqueous solutions. Langmuir 10(4):1008–1011
Hill RM, Lin Z, He M, Scriven LE, Davis HT (1994) Comparison of the liquid crystal phase behavior of four trisiloxane superwetter surfactants. Langmuir 10(6):1724–1734
Hill RM, Snow SA (1994) Silicone vesicles and entrapment. US Patent 5,364,633
Hill RM, Snow SA (1995) Silicone vesicles and entrapment. US Patent 5,411,744
Kickelbick G, Bauer J, Huesing N, Andersson M, Holmberg K (2003) Aggregation behavior of short-chain PDMS-b-PEO diblock copolymers in aqueous solutions. Langmuir 19:10073–10076
Kickelbick G, Bauer J, Husing N, Andersson M, Palmqvist A (2003) Spontaneous vesicle formation of short-chain amphiphilic polysiloxane-b-poly(ethylene oxide) block copolymers. Langmuir 19:3198–3201
Yao D, Bender T, Gerroir PJ, Sundararajan PR (2005) Self-assembled vesicular nanostructures of perylene end-capped poly(dimethylsiloxane). Macromolecules 38(16):6972–6978
Yan Y, Hoffmann H, Drechsler M, Talmon Y, Makarsky E (2006) Influence of a hydrocarbon surfactant on the aggregation behavior of a silicone surfactant: observation of intermediate structures in the vesicle-micelle transition. J Phys Chem B 110(11):5621–5626
Wang GY, Du ZP, Li QX, Zhang W (2010) Carbohydrate modified siloxane surfactants and their adsorption and aggregation behavior in aqueous solution. J Phys Chem B 114:6872–6876
Wang G, Qu W, Du Z, Cao Q, Li Q (2011) Adsorption and aggregation behavior of tetrasiloxane-tailed surfactants containing oligo(ethylene oxide) methyl ether and a sugar moiety. J Phys Chem B 115(14):3811–3818
Postiaux S, Lin S (2005) Preparation of vesicle composition comprises combining organopolysiloxane, water miscible volatile solvent, optionally silicone or organic oil and personal care or health care active with water and mixing the obtained aqueous dispersion. World Patent Filing # WO2005102248
Lin S (2005) Preparing vesicle composition useful for personal and health care product involves mixing organopolysiloxane, water miscible volatile solvent and water to form vesicles, and optionally removing the solvent from the vesicles. World Patent Filing # WO2005103157
Lin S, Nguyen K (2005) Aqueous composition for use in making personal, household, and health care composition used in, e.g. antiperspirants, deodorants, comprises dispersed particles with block silicon polyether copolymer. World Patent Filing WO2005103118
Lin S, Leaym T (2006) Polyoxyalkylene-alkyl functional siloxane resin for manufacturing aqueous dispersion, vesicle composition, and aqueous composition for entrapment and delivery of personal, household, and healthcare composition, comprises siloxy units. World Patent Filing WO2006091295
Lin S, Newton J, Postiaux S, Thompson J (2007) Preparation of vesicle composition useful in personal care product e.g. deodorant involves mixing dispersions of organopolysiloxane having hydrophilic substituent with hydrophilic active and hydrophobic active component and mixing water. World Patent Filing WO2007053424
Kickelbick G, Bauer J, Huesing N, Andersson M, Holmberg K (2003) Aggregation behavior of short-chain PDMS-b-PEO diblock copolymers in aqueous solutions. Langmuir 19:10073–10076
Kickelbick G, Bauer J, Husing N, Andersson M, Palmqvist A (2003) Spontaneous vesicle formation of short-chain amphiphilic polysiloxane-b-poly(ethylene oxide) block copolymers. Langmuir 19:3198–3201
Kumar A, Uddin MH, Kunieda H, Furukawa H, Harashima A (2001) Solubilization enhancing effect of A-B-type silicone surfactants in microemulsions. J Disp Sci Tech 22(2&3):245–253
Xu A, Yu JC, Zhang H, Zhang L, Kuang D, Fang Y (2003) Continuous formation of supported unusual mesostructured silica films by sol–gel dip coating. Langmuir 18(24):9570–9573
Xu A (2002) Novel surfactants for the synthesis of unusual highly ordered lamellar oxides. J Phys Chem B 106(45):11713–11715
Xu A (2002) Synthesis of highly ordered long-range lamellar silica composites. Chem Lett 9:878–879
Xu A, Cai Y, Zhang H, Zhang L, Yu JC (2002) Hierarchically ordered silica mesophases using mixed surfactant systems as templates. Angew Chem, Int Ed Engl 41(20):3844–3848
Xu A (2002) Synthesis of mesostructured silica using nonionic copolymers as the templates. Chem Lett 10:982–983
Xu A, Yu JC, Cai Y, Zhang H, Zhang L (2002) The preparation of a highly ordered long-range lamellar silica structure with large interlayer spacings. Chem Commun 15:1614–1615
Xu A (2002) Highly ordered lamellar silica/surfactant composites templated from nonionic amphiphilic copolymer. Chem Mater 14(9):3625–3627
Xu A, Yu JC, Zhang H, Zhang L, Kuang D, Fang Y (2003) Continuous formation of supported unusual mesostructured silica films by sol–gel dip coating. Langmuir 18(24):9570–9573
Gradzielski M, Hoffmann H, Robisch P, Ulbricht W, Gruning B (1990) The aggregation behaviour of silicone surfactants in aqueous solutions. Tenside Surfactants Deterg 27:366–379
Schmaucks G, Sonnek G, Wfistneck R, Herbst M, Ramm M (1992) Effect of siloxanyl groups on the interfacial behavior of quaternary ammonium compounds. Langmuir 8:1724–1730
Hill RM, He M, Lin Z, Davis HT, Scriven LE (1993) Lyotropic liquid crystal phase behavior of polymeric siloxane surfactants. Langmuir 9:2789–2798
Iwanaga T, Kunieda H (2000) Effect of added salts or polyols on the cloud point and the liquid-crystalline structures of polyoxyethylene-modified silicone. J Colloid Interface Sci 227(2):349–355
Kunieda H, Uddin MH, Horii M, Furukawa H, Harashima A (2001) Effect of hydrophilic- and hydrophobic chain lengths on the phase behavior of A–B-type silicone surfactants in water. J Phys Chem B 105(23):5419–5426
Floyd DT (1999) Silicone surfactants: Applications in the personal care Industry. In: Hill RM (ed) Silicone surfactants. Surfactant science series, vol 86. Marcel Dekker, New York, Chap 7
O’Lenick AJ, O’Lenick K (2010) Formulating with surfactant silicones. Cosmet Toilet 125(1):44–49
O’Lenick AJ (2001) PEG/PPG dimethicone: a new name for an old friend. Cosmet Toilet 116(7):49–52
Keil JW (1979) US Patent 4,265,878 and 4,268,499 antiperspirant stick compositions and antiperspirant emulsion compositions
Starch MS (1979) US Patent 4,311,695, Personal care emulsions comprising a siloxane-oxyalkylene copolymer, December
Gruning B, Bungard A (1999) Silicone surfactants: Emulsification. In: Hill RM (ed) Silicone surfactants. Surfactant science series, vol 86. Marcel Dekker, New York, Chap 8
O’Lenick AJ (1979) Applying the three dimensional HLB system. Cosmet Toilet 112(11):59–60, 65
Zombeck A, Dahms G (1996) Novel formulations based on non-aqueous emulsions of polyols in silicones. In: 19th IFSCC Congress, Sydney
Dahms G, Zombeck A (1995) New formulation possibilities offered by silicone co-polyols. Cosmet Toilet 110(3):91–100
Keil J (1985) US Patent 4,532,132
Araki et al. (2009) In: 9th ASCS conference, Oral Presentation #148
Koini T, Dahms G (2003) O/W Emulsions US Patent Pub No 2003/0202948 A1
Dimitrova T, Saulnier L, Verhelst V, Van Reeth I (2011) Silicone polyethers as stabilizers of water-in-oil emulsions. In: Morgan S, Lochhead R (eds) Polymeric delivery of therapeutics. ACS symposium series, vol 1053. Am Chem Soc, Washington
Dimitrova T, Saulnier L, Van Reeth I, Verhelst V (2008) Stabilization of cosmetic formulations by silicone polyethers. In: 25th ISSCC congress, Barcelona, October 2008
Van Reeth I, Bao X, Durand B, Vervier I, Yasuhiro K, Devalle C (2010) Silicone emulsifiers: new developments and formulation concepts. In: 26th IFSCC congress, Buenos Aires, poster 0050
Van Reeth I, Van Oycke S, Kondo H (2006) New developments in water-in-silicone and water-in-oil silicone based emulsifiers. In: 24th IFSCC congress, Osaka, PC-070
Yahagi K (1992) Silicones as conditioning agents in shampoos. J Soc Cosmet Chem 43(5):275–284
Ostergaard T, Gomes A, Quackenbush K, Johnson B (2004) Silicone quaternary microemulsions: a multifunctional product for hair care. Cosmet Toilet 119(11):45–48, 50, 52
Philip A (1988) Formulating for a close shave. Cosmet Toilet 59(10):53–59
Easton T, Stephens D (1995) Silicone surfactants as performance enhancers in waterborne coatings. Polym Paint Colours J 185(4371):26, 28–30
Perry D (2001) Glorious speed. Polym Paint Colours J September:16–19
Ferritto M, Fornier F, Stanga M, Verineau P, Whitmarsh R, Witucki G (2007) WO patent 2007075927
Semmler H, Heilen W (2000) Silicones fight foam formation. Polym Paint Colours J 190(4431):16–18
Van Dam P (2001) Ending bubble trouble. Polym Paint Colours J 191(4441):30–33
O’Neil V, Zeng J Perry D (2003) New silicone foam control agents for waterborne coatings. Paint and Coatings Industry, BNP Media, October
Fey R, Hill RM (1999) Silicone polymers for foam control and demulsification. In: Hill RM (ed) Silicone surfactants, surfactant science series, vol 86. Marcel Dekker, New York, Chap 6
McGee J, Petroff L, Aizawa K, Shoji H (1995) Silicone foam control compositions. US patent 5,380,464
McGee J, Petroff L, Brecht D, Ollinger W (1996) Silicone foam control compositions, US patent 5,543,082
Tonge L, Kidera H, Okada R, Noro T, Harkness B (2003) US patent application 20030013808 A1
Elms R, Lin F, Severance M (2004) Silicone based foam control compositions stable in detergents EP 1 167 502 A1
Henning J, Muller F, Peggau J (1999) Novel applications of silicone surfactants in cleansers and polishes. Commun J Comm Esp Deterg 29:235–246
Panandiker RJ, Rajan K, Vetter K, Barnabas F, Delplancke P (2009) Fabric care compositions and systems comprising organosilicone microemulsions and methods employing same. US patent 7,608,575 B2
Panandiker R, Vetter K, Combs M, Gladney D, Sheets C (2010) Fabric care composition comprising organosilicone microemulsion and anionic/nitrogen containing surfactant system. US patent 7,678,752 B2
Achwal W (2002) Hydrophilic silicone based softeners. Colourage 49(6):58–59
Czech A (2002) Block, non-(AB) n silicone polyalkyleneoxide copolymers with tertiary amino links. US patent 6,475,568
Schwab P, Favresse P, Maurer T, Pascaly M (2010) Silicone containing graft copolymers of blockwise structure. US patent 7,838,603 B2
Kennan J, Lewis K, Vazquez F (2010) Silicone polyether block copolymers having organofunctional endblocking groups. US patent publication US 2010.0048795 A1
Sabia A, Metzler R (1983) The role of silicones in nonwoven fabric applications. Nonwovens Ind 14(9):16–22
Casado-Dominguez A, Goossens E, Hubesch B (2003) Process for preparing an organomodified-silicone by hydrosilylation reaction. US patent application 20030232947 A1
Robinson P, Dolbear G (2006) Hydrotreating and hydrocracking: fundamentals. In: Hsu C, Robinson P (eds) Practical advances in petroleum processing, vol 1. Springer, Berlin, pp 177–217, Chap 7
Hill RM, Fey KC (1999) Silicone polymers for foam control and demulsification. In: Hill RM (ed) Silicone surfactants. Surfactant science series, vol 86. Marcel Dekker, New York, Chap 6
Callagahan I, Gould C, Grabowski W (1987) Method for the separation of gas from oil. US patent 4,711,714
Dalmazzone Noik C (2005) Mechanism of crude-oil/water interface destabilization by silicone demulsifiers. SPE J March:44–53
Owen MJ (1972) Siloxane surfactants as demulsifiers. In: Proc VI int cong surf act, Switzerland, pp 623–630
Theile H, Hoffman H, Rossmy G, Koerner G, Zaske P (1980) Use of demulsifying mixtures for breaking petroleum emulsions. US Patent 4,183,820
Fink H, Koerner G, Rossmy G (1977) De-emulsifier for breaking petroleum emulsions. US Patent 4,029,596
Graham D, Lidy W, McGrath P, Thompson R (1986) Demulsification process. US patent 4,596,653
David D, Le Folletec A, Pezreon I, Dalmazzone C, Noik C, Barre L, Komunjer L (2008) Destabilization of water in crude oil emulsions by silicone copolymer demulsifiers. Oil Gas Sci Technol Rev IFP 63(1):165–173
Phukan M, Koczo K, Falk B, Palumbo A (2010) New silicon copolymers for efficient demulsification. SPE paper 128553, SPE oil and gas conference, Mumbai, India
Alen R (2000) Basic chemistry of wood delignification. In: Gullichsen J, Paulapuro H, Stenium P (eds) Forest products chemistry. Papermaking science and technology, vol. 3, pp 59–103. Tappi Fapet Oy, Chap 2
Burger W, Beubig O, Lappalainen K, Wahlberg H (2003) Chemical digestion process using organosilicone compounds. US Patent 6,521,084
Habermehl J (2005) Silicone processing. Pulp paper technol, summer:59–62
Wilson D (2005) Silicone’s applications. Pulp paper technol, summer:37–40
Ikeda T, Takewaki K (2006) Defoaming composition. Japan patent 2006320837
Pease J, McKendree G (2009) Felt and equipment surface conditioner. US patent 7,534,324
Nellesen B, Northfleet C (2006) Method of deinking. US patent publication 2006/0102298
Nellesen B (2006) Practical experience with the use of silicone derivatives for the detachment and removal of ink. In: Proceedings of 12th PTS/CTP deinking symposium, Leipzig, 25–17 April 2006
Battice D, Fey K, Petroff L, Stanga M (1998) Silicone foam control agents for hydrocarbon liquids—displays consistent compatibility and miscibility with other frequently present fuel additives, especially in diesel or jet fuels. US patent 5,767,192
Fey KC, Combs CS (1995) Middle distillate hydrocarbon foam control agents from cross-linked organopolysiloxane-polyoxyalkylenes. US patent 5,397,367
Penner D, Burow R, Roggenbuck F (1999) Use of organosilicone surfactants as agricultural adjuvants. In: Hill R (ed) Silicone surfactants. Surfactant science series, vol 86. Marcel Dekker, New York, Chap 9
Leatherman M, Policello G, Peng W, Zheng L, Wagner R, Rajaraman S, Xi Z (2009) Hydrolysis resistant organomodified disiloxane ionic surfactant. US patent publication 2009/0173913
Leatherman M, Policello G, Peng W, Zheng L, Wagner R, Rajaraman S, Xia Z (2009) Mixtures of hydrolysis resistant organomodified trisiloxane ionic surfactants. US Patent Publication 2009/0173912
Klein K, Wilkowski S, Selby J (1995) Silane surfactants, novel adjuvants for agricultural applications. In: Gaskin R (ed) 4th international symposium on adjuvants for agrichemicals, Oct 3–6, Melbourne, Aus. NZ Forest Research Institute Bulletin, vol 193, pp 27–31
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Petroff, L.J., Snow, S.A. (2012). Silicone Surfactants. In: Owen, M., Dvornic, P. (eds) Silicone Surface Science. Advances in Silicon Science, vol 4. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-3876-8_9
Download citation
DOI: https://doi.org/10.1007/978-94-007-3876-8_9
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-3875-1
Online ISBN: 978-94-007-3876-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)